Abstract
Objectives
To compare the effectiveness of two different interventions that promote physical activity in individuals with traumatic spinal cord injury (SCI) and determine the effect of relapse prevention.
Methods
A sequential, multiple assignment, randomized trial was conducted at a universally designed community-based exercise facility. Participants were individuals with traumatic SCI, >3 months post injury, levels C5 to T12, age ≥18 years (N = 79). After randomization, Bridge Program participants completed an 8-week personalized, less intense, exercise program informed by American College of Sports Medicine (ACSM) guidelines and supported with hands-on peer mentoring, exercise of choice, and caregiver training. Structured Exercise participants completed an 8-week program in a group format based on ACSM guidelines. After intervention, participants were randomized to receive or not receive relapse prevention for 6 months. The time and intensity of physical activity and psychological change in depression, anxiety, self-efficacy, and function were assessed with self-reported measures.
Results
Compared to baseline, physical activity increased post intervention for both the Bridge and Structured Exercise programs. Compared to baseline, participants in the Bridge Program recorded fewer anxiety symptoms. No significant changes were noted for either program in depressive symptoms, self-efficacy, or function. There was no difference in relapse prevention between the two groups at 6 months.
Conclusions
The Bridge Program, a novel personalized exercise program with peer support, exercise of choice, and caregiver training, and a structured exercise program both improved self-reported physical activity, but the Bridge Program also reduced anxiety symptoms. This study provides important insight into the limitations of commonly used measures of physical activity and psychosocial domains in people with SCI.
Keywords: community participation, exercise, social participation, spinal cord injuries, therapeutic adherence
Introduction
Increasing and sustaining physical activity are major health and wellness challenges for people living with traumatic spinal cord injury (SCI). Physical activity is a key component of wellness for all people and has been shown to protect against heart disease, stroke, and other major chronic diseases such as type 2 diabetes, obesity, and depression.1 People with SCI are typically more sedentary; have an increased incidence of cardiovascular disease, type 2 diabetes, and osteoporosis; and are less likely to engage in sustained physical activity and community participation than the general population.2-5 Guidelines for physical activity for people with SCI have been established,6-9 and several physical activity protocols involving aerobic and resistive exercises have been trialed.10,11 Twenty minutes of moderate to vigorous aerobic activity two times per week is recommended for cardiovascular fitness, and three sets of strength exercise for each major functioning muscle group two times per week is recommended for muscle strength. Improvements in aerobic capacity and strength have been noted with these protocols.3 However, studies have had variable participation rates, have focused on the initial structured intervention period, and generally have not shown the long-term sustainability of physical activity.12 This study compares the outcomes of two interventions, a group-based structured exercise program (Structured Exercise program) with a novel individualized program (Bridge Program) that includes exercise of choice, peer mentors, and caregiver training
The factors affecting sustained participation in physical activity for people with disabilities are not well understood but may be similar to those affecting full participation in society.13 Barriers and facilitators to participation have recently been systematically identified14,15 and a social ecological theoretical framework has been proposed to understand the relationship between environmental factors and participation.15,16 Eight major categories of environmental factors that influence participation have been described in a population of people with disabilities including SCI. Those areas with some specific examples for people with SCI are (1) built environment (accessible wheelchair entrance, locker room and exercise equipment); (2) natural environment (access through rain and snow); (3) transportation (accessible public transportation and close accessible parking); (4) systems, services, and policy (staff training and policies to serve people with disabilities); (5) economic (sliding scale fees and scholarships); (6) social support and societal attitudes (peer support and staff training); (7) information and technology access (online registration and program information); and (8) assistive technology (adapted exercise equipment).14 However, there have been no specific efforts to examine the long-term adoption and sustainability of physical activity programs designed using a social ecological approach to systematically address the barriers and facilitators to participation.
We compared a novel approach, the Bridge Program, to a Structured Exercise program. Using a sequential, multiple assignment, randomized trial (SMART)17 design, we also assessed the effectiveness of a relapse prevention program to sustain physical activity. The Bridge Program was intended to accommodate the thematic category of social support as an identified barrier to participation with peer mentoring and caregiver training.14 It was informed by social cognitive theory, which suggests that when people observe a model performing a behavior, they use the experience to guide future behavior.18 The Bridge Program was also informed by the role of choice in establishing control over one’s life.19 To reduce confounding factors influencing longer term sustained participation in exercise, the study was conducted in an exercise facility specifically designed from a social ecological perspective to reduce other accessibility barriers (e.g., cost, parking, nonaccessible equipment) and enhance other facilitators (e.g., peer support, trained staff, sliding scale fees) to participation in physical activity.14 The specific aims of this study were to (1) evaluate the effectiveness of the two programs, Bridge and Structured Exercise, to improve physical activity and psychosocial outcomes for individuals with SCI and (2) determine the effect of relapse prevention.
Methods
Participants
Participants with traumatic SCI were recruited with flyers and newsletters from hospital multidisciplinary clinics, physician offices, and support groups. Inclusion criteria were history of traumatic SCI >3 months, injury level C5 to T12, age 18 years or older, medical clearance if aged 65 or older, sufficient cognitive ability to provide informed consent and follow directions for exercise instruction, and caregiver availability if needed. Exclusion criteria were prior history of treatment for depression or psychiatric illness, uncontrolled hypertension or diabetes, current treatment for a pressure sore or ulcer, shoulder pain limiting movement, and current participation in an organized exercise program or sports team. Participants with other premorbid or comorbid conditions were considered eligible if they met inclusion criteria and had a willingness to participate in an exercise program. Prior to the first visit, participants discussed their method of transportation and entry into the facility. If transportation was a concern for the participant, the first session was used to address transportation barriers such as scheduling transportation or accessing public transportation.
The study was approved by the institutional review board at the governing institution, and all participants provided signed informed consent.
Facility
Bridge and Structured Exercise interventions were provided at a new universally designed exercise facility. The facility was designed for accessibility of the built environment, including no curbs on entry; ramps and elevators in place of stairs; and wheelchair accessible doors, bathrooms, changing facilities, and exercise equipment. Facility access fees were paid by the study during the intervention period to address the barrier of cost to the participants. Continued facility membership was available on a self-pay sliding scale charge.
Interventions
Bridge Program
The Bridge Program emphasized an individual approach with a peer mentor, caregiver training, and personal choice of exercise regimen informed by American College of Sports Medicine (ACSM) guidelines. The peer mentor demonstrated exercise performance, promoted self-efficacy, and provided encouragement. One peer mentor worked with all participants, received no specific training, and was unique to this study. After initial involvement, the peer mentor involvement ranged from “exercise buddy” to on-call resource depending on participant choice. Both programs utilized the knowledge and skill of a personal Certified Therapeutic Recreation Specialist (CTRS) who was also a Certified Inclusive Fitness Trainer (CIFT) by the ACSM.20,21 Instruction from the CTRS, who was knowledgeable about exercise for persons living with SCI, addressed the barriers of intimidation and lack of facility-based trainers. The Bridge Program also provided a flexible individualized exercise program allowing individual choice and peer support and included training and participation for any caregiver or attendant. The Bridge Program consisted of six to eight visits over the course of 8 weeks. One-hour visits were scheduled to accommodate participant needs and availability with a typical frequency of one to two visits per week. Participants’ caregivers were encouraged to be present for Bridge Program visits and to become involved to support the program. Participants completed an intake questionnaire to identify fitness and recreation interests to help the CTRS tailor the Bridge Program activities to their interests and incorporate activities available at the facility or through a wheelchair adaptive sports program. Exercise activities were individualized but informed by ACSM guidelines of 20 minutes of moderate intensity aerobic activity and strength training two times per week
Structured Exercise program
The Structured Exercise program incorporated cardiovascular fitness and strength training in a group program format for three sessions per week for 8 weeks. Exercise sessions were led by the same CIFT-trained CTRS leading the Bridge Program. Days and times for group exercise varied depending on participant and instructor availability. Caregivers for participants in the Structured Exercise Program could observe the program but had no defined role. The instructor could encourage group participation but not individualize the exercise program. Exercise group sessions lasted for 1 hour and included up to five participants. Each session utilized ACSM SCI-specific guidelines for physical activity and resistance training.6,7 The program included 20 minutes of aerobic activity, 20 minutes circuit training for strengthening, and 20 minutes combined warm up and cool down that included flexibility exercises. Although the Structured Exercise program did not emphasize individualized physical activity, participants were still provided education and modifications were made to equipment to ensure accessibility.
Both groups were provided standard education materials from the ACSM on safe exercise practices, guidelines to exercising, and expectations regarding physical limitations. Researchers suggested educational materials after the conclusion of the active intervention to aid in exercise adherence. Free access to the exercise facility was provided to both groups outside the planned interventions.
Relapse prevention
Relapse prevention was operationalized as a brief, monthly telephone call lasting approximately 15 minutes, and follow-up continued for 6 months. The calls were conducted by a member of the research team familiar with the participant, their individual goals, their motivational level, and their recommended exercise regimen. The call was not scripted, and the facilitator worked individually with all relapse prevention participants to solve any barriers or challenges that they encountered with their exercise program, to engage in goal-setting activities, and to provide positive motivation and encouragement in a nonjudgmental manner.
Outcome measures
Primary outcome measures were time and intensity of postintervention physical activity and psychosocial assessments of depression, anxiety, self-efficacy, and function. Physical activity was assessed with the 10-item Physical Activity Scale for Individuals with Physical Disabilities (PASIPD). The PASIPD is a self-report measure to assess time and intensity spent in physical activity.22,23 It includes items related to leisure time, physical activity, and exercise. Psychosocial measures utilized self-report instruments validated for use with the SCI population. Depression was assessed using the nine-item Patient Health Questionnaire 9 (PHQ-9)24; anxiety was assessed with the Generalized Anxiety Disorder Scale 7 (GAD-7)25; self-efficacy was assessed using the 16-item Moorong Self-Efficacy Scale (MSES)26; and participation was measured with the Craig Handicap Assessment and Reporting Technique-Short Form (CHART-SF).27
Secondary outcome measure was cardiovascular fitness measured with the Fatigue Index28 derived from the Wingate Anaerobic Test.28 The Fatigue Index is a measure of endurance during anaerobic exercise. Peak anaerobic power from the Wingate Anaerobic Test could not be compared between groups because of a mid-study equipment failure that required a replacement machine. Baseline data were collected at enrollment and postintervention (Figure 2). The Wingate Anaerobic Test28 was performed on a Monarch Arm Ergometer (COSMED Inc., Chicago, Illinois)
Data collection
REDCap (Research Electronic Data Capture), Vanderbilt University (Nashville, TN), was used for data collection. The surveys were done face to face or by phone by a research assistant, and then the data was entered into the REDCap database.
Statistical analysis
Demographic characteristics were aggregated with descriptive statistics. Rasch analysis29 was used to transform ordinal scales to interval measures for the PHQ-9, GAD-7, CHART-SF, PASIPD, and MSES. To assess Aim 1, paired t tests were conducted from baseline to week 8 within each exercise group on the Rasch transformed CHART-SF, PHQ-9, and MSES. Wilcoxon signed-rank tests were conducted on the Rasch transformed PASIPD and Fatigue Index. For Aim 2, relapse and no relapse prevention groups were compared by conducting Mann-Whitney U tests on the score differences between 8 weeks and 6 months on the Rasch transformed GAD-7, PHQ-9, MSES, PASIPD, and Fatigue Index. Nonparametric statistics were utilized for nonnormally distributed data. The following statistical programs or models were used to conduct analyses: WINSTEPS version 4.8.0.0 (Winsteps. com, Portland, OR), SAS Enterprise Guide 8.3 (SAS Institute Inc, Cary, NC), and R Studio version 4.2.0 (RStudio, Boston, MA).
Results
We assessed 175 participants for eligibility; 79 were randomized into our two intervention groups: Bridge Program (n = 42) and Structured Exercise (n = 37) (Figure 1). Ninety-six individuals who were not included did not meet inclusion criteria or chose not to participate. Participant characteristics were similar, except the Structured Exercise group contained more people using powered mobility (see Table 1). Most participants were years post injury (mean = 12 years) and driving independently, suggesting an independent lifestyle.
Figure 1.
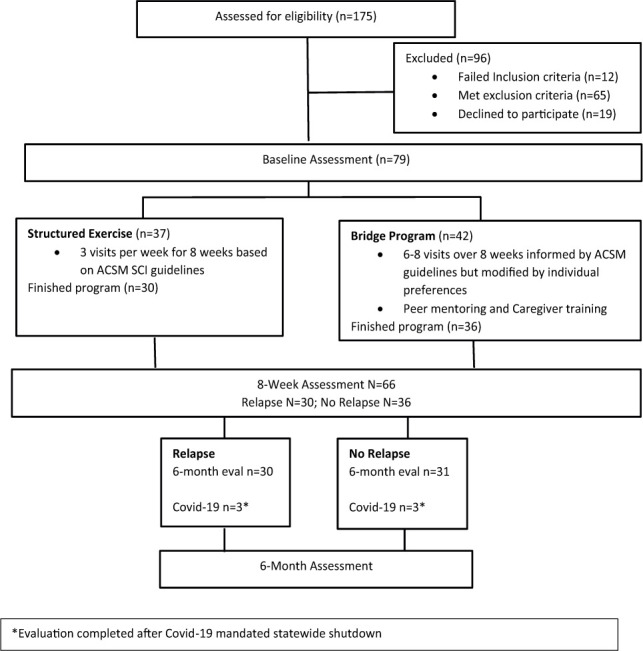
Flow diagram.
Table 1.
Participant characteristics
| Characteristic | Bridge Program (n = 36) | Structured Exercise program (n = 30) | P value |
|---|---|---|---|
| Age, mean (SD) | 46.8 (14.38) | 42.27 (13.78) | .199 |
| Gender | |||
| Male, n (%) | 27(75) | 20 (67) | .457 |
| Race and ethnic group,n (%) | .564 | ||
| Non-Hispanic white | 28 (78) | 23(77) | |
| Non-Hispanic black | 5 (14) | 2 (7) | |
| Hispanic | 2 (6) | 3 (10) | |
| Multiracial | 1 (3) | 2 (7) | |
| BMI, mean (SD) | 27.92 (5.69) | 26.72 (6.18) | .631 |
| Injury type,n (%) | .450 | ||
| Tetraplegia, complete | 12 (33) | 7 (23) | |
| Tetraplegia, incomplete | 7 (19) | 3 (10) | |
| Paraplegia, complete | 7 (19) | 8 (27) | |
| Paraplegia, incomplete | 10 (28) | 12 (40) | |
| Years post injury, | 12.0 (9.4), 10.4 | 12.1 (13.4), 6.7 | .483 |
| mean (SD), median [25th, 75th] | [3.4, 19.5] | [2.4, 19.8] | |
| Mobility level, n (%) | .031 | ||
| Ambulatory | 6 (17) | 3 (10) | |
| Manual wheelchair | 24 (67) | 13 (43) | |
| Power wheelchair | 6 (17) | 14 (47) | |
| COVID | |||
| Affected, n (%) | 3 (8) | 3 (10) | .313 |
Note: BMI = body mass index.
One primary outcome for Aim 1, physical activity measured by the PASIPD, was significantly increased postintervention for both the Bridge Program and the Structured Exercise program compared to their group baselines (see Table 2; p < .0001). In the other primary outcome for Aim 1, psychosocial measures, the Bridge Program recorded a statistical reduction in anxiety symptoms on the GAD-7 (see Table 2; p < .035). Neither program showed change on the PHQ-9, MSES, of CHART-SF from baseline to 8 weeks.
Table 2.
Eight-week results of pairedt tests and Wilcoxon signed rank test
| Measure | Exercise program | Mean difference (SD) | t (p value) |
|---|---|---|---|
| CHART-SF | Bridge | -0.9 (9.9) | -0.5 (.604) |
| Structured | 2.2 (7.6) | 1.6 (.130) | |
| MSES | Bridge | 4.7 (28.0) | 1.0 (.324) |
| Structured | -2.6 (11.5) | -1.3 (.220) | |
| PHQ-9 | Bridge | -6.8 (23.6) | -1.7 (.094) |
| Structured | -2.5 (21.4) | -0.6 (.533) | |
| GAD-7 | Bridge | -7.8 (21.2) | -2.2 (.035) |
| Structured | -2.1 (18.2) | -0.6 (.526) | |
| Median difference | W (p value) | ||
| Fatigue Index | Bridge | -3.2 | 105 (<.0001)* |
| Structured | 2.3 | 105 (<.0001)* | |
| PASIPD | Bridge | 4.0 | 248 (<.0001)* |
| Structured | 6.0 | 162.5 (<.0001)* | |
Note: CHART-SF = Craig Handicap Assessment and Reporting Technique-Short Form; GAD-7 = Generalized Anxiety Disorder Scale-7; MSES = Moorong Self-Efficacy Scale; PHQ-9 = Patient Health Questionnaire-9; PSASIPD = Physical Activity Scale for Individuals with Physical Disabilities.
Secondary outcome for Aim 1, cardiovascular fitness, was measured with the Fatigue Index obtained from the Wingate Anaerobic Test. Participants in the Bridge Program showed improved power decline (decreased Fatigue Index) (see Table 2; p < .0001), whereas the Structured Exercise program had a worse power decline (increased Fatigue Index) (see Table 2; p < .0001) at 8 weeks.
For Aim 2, neither the relapse prevention group nor the control group showed any significant change in any of the measures at 6 months.
Discussion
Both programs increased physical activity after 8 weeks of training, but the Bridge Program also reduced symptoms of anxiety. Improvement in self-reported physical activity with both programs suggests that a less vigorous and more personal program with social support and choice can provide benefits to persons living with SCI. Reduction in anxiety has been reported with focus-of-attention behavioral therapy promoting external focus versus self-focus.30 External focus of attention is a form of cognitive behavioral therapy based on the premise that a change in behavior (an external focus on exercise vs. a self-focus on feelings) leads to a change in emotions.30 The peer mentor encouraged exercise focus during Bridge Program activity, and this external focus of attention versus self-focus may have contributed to the reduction of anxiety symptoms. The improvement in cardiovascular fitness measured by the Fatigue Index for the Bridge Program may relate to the same factors; however, the worsening of the Fatigue Index with the Structured Exercise program was unexpected and could be related to a number of factors. The structured program was more intense and frequent, allowing less time for recovery; more participants in this program used power chairs, suggesting they may have less endurance; and measurement error is also a possibility.
Our peer mentor was selected because he was a wheelchair athlete with an outgoing personality and proven problem-solving skills in living with SCI. However, one study suggests that the effectiveness of the mentorship might be improved by matching specific lived experiences and specific shared interests between the mentor and participant.31 Choice and control are important determinants for full participation in life activities. Choice is associated with power over one’s life, and further exploration of whether enhancing choice of exercise routine would increase sustainability is warranted.19 Neither the relapse prevention group nor the control group showed significant change in any of the outcome measures from postintervention to 6 months. The COVID-19 pandemic and the state-mandated shutdown of all nonessential services may have affected these results.
Several issues emerged with our choice of psychosocial measures. All instruments use ordinal scales. We elected to use a Rasch rating scale analysis on the PHQ-9, GAD-7, MSES, and PASIPD to examine the item content and rating scale structure and to transform ordinal scoring to interval level. The PHQ-9 is an established screening tool for major depressive disorder; it has been used to monitor treatment of depression32 and is the primary depression outcome measure for the Model Spinal Cord Injury Care System.33 However, for our population where a history of depression was an exclusion factor, scores clustered at the low end of the scale well below the cut point indicating floor effects at all time points. For the GAD-7, even though we showed a reduction in anxiety symptoms with the Bridge Program, scores were predominantly low with evidence of a floor effect. The MSES has established clinical utility, and good self-efficacy can be associated with a better health-related quality of life.34,35 However, there is uncertainty about the degree to which self-efficacy is modifiable, how much time is required for change, and which interventions are most effective in inducing change after SCI. The CHART-SF is the most widely used participation instrument in rehabilitation research. However, it does not assess respondents’ subjective assessment or satisfaction with life roles or allow consideration of personal preferences.36 The Participation Assessment with Recombined Tools-Objective (PART-O) has a number of advantages over the CHART-SF and should be considered in future studies.37
In retrospect, in a selected population of highly functioning people with SCI interested in exercise, changes in self-efficacy and symptoms of depression and anxiety may not reflect the overall psychological benefits of exercise. Anecdotally some participants reported improved ability to transfer, feeling more energetic, and renewed interest in other activities. We suggest that future studies measuring the benefit of social support on exercise participation include more direct participation measures such as facility attendance over time and socialization measures including interactions with people. We also recommend critical appraisal of the valid use of measurement instruments for the target population with specific consideration to eligibility criteria and potential for floor or ceiling effects.
A state-mandated shutdown of all nonessential services related to the COVID-19 pandemic interrupted the study. Enrollment was stopped, and follow-up data collection was difficult. Participant dropout caused suspension of the planned 12-month follow-up and modification of the planned SMART design analysis. Three participants in phase one were directly affected by the COVID-19 shutdown resulting in termination of exercise sessions and restriction of outcome data collection to phone interview (i.e., no cardiovascular testing). Participants in phase 2 (postintervention to 6 months) became cautious about leaving home and continuing independent exercise at the start of the pandemic; they were unable to attend any exercise facility after the shutdown. Six participants were affected by the shutdown in phase two, with data collection only by phone and no cardiovascular testing. No participants developed COVID-19. Follow-up testing for the psychosocial measures and social support was continued by phone during the shutdown. After the mandated shutdown, we initiated a structured interview by telephone to assess the effect of the COVID-19 pandemic and the mandated shutdown on our still active participants. In general, our participants were affected in ways like the general population (see Appendix).
Limitations
The sample size was small, in part due to the COVID-19 shutdown, and included varying levels of function with complete and incomplete injury, but it reflected our regional population of people with SCI. Comparisons for some of our outcome measures may have been limited by floor effects. The two randomly selected groups were similar, except the Structured Exercise group contained more people using powered mobility. Dropout and the COVID-19 interruption complicated the randomization for the SMART design. Bias and treatment contamination may have been introduced by not blinding the CTRS to the exercise groups, but consistency of approach was achieved. Groups in the Structured Exercise program could contain two to five people, perhaps providing a more personal experience than was intended, similar to the more individualized Bridge Program. Both exercise groups met in an exercise facility that was universally designed to reduce barriers to participation in the built environment. The past focus on universal design to serve people with disabilities at the facility may have influenced staff to provide some level of social support to both exercise groups. The COVID-19 pandemic shutdown occurred during the study and affected some outcome data collection and opportunity for participants to continue facility-based exercise.
Conclusion
The Bridge Program, a novel personalized exercise program with peer support, choice of exercise, and caregiver training, and a Structured Exercise program both improved self-reported physical activity at 8 weeks, but the Bridge Program also reduced anxiety symptoms. Results from relapse prevention were not different from controls at 6 months. This study provides important insights into the limitations of commonly used measures of physical activity and psychosocial domains in people with SCI. It also shows that peer support and a personalized exercise program of less intensity than guidelines can improve physical activity and reduce symptoms of anxiety.
APPENDIX
Results of the Structured Interview to Assess the Effects of the COVID-19 Pandemic on Participants (N = 28)
-
General Post-COVID-19 Shutdown Questions
86% described their general health as good or very good
43% reported their general health was worse and 54% unchanged
-
64% reported life disrupted a fair amount or a lot and 32% a little
a. Not interacting with friends/family as much 75% b. Not leaving my house as much 72% c. Not getting as much exercise 64% d. Started connecting with family/friends by computer/phone 64% e. Started having food delivered from restaurants 36% f. Started having groceries delivered 29% g. Relying more on family/friends to do things 25% h. Started having medicines delivered 7% -
12 of 28 responded to mood questions
50% reported no days of little interest or pleasure in doing things while 33% reported some days and 17% more than half the days
67% reported no days of feeling down, depressed, or hopeless while 17% reported several days, 8% more than half the days, and 8% nearly every day
71% drove their own automobile before the pandemic and 29% relied on family/friend or public transportation
-
Exercise-Specific Questions
60% began exercise at home after COVID-19 shutdown
-
Of those who began exercise at home:
Most relied on prior information/handouts from therapists or social media
About half purchased equipment usually for less than $50 (e.g., bands, weights)
Barriers were space, equipment, and motivation
Personal fitness and health goals were the primary motivation for those continuing exercise
Footnotes
Financial Support
Financial support was provided by Craig H. Neilsen Foundation grant 441124.
REFERENCES
- 1.Berra K, Rippe J, Manson JE. Making physical activity counseling a priority in clinical practice: The time for action is now. JAMA . 2015;314(24):2617–2618. doi: 10.1001/jama.2015.16244. https://www.ncbi.nlm.nih.gov/pubmed/26662069 [DOI] [PubMed] [Google Scholar]
- 2.Tanhoffer RA, Tanhoffer AI, Raymond J, Hills AP, Davis GM. Exercise, energy expenditure, and body composition in people with spinal cord injury. J Phys Act Health . 2014;11(7):1393–1400. doi: 10.1123/jpah.2012-0149. [DOI] [PubMed] [Google Scholar]
- 3.Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: A systematic review. Spinal Cord . 2011;49(11):1103–1127. doi: 10.1038/sc.2011.62. [DOI] [PubMed] [Google Scholar]
- 4.Gorla JI, Costa e Silva Ade A, Borges M, et al. Impact of wheelchair rugby on body composition of subjects with tetraplegia: A pilot study. Arch Phys Med Rehabil . 2016;97(1):92–96. doi: 10.1016/j.apmr.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Martin Ginis KA, Latimer AE, Arbour-Nicitopoulos KP, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part I: Demographic and injury-related correlates. Arch Phys Med Rehabil . 2010;91(5):722–728. doi: 10.1016/j.apmr.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Martin Ginis KA, Hicks AL, Latimer AE, et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord . 2011;49(11):1088–1096. doi: 10.1038/sc.2011.63. [DOI] [PubMed] [Google Scholar]
- 7.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc . 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 8.Vincent HK. American College of Sports Medicine; 2013. [Accessed June 30, 2016]. Resistance exercise for persons with spinal cord injury. www.acsm.org/docs/brochures/spinal-cord-injury.pdf?sfvrsn=4. [Google Scholar]
- 9.Tweedy SM, Beckman EM, Geraghty TJ, et al. Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury. J Sci Med Sport . 2017;20(2):108–115. doi: 10.1016/j.jsams.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Gainforth HL, Latimer-Cheung AE, Athanasopoulos P, Martin Ginis KA. Examining the feasibility and effectiveness of a community-based organization implementing an event-based knowledge mobilization initiative to promote physical activity guidelines for people with spinal cord injury among support personnel. Health Promot Pract . 2015;16(1):55–62. doi: 10.1177/1524839914528210. [DOI] [PubMed] [Google Scholar]
- 11.Crane DA, Hoffman JM, Reyes MR. Benefits of an exercise wellness program after spinal cord injury. J Spinal Cord Med . 2017;40(2):154–158. doi: 10.1179/2045772315Y.0000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai B, Kim Y, Wilroy J, Bickel CS, Rimmer JH, Motl RW. Sustainability of exercise intervention outcomes among people with disabilities: A secondary review. Disabil Rehabil . 2019;41(13):1584–1595. doi: 10.1080/09638288.2018.1432704. [DOI] [PubMed] [Google Scholar]
- 13.Hammel J, Magasi S, Heinemann A, Whiteneck G, Bogner J, Rodriguez E. What does participation mean? An insider perspective from people with disabilities. Disabil Rehabil . 2008;30(19):1445–1460. doi: 10.1080/09638280701625534. [DOI] [PubMed] [Google Scholar]
- 14.Hammel J, Magasi S, Heinemann A, et al. Environmental barriers and supports to everyday participation: a qualitative insider perspective from people with disabilities. Arch Phys Med Rehabil . 2015;96(4):578–588. doi: 10.1016/j.apmr.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Martin Ginis KA, Ma JK, Latimer-Cheung AE, Rimmer JH. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psychol Rev . 2016;10(4):478–494. doi: 10.1080/17437199.2016.1198240. [DOI] [PubMed] [Google Scholar]
- 16.Magasi S, Wong A, Gray DB, et al. Theoretical foundations for the measurement of environmental factors and their impact on participation among people with disabilities. Arch Phys Med Rehabil . 2015;96(4):569–577. doi: 10.1016/j.apmr.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” design for building individualized treatment sequences. Ann Rev Clin Psychol . 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandura A. Social cognitive theory: An agentic perspective. Ann Rev Psychol . 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Murray CM, Van Kessel G, Guerin M, Hillier S, Stanley M. Exercising choice and control: A qualitative meta-synthesis of perspectives of people with a spinal cord injury. Arch Phys Med Rehabil . 2019;100(9):1752–1762. doi: 10.1016/j.apmr.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 20.American College of Sports Medicine ACSM specialty certifications & certificates. [Accessed May 24, 2022]. https://www.acsm.org/certification/specialized
- 21.National Center on Health Physical Activity and Disability Certified inclusive fitness trainer. https://nchpad.org/614/2577/Certified-Inclusive-Fitness-Trainer
- 22.Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: Sevelopment and evaluation. Arch Phys Med Rehabil . 2002;83(2):193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- 23.de Groot S, van der Woude LH, Niezen A, Smit CA, Post MW. Evaluation of the physical activity scale for individuals with physical disabilities in people with spinal cord injury. Spinal Cord . 2010;48(7):542–547. doi: 10.1038/sc.2009.178. [DOI] [PubMed] [Google Scholar]
- 24.Williams RT, Heinemann AW, Neumann HD, et al. Evaluating the psychometric properties and responsiveness to change of 3 depression measures in a sample of persons with traumatic spinal cord injury and major depressive disorder. Arch Phys Med Rehabil . 2016;97(6):929–937. doi: 10.1016/j.apmr.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med . 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 26.Middleton JW, Tate R.L., Geraghty T.J. Self-efficacy and spinal cord injury: Psychometric properties of a new scale. Rehabil Psychol . 2003;48(4):281–288. [Google Scholar]
- 27.Hall K, Dijkers M, Whiteneck G, Brooks CA, Krause JS. The Craig Handicap Assessment and Reporting Technique (CHART): Metric properties and scoring. Top Spinal Cord Inj Rehabil . 1998;4(1):16–30. [Google Scholar]
- 28.Bar-Or O. The Wingate Anaerobic Test--An update on methodology, reliability and validity. Sports Med . 1987;4(6):381–394. doi: 10.2165/00007256-198704060-00001. [DOI] [PubMed] [Google Scholar]
- 29.Boone WJ. Rasch analysis for instrument development: Why, when, and how? CBE Life Sci Educ . 2016;15(4):rm4. doi: 10.1187/cbe.16-04-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renner KA, Valentiner DP, Holzman JB. Focus-of-attention behavioral experiment: an examination of a therapeutic procedure to reduce social anxiety. Cogn Behav Ther . 2017;46(1):60–74. doi: 10.1080/16506073.2016.1225814. [DOI] [PubMed] [Google Scholar]
- 31.Gainforth HL, Giroux EE, Shaw RB, et al. Investigating characteristics of quality peer mentors with spinal cord injury. Arch Phys Med Rehabil . 2019;100(10):1916–1923. doi: 10.1016/j.apmr.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care . 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 33.National Spinal Cord Injury Statistical Center Annual statistical report. 2018. [Accessed May 25,2022]. https://www.nscisc.uab.edu/Public/2018%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
- 34.Middleton J, Tran Y, Craig A. Relationship between quality of life and self-efficacy in persons with spinal cord injuries. Arch Phys Med Rehabil . 2007;88(12):1643–1648. doi: 10.1016/j.apmr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Guest R, Craig A, Tran Y, Middleton J. Factors predicting resilience in people with spinal cord injury during transition from inpatient rehabilitation to the community. Spinal Cord . 2015;53(9):682–686. doi: 10.1038/sc.2015.32. [DOI] [PubMed] [Google Scholar]
- 36.Magasi SR, Heinemann AW, Whiteneck GG, Quality of Life/Participation C. Participation following traumatic spinal cord injury: An evidence-based review for research. J Spinal Cord Med . 2008;31(2):145–156. doi: 10.1080/10790268.2008.11760705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whiteneck GG, Gassaway J, Ketchum JM. Transforming a Traumatic brain injury measure of participation into a psychometrically sound spinal cord injury participation measure. Arch Phys Med Rehabil . 2019;100(12):2293–2300. doi: 10.1016/j.apmr.2019.06.020. [DOI] [PubMed] [Google Scholar]


