Abstract
Modulation of probiotic performances represents a tool to avoid the probiotic off-flavor in probiotic food. Microencapsulation and sonication were evaluated in slowing down the Lacticaseibacillus casei ATCC 393 induced acidification. Firstly, the influence of alginate concentration and chitosan coating on acidification rate were tested. Microcapsule morphology and the entrapment efficacy were also evaluated. Then, two time of exposure to ultrasound, 6 and 8 min, were applied for L. casei attenuation. Finally, sonicated cells were encapsulated. ΔpH after 6 and 24 h of incubation at 37 °C revealed that chitosan-alginate microcapsules and the 8-min sonicated probiotic presented a significant delayed acidification. When all the systems were compared, the encapsulation of 8-min sonicated L. casei in chitosan-alginate microcapsules significantly improved the results obtained with the single technologies. These results suggest that by modulating the operating parameters and combining these two technologies an increasingly efficient attenuation system can be developed.
Keywords: Fermentative metabolism, Acidification, alginate, chitosan, Coating
Highlights
-
•
Modulating probiotic performance is an innovative food probiotication strategy.
-
•
Alginate's porous structure doesn't attenuate the probiotic, even at 1.5 %.
-
•
Chitosan-alginate interactions create an efficient attenuating microcapsule.
-
•
Sonication intensity determines the attenuation efficacy.
-
•
Sonicated microencapsulated cells showed a completely different acidification pattern.
1. Introduction
Over the years, the knowledge about probiotics and the role of the intestinal microbiota in human health increased. At the same time, more and more bacteria species were included in the category of probiotics. Therefore, the interest in the use of probiotics as a therapy to improve human health also increased [1]. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [2]. Probiotics exert their beneficial role via the production of metabolites, such as short-chain fatty acids (SCFAs), vitamins and antimicrobial compounds [3], protection from pathogen colonization through competition for nutrients and binding sites [4], modulation of the epithelium mucus properties [5] and of the immune system [6]. Probiotics of the Lactic Acid Bacteria group (LAB), specifically, several species belonging to the ex-Lactobacillus genus have been extensively studied for their commercial, industrial and therapeutic potential. The deep knowledge of the genus led to the recent reorganization in 23 new genera [7]. The Lacticaseibacillus casei group comprehends species strictly related to each other: casei, paracasei and rhamnosus [8]. Probiotic strains belonging to these species are knowing for direct or indirect beneficial effects on human health. Lacticaseibacillus casei ATCC 393 has recently been used for selenium (Se) bioremediation, producing selenium nanoparticles (SeNPs). These SeNPs and SeNP-enriched L. casei ATCC 393 in mice have been shown to improve intestinal barrier function, to inhibit colon cancer cell growth and to prevent cognitive dysfunction [[9], [10], [11]]. It is also known to delay the development of colon cancer, enhance the anti-cancer immune response and have anti-proliferative, growth inhibitory and pro-apoptotic effects on cancer model cells [12,13]. L. casei ATCC 393 is also used in various food products. Abdel-Hamid et al. [14] found that the strain releases ACE inhibitors, antioxidants and anticancer peptides during milk fermentation. Additionally, L. casei ATCC 393 has been employed for lactic acid production from agri-food waste, as shown by Dedenaro et al. [15] and Costa et al. [16]. When incorporated into yoghurt or feta-type cheeses it increased the complexity of the flavor profile [17,18]. Furthermore, Sidira et al. [19] employed this probiotic in the production of probiotic dry-fermented sausages, which improved microbial safety, taste and shelf life, especially in high-sugar environments.
The modern market trend is to use probiotics to enrich several categories of food and beverage. Besides the traditional dairy products, other matrix used as probiotic carriers include cereal-based products, legume-based products, vegetable-based products, fruit-based products [20,21], bakery products [22] and chocolate [23]. However, the probiotication of foods represents a challenge due to the sensory acceptance [24,25] and the delivery of live probiotics in adequate amounts to the target site, the colon [26]. Research efforts have been focused on developing systems capable of preserving cell viability rather than preserving the sensory characteristics of the product. Attenuation technologies comprise all the technologies applied on microorganisms to modulate or to control their metabolism. Recently, this term was applied in the probiotic field to avoid and/or minimize nutrients utilization in food and the release of metabolites that modify its sensory characteristics [27,28].
Ultrasound has proved to be a suitable technology for this purpose [28,29]. Mechanical and chemical events, originated from the phenomenon of cavitation, lead to an alteration of the normal physiology and functionality of the cell [30]. However, depending on the strain and treatment parameters, the attenuation effects may occur over shorter or longer periods of time and probiotic viability could be compromised [29]. Therefore, other technologies that avoid the loss of viability need to be investigated.
Microencapsulation is a well-known technology used in the food and probiotic field. It is a process where the probiotic is incorporated into a specific material that has the ability to reduce cell injury or cell loss, derived from environmental factors, with a controlled-release rate under specific conditions [31]. As primary goal, microencapsulation is used to protect the probiotics from harsh environments, such as the food matrix and the gastrointestinal tract. Microcapsules protection is given by the physical barrier between the cell and the external environment. Thus, the microcapsules also control the rate of nutrient uptake and metabolite release, slowing them down enabling minimal cell-environment interactions [32,33]. From this point of view, microencapsulation can be considered an attenuation technology.
The efficacy of microencapsulation to attenuate probiotic metabolism depends on the chemical properties and on the mechanical characteristics of the microcapsule. The matrix of microencapsulation determines the functional properties of the system [34]. Thus, a proper evaluation and selection of matters is required. Hydrogels derived from natural biopolymer cover the requirement for probiotic encapsulation and food industry application. The suitability of hydrogels for microencapsulation process mainly depends on the gelling and swelling properties [35]. Alginate and chitosan are the most common materials used for probiotic microencapsulation. The GRAS status, non-toxicity and biocompatibility of these polymers define their suitability for use in pharmaceuticals, foods and for microorganisms’ encapsulation. Moreover, the complementary polyelectrolyte nature of these polysaccharides leads to the formation of microcapsule chemically stable with high mechanical strength. Alginate microcapsules are characterized of porous structure due to the instantaneous external ionic gelation in presence of divalent cations as Ca2+. When an external layer of chitosan is deposited on the microcapsule surface, a phenomenon of complexation occurs between the two polysaccharides. The negative charged carboxyl group of alginate and the positive charged ammino group of chitosan established high electrostatic interactions. As a consequence, the microcapsule porosity and also its permeability to water soluble molecules is reduced [1,31,35].
The aim of this work is to evaluate the feasibility of microencapsulation and ultrasound as attenuation technology. Firstly, the hypothesis of a correlation between attenuation efficacy and alginate concentration was investigated. Also, an external chitosan layer was added to improve the capsule barrier properties. Microencapsulation efficacy and microcapsules morphology were evaluated. Two sonication treatments were applied to underline the role of sonication intensity on the probiotic. The two approaches were studied alone as a mean for the attenuation of the acidification ability of the strain. Then, microencapsulation, sonication and the combination microencapsulation-sonication were compared to find the best option to attenuate the fermentative metabolism of the probiotic Lacticaseibacillus casei ATCC 393.
The approach applied in the study led to a preliminary understanding of the use of microencapsulation alone and in combination with ultrasound in probiotic attenuation. Compared to what is already known about viability preservation and delivery, this initiates the use of microencapsulation in a new way. In addition, this study opens up the possibility of a more in-depth investigation of the approaches used and a specific application in a food system.
2. Materials and methods
2.1. Probiotic growth conditions
Lacticaseibacillus casei ATCC 393 was acquired by the American Type Culture Collection. It was routinely cultivated in MRS broth (OXOID Ltd., Basingstoke, Hampshire, UK) and incubated at 37 °C for 24 h. MRS agar plates were used to check its purity and to assess viable counts after 48 h of incubation at 37 °C in anaerobic conditions.
2.2. Sonication
L. casei ATCC 393 sonication was performed using the LABSONIC U (B. Braun, Melsungen, Germany) equipment. The ultrasound generation probe was cleaned with 70 % ethanol prior to use. Two treatments were applied on the probiotic suspension in deionized water (30 ml). A fixed frequency (20 kHz), duty cycle (50 %) and power (57 W) were used. The probiotic was exposed to ultrasound for a time of 6- and 8-min. Finally, sonication was performed in an ice box to control the temperature rise and the samples were kept on ice until use.
2.3. Encapsulation
2.3.1. Encapsulation process
Sodium alginate (Sigma, product n. A2033) with medium viscosity and a molecular weight between 250,000 and 350,000 g/mol, as indicated by the producer, was used as biopolymer to encapsulate the probiotic. Four alginate solutions were tested to evaluated the effect of alginate concentration on attenuation efficacy of microencapsulation. Solutions with 0.8, 1.0, 1.2 and 1.5 % of alginate were prepared by dissolving sodium alginate powered in deionized water mildly heated, filter through a gauze and finally sterilized by autoclave.
Non sonicated and sonicated L. casei were microencapsulated by vibrating technology, using the Encapsulator B-395 Pro (BÜCHI Labortechnik, Flawil, Switzerland) as described by De Prisco et al. [36]. Briefly, probiotic fresh culture and sonicated suspension were centrifuged at 6000×g for 10 min and washed twice with an equal volume of quarter-Strength Ringer solution (OXOID). The collected pellet was resuspended in the same volume of alginate solution and loaded in a sterile syringe that was used to feed the encapsulator. A mechanical pump at rate of 2.50 ml/min forced the alginate cell suspension in a microfluidic circuit. The superimposition of a vibrating frequency of 1300 Hz to the microfluid generated segments that became spheric droplets after the extrusion through a nozzle of 120 μm. Droplets were separated by an electric field of 1800 mV and collected in a 0.5 M calcium chloride (CaCl2) gelling bath. For proper formation, microcapsules were left in contact with CaCl2 for at least 20 min in stirring and then collected by sedimentation.
2.3.2. Chitosan coating of alginate microcapsules
After assessing the alginate microcapsules in attenuation of L. casei ATCC 393 metabolism, an external layer of chitosan (0.7 g/l) (Sigma, product no. 448869) was added on 1.5 % alginate microcapsules (MC). Chitosan was dissolved in acetic acid solution 0.14 M, pH 3.2 at 50 °C and sterilized in autoclave. Alginate microcapsules were suspended in chitosan solution in ratio 1:5 (w/v) and stirred at 4000 rpm for 15 min at room temperature. Chitosan-alginate microcapsules (CMC) were collected by sedimentation and washed with quarter-Strength Ringer solution to remove the chitosan excess.
2.3.3. Entrapment efficacy
The entrapment efficacy (EE) was determined on L. casei microcapsules. The microcapsules were broken to count the cells entrapped inside. A sodium citrate solution (0.2 M) was used to break the microcapsules. Finally, the EE was calculated as showed in formula (1):
| (1) |
N0 is the probiotic cell count assessed prior to microencapsulation and Nd is the probiotic cell count assessed after microcapsule disruption.
2.3.4. Microcapsule light microscopy observation
The light microscope Nikon Eclipse E400 at 400× magnification was used to ensure the correct formation of the alginate microcapsules and the chitosan coating.
2.4. Fermentative metabolism attenuation
The attenuation of L. casei fermentative metabolism was evaluated as described by Racioppo et al. [28]. All the samples were added (1 %) in MRS broth and incubated at 37 °C. The broth pH was monitored before inoculation and after 6 and 24 h of incubation. Obtained data were reported as pH decrease (ΔpH).
2.5. Statistical analysis
SPSS Software (version 28.0.1.1) was used to assess the significant differences between the collected data. One-way ANOVA or paired and unpaired sample t-test were applied when necessary and the significance was declared at p < 0.05. Data were preliminarily analyzed to assess the normal and homogeneity distribution of data by the Levene test of SPSS at p < 0.05. All the experiments were repeated three times (n = 3) and results are showed as mean value ± standard deviation.
3. Results and discussions
3.1. Evaluation of operating parameters: microcapsules morphology and microencapsulation efficacy
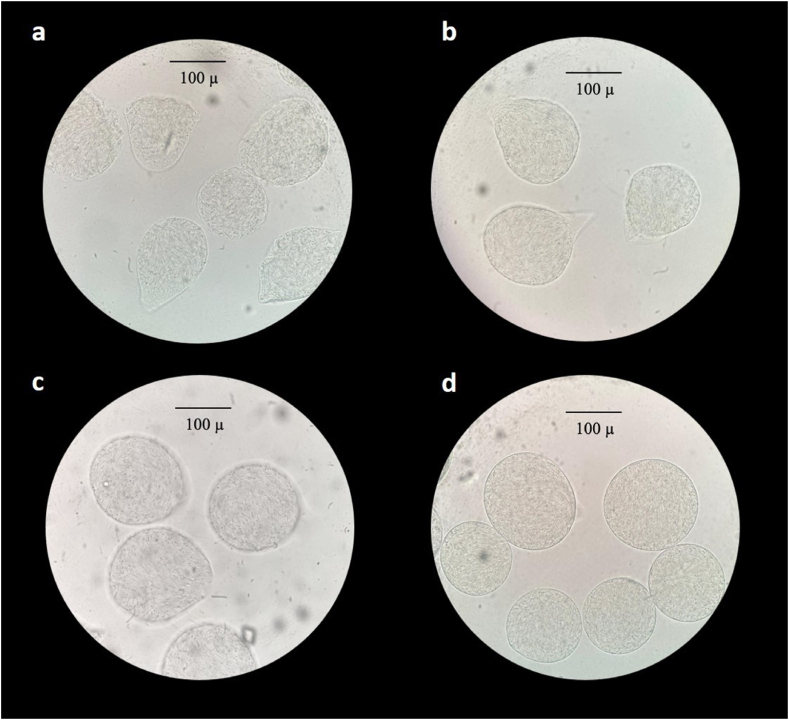
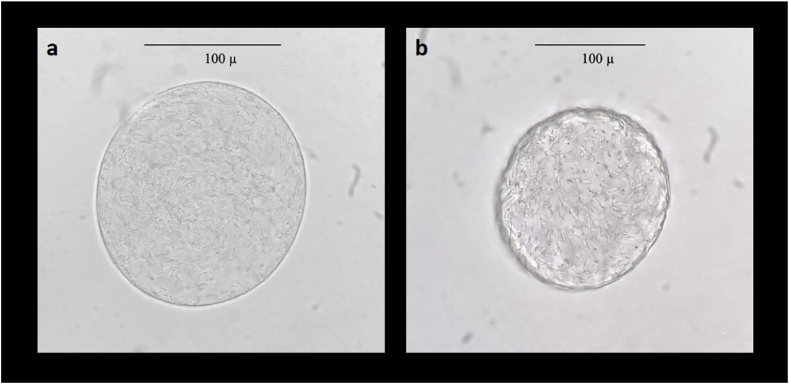
Fig. 1, Fig. 2 shows the light microscope images of the alginate microcapsules and chitosan-alginate microcapsules.
Fig. 1.
Light microscope images (400x) of microcapsules with different alginate concentration. a: 0.8 %; b: 1.0 %; c: 1.2 %; d: 1.5 %.
Fig. 2.
Light microscope images (400x) of 1.5 % alginate microcapsules (MC, a) and chitosan-alginate microcapsules (CMC, b).
In Fig. 1a, the 0.8 % alginate microcapsules appear with variable and irregular morphology. A slight better shaped microcapsules are obtained when the alginate concentration reaches 1 % (Fig. 1b). However, a pedunculated morphology is visible. As showed in Fig. 1c, increasing the concentration of sodium alginate to 1.2 % leads to an improvement of the aesthetics of the microcapsules. Finally, the microcapsules obtained with the most concentrated solution (Fig. 1, Fig. 2a) have a perfectly spherical shape and a smooth surface. On the other hand, chitosan-alginate microcapsules appear with a wrinkled and rougher surface (Fig. 2b).
Optical microscope observation proved that alginate concentration affects the microcapsule shape. Our results are in agreement with the previous findings of Shi et al. [37]. The authors studied the correlation between alginate concentration and microcapsule shape. The lower alginate concentration of 0.5 % lead to tear-like beads, while the higher alginate concentration of 8 % lead to sperm-like beads. The optimum alginate concentration was 2 % with round shape microcapsules. The alginate concentration influences the solution viscosity. A low-viscosity solution leads to the deformation of the microcapsules as they collide with the gelling bath. On the other hand, high-viscosity solution prevents a regular flowing of the droplets out of the nozzle resulting in the formation of a tail [[37], [38], [39]]. In their study, Davarcı et al. [40] found that correctly defining the flow rate of the cell-alginate suspension enables the production of spherical beads as they are released from the nozzle. Thus, one main problem in the capsule shape is the collision with the gelling bath. When the penetration depth falls below a certain threshold, it prevents the surface of the CaCl2 gelling bath from breaking or opening, which in turn leads to droplets remaining attached to its surface. The increase in the cross-linker viscosity reduces the capsule deformation after the collision. Beyond aesthetics, the bead shape is an important parameter to taking into account during the microencapsulation process. In fact, it is proved that the spherical shape has significant effects on mechanical and chemical stability of the microcapsule [38].
The microcapsule size is also an important parameter to consider for the probiotic attenuation. The resulting microcapsules are characterized by a diameter of approximately twice the nozzle diameter (240 μm). The efflux rate of nutrients, metabolites and waste into and outside the beads depends on its size. Lee et al. [38] postulated that diffusion limitation occurs in larger beads, hence slowing cellular metabolism. Latnikova and Jobmann [41] also found a slow release of metabolites in large capsules and a faster release in smaller ones. Therefore, we can assume that the larger the microcapsule, the greater the attenuation effect. In addition, the selection of process variables for the extrusion system can be tailored to achieve the desired size distribution, size range, shape type, and sample size of Ca-alginate beads, depending on the intended application [38].
Probiotic cultivability and microencapsulation entrapment efficacy data are reported in Table 1 and Table 2. Following the exposure to the sonication conditions, around 1- and 3-Log reduction (p < 0.05) was found for LC_S6 and LC_S8, respectively. The obtained data also showed no significant differences (p > 0.05) between L. casei ATCC 393 plate counts before and after alginate encapsulation with an EE ranging from 96.98 to 99.38 %. However, a significant (p < 0.05) Log reduction was found after chitosan coating and, therefore, the EE for chitosan-alginate microcapsules was lower than for the alginate microcapsules, ranging from 80.14 to 91.42 %.
Table 1.
Cell counts in Log CFU/ml of Lacticaseibacillus casei ATCC 393. LC: L. casei non sonicated; LC_S6: L. casei 6-min sonicated; LC_S8: L. casei 8-min sonicated; dMC: disrupted 1.5 % alginate microcapsules; dCMC: disrupted chitosan-alginate (1.5 %) microcapsules.
| Samples | Free* | dMC* | dCMC* |
|---|---|---|---|
| LC | 9.24 ± 0.05Aa | 9.13 ± 0.06Aa | 8.45 ± 0.41Ab |
| LC_S6 | 8.63 ± 0.16Ba | 8.58 ± 0.16Ba | 8.10 ± 0.08Ab |
| LC_S8 | 6.55 ± 0.12Ca | 6.35 ± 0.16Ca | 5.25 ± 0.29Bb |
* Data are reported as mean values ± standard deviation (n = 3). Different capital letter in the same column indicates significant difference (p < 0.05); different lowercase letter in the same row indicates significant difference (p < 0.05).
Table 2.
Microencapsulation entrapment efficacy (EE) for disrupted 1.5 % alginate microcapsules (dMC) and disrupted chitosan alginate microcapsules (dCMC). LC: L. casei non sonicated; LC_S6: L. casei 6-min sonicated; LC_S8: L. casei 8-min sonicated.
| Samples | dMC* | dCMC* |
|---|---|---|
| LC | 98.75 ± 0.43Aa | 91.42 ± 4.72Aa |
| LC_S6 | 99.38 ± 0.35ABa | 93.13 ± 3.03Ab |
| LC_S8 | 96.98 ± 1.22ACa | 80.14 ± 3.93Bb |
* Data are reported as mean values ± standard deviation (n = 3). Different capital letter in the same column indicates significant difference (p < 0.05); different lowercase letter in the same row indicates significant difference (p < 0.05).
The goal of attenuation is to regulate probiotic metabolism while preserving its viability without inducing any adverse effects. However, the increase in ultrasound exposure caused the loss of bacteria cultivability. Hypotheses of ultrasound modulation of L. casei ATCC 393 cultivability were well discuss in our previous work [29]. Briefly, multiple unknown reactions start into the cell upon sonication treatment. The reduction in plate count could be due to loss of viability or the cell entering the Viable But Non Culturable (VBNC) state, which is activated by the cell as a protective strategy.
Although vibration technology enables the microencapsulation of probiotics under mild conditions, it is a relatively complex process due to the variety of parameters to control. EE is a useful criterion to define the success or not of the encapsulation process. The high encapsulation yield confirmed that the operating conditions are properly defined. Moreover, obtained results suggested that deposition of the external layer of chitosan affects the probiotic viability. Even though chitosan is a biocompatible polymer it has some antimicrobial properties [31]. In addition, the lowest counts and EE after chitosan coating was recorded for LC_S8. These data suggest a higher susceptibility of the 8-min sonicated cells to chitosan antimicrobial activity. In fact, when ultrasound interact with bacteria membrane small random pores are formed, thus increasing the membrane permeability. Therefore, we can suppose that during the coating smaller chitosan particles diffuse into the microcapsule interacting with the probiotic. A further direct probiotic-chitosan interaction occurs during breakdown of the microcapsule in sodium citrate solution. These findings were previously observed by Malmo et al. [42] while Graff et al. [43] did not find any different in cell counts during chitosan coating.
3.2. Attenuation efficacy of microencapsulation
Both after 6 and 24 h of incubation no significant differences (p > 0.05) were found between the acidification capabilities of the probiotic in free and encapsulated form, regardless of alginate concentration (data not shown).
Microencapsulation-induced attenuation depends on the bead's structure and permeability, solute chemistry and the medium in which they are added. It is assumed that the action of the microcapsules on the entrapped bacteria is basically a physical protection. However, the microcapsule creates an internal well-defined microenvironment where the probiotic is an important component. Obtained data suggested that the alginate concentration does not affect the rate exchange of molecules between the inside and outside of the microcapsule. Li et al. [44] studied the impact of microcapsules diameter, alginate concentration and physical state of microcapsule core on the production and retention of signal molecules. They found that a high diameter, high alginate concentration and a hydrogel core increase the resistance to mass transport and limit the external diffusion of signal molecules. We can assume this as a general rule for mass exchange between the inside of the microcapsule and the surroundings. In addition, it important to underline that the diffusion of nutrients and metabolites depends on the intrinsic characteristics of the alginate and is facilitated by the high-water content [35] and high porosity of the alginate beads. The higher the concentration of alginate, the more carboxyl sites will be available to interact with CaCl2 [44]. Therefore, the calcium alginate structure will be less lax. Although we increased the alginate concentration to 1.5 %, probably, this is not enough to reduce the rate of mass transport. Moreover, some authors described a degradation process of alginate microcapsules in acidic environment [34]. We can also hypothesize that as the acidification increases, the structure of the microcapsule is compromised. Therefore, the main disadvantages are the rapid release of the encapsulated bacteria that can metabolize external nutrient and reduce the medium pH.
Considering these results and the morphological evaluations, a chitosan coating was added on the 1.5 % alginate microcapsules. Data of chitosan-alginate microcapsules attenuation are reported in Table 3. Significant differences (p < 0.05) were found between the L. casei ATCC 393 free and the encapsulated ones after 6 h of incubation. In fact, free L. casei reduced the pH of the broth of about 0.48 while in alginate and chitosan-alginate microcapsules reduced the pH of about 0.26 and 0.22, respectively. Moreover, no significant differences (p > 0.05) were found between the uncoated and coated microcapsules. Interesting, after 24 h of incubation the chitosan coated microcapsules were significant different (p < 0.05) from both the control and the alginate beads. The ΔpH was 2.17, 2.04 and 1.54 for LC, MC and CMC, respectively.
Table 3.
pH decrease* of MRS broth after Lacticaseibacillus casei ATCC 393 inoculum in free form (LC), in 1.5 % alginate microcapsules (MC) and in chitosan-alginate microcapsules (CMC).
| Samples | Time of incubation (h) |
|
|---|---|---|
| 6 | 24 | |
| LC | 0.48 ± 0.03a | 2.17 ± 0.04a |
| MC | 0.26 ± 0.01b | 2.04 ± 0.02a |
| CMC | 0.22 ± 0.05b | 1.54 ± 2.24b |
* Data are reported as mean values ± standard deviation (n = 3) and different letter in the same column indicates significant difference (p < 0.05).
Many attempts have been done to improve the alginate delivery system of probiotic. Among the others, chitosan is the most used one within several application [31,45]. Collected data showed that alginate is not a suitable matrix for probiotic attenuation. On the other hand, different results were obtained with a chitosan coating. Alginate-chitosan high electrostatics interactions confer to the microcapsule new permeability properties producing a semipermeable membrane with a more homogeneous surface and reduced porosity [31]. From our results, we can hypostasize that the greater attenuation efficacy of chitosan-alginate microcapsules depends on the reduced permeability. In addition, the thickness of the microcapsule wall also affects the molecules diffusion [41]. The deposition of an additional external layer increases the microcapsule thickness thus reducing the diffusion rate.
These finding highlight that microcapsules with adequate barrier properties are the key factor to develop an efficient attenuation system.
3.3. Combination of ultrasound and microencapsulation
Table 4 shows the results of the attenuation induced by the combination of ultrasound and microencapsulation. After 6 h of incubation, not sonicated L. casei ATCC 393 in free and encapsulated form was significantly different (p < 0.05) from sonicated samples. Instead, no significant differences (p > 0.05) were found between LC_S6 and LC_S8, regardless the form of addition. After 24 h of incubation the 8-min sonicated sample was significant different (p < 0.05) from the un-sonicated probiotic and the 6-min sonicated one, both in free and encapsulated forms. Finally, chitosan-alginate microcapsules were significantly different (p < 0.05) from free cells and alginate microcapsules for all the samples. The ΔpH of CMC was 1.54, 1.49 and 0.31 for LC, LC_S6 and LC_S8, respectively. However, only for the 8-min sonicated sample the three systems were all significantly different (p < 0.05).
Table 4.
pH decrease* of MRS broth after Lacticaseibacillus casei ATCC 393 inoculum in free form, in 1.5 % alginate microcapsules (MC) and in chitosan-alginate microcapsules (CMC), not sonicated (LC), 6-min sonicated (LC_S6), 8-min sonicated (LC_S8).
| Samples | Free form |
MC |
CMC |
|---|---|---|---|
| 6 h of incubation | |||
| LC | 0.48 ± 0.03Aa | 0.26 ± 0.01Ab | 0.22 ± 0.05Ab |
| LC_S6 | 0.14 ± 0.02Ba | 0.16 ± 0.01Ba | 0.16 ± 0.01Ba |
| LC_S8 | 0.11 ± 0.03Ba | 0.15 ± 0.01Ba | 0.15 ± 0.01Ba |
| 24 h of incubation | |||
| LC | 2.17 ± 0.04Aa | 2.04 ± 0.05Aa | 1.54 ± 0.09Ab |
| LC_S6 | 2.10 ± 0.02Aa | 1.83 ± 0.07Aa | 1.49 ± 0.04Ab |
| LC_S8 | 1.04 ± 0.03Ba | 0.77 ± 0.01Bb | 0.31 ± 0.03Bc |
* Data are reported as mean values ± standard deviation (n = 3). Different capital letter in the same column indicates significant difference (p < 0.05) within 6 or 24 h of incubation; different lowercase letter in the same row indicates significant difference (p < 0.05).
Sonication and microencapsulation were combined to develop an efficient attenuation system. Collected data suggested that sonication-induced attenuation is significantly improved when a chitosan-alginate microcapsule is built around the cells. Complex multiple events driven by ultrasound modulate the probiotic activity. As previously observed [29], a correlation between treatment intensity and the obtained results was found. Taking into account the loss of bacteria cultivability it is not possible to further increase ultrasound intensity to improve the effectiveness of sonication for metabolism attenuation. It is therefore necessary to consider other approaches. As explained before, the microcapsule physically modulates the interaction between the probiotic and the external environment without compromises any cell functions. Interesting, microencapsulation is recently proposed as a way to modulate bacteria metabolism by the modulation of Quorum Sensing (QS) activity. The composition of microenvironment inside the microcapsule has a central role in this mechanism [44,46]. A deeper understanding of encapsulation-induced attenuation could be provided by studying this phenomenon in relation to the production and activity of enzymes involved in sugar metabolism within this microenvironment.
4. Conclusion
Microencapsulation and sonication are multiparametric technologies that can be applied on several bacteria species to achieve different goals. To control the probiotic metabolism to avoid sensory characteristics deviation in food could be a promising approach to ensure the success of a new probiotic product among the consumers.
The present work aimed to study the potential of microencapsulation and sonication in L. casei ATCC 393 metabolic attenuation, both alone and in combination. Our results showed that chitosan-alginate microcapsules and 8-min sonicated were both efficient approaches in the purpose. Moreover, the attenuation effect was significantly improved when the two technologies were used together.
To our knowledge, microencapsulation has never been used to modulate the fermentative metabolism of probiotics until now while some results about ultrasound attenuation have already been presented. Operating parameters for both microencapsulation and sonication can be changed and used in several combination. Therefore, this opens up the possibility to build unlimited systems with specific attenuation abilities.
Funding statement
Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 - Call for proposals No. 341 of March 15, 2022 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Project code PE00000003, Concession Decree No. 1550 of October 11, 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title "ON Foods - Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods".
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Irene Giordano: Writing - original draft, Methodology, Formal analysis. Diamante Maresca: Methodology, Investigation, Formal analysis. Gianluigi Mauriello: Writing - review & editing, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Gianluigi Mauriello reports financial support was provided by Government of Italy Ministry of Education University and Research.
References
- 1.Kowalska E., Ziarno M., Ekielski A., Żelaziński T. Materials used for the microencapsulation of probiotic bacteria in the food industry. Molecules. 2022;27(10):3321. doi: 10.3390/molecules27103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B.…Sanders M.E. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014 doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 3.Chugh B., Kamal-Eldin A. Bioactive compounds produced by probiotics in food products. Cur. Opin. Food Sci. 2020;32:76–82. doi: 10.1016/j.cofs.2020.02.003. [DOI] [Google Scholar]
- 4.Khaneghah A.M., Abhari K., Eş I., Soares M.B., Oliveira R.B., Hosseini H.…Sant'Ana A.S. Interactions between probiotics and pathogenic microorganisms in hosts and foods: a review. Trends Food Sci. Technol. 2020;95:205–218. doi: 10.1016/j.tifs.2019.11.022. [DOI] [Google Scholar]
- 5.La Fata G., Weber P., Mohajeri M.H. Probiotics and the gut immune system: indirect regulation. Probiotics Antimicrob. Proteins. 2018;10:11–21. doi: 10.1007/s12602-017-9322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yousefi B., Eslami M., Ghasemian A., Kokhaei P., Salek Farrokhi A., Darabi N. Probiotics importance and their immunomodulatory properties. J. Cell. Physiology. 2019;234(6):8008–8018. doi: 10.1002/jcp.27559. [DOI] [PubMed] [Google Scholar]
- 7.Zheng J., Wittouck S., Salvetti E., Franz C.M., Harris H.M., Mattarelli P.…Lebeer S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 8.Hill D., Sugrue I., Tobin C., Hill C., Stanton C., Ross R.P. The Lactobacillus casei group: history and health related applications. Front. Microbiol. 2018;2107 doi: 10.3389/fmicb.2018.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spyridopoulou K., Tryfonopoulou E., Aindelis G., Ypsilantis P., Sarafidis C., Kalogirou O., Chlichlia K. Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo. Nanoscale Adv. 2021;3(9) doi: 10.1039/D0NA00984A. 2516-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao L., Chen Y., Song X., Dou X., Xu C. Selenium nanoparticles-enriched Lactobacillus casei ATCC 393 prevents cognitive dysfunction in mice through modulating microbiota-gut-brain axis. Int J Nanomedicine. 2022:4807–4827. doi: 10.2147/IJN.S374024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song X., Qiao L., Chang J., Dou X., Zhang X., Pi S., Xu C. Dietary supplementation with selenium nanoparticles-enriched Lactobacillus casei ATCC 393 alleviates intestinal barrier dysfunction of mice exposed to deoxynivalenol by regulating endoplasmic reticulum stress and gut microbiota. Ecotoxicol. Environ. Saf. 2022;248 doi: 10.1016/j.ecoenv.2022.114276. [DOI] [PubMed] [Google Scholar]
- 12.Tiptiri-Kourpeti A., Spyridopoulou K., Santarmaki V., Aindelis G., Tompoulidou E., Lamprianidou E.E.…Chlichlia K. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0147960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aindelis G., Tiptiri-Kourpeti A., Lampri E., Spyridopoulou K., Lamprianidou E., Kotsianidis I.…Chlichlia K. Immune responses raised in an experimental colon carcinoma model following oral administration of Lactobacillus casei. Cancers. 2020;12(2):368. doi: 10.3390/cancers12020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Hamid M., Romeih E., Gamba R.R., Nagai E., Suzuki T., Koyanagi T., Enomoto T. The biological activity of fermented milk produced by Lactobacillus casei ATCC 393 during cold storage. Int. Dairy J. 2019;91:1–8. doi: 10.1016/j.idairyj.2018.12.007. [DOI] [Google Scholar]
- 15.Dedenaro G., Costa S., Rugiero I., Pedrini P., Tamburini E. Valorization of agri-food waste via fermentation: production of L-lactic acid as a building block for the synthesis of biopolymers. Appl. Sci. 2016;6(12):379. doi: 10.3390/app6120379. [DOI] [Google Scholar]
- 16.Costa S., Summa D., Semeraro B., Zappaterra F., Rugiero I., Tamburini E. Fermentation as a strategy for bio-transforming waste into resources: lactic acid production from agri-food residues. Ferment. 2020;7(1):3. doi: 10.3390/fermentation7010003. [DOI] [Google Scholar]
- 17.Dimitrellou D., Kandylis P., Sidira M., Koutinas A.A., Kourkoutas Y. Free and immobilized Lactobacillus casei ATCC 393 on whey protein as starter cultures for probiotic Feta-type cheese production. J. Dairy Sci. 2014;97(8):4675–4685. doi: 10.3168/jds.2013-7597. [DOI] [PubMed] [Google Scholar]
- 18.Dimitrellou D., Kandylis P., Kourkoutas Y. Assessment of freeze-dried immobilized Lactobacillus casei as probiotic adjunct culture in yogurts. Foods. 2019;8(9):374. doi: 10.3390/foods8090374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidira M., Mitropoulou G., Galanis A., Kanellaki M., Kourkoutas Y. Effect of sugar content on quality characteristics and shelf-life of probiotic dry-fermented sausages produced by free or immobilized Lactobacillus casei ATCC 393. Foods. 2019;8(6):219. doi: 10.3390/foods8060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasika D.M.D., Vidanarachchi J.K., Luiz S.F., Azeredo D.R.P., Cruz A.G., Ranadheera C.S. Probiotic delivery through non-dairy plant-based food matrices. Agriculture. 2021;11(7):599. doi: 10.3390/agriculture11070599. [DOI] [Google Scholar]
- 21.Pulido V.M., Castro R., Durán-Guerrero E., Lasanta C., Díaz A.B. Alternative beverages for probiotic foods. Eur. Food Res. Technol. 2022;248(2):301–314. doi: 10.1007/s00217-021-03904-w. [DOI] [Google Scholar]
- 22.Arepally D., Reddy R.S., Goswami T.K., Coorey R. A review on probiotic microencapsulation and recent advances of their application in bakery products. Food Bioprocess Technol. 2022;15(8):1677–1699. doi: 10.1007/s11947-022-02796-2. [DOI] [Google Scholar]
- 23.Hossain M.N., Ranadheera C.S., Fang Z., Ajlouni S. Healthy chocolate enriched with probiotics: a review. Food Sci. Technol. 2020;41:531–543. doi: 10.1590/fst.11420. [DOI] [Google Scholar]
- 24.Cruz A.G., Cadena R.S., Walter E.H., Mortazavian A.M., Granato D., Faria J.A., Bolini H.M. Sensory analysis: relevance for prebiotic, probiotic, and synbiotic product development. Compr. Rev. Food Sci. Food Saf. 2010;9(4):358–373. doi: 10.1111/j.1541-4337.2010.00115.x. [DOI] [PubMed] [Google Scholar]
- 25.Tuorila H., Hartmann C. Consumer responses to novel and unfamiliar foods. Curr. Opin. Food Sci. 2020;33:1–8. doi: 10.1016/j.cofs.2019.09.004. [DOI] [Google Scholar]
- 26.How Y., Pui L. Survivability of microencapsulated probiotics in nondairy beverages: a review. J. Food Process. Preserv. 2021;45(7) doi: 10.1111/jfpp.15641. [DOI] [Google Scholar]
- 27.Bevilacqua A., Casanova F.P., Petruzzi L., Sinigaglia M., Corbo M.R. Using physical approaches for the attenuation of lactic acid bacteria in an organic rice beverage. Food Microbiol. 2016;53:1–8. doi: 10.1016/j.fm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Racioppo A., Corbo M.R., Piccoli C., Sinigaglia M., Speranza B., Bevilacqua A. Ultrasound attenuation of lactobacilli and bifidobacteria: effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017;243:78–83. doi: 10.1016/j.ijfoodmicro.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Giordano I., Mauriello G. Ultrasound attenuation improves some surface properties of the probiotic strain Lacticaseibacillus casei ATCC 393. Microorganisms. 2023;11(1):142. doi: 10.3390/microorganisms11010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zupanc M., Pandur Ž., Perdih T.S., Stopar D., Petkovšek M., Dular M. Effects of cavitation on different microorganisms: the current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019;57:147–165. doi: 10.1016/j.ultsonch.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Călinoiu L.F., Ştefănescu B.E., Pop I.D., Muntean L., Vodnar D.C. Chitosan coating applications in probiotic microencapsulation. Coatings. 2019;9(3):194. doi: 10.3390/coatings9030194. [DOI] [Google Scholar]
- 32.Yao M., Xie J., Du H., McClements D.J., Xiao H., Li L. Progress in microencapsulation of probiotics: a review. Compr. Rev. Food Sci. Food Saf. 2020;19(2):857–874. doi: 10.1111/1541-4337.12532. [DOI] [PubMed] [Google Scholar]
- 33.Sun C., Wang S., Yang L., Song H. Advances in probiotic encapsulation methods to improve bioactivity. Food Biosci. 2023;102476 doi: 10.1016/j.fbio.2023.102476. [DOI] [Google Scholar]
- 34.Razavi S., Janfaza S., Tasnim N., Gibson D.L., Hoorfar M. Microencapsulating polymers for probiotics delivery systems: Preparation, characterization, and applications. Food Hydrocolloids. 2021;120 doi: 10.1016/j.foodhyd.2021.106882. [DOI] [Google Scholar]
- 35.Smith A.M., Senior J.J. In: Tunable Hydrogels. Lavrentieva A., Pepelanova I., Seliktar D., editors. vol. 178. Springer; Cham: 2021. Alginate hydrogels with Tuneable properties; pp. 37–61. (Advances in Biochemical Engineering/Biotechnology). [DOI] [PubMed] [Google Scholar]
- 36.De Prisco A., Maresca D., Ongeng D., Mauriello G. Microencapsulation by vibrating technology of the probiotic strain Lactobacillus reuteri DSM 17938 to enhance its survival in foods and in gastrointestinal environment. LWT Food Sci. Technol. 2015;61(2):452–462. doi: 10.1016/j.lwt.2014.12.011. [DOI] [Google Scholar]
- 37.Shi P., He P., Teh T.K., Morsi Y.S., Goh J.C. Parametric analysis of shape changes of alginate beads. Powder Technol. 2011;210(1):60–66. doi: 10.1016/j.powtec.2011.02.023. [DOI] [Google Scholar]
- 38.Lee B.B., Ravindra P., Chan E.S. Size and shape of calcium alginate beads produced by extrusion dripping. Chem. Eng. Technol. 2013;36(10):1627–1642. doi: 10.1002/ceat.201300230. [DOI] [Google Scholar]
- 39.Li Z.Q., Hou L. da, Li Z., Zheng W., Li L. Study on shape optimization of calcium-alginate beads. Adv. Mater. Res. 2013;648:125–130. doi: 10.4028/www.scientific.net/AMR.648.125. [DOI] [Google Scholar]
- 40.Davarcı F., Turan D., Ozcelik B., Poncelet D. The influence of solution viscosities and surface tension on calcium-alginate microbead formation using dripping technique. Food Hydrocolloids. 2017;62:119–127. doi: 10.1016/j.foodhyd.2016.06.029. [DOI] [Google Scholar]
- 41.Latnikova A., Jobmann M. Towards microcapsules with improved barrier properties. Top. Curr. Chem. 2017;375:1–18. doi: 10.1007/s41061-017-0152-5. [DOI] [PubMed] [Google Scholar]
- 42.Malmo C., La Storia A., Mauriello G. Microencapsulation of Lactobacillus reuteri DSM 17938 cells coated in alginate beads with chitosan by spray drying to use as a probiotic cell in a chocolate soufflé. Food Bioproc. Tech. 2013;6:795–805. doi: 10.1007/s11947-011-0755-8. [DOI] [Google Scholar]
- 43.Graff S., Hussain S., Chaumeil J.C., Charrueau C. Increased intestinal delivery of viable Saccharomyces boulardii by encapsulation in microspheres. Pharm. Res. (N. Y.) 2008;25:1290–1296. doi: 10.1007/s11095-007-9528-5. [DOI] [PubMed] [Google Scholar]
- 44.Li C., Gao M., Zheng G., Ma X., Liu X., Yu W. Enhanced quorum sensing capacity via regulating microenvironment to facilitate stress resistance of probiotic in alginate-based microcapsules. Int. J. Biol. Macromol. 2023;225:605–614. doi: 10.1016/j.ijbiomac.2022.11.119. [DOI] [PubMed] [Google Scholar]
- 45.Meyer-Déru L., David G., Auvergne R. Chitosan chemistry review for living organisms encapsulation. Carbohydr. Polym. 2022 doi: 10.1016/j.carbpol.2022.119877. [DOI] [PubMed] [Google Scholar]
- 46.Gao M., Zheng H., Ren Y., Lou R., Wu F., Yu W.…Ma X. A crucial role for spatial distribution in bacterial quorum sensing. Sci. Rep. 2016;6(1) doi: 10.1038/srep34695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.