Abstract
Background
The empirical dietary index for hyperinsulinemia (EDIH) and empirical dietary inflammatory pattern (EDIP) are novel measures of dietary quality associated with insulin hypersecretion or chronic inflammation, respectively, whereas the Healthy Eating Index (HEI-2015) measures adherence to the Dietary Guidelines for Americans (DGA). We evaluated associations of EDIH, EDIP and HEI-2015 on the risk of both kidney cancer development and mortality.
Methods
We calculated the dietary scores from baseline food frequency questionnaires among 115,830 participants aged 50–79 years in the Women’s Health Initiative. Multivariable-adjusted Cox regression was used to estimate hazard ratios (HR) and 95% confidence intervals (95%CI) for kidney cancer risk, kidney cancer-specific mortality and all-cause mortality, per 1-standard deviation increment in dietary pattern scores.
Results
Higher EDIH was associated with greater risk of kidney cancer development [HR, 1.12; 95%CI, (1.01,1.23)], kidney cancer-specific death [1.22(0.99,1.48)], and all-cause mortality, [1.05(1.02,1.08)]. Higher HEI-2015 was associated with lower risk of kidney cancer development, [0.85(0.77, 0.94)], kidney cancer-specific death, [0.84(0.69,1.03)] and all-cause mortality, [0.97(0.95,1.00)]. However, EDIP was not significantly associated with outcomes. Associations did not differ by BMI categories.
Conclusions
Low-insulinemic dietary patterns and higher quality diets, are worthy of testing in dietary pattern intervention trials for kidney cancer prevention and improved survivorship.
Subject terms: Cancer prevention, Lifestyle modification
Introduction
Kidney cancer is a common genitourinary cancer arising from the renal parenchyma or renal pelvis, and incidence has risen over the past two decades [1]. Despite recent advances in treatments leading to improved survival, kidney cancer is among the leading causes of morbidity and mortality in U.S. adults. In 2022, 79,000 adults were estimated to have been diagnosed with kidney cancer, and 13,920 estimated deaths [2]. Globally, kidney cancer cases increased from 18.3 million in 2007 to 24.5 million in 2017 [3], and accounted for 9.6 million deaths in 2017, or 17% of all deaths [4].
Large-scale studies have identified several risk factors for developing kidney cancer, including non-modifiable factors such as age [5], male gender [6], and genetics [2, 7]. In addition, modifiable risk factors including smoking [8], alcohol consumption (lower risk) [8], physical inactivity [9], obesity [10], diabetes [11], and hypertension [11] have been associated with kidney cancer risk and mortality. For example, cigarette smoking increases the risk of kidney cancer development by about 30% among current smokers and about 15% among former smokers, compared to never smokers [8]. Higher physical activity and moderate alcohol intake have been associated with lower kidney cancer risk [12, 13], whereas obesity, which has emerged as a global public health crisis, has been associated with many chronic health conditions, including increased incidence of kidney cancer [10, 14–18]. Several meta-analyses have shown that patients with overweight and obesity have an increased risk of developing kidney cancer ranging from 28% to 77%, compared to those with normal body weight [19]. Paradoxically, overweight and obese patients appear to have improved survival than patients with normal BMI, referred to, as the “obesity paradox” [20].
In addition to obesity, dietary patterns have been associated with cancer risk and mortality [21, 22], yet there is a paucity of dietary pattern studies specifically on kidney cancer. A recent meta-analysis that included 49 prospective cohort studies and randomized clinical trials did not identify any previous study evaluating dietary pattern interventions among kidney cancer patients [23]. This is in contrast to survivors of other cancers such as breast, prostate and colorectum that are more widely studied to identify dietary and lifestyle interventions to improve overall health and cancer survivorship. In addition, very few studies have examined the association of specific dietary pattern with kidney cancer-specific mortality [24]. People with diets rich in fruits and vegetables have been shown to have a lower risk of kidney cancer, while dietary patterns rich in red meat are associated with higher risk [12]. Similarly, a World Cancer Research Fund /American Institute for Cancer Research dose-response meta-analysis of eight studies, showed a decreased risk of kidney cancer per 10 gram of alcohol intake per day [25]. Diet is thought to exert effects through chronic inflammation and/or chronic insulin hypersecretion, among other possible mechanisms [26]. To evaluate the inflammatory and insulinemic potentials of the dietary pattern, our group previously used circulating C-peptide and inflammatory biomarker data to develop the empirical dietary index for hyperinsulinemia (EDIH) score and the empirical dietary inflammatory pattern (EDIP) score [27]. Healthy Eating Index (HEI-2015), defined as adherence to the Dietary Guidelines for Americans (DGA), is another modality to assess dietary patterns. A poor-quality diet, as reflected by higher EDIH, higher EDIP, or lower HEI, has consistently been associated with higher risk of developing multiple diseases including diabetes [28–30], cardiovascular disease [31, 32], cancer [30], and with mortality [32]. However, EDIH and EDIP have not been specifically studied in relation to kidney cancer risk and mortality. In the present study, we calculated the EDIH, EDIP, and HEI-2015 scores using the baseline food frequency questionnaire in the Women’s Health Initiative (WHI) and evaluated their associations with the development of kidney cancer, kidney cancer-related mortality and all-cause mortality in WHI participants.
Materials and methods
Study population
The WHI study design has been previously described [33]. Briefly, the WHI enrolled 161,808 postmenopausal women aged 50–79 years during 1993–1998 in the United States. The WHI consisted of an Observational Study (OS), and three Clinical Trial (CT) components including a Dietary Modification trial (DM), two Hormone Therapy trials (HT), and a Calcium and Vitamin D trial (CaD). The WHI-OS enrolled postmenopausal women who were ineligible or unwilling to participate in the clinical trials (n = 93,676). The WHI-DM (1993–2005) investigated whether a low-fat dietary pattern could reduce the risk of breast and colorectal cancers and cardiovascular diseases among postmenopausal women. The WHI-HT were trials to evaluate the effects of postmenopausal hormone therapy on women’s risk of coronary heart disease, on hip and other fractures, and breast cancer. The WHI-CaD trial (n = 36,282) investigated the effect of calcium and vitamin D on women’s risk of hip fracture.
Details of the analytic datasets are shown in Fig. S1. We excluded women in the intervention arm of the DM trial as diet from these participants had been altered and therefore not representative of habitual dietary intake, those with prevalent cancer at baseline (except non-melanoma skin cancer), implausible dietary energy intake ( < 600 or >5000 kcal) based on FFQ, extreme BMI ( < 15 or >50 kg/m2), women with missing outcome status, time-to-event information, or missing dietary information. We applied a four-year lag excluding specific outcome cases that occurred within 4 years from baseline. The final analytic dataset for each analyses included 115,830 women for the kidney cancer risk analyses, 117,870 for kidney cancer-specific mortality analyses, 115,918 for all-cause mortality analyses.
Dietary assessment and calculation of dietary indices
To calculate the dietary indices, we used dietary data from baseline, self-administered food frequency questionnaire (FFQ) that represents habitual dietary intake in the preceding three months [34]. The FFQ data were processed using the University of Minnesota Nutrition Coordinating Center food and nutrient database (version 2005) [35]. A previous study evaluated the WHI FFQ for bias and precision by comparing with four 24-hour dietary recalls and 4-day food records. This study found that the mean intake values of most nutrients from the FFQ within 10% of the recalls or records and energy-adjusted correlation coefficients ranging from 0.2 (vitamin B12) to 0.68 (magnesium) [34].
The development and validation of the EDIH and EDIP scores have been published [27, 36]. Briefly, both the EDIH and EDIP are a weighted sum of 18 food groups (different foods in each index), which were most predictive of C-peptide concentrations for EDIH, and three inflammatory biomarkers (interleukin-6, C-reactive protein, and tumor necrosis factor α receptor 2) for EDIP, respectively. Components of both scores in the WHI FFQ have been previously described [37]. The HEI-2015 represents adherence to the recommendations of the Dietary Guidelines for Americans (DGA), and was calculated as the sum score of a total of 13 food and nutrient components in the WHI and ranged from 0 to 100 [38]. All three dietary scores were adjusted for total energy intake using the residual method [39].
Ascertainment of incident cancer and mortality
Study outcomes were incident kidney cancer, kidney cancer-specific death, and all-cause death. The WHI-CT and OS participants were contacted semi-annually and annually, respectively, to identify cancer diagnoses via self-reports. In the outcomes adjudication process, information on cancer incidence was first verified by medical records and pathology reports, then underwent local and central adjudications by trained physician adjudicators [40]. Kidney cancer was defined as the first occurrence of primary kidney cancer using SEER morphology codes. The WHI recorded kidney cancer specific death and all-cause death. The cause of death was determined by death certificate review or medical record and, in some cases, by a relative’s report. Information on cause of death was enhanced by linkage to the National Death Index [40].
Time to cancer development, cancer-specific death, and all-cause death was defined as number of days from enrollment to the return of the follow-up questionnaire in which the event was reported. Participants were followed from enrollment to death, lost to follow-up, or to the most recent follow-up, whichever was first. The study includes follow-up through March 1, 2019.
Statistical analysis
We used Cox proportional hazards regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) of the relative associations of each dietary index and risk of kidney cancer development, kidney cancer-specific death or all-cause death, using the lowest dietary index quintiles as reference. Lowest EDIH/EDIP quintiles represent the highest dietary quality based on its low insulinemic or inflammatory potential, respectively, whereas the lowest HEI-2015 quintile represents the lowest overall dietary quality. We conducted two models – an age-stratified model and a multivariable-adjusted (MV) model further stratified by educational level, race and ethnicity, comorbidity score, hormone therapy study arm (Not randomized to HRT, E-alone intervention, E-alone control, E + P intervention, E + P control), baseline hormone therapy ever, number of hormones used, age at enrollment, and adjusted for the following baseline covariates: non-steroidal anti-inflammatory drug use, family history of cancer, total alcohol intake, coffee/tea, physical activity, pack-years of smoking, number of supplements used and oral contraceptive duration. The proportional hazard assumption was assessed using Schoenfeld residual method and the time dependent covariate method. Because BMI and type 2 diabetes may strongly mediate the association of the dietary patterns and cancer risk, we additionally adjusted for these mediators in separate models. We calculated p-values for linear trend by assigning the quintile specific median value of each dietary pattern to all participants in the quintile and modeling as an ordinal variable [41].
In addition to the relative risk estimates, we calculated multivariable-adjusted absolute risk (incidence rate) of cancer in quintiles of each dietary index. Using the residual method [39], each dietary index was sequentially adjusted for each of the covariates included in the Cox models, then categorized into quintiles. Incidence rates per 100,000 person-years were then calculated using the number of kidney cancer cases within a quintile of the dietary score divided by the sum of follow-up time within that dietary score quintile. Furthermore, for the mortality analyses, we calculated multivariable-adjusted Kaplan-Meier survival curves using the fully adjusted dietary indices and categorized the scores at the median for analyses ( < median, ≥median). Group differences were then tested using the Log-rank test.
We explored associations within subgroups of potential effect modifiers including hypertension (yes vs. no), type 2 diabetes (yes vs. no), cigarette smoking (current vs. former/never) and BMI (normal weight, BMI < 25 kg/m2 vs. overweight/obese, BMI > = 25 kg/m2). Tests of interaction were conducted using the likelihood ratio test for the difference between the models with and without the interaction term. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, North Carolina). P-values < 0.05 were considered statistically significant.
Results
Table 1 shows the distribution of participant characteristics in quintiles of the dietary indices. Comparing participants in the highest EDIH and EDIP quintiles (lower quality diets) to those in the lowest quintiles, showed that a higher proportion of African Americans and Hispanics/ Latino women reported to consume low-quality diets, as reflected by higher EDIH and EDIP, and lower HEI-2015, compared to European American women who reported higher quality dietary patterns. Participants who reported lower quality diets also had higher obesity and lower physical activity.
Table 1.
Distribution of participant characteristics.
| Empirical Dietary Index for Hyperinsulinemic (EDIH) scorea,b | Empirical Dietary Inflammatory Pattern (EDIP) scorea,c | Health Eating Index 2015 (HEI-2015)a,d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 |
| n = 115,830 | 23166 | 23166 | 23166 | 23166 | 23166 | 23166 | 23166 | 23166 | 23166 |
| Race, % | |||||||||
| Black | 906 (3.91) | 1569 (6.77) | 3026 (13.06) | 717 (3.10) | 1401 (6.05) | 3635 (15.69) | 2541 (10.97) | 1697 (7.33) | 1249 (5.39) |
| American Indian or Alaskan Native | 48 (0.21) | 83 (0.36) | 102 (0.44) | 55 (0.24) | 60 (0.26) | 117 (0.51) | 123 (0.53) | 71 (0.31) | 46 (0.2) |
| Asian or Pacific Islander | 500 (2.16) | 710 (3.06) | 561 (2.42) | 273 (1.18) | 524 (2.26) | 1147 (4.95) | 595 (2.57) | 676 (2.92) | 544 (2.35) |
| White | 21122 (91.18) | 20154 (87.00) | 18492 (79.82) | 21644 (93.43) | 20589 (88.88) | 16923 (73.05) | 18969 (81.88) | 19972 (86.21) | 20822 (89.88) |
| Native Hawaiian/Other PI | 13 (0.06) | 6 (0.03) | 33 (0.14) | 13 (0.06) | 6 (0.03) | 42 (0.18) | 27 (0.12) | 17 (0.07) | 12 (0.05) |
| More than one race | 221 (0.95) | 231 (1) | 327 (1.41) | 225 (0.97) | 246 (1.06) | 366 (1.58) | 280 (1.21) | 267 (1.15) | 231 (1) |
| Unknown/Not reported | 356 (1.54) | 413 (1.78) | 625 (2.7) | 239 (1.03) | 340 (1.47) | 936 (4.04) | 631 (2.72) | 466 (2.01) | 262 (1.13) |
| Ethnicity, % | |||||||||
| Not Hispanic/Latino | 22301 (96.27) | 22072 (95.28) | 21644 (93.43) | 22573 (97.44) | 22305 (96.28) | 20804 (89.80) | 21515 (92.87) | 22007 (95) | 22470 (97) |
| Hispanic/Latino | 717 (3.1) | 891 (3.85) | 1289 (5.56) | 475 (2.05) | 716 (3.09) | 2001 (8.64) | 1388 (5.99) | 944 (4.07) | 545 (2.35) |
| Unknown/Not reported | 148 (0.64) | 203 (0.88) | 233 (1.01) | 118 (0.51) | 145 (0.63) | 361 (1.56) | 263 (1.14) | 215 (0.93) | 151 (0.65) |
| Age, years, mean ± sd | 63.15 ± 7.24 | 63.77 ± 7.26 | 62.05 ± 7.16 | 62.80 ± 7.08 | 63.62 ± 7.24 | 62.57 ±7.34 | 62.04 ±7.18 | 63.12 ±7.24 | 64.36 ± 7.17 |
| BMI, kg/m2, mean ± sd | 25.98 ± 4.94 | 27.07 ±5.36 | 29.62 ±6.30 | 26.53 ±5.10 | 27.18 ±5.44 | 28.99 ±6.32 | 28.90 ± 6.16 | 27.44 ±5.54 | 25.96 ±4.98 |
| Under/Normal weight (15 ≤ BMI < 25), % | 11340 (48.95) | 9153 (39.51) | 5750 (24.82) | 10215 (44.09) | 9102 (39.29) | 6745 (29.12) | 6698 (28.91) | 8646 (37.32) | 11479 (49.55) |
| Overweight (25 ≤ BMI < 30), % | 7646 (33.01) | 8214 (35.46) | 7391 (31.9) | 7990 (34.49) | 8071 (34.84) | 7396 (31.93) | 7657 (33.05) | 8140 (35.14) | 7503 (32.39) |
| Obese (BMI ≥ 30), % | 4180 (18.04) | 5799 (25.03) | 10025 (43.27) | 4961 (21.42) | 5993 (25.87) | 9025 (38.96) | 8811 (38.03) | 6380 (27.54) | 4184 (18.06) |
| Physical activity, MET-hours/week, mean ± sd | 16.91 ± 15.72 | 12.92 ± 13.32 | 9.04 ± 11.45 | 15.56 ± 14.94 | 13.03 ± 13.25 | 9.84 ± 12.30 | 8.36 ± 11.14 | 12.94 ± 13.53 | 17.37 ± 15.17 |
| Pack years of smoking, mean ± sd | 10.86 ± 18.33 | 9.25 ± 17.46 | 10.58 ± 19.41 | 12.98 ± 20.22 | 9.41 ± 17.59 | 8.23 ± 17.07 | 12.59 ± 20.87 | 9.53 ± 17.68 | 8.18 ±15.76 |
| Current smoking, % | 1387 (5.99) | 1392 (6.01) | 2223 (9.6) | 1968 (8.50) | 1424 (6.15) | 1621 (7.00) | 2964 (12.79) | 1328 (5.73) | 682 (2.94) |
| Aspirin/NSAIDs use, % | 3211 (13.86) | 3083 (13.31) | 2972 (12.83) | 3324 (14.35) | 3234 (13.96) | 2804 (12.10) | 3045 (13.14) | 3156 (13.62) | 3158 (13.63) |
| Statin use, % | 493 (2.13) | 590 (2.55) | 496 (2.14) | 450 (1.94) | 576 (2.49) | 563 (2.43) | 426 (1.84) | 596 (2.57) | 570 (2.46) |
| Hypercholestrolemia,% | 2905 (12.54) | 3397 (14.66) | 3396 (14.66) | 2784 (12.02) | 3432 (14.81) | 3687 (15.92) | 2893 (12.49) | 3360 (14.5) | 3707 (16) |
| Educational level, % | |||||||||
| Didn’t go to school/grade school (1–8 years) | 393 (1.7) | 483 (2.08) | 669 (2.89) | 266 (1.15) | 362 (1.56) | 996 (4.30) | 781 (3.37) | 467 (2.02) | 298 (1.29) |
| Some high school (9–11 years)/High school diploma/GED/Vocational or training school/ Some college/associate degree | 5463 (23.58) | 7369 (31.81) | 9214 (39.77) | 5976 (25.80) | 7107 (30.68) | 8960 (38.68) | 9779 (42.21) | 6993 (30.19) | 5332 (23.02) |
| Some post-graduate or professional/College graduate/Baccalaureate/Master’s/Doctoral Degree | 17072 (73.69) | 15178 (65.52) | 13172 (56.86) | 16720 (72.17) | 15559 (67.16) | 13081 (56.47) | 12470 (53.83) | 15576 (67.24) | 17351 (74.9) |
| Total alcohol intake, alcohol servings/weeke | 4.83 ± 7.60 | 1.88 ± 3.74 | 1.49 ± 3.68 | 5.30 ± 7.89 | 1.96 ± 3.66 | 0.84 ± 2.61 | 1.69 ± 4.00 | 2.55 ± 5.05 | 3.00 ± 5.56 |
| Macronutrients | |||||||||
| Percent calories from carbohydrates/day | 54.40 ± 9.82 | 52.11 ± 8.48 | 44.86 ± 8.51 | 50.50 ± 9.86 | 51.46 ± 9.19 | 49.86 ± 9.47 | 45.74 ± 8.92 | 50.78 ± 8.78 | 56.41 ± 8.18 |
| Percent calories from fat per day | 27.57 ± 8.14 | 31.17 ± 7.66 | 37.81 ± 7.33 | 29.97 ± 8.59 | 31.69 ± 8.21 | 34.45 ± 8.31 | 38.16 ± 7.36 | 31.77 ± 7.49 | 25.91 ± 6.89 |
| Percent calories from saturated fats per day | 9.26 ± 3.34 | 10.30 ± 3.04 | 12.64 ± 3.05 | 10.01 ±3.42 | 10.53 ± 3.24 | 11.43 ± 3.25 | 13.62 ± 3.17 | 10.53 ± 2.62 | 7.77 ±2.08 |
| Percent calories from polyunsaturated fatty acids per day | 5.83 ± 2.01 | 6.62 ± 2.09 | 7.57 ± 2.15 | 6.27 ± 2.11 | 6.66 ± 2.11 | 7.11 ± 2.29 | 7.22 ± 2.15 | 6.65 ± 2.16 | 6.12 ± 2.02 |
| Percent calories from monounsaturated fatty acids per day | 10.35 ± 3.36 | 11.78 ± 3.22 | 14.49 ± 3.04 | 11.32 ± 3.55 | 11.99 ± 3.41 | 13.13 ± 3.47 | 14.32 ± 3.06 | 12.05 ± 3.24 | 9.93 ±3.10 |
| Percent calories from protein per day | 15.93 ± 2.95 | 16.87 ± 3.01 | 17.37 ± 3.64 | 16.69 ± 3.19 | 16.92 ± 3.08 | 16.59 ± 3.53 | 15.60 ± 3.30 | 16.91 ± 3.08 | 17.69 ± 3.05 |
| Dietary animal protein/dietary vegetable protein | 2.05 ± 1.03 | 2.40 ± 1.04 | 3.27 ± 1.59 | 2.38 ± 1.15 | 2.48 ± 1.17 | 2.78 ± 1.44 | 2.92 ± 1.69 | 2.53 ± 1.12 | 2.11 ± 0.92 |
| Micronutrients | |||||||||
| Calcium (mg per 1000 kcal/d) | 577.64 ± 216.83 | 529.23 ± 203.89 | 409.95 ± 155.34 | 533.35 ± 205.07 | 526.28 ± 201.94 | 459.49 ± 201.04 | 419.22 ± 167.50 | 508.43 ± 196.27 | 614.43 ± 212.50 |
| Magnesium (mg per 1000 kcal/d) | 180.29 ± 35.09 | 166.92 ± 33.01 | 133.64 ± 27.71 | 177.29 ± 34.27 | 164.64 ± 33.58 | 141.00 ± 35.13 | 126.27 ± 24.30 | 162.32 ± 26.49 | 197.31 ± 29.47 |
| Potassium (mg per 1000 kcal/d) | 1861.79 ± 426.42 | 1730.31 ± 388.50 | 1384.47 ± 318.98 | 1884.01 ± 416.11 | 1712.72 ± 376.96 | 1398.23 ± 370.66 | 1297.53 ± 295.07 | 1689.92 ± 336.18 | 2031.69 ± 360.05 |
| Iron (mg/1000 kcal/d) | 7.97 ± 2.47 | 8.20 ± 2.76 | 7.32 ± 2.03 | 7.74 ± 2.30 | 8.02 ± 2.54 | 7.84 ± 2.57 | 7.07 ± 2.11 | 7.99 ± 2.53 | 8.63 ± 2.61 |
| Folate (mcg/1000 kcal/d) | 183.99 ± 64.66 | 174.98 ± 64.51 | 135.98 ± 50.55 | 184.60 ± 62.42 | 172.02 ± 62.23 | 142.16 ± 61.10 | 129.49 ± 51.84 | 169.82 ± 59.26 | 202.74 ± 62.62 |
| Vitamin A (mcg RAE/1000 kcal/d) | 483.41 ± 193.79 | 486.49 ± 197.79 | 423.52 ± 196.40 | 490.42 ± 205.48 | 481.05 ± 188.42 | 426.36 ± 209.82 | 385.91 ± 182.60 | 469.61 ± 195.29 | 553.39 ± 192.72 |
| Vitamin C (mg/1000 kcal/d) | 74.02 ± 39.78 | 71.88 ± 39.26 | 48.70 ± 27.81 | 70.08 ± 38.51 | 70.40 ± 38.84 | 54.32 ± 33.52 | 40.83 ± 26.41 | 68.63 ± 35.55 | 88.34 ± 38.22 |
| Vitamin D (mcg/1000 kcal/d) | 2.80 ± 1.77 | 2.81 ± 1.63 | 2.41 ± 1.26 | 2.59 ± 1.60 | 2.79 ± 1.58 | 2.65 ± 1.58 | 2.14 ± 1.26 | 2.67 ± 1.53 | 3.39 ± 1.80 |
| Vitamin E (IU/1000 kcal/d) | 5.91 ± 3.49 | 6.17 ± 4.07 | 5.39 ± 2.59 | 5.92 ± 3.22 | 6.06 ± 3.66 | 5.48 ± 3.39 | 5.09 ± 2.58 | 6.00 ± 3.66 | 6.52 ± 4.02 |
| Stage at diagnosis, % | |||||||||
| In Situ or localized | 31(0.13) | 47 (0.2) | 75 (0.32) | 49 (0.21) | 43 (0.19) | 62 (0.27) | 71 (0.31) | 57 (0.25) | 37 (0.16) |
| Regional | 16(0.07) | 16 (0.07) | 18 (0.08) | 13 (0.06) | 13 (0.06) | 14 (0.06) | 18 (0.08) | 9 (0.04) | 14 (0.06) |
| Distant | 10(0.04) | 10 (0.04) | 14 (0.06) | 10 (0.04) | 8 (0.03) | 11 (0.05) | 11 (0.05) | 7 (0.03) | 9 (0.04) |
| Number of supplements taken | 1.52 ± 2.05 | 1.30 ± 1.94 | 0.96 ± 1.72 | 1.41 ± 1.99 | 1.30 ± 1.90 | 1.05 ± 1.79 | 0.9 ± 1.67 | 1.29 ± 1.91 | 1.64 ± 2.12 |
aEDIP, EDIH, and HEI2015 scores were adjusted for total energy intake using the residual method. Lower EDIP indicates anti-inflammatory diets while higher EDIP scores indicate pro-inflammatory diets. Lower EDIH indicates anti-hyperinsulinemic diet while a higher score indicates pro-hyperinsulinemic diet. HEI-2015 assessing adherence to the 2015–2020 Dietary Guidelines for Americans – higher HEI-2015 scores are indicative of greater adherence and higher dietary quality.
bThe EDIH component foods (servings/d) in the WHI were: Red meat (ground meat including hamburgers, beef, pork and lamb as a main dish, or as a sandwich; stew, pot pie and casseroles with meat; gravies made with meat drippings); high-energy sugary beverages, (all regular – not diet – soft drinks); low-energy sugary beverages (the WHI FFQ did not assess low-energy beverages separately from other sugar-sweetened beverages); cream soup (such as chowders, potato, tomato, cheese, ajiaco); processed meat (hot dogs, chorizo; other sausage, bacon, breakfast sausage, scrapple; lunch meat such as ham, turkey; other lunch meat such as bologna); butter, margarine (butter, margarine or oil, on bread or tortillas; margarine or butter added to cooked cereal or grits; butter, margarine, sour cream, oils, or other fat added to vegetables, beans, rice, and potatoes, after cooking); poultry (poultry); French fries (French fries, fried potatoes, fried rice, fried cassava and fritters); non-dark or non-oily fish (fried fish, shrimp, lobster, crab and oysters, canned tuna, tuna salad, and tuna casserole, white fish such as sole, snapper, cod); tomatoes (fresh tomato, tomato juice, tomato sauce, cooked tomato, salsa and salsa picante); low-fat dairy (part-skim or reduced fat cheeses, such as Mexican-type cheeses or mozzarella. Include cheese added to foods and in cooking; low-fat cottage cheese; low-fat or no-fat frozen desserts, such as frozen yogurt, sherbet, ice milk, and low-fat milkshakes; non-fat yogurt (not frozen); all other yogurt (not frozen); low-fat milk; Milk, cream, or creamer in coffee or tea); eggs (eggs); wine (red wine, white wine); coffee or tea (all types); fruits (all types); high-fat dairy (whole milk, evaporated/condense milk, ice cream, cottage cheese and ricotta cheese, other cheese); green leafy vegetables (cooked greens such as spinach, mustard greens, turnip greens, collards; lettuce and plain lettuce salad; mixed lettuce or spinach salad with vegetables).
cThe EDIP component foods (servings/d) in the WHI were: processed meat (hot dogs, chorizo, other sausage, bacon, breakfast sausage, scrapple; lunch meat such as ham, turkey; other lunch meat such as bologna); red meat (ground meat including hamburgers, beef, pork, and lamb as a main dish or as a sandwich; stew, pot pie, and casseroles with meat; gravies made with meat drippings); organ meat (liver, including chicken liver; other organ meats); fish other than dark-meat fish (fried fish, shrimp, lobster, crab and oysters, canned tuna, tuna salad, and tuna casserole, white fish such as sole, snapper, cod); other vegetables (i.e., vegetables other than green leafy vegetables and dark yellow vegetables: red peppers and red chilies, green peppers, green chilies, jalapenos, and green chili salsa, corn, and hominy); refined grains (total grain variable minus whole grain variable, both WHI-computed food groups); high-energy beverages [all regular (not diet) soft drinks]; low-energy beverages (the WHI FFQ did not assess low-energy beverages); tomatoes (fresh tomato, tomato juice, tomato sauce, cooked tomato, salsa and salsa picante); beer (all types); wine (red wine, white wine); coffee or tea (all types); dark-yellow vegetables (carrots, including mixed dishes with carrots; summer squash, zucchini, nopales, and okra; winter squash, such as acorn, butternut, and pumpkin; sweet potatoes and yams; other potatoes, cassava, and yucca—boiled, baked, or mashed); green leafy vegetables (cooked greens such as spinach, mustard greens, turnip greens, collards; lettuce and plain lettuce salad; mixed lettuce or spinach salad with vegetables); pizza (low-fat pizza; other pizza); fruit juice (orange juice and grapefruit juice; other fruit juices such as apple and grape); snacks (snacks such as potato chips, corn chips, tortilla chips, Ritz and cheese crackers; saltines, Snackwell’s, fat-free tortilla chips and fat-free potato chips; popcorn).
dThe HEI2015 component foods (servings/d) in the WHI were: total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, sea food and plant proteins, fatty acids, refined grains, sodium, added sugars, saturated fats.
eAlcohol serving was the sum of: beer (1 glass, 1 bottle, or 1 can), wine (4-oz glass of red wine, white wine), and liquor (1 drink or 1 shot whiskey, gin, etc.).
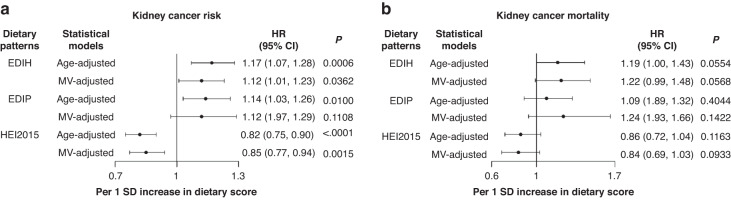
Over a median follow up time of 19.86 years, there were 429 participants diagnosed with kidney cancer. Table 2 shows the multivariable-adjusted absolute risk (incidence rate) and relative risk of future development of kidney cancer in quintiles of the dietary indices. The highest quintile of EDIH (most hyperinsulinemic dietary pattern) compared to the lowest quintile was associated with a 77% greater risk of kidney cancer development (HR, 1.77; 95% CI, 1.26, 2.49; P-trend=0.0017). The risk was elevated for EDIP but did not attain statistical significance. Also, there was a 33% lower risk of kidney cancer among those with the highest dietary quality classified in HEI-2015 quintile 5 compared to those in the lowest quintile, HR, 0.67; 95% CI, 0.49, 0.92; P-trend=0.01. These findings in categorical analyses were well aligned with the findings when the dietary indices were modeled as continuous variables (1-standard deviation increments) as shown in Fig. 1a. The multivariable-adjusted absolute risk difference of consuming a hyperinsulinemic dietary pattern (high-EDIH) or high-quality diet per HEI-2015 was 7 cases per 100,000 person-years between the highest and the lowest quintiles (Table 2).
Table 2.
Multivariable-adjusted absolute and relative risk for the associations of dietary patterns and future development of kidney cancera.
| Dietary pattern | Cancer risk type | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Q5-Q1 (Absolute risk difference)b; P for linear trendc |
|---|---|---|---|---|---|---|---|
| EDIHd | Absolute risk | 18 | 20 | 19 | 24 | 25 | 7 |
| EDIH | Relative risk | 1 (reference) | 1.33 (0.95, 1.85) | 1.28 (0.90, 1.80) | 1.30 (0.92, 1.84) | 1.77 (1.26, 2.49) | <0.01 |
| EDIPd | Absolute risk | 21 | 19 | 22 | 18 | 24 | 4 |
| EDIP | Relative risk | 1 (reference) | 1.15 (0.82, 1.61) | 0.97 (0.67, 1.41) | 1.32 (0.91, 1.93) | 1.37 (0.91, 2.05) | 0.10 |
| HEI-2015d | Absolute risk | 25 | 24 | 18 | 21 | 18 | -7 |
| HEI-2015 | Relative risk | 1 (reference) | 0.88 (0.66, 1.16) | 0.76 (0.56, 1.02) | 0.78 (0.58, 1.05) | 0.67 (0.49, 0.92) | 0.01 |
aValues presented are hazard ratios (HR) and 95% confidence intervals (95% CI) for relative risk and incidence rate per 100,000 person-years for absolute risk. HRs were derived from multivariable-adjusted Cox proportional hazards regression models stratified by age at enrollment, race and ethnicity, cardiovascular diseases status, non-steroidal anti-inflammatory drug use, hormone therapy study arm (Not randomized to HRT, E-alone intervention, E-alone control, E + P intervention, E + P control), and further adjusted for the following baseline covariates: total alcohol intake, coffee/tea, physical activity, educational level, pack-years of smoking, family history of cancer, number of hormones used, comorbidity score, baseline lung disease, number of supplements used, baseline hormone therapy ever, and oral contraceptive duration.
bEach dietary pattern was adjusted for the same covariates using the residual method prior to estimating the incidence rates. Incidence rate per 100,000 person-years were calculated using the number of cases of each cancer within a quintile of the dietary score divided by the sum of year-to-event within that dietary score quintile and then multiply by 100,000. The Q5-Q1 incidence rate per 100,000 person-years was calculated using the incidence rate per 100,000 person-year of the 5th quintile of a dietary score minus that of the 1st quintile of the dietary score.
cThe p value for linear trend was estimated in the same multivariable-adjusted models by assigning the quintile-specific median value of each dietary pattern to all participants in the quintile and modeling as an ordinal variable.
dEDIH, empirical dietary index for hyperinsulinemia score assessing the ability of the dietary pattern to contribute to insulin hypersecretion – higher EDIH scores reflect more hyperinsulinemic dietary patterns; EDIP, empirical dietary inflammatory pattern score assessing the ability of the dietary pattern to contribute to chronic systemic inflammation – higher EDIP scores reflect more pro-inflammatory dietary patterns; HEI-2015, healthy eating index-2015 assessing adherence to the 2015–2020 Dietary Guidelines for Americans – higher HEI-2015 scores are indicative of greater adherence and higher dietary quality. EDIH and EDIP are positively correlated, whereas both scores are inversely correlated with HEI-2015, i.e., more hyperinsulinemic or pro-inflammatory dietary patterns are of lower dietary quality.
Fig. 1. Relative risk estimates for the associations of dietary patterns (per 1 standard deviation increment) and future development of kidney cancer.
a and future kidney cancer-specific death (b). Values presented are hazard ratios (HR) and 95% confidence intervals (95% CI). Dietary score was input as a continuous variable. HRs of age-adjusted models were derived from age-adjusted Cox proportional hazards regression models stratified by age(categorical); HRs of MV-adjusted models were derived from multivariable-adjusted Cox proportional hazards regression models stratified by age at enrollment, race and ethnicity, cardiovascular diseases status, non-steroidal anti-inflammatory drug use, hormone therapy study arm (Not randomized to HRT, E-alone intervention, E-alone control, E + P intervention, E + P control), and further adjusted for the following baseline covariates: total alcohol intake, coffee/tea, physical activity, educational level, pack-years of smoking, family history of cancer, number of hormones used, comorbidity score, baseline lung disease, number of supplements used, baseline hormone therapy ever, and oral contraceptive duration.
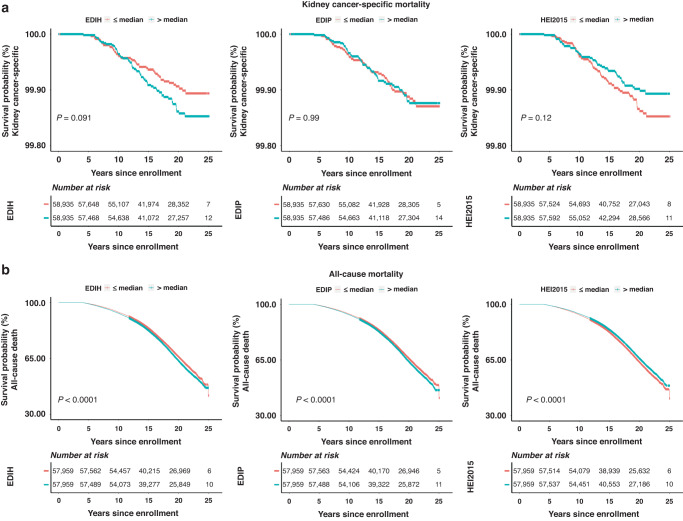
Over the same follow-up period, 41, 333 participants died of all causes including 113 from kidney cancer. As shown in Table 3 and Fig. 1b, the highest compared to the lowest EDIH or EDIP quintiles were associated with greater risk of all-cause death: 20% greater risk for EDIH (HR = 1.20; 95%CI, 1.10, 1.32; P-trend<0.0001) and 13% greater risk for EDIP (HR,1.13; 95%CI, 1.01, 1.27; P-trend=0.007). The same comparison for HEI-2015 was associated with a 9% lower risk (HR, 0.91; 95%CI, 0.84, 0.99; P-trend=0.040). For kidney cancer-specific mortality, a 1-sd increment in EDIH score was associated with a 22% greater risk of dying from kidney cancer (HR: 1.22, CI: 0.99, 1.48, p trend: 0.057). The risk for EDIP was elevated but not statistically significant, whereas the lower risk from a higher dietary quality per the HEI-2015 was marginally significant, HR, 0.84; 95%CI, 0.69, 1.03; P-trend=0.093. For all-cause mortality, multivariable-adjusted Kaplan-Meier curves showed that women who consumed a low insulinemic or anti-inflammatory diet, as demonstrated by EDIH/EDIP less than or equal to the median, or those who consumed higher-overall quality diet, as indicated by HEI-2015 greater than median, had lower risk of mortality from all-cause mortality compared to those classified as consuming lower quality dietary patterns. For kidney cancer-specific mortality, these differences were evident on the curves for EDIH and HEI-2015 but not for EDIP, though the differences did not attain statistical significance (Fig. 2).
Table 3.
Multivariable-adjusted relative risk for the associations of dietary patterns and future development of kidney cancer mortality and all-cause mortalitya,b.
| Dietary pattern | Statistical Model | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | .P for linear trendc | 1-SD increment | P for continuous dietary scored |
|---|---|---|---|---|---|---|---|---|---|
| All cause death | |||||||||
| EDIH | Cases/Person-year | 7713/424934 | 8044/419601 | 8508/412970 | 8612/408662 | 8456/403448 | |||
| Age-adjusted | 1 | 1.04 (1.00, 1.07) | 1.12 (1.08, 1.15) | 1.21 (1.18, 1.25) | 1.39 (1.35, 1.43) | <0.01 | 1.12 (1.11, 1.13) | <0.01 | |
| MV-adjusted | 1 | 1.03 (0.93, 1.13) | 1.04 (0.95, 1.14) | 1.04 (0.95, 1.14) | 1.20 (1.10, 1.32) | <0.01 | 1.05 (1.02, 1.08) | <0.01 | |
| EDIP | Cases/Person-year | 7739/425879 | 7984/422007 | 8328/416174 | 8673/408140 | 8609/397415 | |||
| Age-adjusted | 1 | 1.01 (0.98, 1.04) | 1.05 (1.02, 1.08) | 1.14 (1.11, 1.18) | 1.33 (1.29, 1.37) | <0.01 | 1.10 (1.09, 1.11) | <0.01 | |
| MV-adjusted | 1 | 0.99 (0.89, 1.10) | 1.03 (0.92, 1.14) | 1.01 (0.91, 1.13) | 1.13 (1.01, 1.27) | <0.01 | 1.03 (0.99, 1.07) | 0.17 | |
| HEI 2015 | Cases/Person-year | 8314/404625 | 8318/411222 | 8159/414992 | 8174/418172 | 8368/420604 | |||
| Age-adjusted | 1 | 0.90 (0.87, 0.93) | 0.83 (0.80, 0.85) | 0.78 (0.76, 0.80) | 0.72 (0.70, 0.75) | <0.01 | 0.89 (0.88, 0.90) | <0.01 | |
| MV-adjusted | 1 | 1.00 (0.93, 1.07) | 1.03 (0.95, 1.11) | 0.97 (0.90, 1.05) | 0.91 (0.84, 0.99) | 0.04 | 0.97 (0.95, 1.00) | 0.03 | |
| Kidney cancer specific death | |||||||||
| EDIH | Cases/Person-year | 19/432515 | 19/ 428289 | 26/ 421476 | 21/ 416919 | 28/ 411573 | |||
| Age-adjusted | 1 | 0.99 (0.52, 1.86) | 1.37 (0.76, 2.47) | 1.15 (0.62, 2.14) | 1.70 (0.95, 3.05) | 0.06 | 1.19 (1.00, 1.43) | 0.06 | |
| MV-adjusted | 1 | 1.01 (0.52, 1.94) | 1.36 (0.72, 2.58) | 1.27 (0.65, 2.49) | 1.85 (0.96, 3.55) | 0.04 | 1.22 (0.99, 1.48) | 0.06 | |
| EDIP | Cases/Person-year | 19/ 433705 | 22/ 430286 | 25/424262 | 23/ 416460 | 24/ 406059 | |||
| Age-adjusted | 1 | 1.14 (0.62, 2.11) | 1.28 (0.71, 2.33) | 1.21 (0.66, 2.23) | 1.40 (0.77, 2.55) | 0.27 | 1.09 (0.89, 1.32) | 0.40 | |
| MV-adjusted | 1 | 1.36 (0.69, 2.68) | 1.59 (0.78, 3.26) | 1.68 (0.77, 3.66) | 2.12 (0.92, 4.88) | 0.07 | 1.24 (0.93, 1.66) | 0.14 | |
| HEI 2015 | Cases/Person-year | 26/ 412367 | 28/ 419562 | 16/ 423091 | 17/426714 | 26/429038 | |||
| Age-adjusted | 1 | 1.01 (0.59, 1.72) | 0.55 (0.30, 1.03) | 0.56 (0.31, 1.04) | 0.81 (0.47, 1.40) | 0.14 | 0.86 (0.72, 1.04) | 0.12 | |
| MV-adjusted | 1 | 1.09 (0.63, 1.88) | 0.49 (0.25, 0.94) | 0.56 (0.29, 1.05) | 0.81 (0.45, 1.45) | 0.13 | 0.84 (0.69, 1.03) | 0.09 | |
aValues presented are hazard ratios (HR) and 95% confidence intervals (95% CI) for relative risk. For all-cause deaths: Age-adjusted models were stratified by age categories. Multivariable-adjusted Cox proportional hazards regression models were stratified by, age at enrollment, educational level, race and ethnicity, non-steroidal anti-inflammatory drug use, total alcohol intake, pack-years of smoking, physical activity, number of hormones used, oral contraceptive duration; and further adjusted for the following baseline covariates: hormone therapy study arm (Not randomized to HRT, E-alone intervention, E-alone control, E + P intervention, E + P control), family history of cancer, coffee/tea, baseline hormone therapy ever, comorbidity score (further included baseline lung disease status and cardiovascular disease status compared with the kidney cancer risk analyses), number of supplements used.
For kidney-specific deaths: Age-adjusted models were stratified by age categories. Multivariable-adjusted Cox proportional hazards regression models were stratified by educational level, race and ethnicity, comorbidity score (further included baseline lung disease status and cardiovascular disease status compared with the kidney cancer risk analyses), hormone therapy study arm (Not randomized to HRT, E-alone intervention, E-alone control, E + P intervention, E + P control), baseline hormone therapy ever, number of hormones used, age at enrollment, and further adjusted for the following baseline covariates: non-steroidal anti-inflammatory drug use, family history of cancer, total alcohol intake, coffee/tea, physical activity, pack-years of smoking, number of supplements used and oral contraceptive duration. MV BMI- adjusted models were further adjusted for body mass index (BMI). MV DIAB-adjusted models were further adjusted for baseline status of diabetes.
bEDIH, empirical dietary index for hyperinsulinemia score assessing the ability of the dietary pattern to contribute to insulin hypersecretion – higher EDIH scores reflect more hyperinsulinemic dietary patterns; EDIP, empirical dietary inflammatory pattern score assessing the ability of the dietary pattern to contribute to chronic systemic inflammation – higher EDIP scores reflect more pro-inflammatory dietary patterns; HEI-2015, healthy eating index-2015 assessing adherence to the 2015–2020 Dietary Guidelines for Americans – higher HEI-2015 scores are indicative of greater adherence and higher dietary quality. EDIH and EDIP are positively correlated, whereas both scores are inversely correlated with HEI-2015, i.e., more hyperinsulinemic or pro-inflammatory dietary patterns are of lower dietary quality.
cThe p value for linear trend was estimated in the same multivariable-adjusted models by assigning the quintile-specific median value of each dietary pattern to all participants in the quintile and modeling as an ordinal variable.
dDietary score was input in the corresponding age-adjusted, multivariable-adjusted models as a continuous variable.
Fig. 2. Kaplan–Meier curves of kidney cancer death and all-cause death by binary median cutoffs of baseline multivariable adjusted EDIH, EDIP, and HEI2015 scores.
Log-rank p values were calculated to test differences across median (<=median versus > median). Each dietary score was adjusted for total energy intake, age at enrollment, education, race and ethnicity, non-steroidal anti-inflammatory drug use, hormone therapy study arm (Not randomized to HRT, E-alone intervention, E-alone control, E + P intervention, E + P control), family history of cancer, baseline hormone therapy ever, total alcohol intake, tea/coffee, physical activity, pack-years of smoking, number of hormones, comorbidity score (further included baseline lung disease status and cardiovascular disease status compared with the kidney cancer risk analyses), dietary supplements, oral contraceptive duration.
Subgroup analysis results are presented in Supplementary Table 2 for hypertension and Supplementary Fig. 2, for diabetes, smoking and BMI. Results observed in the overall population were driven mainly by participants without hypertension among whom EDIP was also significantly associated with kidney cancer risk, though interaction p values were not significant. No differences were observed in subgroups defined by diabetes, smoking or BMI. Sensitivity analyses additionally adjusting for hypertension and for smoking status did not materially change the results (Supplementary Tables 3–6).
Discussion
In this study among postmenopausal women conducted in the United States, we examined the association of dietary patterns namely EDIH, EDIP, and HEI-2015 scores with the development of kidney cancer, kidney cancer-related mortality, and overall mortality. We found that a more hyperinsulinemic dietary pattern, reflected by higher EDIH score was associated with higher risk of kidney cancer development, kidney cancer-specific death, and all-cause death. Also, higher adherence to DGA (higher HEI-2015) was associated with lower risk of all three study outcomes. In contrast, EDIP was not significantly associated with outcomes except total mortality. Associations were not significantly different by BMI categories. Kaplan-Meier curves adjusted for multiple potential confounding factors, showed that women consuming low insulinemic or higher quality diets had lower risk and potentially better survival compared to those consuming more hyperinsulinemic or lower quality dietary patterns.
Few previous studies have investigated the association of dietary patterns with kidney cancer [42–44], and this is the first study to examine the role of EDIH, EDIP and HEI-2015 dietary patterns in relation to kidney cancer risk and mortality. One case-control study in Canada identified dietary patterns using principal components analysis (PCA) and found that a pattern high in processed carbohydrate-rich foods (sweets) and one high in animal-based products was associated with higher risk of renal cell carcinoma [43]. A prospective study in a cohort of Swedish women used PCA to identify 3 dietary patterns: plant-based, animal-based and alcohol-based patterns and the alcohol-based pattern was significantly associated with lower risk of renal cell carcinoma, which could be partially explained by the inverse association between alcohol intake and kidney cancer [44]. Specific dietary factors have also been studied in relation to kidney cancer. For example, a meta-analysis of 12 case-control studies and 3 prospective cohort studies did not find an association between fish consumption and kidney cancer risk [45]. Similarly, two meta-analyses of data from prospective cohort studies did not identify significant associations between higher intake of red and processed meat, total fat and total protein and risk of kidney cancer [46, 47]. However, another study found that intake of fiber and fiber-rich plant foods was associated with a significantly lower risk of kidney cancer [48]. Taken in total, the inconsistent findings from studies of single dietary factors warrant a dietary pattern approach. However, most studies have created dietary patterns mainly using the principal component analyses (PCA) method, which creates patterns based on how foods track together to explain variability in the diet between individuals. In addition, the World Cancer Research Fund / American Institute for Cancer 2018 report, which is informed by a global synthesis of the evidence from prospective cohort studies and randomized clinical trials, found that alcohol was associated with lower risk while there was limited evidence on all other single dietary factors. The report did not include data from dietary pattern studies [25].
Though higher inflammatory potential of the diet (higher EDIP) was significantly associated with higher risk of all-cause mortality, EDIP was not associated with kidney cancer risk and mortality. This is in contrast with two previous studies that used a literature-derived nutrient-based dietary inflammatory index (DII) to assess dietary inflammatory potential and examined associations with kidney cancer risk. One case-control study from Italy found that higher DII scores were associated with higher odds of renal cell carcinoma [49], and a prospective study using data from the Iowa Women’s Health study also found that higher DII was associated with higher risk of renal cell carcinoma [50]. It is possible that the inflammatory potential of the diet may be associated with kidney cancer risk, however, it is difficult to directly compare EDIP, which is based exclusively on foods, with DII, which is nutrient-based and highly influenced by nutritional supplements. The previous DII studies did not clearly separate findings for DII from diet and DII from total intake (diet plus supplement), making it difficult to assess the effects of diet independent of supplements.
The alignment of findings between EDIH and HEI-2015 may indicate that higher overall dietary quality may lower kidney cancer risk via dietary modulation of insulin response and insulin sensitivity. EDIH is broadly predictive of the potential of the diet to contribute to hyperglycemia, hyperinsulinemia, insulin resistance and β-cell dysfunction [51]. In addition, higher EDIH has been shown to have a strong association with greater weight gain [29] and type 2 diabetes risk [28, 37], and both are strong risk factors for kidney cancer. Given our finding that EDIH was strongly associated with both kidney cancer incidence and mortality, it is therefore likely that the association of higher BMI with lower kidney cancer risk in previous studies may be more reflective of the limitation of BMI as a measure of body composition, e.g., BMI cannot distinguish between fat mass and muscle mass [52–55].
Our study has several strengths. This is the first study to examine associations of overall dietary quality based on adherence to the DGA and mechanism-based dietary patterns such as EDIH and EDIP. In addition to the usual relative risk estimates, we also estimated multivariable-adjusted incidence rates and K-M curves, providing better clinical and public health context for interpreting the relative risk estimates. Regarding study limitations, though we had a large sample size overall, the sample size for the kidney cancer mortality analysis was small, precluding robust findings especially in the subgroup analysis. Other potential limitations may include the use of self-reported dietary intake which is subject to both systematic and random measurement error, though the FFQ used was previously evaluated for bias and precision [34]. Also, though we adjusted for a large number of potential confounding factors, there may still be residual confounding due to unmeasured or improperly measured variables. In addition, the WHI did not ascertain specific types of kidney cancer, though renal cell carcinoma is the predominant subtype accounting for about 85% to 90% of kidney cancers.
In summary, we found that low-insulinemic dietary patterns and higher overall dietary quality were significantly associated with a lower risk of kidney cancer development, kidney cancer-specific death and all-cause mortality, supporting the implementation of these dietary patterns in clinical intervention trials for kidney cancer prevention and improved survivorship.
Supplementary information
supplementary tables and figures for online publication
Acknowledgements
The WHI program is funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005.
Author contributions
QJ and FKT designed research. QJ conducted research, performed statistical analysis; NS analyzed, and interpreted the data and provided critical output. QJ, JG, and SN wrote initial drafts of the manuscript. SN conducted data reviews for accuracy. FKT provided study oversight. All other authors revised the manuscript and agreed to be accountable for all aspects of the work.
Data availability
The data used in this project were provided by the Women’s Health Initiative. Data will be made available on request via the Women’s Health Initiative manuscript proposal process available at: https://www.whi.org/md/working-with-whi-data.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The WHI protocol was approved by the institutional review boards at the Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center (Seattle, WA) and at each Clinical Center and all women signed written informed consent. WHI is registered at clinicaltrials.gov as NCT00000611.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qi Jin, Jinesh Gheeya, Sushma Nepal.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02469-7.
References
- 1.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–24. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Collaboration GBoDC. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–68. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (2018); 392: 1736-88; e-pub ahead of print 2018/11/30; 10.1016/s0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed]
- 5.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–30. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Scelo G, Li P, Chanudet E, Muller DC. Variability of Sex Disparities in Cancer Incidence over 30 Years: The Striking Case of Kidney Cancer. Eur Urol Focus. 2018;4:586–90. doi: 10.1016/j.euf.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 7.American cancer society. Risk factors for kidney cancer https://www.cancer.org/cancer/kidney-cancer/causes-risks-prevention/risk-factors.html. Accessed July 8, 2022 (2020).
- 8.Cumberbatch MG, Rota M, Catto JWF, La Vecchia C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur Urol. 2016;70:458–66. doi: 10.1016/j.eururo.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Boorjian S. Commentary on “The association between physical activity and renal cancer: systematic review and meta-analysis.” Behrens G, Leitzmann MF, Department of Epidemiology and Preventive Medicine, Regensburg University Medical Center, Regensburg, Germany. Br J Cancer. 2013;108:798–811. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013–1018. doi: 10.1007/s00125-011-2051-6. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CC, Chow WH, Boffetta P, Moore L, Zaridze D, Moukeria A, et al. Dietary risk factors for kidney cancer in Eastern and Central Europe. Am J Epidemiol. 2007;166:62–70. doi: 10.1093/aje/kwm043. [DOI] [PubMed] [Google Scholar]
- 13.Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer. 2013;108:798–811. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conway BN, Han X, Munro HM, Gross AL, Shu XO, Hargreaves MK, et al. The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PLoS One. 2018;13:e0190993. doi: 10.1371/journal.pone.0190993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luhar S, Timaeus IM, Jones R, Cunningham S, Patel SA, Kinra S, et al. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS One. 2020;15:e0229438. doi: 10.1371/journal.pone.0229438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 2021;9:446–61. doi: 10.1016/s2213-8587(21)00118-2. [DOI] [PubMed] [Google Scholar]
- 17.Seo MH, Kim YH, Han K, Jung JH, Park YG, Lee SS, et al. Prevalence of Obesity and Incidence of Obesity-Related Comorbidities in Koreans Based on National Health Insurance Service Health Checkup Data 2006-2015. J Obes Metab Syndr. 2018;27:46–52. doi: 10.7570/jomes.2018.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/s0140-6736(08)60269-x. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2014;135:1673–86. doi: 10.1002/ijc.28813. [DOI] [PubMed] [Google Scholar]
- 20.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18:56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Li Z, Li J, Li Z, Han J. A healthy dietary pattern reduces lung cancer risk: a systematic review and meta-analysis. Nutrients. 2016;8:134. doi: 10.3390/nu8030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couto E, Boffetta P, Lagiou P, Ferrari P, Buckland G, Overvad K, et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br J Cancer. 2011;104:1493–99. doi: 10.1038/bjc.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro-Espin C, Agudo A The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients (2022); 14; e-pub ahead of print 2022/01/22; 10.3390/nu14020348. [DOI] [PMC free article] [PubMed]
- 24.Entwistle MR, Schweizer D, Cisneros R. Dietary patterns related to total mortality and cancer mortality in the United States. Cancer Causes Control. 2021;32:1279–1288. doi: 10.1007/s10552-021-01478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Cancer Research Fund /American Institute for Cancer Research. Continuous Update Project Report Summary. Food, Nutrition, Physical Activity, and the Prevention of Kidney Cancer, a Global Perpective. https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (2018).
- 26.Galland L. Diet and inflammation. Nutr Clin Pr. 2010;25:634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 27.Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE et al. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr (2016): 1-12; e-pub ahead of print 2016/11/09; 10.1017/s0007114516003755. [DOI] [PMC free article] [PubMed]
- 28.Lee DH, Li J, Li Y, Liu G, Wu K, Bhupathiraju S, et al. Dietary Inflammatory and Insulinemic Potential and Risk of Type 2 Diabetes: Results From Three Prospective U.S. Cohort Studies. Diabetes Care. 2020;43:2675–2683. doi: 10.2337/dc20-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabung FK, Satija A, Fung TT, Clinton SK, Giovannucci EL. Long-Term Change in both Dietary Insulinemic and Inflammatory Potential Is Associated with Weight Gain in Adult Women and Men. J Nutr. 2019;149:804–815. doi: 10.1093/jn/nxy319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W, Sui J, Zhao L, Ma Y, Tabung FK, Simon TG, et al. Association of Inflammatory and Insulinemic Potential of Diet and Lifestyle with Risk of Hepatocellular Carcinoma. Cancer Epidemiol Biomark Prev. 2021;30:789–796. doi: 10.1158/1055-9965.EPI-20-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu BC, Tabung FK, Pernar CH, Wang W, Gonzalez-Feliciano AG, Chowdhury-Paulino IM, et al. Insulinemic and Inflammatory Dietary Patterns and Risk of Prostate Cancer. Eur Urol. 2021;79:405–412. doi: 10.1016/j.eururo.2020.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan Y, Tabung FK, Lee DH, Fung TT, Willett WC, Giovannucci EL. Dietary Insulinemic Potential and Risk of Total and Cause-Specific Mortality in the Nurses’ Health Study and the Health Professionals Follow-up Study. Diabetes Care. 2022;45:451–459. doi: 10.2337/dc21-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials (1998); 19: 61-109; e-pub ahead of print 1998/03/11; 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed]
- 34.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement Characteristics of the Women’s Health Initiative Food Frequency Questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 35.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. doi: 10.1016/S0002-8223(21)07997-9. [DOI] [PubMed] [Google Scholar]
- 36.Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, et al. Development and Validation of an Empirical Dietary Inflammatory Index. J Nutr. 2016;146:1560–1570. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Q, Shi N, Aroke D, Lee DH, Joseph JJ, Donneyong M, et al. Insulinemic and Inflammatory Dietary Patterns Show Enhanced Predictive Potential for Type 2 Diabetes Risk in Postmenopausal Women. Diabetes Care. 2021;44:707–14. doi: 10.2337/dc20-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Healthy Eating Index. U.S. Department of Agriculture Food and Nutrition Service. https://www.fns.usda.gov/healthy-eating-index-hei. Accessed 02/17, (2022).
- 39.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 40.Curb JD, Mctiernan A, Heckbert SR, Kooperberg C, Stanford A, Nevitt M, et al. Outcomes Ascertainment and Adjudication Methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/S1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 41.Tabung FK, Wang W, Fung TT, Smith-Warner SA, Keum N, Wu K, et al. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr. 2018;108:363–370. doi: 10.1093/ajcn/nqy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dugue PA, Hodge AM, Brinkman MT, Bassett JK, Shivappa N, Hebert JR, et al. Association between selected dietary scores and the risk of urothelial cell carcinoma: A prospective cohort study. Int J Cancer. 2016;139:1251–60. doi: 10.1002/ijc.30175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handa K, Kreiger N. Diet patterns and the risk of renal cell carcinoma. Public Health Nutr. 2002;5:757–767. doi: 10.1079/PHN2002347. [DOI] [PubMed] [Google Scholar]
- 44.Rashidkhani B, Akesson A, Lindblad P, Wolk A. Major dietary patterns and risk of renal cell carcinoma in a prospective cohort of Swedish women. J Nutr. 2005;135:1757–62. doi: 10.1093/jn/135.7.1757. [DOI] [PubMed] [Google Scholar]
- 45.Bai HW, Qian YY, Shi BY, Li G, Fan Y, Wang Z, et al. The association between fish consumption and risk of renal cancer: a meta-analysis of observational studies. PLoS One. 2013;8:e81939. doi: 10.1371/journal.pone.0081939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander DD, Cushing CA. Quantitative assessment of red meat or processed meat consumption and kidney cancer. Cancer Detect Prev. 2009;32:340–51. doi: 10.1016/j.cdp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Lee JE, Spiegelman D, Hunter DJ, Albanes D, Bernstein L, van den Brandt PA, et al. Fat, protein, and meat consumption and renal cell cancer risk: a pooled analysis of 13 prospective studies. J Natl Cancer Inst. 2008;100:1695–1706. doi: 10.1093/jnci/djn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniel CR, Park Y, Chow WH, Graubard BI, Hollenbeck AR, Sinha R. Intake of fiber and fiber-rich plant foods is associated with a lower risk of renal cell carcinoma in a large US cohort. Am J Clin Nutr. 2013;97:1036–43. doi: 10.3945/ajcn.112.045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shivappa N, Hebert JR, Rosato V, Rossi M, Montella M, Serraino D, et al. Dietary Inflammatory Index and Renal Cell Carcinoma Risk in an Italian Case-Control Study. Nutr Cancer. 2017;69:833–39. doi: 10.1080/01635581.2017.1339815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shivappa N, Blair CK, Prizment AE, Jacobs DR, Jr, Hebert JR. Dietary inflammatory index and risk of renal cancer in the Iowa Women’s Health Study. Eur J Nutr. 2018;57:1207–13. doi: 10.1007/s00394-017-1403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi N, Aroke D, Jin Q, Lee DH, Hussan H, Zhang X, et al. Proinflammatory and Hyperinsulinemic Dietary Patterns Are Associated With Specific Profiles of Biomarkers Predictive of Chronic Inflammation, Glucose-Insulin Dysregulation, and Dyslipidemia in Postmenopausal Women. Front Nutr. 2021;8:690428. doi: 10.3389/fnut.2021.690428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–99. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 54.Heymsfield SB, Cefalu WT. Does body mass index adequately convey a patient’s mortality risk? Jama. 2013;309:87–88. doi: 10.1001/jama.2012.185445. [DOI] [PubMed] [Google Scholar]
- 55.Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L, et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women With Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2019;5:155–63. doi: 10.1001/jamaoncol.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary tables and figures for online publication
Data Availability Statement
The data used in this project were provided by the Women’s Health Initiative. Data will be made available on request via the Women’s Health Initiative manuscript proposal process available at: https://www.whi.org/md/working-with-whi-data.