Abstract
Purpose
Chinese Martial arts is becoming increasingly prevalent worldwide. There is insufficient evidence to prove the health effects of martial arts due to methodological weaknesses. The aim of this study was to better understand the effects of Chinese martial arts on the skeletal and muscular status of the lumbar spine by quantitative magnetic resonance imaging (MRI).
Methods
Nine elite martial arts athletes, eighteen amateurs, and eighteen sedentary volunteers underwent T2 mapping and q-Dixon imaging of the lumbar spine on a 3T (Tesla) system. T2 (Transverse) relaxation times were measured in different regions of interest of the intervertebral disc (IVD). Fat Fraction (FF) was measured in the paraspinal muscles and vertebral body.
Results
Compared to the sedentary controls, martial arts athletes showed: (1) increased T2 times in the nucleus pulposus, especially in the central nucleus pulposus (P = 0.004); (2) decreased FF in the multifidus and erector spinae (all P < 0.04), and increased cross-sectional area in the psoas, quadratus lumborum, and multifidus (all P < 0.03); (3) decreased FF in vertebral body (P = 0.001). There was no significant difference in all quantitative MRI parameters between athletes and amateur enthusiasts. Besides, paraspinal muscle FF was negatively correlated with IVD T2 times (ρ = −0.221; P = 0.014) and positively correlated with vertebral body FF (ρ = 0.314; P < 0.001).
Conclusions
Chinese martial arts has a positive effect on lumbar tissues, manifesting as better disc hydration, paraspinal muscle hypertrophy and reduced fat infiltration, and lower vertebral body fat content. Our study suggests a possible mechanism: martial arts practice enhances paraspinal muscles to maintain spinal stability, indirectly contributing to slowing down bone marrow conversion and promoting IVD hydration.
1. Introduction
Chinese martial arts, also known as Kung fu, is one of the most prevalent traditional sports in China, boasting an estimated participation of over 70 million practitioners [1]. Initially developed on the battlefield for combat and self-defense, Chinese martial arts has gradually matured into a modern competitive sport centered around fighting techniques as its main content, encompassing routines and free sparring as its form of athletic expression, while emphasizing a balance between internal and external cultivation [2,3]. Differing from other Asian martial arts such as taekwondo, it not only focuses on the practice of leg technique, but also highlights the comprehensive application of the entire body and the coordinated movement of various components [4]. Previous studies have reported the beneficial effects of martial arts on the lumbar spine [[5], [6], [7]]. For example, a randomized controlled trail conducted by Hall et al. showed that Tai Chi (a form of Chinese martial arts) exercise can improve symptoms for people with low back pain [8]. However, existing studies primarily focus on the elderly individuals or patients, and the specific effects and mechanisms of martial arts on lumbar tissue in young athletes remain unclear.
T2 mapping, a quantitative magnetic resonance technique (MRI), has been widely used to noninvasive assess biochemical changes in intervertebral discs (IVD) [9,10]. T2 (Transverse) relaxation time is an intrinsic property of tissue that reflects the molecular environment created by water, proteins, collagen, and other solutes [11]. Indeed, the T2 value is susceptible to water content and collagen orientation [12,]. Recently, an increasing body of literature has utilized T2 mapping to quantitatively assess IVD in individuals participating in various sports like running and cycling [14,15]. Nevertheless, the potential impact of martial arts on IVD T2 relaxation time remains an unexplored area.
The q-Dixon is a 6-echo volumetric interpolated breath-hold examination (VIBE) Dixon sequence based on a small flip angle and T2* correction [16]. In one scan, this sequence will generate water, fat, fat percentage, goodness-of-fit, R2* map, T2* map, and water percentage images [17]. Compared to traditional water-fat separated MRI techniques, q-Dixon corrects the T1 bias and T2* effect in fat measurement [18]. Fat fraction (FF) measurement in the liver, kidney, bone marrow, and muscle using q-Dixon has been reported [[18], [19], [20]].
The waist is a vital force site in martial arts training. This study aimed to analyze the biochemical differences of IVD, paraspinal muscle, and vertebral body in Chinese martial arts trainers using T2 mapping and q-Dixon, and determine the impact of martial arts on these tissues.
2. Materials and methods
This cross-sectional study was approved by the Medical Ethics Committee, TJ-IRB20220162, and written informed consent was obtained from all subjects before participating in the study. The committee adhered to the principles of the WMA Declaration of Helsinki.
2.1. Subjects
This study recruited 45 subjects with different sports histories from September 2021 to June 2022. All participants were 18–25 years old to avoid the effects of age-related degeneration on the lumbar spine, and they self-reported the years of physical activity participation as well as the weekly frequency and duration of various physical activities. Subjects were divided into three groups based on different sports histories: (1) Elite martial arts athletes group: including 9 national first-class athletes with over a decade of experience in martial arts routines, training at least 5 times per week, and refraining from engaging in any other form of exercise more than once weekly. (2) Amateur martial arts enthusiasts group: including 18 martial arts enthusiasts from amateur clubs, practicing Tai Chi, Changquan, and Nanquan for a duration of over one year but less than 3 times a week, while participating in other types of exercise at most 2 times per week. (3) Sedentary group: including 18 sedentary healthy volunteers. The short version of the International Physical Activity Questionnaire (IPAQ) was used to ensure that the recruited volunteers were sedentary [21]. The validity and reliability of the IPAQ have been checked. Sedentary is defined as less than 2 h of moderate-to-vigorous physical activity per week (≥4 metabolic equivalents of tasks (METs)). Exclusion criteria included spinal deformity, trauma, fracture, previous surgery, infection, tumor, and MRI-related or absolute contraindications. The basic information of 45 subjects is shown in Table 1.
Table 1.
Participant characteristics.
| characteristics | Elite martial arts athletes | Amateur enthusiasts | Sedentary | P value |
|---|---|---|---|---|
| Number of participants | 9 | 18 | 18 | – |
| Females | 5 | 10 | 10 | – |
| Age (years) | 20.1 ± 1.1 | 21.1 ± 2.0 | 21.4 ± 1.5 | 0.115 |
| Height (cm) | 167.3 ± 8.2 | 169.1 ± 8.3 | 168.3 ± 8.3 | 0.876 |
| Weight (kg) | 61.1 ± 8.7 | 59.1 ± 8.1 | 60.5 ± 9.8 | 0.936 |
| BMI (kg/m2) | 21.7 ± 1.1 | 20.6 ± 1.7 | 21.3 ± 2.1 | 0.281 |
| Years of martial arts participation | 11.6 ± 1.9 | 2.2 ± 1.3 | – | – |
| duration of physical activity (hrs/wk) | 14.7 ± 1.0 | 5.6 ± 1.5 | – | – |
Data are presented as mean ± SD (except the number of participants and females). BMI = body mass index.
2.2. MRI protocol
All MRI scans were performed after 2:00 p.m. to minimize the influence of diurnal change in IVD water content. Before the scanning, participants received comprehensive explanations about the study's purpose, procedures, and potential risks. They were then required to sign an informed consent form and complete a questionnaire related to their sports histories. Following this, participants were guided to sit and rest for at least 30 min in a quiet and temperature-suited room. A 3 T(T) scanner (Magnetom Skyra, Siemens Healthcare, Germany) was used with a dedicated eight-channel spine for all MRI examinations after the rest period. The imaging protocol consisted of conventional MRI, T2 mapping with spin-echo multi-echo sequence, and q-Dixon with gradient echo sequence. The parameters of these sequences are as follows.
Sagittal T2 mapping: repetition time (TR)/echo times (TEs) = 1800/12, 24, 36, 48, 60, 72, 84, 96 ms; voxel size: 0.375 × 0.375 × 2.5 mm3; slice thickness 3 mm; interslice gap 3.3 mm; field of view (FOV) = 280 × 227 mm; flip angle 180°; the number of slices 13; acquisition time 3:50 min [22].
Sagittal q-Dixon: TR/TEs = 20/1.54, 2.97, 4.40, 5.83, 7.26, 8.69 ms, slice thickness 1.1 mm; interslice gap 0 mm; FOV = 306 × 306 mm; flip angle 4°; the number of slices 48; acquisition time 2:15 min. Axial q-Dixon: TR = 12 ms; echo time, slice thickness, interslice gap, flip angle, and FOV were the same as the previous sagittal images; the number of slices 80 (covered three lumbar segments L3-4 to L5-S1); acquisition time 2:21 min.
Conventional MRI protocols included a sagittal T2-weighted with turbo inversion recovery sequence (TR = 3200 ms; TE = 41 ms; inversion time 200 ms; slice thickness 4 mm; interslice gap 4.4 mm; FOV 360 × 360 mm) and an axial T2-weighted with turbo spin-echo sequence (TR = 4700 ms; TE = 25 ms; slice thickness 5 mm; interslice gap 6 mm; FOV 240 × 240 mm).
2.3. Image analysis
ImageJ 1.52v (National Institutes of Health, USA) was used to perform all MRI measurements. The regions of interest (ROI) were drawn by one musculoskeletal radiologist (5 years of experience) under the supervision of an experienced senior expert (10 years of experience). T2 maps were processed in the MR workspace using mono-exponential decay models. In the midsagittal T2 maps, an ROI was drawn to include each disk's central (high signal intensity) region using T2-weighted images as reference, defined as the nuclear region [23]. For disks in which the boundary between the nucleus pulposus and annulus fibrosus was unclear, the investigator selected a region corresponding to the expected location of the nucleus pulposus region [23]. In addition, five equally sized elliptical ROIs on the central slice were drawn manually (Fig. 1). Each ROI was located in the most central portion of the IVD and had an area of 10–20 mm2. The most anterior and most posterior ROIs (ROI 1 and ROI 5) were defined as annulus fibrosus (AF), and the ROIs in between (ROI 2, ROI 3, and ROI 4) were defined as nucleus pulposus (NP) [24,25].
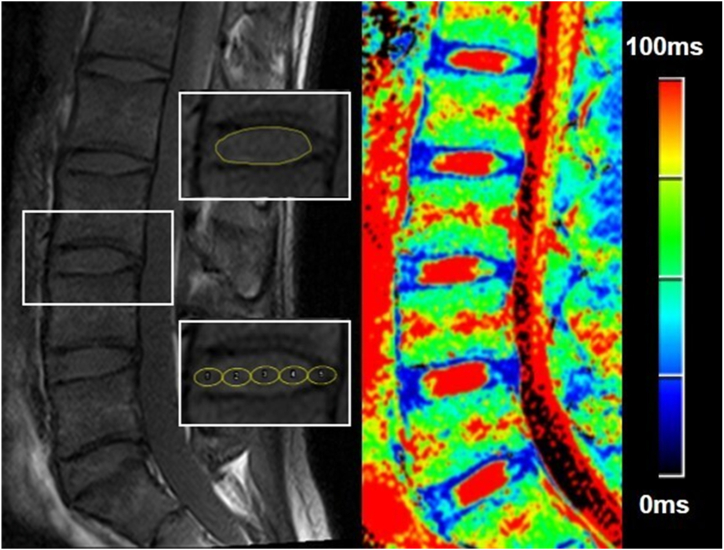
Fig. 1.
Schematic illustration of the regions of interest (ROIs) of the intervertebral discs (IVD) and a color-coded T2 map. The ROI in the upper left image was placed in the nucleus pulposus. The bottom left image showed the division of IVD into five subregions from anterior to posterior (ROI 1 to 5): anterior annulus fibrosus (AAF), anterior nucleus pulposus (ANP), central nucleus pulposus (CNP), posterior nucleus pulposus (PNP), posterior annulus fibrosus (PAF). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
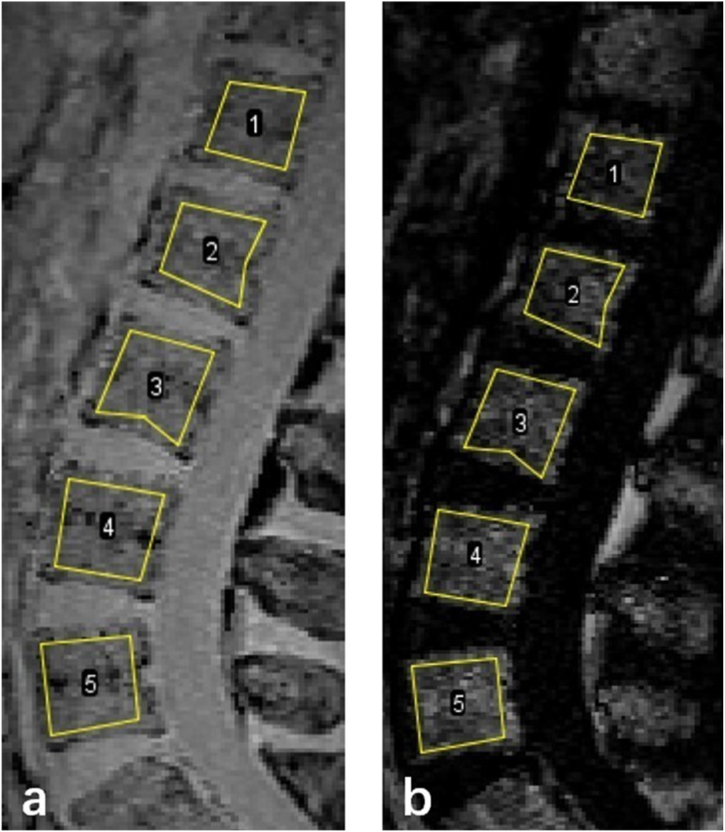
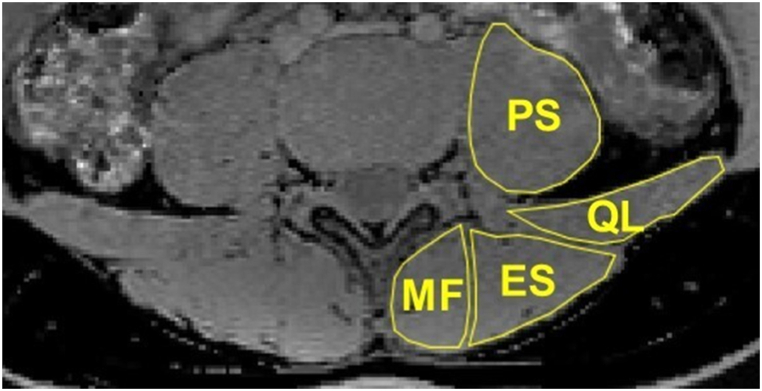
The L1-L5 vertebra was manually segmented. The ROIs were drawn on the mid-sagittal plane and the two para-mid-sagittal planes on the water images (Fig. 2a) and then copied to the fat images (Fig. 2b). The ROIs from axial q-Dixon were drawn around the psoas (PS), quadratus lumborum (QL), multifidus (MF), and erector spinae (ES) muscles on the middle slice and the two adjacent slices at the level of each disc (Fig. 3). To accurately measure intramuscular fat, anatomical boundaries of ROIs do not include what might be considered extra muscular fat, such as fat that is occupying space that approximates bony tissue (spinous processes, laminae, zygapophyseal joint, and transverse processes) [26]. The quadratus lumborum and erector spinae muscles were only measured at IVDs L3-4 to L4-5 levels, as these muscles were poorly identifiable below these levels [26,27]. Cross-sectional area (CSA) was determined from each ROI. FF was calculated from the SI of the fat and water images as SIfat/(SIfat + SIwater) × 100 %. Average FF was calculated for each vertebral body and paraspinal muscle, respectively. Bilateral mean muscle CSA and FF were used in the analysis.
Fig. 2.
Sagittal q-Dixon images and ROI placement for L1 to L5 lumbar vertebra. (a) water image, (b) fat image.
Fig. 3.
Axial q-Dixon-water image of a 21 years old martial arts athlete at L4/5 IVD level. PS = psoas, QL = quadratus lumborum, MF = multifidus, ES = erector spinae.
2.4. Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS Statistics, version 26.0, IBM Corporation, Armonk, NY, USA). The Shapiro-Wilk test evaluated the normality of data. The ANOVA or Kruskal-Wallis test was applied to determine the difference among the three groups, and the Bonferroni test was then used for multiple comparisons. Spearman correlation was used to evaluate the correlations between the years of martial arts practice, IVD T2 time, vertebral body FF, paraspinal muscle FF, and CSA. Paraspinal muscle FF or CSA was defined as the average of all ROIs (including PS, QL, MF, and ES) at one IVD level. The correlations were interpreted as strong (≥0.70), moderate (0.40–0.69), and weak (≤0.40). P < 0.05 was taken for statistical significance.
3. Result
3.1. Effect of martial arts on IVD T2 relaxation time
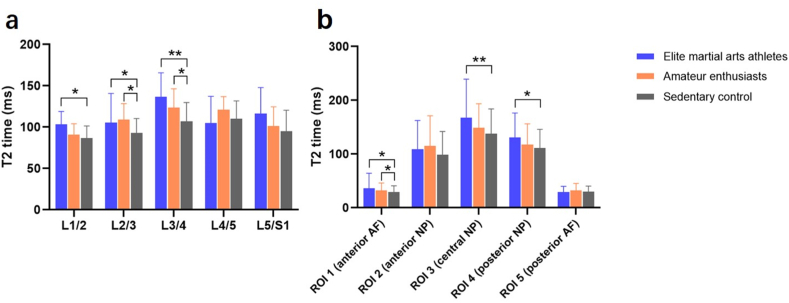
Elite martial arts athletes and amateur enthusiasts showed significantly increased average T2 times in whole nucleus pulposus than sedentary individuals (113.5 ± 30.8 and 109.3 ± 22.3 vs. 98.3 ± 22.2 ms; P = 0.001). Martial arts athletes had longer NP T2 times at L1/2 (103.5 ± 15.4 vs. 86.7 ± 14.7 ms, P = 0.018), L2/3 (105.6 ± 35.1 vs. 92.9 ± 17.5 ms, P = 0.032), and L3/4 (136.7 ± 29.1 vs. 106.9 ± 22.9 ms, P = 0.010, Fig. 4a). For subregional analysis, the effect of martial arts on IVD was strongest in the central nucleus pulposus (ROI3, P = 0.004), followed by the posterior nucleus pulposus (ROI4, P = 0.025, Fig. 4b). There was no statistical difference in T2 times of NP and all subregions when comparing martial arts athletes and amateur enthusiasts (P = 1.000, 0.635, 0.069, 0.082, 0.219, and 0.294).
Fig. 4.
(A) T2 relaxation times of nucleus pulposus (NP) at different intervertebral disc (IVD) levels. The effect of martial arts on NP was predominantly present at upper lumbar levels. (b) T2 relaxation times for different regions of interest (ROIs) of IVD. The effect of martial arts on IVD was strongest in the central nucleus pulposus. Data are presented as mean ± SD. *P < 0.05; **P < 0.01.
3.2. Effect of martial arts on paraspinal muscle fat fraction and cross-sectional area
The fat fraction of MF in elite martial arts athletes and amateur enthusiasts was significantly lower than that in sedentary control (P = 0.003). Elite martial arts athletes showed significantly decreased FF in ES than sedentary control (P = 0.039). The CSA of paraspinal muscles tended to increase after long-term exercise. There were significant differences in CSA of PS, QL, and MF between the three groups (all P < 0.03). The post hoc test showed no significant difference in muscle CSA between elite martial arts athletes and amateur enthusiasts. These results are summarized in Table 2.
Table 2.
T2 relaxation time and cross-sectional area (CSA) of paraspinal muscles.
| Muscles | Elite martial arts athletes | Amateur enthusiasts | Sedentary | P value | Post hoc test |
||
|---|---|---|---|---|---|---|---|
| P12 | P13 | P23 | |||||
| FF (%) | |||||||
| PS | 2.6 (1.7–3.5) | 3.0 (2.5–3.6) | 3.7 (3.2–4.2) | 0.154 | – | – | – |
| QL | 2.8 (1.6–3.9) | 4.0 (2.8–5.2) | 4.3 (3.5–5.1) | 0.07 | – | – | – |
| MF | 2.9 (1.9–3.9) | 4.7 (3.4–6.0) | 5.2 (4.3–6.1) | 0.003 | 0.139 | 0.001 | 0.026 |
| ES | 2.9 (1.7–4.1) | 4.3 (3.0–5.5) | 5.4 (4.2–6.5) | 0.039 | 0.200 | 0.013 | 0.138 |
| CSA (cm2) | |||||||
| PS | 12.6 (11.0–14.2) | 11.4 (10.4–12.3) | 9.9 (8.9–10.8) | 0.004 | 0.248 | 0.002 | 0.019 |
| QL | 5.6 (4.6–6.6) | 5.1 (4.3–5.8) | 4.1 (3.6–4.6) | 0.021 | 0.274 | 0.008 | 0.061 |
| MF | 7.0 (6.1–7.9) | 7.0 (6.5–7.5) | 6.1 (5.6–6.5) | 0.029 | 0.782 | 0.074 | 0.011 |
| ES | 15.1 (12.3–18.0) | 12.1 (10.7–13.5) | 12.3 (11.0–13.6) | 0.087 | – | – | – |
Data are presented as mean (95 % CI). PS = psoas, QL = quadratus lumborum, MF = multifidus, ES = erector spinae.
P values < 0.05 indicate statistically significant results and are marked in bold.
P12 values represent the difference between elite martial arts athletes and amateur enthusiasts.
P13 values represent the difference between elite martial arts athletes and sedentary control.
P23 values represent the difference between amateur enthusiasts and sedentary control.
3.3. Effect of martial arts on vertebral body fat fraction
The vertebral body FF was 30.0 % ± 8.4 % in elite martial arts athletes, 30.0 % ± 11.2 % in amateur enthusiasts, and 34.7 % ± 9.6 % in sedentary control. There was a significant difference in vertebral body FF between the three groups (P = 0.001). The post hoc test showed that the vertebral body FF of martial arts athletes and amateurs was significantly lower than that of sedentary controls (all P < 0.005).
3.4. Correlations
The years of martial arts practice were weakly correlated with IVD T2 time (ρ = 0.270; P < 0.001), vertebral body FF (ρ = −0.257; P < 0.001), paraspinal muscle FF (ρ = −0.336; P < 0.001) and CSA (ρ = 0.355; P < 0.001) in all subjects. There was a significant weak correlation between vertebral body FF and paraspinal muscle FF (ρ = 0.314; P < 0.001), but no correlation was found between vertebral body FF and IVD T2 time. A weak negative correlation was found between paraspinal muscle FF and IVD T2 times (ρ = −0.221; P = 0.014). There was a moderate negative correlation between paraspinal muscle CSA and muscle FF (ρ = −0.622; P < 0.001). And there was a weak positive correlation between paraspinal muscle CSA and IVD T2 time (ρ = 0.209; P = 0.015). No correlation was found between paraspinal muscle CSA and vertebral body FF. The detailed correlation results are shown in Table 3.
Table 3.
Correlation analysis of quantitative MRI parameters.
| IVD T2 time | Vertebral body FF | Paraspinal muscle FF | Paraspinal muscle CSA | |
|---|---|---|---|---|
| Years of martial arts practice | 0.270** | −0.257** | −0.336** | 0.355** |
| IVD T2 time | −0.006 | −0.221* | 0.209* | |
| Vertebral body FF | 0.314** | −0.110 | ||
| Paraspinal muscle FF | −0.622** |
Values are Spearman's correlation coefficient. *P < 0.05; **P < 0.01. IVD = intervertebral disc, FF = fat fraction, CSA = cross-sectional area.
4. Discussion
In this study, T2 mapping and q-Dixon were used to compare differences in the composition of lumbar spine tissue between elite martial arts athletes, amateur enthusiasts, and matched sedentary controls. Correlations between quantitative MRI parameters and correlations between each parameter and the duration of martial arts practice were also analyzed in all subjects. Our main finding was that elite martial arts athletes and amateurs showed better IVD hydration (higher IVD T2 times), larger paraspinal muscle size, and lower paraspinal muscle and vertebral body fat content compared to sedentary controls. The positive effect of martial arts training on muscular and skeletal status has been well reported, which was also proved in our result. A meta-analysis of Tai Chi in treating lumbar spondylosis and back pain by Zhang et al. indicated that Tai Chi individually or with additional treatment along with routine physical exercises might reduce the pain and functional disorders for patients suffering from back pain [28]. Our previous study also demonstrated that regular martial arts exercise could possibly retard the degeneration of lumbar vertebrae and lumbar discs in middle-aged and aged people based on conventional MRI [29]. This study is the first to assess the effects of Chinese martial arts on IVD, vertebral body, and paraspinal muscle in adolescents using quantitative MRI.
IVD consists of NP, peripheral AF, and hyaline cartilaginous endplate. NP is located in the center of IVD and rich in PG, which can attract and bind water molecules. Peripheral AF is rich in collagen (especially type Ⅰ collagen), which contributes to maintaining the integrity and shape of the discs. Early signs of IVD degeneration manifest as increased catabolism, such as PG loss, dehydration, and collagen degradation, ultimately leading to morphologic degradation in the vertebral bodies, endplates, and facet joints [30,31]. In line with the current literature, a healthy IVD is defined as the absence of IVD degeneration. The positive influence on the IVD towards the “healthier state” is the anabolic mechanobiological cues to increase PG production. Numerous studies have demonstrated that the T2 relaxation time of IVD tissue correlates strongly with water and PG content. Therefore, the T2 value is a sensitive indicator for quantifying the disc's healthy state before morphological changes. This study found that martial arts athletes had longer NP T2 values (particularly central NP) than sedentary individuals, indicating greater hydration and higher PG content. In the field of exercise physiology, the idea that certain types of loading may strengthen the IVD is nothing new. A review suggested that dynamic, axial loading of the spine at slow to moderate movement speeds is likely to result in positive adaptations of the disc, whereas prolonged exposure to hypo- or hyper-physiological loading (e.g., extremes of movement, high-impact loads, and sedentary behavior) may be detrimental to the disc [32]. Chinese martial arts is considered a low to moderate-intensity comprehensive exercise emphasizing rigidity and softness [33]. The load characteristic of martial arts on the spine may be the reason for its positive effect on IVD. This effect was predominantly present at upper lumbar levels and central NP.
We found that martial arts athletes also had better paraspinal muscle quality than sedentary people. Increased muscle CSA (except ES) and decreased muscle FF (MF and ES) were observed in martial arts athletes. The paraspinal muscles play a crucial role in maintaining lumbar segment stability and restricting excessive intervertebral movement. Paraspinal muscle atrophy and fatty infiltration have been reported to be associated with low back pain diseases. Exercise-based rehabilitation targets strengthening these muscles. A study indicated that regular physical training enhanced trunk musculature hypertrophy, force, and endurance in adolescent girls and that there was an association between muscle CSA and strength parameters. Another study showed that Dixon MRI was capable to measure training-related changes in muscle area and muscular fat, and both parameters correlated to muscle function [34]. Therefore, our results provide evidence that martial arts training benefits paraspinal muscle. Further studies need to include assessments of muscle function, such as trunk muscle endurance. In addition, a correlation was found between IVD T2 times and paraspinal muscle FF and CSA, indicating that better IVD hydration may be associated with paraspinal muscle strengthening. The relationship between IVD and paraspinal muscles is similar to that between bone and muscle tissue. They are linked anatomically and physiologically, interacting through physical forces and secreted osteokines (derived from bone cells) and myokines (derived from myocytes). This interaction is called muscle-bone crosstalk and plays a vital role in human locomotion and metabolism. Crosstalk may be one of the mechanisms that martial arts training is beneficial for IVD and muscle: exercise load applied to skeletal muscle is transferred to bone, which induces the release of myokines (e.g., ILs, IGF-1, FGF2, and FGF21) and osteokines (e.g., OCN), activates cellular signaling pathways, and together affects muscle metabolism and bone metabolism, thereby increasing muscle protein synthesis and bone formation [[22], [23], [24], [25]].
The bone marrow consists of two types of stem cells: (1) mesenchymal stem cells, which differentiate into osteoblasts or adipocytes, and (2) hematopoietic stem cells, which differentiate into red blood cells, white blood cells, or platelets [35]. At birth, the skeleton is full of active hematopoietic red marrow. It gradually converts into yellow bone marrow in an orderly and centripetal pattern: the conversion begins in the extremities and progresses toward the axial skeleton, and it can finally be completed by approximately 25 years of age [36]. It is well known that bone marrow adipose tissue (MAT) is a distinct fat depot, which plays an essential role in systemic and skeletal metabolism. For example, the inverse relationship between MAT and bone mineral density is well established, which may be one of the pathogenesis of osteoporosis [37]. However, the mechanism regulating bone marrow conversion is unclear. A growing number of studies have paid attention to the relationship between MAT and physical activity. Trudel et al. found that bed rest for 60 days at −6° head-down tilt increased lumbar vertebral FF in men beyond natural involution and resistive exercises effectively prevented vertebral FF accumulation [38]. A study by Belavy et al. also confirmed that specific volumes and types of exercise might influence the age-determined adipose marrow conversion and result in low MAT, which is consistent with our results [39]. The vertebral body FF of martial arts athletes and amateur enthusiasts was 4.7% points lower than that of sedentary controls, equivalent to a seven-year younger MAT level (bone marrow conversion estimated at 7 % per decade of life). Regular exercise requires the steady production of red blood cells and their export from the bone marrow to circulation [39]. Therefore, the reduction of MAT can increase hematopoietic tissue volume to meet exercise needs. Correlation analysis showed a significant correlation between vertebral body FF and paraspinal muscle FF. In theory, spinal biomechanical instability may lead to MAT accumulation in unstable vertebrae [40]. Better paraspinal muscle performance stabilizes the spine and prevents marrow conversion from red to yellow, thus reducing MAT content in the vertebral body. Unlike prior studies, no correlation between IVD T2 times and vertebral body FF was found in our research. The small sample size of athletes and the indirect interaction mechanism between IVD and vertebrae may be a possible explanation. In addition, the correlation between martial arts practice duration and quantitative MRI parameters provides further evidence that martial arts is beneficial for the lumbar spine.
It is worth noting that there was no significant difference in all parameters between martial arts athletes and amateur enthusiasts. This further indicates that lumbar tissue is susceptible to exercise loading and has a lower “threshold” for adaptive changes. Only a small exercise volume or intensity is required to initiate an adaptive response. Increasing the exercise volume has a diminishing effect on further adaptation [41]. A study reported that a loading range of 0.2–0.8 MPa, generating intradiscal pressures of 0.3–1.2 MPa, and a duration of 8 h per day are regarded as the optimal loading conditions for IVD [42]. In this study, we confirmed the beneficial role of martial arts on the lumbar tissue of ordinary enthusiasts; however, the specific exercise frequency, intensity, and duration required to achieve such effects need further investigation.
There are several limitations to this study. Firstly, we conducted quantitative MRI analysis exclusively, without incorporating clinical outcomes or performance measures to validate the results, which may potentially limit our interpretation and generalization of the findings. Secondly, due to the cross-sectional nature of the study and the limited sample size, causal inferences cannot be drawn. Thirdly, the insufficient control over the type, intensity, and duration of extra exercise for amateurs could serve as a potential confounding factor. In the future, well-controlled prospective studies with larger sample sizes are required to validate the current preliminary results.
5. Conclusion
In conclusion, the beneficial effects of Chinese martial arts on the lumbar spine are manifested as follows: better IVD hydration (significant at upper lumbar levels), especially in the central nucleus pulposus; paraspinal muscle hypertrophy and reduced fat infiltration (mainly in the erector spinae and multifidus); and lower vertebral body fat content. Lower paraspinal muscle FF was associated with higher IVD T2 times and lower vertebral body FF. This indicates that Chinese martial arts training may improve muscle quality and maintain spinal stability, indirectly contributing to slowing down bone marrow conversion and promoting IVD hydration. Preliminary findings suggest that lumbar tissue is sensitive to exercise loading, and these effects can be observed even by amateur enthusiasts. Further studies are needed to determine the thresholds and optimal loading conditions for these tissues to adapt to loading.
Data availability statement
The data associated with our study has not been deposited into a publicly available repository. However, it will be made available on request.
CRediT authorship contribution statement
Yao Zhang: Data curation, Writing – original draft. Jun Ran: Conceptualization, Writing – review & editing. Wei Xia: Conceptualization, Methodology. Chanyuan Liu: Formal analysis, Methodology. Chenghu Deng: Data curation, Investigation, Resources. Xiaoming Li: Conceptualization, Funding acquisition, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Xiaoming Li reports financial support was provided by National Natural Science Foundation of China (81930045). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Chenghu Deng, Email: 1186899562@qq.com.
Xiaoming Li, Email: lilyboston2002@sina.com.
References
- 1.Theeboom M., Zhu D., Vertonghen J. ‘Wushu belongs to the world’. But the gold goes to China…: the international development of the Chinese martial arts. Int. Rev. Sociol. Sport. 2016;52:3–23. doi: 10.1177/1012690215581605. [DOI] [Google Scholar]
- 2.Bu B., Haijun H., Yong L., Chaohui Z., Xiaoyuan Y., Singh M.F. Effects of martial arts on health status: a systematic review. J Evid Based Med. 2010;3:205–219. doi: 10.1111/j.1756-5391.2010.01107.x. [DOI] [PubMed] [Google Scholar]
- 3.Han Q., Theeboom M., Zhu D. Chinese martial arts and the Olympics: Analysing the policy of the international wushu federation. Int. Rev. Sociol. Sport. 2020;56:603–624. doi: 10.1177/1012690220957177. [DOI] [Google Scholar]
- 4.Li B., Li R., Qin H., Chen T., Sun J. Effects of Chinese martial arts on motor skills in children between 5 and 6 Years of age: a randomized controlled trial. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph191610204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhagen A.P., Immink M., van der Meulen A., Bierma-Zeinstra S.M. The efficacy of Tai Chi Chuan in older adults: a systematic review. Fam. Pract. 2004;21:107–113. doi: 10.1093/fampra/cmh122. [DOI] [PubMed] [Google Scholar]
- 6.Origua Rios S., Marks J., Estevan I., Barnett L.M. Health benefits of hard martial arts in adults: a systematic review. J. Sports Sci. 2018;36:1614–1622. doi: 10.1080/02640414.2017.1406297. [DOI] [PubMed] [Google Scholar]
- 7.Fong S.S.M., Guo X., Cheung A.P.M., et al. Elder Chinese martial art practitioners have higher radial bone strength, hand-grip strength, and better standing balance control. ISRN Rehabilitation. 2013;2013:1–6. doi: 10.1155/2013/185090. [DOI] [Google Scholar]
- 8.Hall A.M., Maher C.G., Lam P., Ferreira M., Latimer J. Tai chi exercise for treatment of pain and disability in people with persistent low back pain: a randomized controlled trial. Arthritis Care Res. 2011;63:1576–1583. doi: 10.1002/acr.20594. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe S., Quirbach S., Mamisch T.C., Krause F.G., Werlen S., Benneker L.M. Axial T2 mapping in intervertebral discs: a new technique for assessment of intervertebral disc degeneration. Eur. Radiol. 2012;22:2013–2019. doi: 10.1007/s00330-012-2448-8. [DOI] [PubMed] [Google Scholar]
- 10.Stelzeneder D., Welsch G.H., Kovacs B.K., et al. Quantitative T2 evaluation at 3.0T compared to morphological grading of the lumbar intervertebral disc: a standardized evaluation approach in patients with low back pain. Eur. J. Radiol. 2012;81:324–330. doi: 10.1016/j.ejrad.2010.12.093. [DOI] [PubMed] [Google Scholar]
- 11.Xie R., Ruan L., Chen L., et al. T2 relaxation time for intervertebral disc degeneration in patients with upper back pain: initial results on the clinical use of 3.0 Tesla MRI. BMC Med Imaging. 2017;17:9. doi: 10.1186/s12880-017-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinelli N.L., Haughton V.M., Muñoz A., Anderson P.A. T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine (Phila Pa. 2009;34:520–524. doi: 10.1097/BRS.0b013e318195dd44. 1976. [DOI] [PubMed] [Google Scholar]
- 14.Belavy D.L., Quittner M.J., Ridgers N., Ling Y., Connell D., Rantalainen T. Running exercise strengthens the intervertebral disc. Sci. Rep. 2017;7 doi: 10.1038/srep45975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belavy D.L., Quittner M., Ridgers N.D., et al. Beneficial intervertebral disc and muscle adaptations in high-volume road cyclists. Med. Sci. Sports Exerc. 2019;51:211–217. doi: 10.1249/MSS.0000000000001770. [DOI] [PubMed] [Google Scholar]
- 16.Schmeel F.C., Enkirch S.J., Luetkens J.A., et al. Diagnostic accuracy of quantitative imaging biomarkers in the differentiation of benign and malignant vertebral lesions : combination of diffusion-weighted and proton density fat fraction spine MRI. Clin. Neuroradiol. 2021;31:1059–1070. doi: 10.1007/s00062-021-01009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H., Shimakawa A., Hines C.D., et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn. Reson. Med. 2011;66:199–206. doi: 10.1002/mrm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Xie Y., Lu R., et al. Q-dixon and GRAPPATINI T2 mapping parameters: a whole spinal assessment of the relationship between osteoporosis and intervertebral disc degeneration. J Magn Reson Imaging. 2022;55:1536–1546. doi: 10.1002/jmri.27959. [DOI] [PubMed] [Google Scholar]
- 19.Fischer M.A., Donati O.F., Chuck N., et al. Two- versus three-dimensional dual gradient-echo MRI of the liver: a technical comparison. Eur. Radiol. 2013;23:408–416. doi: 10.1007/s00330-012-2614-z. [DOI] [PubMed] [Google Scholar]
- 20.Li S.R., Pui M.H., Guo Y., et al. Efficacy of 3D VIBE Dixon fat quantification for differentiating clear-cell from non-clear-cell renal cell carcinoma. Clin. Radiol. 2018;73:975–980. doi: 10.1016/j.crad.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Craig C.L., Marshall A.L., Sjöström M., et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.mss.0000078924.61453.fb. [DOI] [PubMed] [Google Scholar]
- 22.Liu C., Wang J., Hou B., et al. Diurnal variation in hydration of the cervical intervertebral disc assessed using T2 mapping of magnetic resonance imaging. Korean J. Radiol. 2022;23:638–648. doi: 10.3348/kjr.2021.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinelli N.L., Haughton V.M., Anderson P.A. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. AJNR Am J Neuroradiol. 2010;31:1278–1282. doi: 10.3174/ajnr.A2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stelzeneder D., Kovacs B.K., Goed S., et al. Effect of short-term unloading on T2 relaxation time in the lumbar intervertebral disc--in vivo magnetic resonance imaging study at 3.0 tesla. Spine J. 2012;12:257–264. doi: 10.1016/j.spinee.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon M.A., Hong S.J., Kang C.H., Ahn K.S., Kim B.H. T1rho and T2 mapping of lumbar intervertebral disc: correlation with degeneration and morphologic changes in different disc regions. Magn. Reson. Imaging. 2016;34:932–939. doi: 10.1016/j.mri.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Crawford R.J., Cornwall J., Abbott R., Elliott J.M. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet Disord. 2017;18:25. doi: 10.1186/s12891-016-1378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranson C.A., Burnett A.F., Kerslake R., Batt M.E., O'Sullivan P.B. An investigation into the use of MR imaging to determine the functional cross sectional area of lumbar paraspinal muscles. Eur. Spine J. 2006;15:764–773. doi: 10.1007/s00586-005-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F., Zhao J., Jiang N., Zhai Q., Hu J., Zhang J. Meta-analysis of Tai chi chuan in treating lumbar spondylosis and back pain. Appl. Bionics Biomech. 2022;2022 doi: 10.1155/2022/2759977. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Deng C., Xia W. Effect of Tai Chi Chuan on degeneration of lumbar vertebrae and lumbar discs in middle-aged and aged people: a cross-sectional study based on magnetic resonance images. J. Int. Med. Res. 2018;46:578–585. doi: 10.1177/0300060517734115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C., Huang M., Han Z., et al. Quantitative T2 magnetic resonance imaging compared to morphological grading of the early cervical intervertebral disc degeneration: an evaluation approach in asymptomatic young adults. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandit P., Talbott J.F., Pedoia V., Dillon W., Majumdar S. T1rho and T2 -based characterization of regional variations in intervertebral discs to detect early degenerative changes. J. Orthop. Res. 2016;34:1373–1381. doi: 10.1002/jor.23311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belavy D.L., Albracht K., Bruggemann G.P., Vergroesen P.P., van Dieen J.H. Can exercise positively influence the intervertebral disc? Sports Med. 2016;46:473–485. doi: 10.1007/s40279-015-0444-2. [DOI] [PubMed] [Google Scholar]
- 33.Lan C., Chen S.Y., Lai J.S. The exercise intensity of Tai chi chuan. Med. Sport Sci. 2008;52:12–19. doi: 10.1159/000134225. [DOI] [PubMed] [Google Scholar]
- 34.Peltonen J.E., Taimela S., Erkintalo M., Salminen J.J., Oksanen A., Kujala U.M. Back extensor and psoas muscle cross-sectional area, prior physical training, and trunk muscle strength-a longitudinal study in adolescent girls. Eur. J. Appl. Physiol. Occup. Physiol. 1998;77:66–71. doi: 10.1007/s004210050301. [DOI] [PubMed] [Google Scholar]
- 35.Polineni S., Resulaj M., Faje A.T., et al. Red and white blood cell counts are associated with bone marrow adipose tissue, bone mineral density, and bone microarchitecture in premenopausal women. J. Bone Miner. Res. 2020;35:1031–1039. doi: 10.1111/jbmr.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao F., Shen J., Yu X., Zhou H., Lu Z., Yan S. Bone marrow reconversion mimicking pelvis metastases in a patient with rectal cancer: a pitfall on magnetic resonance images. Quant Imaging Med Surg. 2018;8:621–626. doi: 10.21037/qims.2018.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen W., Scherzer R., Gantz M., et al. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J. Clin. Endocrinol. Metab. 2012;97:1337–1346. doi: 10.1210/jc.2011-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trudel G., Coletta E., Cameron I., et al. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. J. Appl. Physiol. 2012;112:1824–1831. doi: 10.1152/japplphysiol.00029.2012. 1985. [DOI] [PubMed] [Google Scholar]
- 39.Belavy D.L., Quittner M.J., Ridgers N.D., Shiekh A., Rantalainen T., Trudel G. Specific modulation of vertebral marrow adipose tissue by physical activity. J. Bone Miner. Res. 2018;33:651–657. doi: 10.1002/jbmr.3357. [DOI] [PubMed] [Google Scholar]
- 40.Quittner M., Rantalainen T., Ridgers N.D., et al. Intervertebral disc status is associated with vertebral marrow adipose tissue and muscular endurance. Eur. Spine J. 2018;27:1704–1711. doi: 10.1007/s00586-018-5567-3. [DOI] [PubMed] [Google Scholar]
- 41.Turner C.H. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23:399–407. doi: 10.1016/s8756-3282(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 42.Chan S.C., Ferguson S.J., Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur. Spine J. 2011;20:1796–1812. doi: 10.1007/s00586-011-1827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with our study has not been deposited into a publicly available repository. However, it will be made available on request.