Abstract
Diabetes and hypertension have been declared as a global health menace of the 21st Century. Thus, the search for potential therapeutic agents from medicinal plants for the management of diabetes and hypertension is important. This study was undertaken to investigate medicinal plants being used in the management of diabetes and hypertension by herbalists in Ghana. Data were obtained from 36 herbalists through questionnaire interviews and conversations. Botanical specimens were collected, processed and identified following standard ethnobotanical methods. Data were analyzed using Fidelity Level (FL) and Informant Consensus Factor (ICF). A total of 39 species of plants belonging to 31 families were reported being used for management of diabetes and hypertension. Eighteen of these plants are used for the treatment of hypertension, 12 species for diabetes, and 9 species for management of both diseases. Informant consensus factor was highest for plants used to treat both diseases (IFC = 0.82) followed by hypertension (ICF = 0.31) and then diabetes (IFC = 0.24). FL values were high for Carica papaya L. Moringa oleifera Lam. and Khaya senegalensis A. Juss. for the management of both diabetes and hypertension. Of the 14 species used for hypertension, Tetrapleura tetraptera (Schum. ex. Thonn.) recorded the highest FL value whiles Momordica charantia L. recorded the highest FL value for antidiabetic plants. Baphia nitida G. Lodd, Luffa aegyptiaca Mill. and Tapinanthus banguwensis (Engl. & k. Krause) Dancing are being mentioned for the first time in the management of hypertension. Herbal medicines for treatments of both diabetes and hypertension were usually prepared from multiple plant prescriptions by boiling the plant parts, and the decoctions drunk for treatments. The results show that there is substantial preclinical evidence to support the usefulness of some of these herbs as an important choice for patients with diabetes and hypertension. However, clinical studies are important to confirm the efficacy and safety of the herbal medicines prescribed by herbalists.
Keywords: Diabetes, Hypertension, Ethnobotany, Herbalist, Ghana
1. Introduction
Since time immemorial medicinal plants have, and continue to play a key role in the healthcare delivery system in the world, particularly in developing countries. It is reported that about 80 % of the population in developing countries depend on traditional medicine, largely herbal medicine, for their primary health care and socio-economic needs [1,2]. In Africa, many areas lack hospitals and even where they are available, they are far from most of the populace. For instance, Torres-León et al. [3] reported that local people have to walk for at least 53 km to access the conventional healthcare system, and thus resorted to plant medicine for their healthcare needs.
Medicinal plants have been reported as being used in the treatment and management of many diseases such as malaria [[4], [5]], tuberculosis [6,7], hepatitis B [8] and typhoid fever [9] in both developing and developed countries of the world. As a result, the World Health Organization has recommended its adoption into the mainstream health system including some herbal products on the essential medicine list [10]. Efforts to improve and consolidate herbal medicine have been enormous with the availability of scientific evidence supporting the efficacy of herbal medicine [[11], [12], [13]]. Studies have explored the importance of medicinal plants in the management of diabetes [14], and hypertension [15] in other jurisdictions. However, in Ghana there is inadequate knowledge about medicinal plants being used by herbalists in different areas in the management of diabetes and hypertension declared as a global health menace of the 21st Century [16,17].
Diabetes mellitus is a chronic endocrine disorder that adversely alters the metabolism of carbohydrates, proteins, fat, electrolytes and water, predominantly due to a derangement in insulin production and/or action [18]. Diabetes is associated with long-term complications such as heart disease, stroke, kidney failure, blindness, nerve damage and neuropathy, atherosclerosis, chronic infections, immune deficiency and peripheral vascular disease that may lead to ulcers, gangrene and amputation [19,20]. Studies have reported on two main types of diabetes mellitus, namely, Type 1 and Type 2 [21,22]. Type 1 diabetes is mostly attributed to inadequate secretion of insulin resulting in chronic hyperglycemia [21]. Type 2 diabetes mellitus is reported to be more prevalent than all types of diabetes and affects about 90 % of the global population [23], which is caused by inefficient metabolic processes or organ damage or malfunction. Bahrami and Gerstein [24] noted that type 2 diabetes mellitus is a serious public health menace as 50 % of people living with it are undiagnosed. It has been estimated that about 7 % of the world's population has diabetes [25,26], and 80 % of such cases are reported in developing countries. The eighth edition of the International Diabetes Federation (IDF) Atlas report noted that Sub-Saharan Africa accounts for 77 % of all diabetes-related deaths in people under 60 years worldwide. Likewise, IDF projected prevalence rate of diabetes in Africa is 156 % by the year 2045 [27]. According to [28] the incidence rate of diabetes in Ghana is 4.1 % and it is expected to rise to 5.0 % by 2030. Similarly, available statistics indicate that of all disease conditions reported in the various hospital across Ghana, diabetes-related complications accounts for about 6 % between 2002 and 2017 [29].
Hypertension is the most common risk factor for acute myocardial infarction, stroke, and peripheral vascular disease, and is the major known factor for cardiovascular disease and its mortality [30,31]. The major causes of hypertension include excessive alcohol intake, obesity, stress, too much salt intake, oral contraceptive drugs, renal failure, tobacco use, lack of exercise and hereditary-related [32,33]. It is reported that about 40 % of the world's adult population (25 years and above) have been identified with hypertension, out of this; Africa leads with 46 % [[21], [34]]. Hypertension has been classified broadly into two main types; primary and secondary hypertension. Primary hypertension results in high Blood Pressure for which no medical reason has been confirmed and constitutes about 90–95 % of all reported cases [35]. While, secondary hypertension is induced by conditions that affect the heart, kidney, arteries or the endocrine system [36].
Diabetes and hypertension are pathophysiologically related diseases. Epidemiological studies have indicated the co-existence of diabetes and hypertension in some patients, and both diseases share common risk factors [37]. There are several commercial drugs available for the treatment or management of diabetes and hypertension. These drugs include Glibenclamide and metformin for the management of diabetes and Amlodipine and Lisinopril for the management of hypertension. However, the intensive and vigorous management regimes associated with the use of orthodox medicine for the management of both diabetes and hypertension coupled with drug resistance, related side effects, and high treatment cost has necessitated a paradigm shift towards plant medicine regime [38,39]. Studies have shown that medicinal plants due to their active ingredients, medicinal and antioxidant compounds have beneficial effects on human health and have a therapeutic effect on various organs of the body and various diseases [4,40,41]. Therefore, it is imperative to explore potential therapeutic agents with minimal side effects from medicinal plants, which may be useful in the management of diseases. As yet, there is a scarcity of studies on medicinal plants being used by herbalists in the management of diabetes and hypertension in Ghana. Thus, this present study aimed to investigate the pharmacotherapeutic potential of medicinal plants that are being used by herbalists in the management of diabetes and hypertension in Ghana. Specifically, the study addresses the following questions: (1) What species of medicinal plants are used in the management of diabetes and hypertension in Ghana? (2) How are the medicinal plants being prepared and used in the management of the diseases; (3) What are the common symptoms for the diagnosis of diabetes and hypertension by herbalists?
2. Materials and methods
2.1. Study area
The present study was conducted at the Centre for Plant Medicine Research (CPMR) in Ghana. The Centre for Plant Medicine Research (CPMR) is one of the leading research institutions in Ghana. It was established by the Government of Ghana in 1975 as an Agency of the Ministry of Health mandated to undertake research into plant medicine. This makes the Centre a unique research institution in Sub-Saharan Africa and beyond. The CPMR is located on Latitude 5°55′04.7″N and Longitude 0°08′ 03.2″ W in the peri-urban town of Akuapem-Mampong in the Eastern Region of Ghana.
Ghana is situated in West Africa bounded by Burkina Faso on the north, Togo on the eastern border, Côte d'ivoire on the west, and the Gulf of Guinea on the south (Fig. 1). The country has a total surface area of 238,540 km2. Total annual rainfall ranges from 780 mm in the dry eastern coastal belt to 2200 mm in the wet southwest corner of the country. Traditional medicine is a major component of the health care delivery system in the country [42]. Thus traditional healers in Ghana such as herbal practitioners, fetish priests, divine healers, psychic practitioners and traditional medicine practitioners are well-organized under an umbrella organization Ghana Federation of Traditional Medicine Healers Association (GHAFTRAM).
Fig. 1.
A map of Ghana showing location regions of respondents.
2.2. Selection of herbalists and ethnobotanical interviews
A total of 36 registered herbalists belonging to GHAFTRAM were involved in this study. The 36 herbalists represented 64 % of the 56 herbalists that participated in a training workshop organized by CPMR in September 2022. Herbalists who participated in this study were located in the Western (44 %), Greater Accra (22 %), Eastern (19 %) and Ashanti (14 %) regions of Ghana.
Ethnobotanical data were acquired from the herbalists through conversation and interviews using a semi-structured questionnaire with predetermined open-ended and direct questions. Pretesting of the questionnaire was undertaken with 20 randomly selected herbalists within the study area and improvements were made to the questionnaire before its use. The questionnaire was designed to capture data on the demographic background of herbalists, vernacular names of the plants, plant parts used, mode of preparation and administration. Face-to-face interviews with herbalists involved an interpreter, a recorder and a botanist. We also followed the face-to-face interviews with telephone conversations with the herbalists to validate information that they have previously provided. Data collection took place between September 2022–January 2023.
Voucher specimens of the species of plants reported being used were collected with the herbalists from the field. The plant specimens collected were pressed and processed following standard ethnobotanical practices [43], and voucher specimens have been deposited at the Centre for Plant Medicine Research herbarium (CPMR). Plant identification was achieved via comparison of the voucher specimens collected with already identified specimens at the CPMR herbarium. Nomenclature and classification of the species of plants follow Plants of the World Online (POWO) and The Plant List databases (http://www.theplantlist.org; and https://powo.science.kew.org/accessed respectively on December 18, 2022 and July 10, 2023).
Ethical statement
The selection of the herbalists and the conduct of interviews were guided by the principles of the Code of Ethics of the International Society of Ethnobiology [43,44]. In this regard, the purpose of the study including methods of data collection and intention to publish data as well as an assurance of the confidentiality of the information to be provided was thoroughly explained to herbalists to enable them to make prior-informed decisions about their participation, as involvement in this study was voluntary. Only herbalists that consented to the objectives of the study were enrolled and consequently interviewed.
2.3. Data analysis
Data were analyzed using two ethnobotanical indices, namely, Fidelity Level (FL) and Informant Consensus Factor (ICF). The Fidelity Level is a way of calculating the percentage of informants who use a particular plant for the same purpose compared to all uses of all plants, and was computed using the formula [45]: ; where Ns is the number of informants that use a particular plant for a specific purpose, and URs is the total number of use reports for the species.
Informant Consensus Factor (ICF) was employed to deduce the homogeneity of the information about plant use to treat a particular category of disease (i.e., diabetes and hypertension). The ICF was calculated using the formula [46]: where “Nur” refers to the total number of use reports for each disease category and “Nt” refers to the total number of species used for that category.
3. Results and discussion
3.1. Background of herbalists
Information about demographic background of the 36 herbalists interviewed is summarized in Table 1. More than half (64 %) of the herbalists interviewed were males, and the majority (47 %) of the herbalists were within the age group of 41–50 years. The herbalists interviewed had attained different levels of education ranging from primary school to university level. The herbalists interviewed included Christians (92 %), traditionalists (5 %), and Muslims (3 %). About 53 % of the herbalists had practice for more than 40 years. The extensive experience (>40 years) of most of the herbalists (53 %) could indicate that the herbalists have adequate knowledge in the management of diseases [47].
Table 1.
Background on herbalists (n = 36) interviewed.
| Categories | Variables | Number of herbalists | % of herbalists |
|---|---|---|---|
| Gender | Male Female |
23 13 |
64 36 |
| Age-groups | 18–30 | 2 | 6 |
| 31–40 | 5 | 14 | |
| 41–50 | 17 | 47 | |
| 51–60 | 12 | 33 | |
| Religion | Christian | 33 | 92 |
| Muslim | 1 | 3 | |
| Traditional | 2 | 5 | |
| Educational level | No formal education | 2 | 6 |
| Basic education | 9 | 25 | |
| Senior High School | 16 | 44 | |
| Tertiary | 9 | 25 | |
| Years of practice | 10–20 | 8 | 22 |
| 21–30 | 2 | 6 | |
| 31–40 | 7 | 19 | |
| >41 | 19 | 53 |
The herbalists diagnosed diabetes mellitus based on symptoms such as unexplained weight loss (36 %), frequent urination (28 %), increased thirst (25 %) and extreme hunger (11 %). These symptoms are among those commonly used by traditional healers in the diagnosis of diabetes mellitus for their treatments [48,49] and used in clinical diagnosis of the disease. In Kenya, some herbalists in addition to the previously mentioned parameters relied on headache, sweating and fever in diagnosing diabetes [50]. Note that in Traditional Chinese medicine patients with chronic diseases such as diabetes are also examined using physical examination, pulse and breath odour, in addition to relying on the history of the patients [51].
Regarding the diagnosis of hypertension, herbalists mentioned irregular heartbeats (35 %), difficulty in breathing (22 %), vision problems (16 %), chest pains (14 %), fatigue (5 %), and blood in the urine (8 %). In the Maritime Region of Togo, traditional healers used a combination of symptoms including nose bleeding, headache, swarming, loss of consciousness, and shortness of breath for diagnosis of hypertension [15]. In addition, the herbalists also relied on an orthodox diagnosis of patients with diabetes and hypertension for their treatments. This is usually the practice if herbalists have the training and the equipment to subject patients to the clinical examination of diabetes and hypertension [50].
About 44 % of the herbalists reported that 10 patients on average per week visited their clinics for treatment of both diseases, and that they received positive feedback from the patients. This suggests that herbalists play a major role in the management of both diabetes and hypertension in Ghana, which also means that the effects of their medicines on patients must be properly investigated.
3.2. Medicinal plants
A total of 39 species of plants belonging to 31 families were reported by the herbalists as being used in the management of hypertension and diabetes (Table 2). Most of the species of plants belong to the Apocynaceae (14 %), Fabaceae (14 %), and the others; Amaranthaceae, Cucurbitaceae, Lamiaceae, Lauraceae, Meliaceae, Musaceae, Rubiaceae and Zingiberaceae have Ten percent respectively. Trees formed the majority (55 %) of the plants being used while shrubs and herbs formed 24 % and 21 %, respectively. Of the 39 species of plants, 18 were being used for the management of only hypertension and 12 species for only diabetes. Nine species of plants, namely, Carica papaya L. Fleurya ovalifolia Schum. & Thonn., Khaya senegalensis A. Juss., Mangifera indica L., Morinda citrifolia L., Morinda lucida A. Gray., Moringa oleifera Lam., Ocimum gratissimum A Forsk. and Gymnanthemum amygdalinum (Delile) Sch.Bip was reported being used in the management of both diabetes and hypertension. The use of the same species of plants in the management of both diabetes and hypertension has been reported [52]. However, the species reported in this case is different from what has been reported elsewhere [52].
Table 2.
Medicinal plants reported used for management of diabetes and hypertension by herbalists in Ghana with information on their vernacular names, conservation status, habits, diseases treated, and how they are being used.
| Species (Voucher #)/Conservation Status | Family (habit) | Local name | Disease type* | Plant parts | Modes of preparation | Modes of administration |
|---|---|---|---|---|---|---|
| Aframomum melegueta K. Schum. (CPMR 4945) | Zingiberaceae (herb) | Fam wisa (Twi) | H | Seeds | Decoction: boil with stem bark of Khaya senegalensis and Zingiber officinale | Oral: drink decoction |
| Alchornea cordifolia (Schumach. & Thonn.) Müll.Arg (CPMR 4920) | Euphorbiaceae (shrub) | Ogyama (Twi) | D | Leaves | Decoction: Boil with leaves of Psidium guajava and Citrus aurantifolia leaves | Oral: drink decoction |
| Alstonia boonei De Wild. (CPMR 4911) | Apocynaceae (Tree) | Nyamedua (Twi) | H | Stem & leaves | Decoction: Boil leaves and stem together and add Zingiber officinale | Oral: drink decoction |
| Alternanthera pungens Kunth (CPMR 4906) | Amaranthaceae (herb) | Nkasienkasie (Asante) | D | Stem bark | Boil with leaves of Taraxacum officinale and moringa oleifera | Oral: drink decoction |
| Anthocleista nobilis G. Don (CPMR 4930) | Loganiaceae (tree) | Awudifo kete (Twi) | D | Stem bark | Boil with whole plant of Paullina pinnata, Carica papaya leaves and add lemon juice | Oral: drink decoction |
| Azadirachta indica A. Juss. (CPMR 4933) | Meliaceae (tree) | Nim (Fante) | D | Root bark | Boil with leaves of Carica papaya, Gossypium arboreum leaves and seeds Xylopia aethiopica | Oral: drink decoction |
| Baphia nitida G. Lodd. (CPMR 4921) | Fabaceae (tree) | Odwene (Twi) | H | Leaves | Boil leaves with Xylopia aethiopica fruits | Oral: drink decoction |
| Bryophyllum pinnatum (Lam.) Oken (CPMR 4925) | Crassulaceae (herb) | Egoro (Twi) | H | Leaves | Boil with fruit Tetrapleura tetraptera | Oral: drink decoction |
| Carica papaya L. (CPMR 4917) | Caricaceae (tree) | Brofre (Twi) | DH | Leaves | Boil with Khaya senegalensis stem bark, whole plant of Paullinia pinnata and Psidium guajava leaves | Oral: drink decoction |
| Cinnamomum zeylanicum Blume. (CPMR 4928) | Lauraceae (tree) | Anoatre dua (Twi) | H | Stem bark | Boil with fruit Monodora myristica and dried Zingiber officinale | Oral: drink decoction |
| Cymbopogon citratus (DC.) Stapf. (CPMR 4924) | Poaceae (grass) | Ti-ahaban (Fante) | H | Whole plant | Boil whole plant | Oral: drink decoction |
| Fleurya ovalifolia (Schumach. & Thonn.) Dandy (CPMR 4943) | Moraceae (tree) | Nsasono (Twi) | DH | Leaves | Grind dried into granules with Carica papaya and Momordica charantia leaves | Oral: drink decoction |
| Gomphrena celosioides Mart. (CPMR 4907) | Amaranthaceae (herb) | Water globe (English) | H | Whole plant | Boil whole plant | Oral: drink decoction |
|
Gossypium arboreum L. (CPMR 4932) NT |
Malvaceae (tree) | Asaawa (Twi) | D | Leaves | Boil with root of Carica papaya, Morinda lucida and leaves of Ocimum gratissimum | Oral: drink decoction |
| Dianthera secunda (Lamb.) Griseb. (CPMR 4905) | Acanthaceae (shrub) | Jehovah witness tree (English) | D | Leaves | Boil leaves with krokrodoso leaves | Oral: drink decoction |
|
Khaya senegalensis (Desr.) A. Juss. (CPMR 4934) VU |
Meliaceae (tree) | Kuntunkuri (Twi) | DH | Stem bark | Boil with dried leaves of Carica papaya, | Oral: drink decoction |
| Kigelia africana (Lam.) Benth. (CPMR 4916) | Bignoniaceae (tree) | Nufuten (Asante) | H | Leaves, Stem bark | Boil stem bark and leaves together | Oral: drink decoction |
| Lantana camara L. (CPMR 4944) | Verbenaceae (shrub) | Anansedokono (Akan) | H | Whole plant | Boil with Tapinanthus banguwensis, Citrus aurantifolia leaves and Zingiber officinale | Oral: drink decoction |
| Luffa cylindrica M. Roem. (CPMR 4918) | Cucurbitaceae (shrub) | Sapo (Twi) | H | Leaves | Infusion: soak fresh leaves in water. | Oral: drink infusion |
| Mangifera indica L. (CPMR 4908) | Anacardiaceae (tree) | Amango (Asante) | DH | Leaves | Boil leaves and bark together with Psidium guajava leaves | Oral: drink decoction |
| Momordica charantia L.CPMR 4919 | Cucurbitaceae (tree) | Nyinya (Twi) | D | Fruit | Mill fruits | Oral: drink decoction |
| Morinda citrofolia L. (CPMR 4940) | Rubiaceae (tree) | Noni (Hawalli) | DH | Fruit, Leaves | Boil dried leaves and ferment the fruits. | Oral: drink decoction |
| Morinda lucida Benth. (CPMR 4939) | Rubiaceae (tree) | Konkroma (Asante) | DH | Leaves, Stem bark | Mill fresh leaves and stem bark and add lemon juice | Oral: drink decoction |
| Moringa oliefera Lam. (CPMR 4935) | Moringaceae (tree) | Moringa (English) | DH | Leaves | Boil freshly together with Launaea taraxacifolia or Mill dried leaves with Launaea taraxacifolia | Oral: drink decoction |
| Musa paradisiaca L. (CPMR 4936) | Musaceae (tree) | Brodea (Twi) | H | Leaves, Stem | Boil with dried leaves of Mangifera indica and maize husks | Oral: drink decoction |
| Musa sapientum f. pruinosa King. (CPMR 4937) | Musaceae (tree) | Kwadu (Twi) | D | Leaves | Boil together with dried leaves of carica papaya and Zingiber officinale | Oral: drink decoction |
| Ocimum gratissimum Forssk. (CPMR 4926) | Lamiaceae (herb) | Nunum (Twi) | DH | Leaves | Boil dried leaves with Morinda lucida leaves | Oral: drink decoction |
| Paullinia pinnata L. (CPMR 4941) | Sapindaceae (shrub) | Toatin (Asante) | D | Whole plant | Boil with Psidium guajava leaves, Carica papaya leaves | Oral: drink decoction |
| Persea americana Mill. (CPMR 4929) | Lauraceae (tree) | Paya (Akan) | H | Seed | Grind dried seeds into powder. | Oral: drink decoction |
| Psidium guajava L. (CPMR 4938) | Myrtaceae (tree) | Oguawa (Twi) | D | Leaves | Boil with Carica papaya and Persea americana leaves | Oral: drink decoction |
| Rauvolfia vomitoria Wennberg (CPMR 4912) | Apocynaceae (tree) | Kakapenpen (Twi) | D | Root bark | Boil with Paullinia pinnata whole plant | Oral: drink decoction |
| Rosmarinus officinalis L. (CPMR 4927) | Lamiaceae (herb) | Rosemary (English) | H | Leaves | Boil with leaves of Alstonia boonei, Zingiber officinale roots and stem bark of Khaya senegalensis | Oral: drink decoction |
| Senna alata (L.) Roxb. (CPMR 4922) | Fabaceae(tree) | Osempe (Twi) | D | Leaves | Boil with Alchornea cordifolia leaves and whole plant of Paullinia pinnata | Oral: drink decoction |
| Strophanthus hispidus DC. (CPMR 4913) | Apocynaceae (shrub) | Omaatwa (Twi) | H | Root bark | Boil roots | Oral: drink decoction |
| Tapinanthus banguwensis (Engl. & k. Krause) Danser (CPMR 4931) | Loranthaceae (Climber) | Nkrapan (Twi) | H | Whole plant | Boil with Launaea taraxacifolia leaves and add Xylopia aethiopica Fruits | Oral: drink decoction |
| Launaea taraxacifolia (Willd.) Amin ex C. Jeffrey (CPMR 4915) | Compositae (herb) | Dandelion (English) | H | Leaves | Boil with Moringa oleifera leaves | Oral: drink decoction |
| Tetrapleura tetraptera (Schumach. &Thonn.) Thaub. (CPMR 4923) | Fabaceae (tree) | Prekese (Twi) | H | Fruits | Boil the dried fruit with leaves of Ocimum gratissimum | Oral: drink decoction |
| Trema orientalis (L.) Blume (CPMR 4942). | Cannabaceae (tree) | Sesea (Asante) | D | Leaves | Decoction: boil together with roots of Morinda lucida | Oral: drink decoction |
| Gymnanthemum amygdalinum (Delile.) Sch. Bip. (CPMR 4914) | Asteraceae (shrub) | Anwonwene (Akan) | DH | Leaves | Decoction: boil with leaves of Persea americana | Oral: drink decoction |
| Xylopia aethiopica (Dunal.) A. Rich (CPMR 4909) | Annonaceae (tree) | Hwentia (Twi) | H | Seed | Decoction: boil with Leaves of Gossypium arboreum, roots of Morinda lucida and Carica papaya | Oral: drink decoction |
| Zanthoxylum zanthoxyloides (Lam.) Zepern.& Timler (CPMR 4910) | Rutaceae (shrub) | Okantor (Twi) | D | Stem bark | Decoction: boil together with Alchornea cordifolia leaves, stem bark of Khaya senegalensis | Oral: drink decoction |
| Zingiber officinale Roscoe (CPMR 4946) | Zingiberaceae (shrub) | Kakaduro (Twi) | H | Root bark | Decoction: boil with Tetrapleura tetraptera | Oral: drink decoction |
NT= Near threatened; VU=Vulnerable; *D = Diabetes; H = hypertension.
The results of literature search showed that all the species of plants reported for the management of diabetes by herbalists have been previously documented for this use [[53], [54], [55], [56], [57], [58], [59], [60], [61], [62]]. However, major differences exist in the way the plants are being used in different areas of Ghana and elsewhere for the management of diabetes. In a recent review of the use of plants in traditional management of diabetes in Nigeria [56], only 21 of the 115 species of plants identified were reported in the present study. Moreover, plants such as Aframomum melegueta K. Schum, Cympopogon citratus (DC.) Stapf and Tapinanthus banguwensis (Engls. and K. Krause) Danscing were reported being used by the herbalists in this study but not in the management of diabetes. In a study of medicinal plants used in the management of diabetes in the Dangme West district of southern Ghana [46], only seven of the species identified were reported used in this study. Only 16 of the 76 plants reported used in the management of diabetes by [54] were reported in this study. Sometimes the plant parts used also differed in different areas. For example, the decoction of whole plant of Momordica charantia L. is used in the Dangme West district while it is the fruits that are used in the management of diabetes in this study. Understanding the use of species in different areas can assist in selecting those plants that justify further studies.
Sixteen of the species of plants have been previously documented in the management of hypertension. These species were Aframomum melegueta K. Schum. [63], Alstonia boonei De Wild [54], Kalanchoe pinnata (Lam.) Pers. [64], Cinnamomum verum J. Presl [65], Cymbopogon citiratus (DC.) Stapf. [55], Gomphrena celesioides Mart. [66], Kigelia africana (Lam.) Benth. [60], Lantana camara f. albiflora Moldenke [67] and Musa paradisiaca L. [68], Persea americana Mill. [69], Rosmarinus officinalis L. [70], Strophanthus hispidus DC. [71], Taraxacum erythrospermum Andrz. ex-Besser [72], Tetrapleura tetraptera (Schumach. & Thonn.) Taub. [73], Xylopia aethiopica (Dunal) A. Rich [74] and Zingiber officinale Roscoe [75]. However, to the best of our knowledge four species; Baphia nitida G. Lodd, Luffa aegyptiaca Mill. and Tapinanthus banguwensis (Engl. & K. Krause) Dancing are being documented for the first time in the management of hypertension (see Table 2).
3.3. Informant consensus and fidelity level
A quantitative analysis of herbalist's agreements about the use of medicinal plants for management of the diseases is presented in Table 3. The highest ICF value (0.82) was recorded for both hypertension and diabetes, followed by 0.31 for only hypertension, and 0.24 for only diabetes mellitus. These results mean that the healers agreed more on the use of plants for the management of hypertension compared to diabetes. A similar study by [76] reported high ICF values for hypertension (ICF = 0.90) and diabetes (0.81) among healers. ICF value may vary due to the abundance, diversity and availability of medicinal plants and their associated knowledge in a particular locality [76]. Also, constraints in the transfer of ethnobotanical knowledge from one generation or locality to another may affect ICF values [76].
Table 3.
Informant Consensus Factor (IFC) about medicinal plants used by herbalists in Ghana for the management of diabetes and hypertension.
| Disease category | Number of use-reports | Number of species | ICF |
|---|---|---|---|
| Diabetes mellitus | 18 | 14 | 0.24 |
| Hypertension | 27 | 19 | 0.31 |
| Diabetes mellitus and hypertension | 45 | 9 | 0.82 |
Fidelity level (FL) values for the 39 medicinal plants regarding the management of the diseases are presented in Table 4, Table 5, Table 6. The species of plants with high FL values for diabetes were Momordica charantia L. (FL = 17.3), and Psidium guajava L. (FL = 13). For hypertension, the highest FL of 15 was recorded for Tetrapleura tetraptera (Schum. ex. Thonn.) Thaub. followed by Persea americana Mill. (12.5) and Launea taraxifolia (Willd.) Amin ex C. Jeffrey (12.5). For both hypertension and diabetes, the highest FL of 23.3 % was recorded for Carica papaya L. (Table 4). These results suggest that herbalists have their preferences regarding the choice of medicinal plants for the management of diseases. It is, however, unclear what informed their choices in the selection of plants for treatments.
Table 4.
Fidelity level for medicinal plants reported by herbalists in Ghana as used in the management of hypertension.
| S/N | Species | Hypertension |
||
|---|---|---|---|---|
| Fidelity level | ||||
| Urs | Ns | FL | ||
| 1. | Aframomum melegueta | 40 | 1 | 2.5 |
| 2. | Alstonia boonei | 40 | 1 | 2.5 |
| 3. | Baphia nitida | 40 | 1 | 2.5 |
| 4. | Bryophyllum pinnatum | 40 | 2 | 5 |
| 5. | Cinnamomum zeylanicum | 40 | 1 | 2.5 |
| 6. | Cymbopogon citiratus | 40 | 1 | 2.2 |
| 7. | Gomphrena celesioides | 40 | 1 | 2.5 |
| 8. | Kigelia africana | 40 | 2 | 5 |
| 9. | Lantana camara | 40 | 1 | 2.5 |
| 10. | Launnea officinale | 40 | 5 | 12.5 |
| 11. | Luffa aegyptiaca | 40 | 1 | 2.5 |
| 12. | Musa paradisiaca | 40 | 2 | 5 |
| 13. | Persea americana | 40 | 5 | 12.5 |
| 14. | Strophanthus hispidus | 40 | 2 | 5 |
| 15. | Tapinanthus banguwensis | 40 | 2 | 5 |
| 16. | Tetrapleura tetraptera | 40 | 6 | 15 |
| 17. | Xylopia aethiopica | 40 | 3 | 7.5 |
| 18. | Zingiber officinale | 40 | 2 | 5 |
Table 5.
Fidelity level for medicinal plants reported by herbalists in Ghana as used in the management of diabetes.
| S/N | Species | Diabetes |
||
|---|---|---|---|---|
| Fidelity level | ||||
| Urs | Ns | FL | ||
| 1. | Alchornea cordifolia | 23 | 1 | 4.3 |
| 2. | Alternanthera pungens | 23 | 1 | 4.3 |
| 3. | Anthocleista nobilis | 23 | 1 | 4.3 |
| 4. | Gossypium americana | 23 | 1 | 4.3 |
| 5. | Momordica charantia | 23 | 4 | 17.3 |
| 6. | Musa sapientum | 23 | 2 | 8.6 |
| 7. | Paullinia pinnata | 23 | 1 | 4.3 |
| 8. | Psidium guajava | 23 | 3 | 13 |
| 9. | Rauvolfia vomitoria | 23 | 1 | 4.3 |
| 10. | Senna alata | 23 | 1 | 4.3 |
| 11. | Trema orientale | 23 | 1 | 2 |
| 12. | Zanthoxylum zanthoxyloides | 23 | 1 | 2 |
Table 6.
Fidelity level for medicinal plants reported by herbalists in Ghana as used in the management of hypertension.
| S/N | Species | Diabetes and Hypertension |
||
|---|---|---|---|---|
| Fidelity level | ||||
| Urs | Ns | FL | ||
| 1. | Carica papaya | 30 | 7 | 23.3 |
| 2. | Fleurya ovalifolium | 30 | 2 | 6.6 |
| 3. | Khaya senegalensis | 30 | 4 | 13.3 |
| 4. | Mangifera indica | 30 | 2 | 6.6 |
| 5. | Morinda citrofolia | 30 | 2 | 6.6 |
| 6. | Morinda lucida | 30 | 3 | 10 |
| 7. | Moringa oliefera | 30 | 4 | 13.3 |
| 8. | Ocimum gratissimum | 30 | 3 | 10 |
| 9. | Vernonia amygdalina | 30 | 3 | 10 |
3.4. Formulation and application of medicinal plants
The herbal medicines for the treatments of both diabetes and hypertension were commonly prepared from multiple plant prescriptions. The multi-component nature of herbal medicines for the treatment of hypertension is known and it is believed to improve efficacy through synergism [77]. Unlike the situation in the Endo state of Nigeria, non-plant materials like snail, potash, earthworm and native chalk were not reported added to the recipes for the management of hypertension [77] in this study. Generally, multiple plant prescriptions are very complex in terms of standardization, identification of bioactive agents, and monitoring of the uses of plants [78]. There were, however, a few instances of single plant prescriptions such as the use of Cympopogon citratus (DC.) Stapf., Gomphrena celosoides Mart., Persea americana Mill., and Strophanthus hispidus DC., for hypertension, Momordica charantia L. for diabetes, and Morinda citrofolia L. and Morinda lucida A. Gray for both diabetes and hypertension. The availability of particular plant species and knowledge of traditional healers are factors that could determine whether species of plants are used singly or in combinations [78].
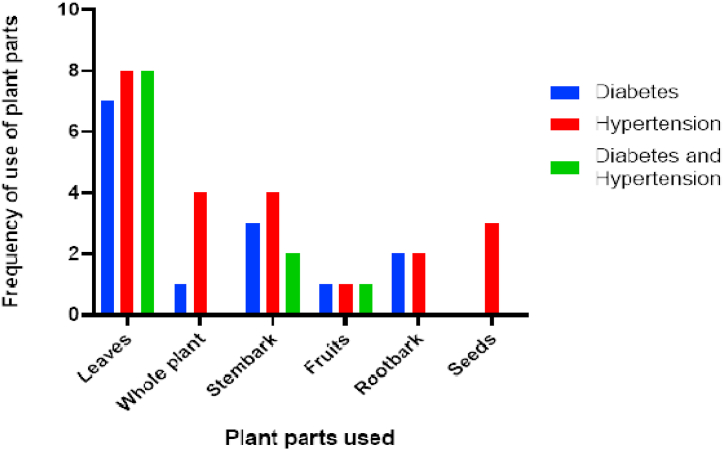
Although the plant' parts used for the management of the diseases differed, the most common parts used were leaves and stem bark (Fig. 2). Similar to the results of this study, stem bark was the most commonly used plant part in the management of diabetes in Guinea [79], and leaves formed the most frequently used plant part in the management of hypertension in nearby Togo [15] and Nigeria [77]. The plant parts used in traditional medicine could depend on the availability of the plants as well as the knowledge of local people. Scientifically, bioactive agents in plants could be related to the plant parts as secondary metabolites differ in concentration in the different plant parts.
Fig. 2.
Plant parts used in the management of diabetes and hypertension by herbalists in Ghana.
Except for the leaves of Luffa cylindrica M. Roem that were prepared in the form of an infusion for the management of hypertension, the herbal medicines were prepared by boiling the plant parts and the decoctions drunk. This observation was not surprising because most traditional medicines are prepared as decoctions for oral administration [80,81]. However, other modes of preparation and administration of herbal medicines for the management of both diabetes and hypertension have been reported elsewhere. For example, in the Dangme West District of Ghana, pulverized plant materials were added to food and eaten for the management of diabetes [48]. Also, concoctions, powders, and essence as modes of preparation of herbal medicines for hypertension have been reported [15].
4. Review of anti-diabetic and anti-hypertensive studies on medicinal plants
The results of the literature review on the bioactivity, phytochemical constituents and clinical studies about the medicinal plants identified are presented in Table 7. Out of the 39 plants reviewed, 38 have been investigated for their biological activities (14 anti-diabetic, 16 anti-hypertensive and 8 for both properties). In the last few decades, the discovery and development of phytochemicals intended for anti-diabetic and anti-hypertensive effects have gained some attention. Anti-diabetic and anti-hypertensive effects of medicinal plants are attributed to the group of phytochemicals or single chemical constituents of plant extracts, which work by various mechanisms. Some phytochemicals with anti-diabetes properties for instance, work through the regulation of glucose and lipid metabolism, stimulation and regeneration of β-cells, insulin secretion, regulation of the NF-ĸB signalling pathway, inhibition of gluconeogenic enzymes and oxidative stress. Also, pharmacological effects such as regulation of potassium and calcium ion channels, adenylate cyclase, nitric oxide (NO) and adrenergic system (α and β receptors), inhibition of angiotensin-converting enzymes (ACE), serotonin (5HT), norepinephrine (NE), prostaglandins (PGs)- pathways, phosphodiesterase (PDE) and sodium ion absorption may underscore the mechanisms used by phytochemicals with anti-hypertensive properties.
Table 7.
Biological activity, bioactivity constituents, and clinical studies on medicinal plants reported used by herbalists in Ghana for management of diabetics and hypertension.
| Species | Biological activity | Phytochemical constituents | Clinical studies |
|---|---|---|---|
| Aframomum melegueta | A. melegueta ethyl acetate fraction (AMEF) treatment at 300 mg/kg showed potent anti-diabetic effect in a T2D model of rats [82]. | Gingeredione, oleanolic acid [63]. | Lowering of cardiovascular indices such as systolic blood pressure (SBP), diastolic blood pressure (DBP). The degree of reduction was found to be within safety limits, indicating its potential usefulness in managing hypertension in young and elderly hypertensive patients [47]. |
| Alchornea cordifolia | Administration of Alchornea cordifolia leaf extract to diabetic animals significantly (p < 0.05) decreased the blood glucose level in the group treated with 200 mg/kg b w and decreased by a higher margin (p < 0.01) in animals treated with 400 and 800 mg/kg b w [83]. | Anthranilic acid, protocatechuic acid, ellagic acid [84]. | No previous report |
| Alstonia boonei | Aqueous extract of the stem bark caused about 44 % and 40 % reduction in the activities of hepatic glu-6-phosphatase and fru-1, 6-phosphatase, respectively. This was accompanied by about 30.5 % increase in glu-6-phosphate dehydrogenase [85]. | Akuammicine, echitamidine, Nα-formylechitamidine, boonein, loganin, lupeol, ursolic acid, and β-amyrin [54]. | No previous report |
| Alternanthera pungens | Treatment with 50, 200 and 300 mg kg–1 of A. pungens extract improved and restored rat serum HDL level [57]. | Glycosides, alkaloids, terpenoids, flavonoids carotenoids [57]. | No previous report |
| Anthocleista nobilis | The stem and root bark extracts significantly (p < 0.5) reduced fasting blood glucose concentration of diabetic animals [53]. | Sweroside, secoiridoid, scopoletin [53] | No previous report |
| Azadirachta indica | Aqueous extracts of neem plant showed antihyperglycemic activity in streptozotocin-induced rats and this effect was due to an increase in glucose uptake and glycogen deposition in isolated rat hemidiaphragm [86]. | Nimbidin, β-sitosterol [11] | No previous report |
| Baphia nitida | No previous report | No previous report | No previous report |
| Bryophyllum pinnatum | Both the aqueous and methanolic leaf extracts of B. pinnatum (BP, 50–800 mg/kg iv. or i.p.) produced dose related, significant (P < 0.05 - 0.001) decrease in arterial blood pressure and heart rates of anaesthetized normotensive and hypertensive rats [87]. | Oleic acid, alpha-d-Glucopyranoside [64]. | Using measured hypercapnic-hypoxic breathing training rehabilitation protocol in patients with mild essential hypertension leads to reduction of high blood pressure indexes [88]. |
| Carica papaya | The methanolic extract of C. papaya elicited angiotensin converting enzyme inhibitory activity. The antihypertensive effects elicited by the methanolic extract of C. papaya were similar to those of enalapril, and the baroreflex sensitivity was normalized in treated spontaneously hypertensive rats [89]. | flavonoids quercetin, rutin, nicotiflorin, clitorin, and manghaslin [89]. | No previous report |
| Cinnamomum zeylanicum | Acute intravenous administration of C. zeylanicum extract (5, 10 and 20 mg/kg) to l-NAME-induced hypertensive rats provoked a long-lasting decrease in blood pressure. Mean arterial blood pressure decreased by 12.5 %, 26.6 % and 30.6 % at the doses of 5, 10 and 20 mg/kg, respectively. In chronic administration, MECZ and captopril significantly prevented the increase in blood pressure and organs' weights, as well as tissue histological damages and were able to reverse the depletion in NO tissue's concentration [90]. | Cinnamaldehyde, eugenol [65] | Improves insulin potentiating activity [91]. |
| Cymbopogon citratus | A fresh leaf aqueous extract of Cymbopogon citratus administered in normal rats lowered the fasting plasma glucose and total cholesterol, triglycerides, low-density lipoproteins and very low-density lipoprotein dose dependently while raising the plasma high-density lipoprotein level in the same dose-related fashion, but with no effect on the plasma triglyceride levels [92]. | Esters, aldehyde and fatty acids [55,106] | No previous report |
| Fleurya ovalifolia | No previous report | No previous report | No previous report |
| Gomphrena celosioides | Ethanolic extract of G. celosioides (EEGC) acutely reduced Mean arterial pressure the dose of 100 mg/kg. In the 4-week assay, EEGC acted as diuretic after acute administration after 1, 2, 3 and 4 weeks of treatment. EEGC also acted as an antihypertensive and it showed significant difference already after 1 week (and after 3 and 4 weeks) compared to control, with its MAP close to pre-surgery values at the end of the experiment [93]. | Aurantiamide, aurantiamide acetate [66] | No previous report |
| Gossypium arboreum | Orally administered diethyl ether and ethanolic extracts of Gossypium arboreum significantly decreased blood glucose level [108. | Flavonoids, phenolic compounds, tannins and alkaloids [94]. | No previous report |
| Justicia secunda | Aqueous extract of J. secunda (AEJs) showed a dose dependent glucose reduction of glucose-induced hyperglycemia where a dose of 3 g/kg BW was substantially identical (P > 0.05) to that of glibenclamide at 10-2 g/kg BW. Examination of the liver-released glucose level of control normoglycemic rats and normoglycemic rats treated with AEJs and glibenclamide showed that aqueous extract of Justicia secunda and glibenclamide decreased the glucose released by the liver [58]. | polyphenols, alkaloids, saponosides and sterols and polyterpenes [58]. | No previous report |
| Khaya senegalensis | Treatment of diabetic groups withv organic fractions of K. senegalensis and and metformin in wistar albino rats showed significant decrease in fasting blood glucose (FBS), ameliorated hepatic and renal damages by decreasing the level of AST, ALT, ALP, Urea and creatinine compared to untreated diabetic rats and stimulated insulin secretion by β cells [95]. | methyl 6-ethyl-7-hydroxy-7,8-dihydrophenanthrene-2-carboxylate [95]. | No previous report |
| Kigelia africana | Significant lowering of the blood glucose levels was also expressed in mice following the administration of 50, 100, 200, and 300 mg/kg body weight of K africana [60]. | phenols, flavonoids, alkaloids, tannins, terpenoids, saponins [60]. | No previous report |
| Lantana camara | Ethanolic extract of Lantana camara leaves reduced workload of heart, maintained isotonic levels by negative chronotropic effect, relaxed the smooth muscles in chick and salt hypertensive rats against renal and vascular injuries [96]. | Glycosides, Saponins, Tannins, Flavonoids, Terpenoids and Steroids [96]. | No previous report |
| Luffa cylindrica | No previous report | No previous report | No previous report |
| Mangifera indica | M. indica leaf extract significantly inhibited alpha-amylase activity in a dose-dependent manner. The inhibitory effect was observed up to (51.4 ± 2.7 %) at a concentration of 200 μg/mL [97]. | Mangiferin [98] | No previous report |
| Momordica charantia | M. charantia improved glucose tolerance and suppressed postprandial hyperglycemia in rats [12]. | Charantin, momordicin, oleanolic acid, vicine [13]. | Hypoglycemic effect in only diabetic patients [99,100] |
| Morinda citrifolia | Aqueous extract of M. citrifolia significantly inhibited rat lens aldose reductase (RLAR) activities and improved free radical scavenging activities in vitro as compared to ethanolic and chloroform extracts thereby indicating a potential to inhibit cataract formation and prevent diabetic complications [101]. | Lucidin, morindone [102]. | No previous report |
| Morinda lucida | Administration of aqueous and methanolic extract of M. lucida significantly (p < 0.05) reduced fasting blood glucose of diabetic animals by 73.5 and 39.0 % of their initial values, respectively [103]. | Steroids, anthraquinone Oleanic acid [104]. | No previous report |
| Moringa oleifera | Ethanolic extract of M. oleifera leaves showed a blood pressure lowering effect in rats, mediated possibly through a calcium antagonist effect [105]. M. oleifera extract showed significant decrease in malonaldehyde and improvements in the inflammatory cytokines (TNF-α and IL-6) compared to control animals in STZ-induced diabetic rats fed with equivalent of 250 mg/kg of MO for 6 weeks [106]. | Nitrile, mustard oil glycosides, thiocarbamate glycosides [105]; quercetin [107], chlorogenic Acid [108], and isothiocyanates [109]. | No previous report |
| Musa paradisiaca | M. paradisiaca extracts inhibited angiotensin-I converting enzyme (ACE) activity in a dose-dependent manner (0–25 μg/ml). Unripe plantain peel (UPP) extract showed had a stronger inhibition (IC50 = 14.93 μg/ml) of ACE activity than over ripe plantain peel (OPP) and ripe plantain peel (RPP) extracts (IC50s of 21.80 μg/ml and 21.32 μg/ml, respectively [110]. | cardiac glycosides, terpenes, deoxy sugar [67]. | No previous report |
| Musa sapientum | Forty-five-day treatment with M. sapientum L extract significantly reduced the fasting blood glucose and the concentration of HbA1c in diabetic rats [111]. | Gallocatechin [112] | No previous report |
| Ocimum gratissimum | O. gratissimum leaf extract at a dose of 200 mg/kg bw decreased the blood glucose of diabetic rats by 20.9 %, at 4 h whereas 250 mg/kg bw was able to decrease the blood glucose of diabetic rats by 29.3 % at 4 h [113]. | Steroids, terpenoids, flavonoids and cardiac glycosides [114]. | No previous report |
| Paullinia pinnata | P. pinnata extract (50 mg/kg) and glibenclamide (2 mg/kg) produced a significant (p < 0.05) oral glucose tolerance effect in both normoglycemic and diabetic rats. The extract produced concentration-dependent increase in antioxidant activity and had its optimum effect at 400 μ μg/mL concentration [59]. | Alkaloids, flavonoids, glycosides, tannins, saponins and terpenes/sterols [59]. | No previous report |
| Persea americana | The hydroalcoholic extract of the leaves of Persea americana reduced blood glucose levels and improved the metabolic state of treated animals. Additionally, PKB activation was observed in the liver and skeletal muscle of treated rats when compared with untreated rats [69]. | p-coumaric acid, quercetin and epicatechin [115]. | No previous report |
| Psidium guajava | Significant blood glucose lowering effects of P. guajava extract were observed after intraperitoneal injection of the extract at a dose of 10 mg/kg in both 1- and 3-month-old Lepr (db)/Lepr(db) mice [116]. | Strictinin, isostrictinin, pedunculagin [117]. | No previous report |
| Rauvolfia vomitoria | At 500, 700 and 1000 mg/kg ethyl acetate of R. vomitoria caused a reduction of blood glucose level of treated normoglycemic rats similar to glibenclamide at 10 mg/kg [61]. | Alkaloids (eg. reserpine), flavonoids, and anthrones, anthraquinones, catechin tannins, saponins and monoterpenoids [61]. | No previous report |
| Rosmarinus officinalis | Rosmarinus officinalis extract on blood glucose and serum insulin levels was studied in alloxan-induced diabetic rabbits. Of the three doses of extract, the highest dose (200 mg/kg) significantly lowered blood glucose level and increased serum insulin concentration in alloxan-diabetic rabbits [70]. | Ursolic acid [118]. | Both blood pressure variables of SBP and DBP reflect the clinically significant anti hypotensive effect of Rosemary essential oil that was maintained throughout the treatment period. After validation of the use of the questionnaire (Cronbach's alpha coefficient 40.82), statistically significant differences have been found between pretreatment and post-treatment values of PSC and MSC, which indicate an improvement in these parameters that is directly related to the variation in blood pressure values [119]. |
| Senna alata | The α-glucosidase inhibitory effect of the crude extract of S. alata was far better than the standard clinically used drug, acarbose (IC (50), 107.31 ± 12.31 μg/ml) [120]. | Kaempferol, kaempferol 3-O-gentiobioside [121]. | No previous report |
| Strophanthus hispidus | There was a protective effect in the case of mean arterial blood pressure where, the 500 mg/Kg and 1000 mg/Kg of the plant extract did reduce the hypertension, 60.0 ± 4.80 and 50.50 ± 6.80, respectively [71]. | Alkaloids, flavonoids, saponins, and cardiac and cyanogenic glycosides [122]. | No previous report |
| Tapinanthus banguwensis | It is the first time it being mention for the treatment of hypertension | No previous report | |
| Launaea taraxacifolia | Results showed that one-off oral administration of L. taraxifolia leaf and L. taraxifolia root significantly (p < 0.05) reduced systolic, diastolic and mean arterial blood pressure. In a sub-chronic study L. taraxifolia leaves extract significantly (p < 0.05) prevented an increase in blood pressure throughout the period of treatment [72]. | Saponins, flavonoids, alkaloids, phenols, triterpenoid [123] | No previous report |
| Tetrapleura tetraptera | Polyphenol-rich hydroethanolic extract of Tetrapleura tetraptera (HET) reduced weight gain, fasting blood glucose and plasma insulin levels as well as homeostasis model assessment of insulin resistance (HOMA-IR) and alleviated obesity and T2DM associated oxidative stress and hypertension in rats [73]. | Hytate, triterpenoid, coumarinic (scopoletin), triterpene glycoside [124]. | No previous report |
| Trema orientale | Aqueous stem bark extract of T. orientalis was reported to have hypoglycemic effects in induced diabetic rats by mechanism different from that of sulfonylurea agents [125]. | Flavonoids, phenolic acids [126]. | No previous report |
| Vernonia amygdalina | The aqueous extract of the leaves of V. amygdalina caused a significant reduction in blood glucose levels of extract only, and allocan plus extract treated animals [127]. | Flavonoids, tannins, saponins, and triterpenoids [128]. | No previous report |
| Xylopia aethiopica | The anti-diabetic effect of Xylopia aethiopica has also been studied. A chloroformic extract of the dried fruits of Xylopia aethiopica administered orally at a dose of 250 mg/kg showed an 82 % reduction in the blood glucose concentration of alloxan monohydrate induced diabetic Wistar albino rats while diabetic non-treated rats and glibenclamide treated rats showed a 6.9 % and 74 % reduction respectively [74]. | Methyl ester, 12-octadecanoic acid, hexadecanoic acid [129]. | No previous report |
| Zanthoxylum zanthoxyloides | Z. zanthoxyloides significantly (p < 0.05) lowered blood glucose in treated animals compared to non-treated groups. Total cholesterol and LDL were significantly lower in Z. zanthoxyloides treated groups, with lowest values found in groups treated with higher concentration. It was also observed that LDH, ALP and ALT showed highest activities at 15 % of Z. zanthoxyloide feed with AST activity lowest at the highest concentration of the feed [122]. | No previous report | No previous report |
| Zingiber officinale | 50 mg/kg/day p.o administration of the petroleum ether and 10 mg/kg/day p.o of toluene fraction reduced the blood pressure in unilateral nephrectomized DOCA salt and fructose-induced hypertensive rats [130]. | Gingerol [131] | Increase insulin receptors and enhanceβ-cell function to decrease insulin resistance of diabetic patients [75]. |
The groups of phytochemicals mostly investigated and reported for these activities include saponins, polyphenols, flavonoids, alkaloids, tannins, glycosides polysaccharides and stilbenes. For this review, the phytochemicals will be classified based on their comparable chemical features, without necessarily considering their common biosynthetic pathways. The classification is as follows; nitrogen-containing compounds (alkaloids), terpenes and terpenoids, polyphenolic compounds, and hydroxyl-containing compounds.
4.1. Alkaloids
Alkaloids are a large family of nitrogen-containing phytochemicals with a lot of medicinal value. The antidiabetic and antihypertensive properties of plants such as Alstonia boonei De Wild., Alternanthera pungens Kunth, Carica papaya L., Gossypium arboreum L., Dianthera secunda (Lam.) Griseb., Kigelia africana (Lam.) Benth., Paullinia pinnata L., Strophanthus hispidus DC. and Launaea taraxacifolia (Willd.) Amin ex C. Jeffrey has been attributed to the presence of phytochemicals including, alkaloids. Some studies have related certain alkaloids with their specific molecular targets in defining their possible mode of action. In the case of antidiabetics, a special reference is made to the insulin-signaling pathway, where there is scientific data to support the activities of compounds like Nα-formylechitamidine, berberine, trigonelline, piperine, oxymatrine, vindoneline, evodiame and neferine in the regulation of insulin-signaling and other related cascades associated with β-cells, adipose tissues, myocytes and the hepatic cells [11].
Akuammicine, purified from the chloroform extract of Picralima nitida (Stapf) T. Durand & T. Durand (seeds), is known to stimulate the uptake of glucose in 3T3-L1 adipose cells [132]. This alkaloid is also detected in plants belonging to the genus Alstonia. This may partly explain why species such as Alstonia boonei De Wild. and Alstonia congensis Engl. have hypoglycemic activity.
There are also reports on alkaloids which exhibit anti-hypertensive properties [54]. reported on echitamine, an alkaloid isolated from Alstonia boonei De Wild. Echitamine lowered arterial blood, relaxed the smooth muscles of the heart and induced negative chronotropic and inotropic responses [87]. Reserpine, another alkaloid isolated from Rauvolfia vomitoria Wennberg is also reported to have antihypertensive activity [88]. Reserpine decreased blood pressure by depletion of catecholamines from the peripheral sympathetic nerve endings thereby regulating heart rate, cardiac contractions and peripheral resistance [133].
4.2. Terpenes and terpenoids
Terpenes are a class of compounds made up of one or more isoprene units. Compounds with an exact sequence of isoprene units are strictly referred to as terpenes while those modified sequences that look like terpenes (with isoprene skeletal pattern and other functional groups) are termed terpenoids. Isoprene or isoprenoid is the simplest unit of terpenes and terpenoids and consists of 5 carbons, connected with some alkene bonds.
Isoprene unit.
Many terpenes and terpenoids isolated from plants have been reported to have anti-diabetic and anti-hypertensive activities. Nimbidin, lupeol, oleanolic acid, β-amyrin, glutinol, ursolic acid are a few known examples. Oleanolic acid is a pentacyclic triterpene present in the fruits, vegetables and herbs of some plants. It has been isolated from ethyl acetate fraction of Aframomum melegueta K. Schum. fruit [63], chloroform fraction of Morinda lucida Benth. ethanolic leaf extract [134] and methanolic extract of Mormodica charantia L. (gourd). The anti-diabetic activity of oleanolic acid, isolated from the fractions of various plants, has been reported [135]. Oleanolic acid, such as thymol and carvacrol extracted from Gymnanthemum amygdalinum (Delile) Sch.Bip. has been reported to show anti-diabetic activities [136,137].
4.3. Phenolic compounds
These are secondary metabolites found in plants and consist of an aromatic ring with one or more hydroxyl groups [138]. Phenolic compounds are mostly bioactive constituents and are grouped as simple phenols, flavonoids, lignins, lignans, tannins, xanthones and coumarin [139]. Scientific evidence shows that some phenolic compounds isolated from medicinal plants such as Carica papaya L., Cinnamomum zeylanicum Blume, Gossypium arboreum, Moringa oleifera, Persea americana and Mangifera indica have cardiovascular protection making them possible anti-hypertensive agents. Phenolic compounds could also be anti-diabetic agents due to their ability to regulate glucose uptake and as well restrict the formation of islet amyloid polypeptide, amylin aggregates (IAPP) [138]. Examples of these phenolic compounds include chlorogenic acid, caffeic acid, coumaric acid, gallic acid, quercetin, isorhamnetin, kaempferol, hesperetin, naringenin, phloretin, enterolactone and enterodio [140]. Quercetin, kaempferol and their glycosides have been detected and isolated from the leaf extracts of Moringa oleifera [141]. Rutin and quercetin have been isolated from the ethanolic leaf extract of Persea americana [142].
Studies by [143] revealed some methoxy phenyl derivatives, isolated from Zingiber officinale, inhibited the enzyme aldose reductase both in vitro and in vivo, by preventing the accumulation of sorbitol and galactitol in human erythrocytes and the lenses of rats, respectively; underscoring the anti-diabetes properties of phenolic compounds.
The aromatic hydroxyl group in the benzo-γ-pyran of most flavonoids has been linked to their antioxidant activity, most especially their ability to scavenge free radicals. This helps to protect against oxidative stress and enable the regeneration of vital cells. For instance, epicatechin and quercetin, two key flavonoids in green tea, protect the islet cells of the pancreas from oxidative stress and also helps regenerate β-cell [56]. Similarly [144], noted that flavonoids can promote the differentiation and mineralization of osteoblasts at a high glucose level.
4.4. Hydroxyl containing compounds
These are non-phenolic but hydroxylated compounds. A number of them have been isolated and purified from medicinal plants, and are shown to be bioactive compounds. The anti-diabetes and anti-hypertension properties of these compounds have been reported. Gingerols, isolated from Zingiber officinale, promote glucose uptake by increased expression of GLUT4 receptor [56]. Also, 6-gingerol was reported to reduce blood pressure by normalizing the expressions of hypertensive biomarkers through peroxisome proliferator-activated receptor delta (PPARδ) [145].
5. Conclusion
Ghana is bestowed with an abundance of plant biodiversity; several are used in managing diabetes and hypertension in Traditional Medicine Practice. This study has documented the current state of indigenous knowledge and medicinal plants used by herbalists for the treatment and management of diabetes and hypertension in Ghana. The results show that there is substantial preclinical evidence to support the usefulness of some of these plants as an important choice for patients with diabetes and hypertension. The reported use of medicinal plants identified in this study confirmed with previous studies could be an indication that the plants might be potent in the treatment of the diseases. Indeed, many of the plants have biological activities, and their bioactive compounds have been identified. However, clinical studies are few, and thus important that the efficacy and safety of the herbal medicines prescribed by herbalists be investigated to validate their medicinal usefulness in people with diabetes and hypertension. Similarly, Baphia nitida G. Lodd, Luffa aegyptiaca Mill. and Tapinanthus banguwensis (Engl. & k. Krause) Dancing being mentioned for the first time in the management of hypertension by the herbalists need to be investigated for their medicinal properties. By doing so, the pharmacotherapeutic potential of these plants could be harnessed towards a possible incorporation into the healthcare system.
Funding statement
This research did not receive any specific grant from funding agency in the public, commercial, or non-profit sectors.
Data availability statement
Data/supplementary material is included and referenced in the article as tables.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to members of the Ghana Federation of Traditional Medicine Practitioners who participated in this study. We are also grateful to Mrs. Susana Oteng-Mintah, Mrs. Mary-Ann Archer and Peter Atta Adjei Junior for their immense contribution to the data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22977.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Neurol. 2014;4(177) doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdez-Solana M.A., Mejıa-Garcıa V.Y., Ellez-Valencia A.T., et al. Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. J. Chem. 2015;2015:p9. [Google Scholar]
- 3.Torres-León Cristian, Fernanda Rebolledo Ramírez, Jorge A., Aguirre-Joya, Ramírez-Moreno Agustina, Mónica L., Chávez-González, Aguillón-Gutierrez David R., Camacho-Guerra Luis, Ramírez-Guzmán Nathiely, Salvador Hernández Vélez, Aguilar Cristóbal N. Saudi Pharmaceut. J. 2023;31(1):21–28. doi: 10.1016/j.jsps.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asase A., Asafo-Agyei T. Plants used for treatment of malaria in communities around the bobiri forest reserve in Ghana. J. Herbs, Spices, Med. Plants. 2011;17(2):85–106. [Google Scholar]
- 5.Asafo-Agyei T., Blagogee H.R., Mintah S.O., Archer M.A., Ayertey F., Sapaty A.C.…Appiah A.A. Ethnobotanical studies of medicinal plants used in traditional treatment of malaria by some herbalists in Ghana. J. Med. Plants Res. 2019;13(16):370–383. [Google Scholar]
- 6.Semenya S.S., Maroyi A. Medicinal plants used for the treatment of tuberculosis by Bapedi traditional healers in three districts of the Limpopo Province, South Africa. Afr. J. Tradit., Complementary Altern. Med.: AJTCAM. 2012;10(2):316–323. doi: 10.4314/ajtcam.v10i2.17.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguta J.M., Appiah-Opong R., Nyarko A.K., Yeboah-Manu D., Addo P.G.A. Medicinal plants used to treat TB in Ghana. International Journal of Mycobacteriology. 2015;4(2):116–123. doi: 10.1016/j.ijmyco.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Philips C.A., Augustine P., Padsalgi G. Herbal medicines and reactivation of chronic hepatitis B virus infection. Hepat. Mon. 2018;18(9) doi: 10.5812/hepatmon.81000. [DOI] [Google Scholar]
- 9.Bekoe E., Agyare C., Sarkodie J., Dadebo D. Herbal medicines used in the treatment of typhoid in the Ga east municipality of Ghana. International Journal of Tropical Disease & Health. 2017;23(4):1–13. doi: 10.9734/IJTDH/2017/31448. [DOI] [Google Scholar]
- 10.World Health Organization . World Health Organization; 2013. WHO Traditional Medicine Strategy: 2014-2023.https://apps.who.int/iris/handle/10665/92455 [Google Scholar]
- 11.Christodoulou M., Tchoumtchoua J., Skaltsounis A., Scorilas A., Halabalaki M. Natural alkaloids intervening the insulin pathway: new hopes for anti-diabetic agents? Curr. Med. Chem. 2019;26(32):5982–6015. doi: 10.2174/0929867325666180430152618. [DOI] [PubMed] [Google Scholar]
- 12.Dineshkumar B., Mitra A., Manjunatha M. A comparative study of alpha amylase inhibitory activities of common anti-diabetic plants at Kharagpur 1 block. Int. J. Green Pharm. 2010;4(2) doi: 10.22377/ijgp.v4i2.131. [DOI] [Google Scholar]
- 13.Abdollahi M., Zuki A.B.Z., Goh Y.M., Rezaeizadeh A., Noordin M.M. The effects of Momordica charantia on the liver in streptozotocin-induced diabetes in neonatal rats. Afr. J. Biotechnol. 2010;9(31):5004–5012. doi: 10.14670/HH-26.13. [DOI] [PubMed] [Google Scholar]
- 14.Torres-León Cristian, Fernanda Rebolledo Ramírez, Jorge A., Aguirre-Joya, Ramírez-Moreno Agustina, Mónica L., Chávez-González, Aguillón-Gutierrez David R., Camacho-Guerra Luis, Ramírez-Guzmán Nathiely, Salvador Hernández Vélez, Aguilar Cristóbal N. Saudi Pharmaceut. J. 2023;31(1):21–28. doi: 10.1016/j.jsps.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gbekley H.E., Katawa G., Karou S.D., Anani S., Tchadjobo T., Ameyapoh Y., Batawila K., Simpore J. Ethnobotanical study of plants used to treat asthma in the Maritime Region in T. Afr. J. Tradit., Complementary Altern. Med.: AJTCAM. 2016;14(1):196–212. doi: 10.21010/ajtcam.v14i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization A global brief on hypertension; silent killer, a global public health crisis. 2013:8–34. (Accessed 3 October 2022) [Google Scholar]
- 17.Delfan B., Saki K., Bahmani M., Rangsaz N., Delfan M., Mohseni N.…Babaeian Z. A study on anti-diabetic and anti-hypertension herbs used in Lorestan province, Iran. Journal of Herb Med Pharmacology. 2014;3(2):71–76. [Google Scholar]
- 18.Farzaei F., Morovati M.R., Farjadmand F., Farzaei M.H. A mechanistic review on medicinal plants used for diabetes mellitus in traditional Persian medicine. Journal of evidence-based complementary & alternative medicine. 2017;22(4):944–955. doi: 10.1177/2156587216686461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beulens J.W.J., Rutters F., Rydén L., et al. Risk and management of pre-diabetes. European Journal of Preventive Cardiology. 2019;26(2_Supplement):47–54. doi: 10.1177/2047487319880041. [DOI] [PubMed] [Google Scholar]
- 20.Boulton A.J., Vileikyte L., Ragnarson-Tennval l.G., Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 21.Chukwuma C.I., Mopuri R., Nagiah S., Chuturgoon A.A., Islam M.S. Erythritol reduces small intestinal glucose absorption, increases muscle glucose uptake, improves glucose metabolic enzymes activities and increases expression of Glut-4 and IRS-1 in type 2 diabetic rats. Eur. J. Nutr. 2017 doi: 10.1007/s00394-017-1516-x. [DOI] [PubMed] [Google Scholar]
- 22.Hu Z., Gao F., Qin L., Yang Y., Xu H. A case-control study on risk factors and their Interactions with prediabetes among the elderly in rural communities of yiyang city, hunan province. J. Diabetes Res. 2019:1–8. doi: 10.1155/2019/1386048. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratley R.E. The early treatment of type 2 diabetes. Am. J. Med. 2013;126:S2–S9. doi: 10.1016/j.amjmed.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Bahrami J., Gerstein H. The prevalence of undiagnosed type 2 diabetes on the general medicine ward. Can. J. Diabetes. 2016;40(5):S49–S50. doi: 10.1016/j.jcjd.2016.08.139. [DOI] [Google Scholar]
- 25.Jangid H., Chaturvedi S., Khinchi M. Asian Journal of Pharmaceutical Research and Development; 2017. An Overview on Diabetes Mellitus; p. 11. [Google Scholar]
- 26.World Health Organisation . 2015. Obesity and Overweight.https://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 27.International Diabetes Federation Diabetes Atlas. eighth ed. 2017. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html Available from: [Google Scholar]
- 28.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Ghana Health Service (Policy Planning Monitoring and Evaluation Division) 2018. The Health sector in Ghana facts and figures 2018. Available at: https://ghanahealthservice.org›Facts+Figures_2018. [Google Scholar]
- 30.Vasan R.S., Beiser A., Seshadri S., Larson M.G., Kannel W.B. The residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 31.Stamler J., Stamler R., Neaton J.D. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch. Intern. Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 32.Douma S., Petidis K., Doumas M., Papaefthimiou P., Triantafyllou A., Kartali N., et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371(9628):1921–1926. doi: 10.1016/S0140-6736(08)60834-X. [DOI] [PubMed] [Google Scholar]
- 33.Edvardsson B., Persson S. Reversible cerebral vasoconstriction syndrome associated with autonomic dysreflexia. J. Headache Pain. 2010;11(3):277–280. doi: 10.1007/s10194-010-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . World Health Organization; Geneva: 2010. Global Status Report on Non-communicable Diseases.https://apps.who.int/iris/handle/10665/44579 2011. Available at: [Google Scholar]
- 35.Carretero O.A., Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101(3):329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 36.Beevers G., Lip G.Y., O'Brien E. ABC of hypertension: the pathophysiology of hypertension. BMJ (Clinical research ed.) 2001;322(7291):912–916. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M.J., Lim N.K., Choi S.J., Park H.Y. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertens. Res.: official journal of the Japanese Society of Hypertension. 2015;38(11):783–789. doi: 10.1038/hr.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kooti W., Farokhipour M., Asadzadeh Z., Ashtary-Larky D., Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron. Physician. 2016;8(1):1832–1842. doi: 10.19082/1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baharvand-Ahmadi B., Asadi-Samani M. A mini-review on the most important effective medicinal plants to treat hypertension in ethnobotanical evidence of Iran. Journal of nephropharmacology. 2016;6(1):3–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Karimian M. Natural remedies for vascular disease. Plant Biotechnol Persa. 2019;1(1):1–3. doi: 10.29252/pbp.1.1.1. [DOI] [Google Scholar]
- 41.Karimi E., Abbasi S., Abbasi N. Thymol polymeric nanoparticle synthesis and its effects on the toxicity of high glucose on OEC cells: involvement of growth factors and integrin-linked kinase. Drug Des. Dev. Ther. 2019;13:2513–2532. doi: 10.2147/DDDT.S214454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barimah K.B., Bonna O. OKAD Publishing; Chalotte NC 28271, USA: 2018. Traditional Medicine of Ghana. [Google Scholar]
- 43.Recommended standards for conducting and reporting ethnopharmacological field studies, J. Ethnopharmacol., Volume 210. Pages 125-132. 10.1016/j.jep.2017.08.018. [DOI] [PubMed]
- 44.The International Society of Ethnobiology (ISE) An alliance of biocultural diversity; code of ethics. 2016. https://www.ethnobiology.net/ Available at:
- 45.Friedman J., Yaniv Z., Dafni A., Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev desert, Israel. J. Ethnopharmacol. 1986;16(2–3):275–287. doi: 10.1016/0378-8741(86)90094-2. [DOI] [PubMed] [Google Scholar]
- 46.Heinrich M., Edwards S., Moerman D.E., Leonti M. Ethnopharmacological field studies: a critical assessment of their conceptual basis and methods. J. Ethnopharmacol. 2009;124(1):1–17. doi: 10.1016/j.jep.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 47.Lawal B., Aderibigbe A., Essiet G., Essien A. Hypotensive and antihypertensive effects of Aframomum melegueta seeds in humans. Int. J. Pharmacol. 2007;3:311–318. doi: 10.3923/ijp.2007.311.318. [DOI] [Google Scholar]
- 48.Asase A., Yohonu D.T. Ethnobotanical study of herbal medicines for management of diabetes mellitus in Dangme West District of southern Ghana. J. Herb. Med. 2016;6:204–209. doi: 10.1016/J.HERMED.2016.07.002. [DOI] [Google Scholar]
- 49.Keter L.K., Mutiso P.C. Ethnobotanical studies of medicinal plants used by traditional health practitioners in the management of diabetes in lower eastern province, Kenya. J. Ethnopharmacol. 2012;139(1):74–80. doi: 10.1016/j.jep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Chege I.N., Okalebo F.A., Guantai A.N., Karanja S., Derese S. 2015.Management of type 2 diabetes mellitus by traditional medicine practitioners in Kenya--Key informant interviews. Pan Afr Med J. 2015 Oct 1;22:90. doi: 10.11604/pamj.2015.22.90.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Covington M.B. Traditional Chinese medicine in the treatment of diabetes. Diabetes Spectr. 2001;14(3):154–159. [Google Scholar]
- 52.Arosemena C.M., Sánchez A.J., Tettamanti M.D., Vasquez C.D., Chang A., et al. Prevalence and risk factors of poorly controlled diabetes mellitus in a clinical setting in guayaquil, Ecuador: a cross-sectional study. Int J Diabetes Clin Res. 2015;2:34. [Google Scholar]
- 53.Madubunyi I.I., Adam K.P., Becker H. Anthocleistol, a new secoiridoid from Anthocleista nobilis. Z. Naturforsch. C. 1994;49:271–272. [Google Scholar]
- 54.Adotey J.K.P., Adukpo G.E., Boahen Y.O., Armah F.A. A review of the ethnobotany and pharmacological importance of Alstonia boonei De Wild (Apocynaceae) Pharmacol. 2012 doi: 10.5402/2012/587160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asif A., Khodadadi E. Medicinal uses and chemistry of flavonoid contents of some common edible tropical plants. JPS Summer. 2013;4(3):119–138. 2013. [Google Scholar]
- 56.Ezuruike U.F., Prieto J.M. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J. Ethnopharmacol. 2014;155(2):857–924. doi: 10.1016/j.jep.2014.05.05. [DOI] [PubMed] [Google Scholar]
- 57.Owa S.O., Oyewale D.G., Taiwo A.A., Edewor-Ikupoyi T.I., Okosun J.A., et al. Antidiabetic and antidyslipidemic effect of ethanolic extract of Alternanathera pungens on alloxan-induced diabetic rats. Asian J. Biochem. 2016;11:82–89. doi: 10.3923/ajb.2016.82.89. [DOI] [Google Scholar]
- 58.Mea A., Ekissi Y.H.R., Abo K.J.C., Kahou Bi G.P. Hypoglycaemiant and anti-hyperglycaemiant effect of Juscticia secunda m. vahl (acanthaceae) on glycaemia in the wistar rat. International Journal of Development Research. 2017;7(6):13178–13184. [Google Scholar]
- 59.Okwudili O.S., Chimaobi N.G., Ikechukwu E.M., Ndukaku O.Y. Antidiabetic and in vitro antioxidant effects of hydromethanol extract of Paullinia pinnata root bark in alloxan-induced diabetic rat. J. Compl. Integr. Med. 2017;15(2) doi: 10.1515/jcim-2015-0017. [DOI] [PubMed] [Google Scholar]
- 60.Njogu S.M., Arika W.M., Machocho A.K., Ngeranwa J.J.N., Njagi E.N.M. In vivo hypoglycemic effect of Kigelia africana (Lam): studies with alloxan-induced diabetic mice. Journal of Evidence-Based Integrative Medicine. 2018;23 doi: 10.1177/2515690x18768727. 2515690X1876872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.N’doua L.A., Abo K.J., Aoussi S., Kouakou L.K., Ehilé E.E. Aqueous extract of rauwolfia vomitoria afzel (Apocynaceae) roots effect on blood glucose level of normoglycemic and hyperglycemic rats. American Scientific Research Journal for Engineering, Technology, and Sciences. 2016;20:66–77. [Google Scholar]
- 62.Adinortey M.B. Biochemico-physiological mechanisms underlying signs and symptoms associated with diabetes mellitus. Adv. Biol. Res. 2019;11(6):382–390. [Google Scholar]
- 63.Mohammed A., Gbonjubola V.A., Koorbanally N.A., Islam M.S. Inhibition of key enzymes linked to type 2 diabetes by compounds isolated from Aframomum melegueta fruit. Pharmaceut. Biol. 2017;55(1):1010–1016. doi: 10.1080/13880209.2017.1286358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchegbu R.I., Ahuchaogu A.A., Amanze O.K., Ibe O.C. Chemical constituents analysis of the leaves of Bryophyllum pinnatum by GC-MS. AASCIT Journal of Chemistry. 2017;3(No. 3):19–22. 2017. [Google Scholar]
- 65.Hosni A.A., Abdel-Moneim E.S., Abdel-Reheim, Mohamed S.M., Helmy H. Cinnamaldehyde potentially attenuates gestational hyperglycemia in rats through modulation of PPARγ, proinflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2017;88:52–60. doi: 10.1016/j.biopha.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 66.Olutola D.O., Onocha P., Ekundayo O., Ali M. Isolation of aurantiamides from Gomphrena celosioides C. Mart. Iran. J. Pharm. Res. (IJPR): IJPR. 2014;13(1):143–147. [PMC free article] [PubMed] [Google Scholar]
- 67.Matta V.K., Pasala P.K., Netala S., Pandrinki S., Konduri P. Anti-Hypertensive Activity of the Ethanolic Extract of Lantana camara leaves on high salt loaded wistar albino rats. Phcog. J. 2015;7(5):289–295. doi: 10.5530/pj.2015.5.7. [DOI] [Google Scholar]
- 68.Akpabio U.D., Udiong D.S., Akpakpan A.E. The physicochemical characteristics of plantain (Musa paradisiaca) and banana (Musa sapientum) pseudo stem wastes. Advances in Natural and Applied Sciences. 2012;6:167–172. [Google Scholar]
- 69.Lima C.R., Vasconcelos C.F.B., Costa-Silva J.H., Maranhão C.A., Costa J., Batista T.M.…Wanderley A.G. Anti-diabetic activity of extract from Persea americana Mill. leaf via the activation of protein kinase B (PKB/Akt) in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2012;141(1):517–525. doi: 10.1016/j.jep.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 70.Bakırel T., Bakırel U., Keleş O.Ü., Ülgen S.G., Yardibi H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008;116(1):64–73. doi: 10.1016/j.jep.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 71.Gundamaraju R., Vemuri R., Singla R., Manikam R., Rao Ar, Sekaran S. Strophanthus hispidus attenuates the Ischemia-Reperfusion induced myocardial Infarction and reduces mean arterial pressure in renal artery occlusion. Phcog. Mag. 2014;10(39):557. doi: 10.4103/0973-1296.139782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aremu O., Tata C., Sewani-Rusike C., Oyedeji A., Oyedeji O., Gwebu E., Nkeh-Chungag B. vol. 74. Transactions of the Royal Society of South Africa; 2019. pp. 132–138. (Acute and Sub-chronic Antihypertensive Properties of Taraxacum officinale Leaf (TOL) and Root (TOR)). [DOI] [Google Scholar]
- 73.Kuate D., Kengne A.P.N., Biapa C.P.N., et al. Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 2015;14:50. doi: 10.1186/s12944-015-0051-0. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fetse J.P., Kofie W., Adosraku R. Ethnopharmacological importance of Xylopia aethiopica (DUNAL) A. RICH (annonaceae) - a review. Br. J. Pharmaceut. Res. 2016;11:1–21. doi: 10.9734/BJPR/2016/24746. [DOI] [Google Scholar]
- 75.Haas C.W. The role of ginger in type 2 diabetes mellitus. Integrative Medicine Alert. 2015;18(8):85–87. [Google Scholar]
- 76.Hossain U., Rahman M.O. Ethnobotanical uses and informant consensus factor of medicinal plants in Barisal district, Bangladesh. Bangladesh J. Plant Taxon. 2018;25(2):241–255. doi: 10.3329/bjpt.v25i2.39530. [DOI] [Google Scholar]
- 77.Gu Q., Burt V.L., Dillon C.F., Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 78.Asase A., Oppong-Mensah G. Traditional antimalarial phytotherapy remedies in herbal markets in southern Ghana. J. Ethnopharmacol. 2012;126(3):492–499. doi: 10.1016/j.jep.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 79.Diallo A., Traore M.S., Keita S.M., Balde M.A., Keita A., Camara M.…Balde A.M. Management of diabetes in Guinean traditional medicine: an ethnobotanical investigation in the coastal lowlands. J. Ethnopharmacol. 2012;144(2):353–361. doi: 10.1016/j.jep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 80.Busia K. vols. 1 & 2. Lightning Source UK Ltd.; Milton Keynes, UK: 2016. (Fundamentals of Herbal Medicine). [Google Scholar]
- 81.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol. Aspect. Med. 2006;27(1):1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Mohammed A., Koorbanally N.A., Islam M.S. Ethyl acetate fraction of Aframomum melegueta fruit ameliorates pancreatic β-cell dysfunction and major diabetes-related parameters in a type 2 diabetes model of rats. J. Ethnopharmacol. 2015;175:518–527. doi: 10.1016/j.jep.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 83.Mohammed R.K., Ibrahim S., Atawodi S.E., Eze E.D., Suleiman J.B. 2012. Anti-diabetic and Haematological Effects of N-Butanol Fraction of Alchornea cordifolia Leaf Extract in Streptozotocin-Induced Diabetic Wistar Rats. [DOI] [Google Scholar]
- 84.Mavar-Manga H., Lejoly J., Quetin-Leclercq, Schmelzer G.H. Protabase Record display; Wageningen, Netherlands: 2007. Alchornea cordifolia (Schumach &Thonn.) Mull.Arg. Plant Resources of Tropical Africa/Ressources vegetales de l‟Afrique tropicale. [Google Scholar]
- 85.Akinloye, Oluseyi Oshilaja R. Hypoglyceamic activity of Alstonia boonei stem bark extract in mice. Agric. Biol. J. N. Am. 2013;4:1–5. doi: 10.5251/abjna.2013.4.1.1.5. [DOI] [Google Scholar]
- 86.Gunjan M. Drug Invention Today; 2010. A Comparative Study of Aloe, Kundru and Neem as an Antidiabetic Agent. [Google Scholar]
- 87.Ojewole J.A.O. 1984. Studies on the Pharmacology of Echitamine, an Alkaloid from the Stem Bark of Alstonia Boonei L. (Apocynaceae). Pharmaceutical Biology. [DOI] [Google Scholar]
- 88.Ruyter C.M., Akram M., Illahi I., Stockigt J. Investigation of the alkaloid con- tent of Rauwolfia serpentina roots from regenerated plants. Planta Med. 1991;57(4):328–330. doi: 10.1055/s-2006-960109. [DOI] [PubMed] [Google Scholar]
- 89.Brasil G., Ronchi S., do Nascimento A., de Lima E., Romão W., da Costa H.…de Andrade T. Antihypertensive effect of Carica papaya via a reduction in ACE activity and improved baroreflex. Planta Med. 2014;80(17):1580–1587. doi: 10.1055/s-0034-1383122. [DOI] [PubMed] [Google Scholar]
- 90.Nyadjeu P., Nguelefack-Mbuyo E.P., Atsamo A.D., et al. Acute and chronic antihypertensive effects of Cinnamomum zeylanicum stem bark methanol extract in L-NAME-induced hypertensive rats. BMC Complement Altern Med. 2013;13:27. doi: 10.1186/1472-6882-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Altschuler J.A., Casella S.J., MacKenzie T.A., Curtis K.M. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. 2007;30(4):813–816. doi: 10.2337/dc06-1871. [DOI] [PubMed] [Google Scholar]
- 92.Adeneye A.A., Agbaje E.O. Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. J. Ethnopharmacol. 2007;112(3):440–444. doi: 10.1016/j.jep.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 93.Shah S., White J.M., Williams S.J. Total syntheses of cis-cyclopropane fatty acids: dihydromalvalic acid, dihydrosterculic acid, lactobacillic acid, and 9,10-methylenehexadecanoic acid. Org. Biomol. Chem. 2014;12(46):9427–9438. doi: 10.1039/c4ob01863j. [DOI] [PubMed] [Google Scholar]
- 94.de Paula Vasconcelos P.C., Tirloni C.A.S., Palozi R.A.C., Leitão M.M., Carneiro M.T.S., Schaedler M.I., Silva A.O., Souza R.I.C., Salvador M.J., Junior A.G., Kassuya C.A.L. Diuretic herb Gomphrena celosioides Mart. (Amaranthaceae) promotes sustained arterial pressure reduction and protection from cardiac remodeling on rats with renovascular hypertension. J. Ethnopharmacol. 2018;224:126–133. doi: 10.1016/j.jep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 95.Velmurugan C., Bhargava A. Anti-diabetic activity of Gossypium herbaceum by alloxan induced model in rats. PharmaTutor. 2014;2(4):126–132. [Google Scholar]
- 96.Alhassan A.J., Ibrahim, Muhammad S., Mohammed W., Alhassan I., Abdullahi Idi, Aminu M., Abubakar S., Mohammed A., Alexander I., Nasir A. Characterization and anti-diabetic activity of dihydrophenantherene isolated from Khaya senegalensis stem bark. Annual Research & Review in Biology. 2017;17:1–17. doi: 10.9734/ARRB/2017/35908. [DOI] [Google Scholar]
- 97.Matta V.K., Pasala P.K., Netala S., Pandrinki S., Konduri P. Anti-Hypertensive Activity of the Ethanolic Extract of Lantana camara leaves on high salt loaded wistar albino rats. Phcog. J. 2015;7(5):289–295. doi: 10.5530/pj.2015.5.7. [DOI] [Google Scholar]
- 98.Ngo Dai-Hung, Dai-Nghiep Ngo, Thi Thanh Nhan Vo, Vo Thanh Sang. Mechanism of action of Mangifera indica leaves for anti-diabetic activity. Sci. Pharm. 2019;87(2):13. doi: 10.3390/scipharm87020013. [DOI] [Google Scholar]
- 99.Harinantenaina L., Tanaka M., Takaoka S., Oda M., Mogami O., Uchida M., et al. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem. Pharm. Bull. (Tokyo) 2006;54:1017–1021. doi: 10.1248/cpb.54.1017. [DOI] [PubMed] [Google Scholar]
- 100.Ooi C.P., Yassin Z., Hamid T.A. Momordica charantia for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2012;8:CD007845. doi: 10.1002/14651858.CD007845.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baldwa V.S., Bhandari C.M., Pangaria A., Goyal R.K. Clinical trial in patients with diabetes mellitus of an insulin-like compound obtained from plant source. Ups. J. Med. Sci. 1977;82(1):39–41. doi: 10.3109/03009737709179057. [DOI] [PubMed] [Google Scholar]
- 102.Gacche R.N., Dhole N.A. Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: an attempt to standardize the botanicals for amelioration of diabetes complications. Food and chemical toxicology an international journal published for the British Industrial Biological Research Association. 2011;49(8):1806–1813. doi: 10.1016/j.fct.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 103.Kamiya K., Hamabe W., Harada S., Murakami R., Tokuyama S., Satake T. Chemical constituents of Morinda citrifolia roots exhibit hypoglycemic effects in streptozotocin-induced diabetic mice. Biological & pharmaceutical bulletin. 2008;31(5):935–938. doi: 10.1248/bpb.31.935. [DOI] [PubMed] [Google Scholar]
- 104.Odutuga A.A., Dairo J.O., Minari J.B.F. Anti-diabetic effect of Morinda lucida stem bark extracts on alloxan-induced diabetic rats. Res. J. Pharmacol. 2010;4:78–82. doi: 10.3923/rjpharm.2010.78.82. [DOI] [Google Scholar]
- 105.Adeyemi T.O.A., Idowu O.D., Ogboru R.O., Iyebor W.E., Owoeye E.A. Phytochemical screening, nutritional and medicinal benefits of Thaumatococcus daniellii Benn (Benth.) Inter. J. Appli. Res. and Technol. 2014;3(8):92–97. [Google Scholar]
- 106.Anwar F., Latif S., Ashraf M., Gilani A.H. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2006;21(1):17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 107.Omodanisi E.I., Aboua Y.G., Oguntibeju O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules. 2017;22(4):439. doi: 10.3390/molecules22040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Atawodi S.E., Atawodi J.C., Idakwo G.A., Pfundstein B., Haubner R., Wurtele G., Bartsch H., Owen R.W. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J. Med. Food. 2010;13(3):710–716. doi: 10.1089/jmf.2009.0057. [DOI] [PubMed] [Google Scholar]
- 109.Adeyemi O.S., Elebiyo T.C. Moringa oleifera supplemented diets prevented nickel-induced nephrotoxicity in wistar rats. Journal of nutrition and metabolism. 2014 doi: 10.1155/2014/958621. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Almatrafi M.M., Vergara-Jimenez M., Murillo A.G., Norris G.H., Blesso C.N., Fernandez M.L. Moringa leaves prevent hepatic lipid accumulation and inflammation in Guinea pigs by reducing the expression of genes involved in lipid metabolism. Int. J. Mol. Sci. 2017;18(7):1330. doi: 10.3390/ijms18071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shodehinde S.A., Oboh G. Distribution and antioxidant activity of polyphenols in boiled unripe plantain (Musa paradisiaca) pulps. J. Food Biochem. 2014;38(3):293–299. [Google Scholar]
- 112.Murthy S.S., Felicia C. Antidiabetic activity of Musa sapientum fruit peel extract on streptozotocin induced diabetic rats. Int. J. Pharma Bio Sci. 2015;6:P537–P543. [Google Scholar]
- 113.Waghmare J., Kurhade A.H. GC-MS analysis of bioactive components from banana peel (Musa sapientum peel) Eur. J. Exp. Biol. 2014;4:10–15. [Google Scholar]
- 114.Okoduwa S.I.R., Umar I.A., James D.B., Inuwa H.M. Anti-diabetic potential of Ocimum gratissimum leaf fractions in fortified diet-fed streptozotocin treated rat model of type-2 diabetes. Medicines (Basel, Switzerland) 2017;4(4):73. doi: 10.3390/medicines4040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Akinmoladun A., Ibukun E., Dan-Ologe I. Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Scientific Research and Essay. 2007;2:191–194. [Google Scholar]
- 116.Ramos-Aguilar A., Ornelas-Paz J., Tapia L., Ruiz-Cruz S., Gardea-Bejar A., Yahia E., Ornelas-Paz J., Rios C., Ibarra-Junquera V. The importnace of the bioactive compounds of avocado fruit (Persea americana Mill) on human health. Biotecnia. 2019;XXI:154–162. doi: 10.18633/biotecnia.v21i3.1047. [DOI] [Google Scholar]
- 117.Oh W.K., Lee C.H., Lee M.S., Bae E.Y., Sohn C.B., Oh H., Kim B.Y., Ahn J.S. Antidiabetic effects of extracts from Psidium guajava. J. Ethnopharmacol. 2005;96(3):411–415. doi: 10.1016/j.jep.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 118.Alamgir A.N.M. Biotechnology, in vitro production of natural bioactive compounds, herbal preparation, and disease management (treatment and prevention) Therapeutic Use of Medicinal Plants and their Extracts: Volume 2: Phytochemistry and Bioactive Compounds. 2018;74:585–664. doi: 10.1007/978-3-319-92387-1_7. [DOI] [Google Scholar]
- 119.Borges R.S., Ortiz B.L.S., Pereira A.C.M., Keita H., Carvalho J.C.T. Rosmarinus officinalis essential oil: a review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019;229:29–45. doi: 10.1016/j.jep.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 120.Fernández L.F., Palomino O.M., Frutos G. Effectiveness of Rosmarinus officinalis essential oil as antihypotensive agent in primary hypotensive patients and its influence on health-related quality of life. J. Ethnopharmacol. 2014;151(1):509–516. doi: 10.1016/j.jep.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 121.Varghese G.K., Bose L.V., Habtemariam S. Antidiabetic components of Cassia alata leaves: identification through α-glucosidase inhibition studies. Pharmaceut. Biol. 2013;51(3):345–349. doi: 10.3109/13880209.2012.729066/. [DOI] [PubMed] [Google Scholar]
- 122.Okey A., Ojiako Chidi, Igwe U. A time-trend hypoglycemic study of ethanol and chloroform extracts of Strophanthus hispidus. J. Herbs, Spices, Med. Plants. 2009;15(1):1–8. doi: 10.1080/10496470902787386. [DOI] [Google Scholar]
- 123.Mir M.A., Sawhney S.S., Jassa M.M.S. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. J. Pharm. Pharmacol. 2013;2(1):1–5. [Google Scholar]
- 124.Prangthipa P., Surasianga R., Charoensiria R., Leardkamolkarnb V., Komindrc S., Yamborisuta U., et al. Amelioration of hyperglycemia, hyperlipidemia, oxidative stress and inflammation in steptozotocin-induced diabetic rats fed a high fat diet by riceberry supplement. J. Funct.Foods. 2013;5:195–203. doi: 10.1016/j.jff.2012.10.005. [DOI] [Google Scholar]
- 125.Dimo T., Ngueguim F.T., Kamtchouing P., Dongo E., Tan P.V. Glucose lowering efficacy of the aqueous stem bark extract of Trema orientalis (Linn) Blume in normal and streptozotocin diabetic rats. Pharmazie. 2006;61(3):233–236. [PubMed] [Google Scholar]
- 126.Noungoue T.D., Cartier G., Dijoux F.M., Tsamo E., Mariotte A. Xanthones and other constituents of Trema orientalis. Pharm. Biol. 2001;39:202–205. [Google Scholar]
- 127.Adejuwon S.A., Osinubi A.A., Noronha C.C., Okanlawon A.O. vol. 8. West Afr J Anat; 2005. pp. 161–168. (Acute Effects of Vernonia Amygdalina on Blood Glucose Levels in Normoglycaemic and Alloxan-Induced Diabetic Male Sprague-Dawley Rats). [Google Scholar]
- 128.Atangwho I.J., Egbung G.E., Ahmad M., Yam M.F., Asmawi M.Z. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem. 2013;141(4):3428–3434. doi: 10.1016/j.foodchem.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 129.Okagu I.U., Ngwu U.E., Odenigbo C.J. Bioactive constituents of methanol extract of Xylopia aethiopica (UDA) fruits from nsukka, enugu state, Nigeria. Open Access Library Journal. 2018;5 doi: 10.4236/oalib.1104230. [DOI] [Google Scholar]
- 130.Aloke C., Ngwu N., Ugwuja E., Idenyi J., Nwachi O., Oga I.O. Effects of Zanthoxylum zanthoxyloides leaves on blood glucose, lipid profile and some liver enzymes in alloxan induced diabetic rats. International Journal of Science and Nuature. 2012;3:497–501. [Google Scholar]
- 131.Mohan M., Balaraman R., Kasture S. Kyung Hee Oriental Medicine Research Center; 2007. Antihypertensive Activity of Zingiber officinale and Korean Ginseng in Experimentally Induced Hypertension in Rats,” Oriental Pharmacy and Experimental Medicine. [DOI] [Google Scholar]
- 132.Shittu H., Gray A., Furman B., Young L. Glucose uptake stimulatory effect of akuammicine from Picralima nitida (Apocynaceae) Phytochem. Lett. 2010;3(1):53–55. doi: 10.1016/j.phytol.2009.11.003. [DOI] [Google Scholar]
- 133.Saseen J.J. In: Cardiovascular Therapeutics: A Companion to Braunwald's Heart Disease. fourth ed. Antman E.M., Sabatine M.S., editors. 2012. Pharmacologic management of hypertension; pp. 474–489. [Google Scholar]
- 134.Kwofie K.D., Tung N.H., Suzuki-Ohashi M., Amoa-Bosompem M., Adegle R., Sakyiamah M.M., Ayertey F., Owusu K.B., Tuffour I., Atchoglo P., Frempong K.K., Anyan W.K., Uto T., Morinaga O., Yamashita T., Aboagye F., Appiah A.A., Appiah-Opong R., Nyarko A.K., Yamaguchi Y.…Ayi I. Antitrypanosomal activities and mechanisms of action of novel tetracyclic Iridoids from Morinda lucida Benth. Antimicrobial agents and chemotherapy. 2016;60(6):3283–3290. doi: 10.1128/AAC.01916-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Camer D., Yu Y., Szabo A., Huang X.F. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol. Nutr. Food Res. 2014;58(8):1750–1759. doi: 10.1002/mnfr.201300861. [DOI] [PubMed] [Google Scholar]
- 136.Saravanan S., Pari L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chem. Biol. Interact. 2016;245:1–11. doi: 10.1016/j.cbi.2015.11.033. 2016. [DOI] [PubMed] [Google Scholar]
- 137.Erukainure O.L., Oyebode O.A., Ibeji C.U., Koorbanally N.A., Islam M.S. Vernonia Amygdalina Del. stimulated glucose uptake in brain tissues enhances antioxidative activities; and modulates functional chemistry and dysregulated metabolic pathways. Metab. Brain Dis. 2019;34:721–732. doi: 10.1007/s11011-018-0363-7. [DOI] [PubMed] [Google Scholar]
- 138.Torres-León Cristian, Fernanda Rebolledo Ramírez, Jorge A., Aguirre-Joya, Ramírez-Moreno Agustina, Mónica L., Chávez-González, Aguillón-Gutierrez David R., Camacho-Guerra Luis, Ramírez-Guzmán Nathiely, Salvador Hernández Vélez, Aguilar Cristóbal N. Saudi Pharmaceut. J. 2023;31(1):21–28. doi: 10.1016/j.jsps.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang W.Y., Cai Y.Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr. Cancer. 2010;62(1):1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 140.Ghosh D., Scheepens A. Vascular action of polyphenols. Mol. Nutr. Food Res. 2009;53(3):322–331. doi: 10.1002/mnfr.200800182. [DOI] [PubMed] [Google Scholar]
- 141.Makita C., Chimuka L., Steenkamp P., Cukrowska E., Madala E. Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. South Afr. J. Bot. 2016;105:116–122. doi: 10.1016/j.sajb.2015.12.007. [DOI] [Google Scholar]
- 142.Owolabi M.A., Coker H.A.B., Jaja S.I. Bioactivity of the phytoconstituents of the leaves of Persea americana. J. Med. Plants Res. 2010;4(12):1130–1135. doi: 10.5897/JMPR09.429. [DOI] [Google Scholar]
- 143.Sowmya N., Singh C.R., Patel K., Patel H., Dodiya T., Raja M.K.M.M. In-vitro and in vivo studies of traditionally prepared Zingiber officinale juice for Antimitotic and Lethality activity. Res. J. Pharm. Technol. 2021;14(9):4652–4656. [Google Scholar]
- 144.Abbasi E., Nassiri-Asl M., Shafeei M., Sheikhi M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem. Biol. Drug Des. 2012;80(2):274–278. doi: 10.1111/j.1747-0285.2012.01400.x. [DOI] [PubMed] [Google Scholar]
- 145.Lee Y.J., Jang Y.N., Han Y.M., Kim H.M., Seo H.S. 6-Gingerol normalizes the expression of biomarkers related to hypertension via PPARδ in HUVECs, HEK293, and differentiated 3T3-L1 cells. PPAR Res. 2018 doi: 10.1155/2018/6485064. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data/supplementary material is included and referenced in the article as tables.