Abstract
BACKGROUND
Impaired glucose tolerance (IGT) is a homeostatic state between euglycemia and hyperglycemia and is considered an early high-risk state of diabetes. When IGT occurs, insulin sensitivity decreases, causing a reduction in insulin secretion and an increase in glucagon secretion. Recently, vascular endothelial growth factor B (VEGFB) has been demonstrated to play a positive role in improving glucose metabolism and insulin sensitivity. Therefore, we constructed a mouse model of IGT through high-fat diet feeding and speculated that VEGFB can regulate hyperglycemia in IGT by influencing insulin-mediated glucagon secretion, thus contributing to the prevention and cure of prediabetes.
AIM
To explore the potential molecular mechanism and regulatory effects of VEGFB on insulin-mediated glucagon in mice with IGT.
METHODS
We conducted in vivo experiments through systematic VEGFB knockout and pancreatic-specific VEGFB overexpression. Insulin and glucagon secretions were detected via enzyme-linked immunosorbent assay, and the protein expression of phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) was determined using western blot. Further, mRNA expression of forkhead box protein O1, phosphoenolpyruvate carboxykinase, and glucose-6 phosphatase was detected via quantitative polymerase chain reaction, and the correlation between the expression of proteins was analyzed via bioinformatics.
RESULTS
In mice with IGT and VEGFB knockout, glucagon secretion increased, and the protein expression of PI3K/AKT decreased dramatically. Further, in mice with VEGFB overexpression, glucagon levels declined, with the activation of the PI3K/AKT signaling pathway.
CONCLUSION
VEGFB/vascular endothelial growth factor receptor 1 can promote insulin-mediated glucagon secretion by activating the PI3K/AKT signaling pathway to regulate glucose metabolism disorders in mice with IGT.
Keywords: Vascular endothelial growth factor B, Insulin-mediated, Glucagon secretion, Prediabetes, Impaired glucose tolerance
Core Tip: Impaired glucose tolerance (IGT) is an abnormal metabolic stage between the normal state and diabetes, which belongs to prediabetes. Therefore, intervention in IGT can effectively reduce the incidence rate of diabetes. The pathological mechanism of IGT is related to glucose homeostasis imbalance and decreased insulin sensitivity. Currently, vascular endothelial growth factor B (VEGFB) has been reported to have the effect of restoring glucose tolerance and improving insulin sensitivity. Therefore, the use of VEGFB as a target for intervention has become the focus of current research. This research mainly illustrates the role of VEGFB in promoting insulin and glucagon secretion to alleviate IGT and its potential molecular mechanism.
INTRODUCTION
Impaired glucose tolerance (IGT) is a homeostatic state between euglycemia and hyperglycemia, indicating a fasting blood glucose (FBG) level of < 7.0 mmol/L and/or postprandial blood glucose (PBG) level of 7.8-11.1 mmol/L after 2 h of oral glucose administration (75 g)[1]. IGT is considered a high-risk state in the early stage of type 2 diabetes (T2DM)[2]. Although only slight abnormal glucose metabolism is observed in this stage, the secretion function of β cells has been reported to be defective, leading to insulin resistance[3]. It is estimated that approximately 70% of IGT cases progress to T2DM[4]. When IGT occurs, insulin sensitivity decreases, causing reduced insulin secretion; decreased insulin levels can increase glucagon secretion[5]. Glucose metabolism disorders occur due to the abnormal production and release of insulin or glucagon[6].
Owing to the high reversibility of IGT, interventions to improve insulin sensitivity in patients with IGT can effectively prevent or delay the progression of IGT to T2DM, thereby reducing the incidence rate of diabetes and its complications[7]. Recently, vascular endothelial growth factor B (VEGFB) has been demonstrated to play a regulatory role in improving glucose metabolism and insulin sensitivity[8]. Animal experiments have confirmed that VEGFB can increase insulin supply and insulin sensitivity in high-fat diet (HFD)-fed mice[9]. Specific VEGFB overexpression in mice can affect insulin secretion and improve diabetes[10]. Notably, we previously reported a decline in insulin secretion in VEGFB knockout mice[11].
Various signal pathways participate in several biological activities such as glucose homeostasis, including the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signal pathway[12]. Activation of the PI3K/AKT pathway regulates insulin secretion in β cells[13]. All IGT types are accompanied with insulin resistance[14]. Alleviation of insulin resistance can promote insulin secretion and inhibit glucagon release, thereby improving IGT[15]. The combination of VEGFB and VEGF receptor 1 (VEGFR1) can activate downstream pathways, including the PI3K/AKT pathway related to the proliferation, differentiation, and metabolism of cells[16]. However, it remains unclear whether VEGFB regulates glucagon secretion and improves IGT via the PI3K/AKT pathway.
In this study, we established an HFD-induced mouse model of IGT and constructed VEGFB knockout and pancreatic-specific VEGFB overexpressed mice to determine the role of VEGFB in mice with IGT. This study aimed to explore the potential mechanism and regulatory effects of VEGFB on insulin-mediated glucagon secretion in mice with IGT. We observed an increase in glucagon secretion and blood glucose levels in mice with IGT and VEGFB knockout; the reduced glucagon level in mice was due to the activated PI3K/AKT pathway. Therefore, we hypothesized that VEGFB can regulate insulin-medicated glucagon secretion in IGT.
MATERIALS AND METHODS
Experimental animals
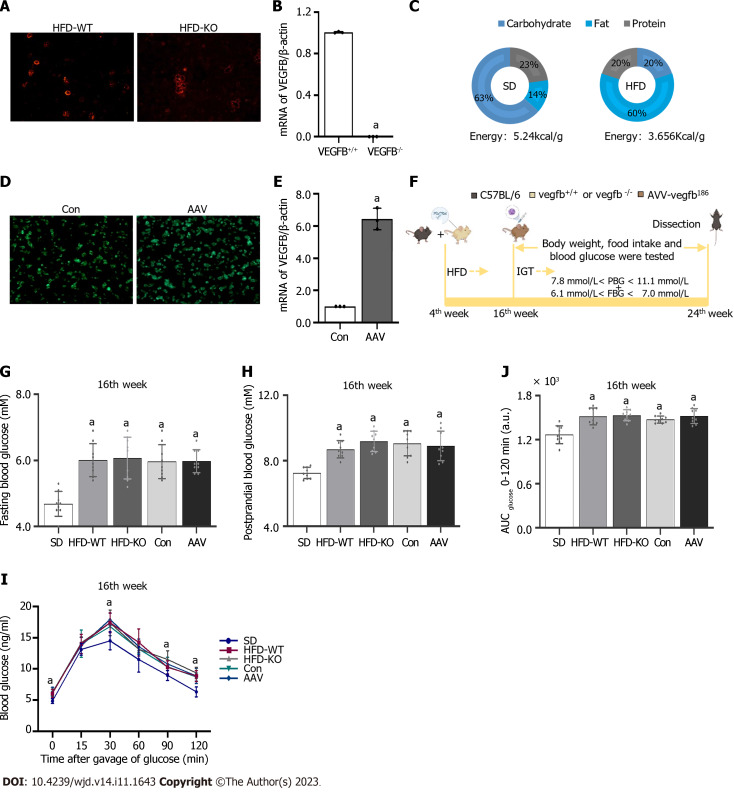
Male C57BL/6 mice were purchased from Jinan PengYue Experimental Animal Breeding Co., Ltd. All mice were acclimatized to laboratory conditions (24 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 1 wk prior to experimentation. All animal experiments were under the approval of the animal ethics committee of Binzhou Medical University (IACUC protocol number: 2023-170). The VEGFB gene knockout mouse model was constructed by CRISPR/Cas 9[17]. Mouse genotypes were identified by agarose gel electrophoresis. The islet cell mass was extracted to detect the expression of VEGFB at the mRNA level in VEGFB+/+ and VEGFB-/- mice (Figure 1A and B). Mice for the experiment were divided into 5 groups (n = 9, total = 45): Standard diet (SD), HFD-WT, HFD-KO, Con, and adeno-associated virus (AAV) group. VEGFB+/+ mice and VEGFB-/- mice were selected randomly for the experiment. Nine VEGFB+/+ mice fed with a standard diet were named the SD group. Twenty-seven VEGFB+/+ mice and nine VEGFB-/- mice were fed with a HFD for 20 wk (Figure 1C). Nine VEGFB+/+ and nine VEGFB-/- mice with HFD were defined as the HFD-WT and HFD-KO groups. Nine VEGFB+/+ mice injected with targeting VEGFB186 AAV were named as AAV group, and nine VEGFB+/+ mice injected with the non-targeting AAV were defined as the Con group. All mice were sacrificed and dissected in the 24th wk through cervical dislocation.
Figure 1.
The construction of impaired glucose tolerance mice with vascular endothelial growth factor B gene knockout and overexpressed. A: Active Stain of the islet cell mass in high-fat diet (HFD)-WT and HFD-KO mice; B: The mRNA expression of vascular endothelial growth factor B (VEGFB) in the islet of mice in the HFD-WT and HFD-KO group; C: The ingredients of standard diet (SD) and HFD; D: Green fluorescence staining of the islet with adeno-associated virus (AAV) infection in Con and AAV mice; E: The mRNA expression of VEGFB in the islet of mice in the Con and AAV group; F: The flow chart of animal experiments; G: Fasting blood glucose at the 16th wk; H: PBG at the 16th wk; I: Oral glucose tolerance test at the 16th wk; J: Area under the curve at the 16th wk. aP < 0.05 vs vegfb+/+; aP < 0.05 vs Con; aP < 0.05 vs SD. HFD: High-fat diet; AAV: Adeno-associated virus; SD: Standard diet; VEGF: Vascular endothelial growth factor.
AAV injection of VEGFB in IGT mouse
The transcript and vector of AAV were constructed from OBIO Technology Co. LTD. (Shanghai, China). The VEGFB gene was overexpressed in the AAV group by injection of pAAV-CAG-VEGFB186-P2A-EGFP-3xFLAG-WPRE vector, pAAV-CAG-EGFP-3xFLAG-WPRE was regarded as a control group. The frozen section of the pancreas with GFP green fluorescence was observed by fluorescence microscopy after 1 mo of injection to detect the efficiency of infection. The expression of VEGFB in islet cells was detected by Q-PCR (Figure 1D and E)[18].
IGT mouse model
FBG was tested after 12 h of starvation and PBG was tested 2 h after being fed. FBG and PBG were detected after 12 wk of HFD. Tail vein blood was detected from the 16th to the 24th wk. In the 16th wk, the FBG and PBG of IGT mice were higher than those of normal mice, but there are no differences between FBG, PBG, and oral glucose tolerance test (OGTT) of IGT mice in the rest four groups. Mice with 7.8 mmol/L < PBG < 11.1 mmol/L and 6.1 mmol/L < FBG < 7.0 mmol/L were regarded as the IGT animal model. The body weight and food intake of mice were measured once every two weeks from the 16th to the 24th wk (Figure 1F-H)[19].
Oral glucose tolerance test and intraperitoneal insulin tolerance test
In the oral glucose tolerance test (OGTT) experiment, mice were given glucose through gavage by a standard dosage of 2.0 mg/kg after fasting for over 12 h. While in the intraperitoneal insulin tolerance test (IPITT) experiment, mice were taken by intraperitoneal injection of insulin (0.5 U/kg) after over 6 h-starvation in advance. Both OGTT and IPITT curve was performed and the area under the curve (AUC) was calculated (Figure 1I and J)[20].
Isolation of islet cells
The pancreas was taken out, and the peripheral adipose tissue was isolated and placed in Hank’s buffer after the mice’s death. Injected 0.5 mg/mL collagenase P through the pancreatic duct and digested for 10 min after complete expansion of the pancreas. Hank’s was pre-cooled at 4 °C to stop digestion, and cell mass was selected under the stereoscopic microscope (OLYMPUS-SZ61, Japan). Then the active stain of the islet cell mass was performed (Figure 1A)[21].
Hormonal analysis using Enzyme-linked Immunosorbent Assay
The blood was gained before the mice were sacrificed. And the serum was collected by centrifuge at 1000 g for 10 min to detect the glucagon, glycosylated hemoglobin (GHb), and serum insulin content using Enzyme-linked Immunosorbent Assay (ELISA) according to the instructions of the manufacturer. Measured the OD values at 450 nm with a microplate reader (BioTek, China)[22].
Hematoxylin and Eosin (H&E) staining
The pancreas tissue sample was washed with run water overnight in an embedding box to wipe off 4% paraformaldehyde which was fixed for 24 h. After gradient dehydration, the embedded box was put into soft wax for 1 h, and then into hard wax for 1 h. And then embedded tissue was sliced and baked at 60 °C for 1.5 h for gradient dewaxing and rehydration. Hematoxylin staining was performed for 5 min. Hydrochloric acid alcohol differentiation solution was used for 10 s and then followed by a water rinse. Stained with eosin for 1 min and then continue to gradient dehydration, sealed the cover glass for observation[23].
Transmission electron microscopy
Pancreas tissue was fixed with 2.5% glutaraldehyde solution and washed with PBS, and repeated overnight. Washed and fixed with 1% osmotic acid fixed buffer, then gradient dehydrated with ethanol, soaked with acetone and embedding agent at room temperature overnight. Embedded at gradient temperature and polymerized the sample blocks for quick trimming and ultra-thin cutting. After staining with uranium diacetate and lead citrate, the section was photographed by the transmission electron microscope (JEM-1400, Japan)[24].
Immunofluorescence
The baked slide was dewaxed and rehydrated by using xylene, alcohol, and distilled water. And then immersed in the citric acid buffer in an antigen repair box to execute antigen repair at 100 °C. After washing with PBS, the sections were added with 3% hydrogen peroxide and incubated with serum. Added the primary antibody of insulin (1:200, Proteintech, 66198-1-I g) and glucagon (1:100, Abcam, ab92517) to the tissue. The next day, the tissue was completely covered by drops of fluorescence enzyme-labeled Goat anti-mouse (1:100, ZSGB-BIO, ZF-0314) in a wet box. Incubated with DAPI in a dark box and rinsed with PBS four times and observed under the fluorescence microscope (Zeiss, LSM880, Germany)[25].
Western blot
The pancreas tissue was weighed 20 mg and added RIPA (Solarbio, R0010, Beijing) lysate containing protease inhibitor cocktail (Solarbio, PO100, Beijing), centrifuged at 4°C at 12000 r/min after 30 min of ice lysed, the supernatant was taken and added the loading buffer (Solarbio, D1020-5, Beijing), heated it at 99°C for 10 min. The protein content was analyzed with a Bradford protein quantitative kit. The proteins were transferred onto the PVDF membrane. The PVDF membrane was sealed and incubated with the primary antibody overnight. Incubated secondary antibody and observed with ECL hypersensitivity exposure (Tanon5200, Tanon Science & Technology)[22].
RNA extraction and quantitative real-time PCR
The total RNA from the pancreas was extracted by isopropyl alcohol and RNA isolator (R401-01-AA, Vazyme Biotech Co., Ltd.). The genomic DNA removal reaction system was configured with 4 × gDNA wiper Mix (R223-01, Vazyme Biotech Co., Ltd.) and DEPC water according to the concentration of RNA samples. The reverse transcription reaction system was prepared using a 5 × HiScript II qRT SuperMix II. And then cDNA was executed with 2 × ChamQ Universal SYBR qPCR Master Mix (Q711-02, Vazyme Biotech Co., Ltd.) by PCR QuantStudio 3 (Thermo Fisher Scientific, Inc.). Primer sequences were: Forkhead box protein O1 (FOXO1), F: 5’-CCCAGGCCGGAGTTTAACC-3’, R: 5’-GTTGCTCATAAAGTCGGTGCT-3’; Phosphoenolpyruvate carboxykinase (PEPCK), F: 5’- CTGCATAACGGTCTGGACTTC-3’, R: 5’-CAGCAACTGCCCGTACTCC-3’; glucose-6 phosphatase (G6Pase), F: 5’-CGACTCGCTATCTCCAAGTGA-3’, R: 5’-GTTGAACCAGTCTCCGACCA-3’[26].
Data analysis
By SPSS 20.0 statistics software to carry on the analysis, all data were calculated as mean ± SD. One-way analysis of variance (ANOVA) was used to compare the means of multiple samples and t-test was used to compare the means of two samples. Statistically, a significant difference was described as P < 0.05.
RESULTS
Effects of VEGFB on glucose metabolism, glucose tolerance, and insulin sensitivity in IGT mice
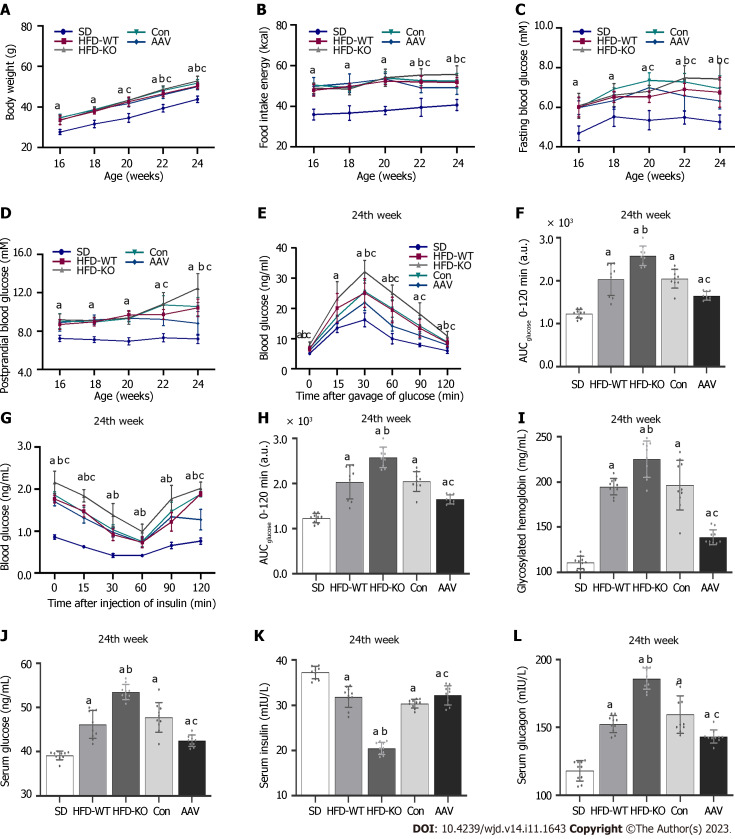
The body weight and food intake in HFD-KO were higher than in HFD-WT mice from the 22nd wk. Compared with mice in the Con group, the body weight and food intake of mice decreased after the injection of AAV (Figure 2A and B). FBG of HFD-KO mice was significantly higher than that of HFD-WT mice from the 22nd wk. After AAV injection, the FBG and PBG of mice were ameliorated dramatically from the 20th wk and the 22nd wk (Figure 2C and D). OGTT and IPITT showed that glucose tolerance and insulin sensitivity in the AAV group were increased, while in the HFD-KO group were decreased after 12 h fasting in the 24th wk (Figure 2E-H). Meanwhile, the contents of GHb, serum glucose, and serum glucagon increased in HFD-KO mice and decreased in AAV group significantly, while the content of serum insulin decreased in HFD-KO group and increased in the AAV group significantly (Figure 2I-L).
Figure 2.
Effects of vascular endothelial growth factor B on body weight and blood glucose level. A: Body weight from the 16th to the 24th wk; B: Food intake energy from the 16th to the 24th wk; C: Fasting blood glucose from the 16th to the 24th wk; D: PBG from the 16th to the 24th wk; E and F: Oral glucose tolerance test and area under the curve (AUC); G and H: Intraperitoneal insulin tolerance test and AUC; I: GHb; J: Serum glucose; K: Serum insulin; L: Serum glucagon. aP < 0.05 vs standard diet; bP < 0.05 vs high-fat diet-WT; cP < 0.05 vs Con. HFD: High-fat diet; AAV: Adeno-associated virus; SD: Standard diet.
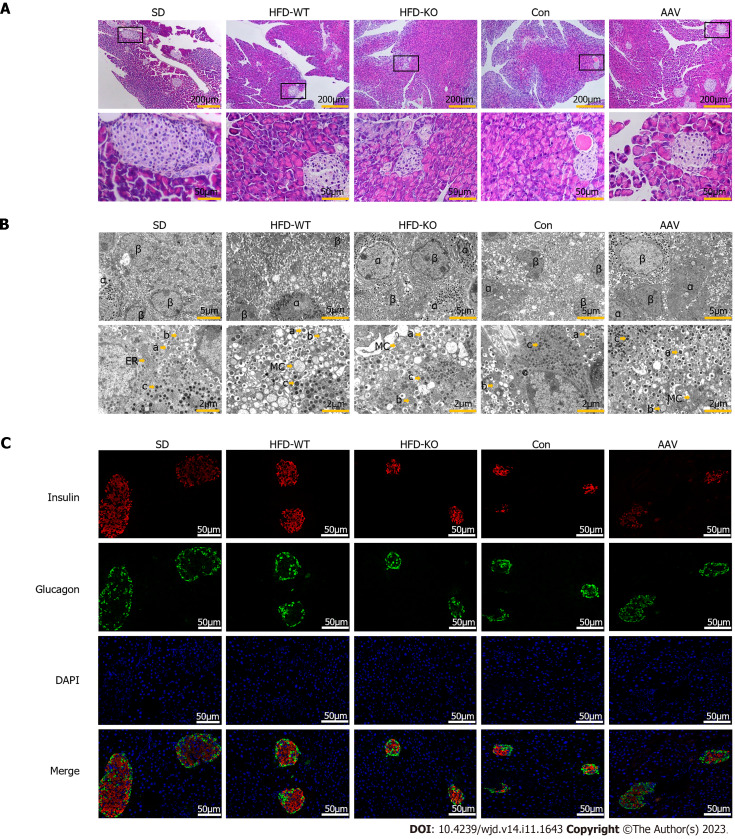
Effects of VEGFB on the morphology of pancreatic islets, number of secretory granules of islet α cells, and β cells
Scattered islet cell clusters with different sizes and shallow staining can be seen in the exocrine pancreas of mice. Compared with mice fed with SD, IGT mice have smaller islet cell clusters with irregular edges and smaller volumes (Figure 3A). It can be seen under the transmission electron microscope that the quantity of β cells is large and the volume is small, and the β cells contain different sizes of secretory granules. When IGT occurs, the number of secretory granules decreased in β cells. The α cells are relatively large in volume, and large secretory granules can be seen in the α cells (Figure 3B). After immunofluorescence labeling, red fluorescent-labeled insulin was mostly concentrated in the center of the islet, while green fluorescent-labeled glucagon was mostly located in the periphery of the islet (Figure 3C).
Figure 3.
Effects of vascular endothelial growth factor B on the cellular structure of islet. A: The HE staining of the pancreas under the light microscope (scale bar = 200 μm and 50 μm); B: The ultrastructure of the islet cell under the transmission electron microscope (scale bar = 5 μm and 2 μm); C: The immunofluorescence of islet cells under the fluorescence microscope (scale bar = 50 μm). HFD: High-fat diet; SD: Standard diet.
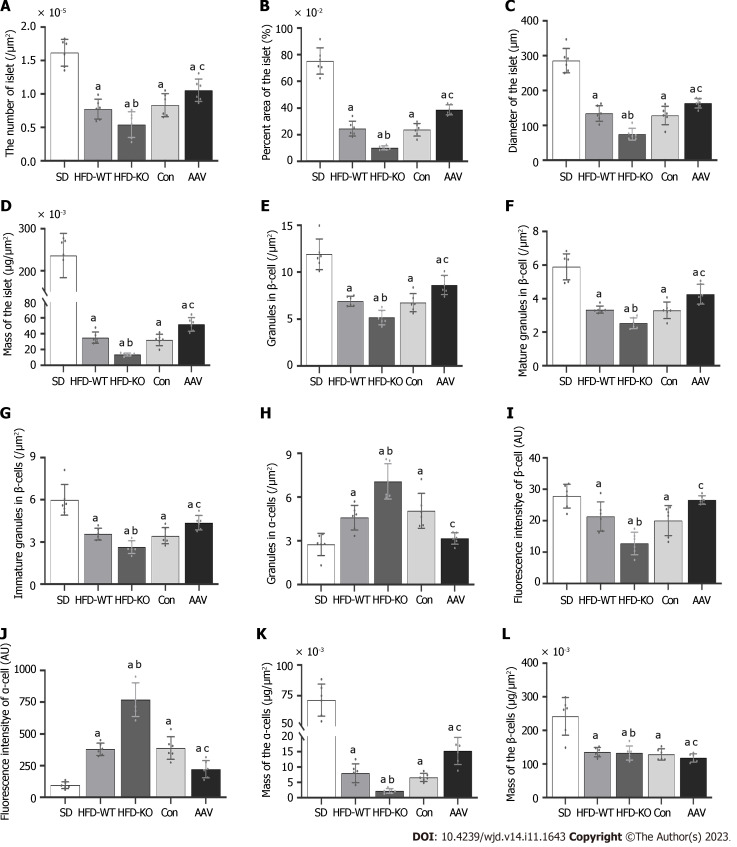
Compared with HFD-WT mice, the number, area, diameter, and mass of islets in HFD-KO mice decreased significantly. Compared with the Con mice, the number, area, diameter, and mass of pancreatic islets in AAV mice were significantly increased (Figure 4A-D). The number of mature and immature secretory granules decreases with the knockout of the VEGFB gene and increases after AAV injection (Figure 4E-G). Unlike β cells, after the VEGFB gene is knocked out, the secretory granules in α cells increased, while secretory granules declined after overexpression of VEGFB by AAV injection (Figure 4H).
Figure 4.
The quantitative analysis of vascular endothelial growth factor B affects the cellular structure of islets. A: The number of islets; B: The relative area of the islet; C: Diameter of the islet; D: Mass of the islet; E: The number of granules in β cells; F: The number of mature granules in β cells; G: The number of immature granules in β cells; H: The number of granules in α cells; I: Mean fluorescence intensity of β cells; J: Mean fluorescence intensity of α cells; K: Mass of the β cells; L: Mass of the α cells. aP < 0.05 vs standard diet; bP < 0.05 vs high-fat diet-WT; cP < 0.05 vs Con. HFD: High-fat diet; AAV: Adeno-associated virus; SD: Standard diet.
The fluorescence intensity of insulin secreted by β cells and the mass of the β cells in HFD-KO mice reduced significantly, while in α cells the fluorescence intensity of glucagon secreted by α cells and the mass of the α cells significantly increased (Figure 4I-L).
Protein-protein interaction network creation
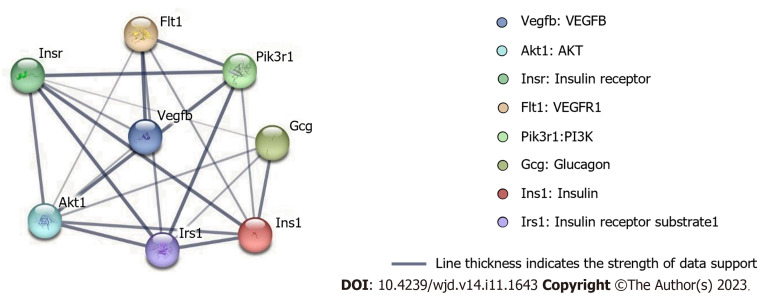
To examine the most significant clusters of the diversely expressed genes (DEGs), the protein-protein interaction (PPI) network of DEGs was constituted by Search Tool for the Retrieval of Interacting Genes (STRING11.5; https://string-db.org/). There were 8 nodes and 21 edges shown in the PPI network, and which PPI enrichment P value is 1.4E-09 (Figure 5).
Figure 5.
Cluster analysis of the protein-protein interaction network. There were 8 nodes and 21 edges shown in the protein-protein interaction network. VEGF: Vascular endothelial growth factor.
VEGFB/VEGFR1 regulates insulin secretion of β cells in IGT mice
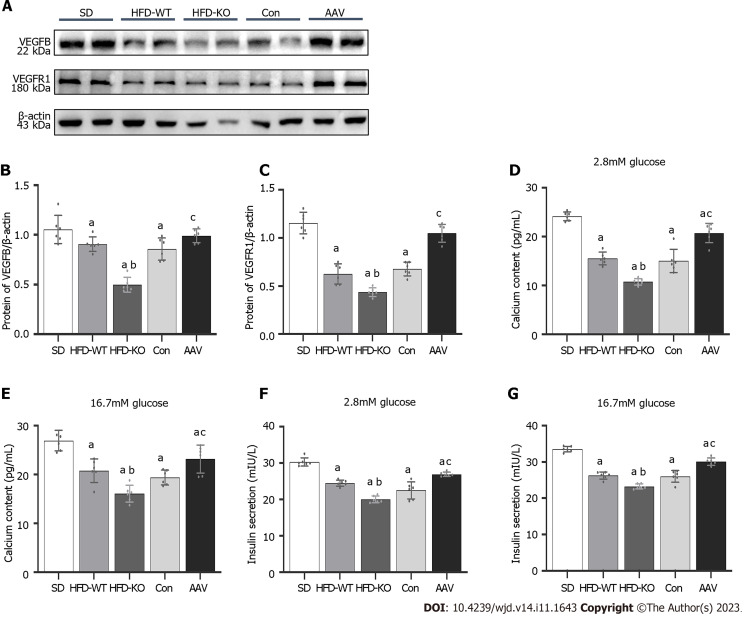
In order to confirm that VEGFB plays a role by combining its receptor, VEGFR1, we detect the expression of VEGFB and VEGFR1 with the western blot method. We observed that the VEGFR1 expressed highly in the AAV group and had a low expression in HFD-KO mice as the VEGFB gene was overexpressed or knocked out (Figure 6A-C). We extracted the islet mass from the pancreas islet of IGT mice. We observed that the concentration of calcium and insulin declined in the isolated islet mass of HFD-KO mice, but went high in AAV mice with the glucose incubation when compared with Con mice (Figure 6D-G).
Figure 6.
Vascular endothelial growth factor B regulates the content of Ca2+ and insulin via combining with vascular endothelial growth factor receptor 1. A: The protein expression of vascular endothelial growth factor B (VEGFB); B: The protein expression of VEGFB/β-actin; C: The protein expression of VEGF receptor 1/β-actin; D and E: The content of Ca2+ with 2.8 mmol/L and 16.7 mmol/L glucose; F and G: The content of insulin with 2.8 mmol/L and 16.7 mmol/L glucose. aP < 0.05 vs standard diet. bP < 0.05 vs high-fat diet-WT; cP < 0.05 vs Con. HFD: High-fat diet; AAV: Adeno-associated virus; SD: Standard diet.
VEGFB regulates glucagon secretion of α cells via PI3K/AKT signal pathway in IGT mice
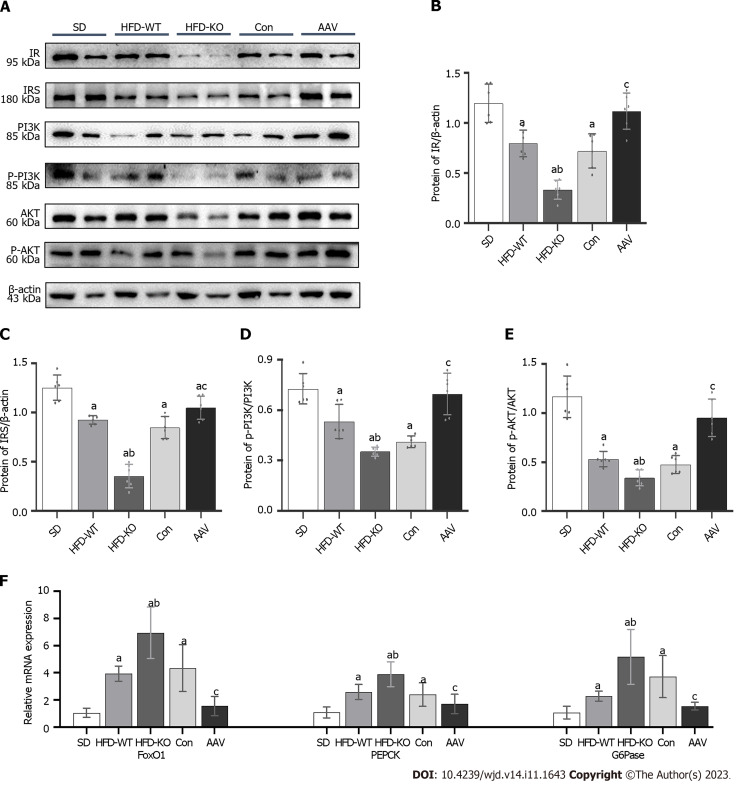
The protein expression of insulin resistance (IR) and insulin resistance substrate (IRS) was inhibited in IGT mice when compared with SD mice. IR and IRS expression were at the lowest levels in HFD-KO mice and increased dramatically after AAV injection. PI3K/AKT was considered to have a relationship with glucagon secretion, therefore we detected the relative protein expression levels through the signal pathway. PI3K and AKT expression levels remained no obvious changes while the phosphorylation of PI3K and AKT changed obviously in HFD-KO and AAV mice (Figure 7A-E). Moreover, glucagon secretion-related genes like FOXO1, G6Pase, and PEPCK were examined by Q-PCR, which demonstrated high expressions level in HFD-KO mice and low expression in AAV mice compared with Con mice (Figure 7F).
Figure 7.
Vascular endothelial growth factor B regulates glucagon secretion via PI3K/AKT signal pathway and relative genes. A: The protein expression of IR, insulin resistance substrate (IRS), PI3K, p-PI3K, AKT, and p-AKT; B: The protein expression of IR/β-actin; C: The protein expression of IRS/β-actin; D: The protein expression of p-PI3K/PI3K; E: The protein expression of p-AKT/AKT; F: The mRNA expression of forkhead box protein O1, PEPCK, and G6Pase. aP < 0.05 vs standard diet; bP < 0.05 vs high-fat diet-WT; cP < 0.05 vs Con. HFD: High-fat diet; AAV: Adeno-associated virus; SD: Standard diet; IRS: Insulin resistance substrate; FOXO: Forkhead box protein O1.
DISCUSSION
Seven members of the VEGF family have been identified to date, including VEGFA, VEGFB, VEGFC, VEGFD, VEGFE, VEGFF, and placental growth factor. These subtypes play different roles in many biological activities. VEGFA and VEGFC deletion can cause pathological changes and biological function loss. VEGFC and VEGFD are mainly involved in the regulation of lymphangiogenesis.
VEGFB is primarily expressed in tissues such as the heart, skeletal muscle, vascular smooth muscle, and pancreas[27]. Unlike other members, the role of VEGFB in the vascular system remains unclear[28]. Recently, VEGFB has been reported to affect glucose metabolism and insulin sensitivity. Robciuc et al[9] revealed that after injection of AAV-VEGFB186, glucose tolerance and peripheral insulin sensitivity improved significantly in HFD-fed mice. Moreover, Shang et al[10] demonstrated that specific overexpression of VEGFB in mice can enhance insulin sensitivity and secretion. The novel role of VEGFB in regulating glucose and insulin homeostasis provides an innovative approach for preventing the progression of IGT to T2DM.
We previously indicated that VEGFB has a similar regulatory effect on insulin secretion in MIN6 cells. After constructing recombinant VEGFB protein, the calcium content and insulin secretion in MIN6 cells increased with glucose stimulation[29]. Impaired fasting glucose (IFG) and IGT are currently considered to be the prediabetes state. According to the prediction of the International Diabetes Federation (IDF), > 470 million people will have prediabetes in 2030[30]. Active intervention in these two prediabetes states is of great significance to prevent the onset of diabetes and improve its long-term prognosis. Therefore, an increasing number of studies are focusing on the regulation mechanism of glucose tolerance in prediabetes.
According to the criteria of the American Diabetes Association, IFG refers to an FBG level of 5.6-6.9 mmol/L[31]. When IFG occurs, insulin resistance reduces the inhibitory effect of insulin on endogenous glucose production[32]. Although islet β cells undergo functional impairment, insulin secretion remains normal in the early stage[33]. After glucose stimulation, glucose abnormalities commonly occur during fasting conditions. The criteria for IGT include an FBG level of > 5.6 mmol/L and PBG level of 7.8-11.0 mmol/L[34]. An abnormal increase in PBG level is associated with peripheral insulin resistance[35]. After glucose stimulation, it is difficult to maintain PBG balance due to a significant decline in insulin secretion in the early stage, thus resulting in PBG abnormalities.
IGT is also associated with obesity[36]. Obesity leads to the excessive release of free fatty acids from adipocytes in the body, inducing insulin resistance, promoting lipid aggregation of islet β cells, and damaging the function of islet β cells. These changes further affect the secretion of glucose and insulin, leading to IGT[36]. Kim et al[37] revealed that patients with obesity and fatty insulin resistance are 4.3 times more likely to have IGT than normal patients. Bacha et al[38] reported that the visceral and subcutaneous fat proportion in adults with IGT was significantly higher than that in healthy adults[38]. We previously demonstrated that the mouse body weight as well as subcutaneous fat, inguinal fat, triglyceride, and cholesterol contents significantly increased after 10 wk of HFD feeding[17]. Moreover, significant increases in FBG and PBG levels were positively correlated with weight gain. At 20 wk of HFD feeding, mice with FBG levels of > 7 mmol/L and PBG levels of > 16.7 mmol/L developed T2DM. In the current study, we constructed the mouse model of IGT via HFD. After 12 wk of HFD feeding, FBG and PBG levels were measured in 16-wk-old mice. Mice with T2DM or IFG alone were excluded, and those with FBG levels of 5.6-6.9 mmol/L and PBG levels of 7.8-11.0 mmol/L were selected as mice with IGT. In mice with IGT and VEGFB knockout, FBG and PBG levels increased gradually with increasing body weight, whereas VEGFB overexpression significantly improved the body weight and blood glucose levels of mice with IGT.
IGT is associated with glucose tolerance and insulin sensitivity[39]. The OGTT and IPITT are used to examine glucose tolerance and insulin sensitivity in the clinical diagnosis of diabetes, respectively[40]. Currently, OGTT is considered the gold standard for evaluating IGT[41]. Using OGTT and IPITT, we further revealed that VEGFB knockout can increase the AUC values of OGTT and IPITT in mice with IGT, whereas VEGFB overexpression can significantly reduce the AUC values. Wu et al[42] revealed significant differences in plasma VEGFB expression between patients with IGT and those with normal glucose tolerance. Hagberg et al[43] confirmed that VEGFB regulates glucose tolerance in db/db mice, which is consistent with our results.
Abnormal elevation in glycosylated hemoglobin (GHb) levels is another measure of IGT[44]. Ye et al[45] reported a significantly strong association between VEGFB and GHb. We observed that the effect of VEGFB on GHb was consistent with that of FBG and PBG. After 8 wk of intervention with AAV overexpressing VEGFB in mice with IGT, the serum GHb levels decreased significantly. IGT is characterized by decreased insulin and glucagon secretion. Under hyperglycemic conditions, β cells release adequate insulin, but α cells release relatively reduced levels of glucagon, prompting α cells to absorb or utilize glucose from the blood[46]. During the abnormal production or release of insulin or glucagon, the body continues to remain hyperglycemic due to inconsistent physiological functions. Our research suggested that VEGFB improves blood glucose balance in mice with IGT. List et al[47] showed that after the occurrence of IFT, the average size of pancreatic islets decreased significantly in VEGFB−/− mice with IGT, along with a decrease in the number, size, area, and mass of pancreatic islets. After 4 wk of VEGFB overexpression, the size and number of pancreatic islets partially recovered as blood glucose levels improved.
Furthermore, fluorescence intensity analysis of β cells demonstrated that the effect of VEGFB was related to the number of islet cells. The IGT-induced decrease in insulin secretion is related to endocrine granules in β cells. β cells contain mature and immature secretory granules. Proinsulin and other soluble proteins are encapsulated in immature granules[48]. Immature granules are transformed into mature granules through a series of regulatory steps. Mature granules are stored in the cisterns or transported near the cell membrane[49]. When the level of blood glucose increases in the body, mature granules fuse with the cell membrane to release insulin. Similar to insulin, glucagon-related proteins accumulate in α cells in the form of granules to release glucagon in a paracrine manner[50]. Enhanced insulin secretion inhibits the secretion pathway of α cells, which reduces proglucagon synthesis in the endoplasmic reticulum, thereby reducing glucagon secretion. Studies have confirmed that insulin-mediated inhibition of glucagon secretion in patients with obesity is impaired as the severity of IGT is similar to that of insulin resistance and cellular dysfunction. We observed that the number of mature and immature granules in islet β cells decreased significantly. Furthermore, the number of granules in α cells increased significantly after VEGFB knockout, whereas the number of granules was significantly affected by VEGFB overexpression in mice with IGT. It is inferred that the effect of VEGFB on IGT hyperglycemia may be related to the activation of insulin-mediated inhibition of glucagon secretion.
Multiple signaling pathways regulate the secretion of insulin and glucagon[51]. Bioinformatics results regarding protein-protein interaction revealed that proteins related to insulin and glucagon secretion, such as insulin receptor (IR), IR substrate (IRS), PI3K, and AKT, are biologically related to VEGFB and VEGFR1. The combined signaling pathways of VEGFB and VEGFR1 can activate various biological reactions (e.g., VEGFB promoting insulin secretion in MIN6 cells by binding to VEGFR1)[29]. Glucagon secretion in α cells critically depends on insulin secretion in β cells[52]. The PI3K/AKT pathway is a typical pathway in insulin signal and is regulated by multiple factors such as insulin and IR; moreover, it is involved in the regulation of glucagon secretion in α cells. When the glucose level increases, insulin is secreted in β cells.
After insulin binds to IR on the membranes of α cells, the conformation of IR changes, leading to the recruitment of IRS proteins[53]. P13K and AKT are downstream proteins of IRS[54]. When PI3K receives the upstream IRS protein signal, its subunit p85 binds to IRS to promote the phosphorylation of PI3K[55]. After PI3K phosphorylation, AKT aggregates in the cell membrane and changes its structure to induce activity, inhibiting the gene encoding glucagon through AKT phosphorylation, thus reducing glucagon secretion in α cells[26]. Mancuso et al[22] utilized in vitro α cell models and revealed that the activation of the PI3K/AKT pathway can inhibit glucagon secretion. In conclusion, VEGFB/VEGFR1 can promote β cells to secrete insulin and activate PI3K/AKT signaling pathways in α cells.
Hyperglycemia in T2DM caused by increased glucagon levels is often accompanied with abnormal gluconeogenesis[56]. PEPCK and G6Pase are two rate-limiting enzymes in gluconeogenesis[57]. Various hormones and signaling pathways affect the transcription and expression of these two key enzymes, thereby affecting glucagon secretion[58]. Among them, insulin affects the secretory function of glucagon in α cells via PEPCK and G6Pase regulation[59]. The PI3K/AKT signaling pathway and its downstream FOXO1 gene regulate the expression of PEPCK and G6Pase[60]. FOXO1 is an important member of the fork-helix transcription factor family, closely related to the proliferation, apoptosis, differentiation, and growth of cells[61]. FOXO1 regulates PEPCK and G6Pase by directly binding to its target DNA sequence and interacting with nuclear receptors[62]. Stavroula indicated that insulin activates the PI3K/AKT signaling pathway and inhibits FOXO1 activity, thereby promoting glucose metabolism[63].
CONCLUSION
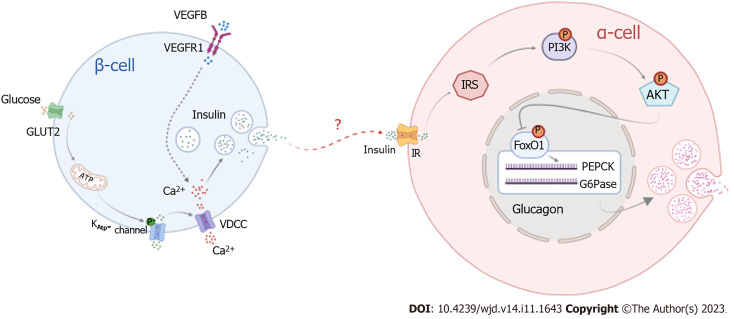
Our research demonstrates that the binding of VEGFB to VEGFR1 promotes insulin release, and insulin binding to its receptor activates PI3K/AKT signaling to inhibit the expression of FOXO1, PEPCK, and G6Pase, thereby suppressing glucagon secretion. Our experiments confirmed that combining VEGFB and VEGFR1 can stimulate insulin-mediated glucagon secretion in α cells by activating the PI3K/AKT signaling pathway to regulate glucose metabolism disorders in mice with IGT (Figure 8). This provides not only new avenues for investigating the molecular mechanism of IGT but also a more theoretical and experimental basis for diagnosing and treating diabetes in the early stage and preventing its development into T2DM.
Figure 8.
Pattern diagram of vascular endothelial growth factor B regulates insulin-medicated glucagon secretion through PI3K/AKT signal pathway. IRS: Insulin resistance substrate; VEGF: Vascular endothelial growth factor; VDCC: Voltage dependent calcium channels.
ARTICLE HIGHLIGHTS
Research background
The results showed that after vascular endothelial growth factor B (VEGFB) overexpression, serum glucose, glucose tolerance, and insulin sensitivity in impaired glucose tolerance (IGT) mice were improved, and the number of secretory granules of β cells was increased by activating the PI3K/AKT signal pathway.
Research motivation
VEGFB can promote insulin-mediated glucagon secretion by activating the PI3K/AKT signaling pathway to improve glucose metabolism in mice with IGT.
Research objectives
This study illustrated the role of VEGFB in regulating insulin-mediated glucagon secretion in mice with IGT by regulating the PI3K/AKT signal pathway, which indicated the regulatory function of VEGFB in improving the IGT condition of mice and preventing the onset of type 2 diabetes in the body. This study proved the molecular mechanism of VEGFB regulating IGT and provided theoretical basis for the treatment of prediabetes.
Research methods
The research was conducted by Crispr Cas9 and high-fat diet feeding to construct the animal model. Western blot and qRT-PCR were used to detect the expression of proteins and genes. Bioinformatics was used to analyze the correlation between relative proteins in the PI3K/AKT signal pathway.
Research results
To specify the underlying mechanism of VEGFB effects on insulin-mediated glucagon secretion in impaired glucose tolerance.
Research conclusions
Type 2 diabetes (T2D) can be prevented in pre-diabetic individuals with impaired glucose tolerance. Therefore, converting IGT into a normal condition is critical to prevent the onset of diabetes.
Research perspectives
Diabetes is a worldwide health problem, affecting about 415 million people globally. Among them, the number of patients with type 2 diabetes accounts for about 90% of the number of patients with diabetes with a population of 373 million. Pre-diabetes is the main risk factor for progression to type 2 diabetes. Impaired glucose tolerance (IGT) is a pre-diabetes state, and it is mainly manifested as fast blood glucose (FBG) level of<7.0 mmol/L and/or posttraumatic blood glucose (PBG) level of 7.8 – 11.1 mmol/L after 2 h of oral glucose administration. Long-term IGT will greatly increase the risk of type 2 diabetes. Therefore, precision intervention can improve the insulin sensitivity of patients with IGT and effectively prevent or delay the progression to type 2 diabetes. VEGFB, as a novel metabolic regulatory target, has received much attention for its role in regulating insulin sensitivity. Therefore, we successfully constructed a mouse model with IGT, and intervened in the upregulation and downregulation of the VEGFB gene at the gene level, so as to explore that VEGFB regulates insulin-mediated Glucagon secretion, and thus improves IGT symptoms in pre-diabetes.
Footnotes
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Animal Ethics Committee of Binzhou Medical University (Approval No. 2023-170).
Conflict-of-interest statement: The authors have declared no conflicts of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 28, 2023
First decision: July 4, 2023
Article in press: September 6, 2023
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Balbaa ME, Egypt; Beg MMA, Kyrgyzstan; Liao Z, Singapore; Yan LJ, United States; Islam S, Australia S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
Contributor Information
Yu-Qi Li, Department of Pathophysiology, School of Basic Medicine, Binzhou Medical University, Yantai 264000, Shandong Province, China.
Lu-Yang Zhang, Department of Rheumatology and Immunology, Yantaishan Hospital, Yantai 264000, Shandong Province, China.
Yu-Chi Zhao, Department of Surgery, Yantaishan Hospital, Yantai 264000, Shandong Province, China.
Fang Xu, Department of Pathophysiology, School of Basic Medicine, Binzhou Medical University, Yantai 264000, Shandong Province, China.
Zhi-Yong Hu, School of Public Health and Management, Binzhou Medical University, Yantai 264000, Shandong Province, China.
Qi-Hao Wu, The First School of Clinical Medicine, Binzhou Medical University, Yantai 264000, Shandong Province, China.
Wen-Hao Li, Department of Pathophysiology, School of Basic Medicine, Binzhou Medical University, Yantai 264000, Shandong Province, China.
Ya-Nuo Li, Department of Pathophysiology, School of Basic Medicine, Binzhou Medical University, Yantai 264000, Shandong Province, China. liyanuo@bzmc.edu.cn.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at liyanuo@bzmc.edu.cn.
References
- 1.Morrison KM, Xu L, Tarnopolsky M, Yusuf Z, Atkinson SA, Yusuf S. Screening for dysglycemia in overweight youth presenting for weight management. Diabetes Care . 2012;35:711–716. doi: 10.2337/dc11-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beulens J, Rutters F, Rydén L, Schnell O, Mellbin L, Hart HE, Vos RC. Risk and management of pre-diabetes. Eur J Prev Cardiol . 2019;26:47–54. doi: 10.1177/2047487319880041. [DOI] [PubMed] [Google Scholar]
- 3.de Mello VD, Lindström J, Eriksson J, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Sundvall J, Laakso M, Tuomilehto J, Uusitupa M. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: the Finnish Diabetes Prevention Study. Diabetes Care . 2012;35:211–217. doi: 10.2337/dc11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Kong L, Wen X, Li M, Su S, Ni Y, Gu J. The Positive Effect of 6-Gingerol on High-Fat Diet and Streptozotocin-Induced Prediabetic Mice: Potential Pathways and Underlying Mechanisms. Nutrients . 2023;15 doi: 10.3390/nu15040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahrén B. Beta- and alpha-cell dysfunction in subjects developing impaired glucose tolerance: outcome of a 12-year prospective study in postmenopausal Caucasian women. Diabetes . 2009;58:726–731. doi: 10.2337/db08-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol . 2013;24:11–21. doi: 10.1016/j.semcdb.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Gastaldelli A, Henry RR, Kitabchi AE, Mudaliar S, Ratner RE, Stentz FB, Musi N, Reaven PD ACT NOW Study. Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes . 2013;62:3920–3926. doi: 10.2337/db13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning FC, Jensen N, Mi J, Lindström W, Balan M, Muhl L, Eriksson U, Nilsson I, Nyqvist D. VEGF-B ablation in pancreatic β-cells upregulates insulin expression without affecting glucose homeostasis or islet lipid uptake. Sci Rep . 2020;10:923. doi: 10.1038/s41598-020-57599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robciuc MR, Kivelä R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppänen VM, Käkelä R, Eklund L, Wasserman DH, Groen AK, Alitalo K. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell Metab . 2016;23:712–724. doi: 10.1016/j.cmet.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang R, Lal N, Lee CS, Zhai Y, Puri K, Seira O, Boushel RC, Sultan I, Räsänen M, Alitalo K, Hussein B, Rodrigues B. Cardiac-specific VEGFB overexpression reduces lipoprotein lipase activity and improves insulin action in rat heart. Am J Physiol Endocrinol Metab . 2021;321:E753–E765. doi: 10.1152/ajpendo.00219.2021. [DOI] [PubMed] [Google Scholar]
- 11.Luo X, Li RR, Li YQ, Yu HP, Yu HN, Jiang WG, Li YN. Reducing VEGFB expression regulates the balance of glucose and lipid metabolism in mice via VEGFR1. Mol Med Rep . 2022;26 doi: 10.3892/mmr.2022.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao R, Wang C, Stöhr O, Qiu W, Hu Y, Miao J, Dong XC, Leng S, Stefater M, Stylopoulos N, Lin L, Copps KD, White MF. Inactivating hepatic follistatin alleviates hyperglycemia. Nat Med . 2018;24:1058–1069. doi: 10.1038/s41591-018-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci . 2018;14:1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz BM, Muñoz B, Yin F, Bauchle C, Atwood BK. A High-fat, High-sugar 'Western' Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience . 2018;372:1–15. doi: 10.1016/j.neuroscience.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pscherer S, Heemann U, Frank H. Effect of Renin-Angiotensin system blockade on insulin resistance and inflammatory parameters in patients with impaired glucose tolerance. Diabetes Care . 2010;33:914–919. doi: 10.2337/dc09-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling M, Quan L, Lai X, Lang L, Li F, Yang X, Fu Y, Feng S, Yi X, Zhu C, Gao P, Zhu X, Wang L, Shu G, Jiang Q, Wang S. VEGFB Promotes Myoblasts Proliferation and Differentiation through VEGFR1-PI3K/Akt Signaling Pathway. Int J Mol Sci . 2021;22 doi: 10.3390/ijms222413352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Li Y, Yang X, Hu Y, Yu H. Reducing VEGFB accelerates NAFLD and insulin resistance in mice via inhibiting AMPK signaling pathway. J Transl Med . 2022;20:341. doi: 10.1186/s12967-022-03540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Pan D, Chen Q, Zhu LJ, Ou J, Wabitsch M, Wang YX. Transcription factor Hlx controls a systematic switch from white to brown fat through Prdm16-mediated co-activation. Nat Commun . 2017;8:68. doi: 10.1038/s41467-017-00098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philippaert K, Pironet A, Mesuere M, Sones W, Vermeiren L, Kerselaers S, Pinto S, Segal A, Antoine N, Gysemans C, Laureys J, Lemaire K, Gilon P, Cuypers E, Tytgat J, Mathieu C, Schuit F, Rorsman P, Talavera K, Voets T, Vennekens R. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat Commun . 2017;8:14733. doi: 10.1038/ncomms14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo F, Xu S, Zhu Y, Zheng X, Lu Y, Tu J, He Y, Jin L, Li Y. PPARγ Transcription Deficiency Exacerbates High-Fat Diet-Induced Adipocyte Hypertrophy and Insulin Resistance in Mice. Front Pharmacol . 2020;11:1285. doi: 10.3389/fphar.2020.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, de Chavez V, Vuguin PM, Charron MJ, Powers AC, Drucker DJ. Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes . 2013;62:1196–1205. doi: 10.2337/db11-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancuso E, Mannino GC, Fuoco A, Leo A, Citraro R, Averta C, Spiga R, Russo E, De Sarro G, Andreozzi F, Sesti G. HDL (High-Density Lipoprotein) and ApoA-1 (Apolipoprotein A-1) Potentially Modulate Pancreatic α-Cell Glucagon Secretion. Arterioscler Thromb Vasc Biol . 2020;40:2941–2952. doi: 10.1161/ATVBAHA.120.314640. [DOI] [PubMed] [Google Scholar]
- 23.Mita Y, Nakayama K, Inari S, Nishito Y, Yoshioka Y, Sakai N, Sotani K, Nagamura T, Kuzuhara Y, Inagaki K, Iwasaki M, Misu H, Ikegawa M, Takamura T, Noguchi N, Saito Y. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat Commun . 2017;8:1658. doi: 10.1038/s41467-017-01863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han F, Li X, Yang J, Liu H, Zhang Y, Yang X, Yang S, Chang B, Chen L. Salsalate Prevents β-Cell Dedifferentiation in OLETF Rats with Type 2 Diabetes through Notch1 Pathway. Aging Dis . 2019;10:719–730. doi: 10.14336/AD.2018.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cigliola V, Ghila L, Thorel F, van Gurp L, Baronnier D, Oropeza D, Gupta S, Miyatsuka T, Kaneto H, Magnuson MA, Osipovich AB, Sander M, Wright CEV, Thomas MK, Furuyama K, Chera S, Herrera PL. Pancreatic islet-autonomous insulin and smoothened-mediated signalling modulate identity changes of glucagon(+) α-cells. Nat Cell Biol . 2018;20:1267–1277. doi: 10.1038/s41556-018-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Zhang FG, Zhang WS, Pan A, Yang YL, Liu JF, Li P, Liu BL, Qi LW. Ginsenoside Rg1 Inhibits Glucagon-Induced Hepatic Gluconeogenesis through Akt-FoxO1 Interaction. Theranostics . 2017;7:4001–4012. doi: 10.7150/thno.18788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Kumar A, Zhang F, Lee C, Tang Z. Complicated life, complicated VEGF-B. Trends Mol Med . 2012;18:119–127. doi: 10.1016/j.molmed.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Caballero B, Sherman SJ, Falk T. Insights into the Mechanisms Involved in Protective Effects of VEGF-B in Dopaminergic Neurons. Parkinsons Dis . 2017;2017:4263795. doi: 10.1155/2017/4263795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia JD, Jiang WG, Luo X, Li RR, Zhao YC, Tian G, Li YN. Vascular endothelial growth factor B inhibits insulin secretion in MIN6 cells and reduces Ca(2+) and cyclic adenosine monophosphate levels through PI3K/AKT pathway. World J Diabetes . 2021;12:480–498. doi: 10.4239/wjd.v12.i4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet . 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinidiyapathirage MJ, Kasturiratne A, Ranawaka UK, Gunasekara D, Wijekoon N, Medagoda K, Perera S, Takeuchi F, Kato N, Warnakulasuriya T, Wickremasinghe AR. The burden of diabetes mellitus and impaired fasting glucose in an urban population of Sri Lanka. Diabet Med . 2013;30:326–332. doi: 10.1111/dme.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes . 1999;48:2197–2203. doi: 10.2337/diabetes.48.11.2197. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Wang M, Tong J, Dong Z, Deng M, Ren X, Li H, Yang J, Meng Z, Sun J, He Q, Liu M. Impaired Glucose-Stimulated Proinsulin Secretion Is an Early Marker of β-Cell Impairment Before Prediabetes Stage. J Clin Endocrinol Metab . 2019;104:4341–4346. doi: 10.1210/jc.2019-00549. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Gao Y, Li Y, Teng D, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ba J, Chen B, Du J, He L, Lai X, Teng X, Shi X, Chi H, Liao E, Liu C, Liu L, Qin G, Qin Y, Quan H, Shi B, Sun H, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Teng W, Shan Z, Li J. The Presence of Serum TgAb Suggests Lower Risks for Glucose and Lipid Metabolic Disorders in Euthyroid General Population From a National Survey. Front Endocrinol (Lausanne) . 2020;11:139. doi: 10.3389/fendo.2020.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucidi P, Rossetti P, Porcellati F, Pampanelli S, Candeloro P, Andreoli AM, Perriello G, Bolli GB, Fanelli CG. Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: the role of lipolysis. Diabetes . 2010;59:1349–1357. doi: 10.2337/db09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenzo C, Lee R, Haffner SM. Impaired glucose tolerance and obesity as effect modifiers of ethnic disparities of the progression to diabetes: the San Antonio Heart Study. Diabetes Care . 2012;35:2548–2552. doi: 10.2337/dc11-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JY, Bacha F, Tfayli H, Michaliszyn SF, Yousuf S, Arslanian S. Adipose Tissue Insulin Resistance in Youth on the Spectrum From Normal Weight to Obese and From Normal Glucose Tolerance to Impaired Glucose Tolerance to Type 2 Diabetes. Diabetes Care . 2019;42:265–272. doi: 10.2337/dc18-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care . 2009;32:100–105. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: II. Observations Using the Oral Glucose Tolerance Test. Diabetes Care . 2018;41:1707–1716. doi: 10.2337/dc18-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uusitupa MI, Stancáková A, Peltonen M, Eriksson JG, Lindström J, Aunola S, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Tuomilehto J, Laakso M. Impact of positive family history and genetic risk variants on the incidence of diabetes: the Finnish Diabetes Prevention Study. Diabetes Care . 2011;34:418–423. doi: 10.2337/dc10-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savoye M, Caprio S, Dziura J, Camp A, Germain G, Summers C, Li F, Shaw M, Nowicka P, Kursawe R, Depourcq F, Kim G, Tamborlane WV. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care . 2014;37:317–324. doi: 10.2337/dc13-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Wei H, Qu H, Feng Z, Long J, Ge Q, Deng H. Plasma vascular endothelial growth factor B levels are increased in patients with newly diagnosed type 2 diabetes mellitus and associated with the first phase of glucose-stimulated insulin secretion function of β-cell. J Endocrinol Invest . 2017;40:1219–1226. doi: 10.1007/s40618-017-0677-z. [DOI] [PubMed] [Google Scholar]
- 43.Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Ylä-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature . 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 44.Randrianarisoa E, Lehn-Stefan A, Hieronimus A, Rietig R, Fritsche A, Machann J, Balletshofer B, Häring HU, Stefan N, Rittig K. Visceral Adiposity Index as an Independent Marker of Subclinical Atherosclerosis in Individuals Prone to Diabetes Mellitus. J Atheroscler Thromb . 2019;26:821–834. doi: 10.5551/jat.47274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye X, Kong W, Zafar MI, Zeng J, Yang R, Chen LL. Plasma vascular endothelial growth factor B is elevated in non-alcoholic fatty liver disease patients and associated with blood pressure and renal dysfunction. EXCLI J . 2020;19:1186–1195. doi: 10.17179/excli2020-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asadi F, Dhanvantari S. Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC1-6 Cells. Front Endocrinol (Lausanne) . 2018;9:792. doi: 10.3389/fendo.2018.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.List EO, Berryman DE, Buchman M, Jensen EA, Funk K, Duran-Ortiz S, Qian Y, Young JA, Slyby J, McKenna S, Kopchick JJ. GH Knockout Mice Have Increased Subcutaneous Adipose Tissue With Decreased Fibrosis and Enhanced Insulin Sensitivity. Endocrinology . 2019;160:1743–1756. doi: 10.1210/en.2019-00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Wright J, Guo H, Xiong Y, Arvan P. Proinsulin entry and transit through the endoplasmic reticulum in pancreatic beta cells. Vitam Horm . 2014;95:35–62. doi: 10.1016/B978-0-12-800174-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 49.Omar-Hmeadi M, Idevall-Hagren O. Insulin granule biogenesis and exocytosis. Cell Mol Life Sci . 2021;78:1957–1970. doi: 10.1007/s00018-020-03688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yip L, Taylor C, Whiting CC, Fathman CG. Diminished adenosine A1 receptor expression in pancreatic α-cells may contribute to the pathology of type 1 diabetes. Diabetes . 2013;62:4208–4219. doi: 10.2337/db13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutchens T, Piston DW. EphA4 Receptor Forward Signaling Inhibits Glucagon Secretion From α-Cells. Diabetes . 2015;64:3839–3851. doi: 10.2337/db15-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology . 2012;153:1039–1048. doi: 10.1210/en.2011-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaki T. Age-Associated Weight Gain, Leptin, and SIRT1: A Possible Role for Hypothalamic SIRT1 in the Prevention of Weight Gain and Aging through Modulation of Leptin Sensitivity. Front Endocrinol (Lausanne) . 2015;6:109. doi: 10.3389/fendo.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, Johnson AMF, Sears D, Shen Z, Cui B, Kong L, Hou S, Liang X, Iovino S, Watkins SM, Ying W, Osborn O, Wollam J, Brenner M, Olefsky JM. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell . 2016;167:973–984.e12. doi: 10.1016/j.cell.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Liu B, Han H, Yuan Q, Xue M, Xu F, Chen Y. Acute Hepatic Insulin Resistance Contributes to Hyperglycemia in Rats Following Myocardial Infarction. Mol Med . 2015;21:68–76. doi: 10.2119/molmed.2014.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramakrishnan SK, Zhang H, Takahashi S, Centofanti B, Periyasamy S, Weisz K, Chen Z, Uhler MD, Rui L, Gonzalez FJ, Shah YM. HIF2α Is an Essential Molecular Brake for Postprandial Hepatic Glucagon Response Independent of Insulin Signaling. Cell Metab . 2016;23:505–516. doi: 10.1016/j.cmet.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng Y, Huang SL, Dou W, Zhang S, Chen JH, Shen Y, Shen JH, Leng Y. Emodin, a natural product, selectively inhibits 11beta-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice. Br J Pharmacol . 2010;161:113–126. doi: 10.1111/j.1476-5381.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao J, Zheng R, Chang X, Zhao Y, Zhang D, Gao M, Yin Z, Jiang C, Zhang J. Cyclocarya paliurus triterpenoids suppress hepatic gluconeogenesis via AMPK-mediated cAMP/PKA/CREB pathway. Phytomedicine . 2022;102:154175. doi: 10.1016/j.phymed.2022.154175. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Yang K, Yang J, Xiao W, Le Y, Yu F, Gu L, Lang S, Tian Q, Jin T, Wei R, Hong T. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine . 2019;41:73–84. doi: 10.1016/j.ebiom.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park SJ, Lee D, Kim D, Lee M, In G, Han ST, Kim SW, Lee MH, Kim OK, Lee J. The non-saponin fraction of Korean Red Ginseng (KGC05P0) decreases glucose uptake and transport in vitro and modulates glucose production via down-regulation of the PI3K/AKT pathway in vivo. J Ginseng Res . 2020;44:362–372. doi: 10.1016/j.jgr.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sidhu A, Miller PJ, Hollenbach AD. FOXO1 stimulates ceruloplasmin promoter activity in human hepatoma cells treated with IL-6. Biochem Biophys Res Commun . 2011;404:963–967. doi: 10.1016/j.bbrc.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 62.Yan F, Chen Y, Azat R, Zheng X. Mulberry Anthocyanin Extract Ameliorates Oxidative Damage in HepG2 Cells and Prolongs the Lifespan of Caenorhabditis elegans through MAPK and Nrf2 Pathways. Oxid Med Cell Longev . 2017;2017:7956158. doi: 10.1155/2017/7956158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone . 2012;50:437–443. doi: 10.1016/j.bone.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at liyanuo@bzmc.edu.cn.