Abstract
Introduction
Individuals diagnosed with atherosclerotic cardiovascular disease (ACD) are exposed to an increased risk of cardiovascular events. Reducing low-density lipoprotein cholesterol (LDL-C) levels has been established as an effective approach to mitigate these risks. However, a comprehensive and up-to-date meta-analysis investigating the LDL-C-lowering effectiveness and the impact on coronary atherosclerotic plaque compositions of Ezetimibe has been lacking.
Methods
We conducted a systematic review by meticulously analyzing databases such as MEDLINE, EMBASE, and the Cochrane CENTRAL for randomized controlled trials that evaluated the efficacy of ezetimibe in lowering LDL-C levels and its influence on coronary atherosclerotic plaques among individuals with ACD. This review encompassed studies available until August 1, 2023. In our analysis, we employed the weighted mean difference (WMD) as the aggregated statistical measure, accompanied by the corresponding 95% confidence interval (CI).
Results
We encompassed a total of 20 eligible studies. Our findings unveiled that the combined therapy involving ezetimibe alongside statins led to a more substantial absolute decrease in LDL-C in comparison to using statins alone. This difference in means amounted to (−14.06 mg/dl; 95% CI −18.0 to −10.0; p = 0.0001). Furthermore, upon conducting subgroup analyses, it became evident that the intervention strategies proved effective in diminishing the volume of dense calcification (DC) in contrast to the control group.
Conclusions
Our study findings indicate that the inclusion of ezetimibe in conjunction with statin therapy leads to a modest yet meaningful additional reduction in LDL-C levels when compared to using statins in isolation. Importantly, the introduction of ezetimibe resulted in a significant decrease in the volume of DC. However, it is worth noting that further investigation is warranted to delve deeper into this phenomenon.
Keywords: low-density lipoprotein cholesterol, ezetimibe, systematic review, cardiovascular disease, meta-analysis
Introduction
Atherosclerotic cardiovascular disease (ACD) stands as the foremost contributor to global mortality. Elevated levels of low-density lipoprotein cholesterol (LDL-C) predominantly underlie the development of ACD (1, 2). To prevent future cardiovascular events, it is highly recommended to focus on the development of novel drugs that target and effectively lower lipoprotein levels. The use of tolerated statins is recommended for reducing LDL-C in patients with ACD. However, it is important to note that even with statin therapy, additional lipid-lowering therapies may be necessary for many patients with clinical ACD. Ezetimibe, a new cholesterol absorption inhibitor drug, has demonstrated the ability to further lower LDL-C levels (3–5). In a randomized controlled trial, it was demonstrated that the combination of ezetimibe with statins significantly reduces levels of LDL-C (6). Additionally, the combination of ezetimibe and statins leads to a substantial decrease in coronary plaque volume compared to statin treatment alone (7). Although the effectiveness of ezetimibe in treating ACD has been acknowledged, there is an absence of a comprehensive and up-to-date meta-analysis regarding the efficacy of ezetimibe in LDL-C lowering in ACD patients. Therefore, this study aimed to systematically assess the efficacy of ezetimibe in reducing LDL-C levels among individuals diagnosed with ACD.
Methods
Search strategy
We performed an extensive exploration of PubMed/MEDLINE, EMBASE, and the Cochrane CENTRAL databases to identify studies available until August 1, 2023, that reported on the efficacy and effectiveness of ezetimibe in patients with ACD. The search terms used were ezetimibe, coronary artery, atherosclerosis, and randomized controlled trial. Only studies published in English were included. This study adhered to the PRISMA statement for its design and reporting (Prospero pending ID: 449721) (8).
Study selection
The collected records from the database searches were merged, and duplicates were removed through the utilization of EndNote X7 (Thomson Reuters, Toronto, ON, Canada). Two reviewers, MR and MJN, conducted a thorough assessment of the records individually, utilizing the title/abstract and full-text screening process to exclude any studies that did not align with the study's objectives.
The studies included in the analysis met the following criteria:
Participants: The studies encompassed individuals diagnosed with ACD.
Intervention: The intervention being investigated was the use of ezetimibe therapy, either as a standalone treatment or in combination with other lipid-lowering therapies.
Comparison: patients who received standard treatment or in combination with other lipid-lowering therapies.
Outcome: The primary outcome was the average change observed in LDL-C levels when compared to the baseline measurements.
Data extraction
Two reviewers, namely MR and MJN, collaboratively devised a structured data extraction form and proceeded to extract information from all qualifying studies. Discrepancies were addressed through mutual agreement. The extracted data encompassed several aspects, including the primary author's name, publication year, study duration, study type, geographical location(s) of the study, ACD patient count, patient age, treatment regimens, demographic details (comprising age, gender, and nationality), and treatment outcomes.
Quality assessment
The quality assessment of the included studies was conducted by two reviewers (MR and PM) using the Cochrane tool (9). In case of any discrepancies, a third reviewer (MJN) was involved. This assessment tool encompasses several domains, encompassing random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, completeness of outcome data, as well as additional factors like selective reporting and potential biases. Each study underwent categorization based on bias risk: low risk of bias when no bias concerns were detected, high risk of bias when bias concerns were present, or unclear risk of bias when there was an insufficient amount of information available for evaluation.
Data analysis
The statistical analysis was performed using Comprehensive Meta-Analysis software, version 3.0 (Biostat Inc., Englewood, NJ, USA). The weighted mean difference (WMD) was used as the pooled statistic, with a corresponding 95% confidence interval (CI). The degree of heterogeneity among the studies was assessed using the I2 value and p-value. In cases where the statistical heterogeneity between the studies was low (I2 ≤ 50% or p ≥ 0.1), the fixed-effect model was utilized. Conversely, if a significant level of inter-study heterogeneity was observed (I2 > 50% or p < 0.1), the random-effects model was employed. Cochran's Q test and the I2 statistic were used to assess between-study heterogeneity. Funnel plots, Egger's and Begg's tests were performed to access the publication bias of studies.
Results
Figure 1 illustrates the flow diagram of the systematic review process. This thorough review yielded a total of 20 records that met the specified eligibility criteria. These records reported the primary outcome, which included variations in LDL-C levels from baseline or the LDL-C level after the study, or both. Importantly, these records contained sufficient independent information that enabled the subsequent meta-analysis.
Figure 1.
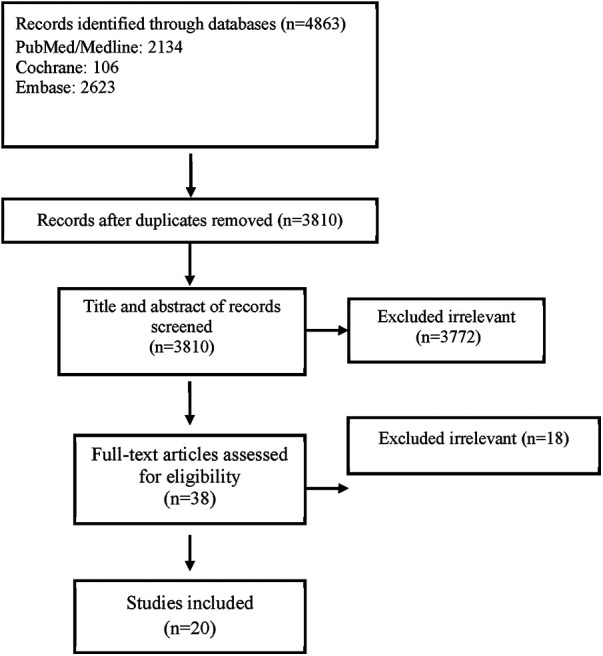
Flow chart of study selection for inclusion in the systematic review and meta-analysis.
The rationale for including or excluding records is succinctly summarized, and the details of the 20 studies incorporated into the meta-analysis are presented in Table 1. This table provides comprehensive insights into various aspects, such as study design, duration, and baseline demographic characteristics.
Table 1.
Demographics and baseline characteristics of patients included in the meta-analysis according to study and treatment group.
| Authors | Study design | Sample size T/C | Age T | Intervention T | Control C | Duration | % of male |
|---|---|---|---|---|---|---|---|
| Hougaard (10) | RCT | 43/44 | 55.3 ± 11.0 | EZ (10) + AT (80) | PL (10) + AT (80) | 12 months | T (90.7) C (81.8) |
| Jung (11) | RCT | 34/36 | 60.9 ± 10.9 | EZ (10) + SI (40) | PR (20) | 03 months | T (79.4) C (75) |
| Kovarnik (12) | RCT | 42/47 | 63.5 ± 9.3 | EZ (10) + AT (80) | AT (10) | 12 months | T (78.6) C (66) |
| Brohet et al. (13) | RCT | 208/210 | 63.6 ± 11.1 | EZE 10 mg QD + SIM 10/20 mg QD | SIM 10/20 mg QD | 6 weeks | T (69.7) C (75.2) |
| Cannon et al. (14) | RCT | 9,067/9,077 | 63.6 ± 9.7 | EZE 10 mg QD + SIM 40–80 mg QD | IM 40–80 mg QD | 6 years | T (75.5) C (75.9) |
| Hibi et al. (15) | RCT | 50/53 | 63 ± 10.0 | EZE 10 mg QD + PITA 2 mg QD | PITA 2 mg QD | 10 months | T (82) C (77) |
| Joshi et al. (16) | RCT | 40/40 | 60.3 ± 9.8 | EZE 10 mg QD + ROSU 10 mg QD | ROSU 10 mg QD | 24 weeks | T (55) C (62.5) |
| Masuda et al. (17) | RCT | 21/19 | 64.0 ± 7.9 | EZE 10 mg QD + ROSU 5 mg QD | ROSU 5 mg QD | 6 months | T (90.5) C (84.2) |
| Ran et al. (18) | RCT | 42/42 | 60.4 ± 8.2 | EZE 10 mg QD + ROSU 10 mg QD | ROSU 10 mg QD | 10 weeks | T (76.2) C (73.8) |
| Ren et al. (19) | RCT | 55/58 | 57.3 ± 1.5 | EZE 10 mg QD + ROSU 10 mg QD | ROSU 10 mg QD | 12 months | T (87.3) C (79.3) |
| Ueda et al. (20) | RCT | 54/54 | 71 ± 8.0 | EZE 10 mg QD + ATOR 10–20 mg QD | ATOR 10–20 mg QD | 9 months | T (76) C (81) |
| Wang et al. (21) | RCT | 51/49 | 58 ± 10.0 | EZE 10 mg QD + ATOR 20 mg QD | ATOR 20 mg QD | 12 months | T (60) C (61) |
| Wang et al. (22) | RCT | 50/48 | 63 ± 10.0 | EZE 10 mg QD + ROSU 10 mg QD | ROSU 10 mg QD | 12 months | T (72) C (73) |
| West et al. (23) | RCT | 18/33 | 62 ± 8.0 | EZE 10 mg QD + SIM 40 mg QD | EZE 10 mg QD | 2 years | T (56) C (48) |
| Zou (24) | RCT | 40/40 | 69.3 ± 5.8 | EZE 10 mg QD + ATOR 10 mg QD | ATOR 10 mg QD | 12 months | T ( |
| El-Tamalawy et al. (25) | RCT | 33/32 | 61 ± 7.1 | EZE 10 mg/day + ATOR 40 mg daily | ATOR 80 mg daily | 3 months | T (55) C (70) |
| Klassen et al. (26) | RCT | 10/10 | 62 (59–64) | EZE 10 mg QD + ROSU 20 mg QD or SIM 40 mg | ROSU 20 mg QD | 1 month | T (80) C (70) |
| Oh et al. (27) | RCT | 18/19 | 56.3 ± 7.1 | ATO10 mg + EZE10 mg | ATO 40 mg | 12 months | T (72) C (79) |
| Pinto et al. (28) | RCT | 50/51 | 59 (52–65) | EZE 10mg + SIM 40 mg | ROSU 20 mg | 1 month | T (76) C (72) |
| Blom et al. (29) | RCT | 63/73 | 55.9 ± 9.0 | EZE 10 mg + ATOR 80 mg | ATOR 80 mg | 1–2 months | T (52.4) C (45.2) |
T, treatment group; C, control group; EZ, ezetimibe; ATOR, atorvastatin; SIM, simvastatin.
It is worth noting that while the intended focus was to assess the impact of ezetimibe therapy on LDL-C reduction, either alone or in combination with other lipid-lowering therapies, all the studies included in the analysis contrasted the combined treatment of ezetimibe and statin with statin monotherapy. Participant age across these studies ranged from 57 to 71 years on average, with variations depending on the specific treatment group (Table 1).
Quality assessment
Regarding the risk of bias across the 20 trials, insufficient detail was provided about the randomization methodology, resulting in an unclear risk of selection bias for random sequence generation and allocation concealment. Other biases were assessed as low in the trials (Table 2).
Table 2.
Quality assessment.
| Author | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data |
|---|---|---|---|---|---|
| Brohet et al. (13) | L | U | L | U | L |
| Cannon et al. (14) | U | U | U | U | L |
| Hibi et al. (15) | L | U | H | L | L |
| Joshi et al. (16) | L | U | U | U | L |
| Masuda et al. (17) | L | L | H | H | L |
| Ran et al. (18) | L | U | H | U | L |
| Ren et al. (19) | L | U | U | U | L |
| Ueda et al. (20) | L | U | H | L | L |
| Wang et al. (21) | U | U | U | U | L |
| Wang et al. (22) | L | U | U | U | L |
| West et al. (23) | L | U | L | L | L |
| Zou (24) | U | U | U | U | L |
| El-Tamalawy et al. (25) | L | U | L | L | L |
| Klassen et al. (26) | L | U | L | L | L |
| Oh et al. (27) | L | U | L | L | L |
| Pinto et al. (28) | L | U | L | L | L |
| Blom et al. (29) | L | U | L | L | L |
| Mikkle et al. (10) | L | U | L | L | L |
| Jung et al. (11) | L | U | L | L | L |
| Kovarnik et al. (12) | L | H | L | L | L |
L, low; H, high; U, unclear.
Efficacy of LDL-C lowering of ezetimibe
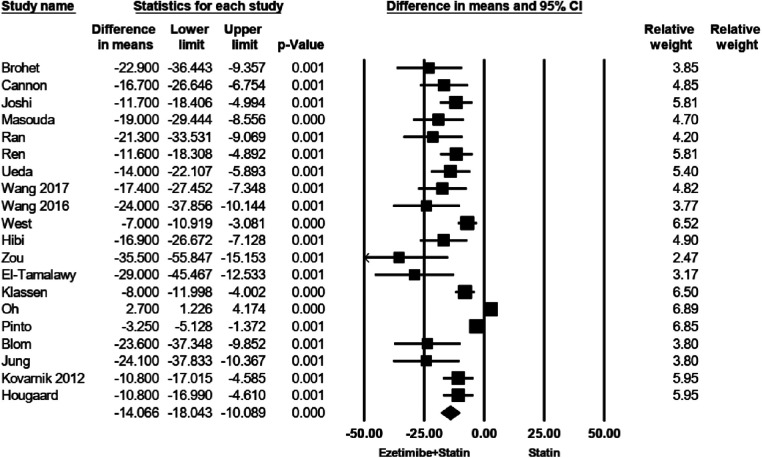
As indicated in Figure 2, patients receiving a combination of statin and ezetimibe were found to have a significant additional reduction in LDL-C (WMD = −14.06 mg/dl; 95% CI −18.0 to −10.0, p < 0.0001) than those receiving statin alone. As shown in Supplementary Figure S1, some evidence for publication bias was observed (P = 0.02 for Begg rank correlation analysis; P = 0.03 for Egger weighted regression analysis).
Figure 2.
Treatment difference in mean LDL-C change (mg/dl).
Fibro-fatty plaque (FFP) volume
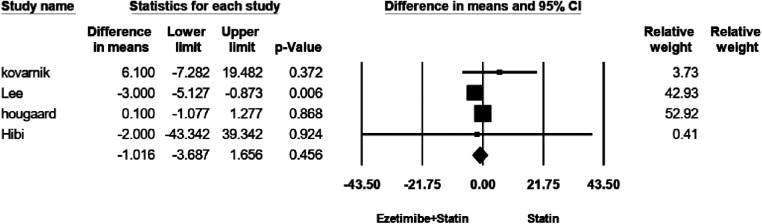
The efficacy of FFP was evaluated in four studies. No significant heterogeneity was observed among the studies (I2 = 58.4%, p = 0.065). Using a random-effects model for analysis, our findings revealed that the treatment interventions within the study group did not result in a significant reduction in FFP volume when compared to the control group [WMD = −1.01, 95% CI −3.6–1.6, p = 0.45], as illustrated in Figure 3. As shown in Supplementary Figure S2, no evidence for publication bias was observed (P = 0.2 for Begg rank correlation analysis; P = 0.4 for Egger weighted regression analysis).
Figure 3.
The forest plot for fibro-fatty plaque (FFP) volume.
Necrotic core (Nc) volume
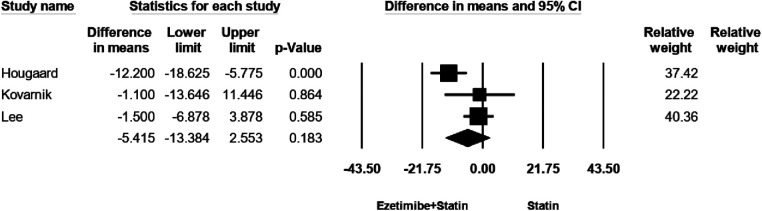
The effectiveness of NC was documented in three studies. Notably, there was no significant heterogeneity observed among these studies (I2 = 70.4%, p = 0.03). Employing a random-effects model analysis, the outcomes revealed no substantial disparity in the reduction of NC between the treatment group and the control group. The WMD equated to −5.41, with a 95% CI spanning from −13.3 to 2.5, yielding a p-value of 0.18, as depicted in Figure 4. As shown in Supplementary Figure S3, no evidence for publication bias was observed (P = 0.1 for Begg rank correlation analysis; P = 0.5 for Egger weighted regression analysis).
Figure 4.
The forest plot for necrotic core (NC) volume.
Change dense calcification (Dc) volume
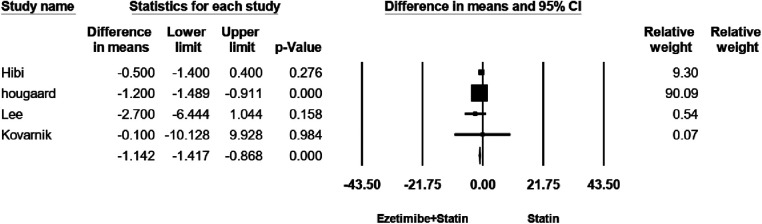
The efficacy of change DC was assessed in four studies. There was no notable heterogeneity observed among these studies (I2 = 0%, p = 0.42). Employing a fixed-effects model analysis, the findings demonstrated a significant distinction in the reduction of change DC between the treatment group and the control group. The WMD amounted to −1.14, accompanied by a 95% CI ranging from −1.4 to −0.8, resulting in a p-value of 0.00, as presented in Figure 5. As shown in Supplementary Figure S4, no evidence for publication bias was observed (P = 0.3 for Begg rank correlation analysis; P = 0.4 for Egger weighted regression analysis).
Figure 5.
The forest plot for dense calcification (DC) volume.
Discussion
Principal findings
This meta-analysis has showcased that the inclusion of ezetimibe alongside statin therapy yields an extra reduction in LDL-C levels when dealing with patients diagnosed with ACD, in contrast to using statin therapy alone.
Comparisons with other studies
Our findings align with existing evidence concerning lipid-lowering treatments. For instance, a previous meta-analysis conducted by Shaya et al. also indicated a more significant relative reduction in LDL-C levels with the incorporation of ezetimibe alongside statin therapy, as opposed to using statins alone (30). Another meta-analysis demonstrated that ezetimibe significantly decreased coronary atherosclerotic plaque compared to the control group (placebo or statin monotherapy) (31). Similarly, the research conducted by Toyota et al. corroborates the notion that all three approaches aimed at enhancing LDL-C reduction, namely intensifying statin therapy, incorporating ezetimibe, and introducing PCSK9 inhibitors, have the potential to enhance clinical outcomes among individuals with high atherosclerotic cardiovascular disease (32). Strilchuk et al. also highlights the effectiveness of combining rosuvastatin and ezetimibe for treating hypercholesterolemia and mixed dyslipidemia (33).
Strengths and limitations of this study
The outcome of our study is in line with the 2020 meta-analysis by Shaya et al., which demonstrated a reduced risk of ACD in patients who underwent ezetimibe treatment (30). Nonetheless, our study boasts certain strengths and distinctive aspects. We delved into studies specifically addressing FFP volume, NC volume, and DC volume. To mitigate the potential influence of confounding factors, we excluded studies involving populations with particular comorbidities. Moreover, articles lacking full text were omitted. On the contrary, we incorporated eight additional studies that were not part of the previous analysis. Our calculated WMD of (−14.06, 95% CI 18.0–10.0) was slightly higher than that of Shaya et al. (−21.8, 95% CI −26.5 to −17.1), while maintaining alignment in terms of direction and statistical significance.
The primary limitation of our study lies in its lack of originality, as the data regarding the efficacy of ezetimibe on LDL-C has been well-established and recognized for a considerable period, even documented in international guidelines. Nevertheless, revisiting and updating the scientific evidence can still hold significance. Several other limitations pertain to the studies we incorporated and are detailed as follows:
Firstly, the absence of recent clinical trial studies is notable, with the most recent one dating back to 2021. This underscores the necessity for further investigations with substantial participant populations to provide deeper insights into the subject.
Secondly, the included studies exhibited confounding factors, and due to limited and inconsistent data, we were unable to perform subgroup analyses to address these confounders adequately.
Thirdly, investigating the mortality benefits of ezetimibe, despite its robust LDL-C lowering effects, presents a notable challenge. Unraveling potential survival advantages hinges on various factors, encompassing drug efficacy, competing risks, off-target effects, baseline cardiovascular risk, and the duration of follow-up in the studies. The fourth limitation of our study is the significant variability in the duration of the included studies, potentially leading to uncertainty in the conclusions we have drawn.
Clinical implications
This comprehensive systematic review delivers essential insights to decision-makers regarding the advantageous impact of ezetimibe on significant cardiovascular outcomes. Notably, the prominent observations highlight the prevalence of moderate to high certainty evidence that supports the effectiveness of ezetimibe in reducing LDL-C levels. Additionally, these agents contribute to the reduction in the volume of DC.
Conclusions
Our study indicates that the addition of ezetimibe to statin therapy results in a modest yet significant further reduction in LDL-C compared to statin monotherapy. Ezetimibe led to a significant reduction in DC volume; however, there were no statistically significant differences observed for NC, or change FFP volumes.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Author contributions
FO: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft. MR: Investigation, Methodology, Software, Writing – original draft. AS: Methodology, Validation, Resources, Writing review & editing. PM: Conceptualization, Investigation, Writing – original draft. TS: Conceptualization, Supervision, Validation, Writing – original draft. MN: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1269172/full#supplementary-material
References
- 1.Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein (a) in atherosclerotic cardiovascular disease and aortic stenosis: a European atherosclerosis society consensus statement. Eur Heart J. (2022) 43(39):3925–46. 10.1093/eurheartj/ehac361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. Lancet. (2020) 396(10263):1644–52. 10.1016/S0140-6736(20)32233-9 [DOI] [PubMed] [Google Scholar]
- 3.Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (ewtopia 75) a randomized, controlled trial. Circulation. (2019) 140(12):992–1003. 10.1161/CIRCULATIONAHA.118.039415 [DOI] [PubMed] [Google Scholar]
- 4.Phan BAP, Dayspring TD, Toth PP. Ezetimibe therapy: mechanism of action and clinical update. Vasc Health Risk Manag. (2012) 8:415–27. 10.2147/VHRM.S33664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vavlukis M, Vavlukis A. Adding ezetimibe to statin therapy: latest evidence and clinical implications. Drugs Context. (2018) 7:1–9. 10.7573/dic.212534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, et al. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT trial. J Am Coll Cardiol. (2016) 67(4):353–61. 10.1016/j.jacc.2015.10.077 [DOI] [PubMed] [Google Scholar]
- 7.Lewek J, Niedziela J, Desperak P, Dyrbuś K, Osadnik T, Jankowski P, et al. Intensive statin therapy versus upfront combination therapy of statin and ezetimibe in patients with acute coronary syndrome: a propensity score matching analysis based on the PLACS data. J Am Heart Assoc. (2023) 12(18):e030414. 10.1161/JAHA.123.030414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151(4):264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:1–9. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hougaard M, Hansen HS, Thayssen P, Antonsen L, Junker A, Veien K, et al. Influence of ezetimibe in addition to high-dose atorvastatin therapy on plaque composition in patients with ST-segment elevation myocardial infarction assessed by serial: intravascular ultrasound with iMap: the OCTIVUS trial. Cardiovasc Revasc Med. (2017) 18(2):110–7. 10.1016/j.carrev.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Shin DH, Kim BK, Ko YG, Choi D, Jang Y, et al. Early effects of intensive lipid-lowering treatment on plaque characteristics assessed by virtual histology intravascular ultrasound. Yonsei Med J. (2016) 57(5):1087–94. 10.3349/ymj.2016.57.5.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J, Skulec R, et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: hEAVEN study. Circ J. (2012) 76(1):176–83. 10.1253/circj.CJ-11-0730 [DOI] [PubMed] [Google Scholar]
- 13.Brohet C, Banai S, Alings AM, Massaad R, Davies MJ, Allen C. LDL-C goal attainment with the addition of ezetimibe to ongoing simvastatin treatment in coronary heart disease patients with hypercholesterolemia. Curr Med Res Opin. (2005) 21(4):571–8. 10.1185/030079905X382004 [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. (2015) 372(25):2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 15.Hibi K, Sonoda S, Kawasaki M, Otsuji Y, Murohara T, Ishii H, et al. Effects of ezetimibe-statin combination therapy on coronary atherosclerosis in acute coronary syndrome. Circ J. (2018) 82(3):757–66. 10.1253/circj.CJ-17-0598 [DOI] [PubMed] [Google Scholar]
- 16.Joshi S, Sharma R, Rao HK, Narang U, Gupta N. Efficacy of combination therapy of rosuvastatin and ezetimibe vs. rosuvastatin monotherapy on lipid profile of patients with coronary artery disease. Journal of Clinical & Diagnostic Research. (2017) 11(12):1–12. 10.7860/JCDR/2017/30458.11004 [DOI] [Google Scholar]
- 17.Masuda J, Tanigawa T, Yamada T, Nishimura Y, Sasou T, Nakata T, et al. Effect of combination therapy of ezetimibe and rosuvastatin on regression of coronary atherosclerosis in patients with coronary artery disease. Int Heart J. (2015) 56(3):278–85. 10.1536/ihj.14-311 [DOI] [PubMed] [Google Scholar]
- 18.Ran D, Nie HJ, Gao YL, Deng SB, Du JL, Liu YJ, et al. A randomized, controlled comparison of different intensive lipid-lowering therapies in Chinese patients with non-ST-elevation acute coronary syndrome (NSTE-ACS): ezetimibe and rosuvastatin versus high-dose rosuvastatin. Int J Cardiol. (2017) 235:49–55. 10.1016/j.ijcard.2017.02.099 [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, Zhu H, Fan Z, Gao Y, Tian N. Comparison of the effect of rosuvastatin versus rosuvastatin/ezetimibe on markers of inflammation in patients with acute myocardial infarction. Exp Ther Med. (2017) 14(5):4942–50. 10.3892/etm.2017.5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda Y, Hiro T, Hirayama A, Komatsu S, Matsuoka H, Takayama T, et al. Effect of ezetimibe on stabilization and regression of intracoronary plaque- the ZIPANGU study. Circ J. (2017) 81(11):1611–9. 10.1253/circj.CJ-17-0193 [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Ai XB, Wang F, Zou YW, Li L, Yi XL. Efficacy of ezetimibe combined with atorvastatin in the treatment of carotid artery plaque in patients with type 2 diabetes mellitus complicated with coronary heart disease. Int Angiol. (2017) 36(5):467–73. 10.23736/S0392-9590.17.03818-4 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Zhao X, Li L, Yao H, Jiang Y, Zhang J. Effects of combination of ezetimibe and rosuvastatin on coronary artery plaque in patients with coronary heart disease. Heart Lung Circ. (2016) 25(5):459–65. 10.1016/j.hlc.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 23.West AM, Anderson JD, Meyer CH, Epstein FH, Wang H, Hagspiel KD, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atherosclerosis. (2011) 218(1):156–62. 10.1016/j.atherosclerosis.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou Y. Effect of ezetimibe combined with low-dose atorvastain calcium on carotid atherosclerosis in elderly patients with coronary heart disease. J Am Geriatr Soc. (2016) 64:S328–S328. [Google Scholar]
- 25.El-Tamalawy MM, Ibrahim OM, Hassan TM, El-Barbari AA. Effect of combination therapy of ezetimibe and atorvastatin on remnant lipoprotein versus double atorvastatin dose in Egyptian diabetic patients. J Clin Pharmacol. (2018) 58(1):34–41. 10.1002/jcph.976 [DOI] [PubMed] [Google Scholar]
- 26.Klassen A, Faccio AT, Picossi CR, Derogis PB, dos Santos, Ferreira CE, Lopes AS, et al. Evaluation of two highly effective lipid-lowering therapies in subjects with acute myocardial infarction. Sci Rep. (2021) 11(1):15973. 10.1038/s41598-021-95455-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh PC, Jang AY, Ha K, Kim M, Moon J, Suh SY, et al. Effect of atorvastatin (10 mg) and ezetimibe (10 mg) combination compared to atorvastatin (40 mg) alone on coronary atherosclerosis. Am J Cardiol. (2021) 154:22–8. 10.1016/j.amjcard.2021.05.039 [DOI] [PubMed] [Google Scholar]
- 28.Pinto LC, Mello AP, Izar MC, Damasceno NR, Neto AM, França CN, et al. Main differences between two highly effective lipid-lowering therapies in subclasses of lipoproteins in patients with acute myocardial infarction. Lipids Health Dis. (2021) 20(1):124. 10.1186/s12944-021-01559-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. (2014) 370(19):1809–19. 10.1056/NEJMoa1316222 [DOI] [PubMed] [Google Scholar]
- 30.Shaya FT, Sing K, Milam R, Husain F, Del Aguila MA, Patel MY. Lipid-lowering efficacy of ezetimibe in patients with atherosclerotic cardiovascular disease: a systematic review and meta-analyses. Am J Cardiovasc Drugs. (2020) 20:239–48. 10.1007/s40256-019-00379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai B, Shen Y, Li Y, Wang X. Meta-analysis and trial sequential analysis of ezetimibe for coronary atherosclerotic plaque compositions. Front Pharmacol. (2023) 14:1166762. 10.3389/fphar.2023.1166762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyota T, Morimoto T, Yamashita Y, Shiomi H, Kato T, Makiyama T, et al. More- versus less-intensive lipid-lowering therapy. Circ Cardiovasc Qual Outcomes. (2019) 12(8):e005460. 10.1161/CIRCOUTCOMES.118.005460 [DOI] [PubMed] [Google Scholar]
- 33.Strilchuk L, Tocci G, Fogacci F, Cicero AF. An overview of rosuvastatin/ezetimibe association for the treatment of hypercholesterolemia and mixed dyslipidemia. Expert Opin Pharmacother. (2020) 21(5):531–9. 10.1080/14656566.2020.1714028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.