Abstract
The expression of the srf operon of Bacillus subtilis, encoding surfactin synthetase and the competence regulatory protein ComS, was observed to be reduced when cells were grown in a rich glucose- and glutamine-containing medium in which late-growth culture pH was 5.0 or lower. The production of the surfactin synthetase subunits and of surfactin itself was also reduced. Raising the pH to near neutrality resulted in dramatic increases in srf expression and surfactin production. This apparent pH-dependent induction of srf expression required spo0K, which encodes the oligopeptide permease that functions in cell-density-dependent control of sporulation and competence, but not CSF, the competence-inducing pheromone that regulates srf expression in a Spo0K-dependent manner. Both ComP and ComA, the two-component regulatory pair that stimulates cell-density-dependent srf transcription, were required for optimal expression of srf at low and high pHs, but ComP was not required for pH-dependent srf induction. The known negative regulators of srf, RapC and CodY, were found not to function significantly in pH-dependent srf expression. Late-growth culture supernatants at low pH were not active in inducing srf expression in cells of low-density cultures but were rendered active when their pH was raised to near neutrality. ComQ (and very likely the srf-inducing pheromone ComX) and Spo0K were found to be required for the extracellular induction of srf-lacZ at neutral pH. The results suggest that srf expression, in response to changes in culture pH, requires Spo0K and another, as yet unidentified, extracellular factor. The study also provides evidence consistent with the hypothesis that ComP acts both positively and negatively in the regulation of ComA and that both activities are controlled by the ComX pheromone.
Certain strains of Bacillus subtilis produce surfactin, a secondary metabolite composed of seven amino acids and a β-hydroxy fatty acid which together constitute an eight-membered cyclic lipopeptide. Surfactin is one of several microbially produced biosurfactants which are amphipathic molecules having many potential commercial applications (7). It is also endowed with antibacterial, antimycoplasma, antiviral, and hemolytic activity (1, 2, 47, 51, 52). Production of surfactin requires the products of the srf operon, encoding the three subunits of surfactin synthetase that catalyze the thiotemplate mechanism of nonribosomal peptide synthesis to incorporate the seven amino acids into the surfactin lipopeptide (5, 12, 15, 27, 28, 48–50). srf also contains the competence regulatory gene comS, which lies within and out-of-frame with the second gene of the operon, srfB (6, 18). A possible objective accomplished by this unusual association is the coregulation of the production of a lytic agent (surfactin) with a physiological state (genetic competence) designed for the uptake of a substance released from lysed cells (DNA).
The production of surfactin is but one example of a situation where an antibiotic biosynthesis gene or operon is activated by a regulatory system coupled to the accumulation of cell-derived extracellular signals. The production of streptomycin by Streptomyces griseus, of phenazine by pseudomonads, and of carbepenems by Erwinia spp. is regulated by extracellular factors mediating quorum sensing (13). The biological and/or ecological objective for regulating antibiotic production in this fashion would be to eliminate competition for scarce resources and coordinate the high level of production of antimicrobial agents among the members of a large, concentrated population so as to maximize the concentration and, thus, the impact of the secreted product. Biosurfactants, like the lipopeptide surfactin, might be produced by high-cell-density populations of Bacillus spp. so as to dispatch a lipophilic agent in high concentration to disperse hydrophobic aggregates, rendering their substituents susceptible to degradation and assimilation. Lipopeptides with similar structure to that of surfactin promote swarming motility in Serratia marcescens and, perhaps, Proteus mirabilis, a process necessarily carried out by dense populations of cells (14, 26).
Two pheromones, ComX and CSF, accumulate to high concentrations in late-growth cultures and are known to stimulate the transcription of srf (23, 25, 44, 45) (Fig. 1). ComX activates the signal transduction system composed of the two-component regulatory proteins ComP and ComA (25, 53, 54). The histidine protein kinase ComP donates a phosphate to the response regulator ComA, which, thus activated, stimulates the transcription of the srf operon (17, 30, 32–34, 40). CSF (competence-stimulatory factor) is encoded by the phrC gene, which is a member of the phr family of genes that encodes extracellular peptide factors that participate in the regulation of sporulation and other late-growth processes in B. subtilis by inhibiting the activity of aspartyl phosphate phosphatases of the Rap family (21, 35–37, 39, 44). CSF is believed to be imported via the oligopeptide permease encoded by spo0K (38, 41) and to negatively control the activity of the aspartyl phosphate phosphatase RapC. RapC is believed to negatively regulate srf transcription by removing the phosphate from ComA-P, thereby rendering ComA inactive (23, 44).
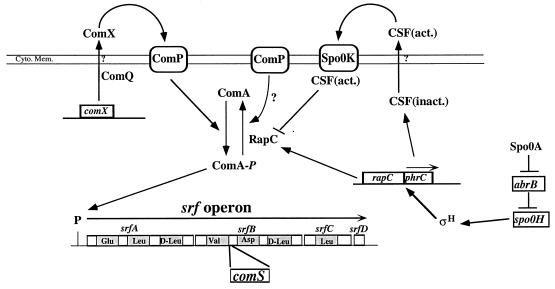
FIG. 1.
Diagram showing the relationships among the regulatory factors governing srf transcription initiation. The srf operon as well as the regions of the srf genes encoding the amino-acid-activating modules of the peptide synthesis enzyme surfactin synthetase is shown. The comS gene, encoding the regulator of competence development, lies within the srfB gene of srf. Two pheromone-dependent pathways are shown. One pathway requires the ComX peptide which is processed by ComQ and which activates srf transcription through its interaction with ComP. ComP activates ComA by donating a phosphate, but it might also exert negative control by dephosphorylating ComA in the absence of ComX (see Discussion). The pheromone CSF activates srf transcription by inhibiting the ComA-phosphate phosphatase RapC. Import of processed, active CSF [CSF(act.)] requires the oligopeptide permease Spo0K. The inactive form of CSF [CSF(inact.)] is encoded by phrC, transcription of which requires the ςH form of RNA polymerase. ςH is encoded by spo0H, the transcription of which is regulated by Spo0A. Spo0A represses the abrB gene, which encodes a negative regulator of spo0H transcription. Cyto. Mem., cytoplasmic membrane.
srf transcription is also regulated through the DNA-binding protein CodY in response to changes in the nutritional environment (42, 43). High external concentrations of amino acids promote CodY-dependent repression of srf transcription initiation. The CodY protein has been shown to interact specifically with the srf promoter DNA (42).
The addition of excess glucose and glutamine to growth medium inhibits the expression of many late-growth genes including srf (4, 10, 11). We have recently reported that increasing the culture medium pH of late-exponential-phase cultures of B. subtilis relieves glucose-glutamine-dependent repression of genes that require the alternative RNA polymerase sigma subunit ςH (4). We have examined the effect of culture pH on the production of surfactin by B. subtilis grown in excess glucose and glutamine and show that raising the culture pH relieves glucose-glutamine repression of surfactin production. This is the result of elevated srf transcription and is dependent on ComQ, ComA, and Spo0K but does not involve CodY-dependent negative control. Although the sensor histidine kinase ComP is required for maximal expression of srf at both low and neutral pHs, it is not required for induction of srf in response to pH elevation. Interestingly, the known regulators that function in the spo0K-dependent pathway of srf transcriptional activation, RapC and CSF, do not significantly function in pH-dependent srf transcriptional regulation. This suggests that there exist other mechanisms that influence srf expression in a spo0K-dependent manner.
MATERIALS AND METHODS
Bacterial strains.
All B. subtilis strains are listed in Table 1 and are derivatives of JH642. LAB452 was constructed by transforming JH642 with pXL5 (30), a plasmid containing a lacZ fusion to the 3.5-kb EcoRV-SalI fragment bearing the 5′ end of the srfA gene and the srf promoter region, with selection for chloramphenicol resistance (Cmr). LAB2583 was constructed by transforming LAB452 (30) with pJL62 (24) linearized with PstI with selection for spectinomycin resistance (Spcr) and screening for chloramphenicol sensitivity. LAB2690 and LAB2691 were produced by transforming LAB2583 with DNA from JMS750 and JMS751 (23) and selecting for Spcr Cmr and for Spcr and macrolide, lincosamide, and streptogramin B resistance (MLSr), respectively. LAB2692 was constructed by transforming LAB452 with DNA from JRL350 (24) and selecting for Cmr and MLSr. LAB2693 and LAB2694 were constructed by transforming LAB452 with DNA from LAB590 (ΔcomP sfp) (31, 54) and PS37 (codY) (42) and selecting for Cmr and neomycin resistance (Neor) and for Cmr Spcr, respectively.
TABLE 1.
B. subtilis strains
| Strain | Genotype and antibiotic resistance phenotype | Reference or source |
|---|---|---|
| LAB452 | trpC2 pheA1 srfA-lacZ::pXL5 Cmr | 30 |
| LAB991 | trpC2 pheA1 comA::Tn917 SPβc2Δ2::Tn917::srfA-lacZ(pXL5) Spcr | 33 |
| LAB2583 | trpC2 pheA1 srfA-lacZ::pXL5 Spcr | This study |
| LAB2690 | trpC2 pheA1 srfA-lacZ::pXL5 SpcrrapC::cat Spcr Cmr | This study |
| LAB2691 | trpC2 pheA1 srfA-lacZ::pXL5 ΔphrC MLSr Spcr | This study |
| LAB2692 | trpC2 pheA1 srfA-lacZ::pXL5 Δspo0K Cmr MLSr | This study |
| LAB2693 | trpC2 pheA1 srfA-lacZ::pXL5 ΔcomP Cmr Neor | This study |
| LAB2694 | trpC2 pheA1 srfA-lacZ::pXL5 ΔcodY Cmr Spcr | This study |
| JMS755 | trpC2 pheA1 ΔphrC comQ::spc Spcr Ermra | |
| OKB105 | pheA1 sfp | 29 |
| OKB192 | trpC2 pheA1 ΔcomQXPA::Tn917 | 32 |
Erm, erythromycin.
Transformation.
Competent B. subtilis cells were prepared as described previously (8, 42).
Culture medium.
Difco sporulation medium (DSM) was made as described previously (29) and was routinely used for all B. subtilis strains. DSM-GG was made by adding sterile 50% glucose and 2.5% glutamine to final concentrations of 1.9 and 0.1%, respectively, to sterile DSM.
Culture growth and β-galactosidase assays.
Inocula and DSM-GGTris (DSM-GG plus Tris-HCl) were prepared and sample collection for β-galactosidase assays was performed as described previously (4).
Construction of B. subtilis strain expressing epitope-tagged srfB.
Plasmid pOH9 bearing the carboxy-terminal end of srfB fused to the DNA encoding the influenza virus hemagglutinin 1 (HA1) epitope was constructed. pOH9 also contains the srf promoter region upstream from the srfB-HA sequence so as to drive the transcription of the srfC gene upon integration of the plasmid into the srf operon locus. First, the BamHI-PvuII fragment of pMMN50 (28) containing the Psrf promoter was inserted into BamHI-StuI-cleaved pBSK-HA (46). The resulting plasmid, pOH7, was then opened with EcoRI and ligated with the 1.8-kb fragment of pMMN7 (28) containing the cat gene of pC194 (19), yielding pOH8. The EcoRV fragment of p223-21K (28) containing the 3′ end of srfB was then inserted into the NruI site of pOH8, creating pOH9 bearing the srfB coding sequence fused with the DNA encoding the HA epitope. Competent cells of B. subtilis OKB105 (29) were transformed with pOH9 with selection for Cmr. A single Campbell recombination event was expected to result in integration of the srfB-HA sequence into the 3′ end of the chromosomal srfB gene and positioning of the srf promoter upstream of srfC. Several transformants were observed to be Srf− despite the presence of the Psrf-srfC fusion. The proper location of the integrated plasmid was confirmed by transformational linkage to srfC::Tn917 of strain LAB223 (28). SrfB-HA protein from the Srf− transformants was detected by Western immunoblot analysis using monoclonal antibody 12CA5 (55) (see Fig. 3C). Western blot analysis was performed as previously described (4, 11).
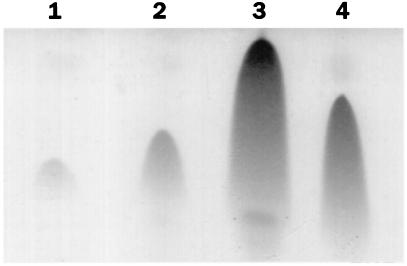
FIG. 3.
Effect of Tris-HCl addition on surfactin production during B. subtilis growth. A TLC analysis showing the amounts of surfactin initially in the medium (lane 1), in the negative control at 3 h after addition of water (lane 2), and at 3 h after addition of Tris-HCl (lane 3) is presented. Lane 4 contains a surfactin standard. Sample volumes analyzed were specific to the total amount of cell protein in each sample.
Protein analysis.
Cells from 10 ml of broth culture were suspended in 500 μl of 50 mM Tris-HCl, pH 7.8. After lysozyme treatment at 37°C for 15 min the cells were sonicated for 2 min. The cell debris was precipitated, and streptomycin sulfate was added to the crude extract to a final concentration of 1% (wt/vol). After 20 min of incubation on ice the nucleic acids were separated from the crude extract by centrifugation. Protein concentration was measured by using the procedure of Bradford (3). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed in 5% polyacrylamide gels according to the method of Laemmli (22).
Surfactin production analysis.
Surfactin was precipitated from 20 ml of culture supernatant by acidification at pH 2. The precipitate was extracted three times with methanol, and the collected extracts were evaporated prior to thin-layer chromatography (TLC) to increase surfactin concentration. A mixture of chloroform-methanol-H2O (65:25:4) was used as the eluent. The TLC matrix was silica gel 60 from Fisher Scientific (Pittsburgh, Pa.). Surfactin was identified by its characteristic Rf of 0.64 (29) after spraying and charring with H2SO4.
Assay of srfA-directed β-galactosidase in cultures treated with conditioned medium.
Stimulation of srfA-lacZ expression by cell-free supernatants was performed essentially as described by Solomon et al. (44). JH642, LAB2691 (ΔphrC), and OKB192 (ΔcomX) cultures used for supernatant harvest were grown to T2 (2 h after the end of exponential growth) in DSM-GG or DSM-GGTris at 37°C with shaking, which corresponded to an A595 of approximately 4.0 or 6.0, respectively. At that time, samples were collected and centrifuged at 5,000 rpm in a Sorvall SA600 rotor at 4°C, and the supernatants were filter sterilized and stored at −20°C. Before use in the assay the supernatants had their pHs adjusted to approximately 6.1 or 5.0 with NaOH or HCl, respectively. Control supernatants were collected from JH642 cultures grown in DSM-GG to an A595 of about 0.6 and were treated as described above. LAB452, LAB2692 (Δspo0K), and LAB2693 (ΔcomP) cultures for the srfA-lacZ assays were grown in DSM-GG at 37°C to an A595 of approximately 1.0 to 1.2 and then were mixed 1:1 with the pH-adjusted supernatants and reincubated at 37°C. Samples (1.0 ml) were then collected at 20-min intervals to test for β-galactosidase activity as described above.
RESULTS
Elevating the pH in DSM-GG cultures results in increased srfA-lacZ expression and surfactin production.
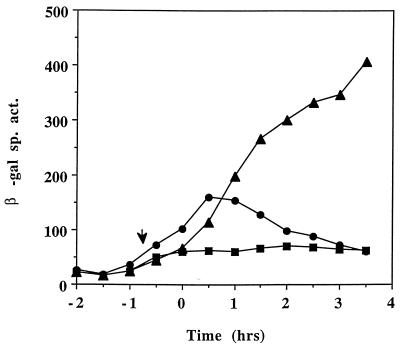
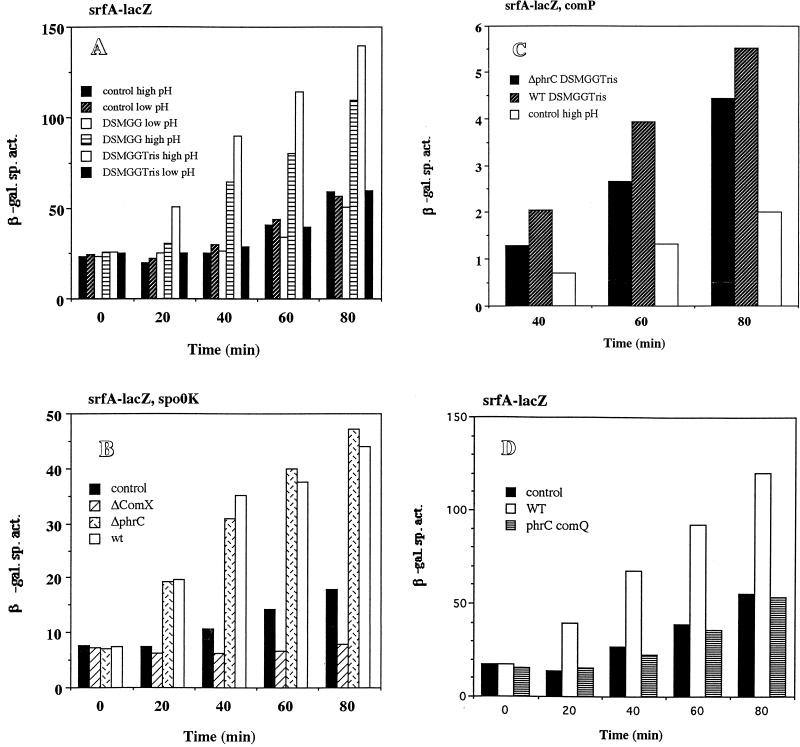
We had previously reported the inhibitory effect of high external glucose and glutamine concentrations on sporulation gene expression (4). A similar repressive effect was observed when srf-lacZ expression was examined in cells growing in glucose-glutamine medium (11), indicating that other late-growth processes were suppressed when the preferred carbon and nitrogen sources were present in excess. But rather than this being a form of catabolite control, we have found that the repression observed might be due to the reduced culture pH, resulting, presumably, from the accumulation of acidic glycolytic end products as the glucose-glutamine-supplemented culture reached the end of exponential growth. Simply raising the pH of the culture by the addition of Tris-HCl or MOPS (morpholinepropanesulfonic acid) buffer resulted in dramatic derepression of late-growth genes, particularly those requiring the RNA polymerase sigma subunit ςH for their transcription. As the expression of srf is also activated in late-growth cultures and also depends in part on spo0H (encoding ςH), we examined the effect of raising the pH of the cultures grown in DSM-GG on the expression of a srf-lacZ operon fusion. In the DSM-GG-grown culture the pH drops to 5.0 near the end of the exponential phase of growth (4). The culture medium pH was raised to about 6.5 when Tris-HCl was added and did not fall below 5.5 during the period when samples were collected for assay of srf-directed β-galactosidase activity. Figure 2 shows that the presence of glucose and glutamine in DSM suppresses srfA-lacZ expression, but adjustment of culture pH with Tris-HCl increased the expression approximately fivefold above that observed in the untreated culture. The accumulation of the srf gene products (SrfA, SrfB, and SrfC [50]) as well as the production of surfactin also increased following adjustment of the DSM-GG pH. Figure 3 shows the relative levels of surfactin, as determined by TLC, immediately prior to Tris-HCl addition (T = 0) to a culture of OKB105 (Srf+) cells in DSM-GG and after Tris-HCl addition (T = 3.5) to the DSM-GG-grown culture and a mock-treated DSM-GG culture. The amounts of surfactin synthetase proteins produced at the above times were also examined in cells of DSM-GG and Tris-HCl-treated DSM-GG cultures. More SrfA, -B, and -C proteins were produced in the Tris-treated culture than in the untreated DSM-GG culture medium (data not shown). Cells of strain LAB2426 containing an influenza virus HA epitope DNA-tagged allele of srfB were also tested for pH-dependent expression of srf. A higher level of SrfB-HA, as indicated by the presence of protein reacting with the 12CA5 monoclonal antibody (55), in the Tris-HCl-treated cells than in the untreated cells was observed (data not shown).
FIG. 2.
Expression of srfA-lacZ in LAB452 grown in DSM-GG (squares), DSM-GGTris (triangles), and DSM without GG (circles). T0 denotes the onset of stationary phase. The arrow indicates the time of Tris-HCl addition. β-Gal, β-galactosidase.
Tris-HCl addition raises srfA-lacZ activity in a comP mutant.
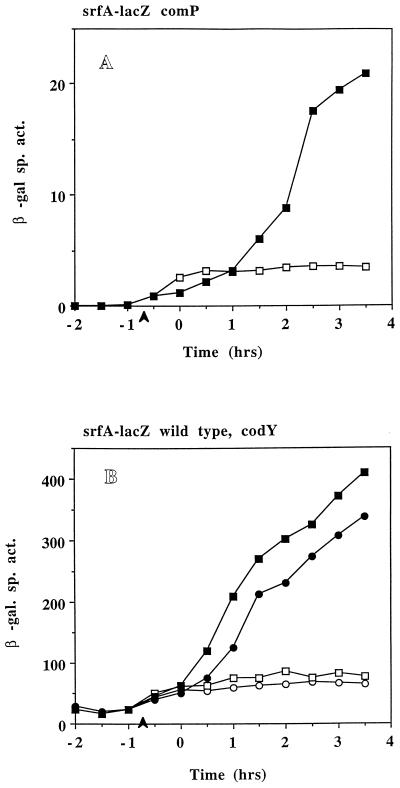
In order to determine if the ComP-ComA sensory transduction system (9) was the target of pH-dependent regulation of srf transcription, the expression of srfA-lacZ was examined in comP and comA mutants. Deletions in comP (Fig. 4A) and comA (data not shown) cause a large decrease in srfA-lacZ activity as has been previously observed. However, increasing the culture pH produces a fourfold increase in srfA-lacZ activity in the comP mutant compared to that in the cultures grown in DSM-GG without pH adjustment (Fig. 4A). A slight increase in srfA-lacZ activity was seen in a comA mutant (LAB991) when Tris-HCl was added to the culture medium, but the activity was too low in this strain for reliable calculation. These results indicate that optimum expression of srf after pH adjustment requires the ComP-ComA system, but since induction is still observed, the sensor kinase, ComP, is likely not the target of the pH-dependent effect.
FIG. 4.
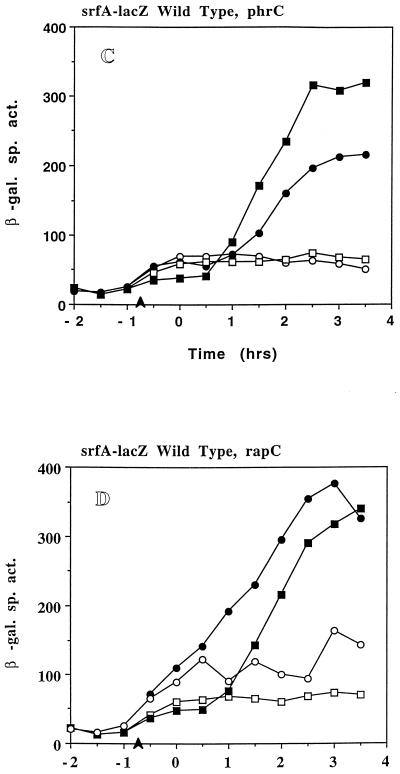
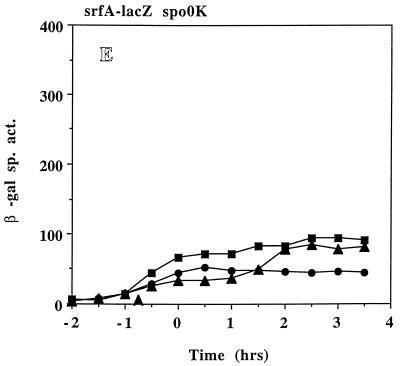
(A) Expression of srfA-lacZ in LAB2693 (srfA-lacZ ΔcomP) grown in DSM-GG (open squares) and DSM-GGTris (filled squares). (B) Expression of srfA-lacZ in LAB452 (srfA-lacZ) (squares) and in LAB2694 (srfA-lacZ ΔcodY) (circles) grown in DSM-GG (open symbols) and DSM-GGTris (filled symbols). (C) Expression of srfA-lacZ in LAB2691 (srf-lacZ ΔphrC) grown in DSM-GG (open circles) and DSM-GGTris (filled circles) and in LAB2583 (SPβsrfA-lacZ) grown in DSM-GG (open squares) and DSM-GGTris (filled squares). (D) Expression of srfA-lacZ in LAB2690 (srfA-lacZ ΔrapC) grown in DSM-GG (open circles) and DSM-GGTris (filled circles) and in LAB2583 grown in DSM-GG (open squares) and DSM-GGTris (filled squares). (E) Expression of srfA-lacZ in LAB2692 (srfA-lacZ Δspo0K) grown in DSM-GG (circles) and DSM-GGTris (triangles) and in LAB452 (srfA-lacZ) grown in DSM-GG (squares). Refer to Fig. 1 for details.
A deletion in codY does not relieve the pH-dependent reduction of srfA-lacZ activity.
It was necessary to determine if the repression of srf observed at low pH was due to the known transcriptional repressor of srf transcription, CodY (42). Hence, srfA-lacZ expression was examined in a codY mutant. No relief of repression was observed for a codY mutant in a low-pH culture (Fig. 4B), indicating that CodY was not functioning in pH-dependent srf repression. Figure 4B shows that a deletion in codY causes a slight but reproducible decrease in srfA-lacZ activity when DSM-GG cultures are treated with Tris-HCl. It is possible that the codY product may also have an indirect role in srfA expression when B. subtilis is grown in DSM-GGTris.
Mutation in spo0K severely impairs pH-dependent induction of srfA-lacZ.
Neither ComP nor CodY appeared to be targets of the apparent pH-dependent control of srf. The role of the CSF/Spo0K/RapC system (23, 44) in srf regulation was next investigated. Figure 4C to E shows srfA-lacZ expression in DSM-GG and DSM-GGTris cultures of strains containing lesions in phrC, the gene which encodes CSF, rapC, which encodes the putative target of CSF, i.e., the Rap phosphatase that inactivates ComA, and spo0K, the oligopeptide permease gene, respectively. Expression of srfA-lacZ in LAB 2691 (ΔphrC) in DSM-GG (Fig. 4C) is comparable to that of the wild-type parent but shows a fourfold induction following Tris-HCl addition and about 65% of the induced activity observed in the wild-type cells. LAB2690 (ΔrapC) shows a slight increase in srfA-lacZ expression above that of LAB2583 (RapC+) in DSM-GG (Fig. 4D) but a five- to sixfold induction of srfA-lacZ expression after Tris-HCl addition, comparable to that observed in wild-type cells. In DSM-GGTris, srfA-lacZ expression in LAB2690 begins about 1.0 to 1.5 h earlier, and overall expression is approximately 20% greater than that in LAB2583. Both LAB2690 and LAB2691 showed cell yields and culture pH profiles similar to those of LAB2583 (wild type) in both media (data not shown). Unlike what was found for strains LAB2690 and -2691, Tris-HCl addition to DSM-GG cultures of LAB2692 (Δspo0K) failed to increase srfA-lacZ expression above that of the wild type in DSM-GG (Fig. 4E). A less than twofold induction was observed in the spo0K mutant when Tris-HCl was added to the culture. The fold increases in srfA-lacZ expression upon pH elevation were determined for all of the mutant strains tested along with those of the wild-type parent cultures run in parallel (Table 2). All strains showed a three- to eightfold increase in srfA-lacZ expression except for the spo0K mutant, which exhibited a less than twofold increase in expression upon pH adjustment. This suggests that the Spo0K peptide is involved in the pH-dependent induction of srf expression.
TABLE 2.
Fold increase of srfA-lacZ expression in DSM-GG by pH adjustment with Tris-HCla
| Type of strain | Expt | Fold increase (SD) in srfA-lacZ expression in strain containing:
|
||||
|---|---|---|---|---|---|---|
| comP | rapC | phrC | spo0K | codY | ||
| Mutant | 1 | 8.1 (1.8) | 2.8 (0.9) | 3.7 (0.6) | 1.5 (0.1) | 4.7 (0.6) |
| 2 | 5.4 (0.6) | 3.2 (0.4) | 3.5 (0.3) | 1.8 (0.1) | 5.6 (0.6) | |
| Wild type | 1 | 5.0 (0.6) | 4.5 (0.4) | 4.6 (0.3) | 3.4 (0.4) | 4.7 (0.4) |
| 2 | 2.8 (0.1) | 4.4 (0.5) | 5.2 (0.6) | 3.6 (0.5) | 5.6 (0.8) | |
Ratios of srfA-lacZ expression (Miller units) in DSM-GGTris cultures to that in DSM-GG cultures were calculated for samples of each strain collected at T2.5, T3, and T3.5. Data are averages from two sets of experiments. As controls, wild-type srf-lacZ strains were analyzed in parallel with each mutant.
srfA-lacZ-inducing substance in late-growth culture supernatant has low level of activity at low pH.
The involvement of Spo0K oligopeptide permease in the pH-dependent induction of srf, along with the modest effects the phrC and rapC mutations had on the observed pH-dependent increase of srfA-lacZ, suggested that there exists another substance, apart from CSF and ComX, in conditioned medium of late-growth cultures that could stimulate srf transcription. To examine this possibility, the srf-inducing activities of conditioned culture media were tested by diluting early-growth cultures one to one in cell-free medium supernatant obtained from late-growth DSM-GG and DSM-GGTris cultures. Supernatant samples were collected from the following centrifuged cultures: (i) late-growth DSM-GG at a pH of ∼5.0, (ii) DSM-GGTris at a pH of ∼6.6, and (iii) early-growth cultures in DSM-GG. Each of the three supernatant samples was split, and one half was subjected to pH adjustment. Thus, half of the first supernatant was treated with NaOH to raise the pH to 6.1. Half of the second supernatant was treated with HCl to reduce the pH to 5.0. Finally, the early-growth supernatant was divided into high-pH (6.1) and low-pH fractions (5.0). srf-activating pheromones had been shown to accumulate in high cell density late in culture growth and to be in low concentration in early-growth cultures (16, 25). Therefore, the early-growth supernatants were included as negative controls.
Addition of cell-free supernatant from late-growth cultures grown in DSM-GGTris to low-density cultures resulted in the induction of srfA-lacZ, but this induction was inhibited if the pH of the conditioned medium was reduced to a pH of 5 (Fig. 5A). The low-pH supernatant of a late-growth DSM-GG culture did not stimulate srfA-lacZ, but induction of the fusion was observed if the pH of this supernatant fluid was first adjusted to a pH of 6.1 with NaOH. This suggests that the capacity to extracellularly induce srf transcription is present in the DSM-GG medium but the low pH inhibits this activity. Neither of the control supernatants, from early-growth cultures with either a low or a high pH, stimulated srfA-lacZ to the same extent as either of the late-growth supernatants (Fig. 5A).
FIG. 5.
Effects of DSM-GG and DSM-GGTris culture supernatants on expression of srfA-lacZ. LAB452 (srfA-lacZ), LAB2692 (srfA-lacZ Δspo0K), and LAB2693 (srfA-lacZ ΔcomP) were grown in DSM-GG to a low density and then mixed 1:1 with pH-adjusted supernatants as described in Materials and Methods. Samples were collected every 20 min for β-galactosidase activity assays. Figures are representative of at least two experiments run under identical conditions. (A) Recipient cultures contained cells of strain LAB452 (srfA-lacZ). (B) Culture medium supernatants were collected from late-growth cultures of strains OKB192 (ΔcomX), LAB2691 (ΔphrC), and LAB452 (wild type). All supernatants were adjusted to pH 6.1. Recipient cultures contained cells of strain LAB2692 (srfA-lacZ Δspo0K). (C) All supernatants were adjusted to pH 6.1. Recipient cultures contained cells of strain LAB2693 (srfA-lacZ ΔcomP). (D) Supernatants of late-growth JMS377 (ΔcomQ ΔphrC) culture, JH642 (wild-type parent) late-growth culture, and JH642 early-growth culture adjusted to pH 6.1. Recipient early-growth cultures contained cells of LAB452 (srfA-lacZ).
The induction of srfA-lacZ by the addition of high-pH-conditioned medium was found to depend on Spo0K, as expected from the experiments described above and from previous studies (39, 44, 45). Supernatant samples from late-growth, high-pH cultures of wild-type and ΔphrC cells were found to stimulate srfA-lacZ when added to spo0K cells of a low-density DSM-GG culture (Fig. 5B). The stimulation of srfA-lacZ caused by the ΔphrC supernatant was likely due to the presence of ComX pheromone and the sensory transduction system of ComP-ComA. Indeed, when supernatant was obtained from a late-growth culture of a ΔcomX strain and tested for srfA-lacZ-stimulating activity in a spo0K mutant, none was observed. This might suggest that the spo0K-dependent induction of srf at neutral pH requires an extracellular substance other than CSF.
That this substance is not CSF (PhrC) was shown by adding a high-pH, late-growth supernatant of a ΔphrC culture to cells of a srfA-lacZ comP strain, which should not respond to ComX. The level of srfA-lacZ after treatment of low-density culture with supernatant from a wild-type or ΔphrC culture was higher than that observed with supernatant from a wild-type low-density culture (Fig. 5C), suggesting the presence of an additional extracellular factor controlling srf transcription.
ComQ is required for extracellular induction of srfA-lacZ at neutral pH.
Although the data above indicate the need for Spo0K, and not ComP, for the pH-dependent stimulation of srf expression, they do not rule out the possibility that ComX might somehow function in a Spo0K-dependent induction process. A late-growth culture supernatant from double mutant JMS755 (ΔphrC comQ::spc, a gift from B. Lazazzera) was added to early-growth cultures of LAB452 (srfA-lacZ). As shown in Fig. 5D, the supernatant from the double mutant showed no srfA-lacZ-stimulating activity, while the culture supernatant of the wild type activated srf expression. In a wild-type strain, both Spo0K and ComQ (and very likely ComX) are required for the extracellular activation of srf at neutral pH.
DISCUSSION
The expression of a srfA-lacZ operon fusion is affected by culture medium pH, as shown by an examination of srf-directed β-galactosidase activity in cells grown in nutrient broth sporulation medium supplemented with glucose and glutamine. Under these conditions, the pH of the culture drops to below 5.0 near the end of the exponential phase and srfA-lacZ expression, normally increasing at this point in the growth curve, remains low and approximately at the levels observed in early and mid-log phase. The addition of a pH stabilizer to raise the pH causes sharp increases in srfA-lacZ expression and surfactin production (Fig. 2 and 3). The maximum expression of srf at both low and neutral pH requires the ComP-ComA signal transduction system and presumably ComX, the peptide signal that is believed to mediate the cell-density-dependent activation of ComP (25). The pH-dependent induction of srf requires spo0K, which is known to function in the CSF-mediated activation of srf transcription (44, 45). Interestingly, the elimination of CSF by deletion of the phrC gene does not impair induction of srf expression in response to pH elevation, although expression levels do not reach those of wild-type srfA-lacZ-bearing cells. The negative regulators of srf transcription, RapC and CodY, also appear not to participate in pH-dependent control of srf, as evidenced by the absence of srf derepression in rapC and codY cells under low-pH culture conditions.
Experiments with cell-free culture supernatants added to early-growth cultures of srf-lacZ-bearing cells show that the extracellular factor necessary for induction of srf does not function at low pH. The low pH of the DSM-GG-grown culture could have several effects on the expression of srfA-lacZ. It is possible that the spo0K-encoded oligopeptide permease is not functional when the pH of the external environment is low. It is also possible that the peptide pheromone activating srf transcription through a Spo0K-dependent mechanism is not in the proper ionic state at low pH. Because the culture supernatant of a low-pH culture can be made active with respect to srf stimulation when its pH is raised, it is possible that the peptide is present in the low-pH DSMGG culture but is not able to activate srf induction.
The requirement for ComQ, and hence ComX, for the extracellular, spo0K-dependent induction of srf at neutral pH (Fig. 5D) might suggest that ComX also acts through a mechanism involving the Spo0K oligopeptide permease. This is supported by the data shown in Fig. 4A and 5C which show that ComP, the reported target of ComX, is not required for pH-dependent, extracellular induction of srf expression. However, previous studies from the Grossman laboratory provide evidence that is inconsistent with the hypothesis that ComX functions to induce srf through a Spo0K-dependent mechanism (25, 44, 45). A reasonable explanation of our results is that ComP functions both positively and negatively in the regulation of srf transcription. ComX has two functions, stimulation of ComP autokinase activity, rendering ComP a phosphate donor for ComA, and inhibition of a ComP phosphatase which can dephosphorylate ComA-P (Fig. 1). Histidine protein kinases of the sensor class of two-component regulatory proteins can possess both phosphate-donating and phosphate-removing activities that are affected by ligand binding (20, 21). In a comP mutant, srf expression is low but is still inducible by a pH- and Spo0K-dependent activity. If ComX is absent, due to a mutation of comQ, the product of which functions in ComX processing and secretion, the ComP phosphatase is active and dephosphorylates ComA. Thus, even in the presence of Spo0K and the extracellular factor governing pH-dependent srf induction, ComP phosphatase reduces ComA activity. However, if comP is eliminated (Fig. 4A and 5C) extracellular pH-dependent induction is observed, even though the level of expression of srfA-lacZ, overall, is low. The comP-independent induction of srf expression does not involve CSF (PhrC), as shown in Fig. 5C. An appropriate course of study, based on these results, is to attempt characterization of the extracellular srf induction mechanism in a comP mutant background.
The requirement for Spo0K in srf induction upon pH elevation suggests the involvement of the Phr/Rap system, but Spo0K could mediate the uptake of other peptide factors which might elicite an entirely different response. The oligopeptide permease is not specific for Phr-like factors but has also been implicated in the uptake of other peptides including the peptide antibiotic bialophos (36). Mutations in spo0K render B. subtilis cells bialophos resistant (36). Hence, it is possible that the spo0K-mediated response to pH elevation is a consequence of multiple extracellular peptide factors that activate separate, distinct regulatory response pathways.
ACKNOWLEDGMENTS
We thank A. Grossman and members of his lab as well as A. L. Sonenshein for providing strains used in this study.
The research reported herein was supported by grant GM54898 from the National Institutes of Health and by funds provided by USDA/ARS project no. 6435-41000-062-015.
REFERENCES
- 1.Arima K, Kainuma A, Tamura G. Surfactin, a crystalline peptide lipid surfactant produced by Bacillus subtilis: isolation, characterization, and its inhibition of fibrin clot formation. Biochem Biophys Res Commun. 1968;31:488–494. doi: 10.1016/0006-291x(68)90503-2. [DOI] [PubMed] [Google Scholar]
- 2.Bernheimer A W, Avegad L S. Nature and properties of a cytolytic agent produced by Bacillus subtilis. J Gen Microbiol. 1970;61:361–369. doi: 10.1099/00221287-61-3-361. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Cosby W M, Zuber P. Regulation of Bacillus subtilis ςH (Spo0H) and AbrB in response to changes in external pH. J Bacteriol. 1997;179:6778–6787. doi: 10.1128/jb.179.21.6778-6787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosmina P, Rodriguez F, de Ferra F, Grandi G, Perego M, Venema G, van Sinderen D. Sequence and analysis of the genetic locus responsible for surfactin synthesis in Bacillus subtilis. Mol Microbiol. 1993;8:821–831. doi: 10.1111/j.1365-2958.1993.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 6.Desai J D, Banat I M. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza C, Nakano M M, Zuber P. Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:9397–9401. doi: 10.1073/pnas.91.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971;56:209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- 9.Dubnau D, Hahn J, Roggiani M, Piazza R, Weinrauch Y. Two-component regulators and genetic competence in Bacillus subtilis. Res Microbiol. 1994;145:403–411. doi: 10.1016/0923-2508(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 10.Frisby D, Zuber P. Analysis of the upstream activating sequence and site of carbon and nitrogen source repression in the promoter of an early-induced sporulation gene of Bacillus subtilis. J Bacteriol. 1991;173:7557–7564. doi: 10.1128/jb.173.23.7557-7564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisby D F, Zuber P. Mutations in pts cause catabolite-resistant sporulation and altered regulation of spo0H in Bacillus subtilis. J Bacteriol. 1994;176:2587–2595. doi: 10.1128/jb.176.9.2587-2595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuma S, Fujishima Y, Corbell N, D’Souza C, Nakano M M, Zuber P, Yamane K. Nucleotide sequence of 5′ portion of srfA that contains the region required for competence establishment in Bacillus subtilis. Nucleic Acids Res. 1993;21:93–97. doi: 10.1093/nar/21.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 14.Gaisser S, Hughes C. A locus coding for putative non-ribosomal peptide/polyketide synthase functions is mutated in a swarming-defective Proteus mirabilis strain. Mol Gen Genet. 1997;253:415–427. doi: 10.1007/s004380050339. [DOI] [PubMed] [Google Scholar]
- 15.Galli G, Rodriguez F, Cosmina P, Pratesi C, Nogarotto R, de Ferra F, Grandi G. Characterization of the surfactin synthetase multienzyme complex. Biochim Biophys Acta. 1994;1205:19–28. doi: 10.1016/0167-4838(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 16.Grossman A D, Losick R. Extracellular control of spore formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1988;85:4369–4373. doi: 10.1073/pnas.85.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn J, Dubnau D. Growth stage signal transduction and the requirements for srfA induction in development of competence. J Bacteriol. 1991;173:7275–7282. doi: 10.1128/jb.173.22.7275-7282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamoen L W, Eshuis H, Jongbloed J. A small gene, designated comS, located within the coding region of the fourth amino acid-activating domain of srfA, is required for competence development in Bacillus subtilis. Mol Microbiol. 1995;15:55–63. doi: 10.1111/j.1365-2958.1995.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 19.Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982;150:815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igo M M, Ninfa A J, Stock J B, Silhavy T J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 21.Jin T, Inouye M. Ligand binding to the receptor domain regulates the ratio of kinase to phosphatase activities of the signaling domain of the hybrid Escherichia coli transmembrane receptor, Taz1. J Mol Biol. 1993;232:484–492. doi: 10.1006/jmbi.1993.1404. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lazazzera B A, Solomon J M, Grossman A D. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell. 1997;89:917–925. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- 24.Ledeaux, J., and A. D. Grossman. Personal communication.
- 25.Magnuson R, Solomon J, Grossman A D. Biochemical and genetic characterization of a competence pheromone from Bacillus subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 26.Matsuyama T, Kaneda K, Nakgawa Y, Isa K, Hara-Hotta H, Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992;174:1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menkhaus M, Ullrich C, Kluge B, Vater J, Vollenbroich D, Kamp R M. Structural and functional organization of the surfactin synthetase multienzyme system. J Biol Chem. 1993;268:7678–7684. [PubMed] [Google Scholar]
- 28.Nakano M M, Magnuson R, Myers A, Curry J, Grossman A D, Zuber P. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol. 1991;173:1770–1778. doi: 10.1128/jb.173.5.1770-1778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano M M, Marahiel M A, Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988;170:5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano M M, Xia L, Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991;173:5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano, M. M., and P. Zuber. Unpublished data.
- 32.Nakano M M, Zuber P. Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol. 1989;171:5347–5353. doi: 10.1128/jb.171.10.5347-5353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano M M, Zuber P. Mutational analysis of the regulatory region of the srfA operon in Bacillus subtilis. J Bacteriol. 1993;175:3188–3191. doi: 10.1128/jb.175.10.3188-3191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakano M M, Zuber P. The primary role of comA in the establishment of the competent state in Bacillus subtilis is to activate the expression of srfA. J Bacteriol. 1991;173:7269–7274. doi: 10.1128/jb.173.22.7269-7274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perego M. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc Natl Acad Sci USA. 1997;94:8612–8617. doi: 10.1073/pnas.94.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perego M, Glaser P, Hoch J A. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol Microbiol. 1996;19:1151–1157. doi: 10.1111/j.1365-2958.1996.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 37.Perego M, Hanstein C, Welsh K M, Djavakhishvili T, Glaser P, Hoch J A. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell. 1994;79:1047–1055. doi: 10.1016/0092-8674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 38.Perego M, Higgins C F, Pearce S R, Gallagher M P, Hoch J A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991;5:173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 39.Perego M, Hoch J A. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:1549–1553. doi: 10.1073/pnas.93.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roggiani M, Dubnau D. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J Bacteriol. 1993;175:3182–3187. doi: 10.1128/jb.175.10.3182-3187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudner D Z, Ledeaux J R, Ireton K, Grossman A D. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991;173:1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serror P, Sonenshein A L. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J Bacteriol. 1996;178:5910–5915. doi: 10.1128/jb.178.20.5910-5915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slack F J, Serror P, Joyce E, Sonenshein A L. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol Microbiol. 1995;15:689–702. doi: 10.1111/j.1365-2958.1995.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 44.Solomon J M, Lazazzera B A, Grossman A D. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 1996;10:2014–2024. doi: 10.1101/gad.10.16.2014. [DOI] [PubMed] [Google Scholar]
- 45.Solomon J M, Magnuson R, Srivastava A, Grossman A D. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 1995;9:547–558. doi: 10.1101/gad.9.5.547. [DOI] [PubMed] [Google Scholar]
- 46.Surdej P, Jacobs-Lorena M. Strategy for epitope tagging the protein-coding region of any gene. BioTechniques. 1994;17:560–565. [PubMed] [Google Scholar]
- 47.Tsukagushi N, Tamura G, Arima K. A novel protoplast-bursting factor (surfactin) obtained from Bacillus subtilis IAM. II. The interaction of surfactin with bacterial membranes and lipids. Biochim Biophys Acta. 1970;196:211–214. doi: 10.1016/0005-2736(70)90008-8. [DOI] [PubMed] [Google Scholar]
- 48.van Sinderen D, Galli G, Cosmina P, de Ferra F, Withoff S, Venema G, Grandi G. Characterization of the srfA locus of Bacillus subtilis: only the valine-activating domain of srfA is involved in the establishment of genetic competence. Mol Microbiol. 1993;8:833–841. doi: 10.1111/j.1365-2958.1993.tb01630.x. [DOI] [PubMed] [Google Scholar]
- 49.van Sinderen D, Withof S, Boels H, Venema G. Isolation and characterization of comL, a transcription unit involved in competence development of Bacillus subtilis. Mol Gen Genet. 1990;224:396–404. doi: 10.1007/BF00262434. [DOI] [PubMed] [Google Scholar]
- 50.Vollenbroich D, Mehta N, Zuber P, Vater J, Kamp R M. Analysis of surfactin synthetase subunits in srfA mutants of Bacillus subtilis OKB105. J Bacteriol. 1994;176:395–400. doi: 10.1128/jb.176.2.395-400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vollenbroich D, Pauli G, Özel M, Vater J. Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl Environ Microbiol. 1997;63:44–49. doi: 10.1128/aem.63.1.44-49.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vollenbroich D, Pauli G, Vater F, Özel M, Kamp R M. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals. 1997;3:289–297. doi: 10.1006/biol.1997.0099. [DOI] [PubMed] [Google Scholar]
- 53.Weinrauch Y, Guillen N, Dubnau D A. Sequence and transcription mapping of Bacillus subtilis competence genes comB and comA, one of which is related to a family of bacterial regulatory determinants. J Bacteriol. 1989;171:5362–5375. doi: 10.1128/jb.171.10.5362-5375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinrauch Y, Penchev R, Dubnau E, Smith I, Dubnau D. A Bacillus subtilis regulatory gene product for genetic competence and sporulation resembles sensor protein members of the bacterial two-component signal-transduction systems. Genes Dev. 1990;4:860–872. doi: 10.1101/gad.4.5.860. [DOI] [PubMed] [Google Scholar]
- 55.Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]