Abstract
This study explored the effects of different doses of adenine intake on mice in terms of kidney function, oxidative stress and gut content microbiota to elucidate interactions between adenine-induced kidney function impairment and gut content microbiota disorder. Mice were gavaged with low-dosage adenine suspension (NML), middle-dosage adenine suspension (NMM), high-dosage adenine suspension (NMH) and sterile water (NC). Behaviour, kidney structure and function, colonic structure, oxidative stress and gut content microbiota were detected. Mice in NML, NMM, and NMH groups had significantly lower body weight, anal temperature and food intake, increased water intake, the mice had loose and deformed feces with obvious water stains through the paper. NMM mice presented significantly structural damage to kidney and colonic tissues, considerably higher BUN and Cr, MDA and lower SOD. MDA and SOD levels in NMM and NMH groups were closely associated with Cr and BUN. Moreover, different doses of adenine intake effected the mice gut content microbiota, and enriched the different characteristic bacteria. Characteristic bacteria Lactobacillus and Bifidobacterium presented significant correlations with MDA. Eventually, Lactobacillus and Bifidobacterium mediated oxidative stress pathway involved in the process of adenine-induced kidney injure in mice.
Keywords: Characteristic bacteria, Kidney function, Adenine, Diarrhea, Oxidative stress, Kidney-gut axis
1. Introduction
Adenine, as a nitrogenous heterocyclic compound of purines, damages kidney structure and function at certain doses and causes impairment of energy metabolism [1]. The main manifestation of adenine is the formation of insoluble 2, 8-dihydroxyadenine by the action of xanthine oxidase, which accumulates in the renal tubules and interstitial area, blocking the renal tubules and causing renal impairment. Of course, its toxic effects also hinder the metabolism of glucose, lipids, proteins and other substances in the body and affect the energy metabolism process, causing the body to experience pathological manifestations of kidney-yang deficiency such as chills, mental lethargy, curling up and weight loss [2]. Fang et al. described that the body weight, body temperature, grip strength, and number of voluntary activities were markedly decreased in model rats, indicating a series of manifestations of kideny-yang deficiency in the rats after adenine modeling [3]. Mao et al. confirmed that the kidneys of rats showed pale changes after adenine modeling. The kidney structure was disturbed with obvious dilatation of the renal tubules and significant deposition of collagen fibers [4]. The serum levels of BUN and Cr were obviously increased compared with the control group. Hence, adenine markedly damaged the structure and function of kidney.
Recently, the interaction between gut microbiota and host has become a focus of attention [5]. Gut microbiota not only directly influence gut health, but regulate other organs including the heart, liver and kidneys [[6], [7], [8]]. The "kidney-gut axis" theory proposed in 2011 better illustrated strong link between the gut and the kidney in gut microbiology, inflammatory response, oxidative stress and etc [9]. Several researches have confirmed the association between the gut and the kidney in pathology and physiology [10,11]. Huang et al. reported that patients with chronic renal failure had gut microbiota disorders with significantly lower levels of Lactobacillus and Bifidobacterium, while considerably higher levels of Enterococcus faecalis, and that changes in the abundance of beneficial and harmful microbiota caused further impairment of kidney function in patients [12]. Wang et al. revealed that mice with chronic renal failure had significant structural and functional damage to the kidney and showed altered gut microbiota structure compared to mice in the normal group, in which 17 gut bacteria were strongly associated with kidney index, Cr and urea [13]. Our previous study confirmed that kidney and gut dysfunction of mice caused the onset of diarrhea [14], and its mechanism was closely related to gut content microbiota disorder [15]. This had led to the idea of "kidney-gut interaction", which deserved further discussion.
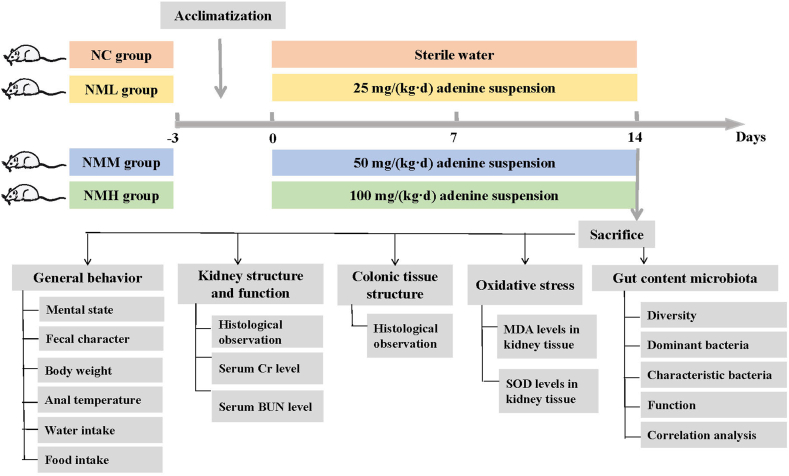
Overall, this study explored effects of different doses of adenine intake on mice in terms of behavioral, kidney function, oxidative stress and gut content microbiota of mice. Association network was constructed by bioinformatics technology to elucidate the interaction among the characteristic bacteria, oxidative stress and kidney function, to interpret "kidney-gut interaction" view from the interaction between adenine-induced kidney dysfunction and gut content microbiota disturbance, and to develop a preliminary "kidney-gut axis-gut bacteria" pathway after adenine intake (Fig. 1).
Fig. 1.
The experimental flow chart.
2. Materials and methods
2.1. Experiments design
Kunming mice (male, n = 24, 4-week-old, 18–22 g) obtained from Slack Jingda Experimental Animal Co, Ltd. (SCXK [Xiang] 2016-0002; Hunan, China). Housing conditions: room temperature (23–25 °C), humidity (50–70 %), 12 h light: 12 h dark cycle. Animals had free access to water and food. Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine was reviewed and accepted the animal experiments (number: LLBH-202108080001). Only male mice were used in this study because of the previous finding that gut microbiota had different effects on the gender of mice [16].
Mice were randomly divided into four groups (n = 6 per group): control (NC) group, low-dosage adenine (NML) group, middle-dosage adenine (NMM) group and high-dosage of adenine (NMH) group. NML、NMM、NMH group were respectively gavaged with adenine suspension (25 mg/(kg·d), 50 mg/(kg·d), 100 mg/(kg·d)), 0.4 mL/each, once a day for 14 days [17]. Equal volume of sterile water gavaged mice in NC group for 14 days. After the experiment, we performed euthanasia for cervical dislocation in mice. The blood, kidney, colon tissue as well as contents of small intestine were harvested for analysis.
2.2. Medicine
Adenine (Changsha Yaer Biology Co., LTD, EZ2811A135). We dissolve adenine in sterile water and proportionally prepare 2.5 mg/mL, 5 mg/mL, 10 mg/mL, daily as needed [17].
2.3. Reagents
MDA assay kit (Beijing Reagan Biotechnology Co., Ltd., 0827A21). SOD assay kit (Beijing Reagan Biotechnology Co., Ltd., 0716A21). BCA protein concentration kit (BIOSHARPLIFESCIENCES, BL521A).
2.4. Mice behaviour
Mice were observed for their mental state, hair form and colour, paw nail colour, faecal form and colour and perianal cleanliness [18]. The water intake and food intake were measured daily in the experiment. Body weight and anal temperature were detected and recorded on days 1, 5, 9 and 13 from the start of the modeling period [19]. We graded the fecal contaminated filter paper according to the size of the stain area [ [20,21]]. tain diameter <1 cm was grade 1, indicated by “+ ”. Stain diameter of 1–1.9 cm was grade 2, indicated by “– ”; diameter of 2–3 cm was grade 3, indicated by “– – ”. Greater than 3 cm was grade 4, indicated by “– – – ”.
2.5. Serum Cr and BUN level detection
Under aseptic conditions, we obtained orbital blood of mice. The serum Cr and BUN levels of mice were measured by automatic biochemical analyzer.
2.6. Histopathological testing of kidney and colon tissues
Mice were executed by cervical dislocation. Connective tissue was removed from the left kidney. Subsequently, colon tissues were collected, and the contents in the colon tissues were cleaned with sterile water.
The treated left kidney and colon tissues were fixed in 4 % buffered paraformaldehyde for 24 h, dewatered for 16 h, routinely embedded for preparation of sections. The sections were subjected to hematoxylin and eosin (H&E) staining.
2.7. MDA and SOD level of kidney tissue detection
Connective tissue was removed from the right kidney of the mice. The MDA level in mouse kidney tissue was measured by TBA microplate method. The SOD level in mouse kidney tissues was measured by NBT riboflavin microplate method.
2.8. Gut content microbiota detection
We collected the content samples in small intestine tissue. The contents of each mouse were labelled and stored in a refrigerator at −80 °C for 16S rRNA high-throughput sequencing. We applied bacterial DNA Kit (OMEGA, USA) to extracted the total genomic DNA samples. The quantity and quality of extracted DNA were determined by NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis. The forward primer 27F (5′-ACTCCTACGGGAGGCAGCA-3′) and reverse primer 1492R (5′-GGACTACHVGGGTWTCTAAT-3′) were used for PCR amplification of 16S rRNA gene. The 16S rRNA gene was amplified by polymerase chain reaction (PCR) using Q5 high-fidelity DNA polymerase (New England BioLabs, USA). The PCR products were detected by 2 % agarose gel electrophoresis, purified and subjected to fluorescence quantification. According to the results, the samples were mixed in proportion to the sequencing requirements of each sample. Sequencing was completed by Paiseno Biological Co., LTD (Shanghai, China).
2.9. Statistical analysis
Statistical analyses were performed using SPSS 21.00 software and data were expressed as mean ± standard deviation. When data conformed to a normal distribution, we applied the one-way ANOVA for comparisons between multiple groups, and the LSD method was applied for two-way comparisons between groups. Non-parametric tests were used if the data did not conform to normal distribution with unequal variances. *P < 0.05 was regarded as a statistically significant difference.
3. Results
3.1. Adenine intake caused mice behavioral changes
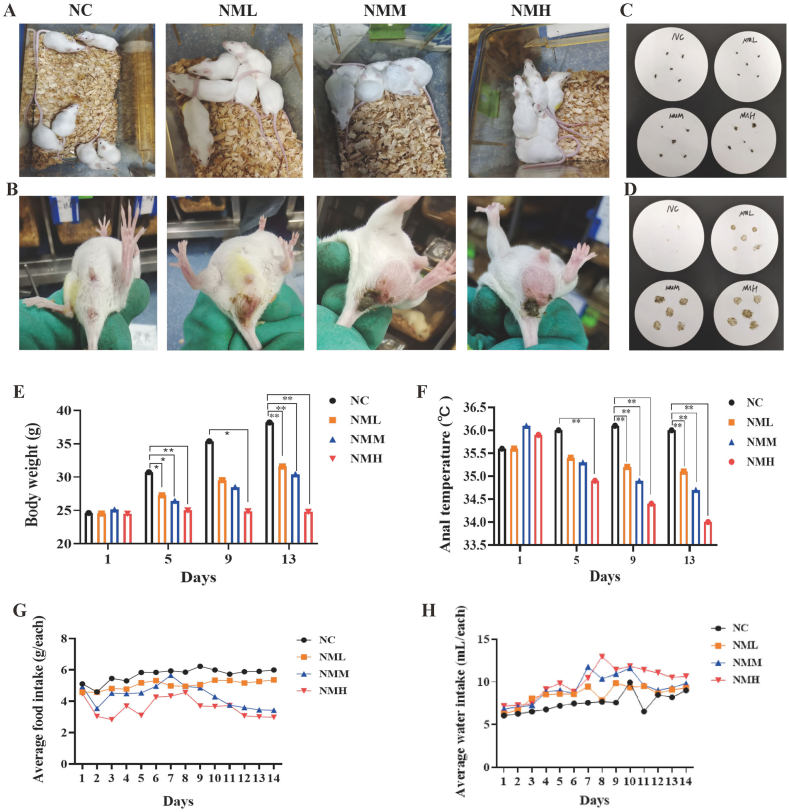
NMM group were slow to respond, curled up with their backs arched, sleepy and lazy, with sparse hair that was easily shed, damp bedding. Fecal adhesion contamination was observed in perianal and tail areas of some mice (Fig. 2A and B). After adenine intake, mice had loose and deformed feces with obvious water stains through the paper, which were more obvious in the NMM and NMH groups (Fig. 2C and D). The degree of wet and dry feces was shown in Table 1. Thus, 50 mg/(kg·d) adenine intake had significantly changed the mental state and autonomic activity of mice, as well as appered symptoms of diarrhea.
Fig. 2.
Effects of different doses of adenine intake on the behaviour of mice. (A) Mental state. (B) Perianal cleansing condition. (C) Fecal character. (D) Fecal water impregnation. (E) Body weight. (F) Anal temperature. (G) Food intake. (H) Water intake. NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group. The values were expressed as mean ± standard deviation (n = 6). *P < 0.05, compared with NC group.
Table 1.
Degree of dry and wet feces of mice.
| Group | Grade |
|---|---|
| NC | + |
| NML | – |
| NMM | – – |
| NMH | – – |
As the modeling time increased, the body weight in the NC, NML and NMM groups increased compared to initial body weight, while the NMH group decreased. During the same period, the body weight in the model groups decreased markedly at 13th day of modeling compared to the NC group (P < 0.05; P < 0.05; P < 0.05) (Fig. 2E). These results indicated that 25 mg/(kg·d) and 50 mg/(kg·d) adenine intake could slowing down the body weight gain, while 100 mg/(kg·d) adenine inhibited the body weight gain.
With the increase of modeling time, the anal temperature of the NML, NMM and NMH groups decreased compared with the initial anal temperature. The anal temperature of mice in the NML, NMM and NMH groups considerably decreased on the 9th and 13th day of modeling compared to the NC group during same period (P < 0.05; P < 0.05; P < 0.05) (Fig. 2F). The results displayed that 25 mg/(kg·d) adenine intake had significantly suppressed the anal temperature of mice.
Compared with NC group, mice in the NML, NMM and NMH groups presented increasing trend in water intake, while NML, NMM and NMH mice all showed a decrease in food intake (Fig. 2G and H). It was suggested that 50 mg/(kg·d) adenine intake showed an increase in water intake and a decrease in food intake.
3.2. Adenine intake damaged kidney structure and function
In the NMM and NMH groups, the kidney of the mice became large and swollen, with a granular feeling when touched, and the kidney of the NMH group were pale in color (Fig. 3A). Mice in the NML, NMM and NMH groups presented different degrees of glomerular atrophy and sclerosis, with inflammatory cell infiltration in renal interstitium. The renal tubules were significantly dilated into cystic shape and disorganized, and the above phenomena were especially prominent in the NMH group (Fig. 3B). The levels of serum Cr and BUN of mice in the NML group were lower compared to those in NC group (P > 0.05; P > 0.05). Serum Cr and BUN in the NMM and NMH groups were enormously elevated (P < 0.05; P < 0.05; P < 0.05; P < 0.05; P < 0.05), with a greater increase in the NMH group (Fig. 3C and D). It was suggested that 50 mg/(kg·d) adenine intake markedly damaged structure and function of kidney, and 100 mg/(kg·d) adenine intake caused more serious damage.
Fig. 3.
Effects of different doses of adenine intake on the kidney structure and function, colonic structure and oxidative stress of mice. (A) Morphological changes of kideny tissue. (B) HE stainin of kidney. (C) Cr level. (D) BUN level. (E) HE stainin of colon tissue. (F) MDA level. (G) SOD level. NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group. The values were expressed as mean ± standard deviation (n = 6). *P < 0.05, compared with NC group.
3.3. Adenine intake caused colon tissue damage
Compared with the NC group, colonic tissues of mice in the adenine dose groups were incomplete, with varying degrees of inflammatory cell infiltration and edema, which were particularly prominent in the NMH group (Fig. 3E). It could be seen that 25 mg/(kg·d) and 50 mg/(kg·d) adenine intake were able to cause structural damage to the colon of mice, while 100 mg/(kg·d) adenine intake caused more serious.
3.4. Adenine intake activated the oxidative stress
Compared with the NC group, MDA level of kidney tissues in the NML group showed an elevated trend (P > 0.05), while in the NMM and NMH groups was considerably higher (P < 0.05; P < 0.05) (Fig. 3F). SOD level of kidney tissues in NML group showed an decreased trend (P > 0.05), while in the NMM and NMH groups was markedly lower (P < 0.05; P < 0.05) (Fig. 3G). Overall, 50 mg/(kg·d) adenine intake had caused the occurrence of the oxidative stress response.
3.5. Adenine intake decreased the diversity and separated microbiota structure
Chao1, Observed_species, and Shannon indexes were significantly lower in the NMM group compared with the NC group (P < 0.05; P < 0.05; P < 0.05), and the above indexes showed a trend of reduction in the NML group (P > 0.05; P > 0.05; P > 0.05) (Fig. 4A–C), suggesting that 50 mg/(kg·d) adenine intake markedly reduced diversity of gut content microbiota of mice. Confidence intervals of the NML, NMH, and NMM groups were clearly separated from the NC group and presented group aggregation (Fig. 4D and E). Mice in the NMM group were statistically different from the NC group in principal coordinate analysis (PCoA) 1 (Fig. 4G) and non-metric multidimensional scaling (NMDS) 1 (Fig. 4I) (P < 0.05; P < 0.05). Subsequent between-group analysis revealed that the within-group differences were smaller than the between-group differences and P < 0.05 (Fig. 4F). These results indicated that community structure of gut content microbiota was significantly altered after adenine intake.
Fig. 4.
Effects of different doses of adenine intake on the diversity of gut content microbiota in mice. (A) Chao1 index. (B) Observed_species index. (C) Shannon index. (D) PCoA analysis. (E) NMDS analysis. (F) Analysis of differences between groups. (G) Statistical analysis of PCoA1. (H) Statistical analysis of PCoA2. (I) Statistical analysis of NMDS1. (J) Statistical analysis of NMDS2. NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group. The values were expressed as mean ± standard deviation (n = 6). *P < 0.05, compared with NC group.
3.6. Adenine intake reshaped the dominant bacteria and enriched the characteristic bacteria
The NC, NML, NMM and NMH groups had a total of 3026, 1993, 812 and 1072 OTUs. Total number of OTUs in four groups was 235 (Fig. 5A). Also, adenine intaking marketedly altered gut content microbiota, with a more pronounced increase in the NMM group (Fig. 5B). We screened the top 10 phyla and genus in relative abundance (Fig. 5C and D). Subsequently, dominant phyla and genus with abundance greater than 1 % were summarized (Fig. 5E and F). Firmicutes (77.89 vs 44.14 %, P = 0.027) and Lactobacillus (43.67 vs 13.54 %, P = 0.025) were enormously less abundant in the NML group compared to the NC group. NMM group had notably higher abundance of Lactobacillus (43.67 vs 86.92 %, P = 5.07e-3) and significantly lower abundance of Facklamia (9.30 vs 0.00 %, P = 0.028) and Halomonas (3.40 vs 0.67 %, P = 0.027). Facklamia (9.30 vs 60.00 %, P = 0.028) and Halomonas (3.40 vs 0.89 %, P = 0.043) were considerably decreased in NMH group (Fig. 5G). It was suggested that dominant bacteria changed at phylum and genus level after different doses of adenine.
Fig. 5.
Effects of different doses of adenine intake on the dominant bacteria of gut content microbiota in mice. (A) Upset diagram. (B) Waffles figure. (C) Horizontal bar diagram of phylum level, genus level (D). (E) Chord chart of phylum level, genus level (F). NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group.
Random forest analysis was applied to analyze the nonlinear relationships between NC group and NML group, NMM group, and NMH group for the top 20 characteristic bacteria in terms of contribution (Fig. 6A–C). ROC analysis of genus-level random forests was subsequently performed (Fig. 6D–F). Among them, the characteristic bacterium Georgenia (AUC = 0.92), Devosia (AUC = 0.81), which contributed more in the NML group. Lactobacillus (AUC = 1) and Shigella (AUC = 0.86) were contributed more in the NMM group. Thermoactinomyces (AUC = 1) and Bifidobacterium (AUC = 0.86) showed larger AUC values in the NMH group. In short, the above characteristic bacteria might serve as potential biomarkers for the pathogenicity of different doses of adenine.
Fig. 6.
Effects of different doses of adenine intake on the characteristic bacteria of gut content microbiota in mice. (A) Genus-level random forests in the NML group, in the NMM group (B) and in the NMH group (C). (D) ROC analysis in the NML group, in the NMM group (E) and in the NMH group (F). NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group.
3.7. Adenine intake changed the function of gut content microbiota
Results found that overall functions of gut content microbiota were divided into 6 classes, with metabolic functions accounting for greater abundance (Fig. 7A). Compared with other metabolic functions, Devosia were negatively correlated with biosynthesis of other secondary metabolites、energy metabolism and etc. Lactobacillus were negatively correlated with energy metabolism. Thermoactinomyces were positively correlated with xenobiotics biodegradation and metabolism. The differences were significant (Fig. 7B–G).
Fig. 7.
Effects of different doses of adenine intake on the KEGG function of gut content microbiota in mice. (A) Predicted abundance of KEGG function. (B) Significantly different metabolic functions in the NML group, in the NMM group (C) and in the NMH group (D). (E) Interaction network of the "characteristic bacteria-metabolic functions" in the NML group, in the NMM group (F) and in the NMH group (G). NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group.
Subsequently, PCoA analysis revealed that the NC group showed significant separation from each sample of the NML, NMM, and NMH groups (Fig. 8A–C). The 140 homologous genes were predicted from the KEGG database in the NML group (10 up-regulated KOs and 9 down-regulated KOs, P < 0.05), 135 homologous genes were predicted in the NMM group (28 up-regulated KOs and 20 down-regulated KOs, P < 0.05), and 136 homologous genes were predicted in the NMH group (3 up-regulated KOs and 3 down-regulated KOs, P < 0.05) (Fig. 8D–I). As indicated in Fig. 7J-L, Devosia in the NML group presented significant negative correlations with ko00960、ko00780、ko00020. In the NML group, Shigella was notably and positively regulated with ko00140. Lactobacillus was enormously and negatively regulated with ko00120、ko00121、ko00900,while positively regulated with ko00340. Thermoactinomyces in the NMH group exhibited a notable positive correlation with ko00280. Bifidobacterium showed a prounced positive correlation with ko00250. On the whole, the above metabolic pathways might be the main pathways through which adenine affected the changes of gut content microbiota.
Fig. 8.
Effects of different doses of adenine intake on the KEGG pathways of gut content microbiota in mice. (A) PCoA diagram. (B) PCoA1 analysis. (C) PCoA2 analysis. (D) NML volcano chart. (E) NMM volcano chart. (F) NMH volcano chart. (G) NML dumbbell chart. (H) NMM dumbbell chart. (I) NMH dumbbell chart. (J) Interaction network of the "characteristic bacteria-KEGG pathway" in the NML group, in the NMM group (K) and in the NMH group (L). NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group. *P < 0.05, compared with NC group.
3.8. Correlation analysis between kidney function-related indexes and oxidative stress-related indexes
We analyzed correlation between kidney function-related indexes and oxidative stress-related indexes in each group of mice by RDA analysis and scatter plots, respectively (Fig. 9A–D). The correlation between kidney function and oxidative stress-related indicators were not significant in the NML group of mice. There were immensely negative regulations between SOD and Cr、BUN in NMM and NMH groups, while there were considerably positive regulations between MDA and Cr、BUN. In brief, there was a significant correlation between the kidney function and oxidative stress in NMM and NMH groups of mice.
Fig. 9.
Correlation analysis between kidney function-related indexes and oxidative stress-related indexes in mice. (A) RDA analysis in the NML group. (B) RDA analysis in the NMM group. (C) RDA analysis in the NMH group. (D) Scatter plot between groups of kidney function and oxidative stress-related indexes. NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group.
3.9. Correlation analysis between oxidative stress-related indexes and characteristic bacteria
Subsequently, we analyzed correlation analysis between oxidative stress-related indexes and characteristic bacteria (Fig. 10A–C). Lactobacillus、Shigella in the NMM group and Bifidobacterium、Thermoactinomyces in the NMH group were significantly positively regulated with MDA (Fig. 10D). It was evident that there was the significantly regulatory effects between the characteristic bacteria of mice gut content and oxidative stress in the NMM and NMH group.
Fig. 10.
Correlation analysis between oxidative stress-related indexes and characteristic bacteria in mice. (A) RDA analysis in the NML group. (B) RDA analysis in the NMM group. (C) RDA analysis in the NMH group. (D) Scatter plot between groups of characteristic bacteria and oxidative stress-related indexes. NC: control group; NML: low-dosage adenine group; NMM: middle-dosage adenine group; NMH: high-dosage adenine group.
4. Discussion
4.1. Adenine intake demaged the kidney structure and function
Study confirmed that adenine was closely related to kidney injury [22]. In the our research, adenine intake was also found to cause damage to kidney structure, with different effects on kidney damage at different doses of intake. Related researches pointed out that serum Cr and BUN reflected glomerular filtration function to some extent and were important indicators for evaluating kideny function [23,24]. When glomerular filtration function was reduced, Cr and BUN increased due to retention. However, the decrease of Cr and BUN in serum of mice in the NML group in the experiment might be due to the dose of adenine used failed to cause kideny function damage. In conjunction with the literature [25], the severity of kidney injury due to different doses of adenine varies, with severe kidney injury producing irreversible lesions in the kidney and mild kidney injury producing a more compensatory self-healing capacity in the kidney. It can be seen that 25 mg/(kg·d) adenine intake might fail to cause significant kidney injury. So, different doses of adenine intake altered kidney structure and function in mice, with 50 mg/(kg·d) adenine intake causing kidney structure and function damage, and 100 mg/(kg·d) adenine intake causing more serious kidney damage in mice.
4.2. Adenine intake changed the structure and function of gut content microbiota
The analysis of diversity showed that middle dose adenine modeling caused a decrease in diversity of gut content microbiota and a significant separation of the community structure, which corroborated the discussion on the alteration of gut microbiota diversity in disease states [26]. During the analysis of relative abundance, different bacteria presented different changes in the NML, NMM and NMH groups. Additionally, we hypothesized that different groups of characteristic bacteria might be key biomarkers for the pathogenicity of different doses of adenine. Focusing on the KEGG metabolic pathway, characteristic bacteria in the NML group were significantly associated with Citrate cycle -TCA cycle in Carbohydrate metabolism, Tropane, piperidine and pyridine alkaloid biosynthesis in Biosynthesis of other secondary metabolites and Biotin metabolism in Metabolism of cofactors and vitamins. Characteristic bacteria in the NMM group were obviously associated with the Primary bile acid biosynthesis, Secondary bile acid biosynthesis, Steroid hormone biosynthesis in Lipid metabolism. Characteristic bacteria in the NMH group were significantly associated with the Valine, leucine and isoleucine degradation and Alanine, aspartate and glutamate metabolism in amino acid metabolism. The colonic tissue of NML group, NMM group and NMH group presented different degree of structural damage. Further, behavioral observation showed that adenine intake caused diarrhea symptoms in mice, and we speculated that this might be related to the disturbance of gut content microbiota. Taken together, different doses of adenine not only damaged the colonic structure of mice and caused diarrhea in mice, but also led to changes in the structure and dominant bacteria of the gut content, as well as the enrichment of different characteristic bacteria The characteristic bacteria of each dose group might participate in the adenine-induced disorder of gut content microbiota through different metabolic pathways.
4.3. Adenine-induced kidney impairment was closely related to disturbance of gut content microbiota
In the previous study, we found that different doses of adenine intake caused structural and functional damage to the kidney of mice, as well as structural and functional disorders of the gut content microbiota. So, is there an association between adenine-induced kidney impairment and disorders of the gut content microbiota? Or is there an association through molecular crosstalk in the "kidney-gut axis"? In this regard, we investigated the interaction between adenine-induced kidney impairment and gut content microbiota disorders by correlation analysis.
It has been reported that the kidney is one of the organs highly sensitive to oxidative stress in the body, and oxidative stress is present throughout the development of kidney disease [27]. Superoxide dismutase (SOD), as the main antioxidant enzyme in cells, scavenges oxygen radicals to protect the body from oxidative damage, and its level reflects body's ability to remove oxygen radicals to some extent [28]. Malondialdehyde (MDA) is the final breakdown product of lipid peroxidation, and changes in its content reflects the degree of cellular damage caused by oxidative stress [29]. In the detection of oxidative stress related indicators, we found that the intake of 50 mg/(kg·d) and 100 mg/(kg·d) adenine caused occurrence of oxidative stress. Recent studies have indicated that oxidative stress related molecules (MDA and SOD) are involved kidney damage in kidney disease. Wang et al. confirmed that serum levels of MDA in patients with type 2 diabetic nephropathy were higher than those of their healthy counterparts, and their values were positively correlated with Cr and BUN [30]. Wang et al. mentioned that SOD levels in patients with acute sepsis were lower than in healthy peer groups, and their values were negatively correlated with Cr and BUN levels [31]. Combined with the results of correlation analysis in the experiment, we found that BUN and Cr of mice in the NMM and NMH groups were markedly negatively correlated with SOD and significantly positively correlated with MDA. On the whole, the kidney function impairment of mice after the intake of 50 mg/(kg·d) and 100 mg/(kg·d) adenine was closely related to the occurrence of oxidative stress.
Besides, we made correlations between the characteristic bacteria of mouse gut content and oxidative stress responses. The differences in regulation between Lactobacillus、Shigella in the NMM group and Thermoactinomyces、Bifidobacterium in the NMH group and MDA were significant. Bifidobacterium, as a probiotic, plays an important role in improving gastrointestinal function [32]. In the functional analysis results, Bifidobacterium presented obvious regulatory effects on the metabolism of alanine, aspartic acid and glutamate. Some reports illustrated that glutamate induced the onset of oxidative stress by generating large amounts of nitrogen-oxygen radicals and peroxidation products through oxidation and nitroxylation [33]. From these, we infered that Bifidobacterium might be indirectly involved in the oxidative stress response through glutamate metabolism. Additionally, Lactobacillus was markedly positively correlated with oxidative stress response induced by medium-dose adenine, which might also involve the interaction between characteristic bacteria. Studies have found that Shigella is a common pathogen of zoonoses causing diarrhea, and its pathogenesis mainly involves intestinal inflammatory response and destruction of intestinal epithelial cells [34]. Functional studies of Thermoactinomyces mainly focused on the production of enzymes and other active substances [35]. However, there are few reports on the relationship between Shigella, Thermoactinomyces and oxidative stress. Combined with the preliminary findings in our study, Shigella and medium-dose adenine-induced oxidative stress response and Thermoactinomyces and high-dose adenine-induced oxidative stress response had obvious regulatory effects, but their related biological properties and mechanisms still need to be further explored. Overall, characteristic bacteria Lactobacillus and Bifidobacterium were closely associated with occurrence of oxidative stress after intake of 50 mg/(kg·d) and 100 mg/(kg·d) of adenine.
In conclusion, differences in the effects of different doses of adenine on behavior, kidney structure and function, oxidative stress, and gut content microbiota in mice. 50 mg/(kg·d) adenine intake was able to cause diarrhea in mice, induce structural and functional damage to kidney and activate oxidative stress in mice, and there was significant correlations between them. Moreover, involvement of the characteristic bacteria Lactobacillus and Bifidobacterium in kidney impairment after adenine intake was closely related to the occurrence of oxidative stress. Thus, we hypothesized that oxidative stress-related molecules as key substances in "kidney-gut axis" crosstalk adenine-induced kidney impairment and gut content microbiota disorders (Fig. 11). At the same time, the "kidney-gut axis-gut bacteria" pathway was formed after adenine intake. This provides directions for subsequent studies on the causal links between specific mechanisms and also provides ideas for targeting specific bacteria to adjust the gut microbiota for disease treatment.
Fig. 11.
The "kidney-gut axis" crosstalk adenine-induced kidney impairment and gut content microbiota disorders.
Availability of data and materials
The data underlying this study was available within the manuscript. The gut content microbiota sequencing data has been uploaded to the NCBI database (https://www.ncbi.nlm.nih.gov/), no. PRJNA854756.
CRediT authorship contribution statement
Xiaoya Li: Writing – original draft, Data curation, Conceptualization. Bo Qiao: Methodology, Data curation. Yueying Wu: Visualization, Investigation. Na Deng: Visualization, Investigation. Zhoujin Tan: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was financially supported by the Natural Science Foundation of Hunan Province (No. 2022JJ30440) and Key Scientific Research Project of Hunan Provincial Education Department (22A0270). Thanks to the Paiseno Biological Co., LTD (Shanghai, China) for providing technical support for this study.
References
- 1.Li W., Wang S.P., Yang F.Y. Research progress on establishment method standred of animal model of kidney yang deficiency syndrome. Jiangxi. J. Trad. Chin. Med. 2021;52:74–76. [Google Scholar]
- 2.Ma Y.R., Xin M.Y., Wu J.L., Wang D.J., Wang H., Wu X.A. Changes in renal excretion pathways in rats with adenine-induced chronic renal failure. J. Chin. Pharmaceut. Sci. 2021;30:319–333. [Google Scholar]
- 3.Mao H.X., Kang T., Wu W.H., Zhu T.T., Zhang L.L., Ou S.T. Expression and significance of HIF-1α, RhoA and SOX9 in rats with adenine-induced chronic kidney disease. Chin. J. Comp. Med. 2022;32:70–77+135. [Google Scholar]
- 4.Fang P., Su J., Chen W., Zhu J.M., Sun C.C., Lv G.Y. Effect of wubishanyao pills on renal lesion of shenyangxu model rats induced by adaenine. Pharm. Clinics. Chin. Mater. 2021;37:11–16. [Google Scholar]
- 5.Kc D., Sumner R., Lippmann S. Gut microbiota and health, Postgrad. Med. 2020;132:274. doi: 10.1080/00325481.2019.1662711. [DOI] [PubMed] [Google Scholar]
- 6.Zaky A., Glastras S.J., Wong M.Y.W., Pollock C.A., Saad S. The role of the gut microbiome in diabetes and obesity-related kidney disease. Int. J. Mol. Sci. 2021;22:9641. doi: 10.3390/ijms22179641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkowski M., Weeks T.L., Hazen S.L. Gut. Microbiota and Cardiovascular Disease, Circ. Res. 2022;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abenavoli L., Procopio A.C., Scarpellini E., Polimeni N., Aquila I. Gut microbiota and non-alcoholic fatty liver disease. Minerva Gastroenterol. 2021;67:339–347. doi: 10.23736/S2724-5985.21.02896-5. [DOI] [PubMed] [Google Scholar]
- 9.Meijers B.K.I., Evenepoel P. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol. Dial. Transplant. 2011;26:759–761. doi: 10.1093/ndt/gfq818. [DOI] [PubMed] [Google Scholar]
- 10.Zou T.J., Peng T. Research progress of TCM prevention and treatment of diabetic nephropathy based on intestinal flora. J. Northwest. Minzu. Univ. 2022;43:58–62. [Google Scholar]
- 11.Liu S.J., Song J., Wang Z.S. Research advances on the mechanism of gut microbiota in renal anemia. J. Clin. Nephrol. 2022;22:685–689. [Google Scholar]
- 12.Huang T.Y., Li C.Y., Liu D.J. Correlation between microecological changes of intestinal flora and renal function in patients with chronic renal failure. Cont. Med. 2021;27:114–115. [Google Scholar]
- 13.Wang R. Hubei Uni Chin Med; 2022. Study on the Efficacy and Molecular Mechanism of Cooked Rhubarb in the Treatment of Mice with Chronic Renal Failure through the Regulation of Intestinal Flora Disorders. [Google Scholar]
- 14.Li X.Y., Zhu J.Y., Wu Y., J Tan Z. Correlation between kidney function and intestinal biological characteristics of adenine and folium SennaeInduced diarrhea model in mice. Turk. J. Gastroenterol. 2023;34:4–12. doi: 10.5152/tjg.2022.211010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X.Y., Peng X.X., Qiao B., Peng M.J., Deng N., Tan Z.J. Gut-kidney impairment process of adenine combined with Folium sennae-induced diarrhea: association with interactions between Lactobacillus intestinalis, Bacteroides acidifaciens and acetic acid, inflammation, and kidney function. Cells. 2022;11:3261. doi: 10.3390/cells11203261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y., Peng X.X., Li X.Y., Li D.D., Tan Z.J., Yu R. Sex hormones influences intestinal microbiota composition in mice. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.964847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X.Y., Deng N., Zheng T., Qiao B., Peng M.J., Xiao N.Q., Tan Z.J. Importance of Dendrobium officinale in improving the adverse effects of high-fat diet on mice associated with intestinal contents microbiota. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.957334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X.Y., Zhu J.Y., Wu Y., Liu Y.W., Hui H.Y., Tan Z.J. Model building and validation of diarrhea mice with kidney-yang depletion syndrome. J. Tradit. Chin. Med. 2022;63:1368–1373. [Google Scholar]
- 19.Qiao B., Liu J., Xiao N.Q., Tan Z.J., Peng M.J. Effects of sweeteners on host physiology by intestinal mucosal microbiota: example-addition sweeteners in Qiweibaizhu Powder on intestinal mucosal microbiota of mice with antibiotic-associated diarrhea. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su X.L., Tang Y.P., Zhang J., Bai Y.B., Shi H.X., Liu Y.J., Chang Y.J., Wei W. Shen warming Pi strengthening method intervened IBS-D rats: an efficacy assessment, Chin. J. Int. Trad. Wes. Med. 2021;34:197–202. [PubMed] [Google Scholar]
- 21.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C. Qiime 2: reproducible, interactive, scalable, and extensible microbiome data science. Peer. J. Preprints. 2018;6 doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z., Li H.Y., Liu J.X., Yu Y.X., Jing T. The effect of shenqi granules on adenine-induced chronic kidney failure in rats. Med. Innov. China. 2022;19:7–11. [Google Scholar]
- 23.Li R., Wang L., Qian H.Y., Zhang F., Dong B.R., Shu W. Research progress of creatinine as biomarkers in disease diagnosis and drug evaluation. Drug. Eval. Res. 2021;44:2007–2012. [Google Scholar]
- 24.Ma Y., Bao G.X. Diagnostic value of combined serum urea nitrogen, creatinine and β2-microglobulin test in elderly patients with essential hypertension with renal injury. Mod. Med. Health, Res. Elec. J. 2021;5:26–28. [Google Scholar]
- 25.Tong J.F.A. Chin. Med. Uni; 2015. Comparative Study of Kidney Yang Deficiency Rat Models of Renal Injury Induced by Different Does of Adenine, Zhejiang. [Google Scholar]
- 26.Gao B., Chi L., Zhu Y.X., Shi X.C., Tu P.C., Li B., Yin J., Gao N., Shen W.S., Schnabl B. An introduction to next generation sequencing bioinformatic analysis in gut microbiome studies. Biomolecules. 2021;11:530. doi: 10.3390/biom11040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao H.Q., Zhang C.Y., Xiao N.Q., Tan Z.J. Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC Microbiol. 2020;20:313. doi: 10.1186/s12866-020-01999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo L., Zheng J.Q., Zhang X.H. A clinical study on the on renal protection effect of the Shenshuai prescription on chronic renal failure under oxidative stress. Clin. J. Chin. Med. 2022;14:61–64. [Google Scholar]
- 29.Zhang S.L., Zhu Z.R., Li J., Zhang S., Ma Y.F. Leonurine protects ischemia-induced brain injury via modulating SOD, MDA and GABA levels. Front. Agri. Sci. Eng. 2019;6:197–205. [Google Scholar]
- 30.Halder T., Upadhyaya G., Basak C., Das A., Chakraborty C., Ray S. Dehydrins impart protection against oxidative stress in transgenic tobacco plants. Front. Plant Sci. 2018;9:136. doi: 10.3389/fpls.2018.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Li J. Detection of serum NF-κB and MDA levels in patients with type 2 diabetic nephropathy and their relationships with renal function, China. J. Mod. Med. 2022;32:21–25. [Google Scholar]
- 32.Liu Y.P., Qian J.J., Zheng W.Q. Advances in the mechanism of oxygen stress response of Bifidobacterium. Industrial. Microbiol. 2021;51:50–55. [Google Scholar]
- 33.Pál B. Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell. Mol. Life Sci. 2018;16:2917–2949. doi: 10.1007/s00018-018-2837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsubo R., Mimuro H., Ashida H. Shigella effector IpaH4.5 targets 19S regulatory particle subunit RPN13 in the 26S proteasome to dampen cytotoxic T lymphocyte activation. Cell Microbiol. 2019;21 doi: 10.1111/cmi.12974. [DOI] [PubMed] [Google Scholar]
- 35.Feng H.J., Zhai L., Cheng K. Research progress of Thermoactinomyces. Food Ferment. Ind. 2017;43:257–261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study was available within the manuscript. The gut content microbiota sequencing data has been uploaded to the NCBI database (https://www.ncbi.nlm.nih.gov/), no. PRJNA854756.