Graphical abstract

Keywords: Porphyromonas gingivalis, Extracellular vesicle, Periodontitis, Memory impairment, Microbiota dysbiosis
Highlight
-
•
Gingivally exposed, but not orally gavaged, Porphyromonas gingivalis (PG) or its extracellular vesicles (pEVs) caused periodontitis and cognitive impairment in mice.
-
•
Gingival exposure to PG or pEVs decreased BDNF and NMDA receptor expression in the hippocampus.
-
•
Gingivally exposed fluorescein-5-isothiocyanate-labeled pEVs were detected in the trigeminal ganglia and hippocampus.
-
•
Gingivally exposed PG or pEVs increased LPS and TNF-α levels in the blood.
-
•
Gingivally exposed, but not orally gavaged, PG or pEVs caused colitis and gingival microbiota dysbiosis.
Abstract
Introduction
Porphyromonas gingivalis (PG)-infected periodontitis is in close connection with the development of Alzheimer’s disease (AD). PG-derived extracellular vesicles (pEVs) contain inflammation-inducing virulence factors, including gingipains (GPs) and lipopolysaccharide (LPS).
Objectives
To understand how PG could cause cognitive decline, we investigated the effects of PG and pEVs on the etiology of periodontitis and cognitive impairment in mice.
Methods
Cognitive behaviors were measured in the Y-maze and novel object recognition tasks. Biomarkers were measured using ELISA, qPCR, immunofluorescence assay, and pyrosequencing.
Results
pEVs contained neurotoxic GPs and inflammation-inducible fimbria protein and LPS. Gingivally exposed, but not orally gavaged, PG or pEVs caused periodontitis and induced memory impairment-like behaviors. Gingival exposure to PG or pEVs increased TNF-α expression in the periodontal and hippocampus tissues. They also increased hippocampal GP+Iba1+, LPS+Iba1+, and NF-κB+Iba1+ cell numbers. Gingivally exposed PG or pEVs decreased BDNF, claudin-5, and N-methyl-D-aspartate receptor expression and BDNF+NeuN+ cell number. Gingivally exposed fluorescein-5-isothiocyanate-labeled pEVs (F-pEVs) were detected in the trigeminal ganglia and hippocampus. However, right trigeminal neurectomy inhibited the translocation of gingivally injected F-EVs into the right trigeminal ganglia. Gingivally exposed PG or pEVs increased blood LPS and TNF-α levels. Furthermore, they caused colitis and gut dysbiosis.
Conclusion
Gingivally infected PG, particularly pEVs, may cause cognitive decline with periodontitis. PG products pEVs and LPS may be translocated into the brain through the trigeminal nerve and periodontal blood pathways, respectively, resulting in the cognitive decline, which may cause colitis and gut dysbiosis. Therefore, pEVs may be a remarkable risk factor for dementia.
Introduction
Dementia is a highly prevalent, neurodegenerative disease [1], [2]. Many patients with dementia, including those with Alzheimer disease (AD), exhibit neuronal inflammation and neurotransmitter imbalance with pathogen infection [3], [4]. Pathogenic components, such as lipopolysaccharide (LPS), have been found in the brain, resulting in neuroinflammation [5], [6], [7]. The induction of neuroinflammation by bacterial LPS, which is produced by gram (−) bacteria, Escherichia coli and Porphyromonas gingivalis (PG), can cause cognitive decline in vivo [6], [7], [8].
PG is a nonmotile, gram (−), rod, and anaerobic pathogen that causes chronic periodontitis [9]. PG produces virulence factors known as LPS and gingipains (GPs) [10], [11]. Oral infection with PG and intraperitoneal injection with PG LPS cause memory impairment in mice [6], [12], [13]. GPs are known to be neurotoxic in vitro and in vivo [11], [14]. GP inhibitors suppress PG-induced neuroinflammation [11]. GPs is detected in the brain of AD patients [11], [15]. PG is suggested to be contributory to the development of dementia with chronic periodontitis [6], [11], [16]. Therefore, PG infection is being considered as a remarkable factor in the AD pathogenesis.
Extracellular vesicles (EVs) are lipid bilayer-delimited nanoparticles secreted from living cells [13]. Bacterial EVs enclose a variety of bacterial components, such as toxins and enzymes, which can be virulent in the host [17], [18]. During bacterial infection, components of bacterial EVs are delivered locally and systematically [19], [20]. In patients with gastric diseases, Helicobacter pylori EVs were detected in the gastric juice [21]. Orally gavaged H. pylori EVs were detected in mouse stomachs. Intraperitoneally injected Filifactor alocis EVs are accumulated in mouse femurs, resulting in systemic bone loss [19]. Orally gavaged Paenalcaligenes hominis EVs were transfected in the brain of mice, resulting in memory impairment [20]. EVs from gram-negative bacteria Escherichia coli and P. hominis induce proinflammatory cytokine expression in vivo through toll-like receptor (TLR)-4-linked NF-κB activation, leading to local and/or systemic inflammation [20], [22]. However, the pathogenicity of PG EVs (pEVs) is not clear.
Therefore, we investigated the roles of PG and its pEVs on the etiology of cognitive impairment and periodontitis in mice. Gingivally infected PG or pEVs caused periodontitis, neuroinflammation, and memory impairment. Gingivally exposed fluorescein-5-isothiocyanate (FITC)-labeled pEVs (F-pEVs) were delivered to the hippocampus. By contrast, orally gavaged pEVs were not delivered to the hippocampus and did not cause memory impairment with neuroinflammation.
Materials and methods
Culture of PG and preparation of pEVs
PG (ATCC 33237) was cultured in hemin-/menadione-contained brain heart infusion medium (HMBHI, BD), as previously reported [23]. Briefly, PG was cultured in HMBHI broth at 37 °C for 2 days (optical density, 0.5–0.8; colony-forming units (CFUs), >5 × 108 CFU/mL) under the anaerobic condition and centrifuged (12,000g, 0.5 h). Precipitated cells were suspended in saline for experiments.
For pEV preparation, the supernatant of the PG culture was successively centrifuged (20,000g, 4 °C, 30 min), filtered through the 0.22-μm millipore-size filter (Millipore Corp.) twice, and recentrifuged (150,000g, 4 °C, 3 h). The precipitate was suspended in saline. pEVs were purified using iodixanol density gradient medium (Sigma), as previously described [19]. Purified pEVs contained 40.11% protein, 0.23% LPS, and 0.03% nucleic acid, which consisted of 39.5% DNA and 60.5% RNA. The weights of pEVs were measured for freeze-dried pEVs. Protein amounts were assayed using a Bradford protein assay kit. Nucleic acid amounts were calculated as the sum of the amounts of purified DNA and RNA using Qiagen’s DNA and RNA purification kits. Eleven milligrams of pEVs were obtained from the supernatant of PG culture (1 l). The size of pEVs was found to be 30–80 nm (round vesicular structures without intact PG) using transmission electron microscopy (Hitachi). The proteins in pEVs were analyzed by SDS-PAGE and LC-MS/MS (ThermoFisher Orbitrap XL with Easy nLC 1000) (Supplement Table S1).
FITC-labeling of pEVs
The FITC labeling of pEVs (2 mg as protein) was performed using the FITC antibody labeling kit (50 μg) according to the manufacturer’s introduction [20]. The FITC-labeled pEV (F-pEV) solution (1.5 mL) was used for in vivo experiments.
Culture of BV2 cells, SH-SY5Y cells, and peritoneal macrophages
SH-SY5Y and BV2 cells were cultured at 37 °C in a 95% air and 5% CO2 in DMEM containing 1% antibiotic–antimycotic and 10% FBS. Peritoneal macrophages, which were isolated according to the method of Ma [24], were cultured at 37 °C in a 95% air and 5% CO2 in RPMI 1640 containing 10% FBS and 1% antibiotics. For the cytotoxicity and brain-derived neurotropic factor expression assay, SH-SY5Y cells (1 × 106 cells/mL) were incubated with pEVs or heat-killed (90 °C, 15 min) PG for 24 h. For assaying TNF-α and IL-1β expression, BV-2 cells (1 × 106 cells/mL) and peritoneal macrophages (2 × 105 cells/mL) were incubated with pEVs or heat-killed PG for 24 h.
Animals
C57BL/6 mice (male, 6-week-old, 18–21 g) were obtained from Koatech Inc. (Yongin-shi, Korea), acclimatized under controlled standard conditions for 1 week, and used in in vivo experiments, which were performed according to the Ethical Policies and Guidelines of Kyung Hee University for Laboratory Animals Care and Use. Animal experiments were approved by the Kyung Hee University Institutional Animal Care and Use Committee of (KUASP(SE)-21007).
To understand whether PG or pEVs were involved in the occurrence of cognitive impairment, PG or pEVs were orally gavaged into mice or exposed to mouse gingiva. Each group consisted of seven mice.
-
[1]
PG (2 × 108 or 1 × 109 CFU/0.1 mL saline/day) was orally gavaged into mice daily for 21 days. And PG (2 × 108 or 1 × 109 CFU/0.05 mL saline/day) was slowly exposed to mouse gingiva daily for 21 days.
-
[2]
pEVs (2 μg or 10 μg as protein/ 0.1 mL saline/day) were orally gavaged into mice daily for 21 days. And pEVs (2 μg or 10 μg as a protein/0.05 mL saline) were slowly exposed to mouse gingiva daily for 21 days.
-
[3]
F-pEVs (2 μg/0.02 mL saline/day) or FITC (0.02 mL/day) was slowly exposed to mouse gingiva daily for 5 days.
-
[4]
F-EVs (2 μg/0.02 mL/day) were injected into the gingiva (right and/or left) of mice with or without the right trigeminal neurotomy daily for 2 days. The right trigeminal neurotomy was performed according to the method of Ginwalla [25].
Memory impairment-like behaviors were measured in the Y-maze task (YMT) and novel object recognition task (NORT) next day after the final PG or pEVs treatment, as previously reported [26]. Mice were thereafter euthanized in a chamber filled with CO2 followed by cervical dislocation. The hippocampus, periodontal, and colon tissues were collected and stored at −80 °C until experimental use.
Enzyme-linked immunosorbent assay (ELISA) and limulus amebocyte lysate assay
Hippocampus, periodontal, and colon tissues were homogenized in RIPA buffer and centrifuged (13,000g, 4 °C, 20 min), as previously reported [27]. Sera were prepared, as previously reported [27]. In the tissue lysate supernatants and sera, cytokine and BDNF levels were determined using commercial ELISA kits. Endotoxin levels were assayed in the blood and stool using a LAL assay kit (Cape Cod Inc.).
Immunofluorescence assay
Hippocampus, periodontal, and colon tissues were isolated from formaldehyde-perfused mice, post-fixed with p-formaldehyde solution, cytoprotected, frozen, and sectioned [20]. The sections were blocked with serum, treated with primary antibodies for NF-κB (1:800, Cell Signaling), CD11c (1:800, Cell Signaling), TLR4 (1:500, Abcam), LPS (1:500), claudin-5 (1:800, Millipore), GP (1:800, Cusabio), Iba1 (1:1000, Abcam), BDNF (1:800, Abcam), and/or NeuN (1:1000, Abcam) overnight, and washed two times. The washed sections were incubated with secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 594 (1:500, Invitrogen) for 2.5 h. The stained sections were observed using a confocal laser microscope.
Microbiota analysis
Bacterial DNA was purified from periodontal tissues and fresh feces of mice using the QIAamp DNA stool mini kit (Qiagen) and amplified using barcoded bacterial 16S rRNA V4 gene region primers [28]. The sequencing of amplicons was carried out using Illumina iSeq 100 and deposited in NCBI (accession number, PRJNA874838).
Quantitative polymearase chain reaction (qPCR)
For the analysis of PG, periodontal and hippocampus tissues were homogenized in 50 mM tris-HCl buffer (pH 7.4) and centrifuged (10,000g, 4 °C, 10 min). Bacterial RNA was isolated using the RNeasy Mini Kit (Qiagen). cDNA was prepared using cDNA synthesis kit (Takara) and random primer (Takara). qPCR was carried out using a Qiagen thermal cycler, as previously reported [20]. PG primer sequence was indicated in Supplement (Table S2).
For the analysis of cytokine, NMDAR, and BDNF levels, hippocampal and periodontal tissues were homogenized in 50 mM tris-HCl buffer (pH 7.4) and centrifuged (10,000g, 4 °C, 10 min). RNA was extracted using the RNeasy Mini Kit. cDNA was prepared using cDNA synthesis kit and oligo dT primer. qPCR was performed, as previously reported [29]. Primers are indicated in Supplement (Table S3).
Thermal cycling was performed at 95 °C for 30 s, followed by 40–42 cycles of denaturation at 95 °C for 5 s and amplification at 72 °C for 30 s. The expression of genes was indicated relative to 16S rRNA or glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analysis
Data are described as mean ± SD using GraphPad Prism 9 (GraphPad Software, Inc.). Significances were analyzed using one-way analysis of variance followed by Dunnett’s multiple range test (p < 0.05).
Results
Oral infection with PG caused periodontitis and cognitive impairment in mice
To investigate whether PG could cause periodontitis in vivo, we gingivally infected or orally gavaged PG into mice and measured proinflammatory cytokines in the periodontal tissue (Fig. 1a–e. Supplement Fig. S1). Gingivally infected PG increased TNF-α and IL-1β expression and NF-κB+CD11c+ and GP+LPS+ cell populations in the periodontal tissue. However, orally gavaged PG did not significantly increase TNF-α, IL-1β, and IL-10 expression and GP+LPS+ and NF-κB+CD11c+ cell numbers in the periodontal tissue. qPCR analysis revealed that gingivally infected PG increased PG 16S rDNA levels in the periodontal tissue, while orally gavaged PG did not (Fig. 1d). Analysis of periodontal microbiota composition through pyrosequencing revealed that gingivally infected PG increased PG as well as Proteobacteria populations (Fig. 1f–i). UniFrac analysis revealed that gingival infection with PG partially shifted the β-diversity of periodontal microbiota, whereas α-diversity remained significantly unchanged.
Fig. 1.
Gingivally infected P. gingivalis (PG) caused periodontitis in mice. Effect on TNF-α (a), IL-1β (b), and IL-10 expression (c) in the periodontal tissue. (d) Effct on PG 16S rDNA level in the periodontal tissue. (e) Effect on GP+LPS+ and NF-κB+CD11c+ cell populations in the periodontal tissue. Effects on periodontal microbiota composition: (f) phylum, (g) family levels, (h) OTUs (α-diversity), and (i) β-diversity (principal coordinate analysis plot based on UniFrac). GI, gingivally exposed; OG, orally gavaged; NC, treated with vehicle; PL, treated with 2 × 108 CFU/mouse/day of PG; PH, treated with 1 × 109 CFU/mouse/day. Arrows in (d) indicate positive cells. Data values were described as mean ± SD (n = 7). #p < 0.05 vs. NC.
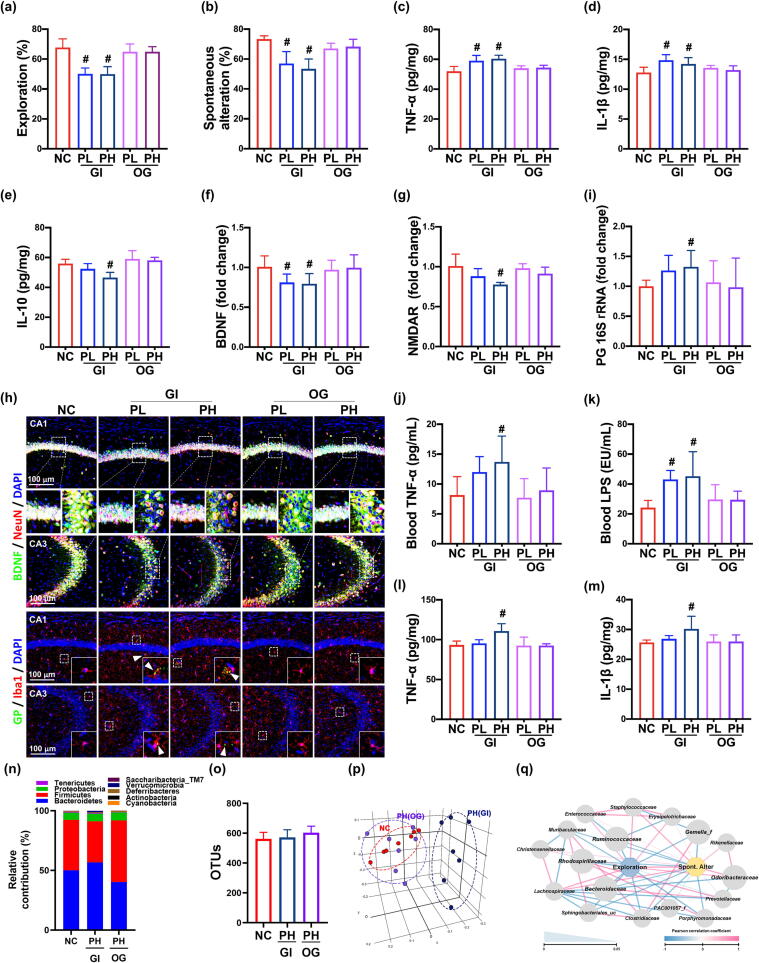
Next, we investigated whether PG could cause memory impairment in mice (Fig. 2a,b). Oral gavage of PG at 2 × 108 CFU/mouse and 1 × 109 CFU/mouse did not significantly increase memory impairment-like behaviors in the YMT and NORT. However, gingival infection with PG at 2 × 108 CFU/mouse increased memory impairment-like behaviors in the YMT and NORT to 77.8% [F(4,30) = 16.45, p < 0.001] and 74.1% [F(4,30) = 23.68, p < 0.001] of control mice, respectively. The memory impairment-like behaviors were more strongly, but not significantly, increased in mice gingivally infected with PG at 1 × 109 CFU/mouse than in mice infected with PG at 2 × 108 CFU/mouse. Furthermore, gingivally infected PG also induced TNF-α and IL-1β expression and GP+Iba1+, NF-κB+Iba1+, LPS+Iba1+, and TLR4+Iba1+ cell numbers and decreased BDNF and IL-10 expression and BNDF+NeuN+ and claudin-5+ cell numbers in the hippocampus (Fig. 2c–h, Supplement Fig. S2). Moreover, gingivally infected PG also suppressed NMDAR expression in the hippocampus. Furthermore, we could detect PG 16S rRNA in the hippocampus of mice gingivally infected with PG, assessed by qPCR (Fig. 2i). However, when the hippocampus homogenate was cultured in PG-culturable broth and agar plate, live PG was not detected. In addition, gingivally infected PG increased TNF-α and LPS levels in the blood (Fig. 2j,k).
Fig. 2.
Gingivally infected P. gingivalis (PG) impaired cognitive function in mice. (a) Effects on spontaneous alternation in NORT (a) and YMT (b). Effects on TNF-α (c), IL-1β (d), IL-10 expression (e) in the hippocampus, assessed by ELISA. Effects on BDNF (f) and NMDAR expression (g) in the hippocampus, assessed by qPCR. (h) Effects on BDNF+NeuN+ cells and GP+Iba1+ cell populations in the hippocampus. (i) Effect on PG (PG) 16S rRNA levels in the hippocampus. Effect on TNF-α (j) and LPS levels (k) in the blood. Effect on TNF-α (l) and IL-1β expression (m) in the colon. Effects on fecal microbiota composition: (n) phylum level, (o) OTUs (α-diversity), (p) β-diversity (principal coordinate analysis plot based on UniFrac), and (q) network of differentially enriched gut microbiota in spontaneous alteration in YMT or exploration in NORT scores. GI, gingivally exposed; OG, orally gavaged; NC, treated with vehicle; PL, treated with 2 × 108 CFU/mice/day of PG; PH, treated with 1 × 109 CFU/mice/day. Arrows in (h) indicate positive cells. Data values were described as mean ± SD (n = 7). #p < 0.05 vs. NC.
Next, we examined whether PG caused colonic inflammation in mice (Fig. 2l,m, Supplement Fig. S3). Gingival infection with PG at 1 × 109 CFU/mouse/day, but not 2 × 108 CFU/mouse/day, weakly increased TNF-α and IL-1β expression and NF-κB+CD11c+ cell population in the colon and caused slight colon shortening. However, oral gavage of PG at 1 × 109 CFU/mouse did not significantly increase TNF-α and IL-1β expression and NF-κB+CD11c+ cell number.
Analysis using 16S rDNA pyrosequencing revealed that, compared to orally gavaged PG or vehicle, gingivally infected PG in part shifted the β-diversity of gut microbiota, increased Bacteroidetes population, and decreased Tenericutes and Muribaculum spp. populations (Fig. 2n-p). In the network analysis, cognitive behaviors, spontaneous alteration in the YMT and exploration in the NORT, were negatively associated with Sphingobacteriales_uc, Clostridiaceae, Porphyromonadaceae, Gemella_f, and Ruminococcaceae populations at the family level, while Lachnospiraceae, Staphylococcaceae, Odoribacteraceae, and Enterococcaceae populations were positively associated (Fig. 2q).
pEVs suppressed BDNF expression in neuron cells and increased TNF-α expression in macrophages
To understand the memory impairment-inducible etiological agent of PG, we isolated pEVs from PG. Transmission electron microscopy revealed that pEVs are round, vesicular structures of 30–80 nm, without intact PG. Dried pEVs consisted of proteins including GPs, LPS, and nucleic acid. Proteomics/LC-MS/MS analysis showed that pEVs contained proteins including GPs (Fig. 3a-c, Supplement Table S1). The contents of neurotoxic GP K and inflammation-inducible LPS were significantly higher 153-fold and 48-fold in pEVs than those in PG, respectively (Supplement Fig. S4). In SH-SY5Y cells, pEVs dose-dependently showed the cytotoxicity and suppressed BDNF expression (Fig. 3d–i). In BV2 cells and peritoneal macrophages, pEVs dose-dependently increased TNF-α and IL-1β expression. In addition, PG also increased TNF-α expression, while BDNF expression decreased (Supplement Fig. S5).
Fig. 3.
P. gingivalis extracellular vesicles (pEVs) suppressed BDNF expression in neuronal SH-SY5Y cells and induced TNF-α in microphage cells. (a) Transmission electron microscope image of pEVs. (b) Sodium-polyacrylamide gel electrophoresis (SDS-PAGE) of pEVs. (c) The identified components of pEVs: A, Mfa1 family fimbria major subunit partial; B, gingipain R [RpgA]; C, gingipain K [Peptidase C25]; D, fimbria protein; E, gingipain K, assessed by LC-MS-MS. Effect of pEVs on the cytotoxicity (d) and BDNF expression (e) in SH-SY5Y cells. Effects on TNF-α (f) and IL-1β expression (g) in peritoneal macrophages. Effects on TNF-α (h) and IL-1β expression (i) in BV2 cells. N, treated with vehicle; EV, treated with 20, 50, 100, 200, and 400 ng/mL of pEVs. Data values were described as mean ± SD (n = 4). #p < 0.05 vs. NC.
Gingivally exposed pEVs caused periodontitis and cognitive decline in mice
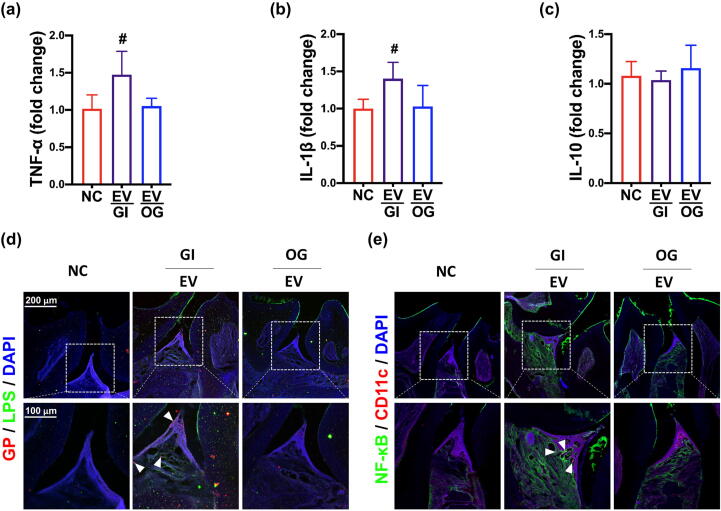
We investigated whether pEVs could cause periodontitis in mice (Fig. 4, Supplement Fig. S6). Gingivally exposed pEVs increased TNF-α and IL-1β expression and NF-κB+CD11c+ cell number in the periodontal tissue. However, orally gavaged pEVs did not significantly affect the expression of proinflammatory cytokine TNF-α and IL-1β and anti-inflammatory cytokine IL-10 and population of NF-κB+CD11c+ cells in the periodontal tissue.
Fig. 4.
Gingivally exposed P. gingivalis extracellular vesicles (pEVs) caused periodontitis in mice. Effects on TNF-α (a), IL-1β (b), and IL-10 (c) in the periodontal tissue. Effects on PG+LPS+ (c) and NF-κB+CD11c+ cell populations (d)in the periodontal tissue. GI, gingivally exposed; OG, orally gavaged; NC, treated with vehicle; EV, treated with 2 μg/mouse/day of pEVs. Arrows in (d) indicate positive cells. Data values were described as mean ± SD (n = 7). #p < 0.05 vs. NC.
Oral gavage of pEVs at 2 μg/mouse/day and 10 μg/mouse/day (as protein) did not cause memory impairment in mice (Fig. 5). However, gingival exposure to pEVs at 2 μg/mouse/day (as protein) increased memory impairment-like behaviors in the YMT and NORT to 80.3% [F(2,18) = 11.29, p < 0.007] and 74.4% [F(2,18) = 93.37, p < 0.001] of control mice (Fig. 5a,b). Gingivally exposed pEVs also increased TNF-α and IL-1β expression GP+Iba1+, NF-κB+Iba1+, LPS+Iba1+, and TLR4+Iba1+ cell numbers in the hippocampus (Fig. 5c-h, Supplement Fig. S7). However, gingivally exposed pEVs decreased BDNF and IL-10 expression and BNDF+NeuN+ and claudin-5+ cell populations in the hippocampus. Orally exposed pEVs also suppressed NMDAR expression in the hippocampus. Moreover, we detected PG 16S rRNA in the hippocampus of mice gingivally exposed to pEVs (Fig. 5i). However, oral gavage of pEVs did not cause memory impairment-like behaviors. Furthermore, orally gavaged pEVs did not affect TNF-α, IL-1β, IL-10, BDNF, and NMDAR expression in the hippocampus. Gingivally exposed pEVs increased TNF-α and LPS levels in the blood, whereas orally gavaged pEVs did not (Fig. 5j,k).
Fig. 5.
Gingivally exposed P. gingivalis extracellular vesicles (pEVs) impaired cognitive function in mice. a) Effects on spontaneous alternation in NORT (a) and YMT (b). Effects on TNF-α (c), IL-1β (d), IL-10 expression (e) in the hippocampus, assessed by ELISA. Effects on BDNF (f) and NMDAR expression (g) in the hippocampus, assessed by qPCR. (h) Effects on BDNF+NeuN+, claudin-5+, NF-κB+Iba1+, LPS+Iba1+, TLR4+Iba1+, and GP+Iba1+ cell populations in the hippocampus. (i) Effect on P. gingivalis (PG) 16S rRNA levels in the hippocampus. Effect on TNF-α (j) and LPS levels (k) in the blood. Effect on TNF-α (l) and IL-1β expression (m) in the colon. Effects on periodontal microbiota composition: (n) phylum level, (o) α-diversity (OTUs), (o) β-diversity (principal coordinate analysis plot based on UniFrac), and (q) network of differentially enriched gut microbiota in spontaneous alteration in YMT or exploration in NORT scores. GI, gingivally exposed; OG, orally gavaged; NC, treated with vehicle; EV, treated with 2 μg/mouse/day of pEVs. Arrows in (h) indicate positive cells. Data values were described as mean ± SD (n = 7). #p < 0.05 vs. NC.
Next, we investigated the effects of pEVs on the etiology of colitis in mice (Fig. 5l,m, Supplement Fig. S8a–c). Gingivally exposed pEVs weakly increased TNF-α and IL-1β expression and NF-κB+CD11c+ cell population in the colon. Oral gavage of pEVs did not significantly affect TNF-α, IL-1β, and IL-10 expression and colon shortening.
Results from 16S rDNA pyrosequencing revealed that, compared to orally gavaged pEVs or vehicle, gingivally exposed pEVs shifted the β-diversity of gut microbiota, increased Bacteroidetes and Verrucomicrobia populations, and decreased Fermicutes and Muribaculum spp. populations (Fig. 5o,n, Supplement Fig. S8d,e). In the network analysis, cognitive behaviors, spontaneous alteration in the YMT or exploration in the NORT, were negatively associated with Coriobacteriaceae, Desulfovibrionaceae, and Ruminococcaceae populations at the family level, while Christensenellaceae and Enterococcaceae populations were positively associated (Fig. 5p).
Gingivally exposed F-pEVs were translocated into the hippocampus through the trigeminal nerve
To understand the translocation pathway of pEVs into the brain, we injected F-pEVs into the gingiva of mice (Fig. 6). The gingivally injected F-pEVs were detected in the pyramidal region of the hippocampus, while orally gavaged F-pEVs were not detected in the hippocampus (Fig. 6a).
Fig. 6.
Trigeminal neurotomy inhibited the translocation of gingivally exposed FITC-labeled pEVs (F-pEVs) into the brain. (a) Effects of administered routes (gingival infection and oral gavage) on the F-pEVs detected in the hippocampus of mice with gingivally exposed to F-pEVs. (b) Effect of right-gingivally injection on the F-pEVs detected in the trigeminal ganglia of mice with gingivally exposed to F-pEVs. (c) Effects of right- and left-gingival injections on the F-pEVs detected on the trigeminal ganglia of mice with right trigeminal neurotomy. NC, treated with vehicle in mice; giEV, gingivally exposed with F-pEVs (2 μg/mouse/day); ogEV, orally gavaged with pEVs (2 μg/mouse/day); EVr, injected with F-pEVs (2 μg/mouse/day) in the right gingiva of mice; EVrl, injected with F-pEVs (2 μg/mouse/day) in the right (R) and left (L) gingiva of mice with the right trigeminal neurotomy. Arrows indicate positive cells.
When FITC-labeled pEVs were injected into the right gingiva of mice, the injected F-pEVs were detected in the right trigeminal ganglia (Fig. 6b, Supplement Fig. S9a). Furthermore, we injected F-pEVs in the right and left gingiva of mice with right trigeminal neurotomy. The injected F-pEVs were detected in the left trigeminal ganglia alone, but not the right trigeminal ganglia (Fig. 6c, Supplement S9b).
Discussion
Patients with PG-induced chronic periodontitis have often been reported to be comorbid for systemic diseases, such as AD, cardiovascular diseases, and rheumatoid arthritis [10], [30], [31]. Infection with pathogens, including PG, into the body activates immune cells, including microglia [32], [33]. The activation of microglial cells by pathogenic byproducts, such as LPSs and toxins, can cause neuroinflammation and neuronal cell death, leading to a leaky blood–brain barrier [11], [33], [34], which accelerates the activation of immune cells in the brain, subsequently causing neurodegeneration [34], [35]. The cognitive decline in AD, with the accumulated intracellular neurofibrillary tangles, is closely associated with chronic intracerebral inflammation [8], [36], [37]. Gingival infection with PG and intraperitoneal injection with PG LPS cause AD-like cognitive decline in mice [6], [12]. Therefore, infection with inflammation-inducing pathogens may cause cognitive decline and deteriorate dementia.
In this present study, we found that gingivally exposed PG or pEVs increased TNF-α and IL-1β expression in the periodontal and hippocampus tissues and memory impairment-like behaviors. In particular, exposure to PG caused detection of PG 16S rDNA in the periodontal microbiota. LPS and GP (LPS+GP+ cells) were also detected in the periodontal tissue of mice gingivally exposed to pEVs. Furthermore, these exposures also induced NF-κB+CD11c+ cell populations in the periodontal tissue and GP+Iba1+, LPS+Iba1+, NF-κB+Iba1+, and TLR4+Iba1+ cell populations in the hippocampus. However, orally gavaged PG or pEVs did not cause periodontitis, neuroinflammation, and memory impairment. In addition, we found that PG produced pEVs, which contained GPs, fimbria protein, and LPS. GPs is neurotoxic [38] and fimbria protein and LPS is inflammation-inducible [8], [39], [40]. PG and pEVs induced TNF-α and IL-1β expression in the BV2 cells and primary macrophages in vitro. They also showed cytotoxicity against neuronal SH-SY5Y cells. These results suggest that gingivally exposed PG or pEVs may cause periodontitis and neuroinflammation through the translocation of inflammation-inducible LPS and fimbria protein and neurotoxic GPs in the periodontium and hippocampus, resulting in memory impairment.
Gingivally exposed PG and pEVs, although weak, also increased TNF-α and IL-1β expression and NF-κB-overexpressed cell populations in the colon, whereas orally gavaged PG and pEVs had no significant effect. Gingivally exposed PG and pEVs fluctuated gut microbiota composition, increasing Bacteroidetes population and decreasing Muribaculum spp. population. However, orally gavaged PG and pEVs did not significantly shift gut microbiota composition. In particular, cognitive behaviors were negatively associated with Sphingobacteriales_uc, Porphyromonadaceae, Gemella_f, and Ruminococcaceae populations positively associated with Lachnospiraceae, Odoribacteraceae, and Enterococcaceae populations in mice gingivally infected with PG. In mice with gingivally infected with pEVs, Coriobacteriaceae, Desulfovibrionaceae, and Ruminococcaceae were negatively assoiated with cognitive behaviors, while Christensenellaceae and Enterococcaceae populations were positively associated. In all mice gingivally infected with PG or pEVs, Enterococcaceae and Ruminococcaceae populations were positively and negatively associated with cognitive behaviors, respectively. Gingivally exposed PG and pEVs suppressed claudin-1 and claudin-5 expression in the periodontal and hippocampus tissues, respectively, whereas orally gavaged PG and pEVs did not. Furthermore, gingivally exposed PG or pEVs caused colonic inflammation and cognitive impairment, whereas orally gavaged PG or pEVs did not. The gut microbiota fluctuation is closely associated with cognitive impairment with gut inflammation through the brain-gut-microbiota axis [22]. These results suggest that orally gavaged PG or pEVs may not be able to reside in the intestine by the hindrance action of gut microbiota prepositioned in the gastrointestinal tract and digestive juice and these may be infectable in the mouth. Moreover, the occurrence of cognitive impairment by gingivally infected PG or pEVs can cause gut inflammation and microbiota dysbiosis, which may deteriorate cognitive impairment through MGB axis.
We also detected PG 16S rDNA in the hippocampus of mice treated with PG or pEVs. However, when the hippocampal lysates were cultured in PG-culturable HMBHI broth and agar plate, PG could not be detected. pEVs isolated from PG contained LPS, proteins (including gingipains and fimbria protein), and nucleic acid (DNA and RNA). Gingival infection with PG or pEVs increased GP-positive and LPS-positive cells in the periodontal tissue and hippocampus. However, orally gavaged EVs or PG did not significantly increase GP+Iba1+ and LPS+Iba1+ cell numbers in the hippocampus. When F-pEVs were gingivally exposed, F-pEVs were detected in the trigeminal ganglia and hippocampus. However, in mice orally gavaged with F-pEVs, F-pEVs were not detected in the trigeminal ganglia and hippocampus. Right trigeminal neurotomy significantly inhibited the translocation of the gingivally (right and left) injected F-pEVs into the right trigeminal ganglia. Dominy et al. detected GPs and PG DNA in the brain of PG-infected mice [11]. Other groups reported that GPs were observed in the brains of AD patients [11], [41], [42]. These findings suggest that, in mice gingivally exposed to PG, the detection of PG 16S rDNA, GPs, and LPS may be due to the translocation of PG-produced pEVs, not PG, from the gingiva (mouth) into the brain through the trigeminal nerve. However, gingival infection with PG or pEVs increased LPS levels in the blood, while oral gavage of PG or pEVs were not fluctuated LPS levels. And we could not detect F-pEVs in the blood of mice gingivally exposed with F-pEVs. These findings suggest that gingival infection with PG or pEVs may translocate the byproducts of PG or pEVs, such as LPS, into the brain through the periodontal blood, resulting in cognitive impairment with neuroinflammation. Nevertheless, further research is needed to identify how pEVs can be translocated into the brain using the anatomic analysis and how PG LPS cause cognitive decline.
Gingivally infected pEVs can activate immune cells, such as macrophages, in the periodontal tissue and microglia in the hippocampus, leading to the periodontitis and neuroinflammation, like gingivally infected PG. Activated immune cells in the periodontitis may activate systemic immune cells, such as microglia, leading to the neuroinflammation, as previously suggested [10], [43], [44]. Neuroinflammation can potentiate neuronal cell damage by accelerating immune cell infiltration into the brain and accumulation of intracellular neurofibrillary tangles (tau and Aβ), resulting in cognitive decline [8], [45]. Our findings revealed that gingivally exposed PG or pEVs suppressed BDNF and NMDAR expression and reduced BDNF+NeuN+ cell population in the hippocampus; however, gingivally exposed PG or pEVs induced TNF-α expression and increased NF-κB+Iba1+ cell number. By contrast, orally gavaged PG or pEVs did not affect BDNF and TNF-α expression. Carreño et al. reported that BDNF activated NMDAR signaling [46]. Afonso et al. reported that BDNF increased synaptic NMDAR abundance in hippocampal neurons [47]. Tanqueiro et al. reported that memantine protected amyloid-β-induced BDNF loss by regulating NMDAR [45]. These results suggest that gingivally infected PG or pEVs may induce cognitive decline by suppressing NMDAR-dependent neuronal BDNF signaling and inducing NF- κB signaling.
Conclusions
Gingivally exposed PG continuously produces a variety of PG byproducts including pEVs and LPS, which can cause periodontitis. pEVs and LPS may be translocated into the brain through the trigeminal nerve and periodontal blood pathways, respectively, resulting in the etiology of cognitive decline by suppressing NMDAR-mediated BDNF expression and induction of NF- κB signaling. PG- or pEVs-mediated cognitive decline thereafter may cause colitis and gut microbiota dysbiosis. The pEVs of PG, a periodontal pathogen, may be a remarkable risk factor for the development of dementia, including AD.
CRediT authorship contribution statement
Xiaoyang Ma: Conceptualization, Methodology, Visualization, Writing – original draft. Yoon-Jung Shin: Writing – original draft, Formal analysis, Visualization. Jong-Wook Yoo: Conceptualization, Methodology, Visualization, Writing – original draft. Hee-Seo Park: Formal analysis. Dong-Hyun Kim: Conceptualization, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This work was supported by the Medical Research Program (2017R1A5A2014768) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
Compliance with Ethics Requirements
This study was approved by the Kyung Hee University Institutional Animal Care and Use Committee of (KUASP(SE)-21007) and performed according to the Ethical Policies and Guidelines of Kyung Hee University for Laboratory Animals Care and Use.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.02.006.
Contributor Information
Xiaoyang Ma, Email: xiaoyangma12@gmail.com.
Yoon-Jung Shin, Email: nayo971111@naver.com.
Jong-Wook Yoo, Email: hooit96@naver.com.
Hee-Seo Park, Email: xlvksl1997@khu.ac.kr.
Dong-Hyun Kim, Email: dhkim@khu.ac.kr.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peila R, Launer LJ. Inflammation and dementia: epidemiologic evidence. Acta Neurol Scand Suppl. 2006;185:102–6. doi:10.1111/j.1600-0404.2006.00693.x. [DOI] [PubMed]
- 3.Zhang Y., Geng R., Tu Q. Gut microbial involvement in Alzheimer's disease pathogenesis. Aging (Albany NY) 2021;13(9):13359–13371. doi: 10.18632/aging.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotz SK, Blackhurst BM, Reagin KL, Funk KE. Microbial infections are a risk factor for neurodegenerative diseases. Front Cell Neurosci. 2021;15:691136. doi:10.3389/fncel.2021.691136. [DOI] [PMC free article] [PubMed]
- 5.Zhao J., Bi W., Xiao S., Lan X., Cheng X., Zhang J., et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9(1):5790. doi: 10.1038/s41598-019-42286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Yu C., Zhang X., Chen H., Dong J., Lu W., et al. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 2018;15(1):37. doi: 10.1186/s12974-017-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang S.E., Lim S.M., Jeong J.J., Jang H.M., Lee H.J., Han M.J., et al. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018;11(2):369–379. doi: 10.1038/mi.2017.49. [DOI] [PubMed] [Google Scholar]
- 8.Kim H.S., Kim S., Shin S.J., Park Y.H., Nam Y., Kim C.W., et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer's disease: pathologic roles and therapeutic implications. Transl Neurodegener. 2021;10(1):49. doi: 10.1186/s40035-021-00273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.How KY, Song KP, Chan KG. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol. 2016;7:53. doi:10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed]
- 10.Bui F.Q., Almeida-da-Silva C.L.C., Huynh B., Trinh A., Liu J., Woodward J., et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333. doi:10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed]
- 12.Ding Y, Ren J, Yu H, Yu W, Zhou Y. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun Ageing 2018;15:6. doi:10.1186/s12979-017-0110-7. [DOI] [PMC free article] [PubMed]
- 13.Bahar B., Singhrao S.K. An evaluation of the molecular mode of action of trans-resveratrol in the Porphyromonas gingivalis lipopolysaccharide challenged neuronal cell model. Mol Biol Rep. 2021;48(1):147–156. doi: 10.1007/s11033-020-06024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryder MI. Porphyromonas gingivalis and Alzheimer disease: Recent findings and potential therapies. J Periodontol. 2020;91 Suppl 1(Suppl 1):S45-s9. doi:10.1002/jper.20-0104. [DOI] [PMC free article] [PubMed]
- 15.Nakanishi H., Nonaka S., Wu Z. Microglial cathepsin B and porphyromonas gingivalis gingipains as potential therapeutic targets for sporadic Alzheimer's disease. CNS Neurol Disord Drug Targets. 2020;19(7):495–502. doi: 10.2174/1871527319666200708125130. [DOI] [PubMed] [Google Scholar]
- 16.Elwishahy A., Antia K., Bhusari S., Ilechukwu N.C., Horstick O., Winkler V. Porphyromonas gingivalis as a risk factor to alzheimer's disease: a systematic review. J Alzheimers Dis Rep. 2021;5(1):721–732. doi: 10.3233/adr-200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. doi:10.1016/j.semcdb.2015.02.006. [DOI] [PubMed]
- 18.Veith P.D., Chen Y.Y., Gorasia D.G., Chen D., Glew M.D., O'Brien-Simpson N.M., et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. 2014;13(5):2420–2432. doi: 10.1021/pr401227e. [DOI] [PubMed] [Google Scholar]
- 19.Kim H.Y., Song M.K., Gho Y.S., Kim H.H., Choi B.K. Extracellular vesicles derived from the periodontal pathogen Filifactor alocis induce systemic bone loss through Toll-like receptor 2. J Extracell Vesicles. 2021;10(12):e12157. doi: 10.1002/jev2.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K.E., Kim J.K., Han S.K., Lee D.Y., Lee H.J., Yim S.V., et al. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8(1):107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi H.I., Choi J.P., Seo J., Kim B.J., Rho M., Han J.K., et al. Helicobacter pylori-derived extracellular vesicles increased in the gastric juices of gastric adenocarcinoma patients and induced inflammation mainly via specific targeting of gastric epithelial cells. Exp Mol Med. 2017;49(5):e330. doi: 10.1038/emm.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang H.M., Lee K.E., Lee H.J., Kim D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci Rep. 2018;8(1):13897. doi: 10.1038/s41598-018-31764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wunsch C.M., Lewis J.P. Porphyromonas gingivalis as a model organism for assessing interaction of anaerobic bacteria with host cells. J Vis Exp. 2015;106:e53408. doi: 10.3791/53408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X., Shin Y.J., Jang H.M., Joo M.K., Yoo J.W., Kim D.H. Lactobacillus rhamnosus and Bifidobacterium longum alleviate colitis and cognitive impairment in mice by regulating IFN-γ to IL-10 and TNF-α to IL-10 expression ratios. Sci Rep. 2021;11(1):20659. doi: 10.1038/s41598-021-00096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginwalla MS. Sugical treatment of trigeminal neuralgia of third division. Oral Surg Oral Med Oral Pathol. 1961;14:1300–4. doi:10.1016/0030-4220(61)90261-4. [DOI] [PubMed]
- 26.Lee H.J., Lee K.E., Kim J.K., Kim D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci Rep. 2019;9(1):11814. doi: 10.1038/s41598-019-48342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang H.M., Kim J.K., Joo M.K., Shin Y.J., Lee C.K., Kim H.J., et al. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci Rep. 2021;11(1):20406. doi: 10.1038/s41598-021-00088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JK, Lee KE, Lee SA, Jang HM, Kim DH. Interplay between human gut bacteria escherichia coli and lactobacillus mucosae in the occurrence of neuropsychiatric disorders in mice. Front Immunol 2020;11:273. doi:10.3389/fimmu.2020.00273. [DOI] [PMC free article] [PubMed]
- 29.Lim S.M., Jeong J.J., Woo K.H., Han M.J., Kim D.H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res. 2016;36(4):337–348. doi: 10.1016/j.nutres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Hajishengallis G., Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim) 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- 32.Mariani M.M., Kielian T. Microglia in infectious diseases of the central nervous system. J Neuroimmune Pharmacol. 2009;4(4):448–461. doi: 10.1007/s11481-009-9170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koutsouras GW, Ramos RL, Martinez LR. Role of microglia in fungal infections of the central nervous system. Virulence 2017;8(6):705–18. doi:10.1080/21505594.2016.1261789. [DOI] [PMC free article] [PubMed]
- 34.Sharma C, Woo H, Kim SR. Addressing blood-brain barrier impairment in Alzheimer's disease. Biomedicines 2022;10(4). doi:10.3390/biomedicines10040742. [DOI] [PMC free article] [PubMed]
- 35.Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer's disease. Neurobiol Dis 2017;107:41–56. doi:10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed]
- 36.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y). 2018;4:575–90. doi:10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed]
- 37.Matsushita K, Yamada-Furukawa M, Kurosawa M, Shikama Y. Periodontal disease and periodontal disease-related bacteria involved in the pathogenesis of Alzheimer's disease. J Inflamm Res 2020;13:275–83. doi:10.2147/jir.S255309. [DOI] [PMC free article] [PubMed]
- 38.Imamura T. The role of gingipains in the pathogenesis of periodontal disease. J Periodontol. 2003;74(1):111–118. doi: 10.1902/jop.2003.74.1.111. [DOI] [PubMed] [Google Scholar]
- 39.Kim KA, Jeong JJ, Yoo SY, Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016;16:9. doi:10.1186/s12866-016-0625-7. [DOI] [PMC free article] [PubMed]
- 40.Uchiya KI, Kamimura Y, Jusakon A, Nikai T. Salmonella Fimbrial Protein FimH is involved in expression of proinflammatory cytokines in a toll-like receptor 4-dependent manner. Infect Immun. 2019;87(3). doi:10.1128/iai.00881-18. [DOI] [PMC free article] [PubMed]
- 41.Ryder MI, Xenoudi P. Alzheimer disease and the periodontal patient: New insights, connections, and therapies. Periodontol 2000. 2021;87(1):32–42. doi:10.1111/prd.12389. [DOI] [PubMed]
- 42.Haditsch U., Roth T., Rodriguez L., Hancock S., Cecere T., Nguyen M., et al. Alzheimer's Disease-Like Neurodegeneration in Porphyromonas gingivalis Infected Neurons with Persistent Expression of Active Gingipains. J Alzheimers Dis. 2020;75(4):1361–1376. doi: 10.3233/jad-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ardura-Fabregat A., Boddeke E., Boza-Serrano A., Brioschi S., Castro-Gomez S., Ceyzériat K., et al. Targeting neuroinflammation to treat Alzheimer's disease. CNS Drugs. 2017;31(12):1057–1082. doi: 10.1007/s40263-017-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantarci A., Tognoni C.M., Yaghmoor W., Marghalani A., Stephens D., Ahn J.Y., et al. Microglial response to experimental periodontitis in a murine model of Alzheimer's disease. Sci Rep. 2020;10(1):18561. doi: 10.1038/s41598-020-75517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laurent C., Buée L., Blum D. Tau and neuroinflammation: What impact for Alzheimer's Disease and Tauopathies? Biomed J. 2018;41(1):21–33. doi: 10.1016/j.bj.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carreño F.R., Walch J.D., Dutta M., Nedungadi T.P., Cunningham J.T. Brain-derived neurotrophic factor-tyrosine kinase B pathway mediates NMDA receptor NR2B subunit phosphorylation in the supraoptic nuclei following progressive dehydration. J Neuroendocrinol. 2011;23(10):894–905. doi: 10.1111/j.1365-2826.2011.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Afonso P, De Luca P, Carvalho RS, Cortes L, Pinheiro P, Oliveiros B, et al. BDNF increases synaptic NMDA receptor abundance by enhancing the local translation of Pyk2 in cultured hippocampal neurons. Sci Signal. 2019;12(586); 10.1126/scisignal.aav3577. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.