Abstract
Background
The REGOBONE multi-cohort study explored the efficacy and safety of regorafenib for patients with advanced bone sarcomas; this report details the Ewing sarcoma (ES) cohort.
Methods
Patients with relapsed ES progressing despite prior standard therapy, were randomised (2:1) to receive regorafenib or placebo. Patients on placebo could crossover to receive regorafenib after centrally confirmed progression. The primary endpoint was the progression-free rate at 8 weeks. With one-sided α of 0.05, and 80% power, at least 14/24 progression-free patients at 8 weeks were needed for success.
Results
From September 2014 to November 2019, 41 patients were accrued. 36 patients were evaluable for efficacy: 23 on regorafenib and 13 on placebo. Thirteen patients (56%; one-sided 95% CI [37.5%–[)) were progression-free at 8 weeks on regorafenib vs. 1 (7.7%; 95% CI [0.4%–[) on placebo. Median PFS was 11.4 weeks on regorafenib, and 3.9 weeks on placebo. Ten placebo patients crossed over to receive regorafenib after progression. The most common grade ≥3 regorafenib-related adverse events were pain (22%), asthenia (17%), thrombocytopenia (13%) and diarrhoea (13%).
Conclusion
Although the primary endpoint was not met statistically in this randomised cohort, there is evidence to suggest that regorafenib might modestly delay tumour progression in relapsed ES after failure of prior chemotherapy.
Subject terms: Cancer, Sarcoma
Background
The prognosis of patients with treatment-refractory or relapsed Ewing sarcoma (ES) of bone remains poor overall. Even after limited localised relapse, the long-term survival is approximately 24% and is even lower for patients with metastatic relapse in whom median overall survival is less than 12 months [1]. When we designed this REGOBONE study, no single-standard chemotherapy regimen has been defined for patients who relapsed following initial therapy [2]. Several combinations of agents had shown promising responses in retrospective or Phase II studies in relapsed Ewing sarcoma, including topotecan plus cyclophosphamide [3], temozolomide plus irinotecan [4], gemcitabine plus docetaxel [5–7], etoposide and carbo-or cisplatin [8] and high-dose ifosfamide [9]. Despite promising preliminary results from early-phase studies, Phase 2 studies of four different monoclonal antibodies against IGF-1-receptor (IGF-1R) reported disappointing efficacy, with objective responses in <15% and median PFS less than 2 months in adults and children with previously treated ES [10, 11]. The Children’s Oncology Group in a randomised Phase 3 trial of the anti-IGF-1R monoclonal antibody, ganitumab, added to interval compressed VDC/IE chemotherapy, also reported no benefit for patients with newly diagnosed metastatic ES [12]. Treatment of patients with recurrent and/or metastatic ES remains an important clinical challenge, and no single “best management strategy” has yet been established with superior
Early clinical data suggest the activity of multikinase inhibitors with anti-VEGF activity, such as sorafenib and sunitinib, in patients with bone sarcomas [13–16]. Regorafenib has demonstrated antitumour activity in pretreated metastatic non-adipocytic soft tissue sarcomas [17], a population for which pazopanib has also demonstrated sufficient activity in prolonging PFS to achieve regulatory approval [18]. Furthermore, compared with placebo, regorafenib also improved PFS in chemotherapy-refractory metastatic osteosarcoma (4.0 versus 1.0 months) in the REGOBONE osteosarcoma cohort [19]. The objective of the present study was to explore the potential antitumour activity of regorafenib in patients with progressive metastatic and/or recurrent ES originating in bone after failure of conventional chemotherapy.
Methods
Study design and participants
REGOBONE, an investigator-initiated signal-seeking trial, is a basket study of five parallel independent cohorts of patients with advanced bone sarcomas separated into different histopathologic subtypes. Parallel cohorts assessed the activity and safety of regorafenib or placebo using a randomised, non-comparative, double-blind, placebo-controlled Phase 2 trial design. This manuscript describes the results of the Ewing sarcoma (ES) of the bone cohort.
The study was approved by an ethical and regulatory committee (French Ethical Committee, Comité de Protection des Personnes Sud Méditerrannée 1, approved on March 26, 2014). All patients provided written informed consent before enrolment, and one study amendment (protocol V6, June 29, 2016) expanded enrolment to include paediatric patients aged >10 years (although ultimately, only one child was enrolled). The trial is registered in the European Clinical Trials Register database (EudraCT N°: 2013-003910-42) and at ClinicalTrials.gov (NCT02389244). Eligible patients were required to have histological diagnosis of ES, and objective disease progression within 3 months prior to study entry measured by RECIST v1.1, both confirmed by centralised-review, as well as measurable disease by RECIST v1.1 not amenable to any curative-intent intervention, and previously treated with at least one but no more than two previous chemotherapy regimens for the treatment of relapsed/refractory disease. The complete list of other eligibility criteria, and the full protocol are available online (http://www.unicancer.fr/protocole-regobone). Patients were randomly assigned (2:1) to receive either oral regorafenib or matched placebo. After centrally confirmed disease progression (per RECIST1.1), patients initially randomised to placebo were offered crossover to open-label regorafenib. Central review of pathology was done by an expert bone sarcoma pathologist from the “Réseau de Relecture en Pathologie des Sarcomes Osseux” in France [20].
Registration and randomisation (2:1) were centralised via a web-based system (IWRS) using permuted blocks design provided by an independent partner (ATLANSAT). Patients, pharmacists, investigators, site study teams, and sponsor were all blinded to the allocated treatment. Treatment allocation was masked until centrally confirmed disease progression. Patients were randomly assigned to receive best supportive care combined with either regorafenib 160 mg orally (four tablets of 40 mg once daily, 3 weeks on and 1 week off), or matched placebo tablets on the same schedule. Best supportive care included any method to preserve the comfort and dignity of the patients and excluded any disease-specific anti-neoplastic agents. Dose interruptions and/or dose reduction recommendations have been previously described [19].
The primary endpoint was the progression-free rate (PFR) at 8 weeks, defined as the proportion of patients without disease progression at 8 weeks, after confirmation by central review of imaging studies per RECIST1.1. Secondary endpoints included: progression-free survival (PFS) per modified RECIST v1.1, objective response rate (ORR), overall survival (OS), duration of overall response (DoR), and safety/tolerability. PFS was measured from the date of randomisation until the date of confirmed radiological progression or death from any cause, whichever occurred first.
For patients who were event-free at the time of the analysis, PFS was censored at the time of the final adequate tumour assessment. Centrally assessed progression was used for the analysis. OS was defined as the time from randomisation to the date of death from any cause and censored at the date of final contact for patients alive. Objective response to treatment corresponded to proportion of patients with the complete or partial response as best response from randomisation. Duration of response, which applies only to responders was measured from the time of first documented response (complete response or partial response) until the first documented disease progression or death. Patients who died from causes other than progression were censored at the date of death.
Statistical analysis
When the REGOBONE study was designed there was a paucity of published data regarding PFS of patients with advanced refractory/recurrent Ewing sarcoma following failure of chemotherapy for relapsed disease. The published literature reported a median PFS with an inactive drug (e.g any IGF-1R inhibitors) of about 6 weeks for Ewing sarcoma [11, 12].
We chose the progression-free rate at 8 weeks as the primary endpoint. We calculated the sample size by A’Hern single-stage design for Phase II trials similar to a Fleming Phase II design but assuming an exact binomial distribution [21].
These hypotheses on median PFS translated into a 67% progression-free rate at 8 weeks, defined as the potential efficacy in the experimental (regorafenib) arm. A lower limit of 40% progression-free patients or less would mean that regorafenib would not warrant further investigation in this setting. A sample size of 23 patients provided 80% power to reject the null hypothesis with a one-sided, type 1 error of 5%, with 14 successful patients being the lower cutoff. To account for a possible non-assessable patient rate of 5%, an additional patient was required in the experimental group (total 24 patients).
The placebo arm was used to strengthen the results obtained in the experimental arm (randomisation avoiding patient selection biases). Hence, more patients were included in the experimental arm, using a 2:1 randomisation ratio. A sample size of 12 evaluable patients was required in the placebo (control) arm. No comparative hypothesis was formulated and no statistical comparison between the control and experimental arms was planned. The placebo group was only included to check the similarity between the enrolled patients and historical controls with respect to clinical outcome when given standard treatment. The primary endpoint and all other efficacy outcomes were analysed by modified intention to treat, including all patients who initiated blinded study drug treatment, with no major protocol violation. Major protocol violations were defined as deviations that could potentially affect efficacy analysis, including patients not meeting important inclusion or exclusion criteria.
The occurrence of adverse events (AE) was analysed in the safety population, defined as all confirmed ES patients who received at least one dose of the intended treatment. The severity of the AE was graded according to the NCI-CTCv4.0. The percentage of progression-free patients at 8 weeks was calculated in each arm with their respective 95% confidence interval. PFS and OS were estimated using the Kaplan–Meier method. We used SAS (version9.4) for all analyses.
Results
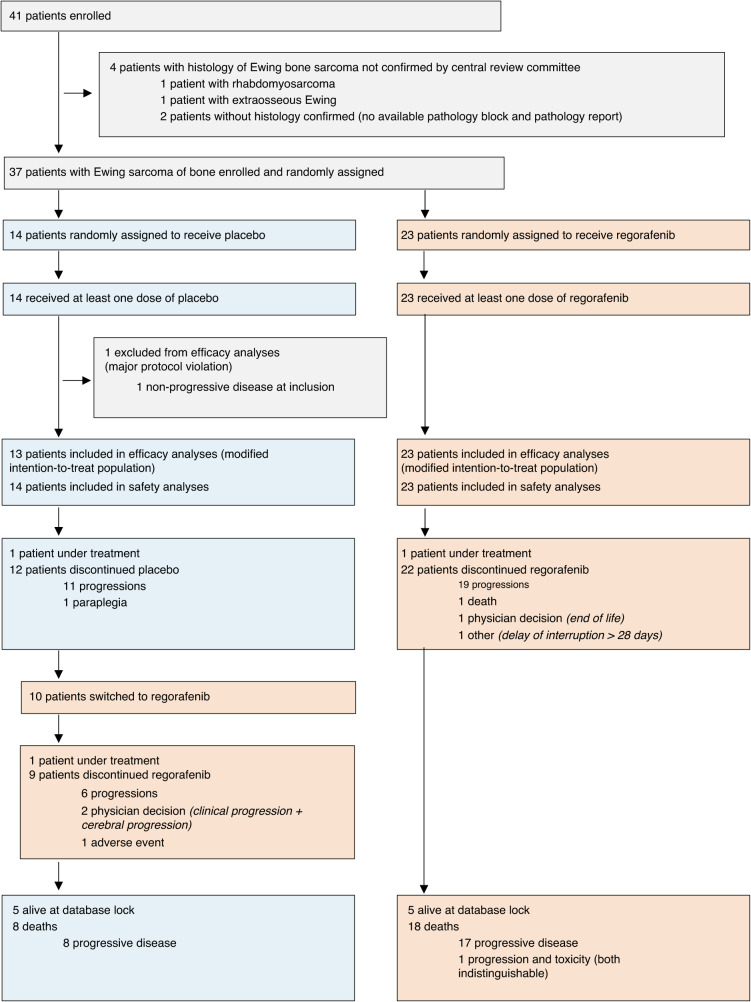
From September 24, 2014 to November 4, 2019, 41 patients were accrued and randomised in this ES cohort, representing the population for safety analysis (Fig. 1). Five patients were excluded from efficacy analyses (three in the regorafenib arm, and two in the placebo arm); four were excluded because the diagnosis of Ewing sarcoma of bone was not histologically confirmed by central review, and one due to non-progressive disease at inclusion. In total, 36 patients with histologically confirmed advanced Ewing sarcoma constituted the population for efficacy analysis: 23 patients were initially randomised to regorafenib and 13 to placebo.
Fig. 1.
Trial profile—study population—consort diagram.
As described in Table 1, the baseline characteristics of 36 patients were well-balanced between the two arms except for small imbalances in ECOG Performance Status, and in the number of previously received chemotherapy regimen for treatment of relapsed disease. All patients received the protocol-required one prior chemotherapy regimen for treatment of relapsed disease; however, 14/24 (61%) received two prior chemotherapy regimens for treatment of relapsed disease in the regorafenib arm, but only 5/13 (38%) received a second-line of prior chemotherapy in the placebo arm.
Table 1.
Baseline patient characteristics.
| Regorafenib (N = 23) | Placebo (N = 13) | Excluded from efficacy analysis (N = 1) | |
|---|---|---|---|
| Age (median, IQR) | 32 (18–59) | 28 (16–59) | 45 |
| Sex (n, %) | |||
| Male | 18 (78) | 10 (77) | 0 (0) |
| Female | 5 (22) | 3 (23) | 1 (100) |
| ECOG PS (n, %) | |||
| 0 | 9 (41) | 4 (33) | 1 (100) |
| 1 | 13 (59) | 8 (67) | 0 (0) |
| Karnofsky*/UK | 1 | 1* | 0 |
| Presence of metastases (n, %) | |||
| No** | 0 | 1 (8) | 0 (0) |
| Yes | 23 (100) | 12 (92) | 1 (100) |
| Main sites of metastases (n, %) | |||
| Lung | 20 (87) | 12 (92) | 1 (100) |
| Bone | 8 (35) | 2 (15) | 0 (0) |
| Lymph node | 3 (13) | 0 (0) | 0 (0) |
| Prior lines of chemotherapy for relapsed/recurrent disease (n, %) | |||
| 1 | 23 (100) | 13 (100) | 1(100) |
| 2 | 14 (61) | 5 (38) | 0 |
| Previous received drugs for relapsed/recurrent disease at entry | |||
| Doxorubicin/Etoposide | 23 (100)/ 22 (95) | 12 (92)/ 12(92) | 1 (100) |
| Ifosfamide/CPM** | 23 (100)/ 10 (43) | 12 (92)/6 (46) | 0 |
| VCR***/Dactinomycin | 23 (100)/19 (82) | 12 (92)/8 (61) | 0 |
| Temo°/Irinotecan | 13 (56)/17 (74) | 7 (54)/8 (61) | 0 |
ECOG Eastern Cooperative Oncology Group.
Data are number of patients (%) or median (range). Patients could have more than one metastasis. *adolescent patient, **CPM: cyclophosphamide, ***VCR: vinvristin, Temo°: temozolomide.
As expected for ES most distant metastases were in the lung. The majority of patients had received for relapsed refractory disease the major drugs used for Ewing sarcoma; ifosfamide, doxorubicin, dactinomycin, vincristin, etoposide, temozolomide and irinotecan.
At the time of the analysis, the median follow-up of surviving patients was 25.9 months (IQR 12.4–26.4), one placebo patient, one regorafenib patient, and another placebo patient on crossover regorafenib after progression were still on study treatment at 8, 51 and 13.5 months, respectively.
Thirteen patients (56%, one-sided 95% CI [37.5%–[) were progression-free at 8 weeks in the regorafenib arm (14 required to be a success) while only one placebo-assigned patient (7.7% one-sided 95% CI [0.4%–[) was progression-free. Three durable partial responses (ORR 13%) were noted in the regorafenib arm (lasting 5.4, 5.5 and 9.9 months, respectively), and one partial response of 7.3 months duration was observed on placebo, still ongoing at the last follow-up.
At the time of the analysis, 19 of 23 patients in the regorafenib arm, and 11 of 13 patients in the placebo arm had progressed, and 26 patients have died; 18 (78.2%) patients in the regorafenib arm, and 8 (61.5%) in the placebo arm. All patients died of disease progression, except one who died of toxicity related to regorafenib (thrombocytopenia with subsequent massive hemoptysis from lung metastasis).
Median PFS was 2.6 months (95% CI 1.1–5.3) in the regorafenib arm and 0.9 months (95% CI 0.8–1.7) on placebo. PFS rates at 3 months and 6 months were 39% (95% CI 20–58) and 26% (95% CI 11–45-, for patients in the regorafenib arm, versus 8% (95% CI 0–29) at 3 and 6 months on placebo respectively. Figure 2 shows the PFS curves per blinded central review.
Fig. 2.
Progression-free survival curves per blinded central review.
In the 13 patients initially randomised to the placebo arm, unblinding procedure occurred in 12: one continued to receive blinded study drug at the time of this analysis. Reasons for unblinding were radiologic progression in 11 patients and paraplegia in one case. After progression, 10 patients from the placebo arm crossed over to open-label regorafenib. Two patients did not crossover; one passed away 14 days after unblinding and the other due to paraplegia associated with disease progression. The median PFS on open-label regorafenib after crossover was 3 months (95% CI 0.9–10.6).
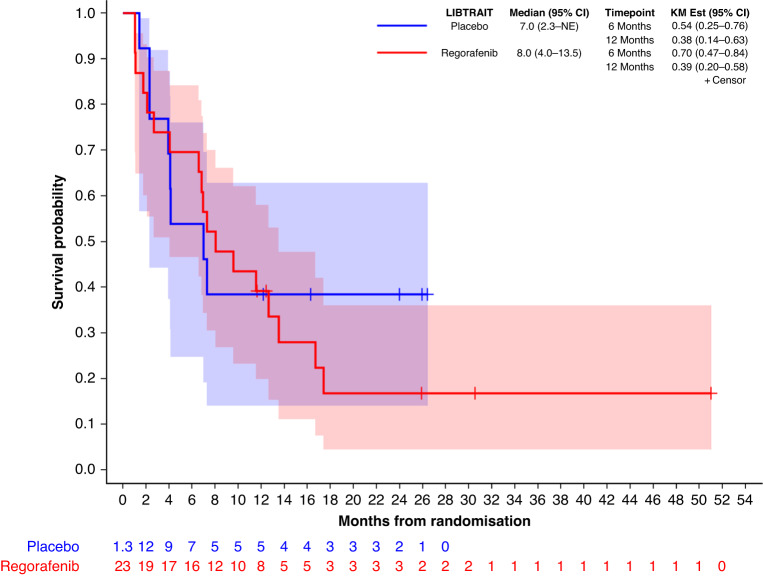
Figure 3 shows the overall survival (OS) curves, including 10 of 13 (77%) of placebo patients who crossed over to open-label regorafenib. Median OS is 8 months (95% CI 4.0–13.5) for patients randomised to regorafenib and 7 months (95% CI 2.3–NE) for those randomised to placebo.
Fig. 3.
Overall survival curves, including crossed over of 77% of placebo patients who received open-label regorafenib.
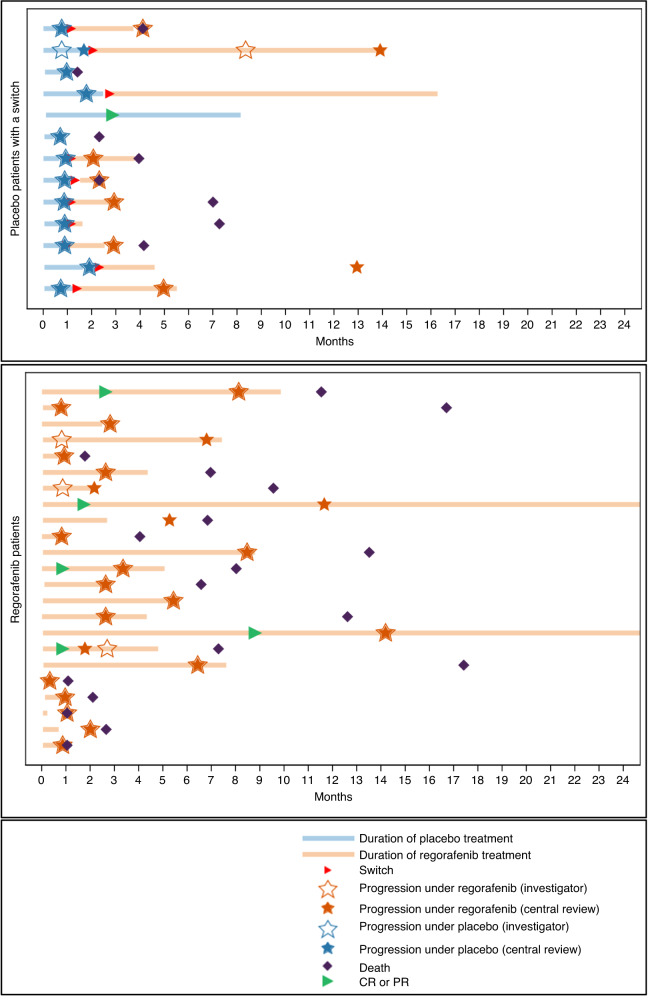
The swimmer plots (Fig. 4) show the PFS while on placebo and PFS after crossover for the ten patients initially randomised to placebo who subsequently received open-label regorafenib. The plots show that rapid disease progression on placebo was slower in the same patients while on subsequent regorafenib. The waterfall plots (Fig. 5) show the tumour responses obtained on blinded treatment and on open-label regorafenib.
Fig. 4.
Swimmer plots showing initial PFS and PFS after crossover for the ten patients initially randomised to placebo who subsequently received open-label regorafenib.
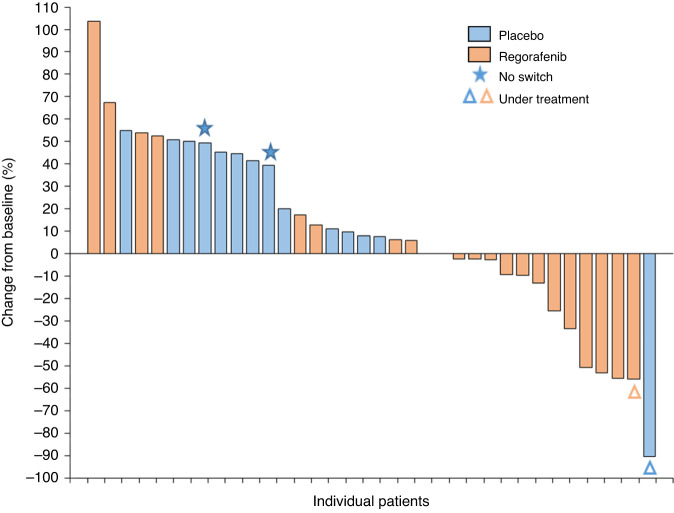
Fig. 5.
Waterfall plots on regorafenib arm and placebo arm, in supplementary materials.
The median treatment duration was 2.7 months (95% CI 0.7–7.4) on regorafenib, and 0.7 months (95% CI 0.7–1.6) on placebo. Transient treatment discontinuation occurred in 11 (48%) of 23 patients in the regorafenib arm but for no patient on placebo. Dose reductions were reported in nine (39%) of 23 patients in the regorafenib arm, due to toxicity in 8 patients. Interestingly, 1 of 14 (8%) patients on placebo had a study drug reduction. Regorafenib was reduced by one dose level to 120 mg/day for six patients (26%) and to 80 mg/day for three patients (13%), while placebo was reduced by one dose level for a single patient.
Table 2 reports the adverse events of grade ≥3 of clinical interest per treatment group to the point of optional crossover for the placebo arm. The most common grade ≥3 regorafenib-related events adverse during the double-blind period were pain (22%), asthenia (17%), thrombocytopenia (13%) and diarrhoea (13%). Supplementary Table S1 reports the adverse events of any clinical grade per treatment group until the optional crossover.
Table 2.
Adverse events of grade ≥3 of clinical interest per treatment group before crossover.
| Regorafenib, N = 23 | Placebo, N = 14 | |||||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 3 | 4 | 5 | |
| Blood and lymphatic system disorders | ||||||
| Febrile neutropenia | 1* (4.3%) | 1* (4.3%) | 0 | 0 | 0 | 0 |
| Leukopenia | 1 (4.3%) | 0 | 0 | 0 | 0 | 0 |
| Lymphopenia | 2 (8.7%) | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 2 (8.7%) | 0 | 1 (4.3%) | 0 | 0 | 0 |
| Gastrointestinal disorders | ||||||
| Diarrhoea | 3 (13.0%) | 0 | 0 | 0 | 0 | 0 |
| Stomatitis | 1 (4.3%) | 0 | 0 | 0 | 0 | 0 |
| General disorders and administration site conditions | ||||||
| Asthenia | 4 (17.4%) | 0 | 0 | 0 | 0 | 0 |
| Fever | 1 (4.3%) | 0 | 0 | 0 | 0 | 0 |
| Mucositis | 2* (8.7%) | 0 | 0 | 0 | 0 | 0 |
| Pain | 5* (21.7%) | 0 | 0 | 1 (7.1%) | 1 (7.1%) | 0 |
| Hepatobiliary disorders | ||||||
| Hepatocellular injury | 1 (4.3%) | 0 | 0 | 0 | 0 | 0 |
| Infections and infestations | ||||||
| Bronchitis | 1 (4.3%) | 0 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 1 (4.3%) | 0 | 0 | 0 |
| Investigations | ||||||
| Blood lactate dehydrogenase increased | 0 | 1 (4.3%) | 0 | 0 | 0 | 0 |
| Metabolism and nutrition disorders | ||||||
| Hypophosphataemia | 1* (4.3%) | 0 | 0 | 0 | 0 | 0 |
| Nervous system disorders | ||||||
| Paraplegia | 0 | 0 | 0 | 1 (7.1%) | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||||
| Dyspnoea | 2 (8.7%) | 0 | 0 | 0 | 1 (7.1%) | 0 |
| Haemoptysis | 1 (4.3%) | 0 | 0 | 0 | 0 | 0 |
| Pneumothorax | 2* (8.7%) | 0 | 0 | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||||
| Hand and foot skin reaction | 3 (13.0%) | 0 | 0 | 0 | 0 | 0 |
| Vascular disorders | ||||||
| Hypertension | 1 (4.3%) | 0 | 0 | 0 | 0 | 0 |
*One patient had a related serious adverse event
Two patients in placebo arm (14.3%) and 19 patients in regorafenib arm (56%) experienced serious adverses events (SAEs). Ten treatment-related SAEs occurred in seven patients in regorafenib arm: febrile neutropenia (two episodes), thrombocytopenia, diverticulitis, stomatitis, mucositis, fever, hypophosphataemia, pain and pneumothorax), while none occurred on placebo. There was one fatal haemorrhage due to severe thrombocytopenia in the regorafenib arm.
Discussion
Previously published data from the osteosarcoma cohort of REGOBONE multi-cohort trial indicated statistically significant activity of regorafenib in delaying progression with that subtype of bone sarcomas [19]. However, the data from the ES cohort of this study did not meet the primary endpoint to be considered “successful”. According to the study design criteria for success, 14/23 (60.9%) progression-free patients at 8 weeks in the regorafenib arm would have been necessary to consider this study successful, while only 13/23 (56.5%) patients remained free of disease progression at the 8-week timepoint in the trial.
Although the primary study endpoint was not met, this small cohort nonetheless suggests an interesting finding of modestly delayed tumour progression with regorafenib in this pretreated population. It is intriguing that the placebo arm confirmed the aggressive nature of recurrent and refractory ES, with only 1 patient of 13 (7.7%) remaining progression-free at 8 weeks, widely below the P0 hypothesis, and with a median PFS (mPFS) of 3.9 weeks on placebo, while the regorafenib arm mPFS was 11.5 weeks. In addition, after rapid progression on placebo, the post-crossover mPFS of 12.9 weeks on open-label regorafenib also supports the hypothesis that regorafenib may exhibit some antitumour activity against recurrent disease in patients with advanced ES after failure of initial chemotherapy for relapse. Furthermore, our study showed a very interesting result with 26% of these pretreated metastatic patients remaining progression-free at 6 months on regorafenib, while only 8% on placebo remained free of progression at that time.
Our results are consistent with those of the Phase 2 SARC024 trial [22] that studied the efficacy and tolerability of regorafenib in the same setting and reported an 8-week PFS rate of 63% (95% CI 46–81%), and a median PFS of 14.8 weeks. In addition, our results are close to those reported with cabozantinib, in the single-arm CABONE study, that reported a 8-week PFS rate of 80%, a median PFS of 4.4 months, and a PFS rate at 26 weeks of 33% [23] in pretreated Ewing sarcoma patients with progressive disease.
We note that regorafenib commonly required dose modification when initiated at full dose (160 mg per day) in 39% of patients. However, our data suggest that patient-specific dose modifications to deliver the drug tailored to individual patient tolerance may nonetheless represent a clinically beneficial dosing strategy. The overall safety profile of regorafenib was consistent with previously published reports and generally amenable to dose modifications. Nonetheless, we did note one event of fatal haemoptysis associated with severe thrombocytopenia in a single patient with lung metastases.
This study has some limitations, since it was statistically non-comparative, done in only one country, included a relatively small number of patients, with adult patients only, except for a single adolescent patient of 16 years who died within one month of study entry. Moreover, there was no correlative biology. This academic study was designed to assess the activity and safety of regorafenib as the primary endpoint and was funded only for the clinical elements of this trial, so it was beyond the scope of our work and planned to study biological correlates.
At this time, there is still no truly optimal standard therapy for patients with relapsed Ewing sarcoma after disease progression following standard first-line combination chemotherapy. The recently presented rEECur collaborative research study reported a multi-arm Phase II/III randomised trial [24] in recurrent Ewing sarcoma. This trial rapidly dropped regimens (e.g., Gemcitabine/Docetaxel and Irinotecan/Tezolomide) based on objective response rate (ORR) as the main endpoint, to move to the Phase III portion which compared high-dose (HD) Ifosfamide versus Topotecan/Cylophosphamide, with event-free survival (EFS) as the main endpoint. Although this trial reported that HD Ifosfamide is more likely to result in better EFS and OS than Cyclophosfamide and Topotecan, the differences were modest. Recurrent Ewing sarcoma remains an aggressive and life-threatening malignancy with overall poor prognosis with any current standard chemotherapy regimen.
Over the last 20 years, several drugs and combinations have been explored in relapsed ES, after failure of any chemotherapy delivered in first-line treatment for relapse, but no major advances has been documented in this challenging setting. Several new biological targets are being explored in ES, including epigenetic therapies using agents such as the LSD1 inhibitor seclidemstat (SP-2577; ClinicalTrials.gov Identifier: NCT03600649) or CDK9 inhibitors (e.g., alvociclib, TP-1297; ClinicalTrials.gov Identifier: NCT03604783), but these are still early in development. It is likely that new combinations of biologically-targeted agents will be necessary to make advances against ES.
We hope that the data from this cohort will help investigators to study other novel strategies which might eventually improve outcomes for patients with recurrent ES, after failure of standard chemotherapy. For example, although activity from single-agent immune checkpoint inhibitor therapy has been only rarely noted in Ewing sarcoma [24–26], regorafenib in combination with anti-PD1 therapy has recently demonstrated very encouraging antitumour activity in patients with advanced gastrointestinal cancers [27, 28], and this strategy might be explored in ES as well. These patients with unmet needs will likely require new drugs with different mechanisms of action for relapsed Ewing sarcoma, and these data with regorafenib may be sufficiently intriguing to merit further research in combination regimens.
Supplementary information
Acknowledgements
We thank the patients and their families for their participation in the study. We thank all the UNICANCER sarcoma staff members involved in the trial management. The data management was conducted by UNICANCER, the analysis was done by Centre Léon Bérard. We thank the patient advocacy group InfoSarcomes. We thank the French National Cancer Institute for funding the labelled networks for the management of sarcoma (e.g., NetSarc, RRePS, RESOS, and InterSarc).
Author contributions
FD and SC designed and supervised the trial and wrote the article; FD, SC and CS analysed the data and contributed to the trial design. All other authors supervised clinical patient management, reviewed and approved the article.
Funding
Bayer Healthcare SAS (Loos, France) supplied regorafenib and placebo tablets and provided funding to UNICANCER. The funder had no role in study design, data collection, monitoring, analysis, and interpretation or writing of the report. Once the trial has been designed, UNICANCER, as the Sponsor for the study, in collaboration with the French Sarcoma Group was responsible for all aspects of the trial. This study was not funded by NIH.
Data availability
The datasets generated and analysed during the current study are not publicly available due to European regulation on data privacy (GDPR). The datasets are also now under licence with Bayer and thus submitted to restrictions. Data are, however, available from the authors upon reasonable request and with permission of Bayer, according to the conditions stated as follows: European regulatory framework: De-identified study data are considered as personal data within the meaning of Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016, known as “GDPR”. French regulatory framework: In addition to GDPR, the transfer of this data by Unicancer is a processing in itself that is subject to Chapter IX and XII of the updated Within the framework of this law, the data processing is permitted by Unicancer’s conformity to the MR-001 Code of conduct.
Competing interests
FD received travel grants from Pharmamar, and Leo Pharma, attended advisory boards for Bayer, Lilly. CC attended advisory boards for Leo Pharma, JYB receives research support and honoraria from Eisei, Eli Lilly, MSD, BMS, GSK, Ignyta, Novartis, Pharmamar and Roche unrelated to this work. AL, AI, PB, EK, NP, CP, VL, EB, ESBB, FB, CS, VL, MC, CS,LM, VV, NG and SC declare no competing interests.
Ethics approval and consent to participate
The study was approved by an ethical and regulatory committee (French Ethical Committee, Comité de Protection des Personnes Sud Méditerrannée 1, approved on March 26, 2014). This study was performed in accordance wuth the Declaration of Helsinki. All patients provided written informed consent before enrolment, and one study amendment (protocol V6, June 29, 2016) expanded enrolment to include paediatric patients aged >10 years (although ultimately only one child was enrolled). The trial is registered in the European Clinical Trials Register database (EudraCT N°: 2013-003910-42) and at ClinicalTrials.gov (NCT02389244).
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02413-9.
References
- 1.Rodríguez-Galindo C, Navid F, Liu T, Billups CA, Rao BN, Krasin MJ. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol. 2008;19:814–20. doi: 10.1093/annonc/mdm521. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, et al. Current management and future approaches through collaboration. JCO. 2015;33:3036–46. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 3.Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jürgens H. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer. 2006;47:795–800. doi: 10.1002/pbc.20719. [DOI] [PubMed] [Google Scholar]
- 4.Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029–34. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 5.Fox E, Patel S, Wathen JK, Schuetze S, Chawla S, Harmon D, et al. Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of Sarcoma Alliance for Research Through Collaboration study 003. Oncologist. 2012;17:321. doi: 10.1634/theoncologist.2010-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oesterheld JE, Reed DR, Setty BA, Isakoff MS, Thompson P, Yin H et al. Phase II trial of gemcitabine and nab-paclitaxel in patients with recurrent Ewing sarcoma : A report form the National Pediatric Cancer Foundation. Pediatr Blood Cancer 2020;67. 10.1002/pbc.28370 [DOI] [PMC free article] [PubMed]
- 7.Navid F, Willert JR, McCarville MB, Furman W, Watkins A, Roberts W, et al. Combination of Gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. C3 Cancer. 2008;113:419–25.. doi: 10.1002/cncr.23586. [DOI] [PubMed] [Google Scholar]
- 8.Rapkin L, Qayed M, Brill P, Martin M, Clark D, George BA, et al. Gemcitabine and Docetaxel (GEMDOX) for the treatment of relapsed and refractory pediatric sarcomas. Pediatr Blood Cancer. 2012;59:854–8. doi: 10.1002/pbc.24101. [DOI] [PubMed] [Google Scholar]
- 9.van Maldegem A, Rutkowski P, Blay JY, van den Berg H, Placzke J, Rasper M, et al. Etoposide and carbo- or cisplatin combination therapy in refrectory or relpased Ewing sarcoma : a large retrospective study. Pediatr Blood Cancer. 2015;61:40–44. doi: 10.1002/pbc.25230. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, del Prever AB, Palmerini E, Staals E, Berta M, Balladelli A, et al. Response to high-dose ifosfamide in patients with advanced/recurrent Ewing sarcoma. Pediatr Blood Cancer. 2009;52:581–4. doi: 10.1002/pbc.21917. [DOI] [PubMed] [Google Scholar]
- 11.Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Calwla SP, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol. 2011;29:4541–7. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappo A, Shreyaskumar P, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumours: results of a phase II sarcoma Alliance for Research Through Collaboration Study. J Clin Oncol. 2011;29:4541–47. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuBois SG, Krailo M, Glade-Bender J, Buxton A, Laack N, Lor Randall R, et al. Randomized phase III trial of ganitumab added to interval compressed chemotherapy for patients with newly diagnosed metastatic Ewing sarcoma: a report from the Children’s Oncology Group (COG) J Clin Oncol. 2023;41:2098–107. doi: 10.1200/JCO.22.01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, Bolontrade MF, Reddy K, Duan X, Guan H, Yu L, et al. Suppression of Ewing’s sarcoma tumor growth, tumor vessel formation, and vasculogenesis following antivascular endothelial growth factor receptor-2 therapy. Clin Cancer Res. 2007;13:4867–73. doi: 10.1158/1078-0432.CCR-07-0133. [DOI] [PubMed] [Google Scholar]
- 15.Reddy K, Zhou Z, Schadler K, Jia SF, Kleinerman ES. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing’s tumor vessels. Mol Cancer Res. 2008;6:929–36. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potikyan G, Savene RO, Gaulden JM, France KA, Zhou Z, Kleinerman ES, et al. EWS/FLI1 regulates tumor angiogenesis in Ewing’s sarcoma via suppression of thrombospondins. Cancer Res. 2007;67:6675–84. doi: 10.1158/0008-5472.CAN-06-4140. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 18.Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:1732–42. doi: 10.1016/S1470-2045(16)30507-1. [DOI] [PubMed] [Google Scholar]
- 19.Van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 20.Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20:120–33. doi: 10.1016/S1470-2045(18)30742-3. [DOI] [PubMed] [Google Scholar]
- 21.Perrier L, Rascle P, Morelle M, Toulmonde M, Ranchere Vince D, Le Cesne A, et al. The cost-saving effect of centralized histological reviews with soft-tissue and visceral sarcomas, GIST, and desmoid tumors: the experiences of the pathologists of the French Sarcoma Group. PLoS ONE. 2018 doi: 10.1371/journal.pone.0193330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–66. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 23.Attia S, Bolejack V, Ganjoo KN, Georges S, Agulnik M, Rushing D, et al. A phase 2 trial of regorafenib (REGO) in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC0024 trial results. Cancer Med. 2022;00:1532–39. doi: 10.1002/cam4.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Italiano A, Mir O, Mathoulin-Plissier S, Penel N, Piperno-Neumann S, Bompas E, et al. Cabozantinib in patients with advanced osteosarcomas and Ewing sarcomas (CABONE): a multicentre, single-arm, phase 2 phase trial. Lancet Oncol. 2020;21:446–55.. doi: 10.1016/S1470-2045(19)30825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe MG, Moroz V, Khan M, Evans A, Fenwick N, Gaspar N, et al. Phase III assessment of topotecan and cyclophosphamide and high-dose ifosfamide in rEECur, an international randomised controlled trial of chemotherapy for the treatment of recurrent and primary refractory Ewing sarcoma (RR-ES) J Clin Oncol. 2022 doi: 10.1200/JCO.2022.40.17_suppl.LBA2. [DOI] [Google Scholar]
- 26.Tawbi H, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493–501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoluzzi L, Cacavio A, Ghesani M, Karambelkar A, Rapkiewicz A, Weber J, et al. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. 2016;6:24. doi: 10.1186/s13569-016-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–26. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to European regulation on data privacy (GDPR). The datasets are also now under licence with Bayer and thus submitted to restrictions. Data are, however, available from the authors upon reasonable request and with permission of Bayer, according to the conditions stated as follows: European regulatory framework: De-identified study data are considered as personal data within the meaning of Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016, known as “GDPR”. French regulatory framework: In addition to GDPR, the transfer of this data by Unicancer is a processing in itself that is subject to Chapter IX and XII of the updated Within the framework of this law, the data processing is permitted by Unicancer’s conformity to the MR-001 Code of conduct.