Abstract
Background
The existence of Type 2 Diabetes Mellitus (DM) in tuberculosis (TB) patients is very dangerous for the health of patients. One of the major concerns is the emergence of MDR-TB in such patients. It is suspected that the development of MDR-TB further worsens the treatment outcomes of TB such as treatment failure and thus, causes disease progression.
Aim
To investigate the impact of DM on the Emergence of MDR-TB and Treatment Failure in TB-DM comorbid patients.
Methodology
The PubMed database was systematically searched until April 03, 2022 (date last searched). Thirty studies met the inclusion criteria and were included in this study after a proper selection process.
Results
Tuberculosis-Diabetes Mellitus patients were at higher risk to develop MDR-TB as compared to TB-non-DM patients (HR 0.81, 95% CI: 0.60–0.96, p < 0.001). Heterogeneity observed among included studies was moderate (I2 = 38%). No significant change was observed in the results after sub-group analysis by study design (HR 0.81, 95% CI: 0.61–0.96, p < 0.000). In the case of treatment failure, TB-DM patients were at higher risk to experience treatment failure rates as compared to TB-non-DM patients (HR 0.46, 95% CI: 0.27–0.67, p < 0.001).
Conclusion
The results showed that DM had a significant impact on the emergence of MDR-TB in TB-diabetes comorbid patients as compared to TB-non-DM patients. DM enhanced the risk of TB treatment failure rates in TB-diabetes patients as compared to TB-non-DM patients. Our study highlights the need for earlier screening of MDR-TB, thorough MDR-TB monitoring, and designing proper and effective treatment strategies to prevent disease progression.
Keywords: tuberculosis, diabetes mellitus, TB-DM comorbidity, multi-drug resistant TB, treatment failure, disease progression
Introduction
The existence of Type 2 Diabetes Mellitus (DM) in tuberculosis (TB) patients causes serious public health concerns (1). People across different regions of the world are becoming affected by this comorbidity (2). The occurrence rate of DM in TB patients is rising globally and causes severe health problems in TB patients (3). DM affected more than 50% of TB patients across all regions of the world (4, 5). This co-morbid condition leads to many unfavorable TB treatment consequences such as treatment failure rates and increased drug resistance cases in TB patients (6, 7).
One of the challenges in controlling this TB-DM comorbid condition is the development of MDR-TB, in which resistance develops for both Isoniazid and Rifampicin (8). The presence of DM enhances the chances of developing MDR-TB and DM was recognized as a predictor for its emergence (9). The prevalence of MDR-TB ranged from 10 to 30% in diabetics (10, 11). The emergence of MDR-TB in TB-DM comorbid patients is very dangerous for the health of people (8). It can be the leading cause of death in people (8). The TB treatment cure rates have been reduced from 96 to 54% due to the presence of MDR in diabetic patients. The reason for reduced TB treatment cure rates and increased treatment failure rates may be due to complex treatment protocols and the adverse effects of alternative medications given to patients with MDR-TB (8). Moreover, it puts an increased financial burden on patients and becomes one of the reasons for disease progression (8, 12).
The “WHO Collaborative Framework for Care and Control of Tuberculosis and Diabetes” helps to guide healthcare professionals in the effective management of both diseases at administrative and clinical levels (13). The link between DM and MDR-TB is not well-studied in the literature. Only 13 studies were included in a previous systematic review and meta-analysis that reported the association between DM and MDR-TB and found that DM is a strong predictor to develop MDR-TB in TB patients and this TB-DM comorbid condition can potentially lead to high treatment failure rates (9). But to the best of the author’s knowledge, there is still a lack of studies on the association between DM and MDR-TB and TB treatment failure results. Moreover, there is also a need for adjusting potential covariates. A more comprehensive systematic assessment of the available literature is needed for further understanding of their association in TB-DM comorbid patients. Thus, this study’s goal was to investigate the impact of DM on the Emergence of MDR-TB and Treatment Failure rates in TB-DM comorbid patients versus TB-non-DM patients.
Methodology
Study design
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were being followed by this study (14).
Literature search
In this review, the PubMed database was systematically searched until April 15, 2023 (the last date of searching). For PubMed Search terms used were as follows: “Diabetes Mellitus” [Mesh]) OR “Diabetes Mellitus, Type 2” [Mesh]) AND “Tuberculosis” [Mesh]) OR “Tuberculosis, Pulmonary” [Mesh]) AND “Treatment Outcome” [Mesh]) AND “Drug resistance” [Mesh]) OR “Multidrug resistance” [Mesh]). The studies that were fulfilling the criteria were also searched from the references of the included studies. The review was only performed on English-language published papers.
Inclusion and exclusion criteria
The inclusion criteria for this study are as follows: the research articles (case–control, cohort or cross-sectional) reporting the association of DM with both MDR-TB and treatment failure rates with adjusted covariates data, studies involving human subjects receiving anti-TB therapy, clinical outcome studies, studies investigating diabetes mellitus’s impact on tuberculosis treatment failure results in tuberculosis patients, and articles published in the English language. The study excluded non-research articles, non-human studies, reports, editorials, studies not analyzing the MDR-TB and treatment failure rates in patients, studies involving pre-DM and type-1 DM patients, and studies for which full text could not be retrieved. Three reviewers independently reviewed papers that fulfilled the selection process. If there were any conflicts between the reviewers, then those were clarified through discussion or the involvement of fourth reviewer.
Study selection
The first step performed was to screen the studies by reading their title and abstracts. The entire content of the selected studies was further retrieved for additional review. For the evaluation of the quality of the individual research articles in the study and to assess the possible risk of bias among studies, the Newcastle-Ottawa Quality Assessment Scale (NOS) was used. The scale comprises of three parts: selection, comparability, and exposure (for cross-sectional or case–control studies) or measurement of outcome (for cohort studies). It is a nine-score quality assessment scale. A score of <3 means having poor quality and high risk of bias, ≥3 means having median quality and moderate risk of bias and a score of ≥7 means having high quality study and a low risk of bias (15).
Data extraction
From the research articles included in this meta-analysis, the relevant information was collected using a data collection form. Author information, year, country of study, study design, participants, inclusion criteria, treatment regimen, covariates, TB treatment outcomes, and regression results with 95% CI and p values were the data of the included studies extracted. The relevant data were extracted independently by two reviewers. The data of included studies extracted were reviewed by a third reviewer. Any conflicts were sorted out through discussion until a final decision was taken.
Statistical analysis
Heterogeneity among studies was assessed by using I2 test. The pooled HR (95% CI) were calculated using the fixed effects model. Subgroup analyses were performed by study design to assess the impact of study design on results. p < 0.01 was considered statistically significant. All statistical analyses were conducted by the meta program in STATA version 12.0 (Stata Corp, College Station, Texas).
Results
Search results
When searching the databases, 2,690 results were retrieved. A total of 1,257 articles qualified for the preliminary screening after 1,433 duplicates were removed. Based on their titles and abstracts, 1,208 studies were irrelevant to our study objective and were excluded. Out of 49 studies that were available for full-text review, 19 studies were excluded out of which seven studies were review articles, two studies were editorials, and eight studies did not use multivariable analysis for adjustment of covariates. Thirty studies were selected for inclusion in this meta-analysis after a complete article overview. Figure 1 depicts the selection process of studies included in the meta-analysis.
Figure 1.
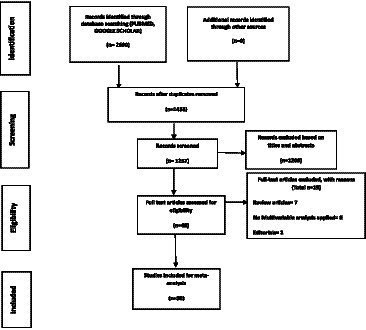
PRISMA flow diagram.
Study characteristics
Among the included studies, 23 were cohort studies including 13 retrospective and 10 prospective and seven were case–control studies. There were six studies conducted in China (16–21), six in Mexico (22–27), three in South Korea (28–30), three in India (31–33) two in United States (25, 34), and one each in Armenia (35), Brazil (36), Spain (37), Indonesia (5), Bangladesh (38), Saudi Arabia (39), Ethiopia (40), Taiwan (41), Georgia (42), and Thailand (43). In the pool analysis, total number of included patients were 225,812, among which 180,619 were TB patients (ranged from 90 to 146,390), while 42,975 were TB-Diabetes comorbid patients (ranged from 24 to 34,988). Table 1 shows all the refined data of included studies.
Table 1.
Summaries of the included studies.
| Author | Year | Country | Research design | TB patients | Patients with DM comorbidity | Covariates | TB treatment outcomes | MDR in TB patients | MDR in TB-DM patients | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Sahakyan et al. (35) | 2020 | Armenia | Retrospective | 585 | 36 | Baseline weight, sputum smear status | Treatment failure, MDR-TB | 90/585 | 5/36 | 0.809 |
| Leung et al. (16) | 2017 | China | Prospective | 18,083 | 3,331 | Demographic factors, socioeconomic factors, medical factors, treatment-related factors, hepatitis, renal disease | MDR-TB | 0.133 | ||
| Lee et al. (30) | 2017 | South Korea | Retrospective | 1,044 | 253 | Gender, age, body mass index (BMI), cavitary disease, previous history of tuberculosis, and presence of diabetes mellitus | Treatment failure, MDR-TB | 25/791 | 15/253 | 0.035 |
| Mi et al. (17) | 2014 | China | Retrospective | 434 | 187 | Age, gender, occupation, resident area, and previous TB treatment | MDR-TB | - | 10/26 | <0.05 |
| Siddiqui et al. (33) | 2016 | India | Prospective | 266 | 37 | Age, sex, BMI, TB history, risk factors, clinical presentation, treatment history, DM status, and adverse drug reaction | Treatment failure, MDR-TB | 4/154 | 0 | <0.05 |
| Yoon et al. (29) | 2017 | South Korea | Prospective | 157 | 108 | Age, smoking status, uncontrolled diabetes, comorbidity, and positive sputum smear | Treatment failure, MDR-TB | 18/401 | 6/94 | 0.466 |
| Gomez-Gomez et al. (27) | 2015 | Mexico | Case–control | 175 | 56 | Age, gender, smoking, malnutrition, alcohol, and other underlying diseases | MDR-TB | 39/139 | 17/36 | <0.05 |
| Gil-Santana et al. (36) | 2016 | Brazil | Retrospective | 273 | 135 | Age, gender | MDR-TB | 3/244 | 12/128 | 0.002 |
| Delgado-Sánchez et al. (26) | 2015 | Mexico | Retrospective | 146,390 | 34,988 | Gender, age, previous TB treatment, year of diagnosis, and malnutrition | Treatment failure, MDR-TB | 630/1614 | 362/672 | <0.001 |
| Hongguang et al. (18) | 2015 | China | Prospective | 944 | 182 | Age, gender, and treatment categorization | Treatment failure, MDR-TB | 4/944 | 2/182 | 0.238 |
| Viswanathan et al. (32) | 2014 | India | Retrospective | 149 | 96 | Severity of Diabetes, glycemic control, and comorbidities | Treatment failure, MDR-TB | 0 | 1/96 | 0.04 |
| Nandhakumar et al. (31) | 2013 | India | Retrospective | 2,449 | 667 | Age, gender, type of TB, smear result, and HIV status | Treatment failure, MDR-TB | 4/427 | 5/677 | 0.04 |
| Fisher-Hoch et al. (25) | 2008 | USA | Retrospective | 1,041 | 401 | Age, gender, alcohol, drug abuse, HIV, and previous TB history | MDR-TB | 31/1041 | 18/401 | <0.05 |
| Fisher-Hoch et al. (25) | 2008 | Mexico | Retrospective | 1,149 | 287 | Age, gender | MDR-TB | - | 287/1436 | <0.05 |
| Suárez-García et al. (37) | 2009 | Spain | Case–control | 655 | 41 | Alcohol, age, and previous TB treatment | MDR-TB | 27/30 | 3/30 | <0.05 |
| Jiménez-Corona et al. (24) | 2013 | Mexico | Prospective | 888 | 374 | Severity of disease, smear status | Treatment failure, MDR-TB | 26/604 | 17/315 | <0.001 |
| Hsu et al. (19) | 2013 | China | Prospective | 804 | 204 | Age, gender | MDR-TB | 24/665 | 5/204 | 0.926 |
| Alisjahbana et al. (5) | 2007 | Indonesia | Prospective | 540 | 94 | Age, gender, BMI, and disease duration | MDR-TB | 13/272 | 1/56 | >0.05 |
| Rifat et al. (38) | 2014 | Bangladesh | Case–control | 917 | 83 | Age group, education, occupation, and smoking | MDR-TB | - | - | 0.001 |
| Singla et al. (39) | 2006 | Saudi Arabia | Retrospective | 505 | 187 | Age, gender | MDR-TB | - | - | 0.001 |
| Bashar et al. (34) | 2001 | USA | Case–control | 102 | 53 | HIV, residency status | MDR-TB | - | - | <0.01 |
| Adane et al. (40) | 2023 | Ethiopia | Prospective | 243 | 24 | Age, BMI, gender, smoking, and alcohol | Treatment failure, MDR-TB | 1/132 | 3/60 | 0.056 |
| Chiang et al. (41) | 2015 | Taiwan | Retrospective | 768 | 705 | Age, gender, sputum smear, drug resistance, and smoking | Treatment failure, MDR-TB | - | - | <0.01 |
| Magee et al. (42) | 2015 | Georgia | Prospective | 281 | 37 | Age, gender, HIV infection, and smoking | Treatment failure, MDR-TB | 32/229 | 11/37 | <0.001 |
| Munoz-Torrico et al. (23) | 2017 | Mexico | Retrospective | 90 | 49 | Age | Treatment failure, MDR-TB | 34/41 | 39/49 | 0.7 |
| Perez-Navarro et al. (22) | 2017 | Mexico | Prospective | 324 | 183 | Age, gender, overcrowding, and smoking | Treatment failure, MDR-TB | 16/324 | 26/183 | <0.001 |
| Wu et al. (20) | 2016 | China | Retrospective | 161 | 40 | Age, gender, history of smoking, lung cavities, smear status, and TB treatment duration | MDR-TB | 4/161 | 4/40 | 0.135 |
| Min et al. (28) | 2005 | South Korea | Case–control | 145 | 52 | Age, smoking | MDR-TB | 41/166 | 11/29 | 0.04 |
| Song et al. (21) | 2015 | China | Case–control | 900 | 54 | Inadequate regimen, insufficient dose, and compliance | MDR-TB | <0.05 | ||
| Jitmuang et al. (43) | 2015 | Thailand | Case–control | 157 | 31 | Age, gender, previous TB, HIV, alcohol, and smear status | MDR-TB | 22/141 | 9/47 | <0.001 |
DOTS, Directly observed treatment, short course strategy; NTP, National Tuberculosis Control Program; and WHO, World Health Organization.
Statistical results
MDR-TB
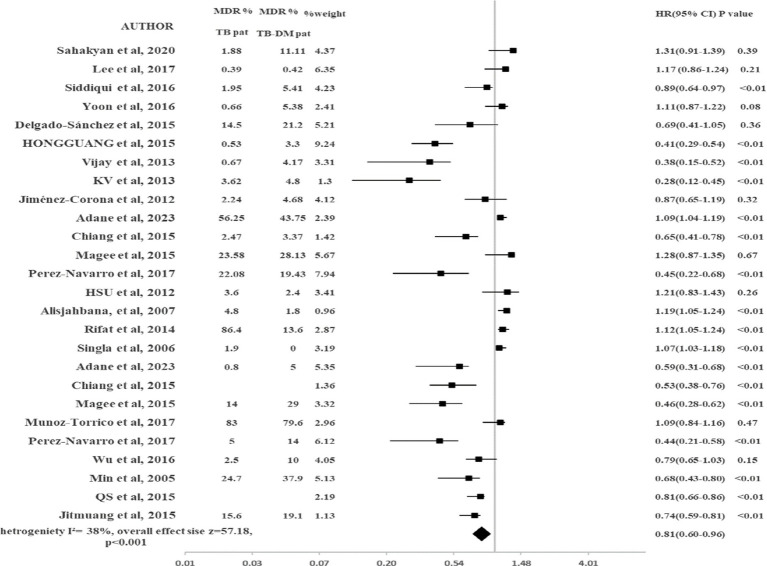
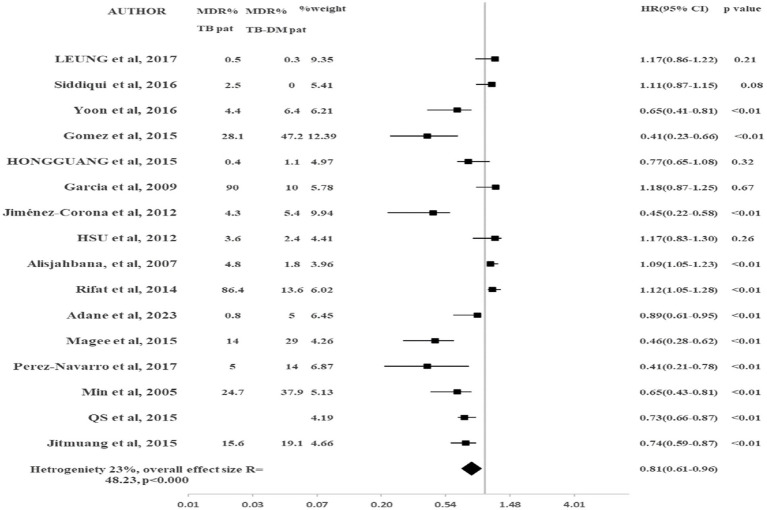
The results of pooled data of MDR-TB for all included studies are shown in Figure 2. This study showed that TB-DM patients were at higher risk to develop MDR-TB as compared to TB-non-DM patients (HR 0.81, 95% CI: 0.60–0.96, p < 0.001). There was 19% reduced risk in TB-non-DM patients for MDR-TB emergence as compared to TB-DM patients. Heterogeneity observed among included studies was moderate (I2 = 38%). To check the impact of the study design on the results, the subgroup analysis was conducted. Only studies that were prospective were included in the sub-group analysis. No significant change was observed in the results (HR 0.81, 95% CI: 0.61–0.96, p < 0.000) as shown in Figure 2. The heterogeneity was reduced (I2 = 23%). The 95% CI was overlapping in both major analysis and subgroup analysis (Figure 3).
Figure 2.
Impact of DM on development of MDR-TB.
Figure 3.
Sub-group analysis by study design.
Treatment failure
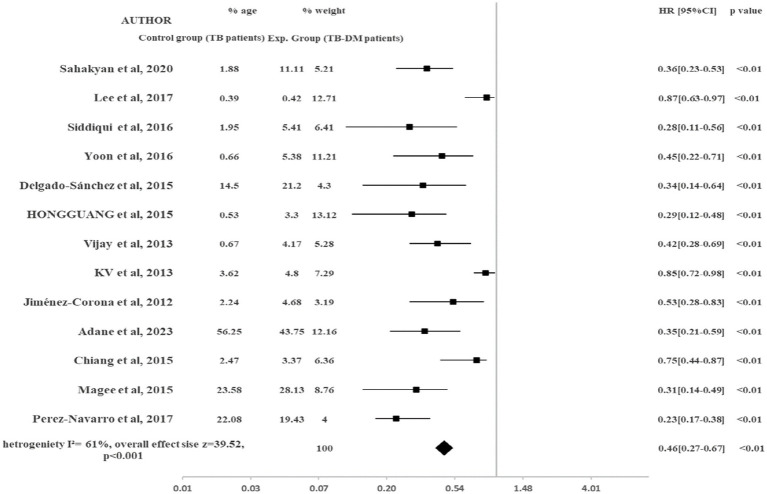
Figure 4 shows the impact of diabetes on treatment failure in TB-Diabetes comorbid patients. TB-DM patients were at higher risk to experience treatment failure rates as compared to TB-non-DM patients (HR 0.46, 95% CI: 0.27–0.67, p < 0.001). TB-non-DM patients were at 46% reduced risk for experiencing treatment failure rates as compared to TB-DM patients. The heterogeneity observed among included studies was (I2 = 61%).
Figure 4.
Impact of DM on TB treatment failure rates.
Risk of bias assessment
The included studies showed NOS score range of 6–9. Out of 30 studies, the quality of 21 studies was high. The criteria for selection domain were fully met by all studies except five studies. The age factor was not adjusted in multivariable analysis in five studies in section of confounding bias. For bias in exposure measurement, the non-response rate was not reported in seven studies by the cases as well as controls. The overall NOS mean score of included studies was seven, indicating low biasness risk and high quality of the included studies. Table 2 provides the risk of bias among included studies.
Table 2.
Risk of bias among included studies.
| Author, Year | Research design | Selection | Comparability | Outcome/Exposure | NOS score | Risk of Bias |
|---|---|---|---|---|---|---|
| Sahakyan et al. (35) | Retrospective | **** | * | * | 6 | Moderate |
| Leung et al. (16) | Prospective | **** | ** | *** | 9 | Low |
| Lee et al. (30) | Retrospective | **** | * | * | 6 | Moderate |
| Mi et al. (17) | Retrospective | **** | ** | *** | 9 | Low |
| Siddiqui et al. (33) | Prospective | *** | ** | * | 6 | Moderate |
| Yoon et al. (29) | Prospective | **** | ** | * | 7 | Low |
| Gomez-Gomez et al. (27) | Case–control | **** | ** | ** | 8 | Low |
| Gil-Santana et al. (36) | Retrospective | **** | ** | * | 7 | Low |
| Delgado-Sánchez et al. (26) | Retrospective | **** | ** | * | 7 | Low |
| Hongguang et al. (18) | Prospective | **** | * | * | 6 | Moderate |
| Viswanathan et al. (32) | Retrospective | *** | * | ** | 6 | Moderate |
| Nandhakumar et al. (31) | Retrospective | **** | ** | *** | 9 | Low |
| Fisher Hoch et al. (25) | Retrospective | **** | ** | ** | 8 | Low |
| Fisher Hoch et al. (25) | Retrospective | **** | ** | ** | 8 | Low |
| Suarez Garcia et al. (37) | Case–control | **** | ** | ** | 8 | Low |
| Jiménez-Corona et al. (24) | Prospective | **** | * | ** | 7 | Low |
| Hsu et al. (19) | Prospective | **** | ** | *** | 9 | Low |
| Alisjahbana, et al. (5) | Prospective | *** | * | ** | 6 | Moderate |
| Rifat et al. (38) | Case–control | **** | ** | ** | 8 | Low |
| Singla et al. (39) | Retrospective | **** | ** | *** | 9 | Low |
| Bashar et al. (34) | Case–control | **** | * | * | 6 | Moderate |
| Adane et al. (40) | Prospective | *** | ** | *** | 8 | Low |
| Chiang et al. (41) | Retrospective | **** | ** | *** | 9 | Low |
| Magee et al. (42) | Prospective | **** | ** | *** | 9 | Low |
| Munoz-Torrico et al. (23) | Retrospective | **** | * | ** | 7 | Low |
| Perez-Navarro et al. (22) | Prospective | *** | ** | * | 6 | Moderate |
| Wu et al. (20) | Retrospective | **** | ** | * | 7 | Low |
| Min et al. (28) | Case–control | **** | ** | * | 7 | Low |
| Song et al. (21) | Case–control | **** | * | ** | 6 | Moderate |
| Jitmuang et al. (43) | Case–control | **** | ** | * | 7 | Low |
Discussion
The TB-DM comorbidity has become more prevalent worldwide and it has become a major challenge for healthcare organizations to control this comorbid condition (44). As it is understood that diabetes in TB patients influences negative TB treatment outcomes including sputum positivity, high risk of relapse, and mortality (17, 39). It is a matter of concern that the development of MDR-TB in TB-DM comorbid patients may further worsen the TB treatment outcomes. It puts an additional financial burden on patients that cause disease progression and ultimately leads to the extensively drug-resistant TB (XDR-TB) (45). This study showed a significant association between DM and MDR-TB.
In this study, there was a lower risk of MDR-TB emergence for TB-non-DM patients when compared with TB-DM comorbid patients (HR 0.81, 95% CI: 0.60–0.96, p < 0.001). Our results were found comparable to the previous studies and meta-analyses that found a significant association between DM and MDR-TB (9). The following factors can be the reason for the emergence of MDR-TB: (1) Due to DM, impaired immune functioning, altered microbial genomics, and uncontrolled glucose levels can enhance the susceptibility to develop MDR-TB in TB patients. (2) DM may also enhance the risk of MDR-TB emergence in TB patients due to DM include phagocytotic activity, chemotactic response, generation of oxidative species, microbe proliferation, altered drug disposition, and non-adherence to treatment (46–50). While results were inconsistent with few previous studies (5, 27, 30–32, 51). The limited number of the patients, geographical variations, and unadjusted covariates may be the reason for these discrepancies in results and should be further addressed.
Our study showed a lower risk of experiencing treatment failure rates in TB-non-DM patients as compared to TB-DM comorbid patients (HR 0.46, 95% CI: 0.27–0.67, p < 0.001). Diabetics are more prone to TB treatment failure rates. The TB treatment failure results in our study were found similar with previous studies (51–53) but discrepancies in results were found in the previous three studies (30, 39, 51). The small sample size and study design used in studies may be the factors causing discrepancies in results. However, most of the studies in our study assessed by the NOS scale were of high quality and there was a lack of lab data for acquiring information on risk factors.
Most of the information was based only on self-reporting or health records of patients, which can lead to assessment error. There should be complete reporting of non-response rates among cases as well as controls to assess reporting-based bias properly. There was moderate heterogeneity across all studies. We performed subgroup analysis by study design, the heterogeneity was reduced. With sub-group analysis by study design for MDR-TB, no change in results was noted when we included prospective studies (HR 0.81, 95% CI: 0.61–0.96, p < 0.000).
There are some limitations present in our study. The studies with different study designs were included that may lead to heterogeneity in results. The heterogeneity in control groups can affect the association to be determined. We assessed overall MDR-TB patients not by primary and secondary subtypes, which may cause differences in risk factors and outcomes. Moreover, there may be the possibility of publication bias, even though the study included a comprehensive set of studies. The statistical techniques used to address the variability in the study design may introduce some uncertainty in the findings. Despite few limitations, the study has several strengths. The main findings of this study given us a valuable understanding of how DM affects emergence of MDR-TB in TB-Diabetes comorbid patients. The strengths of our results lie in comprehensive analysis of a significant number of studies, focusing on a specific patient population. We focused on specific subtypes of DM and TB, providing a more refined understanding of their interaction. The screening for MDR-TB should be prioritized at an earlier stage in TB-DM comorbid patients, which could reduce the economic burden on these patients. There should also be thorough MDR-TB monitoring and necessary steps should be taken to design a proper treatment strategy to prevent the disease progression in these patients. For future concern, this study highlights the importance of conducting more thorough research with large groups of people, using consistent methods, and considering variables that might affect the results. This would help gain a better understanding of how Type 2 DM affects TB-DM comorbid patients.
Conclusion
The results showed that DM had significant impact on emergence of MDR-TB in TB-Diabetes comorbid patients as compared to TB-non-DM patients. DM enhanced the risk of experiencing TB treatment failure rates in TB-Diabetes patients as compared to TB-non-DM patients. Our study highlights the need for earlier screening of MDR-TB, thorough MDR-TB monitoring and designing proper and effective treatment strategies to prevent disease progression.
Author contributions
MK, AR, UM, ML, IA, MR, SS, KH, RH, GA, AA, MA, SAlg, MI, SAlm, SK, and AH made substantial contributions to the conception and design of the study, and analysis and interpretation of the data, and drafted the work or revised it critically for important intellectual content. MK and AR made substantial contributions to the analysis and interpretation of the data. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors are very grateful to the teaching faculty of the Department of Pharmacy Practice, Bahauddin Zakariya University Multan, who gave their valuable feedback on the systematic review and to the library staff for searching and requesting the full-text articles from the authors.
Funding Statement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number: IFP22UQU4290073DSR204.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. (2008) 5:e152. doi: 10.1371/journal.pmed.0050152, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson CR, Critchley JA, Forouhi NG, Roglic G, Williams BG, Dye C, et al. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn. (2007) 3:228–45. doi: 10.1177/1742395307081502, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber-Fiebert JD, Jeon CY, Cohen T, Murray MB. Diabetes mellitus and tuberculosis in countries with high tuberculosis burdens: individual risks and social determinants. Int J Epidemiol. (2011) 40:417–28. doi: 10.1093/ije/dyq238, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balakrishnan S., Vijayan S., Nair S., Subramoniapillai J., Mrithyunjayan S., Wilson N., et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One (2012). 7: e46502. doi: 10.1371/journal.pone.0046502, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff THM, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. (2007) 45:428–35. doi: 10.1086/519841, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. (2009) 9:737–46. doi: 10.1016/S1473-3099(09)70282-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachan RSK, Mistry V, Dholaria M, Rana A, Devgon I, Ali I, et al. Overcoming Mycobacterium tuberculosis drug resistance: novel medications and repositioning strategies. ACS Omega. (2023) 8:32244–57. doi: 10.1021/acsomega.3c02563, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehman Au, Muhammad SA, Tasleem Z, Alsaedi A, Dar M, Iqbal MO, et al. Humanistic and socioeconomic burden of COPD patients and their caregivers in Malaysia. Scientific Reports. (2021) 11:22598. doi: 10.1038/s41598-021-01551-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Li W, Xue M, Chen Y, du X, Wang C, et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: a meta-analysis. Sci Rep. (2017) 7:1090. doi: 10.1038/s41598-017-01213-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanrikulu AC, Hosoglu S, Ozekinci T, Abakay A, Gurkan F. Risk factors for drug resistant tuberculosis in Southeast Turkey. Trop Dr. (2008) 38:91–3. doi: 10.1258/td.2007.070131, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Rasool MF, Rehman AU, Imran I, Abbas S, Shah S, Abbas G, et al. Risk factors associated with medication errors among patients suffering from chronic disorders. Front Public Health. (2020) 8:531038. doi: 10.3389/fpubh.2020.531038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shabrawishi M, AlQarni A, Ghazawi M, Melibari B, Baljoon T, Alwafi H, et al. New disease and old threats: a case series of COVID-19 and tuberculosis coinfection in Saudi Arabia. Clin Case Rep. (2021) 9:e04233. doi: 10.1002/ccr3.4233, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain R, Akram T, Hassali MA, Muneswarao J, Rehman Au, Hashmi F, et al. (2022). Barriers and facilitators to pharmacovigilance activities in Pakistan: A healthcare professionals-based survey. PLoS One. (2022) 17:e0271587. doi: 10.1371/journal.pone.0271587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. (2009) 151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Rehman Au, Hassali MAA, Muhammad SA, Harun SN, Shah S, Abbas S. (2020). The economic burden of chronic obstructive pulmonary disease (COPD) in Europe: results from a systematic review of the literature. Eur J Health Econ. 21:181–194. doi: 10.1007/s10198-019-01119-1 [DOI] [PubMed] [Google Scholar]
- 16.Leung CC, Yew WW, Mok TYW, Lau KS, Wong CF, Chau CH, et al. Effects of diabetes mellitus on the clinical presentation and treatment response in tuberculosis. Respirology. (2017) 22:1225–32. doi: 10.1111/resp.13017, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Mi F, Jiang G, du J, Li L, Yue W, Harries AD, et al. Is resistance to anti-tuberculosis drugs associated with type 2 diabetes mellitus? A register review in Beijing, China. Glob Health Action. (2014) 7:24022. doi: 10.3402/gha.v7.24022, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hongguang C, Min L, Shiwen J, Fanghui G, Shaoping H, Tiejie G, et al. Impact of diabetes on clinical presentation and treatment outcome of pulmonary tuberculosis in Beijing. Epidemiol Infect. (2015) 143:150–6. doi: 10.1017/S095026881400079X, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu A, Lee JJ, Chiang CY, Li YH, Chen LK, Lin CB. Diabetes is associated with drug-resistant tuberculosis in eastern Taiwan. Int J Tuberc Lung Dis. (2013) 17:354–6. doi: 10.5588/ijtld.11.0670, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Guo J, Huang Y, Cai E, Zhang X, Pan Q, et al. Diabetes mellitus in patients with pulmonary tuberculosis in an aging population in Shanghai, China: prevalence, clinical characteristics and outcomes. J Diabetes Complicat. (2016) 30:237–41. doi: 10.1016/j.jdiacomp.2015.11.014, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Song Q. Risk factors for MDR-TB and XDR-TB in Dalian patients. J Dalian Med Univ. (2015) 57:45–8. [Google Scholar]
- 22.Perez-Navarro LM, Restrepo BI, Fuentes-Dominguez FJ, Duggirala R, Morales-Romero J, López-Alvarenga JC, et al. The effect size of type 2 diabetes mellitus on tuberculosis drug resistance and adverse treatment outcomes. Tuberculosis. (2017) 103:83–91. doi: 10.1016/j.tube.2017.01.006, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Muñoz-Torrico M, Caminero-Luna J, Migliori GB, D’Ambrosio L, Carrillo-Alduenda JL, Villareal-Velarde H, et al. Diabetes is associated with severe adverse events in multidrug-resistant tuberculosis. Archiv Bronconeumol. (2017) 53:245–50. doi: 10.1016/j.arbr.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 24.Jiménez-Corona ME, Cruz-Hervert LP, García-García L, Ferreyra-Reyes L, Delgado-Sánchez G, Bobadilla-del-Valle M, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. (2013) 68:214–20. doi: 10.1136/thoraxjnl-2012-201756, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher-Hoch SP, Whitney E, Mccormick JB, Crespo G, Smith B, Rahbar MH, et al. Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis. (2008) 40:888–93. doi: 10.1080/00365540802342372, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado-Sánchez G, García-García L, Castellanos-Joya M, Cruz-Hervert P, Ferreyra-Reyes L, Ferreira-Guerrero E, et al. Association of pulmonary tuberculosis and diabetes in Mexico: analysis of the national tuberculosis registry 2000–2012. PLoS One. (2015) 10:e0129312. doi: 10.1371/journal.pone.0129312, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez-Gómez A, Magaña-Aquino M, López-Meza S, Aranda-Álvarez M, Díaz-Ornelas DE, Hernández-Segura MG, et al. Diabetes and other risk factors for multi-drug resistant tuberculosis in a Mexican population with pulmonary tuberculosis: case control study. Arch Med Res. (2015) 46:142–8. doi: 10.1016/j.arcmed.2015.01.006, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Min J, Park K, Whang S, Kim J. Risk factors for primary multidrug resistant tuberculosis. Tuberc Respir Dis. (2005) 59:600–5. doi: 10.4046/trd.2005.59.6.600 [DOI] [Google Scholar]
- 29.Yoon YS, Jung JW, Jeon EJ, Seo H, Ryu YJ, Yim JJ, et al. The effect of diabetes control status on treatment response in pulmonary tuberculosis: a prospective study. Thorax. (2017) 72:263–70. doi: 10.1136/thoraxjnl-2015-207686, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Lee EH, Lee JM, Kang YA, Leem AY, Kim EY, Jung JY, et al. Prevalence and impact of diabetes mellitus among patients with active pulmonary tuberculosis in South Korea. Lung. (2017) 195:209–15. doi: 10.1007/s00408-017-9978-4, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Nandhakumar KV, Duraisamy K, Balakrishnan S, M S, S JS, Sagili KD, et al. Outcome of tuberculosis treatment in patients with diabetes mellitus treated in the revised national tuberculosis control programme in Malappuram District, Kerala, India. PLoS One. (2013) 8:e76275. doi: 10.1371/journal.pone.0076275, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viswanathan V, Vigneswari A, Selvan K, Satyavani K, Rajeswari R, Kapur A. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis--a report from South India. J Diabetes Complicat. (2014) 28:162–5. doi: 10.1016/j.jdiacomp.2013.12.003, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui AN, Khayyam KU, Sharma M. Effect of diabetes mellitus on tuberculosis treatment outcome and adverse reactions in patients receiving directly observed treatment strategy in India: a prospective study. Biomed Res Int. (2016) 2016:1–11. doi: 10.1155/2016/7273935, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bashar M, Alcabes P, Rom WN, Condos R. Increased incidence of multidrug-resistant tuberculosis in diabetic patients on the Bellevue chest service, 1987 to 1997. Chest. (2001) 120:1514–9. doi: 10.1378/chest.120.5.1514, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Sahakyan S, Petrosyan V, Abrahamyan L. Diabetes mellitus and treatment outcomes of pulmonary tuberculosis: a cohort study. Int J Public Health. (2020) 65:37–43. doi: 10.1007/s00038-019-01277-2 [DOI] [PubMed] [Google Scholar]
- 36.Gil-Santana L, Almeida-Junior JL, Oliveira CAM, Hickson LS, Daltro C, Castro S, et al. Diabetes is associated with worse clinical presentation in tuberculosis patients from Brazil: a retrospective cohort study. PLoS One. (2016) 11:e0146876. doi: 10.1371/journal.pone.0146876, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suárez-García I, Rodríguez-Blanco A, Vidal-Pérez JL, García-Viejo MA, Jaras-Hernández MJ, López O, et al. Risk factors for multidrug-resistant tuberculosis in a tuberculosis unit in Madrid, Spain. Eur J Clin Microbiol Infect Dis. (2009) 28:325–30. doi: 10.1007/s10096-008-0627-y, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Rifat M, Milton AH, Hall J, Oldmeadow C, Islam MA, Husain A, et al. Development of multidrug resistant tuberculosis in Bangladesh: a case-control study on risk factors. PLoS One. (2014) 9:e105214. doi: 10.1371/journal.pone.0105214, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singla R, Khan N, al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. (2006) 10:74–9. PMID: [PubMed] [Google Scholar]
- 40.Adane HT, Howe RC, Wassie L, Magee MJ. Diabetes mellitus is associated with an increased risk of unsuccessful treatment outcomes among drug-susceptible tuberculosis patients in Ethiopia: a prospective health facility-based study. J Clin Tuberc Other Mycobacter Dis. (2023) 31:100368. doi: 10.1016/j.jctube.2023.100368, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang CY, Bai KJ, Lin HH, Chien ST, Lee JJ, Enarson DA, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. (2015) 10:e0121698. doi: 10.1371/journal.pone.0121698, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magee MJ, Kempker RR, Kipiani M, Gandhi NR, Darchia L, Tukvadze N, et al. Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. Int J Tuberc Lung Dis. (2015) 19:685–92. doi: 10.5588/ijtld.14.0811, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jitmuang A, Munjit P, Foongladda S. Prevalence and factors associated with multidrug-resistant tuberculosis at Siriraj hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. (2015) 46:697–706. PMID: [PubMed] [Google Scholar]
- 44.Rehman Au, Shah S, Abbas G, Harun SN, Shakeel S, Hussain R, et al. Assessment of risk factors responsible for rapid deterioration of lung function over a period of one year in patients with chronic obstructive pulmonary disease. Scientific Reports. (2021) 11:13578. doi: 10.1038/s41598-021-92968-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djuretic T, Herbert J, Drobniewski F, Yates M, Smith EG, Magee JG, et al. Antibiotic resistant tuberculosis in the United Kingdom: 1993–1999. Thorax. (2002) 57:477–82. doi: 10.1136/thorax.57.6.477, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RHH, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. (2006) 43:848–54. doi: 10.1086/507543, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Gagneux S, Burgos MV, DeRiemer K, Enciso A, Muñoz S, Hopewell PC, et al. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. (2006) 2:e61. doi: 10.1371/journal.ppat.0020061, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed SM, Ibrahim M, Bayoumi AA. Effect of Ishige Okamurae algae extract on Omentin-1 gene, IL-6 gene, glucose, insulin, and lipid profile in diabetic rats. J Umm Al-Qura Univ Med Sci. (2022) 8:37–43. doi: 10.54940/ms66364380 [DOI] [Google Scholar]
- 49.Shakeel S, Iffat W, Qamar A, Nesar S, Butt F, Siddiqui SN, et al. Assessment of Knowledge, Attitude, and Practice of Obstetricians and Gynecologists Toward Off-Label Medicine Use in Female Reproductive Health Issues. Front. Public Health. (2022) 10:829339. doi: 10.3389/fpubh.2022.829339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.al Daajani MM, Alsahafi AJ, Algarni AM, Moawwad AL, Osman AA, Algaali KYA, et al. Use of smartphone-based video directly observed therapy to increase tuberculosis medication adherence: an interventional study. Galen Med J. (2023) 12:e3067. doi: 10.31661/gmj.v12i.3067 [DOI] [Google Scholar]
- 51.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. (2011) 9:1–15. doi: 10.1186/1741-7015-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang JT, Dou HY, Yen CL, Wu YH, Huang RM, Lin HJ, et al. Effect of type 2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergence of multidrug-resistance. J Formos Med Assoc. (2011) 110:372–81. doi: 10.1016/S0929-6646(11)60055-7, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Kang YA, Kim SY, Jo KW, Kim HJ, Park SK, Kim TH, et al. Impact of diabetes on treatment outcomes and long-term survival in multidrug-resistant tuberculosis. Respiration. (2014) 86:472–8. doi: 10.1159/000348374 [DOI] [PubMed] [Google Scholar]