Abstract
Objectives
To compare acute cardiorespiratory responses during high intensity interval training (HIIT) and moderate intensity continuous training (MICT) on a recumbent handcycle in persons with spinal cord injury (PwSCI).
Methods
Eleven males and nine females with chronic SCI (T3 – L5), aged 23 (9) years, participated in this within-subject design. Based off peak power outputs from an incremental test to exhaustion, participants engaged in a HIIT and MICT session at matched workloads on a recumbent handcycle. Workloads (Joules), time, oxygen uptake (VO2), metabolic equivalent of task (MET), heart rate (HR), and energy expenditure (kcal) were recorded during HIIT and MICT.
Results
Total workload was similar across HIIT (87820 ± 24021 Joules) and MICT sessions (89044 ± 23696 Joules; p > .05). HIIT (20.00 [.03] minutes) was shorter in duration than MICT (23.20 [2.56]; p < .01). Average VO2 (20.96 ± 4.84 vs. 129.38 ± 19.13 mL/kg/min O2), MET (7.54 ± 2.00 vs. 6.21 ± 1.25), and HR (146.26 ± 13.80 vs. 129.38 ± 19.13 beats per minute) responses were significantly greater during HIIT than MICT (p < .01). Participants burned significantly more kilocalories during HIIT (128.08 ± 35.65) than MICT (118.93 ± 29.58; p < .01) and at a faster rate (6.40 ± 1.78 [HIIT] vs. 5.09 ± 1.14 [MICT] kcal/min; p < .01).
Conclusion
HIIT elicits greater increases in oxygen uptake and HR than MICT in PwSCI. In significantly less time, HIIT also burned more calories than MICT.
Keywords: cardiorespiratory, handcycle, high intensity interval training, physical activity, spinal cord injury
Introduction
Because persons with spinal cord injury (PwSCI) derive less optimal benefits from moderate intensity continuous training (MICT) than their able-bodied peers,1-3 novel approaches to exercise are needed to reduce the high prevalence of cardiometabolic disease (CMD).1,4,5 In populations living with CMD, high intensity interval training (HIIT) provokes numerous health benefits6,7 and may be a promising strategy for PwSCI.1 HIIT alternates short, intense submaximal bursts with periods of active recovery.8 In healthy populations, HIIT more efficiently improves cardiorespiratory fitness than traditional modes of endurance training.9-11 For PwSCI, who contend with autonomic and cardiac output limitations, HIIT’s rest periods may compensate for central adaptive deficiencies associated with SCI while also stimulating peripheral muscular adaptations.12,13 HIIT’s reduced time commitment may also entice participation in PwSCI who view exercise as excessively time consuming.14,15
Despite the clear benefits observed in other populations with CMD, existing protocols have not led to enhanced fitness adaptations in PwSCI compared to MICT.16 Graham et al. showed similar cardiorespiratory fitness adaptations in VO2peak following a 6-week intervention of MICT and HIIT.17 Gauthier et al. showed minimal changes in VO2peak following a 6-week HIIT intervention.18 An examination of acute responses to HIIT also revealed suboptimal results, with Astorino and Thum showing that MICT burned more calories than HIIT.19 According to Peters et al., these suboptimal findings may be due to dosing strategies within current HIIT designs (i.e., workload accumulation, intensity levels, interval durations).16 More work is needed to determine the proper dosage of HIIT for PwSCI.16
Exercise volume accumulation, as represented by metabolic equivalent of task (MET), is one of the most important indicators for optimizing health.20 MET can also be viewed as an indirect estimate of energy expenditure.21 Nonetheless, in existing randomized controlled trials (RCT) for PwSCI, exercise volume is greater during MICT than HIIT.16,17 Notably, Graham et al. had participants complete 225% more work during MICT than HIIT. As training adaptations follow a dose-response to volume accumulation,22 HIIT protocols may need to be calibrated with greater overall work outputs and exercise volumes to elicit positive training adaptations.16
In able-bodied handcyclists with matching workloads for HIIT and MICT, HIIT elicited superior fitness adaptations in significantly less time per training session.23 No research has utilized this type of approach in PwSCI. Additionally, no within-subject design has employed HIIT using a handcycle.14,24 Most protocols have utilized research-grade upper-body asynchronous ergometers, which are biomechanically and bioenergetically inefficient25 and may be inaccessible for PwSCI. In contrast to an upper-body ergometer, recumbent handcycling is one of the most popular adapted sports for PwSCI.26
The purpose of this study is to compare the cardiometabolic responses of HIIT and MICT on a stationary recumbent handcycle at matched workloads. We hypothesize that HIIT will elicit a greater cardiorespiratory response than MICT. Cardiorespiratory responses will be measured in oxygen uptake (VO2), MET, energy expenditure (kilocalories or kcal), and heart rate (HR). Additionally, we predict that HIIT sessions will be completed in significantly less time than MICT sessions.24
Methods
Study design
During this within-subjects design, participants were exposed to all experimental conditions. All study procedures were approved by the institutional review board (IRB) at University of Illinois at Urbana-Champaign (UIUC). Before study participation, written informed consent was obtained from all participants.
Participants
Adapted sports athletes at UIUC were recruited between June 2021 and April 2022 to participate in the study. Interested individuals were invited to participate if they met the following inclusion criteria: (1) 18 to 45 years of age, (2) neurologically stable SCI or spinal cord dysfunction at least 12 months post-SCI onset, (3) exposed to vigorous intensity exercise (rating of perceived exertion [RPE] of 15 to 20) within the last 30 days, and (4) meeting American College of Sports Medicine (ACSM) minimum recommendations for physical activity.6 Individuals were excluded from participation if they had orthopedic upper extremity pain, presented signs or symptoms of cardiovascular disease, or had any health conditions or injuries preventing safe participation. Participants were cleared by a medical professional prior to participation. Although not an inclusion criterion, most participants stated to researchers that they had minimal experience with handcycling. All participants were treated in accordance with UIUC and IRB standards of human subject research.
Baseline assessment
Participants attended a baseline assessment to determine handcycle configurations and intensities for HIIT and MICT experimental trials. Participants were asked to refrain from strenuous exercise, caffeine, and alcohol for 24 hours prior to laboratory arrival per ACSM Guidelines for Exercise Testing.6 Upon arrival, participants completed a demographics survey. Then, they transferred from their everyday wheelchair to a recumbent handcycle (Invacare, Elyria, OH). All handcycling activities were conducted on a recumbent handcycle that was connected to an indoor trainer (Lemond-Hoist, Poway, CA). Once comfortably situated in the handcycle, the headset was adjusted so all participants’ elbows were flexed at 15 to 20 degrees during the maximal reach phase of the propulsion cycle. Power production is maximized when the elbows maintain 15 to 20 degrees of flexion during the maximal reach phase of handcycling.27 Once the headset position was determined, researchers measured and recorded the headset shaft distance so handcycle configurations could be replicated for future sessions. Figure 1 represents a depiction of the experimental set-up.
Figure 1.
Experimental set-up.
Participants underwent an incremental test to exhaustion to obtain VO2peak and peak power output (PPO). Given the scarcity of literature involving an incremental test to exhaustion using a handcycle, researchers used a similar protocol to Schoenmakers et al.23 This incremental test began with participants remaining steady on the handcycle for 5 minutes so resting HR could be collected. Once resting HR was collected, participants began a 5-minute warm-up at 30 watts (W). Power was recorded and displayed in real-time for participants using an SRM power meter (SRM, Colorado Springs, CO). Following the warm-up, participants cycled for 1 minute at 30 W and then at an increase of 10 W every minute until exhaustion occurred. To increase power output, participants were instructed to increase their cadence rather than adjust the resistance through the manual gear shift. The resistance on the gear shift of the handcycle was held constant throughout all exercise testing. The test was terminated when participants voluntarily expressed exhaustion or power output targets were not maintained for greater than 10 seconds. Throughout the incremental test, RPE was collected by researchers to ensure that participants were reaching the intended intensity levels. Additionally, participants were connected to an open-circuit indirect calorimetry system (TrueOne; ParvoMedics, Salt Lake City, UT) throughout the incremental test to capture respiratory measures breath by breath (as shown in Figure 1). VO2, HR, METs, and kilocalories were calculated on a 15-second average by the TrueOne software. VO2peak was determined as the highest magnitude VO2 measure recorded by the TrueOne software during the incremental test. PPO was determined using the following equation28:
Where PPO is measured in W; Pfinal is the last exercise intensity completed for 60 seconds (in W); and t was the number of seconds sustained during the final, uncompleted exercise intensity.
Participants remained on the handcycle for 8 to 10 minutes to recover from the incremental test. During this time, participants were given the option to rest or actively recover by cycling at 10 to 30 W. Following this period of recovery, participants familiarized themselves with the HIIT protocol,8,14 which required them to cycle at 90% PPO for 1 minute followed by 1 minute at 10% PPO. Given the varying results in HIIT research for PwSCI16 and the idea that existing HIIT protocols for PwSCI may not elicit an adequate cardiorespiratory response of 80% VO2peak during the high intensity intervals,24,29 we utilized a protocol developed for people with CMD that has proven to be effective in enabling people with CMD to reach high percentages of their VO2peak.8,30 This protocol consisted of ten 1-minute intervals at 90% PPO separated by 1-minute intervals at 10% PPO.
Experimental exercise trial (HIIT)
The baseline and HIIT experimental trials were separated by 2 to 7 days.14 Before the HIIT session, participants were asked to abstain from strenuous exercise, caffeine, and alcohol for 24 hours.6 Participants engaged in a 20-minute HIIT trial, which consisted of ten 1-minute intervals at 90% PPO separated by 1-minute intervals at 10% PPO. Throughout the exercise trial, RPE was recorded by researchers to ensure that participants were reaching the intended intensity levels, and expired gas was recorded breath by breath using open-circuit indirect calorimetry. During the HIIT protocol, total work output was calculated to determine the total duration for the MICT trial. To calculate the total duration for the MICT trial, total work output (Joules) from the HIIT trial was exported from the power meter to MATLAB (MathWorks, Natick, MA). In MATLAB, researchers integrated the power-time curve of the data using a trapezoidal sum to determine total workload of HIIT and standardize the MICT duration.
Experimental exercise trial (MICT)
HIIT and MICT experimental trials were separated by 2 to 7 days.14 Before the MICT session, participants were asked to abstain from strenuous exercise, caffeine, and alcohol for 24 hours.20 During MICT, participants cycled at 45% PPO until the estimated duration (determined from the HIIT protocol) was reached.14,31 Because the purpose of this study was to compare a HIIT-based activity to a traditional mode of exercise for PwSCI, researchers chose a MICT protocol of 45% PPO, which is an output commonly used in other SCI-specific exercise studies available in the literature.14,31 Throughout the MICT trial, RPE was recorded by researchers to ensure that participants were reaching the intended intensity levels, and expired gas was recorded breath by breath using open-circuit indirect calorimetry.
Data analysis
Expired gas data were recorded and averaged into 15-second windows by the TrueOne software. VO2, HR, and energy expenditure were computed directly by the TrueOne software. Heart rate reserve (HRR) was calculated by subtracting the resting HR during the baseline test from the peak HR achieved during the incremental test. To calculate average percentage of HRR (%HRR) during each exercise mode, researchers subtracted the average HR from each exercise by the participant’s resting HR and divided that difference by HRR.6 To calculate SCI-specific MET, researchers multiplied the calculated MET from the TrueOne software by 1.30, in accordance with Collins et al.32
Statistical analysis
Quantitative analysis was performed using IBM SPSS version 28.0. Descriptive statistics were calculated for all variables and tested for normality using the Shapiro-Wilks test. Demographic characteristics such as body mass index (BMI), duration of disability, HRpeak, VO2peak, and PPO were reported as mean ± SD. Age was revealed to be non-normal and was reported as median (IQR). Experimental data such as work output, average VO2, average HR, max %VO2peak, max %HRpeak, average METs, and energy expenditure (kcal) were reported as mean ± SD. Due to the non-normal distribution of time during HIIT, average %HRR during MICT, average %VO2peak during MICT, time, average %HRR, and average %VO2peak were reported as median (IQR). Normally distributed data were analyzed using paired t test. Non-normally distributed data were analyzed using the Wilcoxon signed-rank test. Statistical significance level was set at an alpha level of p ≤ .05.
Results
Demographics
Twenty participants (9 females [45%], 11 males [55%]) with neurologically stable SCI/dysfunction (T3 or lower) completed all three exercise sessions. One participant withdrew due to health reasons unrelated to the study. For participants who completed the entirety of the study, no sessions were aborted prematurely, and no adverse events were reported. Participant demographics and baseline characteristics are detailed in Table 1. Median age for participants was 23 (9) years and mean BMI was 23.21 ± 4.89 kg/m2. Duration of disability was 20.43 ± 6.20 years. Disability types included SCI (n = 9), spina bifida (n = 8), transverse myelitis (n = 3), and cauda equina syndrome (n = 1). Mean VO2peak for participants was 31.04 ± 8.07 mL/kg/min, and mean PPO was 136.33 ± 36.67 W. The mean HRpeak of 177 ± 13 beats per minute (bpm) among participants suggested that our sample did not incur autonomic system disruptions.24
Table 1.
Demographic and baseline characteristics
| ID | Sex | Age, y | BMI, kg/m2 | Disability type | Duration of disability, y | HRpeak bpm | VO2peak, mL/ kg/min | PPO, W |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 45 | 21.70 | SCI, T9 | 26 | 163 | 37.92 | 174 |
| 2 | F | 22 | 28.32 | SB, L3/L4 | 22 | 172 | 19.91 | 108 |
| 3 | F | 23 | 20.47 | TM, T12 | 9 | 157 | 21.98 | 95 |
| 4 | M | 26 | 23.30 | SB, L1/L2 | 26 | 179 | 32.84 | 156 |
| 5 | M | 37 | 22.78 | SCI, T11/L2 | 22 | 185 | 31.21 | 145 |
| 6 | M | 21 | 19.05 | SCI, T3 | 21 | 171 | 39.50 | 140 |
| 7a | F | 33 | 17.01 | SCI, T4/T5 | 32 | 168 | 16.40 | 70 |
| 8 | F | 27 | 20.60 | SCI, L1 | 21 | 195 | 31.37 | 124 |
| 9 | F | 22 | 25.97 | SB, L3/L4 | 22 | 165 | 28.80 | 114 |
| 10 | F | 18 | 15.94 | SB, L1 | 18 | 174 | 24.61 | 80 |
| 11 | M | 20 | 32.12 | SB, L3/L4 | 20 | 177 | 27.58 | 146 |
| 12 | F | 21 | 21.93 | TM, T4 | 10 | 172 | 25.71 | 101 |
| 13 | F | 21 | 26.90 | SB, L3 | 21 | 193 | 22.26 | 90 |
| 14 | F | 24 | 19.48 | CES, T10 | 20 | 175 | 39.73 | 124 |
| 15 | M | 23 | 29.13 | TM, T5 | 13 | 168 | 30.46 | 177 |
| 16 | M | 23 | 22.05 | SB, L3/L4 | 23 | 208 | 41.10 | 192 |
| 17 | M | 32 | 18.56 | SCD, T10 | 32 | 169 | 40.11 | 169 |
| 18 | M | 19 | 27.80 | SB, T10/L5 | 19 | 195 | 40.94 | 179 |
| 19 | M | 35 | 20.25 | SCI, T11 | 23 | 162 | 40.67 | 188 |
| 20 | M | 24 | 20.11 | SCI, T10/T11 | 19 | 197 | 37.75 | 154 |
| 21 | F | 27 | 33.89 | SCI, T9 | 8 | 173 | 20.97 | 137 |
Note: BMI = body mass index; bpm = beats per min; CES = cauda equina syndrome; F = female; L = lumber vertebrae; M = male; SB = spina bifida; SCD = spinal cord dysfunction; SCI = spinal cord injury; T = thoracic vertebrae; TM = transverse myelitis; W = watts; y = years.
Withdrew from participation after session 1 (incremental test).
Cardiorespiratory responses to exercise
Total workload was similar across HIIT and MICT protocols (87820 ± 24021 vs. 89044 ± 23696 Joules; p > .05). HIIT sessions required significantly less time than MICT sessions (20.00 [.03] vs 23.35 [2.77] min; p < .01). Across the exercise sessions, all cardiorespiratory responses were significantly greater during the HIIT session (p < .01). Cardiorespiratory responses to exercise can be found in Table 2.
Table 2.
Acute cardiorespiratory responses to HIIT and MICT
| HIIT | MICT | p value | |
|---|---|---|---|
| Duration, minutes | 20.00 (.03) | 23.20 (2.56) | <.01 |
| Average HR, bpm | 146.26 ± 13.80 | 129.38 ± 19.13 | <.01 |
| Average HRR, %HRR | 69.21 (14.05) | 52.62 (16.36) | <.01 |
| Average VO2 | 20.96 ± 4.84 | 17.08 ± 3.17 | <.01 |
| Average VO2, % of VO2peak | 64.77 (11.27) | 51.54 (10.85) | <.01 |
| Average MET | 7.54 ± 2.00 | 6.21 ± 1.25 | <.01 |
| Energy expenditure, kcal | 128.08 ± 35.65 | 118.93 ± 29.58 | <.01 |
| Energy expenditure, kcal/min | 6.40 ± 1.78 | 5.09 ± 1.14 | <.01 |
Note: Data presented as mean ± SD or median (IQR). bpm = beats per minute; HIIT = high intensity interval training; HR = heart rate; HRR = heart rate reserve; IQR = interquartile range; kcal = kilocalorie; MET = metabolic equivalent of task; MICT = moderate intensity continuous training; VO2 = oxygen uptake; VO2peak = peak oxygen uptake capacity.
Figure 2 shows the time course of %VO2peak and %HRpeak during the HIIT and MICT sessions in a representative subject with SCI (T10). This figure represents the peaks and valleys of one individual’s physiological responses during HIIT and their steady state physiological responses to MICT. This individual’s responses were like the group. As a group, the maximum oxygen uptake as a percentage of VO2peak during HIIT was 90.31 ± 11.22 %VO2peak compared to 65.17 ± 8.36 %VO2peak during MICT (p < .01). The maximum HR as a percentage of HRpeak during HIIT was 96.71 ± 5.87 %HRpeak compared to 80.98 ± 7.49 %HRpeak during MICT (p < .01).
Figure 2.
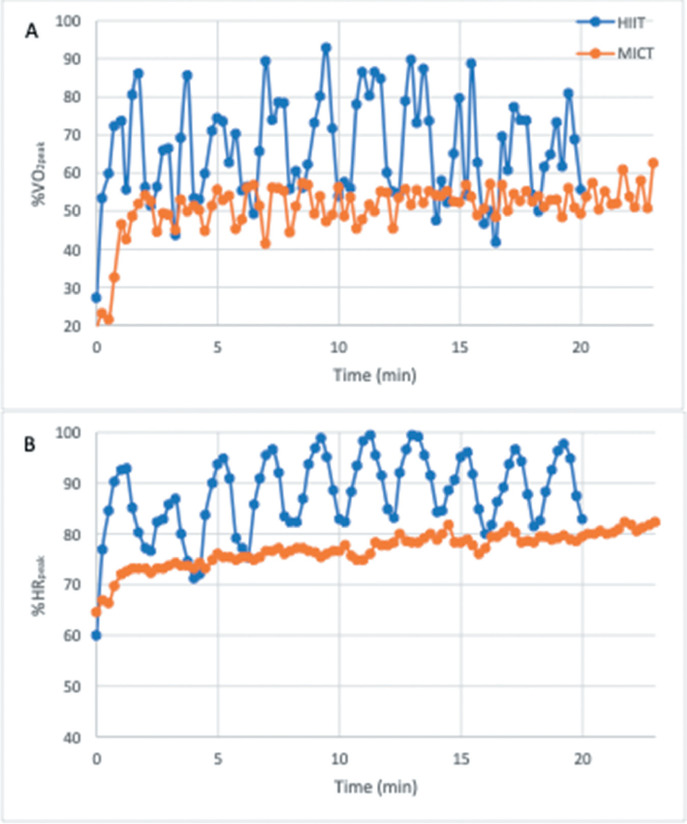
A representative subject’s cardiorespiratory responses across the time course of both exercise trials. HIIT = high intensity interval training; MICT = moderate intensity continuous training; %HRpeak = percentage of peak heart rate; %VO2peak = percentage of peak oxygen uptake capacity.
Discussion
The purpose of this study was to determine the cardiometabolic effectiveness of HIIT compared to MICT on a recumbent handcycle at matched workloads. This study represents the first within-subject design analyzing the cardiorespiratory responses of a HIIT-based handcycling activity in PwSCI. In line with our hypothesis, HIIT led to a greater cardiorespiratory response than MICT. In less time, HIIT burned significantly more calories while eliciting greater overall responses in VO2, MET, and HR.
Our study population was younger than other studies to date, with greater years living with disability. This is likely due to individuals having congenital, nontraumatic SCIs such as spina bifida and cauda equina syndrome. BMI appears to be similar to that found in other studies to date; however, our population may be more active.14,24,33 For example, in McMillan et al.’s HIIT study in PwSCI, the sample had an average VO2peak of 19.2 mL/kg/min compared to 31.04 mL/kg/min in the current study. Given the greater fitness levels within our sample, results may not generalize to broader populations of PwSCI. However, research shows that training effects in trained populations translate well to populations of lower fitness.34 Therefore, even though our population may have been more active than the population in other studies to date, these data represent a promising initial step in determining the optimal use of HIIT for PwSCI.
The study by Schoenmakers et al. showed superior results following HIIT in able-bodied handcyclists using a matched-workload design.23 However, in other able-bodied exercise studies using a matched-workload approach, HIIT has shown only similar fitness improvements to MICT.35,36 In the able-bodied population, MICT is effective in improving fitness; however, because MICT may not be effective for improving vascular health in PwSCI,1,2 HIIT may need to optimize energy expenditures (i.e., kilocalories burnt) and cardiorespiratory responses in comparison to MICT for researchers and clinicians to begin feeling confident prescribing this mode of training. In 15% less time, our results showed that HIIT burned 23% more kilocalories than MICT. Notably, this observed difference between HIIT and MICT may have been even greater if a lower intensity MICT protocol was performed. In the current study, participants performed MICT at 45% PPO, which was noticeably higher than McMillan et al.’s MICT protocol for PwSCI.24
However, these results are similar to McMillan et al.’s findings that showed HIIT was 21% more efficient in burning kilocalories than MICT. Average %VO2peak accumulated during HIIT and MICT sessions within the current study are also similar to results in McMillan et al.’s study.24 Given these similarities, this novel HIIT-based handcycling protocol appears to adequately stimulate the cardiorespiratory system. Results also support that synchronous ergometry can efficiently elicit a large cardiorespiratory response, as stated by Dallmeijer et al.25 Even though a greater number of muscles may be utilized when propelling a synchronous handcycle compared to asynchronous arm ergometry, minimal research has examined the cardiorespiratory responses associated with a synchronous HIIT-based handcycling activity.20 Our protocol enabled participants to burn 120 kcal in less than 20 minutes compared to a recent HIIT protocol using asynchronous ergometry that took over 30 minutes to burn a similar kilocalorie count.24 It should be noted that the expedited burning of kilocalories could have been influenced by the increased intensity in the current study compared to McMillan’s study. Participants in the current study safely engaged in high intensity bouts of 90% PPO compared to 70% PPO in McMillan’s study.24
Currently, minimal data exist regarding habitual physical activity monitoring and health in PwSCI.37 As a physiologically relevant way to quantify energy expenditure, volume, and intensity for the general population,21 the quantification of MET for PwSCI may be a useful metric for tracking health and fitness.32 According to our results, HIIT elicited an average metabolic response of 7.52 MET. Currently, the ACSM recommends accumulating 500 MET-min/week for the general population.6 Therefore, by using these results and a similar protocol, PwSCI may be able to engage in just 66 minutes of HIIT per week to meet ACSM recommendations. Because exercise regimes should be balanced and include aspects of flexibility and resistance training, it would be ill-advised to suggest engaging only in HIIT within an exercise regime.6 Nonetheless, for a population that views exercise as excessively time consuming,14,15 it is encouraging that just an hour of HIIT each week could theoretically enable a PwSCI to meet general exercise guidelines.
Individuals also maintained a higher average HR during HIIT compared to MICT. Even with the integration of rest periods, average HR was higher throughout the HIIT protocol as represented in Figure 2. Additionally, the maximum %VO2peak and %HRpeak reached during HIIT significantly trumped maximal responses during MICT. As exercise modes that maximize cardiovascular strain elicit the greatest fitness adaptations,38 our results support that HIIT will lead to greater fitness adaptations than MICT over time.
It must also be noted that our protocol engaged participants in high intensity bouts of 90% PPO, which was more intense than other HIIT protocols to date. In Astorino and Thum’s study, which revealed HIIT to be less effective in burning kilocalories than MICT, participants engaged in high intensity bursts up to 70% PPO. Although the lower intensity protocol may have been utilized to accommodate for participants with quadriplegia, HIIT protocols that enable PwSCIs to accumulate greater exercise volumes more effectively improve cardiorespiratory health.16 This study further supports that PwSCI may benefit from engaging in HIIT bouts that require a high percentage of maximal power output.
For a population that contends with cardiac output limitations and an inability to sustain prolonged exercise,12 our results suggest that rest periods of 60 seconds may provide enough recovery for PwSCI to reach close to maximal HR during exercise. The ability to reach near-maximal levels may also be critical for enhancing central cardiovascular adaptations. However, it must be noted that participants had primarily lower-level SCIs (i.e., below T6). According Hou and Rabchevsky, SCIs above T6 may cause autonomic nervous system limitations such as blunted HR and sweat response.39 This phenomenon may not have been fully reflected in our study’s sample. Nonetheless, 25% of our participants had thoracic-level SCIs above the T6 region and were able to reach close to maximal HR levels during the high intensity efforts. Future research should examine the effects of HIIT in participants who exhibit autonomic limitations to confirm HIIT’s potency.
To date, this is the largest sample of PwSCI engaging in HIIT. Unlike other within-subject HIIT designs,14,24 this study also had a relatively equal distribution of males (n = 11) and females (n = 9). Given the larger percentage of males who acquire SCI during their life,40 underrepresentation of females in exercise studies for PwSCI is an unfortunate limitation across the literature. Given the different physiological responses to exercise and health outcomes associated with gender,41,42 future studies should examine the influence of gender on acute physiological responses to see if gender plays a role in oxygen uptake kinetics for PwSCI during HIIT.
Limitations
Our population consisted of active PwSCI and may not be representative of the general population living with SCI.33 These participants were likely more fit compared to participants in other studies examining HIIT in PwSCI,24 which may limit generalizability. However, because the current study used a higher intensity protocol compared to related literature in PwSCI, it was important to prioritize safety. Therefore, we elected to recruit a comparatively large sample size of physically active individuals before studying this novel protocol in the general SCI population.6 With no adverse cardiovascular complications occurring during the study, we are better positioned to examine more intense HIIT protocols in larger populations of PwSCI with lower fitness levels. Additionally, although our sample size was among the largest in studies conducted on PwSCI, it was not sufficiently powered to stratify based on neurological level of injury or disability type. This will be critical as this line of research develops. There are also varying protocols for HIIT17,19,23,43 that may elicit different responses to the cardiorespiratory system. We attempted to determine an optimal protocol through examining and comparing existing evidence, but our inability to examine different HIIT protocols within a larger and broader sample makes it difficult to confirm an optimal HIIT protocol for PwSCI. There are also limitations in indirect calorimetry when calculating kilocalorie expenditure during exercises that place an individual in anaerobic metabolism.44 Anaerobic metabolism may have occurred during the intense bursts of HIIT,45 indirectly impacting calorimetry calculations. Additionally, aspects related to sleep and nutrition were not controlled for and may have influenced results. This study’s within-subject design does not verify HIIT as a long-term exercise option. Engaging PwSCI in a 6- to 8-week randomized controlled intervention is needed to confirm HIIT’s effectiveness as a superior exercise option to MICT. This study serves as an initial step for guiding future HIIT RCTs in PwSCI.
Conclusion
PwSCI need effective exercise options that adequately stimulate the cardiometabolic system. However, before researchers can begin to conduct HIIT RCTs, research must prove that HIIT elicits a greater response in VO2 and energy expenditures compared to MICT. Results from this study support HIIT as a superior exercise option to MICT in PwSCI. In significantly less time, HIIT elicited a greater cardiovascular response while burning more kilocalories compared to MICT. Future research should expand upon this design and develop at-home RCT interventions that examine the effectiveness of a handcycling-based HIIT activity in PwSCI.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Nightingale TE, Metcalfe RS, Vollaard NB, Bilzon JL. Exercise guidelines to promote cardiometabolic health in spinal cord injured humans: Time to raise the intensity? Arch Phys Med Rehabil . 2017;98(8):1693–1704. doi: 10.1016/j.apmr.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Totosy de Zepetnek JO, Pelletier CA, Hicks AL, MacDonald MJ. Following the physical activity guidelines for adults with spinal cord injury for 16 weeks does not improve vascular health: A randomized controlled trial. Arch Phys Med Rehabil . 2015;96(9):1566–1575. doi: 10.1016/j.apmr.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Pescatello LS, MacDonald HV, Lamberti L, Johnson BT. Exercise for hypertension: A prescription update integrating existing recommendations with emerging research. Curr Hypertens Rep . 2015;17(11):87. doi: 10.1007/s11906-015-0600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selph SS, Skelly AC, Wasson N, et al. Physical activity and the health of wheelchair users: A systematic review in multiple sclerosis, cerebral palsy, and spinal cord injury. Arch Phys Med Rehabil . 2021;102(12):2464–2481. doi: 10.1016/j.apmr.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 5.LaVela SL, Evans CT, Prohaska TR, Miskevics S, Ganesh SP, Weaver FM. Males aging with a spinal cord injury: prevalence of cardiovascular and metabolic conditions. Arch Phys Med Rehabil . 2012;93(1):90–95. doi: 10.1016/j.apmr.2011.07.201. [DOI] [PubMed] [Google Scholar]
- 6.American College of Sports Medicine ACSM’s Exercise Testing and Prescription . Lippincott Williams & Wilkins; 2017. [Google Scholar]
- 7.Rognmo O, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation . 2012;126(12):1436–1440. doi: 10.1161/CIRCULATIONAHA.112.123117. [DOI] [PubMed] [Google Scholar]
- 8.Currie KD, Dubberley JB, McKelvie RS, MacDonald MJ. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc . 2013;45(8):1436–1442. doi: 10.1249/MSS.0b013e31828bbbd4. [DOI] [PubMed] [Google Scholar]
- 9.Daussin FN, Zoll J, Dufour SP, et al. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: Relationship to aerobic performance improvements in sedentary subjects. Am J Physiol Regul Integr Comp Physiol . 2008;295(1):R264–272. doi: 10.1152/ajpregu.00875.2007. [DOI] [PubMed] [Google Scholar]
- 10.Hood MS, Little JP, Tarnopolsky MA, Myslik F, Gibala MJ. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med Sci Sports Exercise . 2011;43(10):1849–1856. doi: 10.1249/MSS.0b013e3182199834. [DOI] [PubMed] [Google Scholar]
- 11.Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, MacDonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow-mediated dilation in healthy humans [published online April 23, 2008] Am J Physiol Regul Integr Comp Physiol . doi: 10.1152/ajpregu.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devillard X, Rimaud D, Roche F, Calmels P. Effects of training programs for spinal cord injury. Ann Readapt Med Phys . 2007;50(6):490-498–480-489. doi: 10.1016/j.annrmp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Hoeks J, Schrauwen P. Muscle mitochondria and insulin resistance: A human perspective. Trends Endocrinol Metab . 2012;23(9):444–450. doi: 10.1016/j.tem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Astorino TA, Thum JS. Interval training elicits higher enjoyment versus moderate exercise in persons with spinal cord injury. J Spinal Cord Med . 2018;41(1):77–84. doi: 10.1080/10790268.2016.1235754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehn M, Kroll T. Staying physically active after spinal cord injury: A qualitative exploration of barriers and facilitators to exercise participation. BMC Public Health . 2009;9(1):168. doi: 10.1186/1471-2458-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters J, Abou I, Dandeneau K, Rice L, Rice I. The effectiveness of vigorous training interventions on cardiovascular fitness in persons with spinal cord injury: A systematic review and meta-analysis. Spinal Cord . 2021:1–10. doi: 10.1038/s41393-021-00669-7. [DOI] [PubMed] [Google Scholar]
- 17.Graham K, Yarar-Fisher C, Li J, et al. Effects of high-intensity interval training versus moderate-intensity training on cardiometabolic health markers in individuals with spinal cord injury: A pilot study. Top Spinal Cord Inj Rehabil . 2019:25. doi: 10.1310/sci19-00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier C, Brosseau R, Hicks AL, Gagnon DH. Feasibility, safety, and preliminary effectiveness of a home-based self-managed high-intensity interval training program offered to long-term manual wheelchair users. Rehabil Res Pract . 2018;2018:8209360. doi: 10.1155/2018/8209360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astorino TA, Thum JS. Within-session responses to high-intensity interval training in spinal cord injury. Disabil Rehabil . 2018;40(4):444–449. doi: 10.1080/09638288.2016.1260648. [DOI] [PubMed] [Google Scholar]
- 20.Dallmeijer AJ, Zentgraaff ID, Zijp NI, van der Woude LH. Submaximal physical strain and peak performance in handcycling versus handrim wheel-chair propulsion. Spinal Cord . 2004;42(2):91–98. doi: 10.1038/sj.sc.3101566. [DOI] [PubMed] [Google Scholar]
- 21.Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol . 1990;13(8):555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld BJ, Ogborn D, Krieger JW. Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. J Sports Sci . 2017;35(11):1073–1082. doi: 10.1080/02640414.2016.1210197. [DOI] [PubMed] [Google Scholar]
- 23.Schoenmakers P, Reed K, Van Der Woude L, Hettinga FJ. High intensity interval training in handcycling: The effects of a 7 week training intervention in able-bodied men. Front Physiol . 2016;7(638) doi: 10.3389/fphys.2016.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillan DW, Maher JL, Jacobs KA, Nash MS, Bilzon JLJ. Physiological responses to moderate intensity continuous and high-intensity interval exercise in persons with paraplegia [published online July 17, 2020] Spinal Cord . doi: 10.1038/s41393-020-0520-9. [DOI] [PubMed] [Google Scholar]
- 25.Dallmeijer AJ, Ottjes L, de Waardt E, van der Woude LH. A physiological comparison of synchronous and asynchronous hand cycling. Int J Sports Med . 2004;25(8):622–626. doi: 10.1055/s-2004-817879. [DOI] [PubMed] [Google Scholar]
- 26.Koontz AM, Garfunkel CE, Crytzer TM, Anthony SJ, Nindl BC. Feasibility, acceptability, and preliminary efficacy of a handcycling high-intensity interval training program for individuals with spinal cord injury. Spinal Cord . 2021;59(1):34–43. doi: 10.1038/s41393-020-00548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mossberg K, Willman C, Topor MA, Crook H, Patak S. Comparison of asynchronous versus synchronous arm crank ergometry. Spinal Cord . 1999;37(8):569–574. doi: 10.1038/sj.sc.3100875. [DOI] [PubMed] [Google Scholar]
- 28.Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med . 1985;6(4):197–201. doi: 10.1055/s-2008-1025839. [DOI] [PubMed] [Google Scholar]
- 29.Peters J. University of Illinois at Urbana-Champaign; 2022. The feasibility of a high intensity interval training-based handcycling activity in people with spinal cord injury [doctoral dissertation] [Google Scholar]
- 30.Little JP, Gillen JB, Percival ME, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Applied Physiol . 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs KA, Burns P, Kressler J, Nash MS. Heavy reliance on carbohydrate across a wide range of exercise intensities during voluntary arm ergometry in persons with paraplegia. J Spinal Cord Med . 2013;36(5):427–435. doi: 10.1179/2045772313Y.0000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins EG, Gater D, Kiratli J, Butler J, Hanson K, Langbein WE. Energy cost of physical activities in persons with spinal cord injury. Med Sci Sports Exerc . 2010;42(4):691–700. doi: 10.1249/MSS.0b013e3181bb902f. [DOI] [PubMed] [Google Scholar]
- 33.Simmons OL, Kressler J, Nash MS. Reference fitness values in the untrained spinal cord injury population. Arch Phys Med Rehabil . 2014;95(12):2272–2278. doi: 10.1016/j.apmr.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Yang MT, Lee MM, Hsu SC, Chan KH. Effects of high-intensity interval training on canoeing performance. Eur J Sport Sci . 2017;17(7):814–820. doi: 10.1080/17461391.2017.1314553. [DOI] [PubMed] [Google Scholar]
- 35.Ram A, Marcos L, Jones MD, et al. The effect of high-intensity interval training and moderate-intensity continuous training on aerobic fitness and body composition in males with overweight or obesity: A randomized trial. Obesity Med . 2020;17:100187. doi: 10.1038/s41440-019-0392-6. [DOI] [PubMed] [Google Scholar]
- 36.Wewege M, Van Den Berg R, Ward RE, Keech A. The effects of high‐ intensity interval training vs. moderate‐ intensity continuous training on body composition in overweight and obese adults: A systematic review and meta‐analysis. Obesity Rev . 2017;18(6):635–646. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 37.Veerubhotla A, Hong E, Knezevic S, Spungen A, Ding D. Estimation of physical activity intensity in spinal cord injury using a wrist-worn actigraph monitor. Arch Phys Med Rehabil . 2020;101(9):1563–1569. doi: 10.1016/j.apmr.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 38.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol . 2017;595(9):2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou S, Rabchevsky AG. Autonomic consequences of spinal cord injury. Compr Physiol . 2014;4(4):1419–1453. doi: 10.1002/cphy.c130045. [DOI] [PubMed] [Google Scholar]
- 40.White N-H, Black N-H. Spinal Cord Injury (SCI) Facts and Figures at a Glance . Birmingham, AL: National Spinal Cord Injury Statistical Center; 2016. [Google Scholar]
- 41.Raguindin PF, Muka T, Glisic M. Sex and gender gap in spinal cord injury research: Focus on cardiometabolic diseases. A mini review. Maturitas . 2021;147:14–18. doi: 10.1016/j.maturitas.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Gibala M, Gillen J, Percival M. Physiological and health-related adaptations to low-volume interval training: Influences of nutrition and sex. Sports Med (Auckland, NZ) . 2014;44(Suppl 2):127–137. doi: 10.1007/s40279-014-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harnish CR, Daniels JA, Caruso D. Training response to high-intensity interval training in a 42-year-old man with chronic spinal cord injury. J Spinal Cord Med . 2017;40(2):246–249. doi: 10.1080/10790268.2015.1136783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott CB, Leighton BH, Ahearn KJ, McManus JJ. Aerobic, anaerobic, and excess postexercise oxygen consumption energy expenditure of muscular endurance and strength: 1-set of bench press to muscular fatigue. J Strength Condition Res . 2011;25(4):903–908. doi: 10.1519/JSC.0b013e3181c6a128. [DOI] [PubMed] [Google Scholar]
- 45.Jabbour G, Iancu HD, Paulin A. Effects of high-intensity training on anaerobic and aerobic contributions to total energy release during repeated supramaximal exercise in obese adults. Sports Med Open . 2015;1(1):36. doi: 10.1186/s40798-015-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


