Graphical abstract
Keywords: Nischarin/IRAS, Neurodevelopment, Spinal cord injury, Opioid dependence, Anxiety, Depression, Autism
Highlights
-
•
The spatiotemporal specificity and cell selectivity of Nischarin/IRAS expression are closely associated with the regulation of brain development.
-
•
Upregulation of Nischarin/IRAS induced by CNS injuries inhibits axonal repair and affects neuronal apoptosis.
-
•
Nischarin/IRAS promotes the circulation of the μ opioid receptor to regulate opioid dependence and mediates the occurrence of psychiatric diseases.
Abstract
Background
Murine Nischarin and its human homolog IRAS are scaffold proteins highly expressed in the central nervous system (CNS). Nischarin was initially discovered as a tumor suppressor protein, and recent studies have also explored its potential value in the CNS. Research on IRAS has largely focused on its effect on opioid dependence. Although the role of Nischarin/IRAS in the physiological function and pathological process of the CNS has gradually attracted attention and the related research results are expected to be applied in clinical practice, there is no systematic review of the role and mechanisms of Nischarin/IRAS in the CNS so far.
Aim of review
This review will systematically analyze the role and mechanism of Nischarin/IRAS in the CNS, and provide necessary references and possible targets for the treatment of neurological diseases, thereby broadening the direction of Nischarin/IRAS research and facilitating clinical translation.
Key scientific concepts of review
The pathophysiological processes affected by dysregulation of Nischarin/IRAS expression in the CNS are mainly introduced, including spinal cord injury (SCI), opioid dependence, anxiety, depression, and autism. The molecular mechanisms such as factors regulating Nischarin/IRAS expression and signal transduction pathways regulated by Nischarin/IRAS are systematically summarized. Finally, the clinical application of Nischarin/IRAS has been prospected.
Introduction
In 2000, Suresh K. Alahari et al. screened out Nischarin from the mouse embryo gene expression library for the first time [1]. Nischarin is a scaffold protein that specifically binds to the α5β1 subunit of integrins [1]. IRAS is the human homolog of Nischarin. Nischarin [2], [3]and IRAS [4] have partial properties of the I1-imidazoline receptor (IR-1). But recent studies have further argued that Nischarin may not be an IR-1 [5]. Nischarin/IRAS are widely expressed in various organs of the body, and their expression level in neural tissue is much greater than that in other tissues [6], which suggests the potential role of Nischarin/IRAS in CNS.
Research interest in Nischarin/IRAS in the CNS has steadily increased since 2003 [7], [8] (Fig. 1 and Table S1). Related research on Nischarin mainly focuses on the influence of CNS development [6] and the regulation of CNS diseases [9], [10], [11]. Nischarin is expressed in neurons and is a negative regulator of neuronal migration [6]. Low expression of Nischarin in nascent neurons of the cerebral cortex enables them to migrate into the outer cortex [6]. The gradual increase in Nischarin expression during neuronal migration eventually causes neurons to stay at their final destination [6]. According to the latest report, Nischarin appears to be expressed in astrocytes as well [12].
Fig. 1.
Timeline of the main discovery of Nischarin/IRAS in the CNS. After the discovery of Nischarin and IRAS in 2000, a preliminary study on the role of IRAS in the CNS appeared in 2003, and a large number of related studies emerged after 2013. Among them, Nischarin was found to regulate anxiety and blood pressure in 2004 and 2007, respectively. Then in 2013, our laboratory reported the expression characteristics of Nischarin in the rat brain and found the relationship between Nischarin and neurodevelopment. In the same year, the neuroprotective effect of Nischarin was also discovered. Subsequent studies have found the role of Nischarin in neuronal migration, axonal growth, and neuronal apoptosis in animal experiments, and the inhibitory effect of Nischarin on the repair of spinal cord injury has also been found. Compared with the nischarin investigations, the research scope of IRAS is relatively concentrated. After the discovery of the inhibitory effect of IRAS on neuronal apoptosis in 2003, most studies focused on the role of IRAS in the process of opioid dependence and tolerance. The researchers successively discovered the role of IRAS in the process of opioid dependence and tolerance in cells and mice, as well as the binding and action relationship between IRAS and MOR. In 2017, a study examining human tissue samples found dysregulation of the IRAS in the brains of opioid abusers. Another study in the same year reported similar changes in IRAS in the brains of people with depression. CNS: Central nervous system; MOR: μ-opioid receptor.
When neurons are affected by different external factors, the expression of Nischarin increases rapidly. Nischarin up-regulation can promote neuronal apoptosis caused by oxidative stress injury [10], [11], however, it has a protective effect on neurons injured by inflammatory stimuli [9]. Similarly, IRAS overexpression has also been found to have a protective effect on neuronal damage caused by starvation or pro-apoptotic factors [8], [13]. In addition, Nischarin in the rostral ventrolateral medulla (RVLM) of rats negatively regulates blood pressure [14]. During the development of autism, Nischarin in the rat prefrontal cortex (PFC) inhibited the formation of dendritic spines in the neurons [15]. Nischarin in the rat amygdala is down-regulated during anxiety [16]. Up-regulation of IRAS was found in the PFC of patients with depression, while down-regulation of IRAS was found in the Brodmann area 9 (BA9) area of the PFC in patients taking antidepressants [17], [18].
Nischarin was originally discovered as a tumor suppressor protein [19], [20], and more than 20 years of research has also explored its potential value in the CNS. Research on IRAS mainly reveals its inhibitory effect on the dependence and tolerance of opioid drugs in vivo. IRAS inhibits cyclic adenosine monophosphate (cAMP) [21] and calcium signaling [22], which are critical in the process of opioid addiction. At the same time, IRAS can also promote the resensitization of μ opioid receptor (MOR) [23]. The role of Nischarin/IRAS in the physiological function and pathological process of the CNS has gradually attracted attention, and the related research results are supposed to be applied in clinical practice. There is not any systematic review of the role and mechanism of Nischarin/IRAS in the CNS. Here, this study will introduce the role of Nischarin/IRAS in CNS and its related molecular mechanisms in detail.
Molecular structure of Nischarin/IRAS
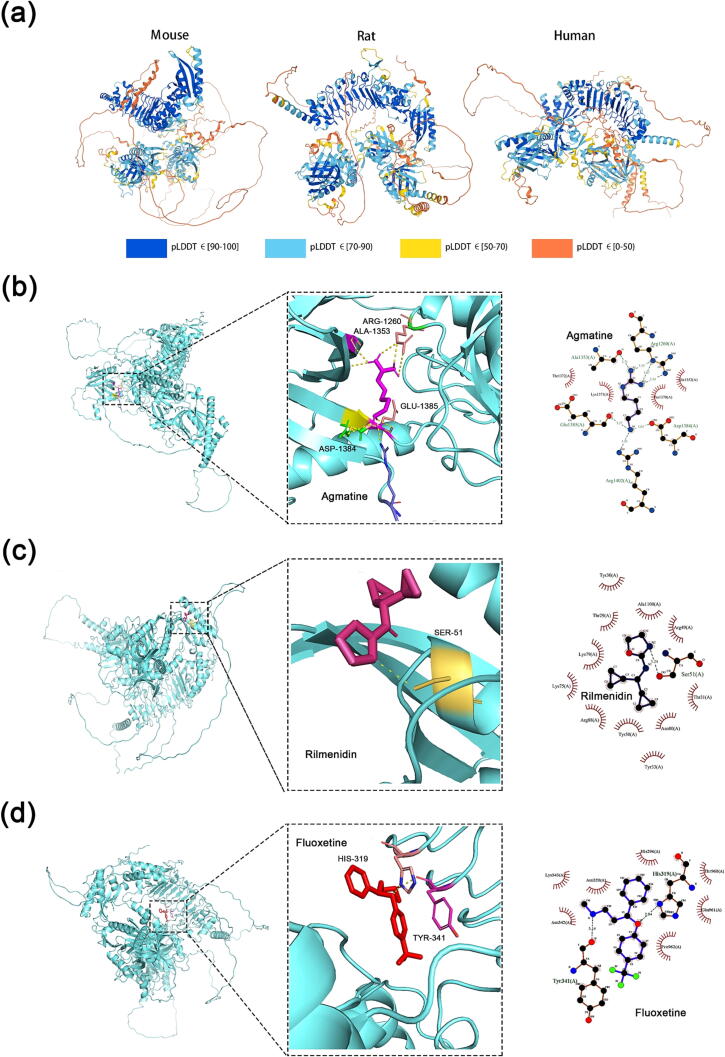
The homology of Nischarin/IRAS nucleic acid sequences in mouse, rat, and human species is higher than 90 %, and the homology of amino acid sequences is higher than 80 % [2], [3]. Previous studies have demonstrated that different regions of Nischarin/IRAS can bind P21 (RAC1) activated kinase 1 (PAK1), liver kinase B1 (LKB1), and other proteins [19], [20]. We used AlphaFold2 Software (AlphaFold Monomer v2.0) [24], [25] to predict the spatial structure of mouse, rat Nischarin and human IRAS, and different colors represent the confidence level of alphafold's structure prediction (Fig. 2). Nischarin/IRAS in all three species consists of three β-helix-rich domains. Among them, rat Nischarin is more similar to mouse Nischarin in structure and slightly different from human IRAS, but still has a great similarity. The high similarity of the spatial structure of Nischarin/IRAS suggests that they have great functional commonality.
Fig. 2.
Molecular structure and drug binding site of Nischarin/IRAS protein. (a) full-length chains of Nischarin/IRAS of mouse, rat, and human are predicted by AlphaFold Monomer v2.0, with different colors indicating the confidence level of AlphaFold 2 in the prediction accuracy. Molecular models of IRAS binding to (b) agmatine, (c) rilmenidine, and (d) fluoxetine are shown as 3D and 2D plots. Model Optimization: AVOGADRO v 1.2.0; Molecular Docking: AUTODOCK VINA v 1.1.2; Conformational Mapping: PyMOL v1.2; Docking Analysis: LIGPLUS v 2.2.4.
Contribution of Nischarin/IRAS in CNS
Nischarin/IRAS and CNS development
The migration of neurons plays an essential role in the development of the cerebral cortex [26]. Early-born neurons stay in the inner layer of the cortex, while later-born neurons migrate outward to form the outer cortical layer, which allows the cortex to form an ordered six-layer structure during development [26] (Fig. 3). The spatiotemporal expression pattern of Nischarin in developing rat brains reveals cell selectivity and regionalization characteristics [6], [9]. The expression of Nischarin was found in all six layers of the mature rat cortex (I-VI), and it was higher in the pyramidal neurons of layers IV-V [6]. Besides, its expression increased with time of cerebral cortex development [6]. In addition, Nischarin expression was also observed in neurons in the hippocampal CA region and Purkinje neurons in the cerebellum of the mature rat, but rarely in glial cells [6]. The expression of Nischarin seems to indicate its close relationship with brain development. It may regulate neuronal migration during the development of the cerebral cortex. When Nischarin was knocked down in primary cortical neurons and mouse neuroblastoma cells (Neuro-2a) in vitro experiment, the migration rate was significantly accelerated [6].
Fig. 3.
Nischarin/IRAS regulates neuronal migration in the cerebral cortex. Neuronal migration plays a central role in the formation of the mature cerebral cortex, which enables the cortex to form an ordered 6-layer structure during development. As a negative regulator of neuronal migration, Nischarin/IRAS plays an important role in the regulation of neuronal migration. Nischarin/IRAS expressed in newborn neurons increases during migration, while gradually increasing Nischarin/IRAS inhibits neuronal migration and keeps neurons at their final destination. PP: Preplate; IZ: Intermediate zone; VZ: Ventricular zone; MZ: Marginal zone; CP: Cortical plate; SP: Subplate.
Vps15, a regulatory subunit of phosphatidylinositol-3-kinase (PI3K), is involved in a variety of cellular and molecular events [27]. Vps15 mutant mice developed severe cortical neuronal migration deficits with upregulation of Nischarin expression [28]. Surprisingly, Nischarin knockdown partially reversed the cortical migration defect caused by acute Vps15 depletion [28]. These results suggest that Nischarin may serve as a negative regulator of neuronal migration.
It is known that changes in dendritic spines are closely related to the process of aging and neurodegeneration in CNS [29]. The results of our laboratory showed that the expression of Nischarin in hippocampal primary neurons increased with the culture time, and simultaneously affected the formation of neuronal dendritic spines (unpublished data). These observations reveal that the effect of Nischarin on neurons may influence the whole process of CNS development from embryo to aging.
IRAS is also highly expressed in the human nervous system, and the expression level in the fetal brain is lower than that in the adult brain [30], [31]. In newborn rats, the expression of Nischarin is also abundant in the brainstem, and the expression of Nischarin is widely observed in many regions including the motor nucleus and sensory nucleus [32]. The neurons in the brainstem send out nerve fibers to form a complex peripheral nerve network. The peripheral neurons ultimately establish connections with the CNS neurons after extensive migration processes [33].
To sum up, the spatiotemporal characteristics and cellular selectivity of Nischarin/IRAS expression in the CNS may determine its function to a great extent. Besides, Nischarin/IRAS was highly enriched in the CNS of mice, rats, and humans [6], [30], suggesting that the functions of Nischarin/IRAS in the CNS of different species might be conserved.
The role of Nischarin/IRAS in CNS injury and repair
Nischarin/IRAS and CNS injury The ability of the CNS to recover and regenerate after an injury is very limited, and different types of injuries can destroy the structure and function of neurons, eventually causing irreversible effects on the CNS [34].
The precise mechanism of CNS injury and repair is still unclear. At present, it is believed that neuronal injury may be related to direct mechanical damage and secondary oxidative stress, inflammatory factors, and cellular metabolic disorders, which jointly lead to cell death [34]. Nischarin/IRAS has been demonstrated to promote apoptosis in breast cancer [35]. However, Nischarin/IRAS in neurons seems to play a more complex role in the process of neuronal apoptosis. In addition, the regeneration of neural networks is also strongly related to the expression of Nischarin/IRAS. After peripheral nerve injury, injured axons generate new growth cones in just a few hours, but this process is strongly inhibited in the CNS [36]. Recent research suggests that it is to a certain extent due to the highly elevated expression of Nischarin/IRAS after the CNS injury, especially the SCI) [37].
Nischarin/IRAS and neuronal apoptosis The relationship between Nischarin/IRAS and neuronal apoptosis was first studied in 2003 [8], [13]. But unlike the pro-apoptotic effect of Nischarin/IRAS in cancer [35], the effect of Nischarin/IRAS on neuronal apoptosis is inconclusive. Different studies revealed that Nischarin/IRAS promoted or inhibited apoptosis in diverse environments (Fig. 4).
Fig. 4.
Nischarin/IRAS mediates neuronal responses to external injuries. The expression of Nischarin/IRAS was significantly increased in a short period when neurons were exposed to different types of harmful stimulations or the animal CNS was subjected to external mechanical damage. At the same time, Nischarin/IRAS mediates or inhibits changes in injury-induced neuronal apoptosis. Under inflammatory stimuli, Nischarin/IRAS expression was slightly upregulated, which ultimately inhibited neuronal apoptosis by activating AKT signaling and thus affecting the mitochondrial apoptosis pathway. In contrast, Nischarin/IRAS expression was significantly upregulated under oxidative stress, which had a pro-apoptotic effect on neuronal apoptosis by inhibiting the wnt signaling pathway. In addition, Nischarin/IRAS also affected the repair process of damaged axons. The high expression of local Nischarin/IRAS inhibited the phosphorylation of cofilin, thereby increasing the depolymerization effect of cofilin on F-actin. AKT: Protein kinase B; BAD: Bcl-xL/Bcl-2 associated death promoter; BAX: Bcl-2-associated X; Bcl-2: B-cell lymphoma-2; BV-2: Murine microglial cells; CNS: Central nervous system; LPS: Lipopolysaccharide; Nis/IRAS: Nischarin/IRAS; PC12: Rat pheochromocytoma cells; TCF: T-cell factor; LEF: Lymphoid enhancing factor.
When cultured rat pheochromocytoma cells (PC12) were incubated with H2O2 to simulate the oxidative stress environment after CNS injury, it was found that the expression of Nischarin/IRAS was greatly up-regulated under oxidative stress, accompanied by the activation of mitochondrial apoptosis signals and the inhibition of wnt signals. Knockdown of Nischarin/IRAS reversed the above effects [10], [11].
When PC12 cells were cultured using a conditioned medium collected after lipopolysaccharide (LPS) stimulation of murine microglial cells(BV-2) to mimic the inflammatory environment of neurons in vivo, Nischarin/IRAS expression in PC12 cells was also up-regulated. Contrary to expectations, the elevated Nischarin/IRAS expression inhibited mitochondrial apoptosis in neurons, which is in contrast to its effect under oxidative stress [9]. In addition to the inflammatory environment, when PC12 cells were subjected to starvation, the increased expression of Nischarin/IRAS also reduced PC12 cell apoptosis, which was related to the PI3K pathway [8], [13]. Notably, Nischarin/IRAS overexpression in PC12 cells caused by exogenous plasmid transfection did not promote or inhibit apoptosis in PC12 cells under normal culture conditions [13].
The above studies were conducted in PC12 cells, which have been widely used in neuroscience research due to their diversity in pharmacological manipulation and ease of culture [38]. Since apoptosis-stimulating conditions are the only known variables in these experiments, we speculate that the effect of Nischarin/IRAS on apoptosis depends on different stimulation conditions. The high expression of Nischarin/IRAS caused by the oxidative stress environment in the above experiments promoted neuronal mitochondrial apoptosis, but the elevated Nischarin/IRAS expression caused by the inflammatory environment inhibited neuronal apoptosis, which seems to be contradictory. After carefully comparing the results of different studies, we found that Nischarin/IRAS expression was increased more significantly and persistently when neurons were damaged by oxidative stress due to direct exposure to H2O2 stimulation [11], whereas the elevated Nischarin/IRAS expression caused by indirect stimulation of conditioned medium was mild and transient [9]. We speculate that there may be a threshold for the effect of Nischarin/IRAS expression on apoptosis and that a mild increase in Nischarin/IRAS expression can protect cells by inhibiting mitochondrial apoptosis through the protein kinase B (AKT)/ Bcl-xL/Bcl-2 associated death promoter (BAD)/ B-cell lymphoma-2(Bcl-2) pathway [9]. However, when its expression is significantly increased beyond this threshold, it can affect the gene transcription process by inhibiting the wnt signaling pathway [11], which eventually promotes mitochondrial apoptosis and accelerates the neuronal death process.
Although further studies are needed to verify, if this hypothesis is plausible, Nischarin/IRAS may become an important therapeutic target for CNS injury. The exogenous injury factors of the CNS are often not limited to a single variable in the experiment, and there are usually multiple factors that work together at different stages to regulate the injury process. Injury-induced Nischarin/IRAS expression readily reaches the threshold. Therefore, high expression of Nischarin/IRAS is unfavorable to damaged neurons, and targeted intervention of Nischarin/IRAS expression may protect these damaged neurons to a certain extent.
In addition, other mechanisms may also be involved in the effect of Nischarin/IRAS on neuronal apoptosis. A new study recently demonstrated that Nischarin/IRAS downregulation is protective in glial cells exposed to excitotoxicity. This was because the presence of Nischarin/IRAS promotes the internalization of glutamate transporter 1 (GLT-1), and the downregulation of GLT-1 led to impaired glutamate clearance in cells [12]. This study revealed the relationship between Nischarin/IRAS and neuronal excitatory injury and opened up new ideas for studying the role of Nischarin/IRAS in neuron injury.
Nischarin and CNS repair after injury The regeneration of neuronal axons is the basis of SCI recovery and an important integration site for multiple regulatory factors in vitro and in vivo [36], [39]. After SCI, damaged axons will break, disintegrate, and retract immediately, and the subsequent repair process is dependent on the formation and extension of growth cones [40]. It is well known that the growth of nerve growth cones mainly depends on the mechanical movement of actin [40]. The depolymerization of actin caused by the highly expressed Nischarin in the CNS is one of the reasons for the difficulty of CNS axon regeneration [37], [41]. Our laboratory found that Nischarin co-localized with filamentous actin (F-actin) in the pseudopodia-like protrusions of Neuro-2a cells, and downregulation of Nischarin promoted neurite growth in Neuro-2a cells and PC12 cells [41]. At the same time, the expression of Nischarin in the injured area of SCI rats increased significantly. When the expression of Nischarin was locally down-regulated by RNA interference technology, the recovery of motor function of SCI rats was greatly improved compared with the control group [37]. High expression of Nischarin in the CNS inhibited the phosphorylation of cofilin through the PAK1/ LIM domain kinase (LIMK)/cofilin pathway and enhanced its depolymerization effect on F-actin. This hinders the establishment and growth of growth cones and ultimately leads to axonal growth inhibition in the CNS [37], [41] (Fig. 4). In addition, the down-regulation of Nischarin also reduced the area of necrosis in the spinal cord of SCI mice. This result suggests that Nischarin is unfavorable for the repair of CNS injury, at least in SCI. Nischarin may play a role in promoting the death of neurons in the injured area [37].
Nischarin/IRAS mediates opioid tolerance and dependence
The relationship between Nischarin/IRAS and opioid tolerance has been studied for more than ten years [21], [22], [23], [42], [43], [44], [45]. Whether in cell experiments or animal experiments, Nischarin/IRAS is closely related to opioid tolerance and dependence, and changes in Nischarin/IRAS expression have even been detected in the brains of opioid abusers [21], [22], [23], [42], [43], [44], [45]. Agmatine is an endogenous ligand of IR-1 and has the effect of relieving the organism's tolerance and dependence on opioids [46]. Efaroxan is an imidazoline receptor antagonist [47]. Nischarin/IRAS is activated by agmatine and antagonized by Efaroxan [21], [22].
Nischarin/IRAS mediates agmatine remission of opioid dependence The intracellular cAMP pathway and Ca2+ pathway are involved in the molecular mechanism of long-term opioid dependence. The endogenous ligand of IRs, agmatine, has a role in the prevention of opioid dependence [21], [22]. In Chinese hamster ovary cells (CHO) expressing MOR, morphine pretreatment followed by naloxone induction increased cytoplasmic calcium and a significant overshoot of cAMP, indicating that the cells were opioid-dependent [21], [22]. Agmatine at low concentrations inhibits cAMP overshoot [21], [22], and these effects can be reversed by Efaroxan [21], [22]. The reversal of opioid dependence by agmatine requires the presence of Nischarin/IRAS. In the presence of Nischarin/IRAS, agmatine can significantly inhibit the increase of calcium ions.
Nischarin/IRAS and the regulation of MOR Like most G protein-coupled receptors, MOR maintains its activity through a cycle of desensitization, endocytosis, and resensitization [23], [48]. Among them, MOR desensitization and endocytosis are critical events in the induction of opioid tolerance and dependence by MOR agonist ligands [23]. Another important mechanism by which IRAS regulates opioid dependence is based on its interaction with MOR. The interaction of MOR through the PX domain of IRAS promotes the cycle of MOR and induces its resensitization [23]. Nischarin/IRAS hinders MOR downregulation induced by the MOR agonist d-Ala2,N-Me-Phe4, Gly5-ol-enkephalin (DAMGO), as Nischarin/IRAS inhibited lysosomal degradation of MOR in the circulation [23] (Fig. 5).
Fig. 5.
Nischarin/IRAS regulates MOR. The binding of Nischarin/IRAS to MOR inhibits the internalization of MOR and the degradation of MOR after internalization, and Nischarin/IRAS promotes the resensitization of MOR. MOR: µ-opioid receptor.
Nischarin/IRAS knockout mice and opioid dependence To study the in vivo effects of Nischarin on opioid dependence, Zhang and his colleagues constructed IRAS knockout (IRAS−/−) mice [49]. IRAS−/− mice showed developmental delays, but the more exciting findings were their changes in opioid dependence and tolerance [49].
There were no significant differences in the analgesic effect of a single morphine injection in IRAS−/− mice compared with wild-type (WT) littermates [45]. However, the development of tolerance in IRAS−/− mice was markedly accelerated after both WT and IRAS−/− mice underwent repeated morphine injections [45]. Meanwhile, IRAS−/− mice also exhibited stronger naloxone-precipitated withdrawal symptoms after long-term morphine treatment [45]. IRAS knockout also altered the MOR cycle in mice. Moreover, IRAS−/− mice showed a down-regulation of MOR in the spinal cord after long-term morphine treatment [45].
While past researchers studying IRAS and morphine withdrawal status have focused primarily on the role of IRAS in the development of physical dependence, a recent study sheds light on its role in the development of psychological dependence [50]. They also found that IRAS modulates morphine reward by activating glutamate receptors in the nucleus accumbens (NAc) of the mouse brain, but not those in the ventral tegmental area (VTA) [50].
Nischarin/IRAS expression in the PFC of opioid abusers Significant increases in IRAS have been reported in the BA9 area of the PFC in chronic opioid and mixed opioid/cocaine abusers [44]. And this up-regulation of IRAS is mainly caused by the elevated expression of membrane IRAS, but not cytoplasm IRAS. The increase of IRAS in the cell membrane is beneficial to its interaction with MOR to relieve opioid dependence and tolerance, so the up-regulation of IRAS may be a compensatory response of the body after long-term opioid abuse [44]. In addition, the MORs in the BA9 region of the PFC of opioid/cocaine abusers were not significantly changed [44], which is consistent with the phenomenon that the MORs in the spinal cord of WT mice did not change after long-term morphine treatment in animal experiments [44], [45].
Nischarin/IRAS and CNS diseases
The expression of Nischarin/IRAS in different regions of the CNS is related to the occurrence, development, and treatment of CNS diseases [14], [15], [16], [17]. Nischarin/IRAS in the amygdala inhibits the occurrence of anxiety [16]; the up-regulation of IRAS in the PFC promotes the occurrence and development of depression and autism [15], [17]. Nischarin/IRAS in the RVLM mediates the hypotensive effect of rilmenidine [14].
Nischarin/IRAS and anxiety In biological evolution, the amygdala has become the main brain region for fear and anxiety regulation [50]. Rho GTPases play the role of molecular switches in the amygdala, and its downstream molecules are also critical in the regulation of emotion [50]. When male rats were exposed to cat odor, the level of Nischarin in the amygdala was down-regulated and the Rho GTPases pathway was activated [16]. Nischarin has been shown to inhibit the Rho GTPases pathway in breast cancer [1], suggesting that Nischarin inhibits the activity of the Rho GTPase pathway in the amygdala to regulate anxiety [16] (Fig. 6).
Fig. 6.
The relationship between Nischarin/IRAS and neurological diseases. Nischarin/IRAS has different effects on different CNS diseases in different regions of the brain. Among them, Nischarin/IRAS in the RVLM is involved in the regulation of blood pressure; Nischarin/IRAS in the amygdala is closely related to the occurrence of anxiety disorders; Nischarin/IRAS expression in the PFC is related to the occurrence of autism and depression. DEHP: Di (2-ethylhexyl) phthalate; ERK1/2: Extracellular regulated protein kinases; RVLM: Rostral ventrolateral medulla; PFC: Prefrontal cortex.
Nischarin/IRAS and depression Regulation of emotion by Nischarin/IRAS is not limited to anxiety, dysregulation of Nischarin/IRAS expression has been found in the BA9 area of the PFC in patients with major depressive disorder (MDD) [17]. Although there was no significant difference in the expression of IRAS in the BA9 region of the PFC between MDD patients and normal people [17]. Surprisingly, when MDD patients were divided into two groups based on whether they were taking antidepressants, MDD patients who were not taking antidepressants showed a significant increase in IRAS in the BA9 area of the PFC compare to the normal [17]. Downregulation of IRAS was found in MDD patients taking depressants [17]. Therefore, Nischarin/IRAS may be involved in the development of MDD and participate in the mechanism of action of antidepressants (Fig. 6).
In addition, there was a significant difference in the expression of Nischarin/IRAS in platelets between patients with cocaine use disorder (CUD)-induced MDD and primary MDD [18]. Clinically, patients with CUD-induced MDD are difficult to diagnose and have a poor prognosis, and Nischarin/IRAS has the potential as a biomarker to differentiate CUD-induced and primary MDD [18].
Nischarin/IRAS and autism Nischarin was found to play a role in autistic spectrum disorder (ASD) induced by prenatal exposure to a plasticizer called di(2-ethylhexyl)phthalate (DEHP) [15]. The loss of dendritic spines in offspring mice prenatally exposed to DEHP was found to be accompanied by overexpression of Nischarin in pyramidal neurons in the PFC, and the loss of dendritic spines was significantly improved after the knockdown of Nischarin. In addition, DEHP has also been reported to have a significant effect on the downstream PAK1/cofilin pathway [15] (Fig. 6). In conclusion, the above results indicate that Nischarin is likely to be involved in the development of ASD, which further highlights the role of Nischarin in neurodevelopment.
Nischarin/IRAS and CNS blood pressure regulation Rilmenidine is an IR-1-selective agonist that exerts its central antihypertensive effect by promoting the phosphorylation of extracellular regulated protein kinases (ERK1/2) [51]. Nischarin can act on IR-1 s in the RVLM and mediate the antihypertensive effect of rilmenidine [14]. The antihypertensive response to rilmenidine was significantly attenuated in rats pretreated with Nischarin antisense ODN and was accompanied by inhibition of ERK1/2 pathway activity [14] (Fig. 6). The above results suggest that Nischarin is involved in CNS blood pressure regulation.
Signaling pathways regulated by Nischarin/IRAS in the CNS
Factors affecting Nischarin/IRAS expression in the CNS
A variety of in vivo and in vitro factors can influence the expression of Nischarin/IRAS in the CNS (Table 1), thereby broadly affecting the downstream pathways of Nischarin/IRAS. Known in vivo stimuli include mechanical SCI [37] and exogenous LPS intraventricular injection [9]. In vitro stimulatory factors include the oxidative stress stimuli H2O2 [10], [11] and the conditioned media collected from LPS-stimulated mouse microglial cells [9]. The above stimuli all led to the up-regulation of Nischarin/IRAS expression.
Table 1.
Regulators of Nischarin/IRAS expression in the CNS and downstream signaling molecules.
| Experiment Type | Stimulus | Experimental subject | Mode of action | IRAS changes | Altered signaling molecules | References |
|---|---|---|---|---|---|---|
| Cell experiment | Oxidative stress environment | PC12 cells | Cells were treated with 100 μM H2O2 | Upregulation | Bcl-2↓, Bax↑, caspase-3↑, GSK-3β↑, TCF-1↑, B-catenin↓ | [10], [11] |
| Inflammatory environment | PC12 cells | Cells were exposed to LPS-treated BV-2 cells for 24 h | Upregulation | pAKT↑, BAD↓, Bcl-2↑, caspase-3↑ | [9] | |
| Animal experiment | Simulate spinal cord injury | Female Sprague-Dawley rat | Cut the right half of the spinal cord with a razor blade after T10 laminectomy | Upregulation | GAP-43↓ | [37] |
| LPS | Male Sprague-Dawley rat | LPS injected into the lateral ventricle of the rat's brain | Upregulation | pAKT↑, BAD↓, Bcl-2↑, caspase-3↑ | [9] | |
| Cat smell exposure | Male rat | Exposure to cloth soaked in predator (cat) odor | Downregulation | Rho GTPase-activating protein ↓, Rho-specific guanine nucleotide exchange factor↓ | [16] | |
| DEHP exposure | Female ICR mice | Daily gavage of DEHP from day 3 to day 17 of pregnancy | Upregulation | cofilin↓ | [15] | |
| Human experiment | Long-term opiate and mixed opiate/cocaine abuse | Human | – | Upregulation | - | [44] |
| Major depressive disorder (MDD) | Human | – | Upregulation | - | [17] | |
| Antidepressant | Human | – | Downregulation | - | [17] |
Various in vitro and in vivo factors in the CNS will affect the expression of Nischarin/IRAS in neurons. Oxidative stress and an inflammatory environment cause Nischarin upregulation in neurons. External mechanical injury and LPS stimulation led to the up-regulation of Nischarin in the spinal cord and brain tissue of animals, respectively. Anxiety and antidepressant usage led to the downregulation of Nischarin expression in the human brain. Meanwhile, the downregulation of Nischarin in the CNS affects downstream signaling molecules.
CNS: Central nervous system; DEHP: Di (2-ethylhexyl) phthalate; LPS: Lipopolysaccharide; PC12: Rat pheochromocytoma cells.
In addition, the mental state or mental illness of humans and animals also affects the expression of Nischarin/IRAS. Under anxiety, the expression of Nischarin is down-regulated in the amygdala of rats [16]. IRAS is up-regulated in the PFC of depression and morphine abusers, and the use of antidepressants such as fluoxetine can significantly down-regulate the expression of IRAS in the BA9 region of human PFC [44], [16], [17], [18].
Nischarin/IRAS affects neuronal migration and axonal growth by regulating the Rho-GTPase signaling pathway
During the development of the cerebral cortex, neuronal migration refers to the process by which neurons migrate from the germinal layer to the outer layers of the cortex [52]. The formation and extension of the cytoskeletal element F-actin are key steps in neuronal migration [53].
In the CNS, pathways regulating neuronal migration and neuronal axon growth include the Rho-GTPase pathway [1], the focal adhesion kinase (FAK) signaling pathway [54], the EphB/ephrinB signaling pathway, and the neurite-inducing factor Netrin signaling pathway, etc [55]. Rho-GTPase, mainly composed of cell division cycle 42 (CDC42), Rac, and ras homolog gene family member A (RhoA), affects cell migration by regulating the cytoskeleton [56].
Nischarin mainly influences the migration of neurons and the outgrowth of neurites by intervening in the Rho-GTPase family and a series of downstream pathways [1]. The downstream molecules of the Rho-GTPase family include PAK1, LIMK, neural Wiskott-Aldrich syndrome protein (N-WASP), actin-related protein 2/3 (Arp2/3), etc [55], [57]. In cancer research, Nischarin inhibits the Rho GTPase family by directly inhibiting its upstream molecule integrin α5β1 [1]. In addition, Nischarin/IRAS can also combine with Rac family small GTPase 1(Rac1) and PAK1 to form a ternary combination [58].
Nischarin is strongly expressed in cerebral cortex IV-V in rat brains [6], almost exclusively in neurons but not in glial cells [6]. The follow-up study of this phenomenon in our laboratory found that Nischarin has a very significant inhibitory effect on neuronal migration and axonal growth [41], [59]. Nischarin can inhibit PAK1 [41] and then hinder its downstream LIMK-cofilin pathway [59], thereby promoting the degradation of F-actin into globular actin (G-actin), which ultimately impedes the axonal growth process of neurons [41], [59] (Fig. 7).
Fig. 7.
Nischarin/IRAS signaling pathways. Nischarin/IRAS inhibits neuronal axon growth and neuronal migration by regulating integrinα5, the Rho-GTPase family, and a series of downstream proteins. Effects of Nischarin/IRAS on L-Ca2+, NGF, MORs, and glutamate receptors inhibit the development of opioid-dependent tolerance in organisms. Meanwhile, the effect of Nischarin/IRAS on the Bcl-2 family mediates the occurrence of neuronal apoptosis. AC: Adenylate cyclase; AMPAR: Viaα-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; AMPK: AMP-activated protein kinase; BAD: Bcl-xL/Bcl-2 associated death promoter; BAX: Bcl2-associated X; BcI-2: B cell lymphoma 2; CDC42: Cell division cycle 42; CREB: cAMP-response element binding protein; DEHP: Di(2-ethylhexyl)phthalate; ERK: Extracellular signal-regulated kinase; GAP-43: Growth-associated protein-43; Gi/Go: Gi and Go protein-coupled receptors; L-Ca2+: l-type Ca2+ channels; LIMK: LIM domain kinase; MOR: µ-opioid receptor; NGF: Nerve growth factor; NMDAR: N-methyl-d-aspartate receptor; PAK1: P21 (RAC1) activated kinase 1; PKA: Protein kinase A; Rac1: Rac family small GTPase 1; RhoA: Ras homolog gene family member A.
It is worth noting that Nischarin can also affect CNS regeneration and repair by inducing the expression of growth-associated protein-43 (GAP-43) [37]. In addition, Nischarin can also be activated by phosphorylation due to EphB1-Fc stimulation [60]. Moreover, DEHP exposure in pregnant mice leads to alteration in Nischarin expression in neonatal mouse brains, accompanied by the loss of dendritic spines, which may also be related to the Rho-GTPase signaling pathway [15].
Nischarin/IRAS affects opioid dependence by regulating MOR and calcium signaling pathway
Morphine, one of the commonly prescribed opioids, can interfere with the physiological activity of neurons through the MOR [61]. After binding to its ligand, MOR stimulates Giα/Goα subunits, which can inhibit adenylate cyclase activity and affect its downstream molecules such as cAMP. In addition, MOR can also form a complex with β-arrestin after being phosphorylated and participate in pathways such as mitogen-activated protein kinase (MAPK) to exert its biological effects in neurons [62].
The biological basis for the induction of cellular opioid tolerance or dependence by chronic exposure to morphine includes molecular, cellular, and neural network adaptations [63]. Molecular adaptation mainly refers to intracellular signal transduction, which involves the cAMP, Ca2+, nitric oxide synthase (NOS) system, etc [63].
Nischarin/IRAS has been shown to block the formation of opioid tolerance or dependence by acting on both MOR and L-Ca2+ channels [64]. On the one hand, Nischarin/IRAS can interact with MOR receptors and block receptor desensitization to alleviate the progression of opioid tolerance [23]. On the other hand, Nischarin/IRAS also directly blocks morphine dependence [21], [65]. Wu et al. observed that Nischarin/IRAS blocked morphine-induced excitatory effects in neurons by stimulating the expression of Nischarin/IRAS with agmatine. They first observed the inhibitory effect of Nischarin/IRAS on cAMP [21] and confirmed that Nischarin/IRAS inhibited the Ca2+/ calmodulin-dependent protein kinases (CAMKs)/rat sarcoma(Ras)/ rapidly accelerated fibrosarcoma(Raf)/mitogen-activated protein kinase kinase(MEK) pathway and cAMP/ERK pathway by negatively regulating intracellular calcium ion concentration [21], [22]. This greatly reduced the activation of ERK1/2 [21], [22], [65]. What’s more, further studies found that the knockdown of Nischarin/IRAS would result in a lower amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) to N-methyl-d-aspartate receptor (NMDAR) ratio, which is associated with psychological addiction [50]. In addition, Piletz et al. found that Nischarin/IRAS also decreased the activation of ERK1/2 by blocking nerve growth factor (NGF) activation [66]. As the most abundant MAPK family member in neurons, ERK1/2 enters the nucleus to activate cyclic-AMP response binding protein (CREB) and then expresses C-Fos, which is an important molecular event in the process of cellular morphine addiction [64]. To sum up, Nischarin/IRAS mainly hinders the development of opioid tolerance or dependence through the above pathways (Fig. 7).
Nischarin/IRAS affects neuronal apoptosis by regulating the Bcl-2/Bax signaling pathway
Neuronal apoptosis is largely involved in various physiological and pathophysiological processes such as CNS development [67], [68], post-injury repair [69], and cognitive impairment [70]. In the process of neuronal apoptosis, the effect of Nischarin/IRAS on apoptosis has two sides.
During neuronal apoptosis, activation of the Bcl-2 family is expected to result in the release of pro-apoptotic substances in mitochondria and ultimately activates multiple caspases to induce apoptosis [67], [71]. The effect of Nischarin/IRAS on apoptosis is based on the targeted regulation of the Bcl-2 family. Nischarin/IRAS activates the Bcl-2 family under oxidative stress to promote neuronal apoptosis. While under inflammatory or starvation conditions, Nischarin/IRAS inhibits the Bcl-2 family and reduces neuronal apoptosis. Either way, it precisely regulates apoptosis-related factors such as Bcl-2, Bax, and BAD to induce or inhibit apoptosis [9], [10], [11]. The inhibitory effect of Nischarin/IRAS on apoptosis is related to its regulation of the PI3K/AKT pathway [9], [13]. Under the environment of oxidative stress, Nischarin/IRAS may induce neuronal apoptosis by inhibiting the Wnt/β-catenin pathway [10], [11]. However, the specific mechanism is still unclear (Fig. 7).
Clinical prospects of Nischarin/IRAS
Treatment for CNS diseases targeting Nischarin/IRAS
In the research on the role of Nischarin/IRAS in the CNS, the techniques of manipulating the expression of Nischarin/IRAS in cells or animals by external interference have been widely used and achieved good results [21], [22], [37], [41], [45]. Suresh K. Alahari et al. have proposed to design peptide-based drugs which simulate the function of Nischarin/IRAS to locally inhibit cancer [19], and similar techniques have now appeared in the nervous system. For example, using nanomaterials to deliver Nischarin-siRNA locally down-regulates the expression of Nischarin in the SCI mouse model greatly promoted the recovery of SCI mice [37]. In addition, overexpression or downregulation of Nischarin/IRAS through exogenous plasmid transfection had significant effects on neuronal apoptosis and opioid dependence or tolerance(Table 2 and Fig. 8).
Table 2.
Techniques and effects of interfering with Nischarin/IRAS expression in the CNS.
| Experiment Type | Technology | Experimental subject | Mode of action | IRAS changes | Effects of IRAS Alterations | References |
|---|---|---|---|---|---|---|
| Cell experiment | PEI-ALG/Nis-siRNA | Female Sprague-Dawley rat | 5 μL microinjection into the central lesion of the spinal cord | Knockdown | Improves motor function in rats with spinal cord injury, reduces the area of spinal cord necrosis, and up-regulates GAP-43 | [37] |
| NISCH siRNA | PC12 cells | Lipofectamine™ 2000 lipofection | Knockdown | Apoptosis decreased, Bcl-2↑, Bax↓, caspase-3↓, GSK-3β↓, TCF-1↓, B-catenin↑ | [10], [11] | |
| Rat Nischarin (NC_005115.3) siRNA | PC12 cells | SuperFectinII lipofection | Knockdown | Apoptosis increased, pAKT↓, BAD↑, Bcl-2↓, caspase-3↑ | [9] | |
| hIRAS cDNA (hIRAS-pcDNA3.1(+)) | CHO cells | Lipofectamine™ 2000 lipofection | Overexpression | IRAS mediates the inhibitory effect of low-concentration agmatine on cellular morphine dependence and tolerance, and directly inhibits cellular morphine tolerance | [42], [21], [22], [23] | |
| IRAS siRNA | CHO cells, Primary cultured rat hippocampal neurons | Lipofectamine™ 2000 lipofection | Knockdown | Reversal of IRAS-mediated inhibition of cellular morphine dependence by agmatine | [43], [79] | |
| Efaroxan | CHO cells, Primary cultured rat hippocampal neurons | – | Inhibition | Reversal of IRAS-mediated inhibitory effects of agmatine on cellular morphine dependence and tolerance | [21], [22], [42], [43] | |
| Moxonidine | CHO cells | – | Activation | Ineffective against cellular morphine dependence | [79] | |
| Rilmenidine | CHO cells | – | Activation | Ineffective against cellular morphine dependence | [79] | |
| Animal experiment | Nischarin siRNA | Male BALB/c mice | JetSI™ lipofection, injection into the brain | Knockdown | Reversal of IRAS-mediated agmatine-induced remission of morphine withdrawal symptoms in mice | [43] |
| Nis-shRNA | Primary cultured PFC neurons | Lipofectamine™ 3000 lipofection | Knockdown | Reversal of dendritic spine loss in prefrontal cortex neurons in offspring mice | [15] | |
|
Nischarin antisense oligodeoxynucleotide (ODN) (AS1 or AS2) |
Sprague-Dawley male rat | Intracisternal administration | Knockdown | Reversal of rilmenidine-induced hypotension and reversal of ERK1/2 activation in the RVLM | [14] | |
| IRAS knockout | EIIa-Cre mice | – | Knockdown | Increased morphine dependence and tolerance in mice | [45], [49] | |
| Efaroxan | Male BALB/c mice | 1 mg/kg intravenously | Inhibition | Reversal of IRAS-mediated agmatine-induced remission of morphine withdrawal symptoms in mice | [43] | |
| Rilmenidine | Sprague-Dawley male rat | Intracisternal administration | Activation | Hypotension and ERK1/2 activation |
Numerous studies have used small interfering RNAs, plasmids, antisense oligonucleotides, or Nischarin/IRAS knockout mice to alter the expression of Nischarin/IRAS in cells or animals to study the function of Nischarin/IRAS in the CNS. The alteration of Nischarin/IRAS expression results in dramatic changes in various CNS functions of organisms.
CNS: Central nervous system; CHO: Chinese hamster ovary cells; PC12: Rat pheochromocytoma cells.
Fig. 8.
The effect of interference of Nischarin/IRAS expression. Knockdown of Nischarin/IRAS promoted the recovery of motor function in mice after spinal cord injury, and down-regulated Nischarin/IRAS also promoted the expression of neuron-specific protein GAP-43. Meanwhile, the knockdown of Nischarin/IRAS promoted neuronal apoptosis under the inflammatory environment but inhibited apoptosis leading to neuronal survival under oxidative stress. In addition, Nischarin/IRAS overexpressed in neurons effectively inhibited the elevation of cAMP and Ca2+ induced by morphine treatment, thereby inhibiting the formation of morphine dependence in neurons. Nis: Nischarin/IRAS; IRAS: Nischarin/IRAS; GAP-43: Growth-associated protein-43; ROS: Reactive oxygen species; cAMP: Cyclic adenosine monophosphate; Ca2+: Calcium.
However, how to deliver exogenous siRNA or plasmid to the correct location and play a role is the key to promoting the clinical translation of Nischarin/IRAS as a therapeutic target. These functional oligonucleotides cannot enter cells spontaneously, and a large number of free oligonucleotides in circulation may lead to inefficient accumulation in non-target organs, eventually leading to off-target effects [75]. We note that a class of nucleic acid nanomaterials known as tetrahedral framework nucleic acids (tFNAs) has recently attracted considerable attention [75]. tFNAs have been reported to have properties superior to those that cross the blood–brain barrier (BBB) [77]. It was also reported that tFNA carrying siRNA was detectable in the brain but not in other organs 1 h after local injection into the ventricles, suggesting that tFNA helps avoid off-target effects [75]. miRNA-22-3p is a microRNA with neuroregenerative potential. Loading of miRNA-22-3p by tFNA ultimately promotes axonal and myelin regeneration after peripheral nervous system injury [76]. These initially demonstrated the therapeutic value of tFNA in the field of neurological disease treatment [76], [77]. Combining Nischarin/IRAS-related targeting functional oligonucleotides with tFNA may be a suitable approach for clinical translation. We believe that future research can focus on using tFNA as a carrier to precisely regulate the expression of local Nischarin/IRAS, which is expected to achieve satisfactory therapeutic effects in a variety of central nervous system diseases.
In addition, there are multiple inhibitors or activators specifically designed for Nischarin/IRAS. In DrugBank (https://go.drugbank.com/), there are at least four activators or inhibitors of Nischarin/IRAS. Among them, agmatine has been used in studies of opioid dependence [21], [22]. Importantly, these Nischarin/IRAS-targeted reagents have all passed clinical trials and have been used in the clinical treatment of other diseases for many years. For example, tizanidine can reduce muscle spasms in patients with post-stroke spastic hemiplegia [72]. Moxonidine's inhibition of the central sympathetic nerve significantly reduces the occurrence of atrial fibrillation in patients with hypertension after radiofrequency ablation [73]. Moreover, the side effect of the reagents is acceptable [72], [73]. With the deepening understanding of the role of Nischarin/IRAS in CNS pathophysiological processes, it is expected that these drugs will generate new therapeutic value in the treatment of CNS diseases.
Drug binding sites of Nischarin/IRAS
In the CNS, agmatine has been found to play a neuroprotective role when the organism is subjected to a variety of external stimuli including mechanical damage, ischemia and hypoxia, and oxidative stress, and it also has clinical application prospects for the treatment of psychiatric diseases [74], [75]. Rilmenidine is a classic central antihypertensive agent [76], and in recent years it has also been found to have therapeutic value in neurodegenerative diseases such as Huntington's disease [77]. Fluoxetine is a widely used antidepressant, and a large number of clinical trials are still carried out to explore the potential value of fluoxetine in the treatment of depression [78].
Nischarin/IRAS can bind to various imidazoline ligands and affect CNS function [14], [79]. The combination of Nischarin/IRAS and agmatine inhibits the dependence and tolerance of opioids [21], [22], and the interaction of Nischarin/IRAS and rilmenidine exerts a blood pressure-lowering effect [14]. In addition, it has been reported in the literature that antidepressants such as fluoxetine and imipramine can down-regulate the expression of Nischarin/IRAS [17]. We speculate that they play the antidepressant effect by binding to Nischarin/IRAS.
Nischarin/IRAS is related to biological opioid dependence and tolerance, CNS blood pressure regulation, and the occurrence and treatment of depression [14], [17], [21], [22]. Therefore, we predicted the binding relationship between Nischarin/IRAS and the drugs clinically used to treat the above-mentioned CNS diseases to further estimate the potential role of Nischarin/IRAS in the treatment of CNS diseases. We used AutoDock Vina software (v 1.1.2) to molecularly dock the Nischarin/IRAS predicted by alphafold 2 with the drug, and then also analyzed this interaction using Ligplus Software (v 2.2.4). As shown in Fig. 2, the Arg1260, Asp1384, Glu1382 and Arg1402 residues of IRAS can form hydrogen bonds with agmatine (binding energy: −5.4 kcal/mol); the Ser51 residues of IRAS form hydrogen bonds with rilmenidine (binding energy: −5.8 kcal/mol); hydrogen bonds are formed between the His319 and Tyr341 residues of IRAS and fluoxetine (binding energy: −7.5 kcal/mol). This indicates that IRAS has a good binding capacity to agmatine, rilmenidine, and fluoxetine [80]. An in-depth understanding of the molecular mechanism of Nischarin/IRAS in the future will hopefully lead to the development of novel therapeutic drugs for various CNS diseases targeting Nischarin/IRAS.
Conclusions
Nischarin/IRAS, as a therapeutic target, will help the treatment of various diseases of the nervous system in the future, but there is still a long way to go for Nischarin-targeted drugs to be applied clinically. At present, the challenge we face is that the molecular mechanisms of Nischarin/IRAS in the development of the nervous system or in the development of different nervous system injuries and diseases are still not clear. For example, the specific role of Nischarin/IRAS in neuronal injury remains controversial. More in-depth studies are needed in the future to determine the specific role of Nischarin/IRAS in neuronal injury. Furthermore, research on the role of Nischarin/IRAS in conditions such as autism, depression, and anxiety appears to be experimental. Systematic studies are needed to reveal the specific mechanisms of Nischarin/IRAS in the development of these diseases. In addition, existing research still has some difficulties in achieving precise regulation of Nischarin/IRAS, although Nischarin/IRAS inhibitors, activators, or plasmids targeting Nischarin/IRAS can regulate the expression of Nischarin/IRAS. Among them, the efficacy of existing Nischarin/IRAS inhibitors or activators is unknown. In the future, it is necessary to understand the intensity, duration, and specific target tissues or organs of these drugs on Nischarin/IRAS expression in vivo. The use of Nischarin/IRAS-targeted plasmids requires finding and testing a suitable vector, which needs to meet multiple requirements such as being able to cross the BBB, being able to be enriched in specific organs, and having biological safety. Nevertheless, the prospect of developing drugs targeting Nischarin/IRAS to treat various neurological diseases in the future is very optimistic.
In summary, with its complex molecular interaction, Nischarin/IRAS extensively regulates a variety of biological behaviors in the CNS from development to aging, from injury to repair [6], [37], [41]. Nischarin/IRAS may also play an important role in the embryonic development of peripheral neurons [32]. With the in-depth research on the relationship between Nischarin/IRAS and brain development, Nischarin/IRAS is also expected to contribute to brain aging and neurodegenerative diseases [11], [41]. Subsequent research is expected to target Nischarin/IRAS to treat and prevent various CNS diseases such as autism [15], anxiety [16], depression [17], [18], etc.
Ethics approval and consent to participate
Not applicable.
CRediT authorship contribution statement
PeijieZheng: Conceptualization, Writing – original & editing, Visualization. ChenshuPan: Writing – original & editing, Visualization. ChuntaoZhou: Visualization, Formal analysis. BinLiu: Visualization. LinlinWang: Conceptualization. ShiweiDuan: Conceptualization, Writing – review & editing. YueminDing: Conceptualization, Writing – review & editing, Funding acquision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Some of the Figures were created through BioRender.com.
Consent for publication
The Authors confirm: (1) that the work described has not been published before; (2) that it is not under consideration for publication elsewhere; (3) that its publication has been approved by all co-authors if any; (4) that its publication has been approved (tacitly or explicitly) by the responsible authorities at the institution where the work is carried out.
Availability of data and materials
Not applicable.
Funding
This research was funded by the Zhejiang Provincial Public Welfare Technology Research Program/Experimental Animal Project (LGD19H090008).
Biographies
Peijie Zheng is currently a senior medical student of the Department of Clinical Medicine, Zhejiang University City College. He is engaged in research related to the effects of Nischarin on cell migration. He has published a review paper in Journal of Experimental & Clinical Cancer Research as the first author.
Chenshu Pan is a senior medical student of the Department of Clinical Medicine, Zhejiang University City College. His is interested in the regulatory mechanism of neuronal cytoskeleton.
Chuntao Zhou is a medical student of the Department of Clinical Medicine, Zhejiang University City College. At present, he is mainly engaged in the research on Nischarin regulating cell proliferation and migration. He has published a review paper in Journal of Experimental & Clinical Cancer Research as a co-author.
Bin Liu is a medical student of the Department of Clinical Medicine, Zhejiang University City College. He is the co-author of the review paper published in Journal of Experimental & Clinical Cancer Research.
Linlin Wang is an associate professor in Zhejiang University School of Medicine. In 2003, she received her PhD from Zhejiang University. From 2003 to 2005, she was a postdoctoral fellow in Hokkaido University, Japan. She is mainly engaged in basic and transformation research on neuronal repair and regeneration after spinal cord injury.

Shiwei Duan is currently a professor at Zhejiang University City College School of Medicine. He received his Ph.D. degree in molecular and cell biology from Shanghai Jiaotong University in 2005. After that, he successively worked at the University of Chicago School of Medicine (2005-2009), the Singapore Agency for Science, Technology, and Research (A*STAR) in Singapore (2009-2011), and Ningbo University School of Medicine (2011-2021). He is a member of the American Association for Cancer Research, a member of the American Society of Human Genetics, a member of the National Pharmacogenomics Professional Committee, Member of the Chinese Pharmacological Society.

Yuemin Ding is currently a professor at Zhejiang University City College, School of Medicine. She graduated from Zhejiang Medical University in 1998 and received her M.D. degree. She received her M.Sc. degree in Clinical Neuroscience from University College London in 2003 and her Ph.D. degree in Physiology from Zhejiang University in 2014. From 2004 to 2006, when she was a visiting scholar at the Health Science Center of Louisiana State University in the United States, she began to follow Dr. Suresh Alahari, the discoverer of Nischarin protein, to study its anti-tumor function. Since then, she has been devoted to studying the function of Nischarin in the central nervous system. She is a member of the Chinese Neuroscience Society.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.01.020.
Contributor Information
Shiwei Duan, Email: duansw@zucc.edu.cn.
Yuemin Ding, Email: dingyuemin@zucc.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Alahari S.K., Lee J.W., Juliano R.L. Nischarin, a novel protein that interacts with the integrin alpha5 subunit and inhibits cell migration. J Cell Biol. 2000;151(6):1141–1154. doi: 10.1083/jcb.151.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J., Abdel-Rahman A.A. Nischarin as a functional imidazoline (I1) receptor. FEBS Lett. 2006;580(13):3070–3074. doi: 10.1016/j.febslet.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z., Chang C.H., Ernsberger P. Identification of IRAS/Nischarin as an I1-imidazoline receptor in PC12 rat pheochromocytoma cells. J Neurochem. 2007;101(1):99–108. doi: 10.1111/j.1471-4159.2006.04413.x. [DOI] [PubMed] [Google Scholar]
- 4.Piletz J.E., Ivanov T.R., Sharp J.D., Ernsberger P., Chang C.H., Pickard R.T., et al. Imidazoline receptor antisera-selected (IRAS) cDNA: cloning and characterization. DNA Cell Biol. 2000;19(6):319–329. doi: 10.1089/10445490050043290. [DOI] [PubMed] [Google Scholar]
- 5.Arnoux A., Aubertin G., Da Silva S., Weiss M., Bousquet P., Monassier L., et al. Nischarin Is Not the Functional I1 Imidazoline Receptor Involved in Blood Pressure Regulation. J Cardiovasc Pharmacol. 2022;79(2):229–234. doi: 10.1097/FJC.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 6.Ding Y., Zhang R., Zhang K., Lv X., Chen Y., Li A., et al. Nischarin is differentially expressed in rat brain and regulates neuronal migration. PLoS One. 2013;8(1):e54563. doi: 10.1371/journal.pone.0054563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alahari S.K. Nischarin inhibits Rac induced migration and invasion of epithelial cells by affecting signaling cascades involving PAK. Exp Cell Res. 2003;288(2):415–424. doi: 10.1016/s0014-4827(03)00233-7. [DOI] [PubMed] [Google Scholar]
- 8.Dontenwill M., Pascal G., Piletz J.E., Chen M., Baldwin J., Rondé P., et al. IRAS, the human homologue of Nischarin, prolongs survival of transfected PC12 cells. Cell Death Differ. 2003;10(8):933–935. doi: 10.1038/sj.cdd.4401275. [DOI] [PubMed] [Google Scholar]
- 9.Wu X., Xu W., Cui G., Yan Y., Wu X., Li L., et al. The expression pattern of Nischarin after lipopolysaccharides (LPS)-induced neuroinflammation in rats brain cortex. Inflamm Resofficial J European Histamine Research Society [et al] 2013;62(11):929–940. doi: 10.1007/s00011-013-0631-2. [DOI] [PubMed] [Google Scholar]
- 10.Guo Z., Yuan Y., Guo Y., Wang H., Song C., Huang M. Nischarin attenuates apoptosis induced by oxidative stress in PC12 cells. Exp Ther Med. 2019;17(1):663–670. doi: 10.3892/etm.2018.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Z., Huang M., Yuan Y., Guo Y., Song C., Wang H., et al. Nischarin downregulation attenuates cell injury induced by oxidative stress via Wnt signaling. Neuroreport. 2020;31(17):1199–1207. doi: 10.1097/WNR.0000000000001536. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Bazargani N, Drew J, Howden JH, Modi S, Al Awabdh S, et al. The non-adrenergic imidazoline-1 receptor protein nischarin is a key regulator of astrocyte glutamate uptake. iScience. 2022;25(4):104127. [DOI] [PMC free article] [PubMed]
- 13.Dontenwill M., Piletz J.E., Chen M., Baldwin J., Pascal G., Ronde P., et al. IRAS is an anti-apoptotic protein. Ann N Y Acad Sci. 2003;1009:400–412. doi: 10.1196/annals.1304.054. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J., Abdel-Rahman A.A. Inhibition of nischarin expression attenuates rilmenidine-evoked hypotension and phosphorylated extracellular signal-regulated kinase 1/2 production in the rostral ventrolateral medulla of rats. J Pharmacol Exp Ther. 2008;324(1):72–78. doi: 10.1124/jpet.107.129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Huang J., Zheng G., Liang J., Hu B., Lou Z., et al. Prenatal exposure to di (2-ethylhexyl) phthalate causes autism-like behavior through inducing Nischarin expression in the mouse offspring. Biochem Biophys Res Commun. 2021;585:29–35. doi: 10.1016/j.bbrc.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Kõks S., Luuk H., Nelovkov A., Areda T., Vasar E. A screen for genes induced in the amygdaloid area during cat odor exposure. Genes Brain Behav. 2004;3(2):80–89. doi: 10.1046/j.1601-183x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 17.Keller B., García-Sevilla J.A. Dysregulation of IRAS/nischarin and other potential I(1)-imidazoline receptors in major depression postmortem brain: Downregulation of basal contents by antidepressant drug treatments. J Affect Disord. 2017;208:646–652. doi: 10.1016/j.jad.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Keller B., Mestre-Pinto J.I., Álvaro-Bartolomé M., Martinez-Sanvisens D., Farre M., García-Fuster M.J., et al. A Biomarker to Differentiate between Primary and Cocaine-Induced Major Depression in Cocaine Use Disorder: The Role of Platelet IRAS/Nischarin (I(1)-Imidazoline Receptor) Front Psych. 2017;8:258. doi: 10.3389/fpsyt.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maziveyi M., Alahari S.K. Breast Cancer Tumor Suppressors: A Special Emphasis on Novel Protein Nischarin. Cancer Res. 2015;75(20):4252–4259. doi: 10.1158/0008-5472.CAN-15-1395. [DOI] [PubMed] [Google Scholar]
- 20.Okpechi S.C., Yousefi H., Nguyen K., Cheng T., Alahari N.V., Collins-Burow B., et al. Role of Nischarin in the pathology of diseases: a special emphasis on breast cancer. Oncogene. 2022;41(8):1079–1086. doi: 10.1038/s41388-021-02150-4. [DOI] [PubMed] [Google Scholar]
- 21.Wu N., Su R.B., Xu B., Lu X.Q., Liu Y., Zheng J.Q., et al. IRAS, a candidate for I1-imidazoline receptor, mediates inhibitory effect of agmatine on cellular morphine dependence. Biochem Pharmacol. 2005;70(7):1079–1087. doi: 10.1016/j.bcp.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Wu N., Su R.B., Liu Y., Lu X.Q., Zheng J.Q., Cong B., et al. Modulation of agmatine on calcium signal in morphine-dependent CHO cells by activation of IRAS, a candidate for imidazoline I1 receptor. Eur J Pharmacol. 2006;548(1–3):21–28. doi: 10.1016/j.ejphar.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Li F., Ma H., Wu N., Li J. IRAS Modulates Opioid Tolerance and Dependence by Regulating μ Opioid Receptor Trafficking. Mol Neurobiol. 2016;53(7):4918–4930. doi: 10.1007/s12035-015-9417-6. [DOI] [PubMed] [Google Scholar]
- 24.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50(D1):D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agirman G., Broix L., Nguyen L. Cerebral cortex development: an outside-in perspective. FEBS Lett. 2017;591(24):3978–3992. doi: 10.1002/1873-3468.12924. [DOI] [PubMed] [Google Scholar]
- 27.Lindmo K., Brech A., Finley K.D., Gaumer S., Contamine D., Rusten T.E., et al. The PI 3-kinase regulator Vps15 is required for autophagic clearance of protein aggregates. Autophagy. 2008;4(4):500–506. doi: 10.4161/auto.5829. [DOI] [PubMed] [Google Scholar]
- 28.Gstrein T., Edwards A., Přistoupilová A., Leca I., Breuss M., Pilat-Carotta S., et al. Mutations in Vps15 perturb neuronal migration in mice and are associated with neurodevelopmental disease in humans. Nat Neurosci. 2018;21(2):207–217. doi: 10.1038/s41593-017-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker C.K., Herskowitz J.H. Dendritic Spines: Mediators of Cognitive Resilience in Aging and Alzheimer's Disease. Neuroscientist: Rev J Bringing Neurobiol, Neurol Psychiatry. 2021;27(5):487–505. doi: 10.1177/1073858420945964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics: MCP. 2014;13(2):397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duff M.O., Olson S., Wei X., Garrett S.C., Osman A., Bolisetty M., et al. Genome-wide identification of zero nucleotide recursive splicing in Drosophila. Nature. 2015;521(7552):376–379. doi: 10.1038/nature14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagakura Y., Ide R., Saiki C., Sato Hashizume N., Imai T. Expression of nischarin, an imidazoline 1 receptor candidate protein, in the ventrolateral medulla of newborn rats. Neurosci Lett. 2021;761 doi: 10.1016/j.neulet.2021.136113. [DOI] [PubMed] [Google Scholar]
- 33.Holt E., Stanton-Turcotte D., Iulianella A. Development of the Vertebrate Trunk Sensory System: Origins, Specification, Axon Guidance, and Central Connectivity. Neuroscience. 2021;458:229–243. doi: 10.1016/j.neuroscience.2020.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Anjum A., Yazid M.D., Fauzi Daud M., Idris J., Ng A.M.H., Selvi Naicker A., et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020;21(20) doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang C., Wei W., Han D., Meng J., Zhu F., Xiao Y., et al. Expression of Nischarin negatively correlates with estrogen receptor and alters apoptosis, migration and invasion in human breast cancer. Biochem Biophys Res Commun. 2017;484(3):536–542. doi: 10.1016/j.bbrc.2017.01.109. [DOI] [PubMed] [Google Scholar]
- 36.Filous A.R., Schwab J.M. Determinants of Axon Growth, Plasticity, and Regeneration in the Context of Spinal Cord Injury. Am J Pathol. 2018;188(1):53–62. doi: 10.1016/j.ajpath.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding Y.M., Li Y.Y., Wang C., Huang H., Zheng C.C., Huang S.H., et al. Nischarin-siRNA delivered by polyethylenimine-alginate nanoparticles accelerates motor function recovery after spinal cord injury. Neural Regen Res. 2017;12(10):1687–1694. doi: 10.4103/1673-5374.217348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiatrak B., Kubis-Kubiak A., Piwowar A., Barg E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells. 2020;9(4) doi: 10.3390/cells9040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Shea T.M., Burda J.E., Sofroniew M.V. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127(9):3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omotade O.F., Pollitt S.L., Zheng J.Q. Actin-based growth cone motility and guidance. Mol Cell Neurosci. 2017;84:4–10. doi: 10.1016/j.mcn.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding Y., Li Y., Lu L., Zhang R., Zeng L., Wang L., et al. Inhibition of Nischarin Expression Promotes Neurite Outgrowth through Regulation of PAK Activity. PLoS One. 2015;10(12):e0144948. doi: 10.1371/journal.pone.0144948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y., Li F., Wu N., Su R.B., Liu Y., Lu X.Q., et al. Effect of agmatine on DAMGO-induced mu-opioid receptor down-regulation and internalization via activation of IRAS, a candidate for imidazoline I(1) receptor. Eur J Pharmacol. 2008;599(1–3):18–23. doi: 10.1016/j.ejphar.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Li F., Wu N., Su R., Chen Y., Lu X., Liu Y., et al. Imidazoline receptor antisera-selected/Nischarin regulates the effect of agmatine on the development of morphine dependence. Addict Biol. 2012;17(2):392–408. doi: 10.1111/j.1369-1600.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 44.Keller B., La Harpe R., García-Sevilla J.A. Upregulation of IRAS/nischarin (I(1)-imidazoline receptor), a regulatory protein of μ-opioid receptor trafficking, in postmortem prefrontal cortex of long-term opiate and mixed opiate/cocaine abusers. Neurochem Int. 2017;108:282–286. doi: 10.1016/j.neuint.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Li S., Wu N., Zhao T.Y., Lu G.Y., Wang Z.Y., Li F., et al. The role of IRAS/Nischarin involved in the development of morphine tolerance and physical dependence. Biochem Biophys Res Commun. 2019;512(3):460–466. doi: 10.1016/j.bbrc.2019.03.055. [DOI] [PubMed] [Google Scholar]
- 46.Wu N., Su R.B., Li J. Agmatine and imidazoline receptors: their role in opioid analgesia, tolerance and dependence. Cell Mol Neurobiol. 2008;28(5):629–641. doi: 10.1007/s10571-007-9164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egan J.A., Filer C.N. Tritium labelling and characterization of the potent imidazoline I1 receptor antagonist [5,7–3H] (+/-)-efaroxan at high specific activity. Appl Radiat Isot: Including Data, Instrument Methods Use Agric, Ind Med. 2003;58(6):675–677. doi: 10.1016/s0969-8043(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 48.Martini L., Whistler J.L. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17(5):556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L., Zhao T.Y., Hou N., Teng Y., Cheng X., Wang B., et al. Generation and primary phenotypes of imidazoline receptor antisera-selected (IRAS) knockout mice. CNS Neurosci Ther. 2013;19(12):978–981. doi: 10.1111/cns.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarowar T., Grabrucker A.M. Rho GTPases in the Amygdala-A Switch for Fears? Cells. 2020;9(9) doi: 10.3390/cells9091972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reid J.L. Rilmenidine: a clinical overview. Am J Hypertens. 2000;13(6 Pt 2):106s–s111. doi: 10.1016/s0895-7061(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 52.Nadarajah B., Brunstrom J.E., Grutzendler J., Wong R.O., Pearlman A.L. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4(2):143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 53.Dogterom M., Koenderink G.H. Actin-microtubule crosstalk in cell biology. Nat Rev Mol Cell Biol. 2019;20(1):38–54. doi: 10.1038/s41580-018-0067-1. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X., Guan J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63(8):610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niftullayev S., Lamarche-Vane N. Regulators of Rho GTPases in the Nervous System: Molecular Implication in Axon Guidance and Neurological Disorders. Int J Mol Sci. 2019;20(6) doi: 10.3390/ijms20061497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridley A.J. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–112. doi: 10.1016/j.ceb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C., Wirth A., Ponimaskin E. Cdc42: an important regulator of neuronal morphology. Int J Biochem Cell Biol. 2012;44(3):447–451. doi: 10.1016/j.biocel.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Alahari S.K., Reddig P.J., Juliano R.L. The integrin-binding protein Nischarin regulates cell migration by inhibiting PAK. EMBO J. 2004;23(14):2777–2788. doi: 10.1038/sj.emboj.7600291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding Y., Milosavljevic T., Alahari S.K. Nischarin inhibits LIM kinase to regulate cofilin phosphorylation and cell invasion. Mol Cell Biol. 2008;28(11):3742–3756. doi: 10.1128/MCB.01832-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darie C.C., Deinhardt K., Zhang G., Cardasis H.S., Chao M.V., Neubert T.A. Identifying transient protein-protein interactions in EphB2 signaling by blue native PAGE and mass spectrometry. Proteomics. 2011;11(23):4514–4528. doi: 10.1002/pmic.201000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moreno-Rius J. Opioid addiction and the cerebellum. Neurosci Biobehav Rev. 2019;107:238–251. doi: 10.1016/j.neubiorev.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Gintzler A.R., Chakrabarti S. Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci. 2006;79(8):717–722. doi: 10.1016/j.lfs.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 63.Williams J.T., Christie M.J., Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 64.Przewlocki R. Opioid abuse and brain gene expression. Eur J Pharmacol. 2004;500(1–3):331–349. doi: 10.1016/j.ejphar.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 65.Bilecki W., Przewłocki R. Effect of opioids on Ca2+/cAMP responsive element binding protein. Acta Neurobiol Exp. 2000;60(4):557–567. doi: 10.55782/ane-2000-1376. [DOI] [PubMed] [Google Scholar]
- 66.Piletz J.E., Wang G., Zhu H. Cell signaling by imidazoline-1 receptor candidate, IRAS, and the nischarin homologue. Ann N Y Acad Sci. 2003;1009:392–399. doi: 10.1196/annals.1304.053. [DOI] [PubMed] [Google Scholar]
- 67.Fricker M., Tolkovsky A.M., Borutaite V., Coleman M., Brown G.C. Neuronal Cell Death. Physiol Rev. 2018;98(2):813–880. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pemberton J.M., Pogmore J.P., Andrews D.W. Neuronal cell life, death, and axonal degeneration as regulated by the BCL-2 family proteins. Cell Death Differ. 2021;28(1):108–122. doi: 10.1038/s41418-020-00654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Egawa N., Lok J., Washida K., Arai K. Mechanisms of Axonal Damage and Repair after Central Nervous System Injury. Transl Stroke Res. 2017;8(1):14–21. doi: 10.1007/s12975-016-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheon S.Y., Cho K.J. Pathological role of apoptosis signal-regulating kinase 1 in human diseases and its potential as a therapeutic target for cognitive disorders. J Mol Med (Berl) 2019;97(2):153–161. doi: 10.1007/s00109-018-01739-9. [DOI] [PubMed] [Google Scholar]
- 71.Pistritto G., Trisciuoglio D., Ceci C., Garufi A., D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8(4):603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maupas E., Marque P., Roques C.F., Simonetta-Moreau M. Modulation of the transmission in group II heteronymous pathways by tizanidine in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry. 2004;75(1):130–135. [PMC free article] [PubMed] [Google Scholar]
- 73.Giannopoulos G., Kossyvakis C., Efremidis M., Katsivas A., Panagopoulou V., Doudoumis K., et al. Central sympathetic inhibition to reduce postablation atrial fibrillation recurrences in hypertensive patients: a randomized, controlled study. Circulation. 2014;130(16):1346–1352. doi: 10.1161/CIRCULATIONAHA.114.010999. [DOI] [PubMed] [Google Scholar]
- 74.Kotagale N.R., Taksande B.G., Inamdar N.N. Neuroprotective offerings by agmatine. Neurotoxicology. 2019;73:228–245. doi: 10.1016/j.neuro.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Watts D., Pfaffenseller B., Wollenhaupt-Aguiar B., Paul Géa L., Cardoso T.A., Kapczinski F. Agmatine as a potential therapeutic intervention in bipolar depression: the preclinical landscape. Expert Opin Ther Targets. 2019;23(4):327–339. doi: 10.1080/14728222.2019.1581764. [DOI] [PubMed] [Google Scholar]
- 76.Safar M.E. Rilmenidine: a novel antihypertensive agent. Am J Med. 1989;87(3c):24s–s29. doi: 10.1016/0002-9343(89)90501-9. [DOI] [PubMed] [Google Scholar]
- 77.Underwood B.R., Green-Thompson Z.W., Pugh P.J., Lazic S.E., Mason S.L., Griffin J., et al. An open-label study to assess the feasibility and tolerability of rilmenidine for the treatment of Huntington's disease. J Neurol. 2017;264(12):2457–2463. doi: 10.1007/s00415-017-8647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davey C.G., Chanen A.M., Hetrick S.E., Cotton S.M., Ratheesh A., Amminger G.P., et al. The addition of fluoxetine to cognitive behavioural therapy for youth depression (YoDA-C): a randomised, double-blind, placebo-controlled, multicentre clinical trial. Lancet Psychiatry. 2019;6(9):735–744. doi: 10.1016/S2215-0366(19)30215-9. [DOI] [PubMed] [Google Scholar]
- 79.Li F., Wu N., Su R.B., Liu Y., Lu X.Q., Li J. Comparison of agmatine with moxonidine and rilmenidine in morphine dependence in vitro: role of imidazoline I(1) receptors. Eur J Pharmacol. 2009;612(1–3):1–8. doi: 10.1016/j.ejphar.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 80.Wan S., Bhati A.P., Zasada S.J., Coveney P.V. Rapid, accurate, precise and reproducible ligand-protein binding free energy prediction. Interface focus. 2020;10(6):20200007. doi: 10.1098/rsfs.2020.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.