Abstract
Light is the most important factor controlling circadian systems in response to day-night cycles. In order to better understand the regulation of circadian rhythms by light in Synechococcus elongatus PCC 7942, we screened for mutants with defective phase shifting in response to dark pulses. Using a 5-h dark-pulse protocol, we identified a mutation in kaiC that we termed pr1, for phase response 1. In the pr1 mutant, a 5-h dark pulse failed to shift the phase of the circadian rhythm, while the same pulse caused a 10-h phase shift in wild-type cells. The rhythm in accumulation of KaiC was abolished in the pr1 mutant, and the rhythmicity of KaiC phosphorylation was reduced. Additionally, the pr1 mutant was defective in mediating the feedback inhibition of kaiBC. Finally, overexpression of mutant KaiC led to a reduced phase shift compared to that for wild-type KaiC. Thus, KaiC appears to play a role in resetting the cellular clock in addition to its documented role in the feedback regulation of circadian rhythms.
The circadian clock is an endogenous biological timing process that exists in organisms from cyanobacteria to green plants and humans. The temporal regulation of various metabolic and behavioral activities by the circadian clock may enhance organism survival in response to regular, daily environmental changes (15). In various model organisms, transcription-translation feedback regulation of specific “clock genes” controls the internal circadian clocks (24).
Cyanobacteria are the simplest organisms that exhibit circadian rhythms (2, 5). Three clock genes, kaiA, kaiB, and kaiC, have been identified in the cyanobacterium Synechococcus elongatus PCC 7942 as essential clock components, and an autoregulatory loop of kaiBC gene expression has been proposed to generate the circadian rhythms (3). Recent studies suggested that KaiC protein coordinates genome-wide gene expression, including its own (12, 22). KaiC protein may also affect DNA condensation or the supercoiling status of Synechococcus chromosomes (11).
In nature, the circadian clock oscillates in exact 24-h cycles in response to variations in light resulting from the natural day-night cycle. Input pathways that receive and transmit environmental signals to the central oscillator differ among organisms, particularly in the perception of light signals. In mammals, membrane-associated opsin proteins in the eyes carrying retinal chromophores encounter light (25). In Drosophila melanogaster, both opsin-based photopigment and the blue-light photoreceptor cryptochrome function as photoreceptors (25). Two putative transcription factors in Neurospora crassa, WC-1 and WC-2, that interact with a critical element of the circadian clock, FRQ, are necessary for light perception (10). Two photoreceptors in higher plants, phytochromes and cryptochrome, transduce light signals to the circadian clock (23).
In cyanobacteria, only CikA (18), a member of the bacteriophytochrome protein family, has been identified as controlling circadian clock resetting. A cikA mutant was originally isolated as giving rise to the abnormal expression of a photosystem II gene, psbA. Further study revealed that expression of mutant cikA impaired the phase shift associated with a 5-h dark pulse. While CikA is essential for the phase resetting of the cyanobacterial clock, it is unlikely that it is solely responsible for all the steps between light perception and clock resetting. In order to better understand the regulation of the circadian clock in Synechococcus, we mutagenized bacteria with ethylmethanesulfonate (EMS) and examined the cellular reaction to a 5-h dark pulse. We identified a bacterial strain that did not undergo a circadian phase shift following a dark pulse. This strain possessed a mutation in the kaiC coding region, and the phase-shift phenotype was rescued by the introduction of the wild-type kai gene cluster. This mutant, designated the pr1 (for kaiC phase response 1) mutant, grew as well as wild-type cells and showed a normal circadian rhythm. In the pr1 mutant, normal cyclic variations in KaiC levels were significantly suppressed and rhythmicity in the KaiC phosphorylation state was reduced. Additionally, the pr1 mutant showed decreased KaiC autoregulatory activity. Our results suggest that KaiC phosphorylation is also important in resetting the cyanobacterial circadian clock.
MATERIALS AND METHODS
Cyanobacterial strains and culture conditions.
S. elongatus PCC 7942 was used as the background strain in this study. A strain that carries the PkaiBC::luxAB bioluminescence reporter construct at neutral site I (S. Kutsuna, M. Isiura, and T. Kondo, submitted for publication) was used as the wild-type strain. All Synechococcus strains were cultured in either modified BG-11 liquid medium or solid BG-11 medium containing 1.5% Bacto agar (Difco) under constant illumination (LL) of 46 μmol m−2 s−1 from white fluorescent lamps at 30°C.
Screening for resetting mutants.
Wild-type cells were mutagenized with EMS to a survival rate of 10%. After mutagenesis, cells were subcultured three times to segregate multiple copies of chromosomes in Synechococcus cells into individual cells. Cells were inoculated onto BG-11 agar plates, incubated under standard LL conditions for 36 h, and subjected to 12 h of darkness to synchronize the clocks of the cells. After the 12-h dark treatment, the plates were returned to LL conditions for 5 h and subjected to a 5-h dark pulse. After the 5-h dark treatment, plates were returned to LL conditions and incubated for 2 or 3 days until the colonies grew up to measurable size. Then the bioluminescence rhythms of each clone were monitored with a cooled charge-coupled device camera system (8) (Fig. 1A).
FIG. 1.
Phase shifting by dark pulses. (A) Standard protocol for phase shifting by dark pulses in LL. Cyanobacterial suspensions were inoculated onto agar plates and kept in LL for 1 or 2 days. After synchronization with a single 12-h dark period, the plates were returned to LL. At various times after the return to LL, samples were subjected to darkness for 4 h. After dark treatment, bioluminescence rhythms were monitored in LL for six or seven circadian cycles. Gray bars, LL conditions; black bar, dark condition; arrows, time points for dark-pulse initiation. (B) Phase response curve for 4-h dark pulses. Phase shifts induced by dark treatment were plotted against time of onset of the treatment in LL. Positive and negative values on the ordinate represent phase advance and delay, respectively.
DNA isolation and sequencing.
We sequenced mutant kai genes after in vitro amplification of kai segments by PCR. Synechococcus cells were mixed in a tube with zircon-silica beads (diameter, 0.1 mm; Biospec Products) and lysed by shaking with a Multi-Beads Shocker (Yasui Kikai). Genomic DNA was isolated by phenol extraction and purified by ethanol precipitation. Segments carrying the kai gene were amplified by PCR using the primer pair 818(3)up (5′-ACCGGCCACGTAGGGCTGTCC-3′) (409 to 389 bp upstream of the kaiA start codon)-715(5)low (5′-GGTGCTCGGGTTGACGACTG-3′) (+53 to +34 bp downstream of the end of the kaiC open reading frame). The DNA sequence was then analyzed with an ABI-PRISM model 310 sequencer.
Complementation by kaiABC.
The pr1 mutant strain was transformed with plasmid pDkaiABC to replace the mutant kaiABC locus with the kanamycin resistance gene. This transformant was then retransformed with plasmid pCkaiABC to express wild-type kaiABC genes (3).
Western blot analysis.
Synechococcus cells were grown in BG-11 liquid medium until the optical density at 730 nm was 0.2. The cells were exposed to two cycles of 12-h light-12-h dark alternation and then cultured under LL conditions. Cells were harvested at various time points after growth in LL. Cells were disrupted by using the Multi-Beads Shocker with zirconium beads for five cycles of 30-s agitation and 30-s resting at 4°C. Following two centrifugations, the supernatants were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and immunoblotted with anti-KaiC antibodies, as described previously (4). KaiC protein was detected with an enhanced chemiluminescence detector (ECL; Amersham). The protein concentration was determined by the bicinchoninic acid method using bovine serum albumin as a standard.
Overexpression of the kaiC gene.
NcoI-BamHI fragments carrying kaiC were ligated with NcoI- and BamHI-digested p322Ptrc (9) to yield p322Ptrc::kaiC. The smaller BglII fragment from p322Ptrc::kaiC was inserted into the BamHI site of pTS2KC (9) to yield pTS2C-Ptrc::kaiC. Wild-type cells were transformed with the plasmid as described previously (9) for specific chromosomal integration (neutral site II [NSII]) (1). KaiC overexpression was performed as described previously (3).
Bioluminescence assays with a photomultiplier tube system.
To analyze the precise bioluminescence profile of clones obtained by the initial screening, Synechococcus cells were inoculated onto BG-11 agar plates in a plastic dish (diameter, 35 mm), incubated under LL conditions (46 μmol m−2 s−1), and allowed to grow to 50 to 100 colonies per plate. Cells were subjected to 12 h of darkness in order to synchronize circadian clocks, and bioluminescence from the agar plates was automatically monitored under LL in the presence of 3% decanal with a photomultiplier-tube-based bioluminescence monitoring system (2). In this paper, the maximum bioluminescence of each experiment was standardized to 100.
RESULTS
Clock resetting followed by dark pulses.
Administration of a light pulse to cells grown in darkness advances or delays the circadian rhythm. This response is consistent among many organisms, and this technique is used to assess the effect of light on the circadian clock (16). However, in the cyanobacterium S. elongatus PCC 7942, the bioluminescence reporter does not work in darkness, making it impossible to examine phase shifting induced by a light pulse. A 4- or 5-h dark treatment (dark pulse) following culture in LL efficiently shifts the phase of circadian rhythms in cyanobacteria, however (K. Okamoto et al., unpublished data). We exposed an S. elongatus PCC 7942 strain expressing a PkaiBC::luxAB bioluminescence reporter to a 4-h dark pulse at various times following synchronization with the 12-h dark treatment. After the dark pulse, bioluminescence rhythms were monitored for six or seven circadian cycles, and peaks of bioluminescence were compared with that of an LL control in order to observe any phase shift induced by the dark pulse. Figure 1B shows the measured phase responses plotted against the time of day when the 4-h dark pulse was administered (phase response curve [PRC]). The phase response oscillated for at least three circadian cycles, with breakpoints from the largest phase delay to the largest advance in the early subjective day. Based on the PRC, we examined various dark pulses that caused significant and stable phase shifting and chose a 5-h dark pulse starting after 5 h of LL (LL5), which causes a stable 10-h phase advance.
Screening for light input mutants.
In screening for mutants with an abnormal phase response to a 5-h dark pulse, we excluded clones with an abnormal period length, because most period mutants have been identified as kai mutants. From an initial pool of 40,000 clones, we isolated 5 candidate clones. However, the periods of four candidates were slightly shorter or longer, resulting in an apparently abnormal phase response. The final mutant, pr1, had a normal bioluminescence rhythm (periods of the pr1 mutant and the wild type, 24.9 ± 0.2 and 24.6 ± 0.4 h, respectively), but the rhythm peak was not changed by a 5-h dark pulse at LL5 (Fig. 2A). Because the pr1 mutant was advanced 3 to 4 h (3.9 ± 1.6 h) relative to wild-type cells (Fig. 2A), we examined the phase response of the pr1 mutant to a 4-h dark pulse at various times of the day and confirmed that it failed to respond to a dark pulse at any time of the day (Fig. 2B).
FIG. 2.
Phase shift in pr1 mutants by dark pulses. (A) Bioluminescence rhythms of wild-type and pr1 cells after dark pulses. Bioluminescence by the PkaiBC::luxAB reporter was plotted against time in LL. Maximum bioluminescence in frame was standardized to 100. Solid circles, bioluminescence of LL control (no dark pulse); open circles, bioluminescence of culture subjected to dark pulse (LL, 5 to 10). Gray bars indicate the time of 5-h dark pulses. (B) Phase response curve for 4-h dark pulses. Solid circles indicate phase shifting of wild-type cells; open circles indicate that of the pr1 mutant. Experimental procedures and presentation of data are the same as for Fig. 1B. (C) Rescue of the pr1 mutant with wild-type kaiABC. Bioluminescence rhythm of the pr1/pCkaiABC strain. Solid circles, bioluminescence of LL control (no dark pulse); open circles, bioluminescence of culture subjected to dark pulse (LL, 5 to 10). Experimental procedures and presentation of data are the same as for panel A.
Mutation of the pr1 mutant.
As noted above, most abnormal clock phenotypes in Synechococcus are caused by mutations in the sequence of kai genes (3). Therefore, we examined the sequence of kai genes in the pr1 mutant and found a single base mutation from C to T at position 1265 of kaiC. This mutation resulted in the substitution of an alanine residue for a valine residue at position 422 of KaiC. We replaced the kaiC gene of the pr1 mutant with wild-type kaiC by using plasmid pCkaiABC containing wild-type kaiC. As shown in Fig. 2C, the bioluminescence rhythm of the rescued pr1 cells exhibited both normal period length and a phase shift following the 5-h dark pulse. Taken together, these data confirm that the mutation in KaiC in the pr1 mutant is responsible for the phenotype observed.
KaiC accumulation in the pr1 mutant.
To better understand the changes arising from the pr1 mutation at a biochemical level, we examined the temporal regulation of KaiC in pr1 and wild-type cells. Soluble protein extracts, prepared at various times following LL, were immunoblotted with an anti-KaiC serum. As shown in Fig. 3A, KaiC levels oscillated dramatically, peaking at the subjective dusk in wild-type cells, while oscillation of KaiC abundance was reduced in pr1 mutant cells. As reported previously, KaiC migrates as an upper nonphosphorylated band and a lower phosphorylated band (6). In wild-type cells, the phosphorylation of KaiC oscillated strongly with the circadian cycle, whereas oscillation of phosphorylation was reduced in the pr1 mutant. To evaluate these rhythmicities of the pr1 mutant, we repeated the assay, and quantification of the results (Fig. 3B to E) indicated that the rhythm of KaiC accumulation was abolished in the pr1 mutant, whereas a weak rhythmicity was still found in KaiC phosphorylation levels. The phase of the weak phosphorylation rhythm was similar to that of the wild type.
FIG. 3.
Accumulation of KaiC in pr1 cells. (A) Western blot analysis of KaiC in wild-type and pr1 mutant cells. Protein levels of KaiC in wild-type and pr1 cells were examined. Cells were collected at the indicated times in LL. Total proteins (1 μg) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels and analyzed by immunoblotting using anti-KaiC antisera. KaiC signals appear as upper phosphorylated (P-KaiC) and lower nonphosphorylated (KaiC) bands. (B and C) KaiC accumulation signals of wild-type (B) or pr1 (C) cells by Western blot analysis (from panel A) were measured by densitometry and plotted against time in LL. A representative profile for two experiments with wild-type cells is shown in panel B. For pr1, each time point was normalized to the mean intensity of the KaiC signal for 13 time points in each experiment. The average KaiC level of the pr1 mutant (1 on the vertical axis) corresponded to 60 to 70% of the average intensity of wild-type cells. (D and E) The phosphorylation ratio of KaiC in wild-type (D) or pr1 (E) cells was calculated as the ratio of phosphorylated KaiC to total KaiC and plotted against time in LL. A representative profile for two experiments with wild-type cells is shown in panel D. For pr1, each time point was normalized to the average ratio of KaiC phosphorylation for 13 time points in each experiment. The average KaiC phosphorylation ratios of the wild type and the pr1 mutant (1 on the vertical axis) were both 50%. Error bars in panels C and E, standard deviations (n = 6).
Overexpression of KaiC and KaiCpr1.
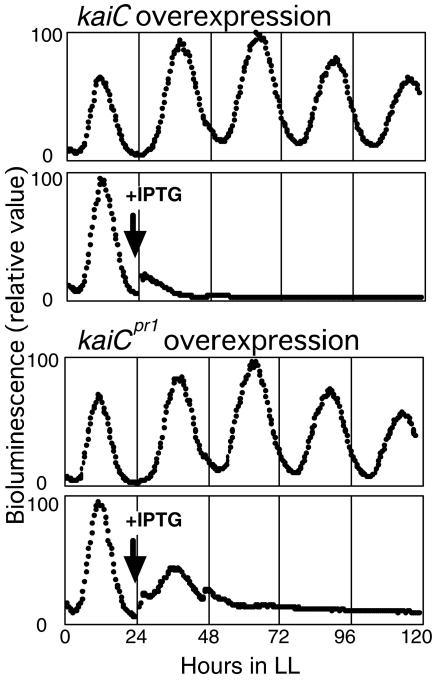
KaiC broadly suppresses gene expression in Synechococcus, and it is thought to function as the essential feedback regulator of circadian oscillations (3, 12). We wished to examine the effect of mutant KaiC expression on gene expression. An Escherichia coli-inducible promoter, Ptrc, was fused to wild-type kaiC or mutant kaiC (C1265T) and introduced into wild-type cells. KaiC overexpression was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG). As shown in Fig. 4, induction of wild-type kaiC nullified the bioluminescence in wild-type cells in about 15 h. In contrast, repression by overexpression of KaiCpr1 (mutant KaiC) was slower and incomplete in wild-type cells. After about 40 h, expression downstream of PkaiBC was suppressed to 10% of the noninduced level, and this level was sustained.
FIG. 4.
Effects of overexpression of kaiC and mutant kaiC (C1265T). Bioluminescence rhythms of the PkaiBC::luxAB reporter of wild-type cells expressing Ptrc::kaiC or Ptrc::kaiC (C1265T) were plotted against time in LL. IPTG (100 μM) was administered at the time point indicated by arrows. Presentation of data is the same as for Fig. 2A.
Resetting of the bioluminescence rhythm by temporal overexpression of KaiC.
Temporal overexpression of KaiC shifts the phase of the bioluminescence rhythm (3), and we examined the ability of KaiCpr1 to cause a phase shift in wild-type cells. As shown in Fig. 5A, a 6-h overexpression of KaiC from LL21 caused an 11-h phase delay, while only a 3-h delay was caused by overexpression of KaiCpr1. To investigate the phase response to temporal overexpression of KaiCpr1 over the circadian cycle, we examined a PRC for a 6-h overexpression pulse of KaiC and KaiCpr1 (Fig. 5B). While 6-h overexpression of wild-type KaiC caused a maximum 10- to 11-h phase delay and an advance in the late subjective night, overexpression of KaiCpr1 caused a smaller shift; the largest phase shift, 5 h, occurred in the early subjective day.
FIG. 5.
Phase shifting by temporal overexpression of kaiC and mutant kaiC (C1265T). (A) Bioluminescence rhythms of the PkaiBC::luxAB reporter of wild-type cells carrying the Ptrc::kaiC or Ptrc::kaiC (C1265T) construct. Solid circles, control (no induction); open circles, rhythms after kaiC overexpression pulse (CT, 21 to 27). Gray bars indicate the time of 6-h overexpression of each kaiC form. Presentation of data is the same as in Fig. 2A. (B) Phase response curve for 6-h overexpression of kaiC. The abscissa indicates the onset of overexpression pulses in LL. Presentation of data are the same as for Fig. 1B.
DISCUSSION
We screened 40,000 clones and found many period-altered or arrhythmic mutants. However, from our experience, it was likely that nearly all EMS mutants with this phenotype had a mutation in the kai gene cluster. We excluded all period mutants and focused on clones that retained normal rhythmicity but responded to a dark pulse abnormally. Only one such mutant was isolated (the pr1 mutant), and it was later identified as a kaiC mutant. Thus, no mutant with a mutation of a novel component in the phototransduction pathway to the central clock system (the so called “input pathway”) was found in this screen. In the primary screening, we found different period or arrhythmic mutants at a ratio of 1/1,000; thus, our mutagenesis strategy is effective at inducing mutations in the cyanobacterial genome. Our failure to identify an input pathway mutant could have occurred for several reasons. The component(s) of the input pathway might also function in basic metabolism, and mutation of these genes could be lethal. On the other hand, if our protocol induced two or more independent processes (such as photosynthetic energy metabolism) that contribute to the resetting, disabling a single process by mutation would be insufficient to abolish resetting. Probably, it would be necessary to develop a new protocol that would exclusively perturb the light input pathway.
More than 100 mutants have been mapped to kaiC, and nearly all give rise to arrhythmia or period abnormalities (3) (unpublished data). The novel mutant identified in this study, the pr1 mutant, failed to respond to a dark pulse but exhibited circadian rhythms with a period and amplitude similar to those of wild-type cells (Fig. 2). Conversely, a mutant with a mutation of cikA, encoding a histidine protein kinase (18), also displays poor clock resetting after a dark pulse, but it also exhibits a low-amplitude bioluminescence rhythm. The robust rhythmicity of the pr1 mutant will allow for the detailed analysis of the mechanism(s) regulating phase resetting. While we recently reported on the KaiC phosphorylation cycle as a pacemaker of the Synechococcus oscillator (21), negative-feedback regulation by KaiC of its own expression is still an important process regulating the Synechococcus oscillator, and any phase-resetting pathway should affect this feedback process. Therefore, the pr1 mutation might affect the ability of KaiC to receive a resetting signal from the phase-resetting pathways. This is the case for the timSL and cryb mutations in the Drosophila circadian system (17, 19).
The pr1 mutation gives rise to a valine-to-alanine substitution at position 422 of KaiC, and this mutation is mapped to a possible KaiA binding domain (20). This is further supported by the recently solved KaiC crystal structure (14). The A422 site is also close to the phosphorylation sites of KaiC (S431 and T432) (13). Thus, the pr1 mutation may affect the phosphorylation of KaiC. KaiC phosphorylation oscillates in vivo, peaking at hour 16 of LL, and KaiA and its binding to KaiC facilitate KaiC phosphorylation (6, 7). Moreover, biochemical studies have revealed that KaiC phosphorylation is required for the formation of the Kai/SasA complex (6, 13), which is thought to regulate global gene expression in Synechococcus. In a recent study, it was demonstrated that the KaiC phosphorylation rhythm could be maintained at least 3 days without transcription-translation feedback (21). These results imply that the oscillation of KaiC phosphorylation is the primary oscillation of the cyanobacterial circadian clock and that changes in the phosphorylation level are required for phase resetting. Indeed, the pr1 mutation reduced KaiC phosphorylation levels (Fig. 3A), but the rhythm of KaiC phosphorylation still persisted even in the pr1 mutant, and the relatively robust rhythmicity observed via the bioluminescence reporter can be ascribed to an unknown process that links the phosphorylation rhythm to gene expression processes. On the other hand, if the rates of phosphorylation and dephosphorylation of KaiC are reduced by the pr1 mutation, this reduction could explain the failure of phase response to a 5-h dark pulse in the pr1 mutant.
Functionally, KaiC is the main repressor of kaiBC expression in the Synechococcus circadian feedback loop. Overexpression of KaiC strongly represses its own promoter activity, and temporal overexpression of KaiC shifts the phase of the circadian rhythm in a phase-dependent manner (3). We confirmed that both functions of KaiC were affected in the pr1 mutant (Fig. 4 and 5). While overexpression of KaiC rapidly represses kaiBC expression almost completely, overexpressed KaiCpr1 reduced promoter activity more slowly and permitted 10 to 20% residual activity (Fig. 4). Thus, the weak suppression of kaiBC by KaiCpr1 supports normal circadian oscillation in LL and resetting by strong stimuli (12-h dark pulse); however, the lower level of repression by KaiCpr1 is insufficient to cause a phase shift following a 5-h dark pulse. Similarly, as shown in Fig. 5, the temporal overexpression of KaiCpr1 incompletely repressed kaiBC-driven expression and reduced the phase shift.
Acknowledgments
This research was supported in part by grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (15GS0308 and 14COEEA01, to T.K.).
REFERENCES
- 1.Andersson, C. R., N. F. Tsinoremas, J. Shelton, N. V. Lebedeva, J. Yarrow, H. Min, S. S. Golden, H. Iwasaki, A. Tanabe, C. H. Johnson, and T. Kondo. 2000. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 305:527-542. [DOI] [PubMed] [Google Scholar]
- 2.Golden, S. S., M. Ishiura, C. H. Johnson, and T. Kondo. 1997. Cyanobacterial circadian rhythms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:327-354. [DOI] [PubMed] [Google Scholar]
- 3.Ishiura, M., S. Kutsuna, S. Aoki, H. Iwasaki, C. R. Andersson, A. Tanabe, S. S. Golden, C. H. Johnson, and T. Kondo. 1998. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281:1519-1523. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki, H., Y. Taniguchi, M. Ishiura, and T. Kondo. 1999. Physical interactions among circadian clock proteins KaiA, KaiB, and KaiC in cyanobacteria. EMBO J. 18:1137-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki, H., and T. Kondo. 2000. The current state and problems of circadian clock studies in cyanobacteria. Plant Cell Physiol. 41:1013-1020. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki, H., T. Nishiwaki, Y. Kitayama, M. Nakajima, and T. Kondo. 2002. KaiA-stimulated KaiC phosphorylation in cyanobacteria. Proc. Natl. Acad. Sci. USA 99:15788-15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitayama, Y., H. Iwasaki, T. Nishiwaki, and T. Kondo. 2003. KaiB functions as an attenuator of KaiC phosphorylation in the cyanobacterial circadian clock system. EMBO J. 22:2127-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo, T., N. F. Tsinoremas, S. S. Golden, C. H. Johnson, S. Kutsuna, and M. Ishiura. 1994. Circadian clock mutants of cyanobacteria. Science 266:1233-1236. [DOI] [PubMed] [Google Scholar]
- 9.Kutsuna, S., T. Kondo, S. Aoki, and M. Ishiura. 1998. A period-extender gene, pex, that extends the period of the circadian clock in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 180:2167-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loros, J. J., and J. C. Dunlap. 2001. Genetic and molecular analysis of circadian rhythms in Neurospora. Annu. Rev. Physiol. 63:757-794. [DOI] [PubMed] [Google Scholar]
- 11.Mori, T., and C. H. Johnson. 2002. Circadian clock protein KaiC forms ATP-dependent hexameric rings and binds DNA. Proc. Natl. Acad. Sci. USA 99:17203-17208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakahira, Y., M. Katayama, H. Miyashita, S. Kutsuna, H. Iwasaki, T. Oyama, and T. Kondo. 2004. Global gene repression by KaiC as a master process of prokaryotic circadian system. Proc. Natl. Acad. Sci. USA 101:881-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishiwaki, T., Y. Satomi, M. Nakajima, C. Lee, R. Kiyohara, H. Kageyama, Y. Kitayama, M. Temamoto, A. Yamaguchi, A. Hijikata, M. Go, H. Iwasaki, T. Takao, and T. Kondo. 2004. Role of KaiC phosphorylation in the circadian clock system of Synechococcus elongatus PCC 7942. Proc. Natl. Acad. Sci. USA 101:13927-13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pattanayek, R., J. Wang, T. Mori, Y. Xu, C. H. Johnson, and M. Egli. 2004. Visualizing a circadian clock protein: crystal structure of KaiC and functional insights. Mol. Cell 15:375-388. [DOI] [PubMed] [Google Scholar]
- 15.Pittendrigh, C. S. 1993. Temporal organization: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 55:17-54. [DOI] [PubMed] [Google Scholar]
- 16.Roenneberg, T., and R. Foster. 1997. Twilight times: light and the circadian system. Photochem. Photobiol. 66:549-561. [DOI] [PubMed] [Google Scholar]
- 17.Rutila, J. E., O. Maltseva, and M. Rosbash. 1998. The timSL mutant affects a restricted portion of the Drosophila melanogaster circadian cycle. J. Biol. Rhythms 13:380-392. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz, O., M. Katayama, S. B. Williams, T. Kondo, and S. S. Golden. 2000. CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science 289:765-768. [DOI] [PubMed] [Google Scholar]
- 19.Stanewsky, R., M. Kaneko, P. Emery, B. Beretta, K. Wanger-Smith, S. A. Kay, M. Rosbash, and J. C. Hall. 1998. The cryb mutation identifies Cryptochrome as a circadian photoreceptor in Drosophila. Cell 95:681-692. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi, Y., A. Yamaguchi, A. Hijikata, H. Iwasaki, K. Kamagata, M. Ishiura, M. Go, and T. Kondo. 2001. Two KaiA-binding domains of cyanobacterial circadian clock protein KaiC. FEBS Lett. 496:86-90. [DOI] [PubMed] [Google Scholar]
- 21.Tomita, J., M. Nakajima, T. Kondo, and H. Iwasaki. 2005. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science 307:251-254. [Online.] doi:10.1126/science.1102540. [DOI] [PubMed] [Google Scholar]
- 22.Xu, Y., T. Mori, and C. H. Johnson. 2003. Cyanobacterial circadian clockwork: roles of KaiA, KaiB and the kaiBC promoter in regulating KaiC. EMBO J. 22:2117-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanovsky, M. J., and S. A. Kay. 2001. Signaling networks in the plant circadian system. Curr. Opin. Plant Biol. 4:429-435. [DOI] [PubMed] [Google Scholar]
- 24.Young, M. W., and S. A. Kay. 2001. Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 9:702-715. [DOI] [PubMed] [Google Scholar]
- 25.Zordan, M. A., E. Rosato, A. Piccin, and R. Foster. 2001. Photic entrainment of the circadian clock: from Drosophila to mammals. Semin. Cell Dev. Biol. 12:317-328. [DOI] [PubMed] [Google Scholar]