Key Points
Question
Among women carrying germline BRCA pathogenic variants, is pregnancy after breast cancer associated with adverse maternal or fetal outcomes?
Findings
This international, hospital-based, retrospective cohort study including 4732 BRCA carriers showed that 1 in 5 patients conceived within 10 years after breast cancer diagnosis. Pregnancy following breast cancer in BRCA carriers was not associated with adverse maternal prognosis or fetal outcomes.
Meaning
The cumulative incidence of pregnancy after breast cancer and disease-free survival in this large international cohort of young BRCA carriers may inform care for affected patients.
Abstract
Importance
Young women with breast cancer who have germline pathogenic variants in BRCA1 or BRCA2 face unique challenges regarding fertility. Previous studies demonstrating the feasibility and safety of pregnancy in breast cancer survivors included limited data regarding BRCA carriers.
Objective
To investigate cumulative incidence of pregnancy and disease-free survival in young women who are BRCA carriers.
Design, Setting, and Participants
International, multicenter, hospital-based, retrospective cohort study conducted at 78 participating centers worldwide. The study included female participants diagnosed with invasive breast cancer at age 40 years or younger between January 2000 and December 2020 carrying germline pathogenic variants in BRCA1 and/or BRCA2. Last delivery was October 7, 2022; last follow-up was February 20, 2023.
Exposure
Pregnancy after breast cancer.
Main Outcomes and Measures
Primary end points were cumulative incidence of pregnancy after breast cancer and disease-free survival. Secondary end points were breast cancer–specific survival, overall survival, pregnancy, and fetal and obstetric outcomes.
Results
Of 4732 BRCA carriers included, 659 had at least 1 pregnancy after breast cancer and 4073 did not. Median age at diagnosis in the overall cohort was 35 years (IQR, 31-38 years). Cumulative incidence of pregnancy at 10 years was 22% (95% CI, 21%-24%), with a median time from breast cancer diagnosis to conception of 3.5 years (IQR, 2.2-5.3 years). Among the 659 patients who had a pregnancy, 45 (6.9%) and 63 (9.7%) had an induced abortion or a miscarriage, respectively. Of the 517 patients (79.7%) with a completed pregnancy, 406 (91.0%) delivered at term (≥37 weeks) and 54 (10.4%) had twins. Among the 470 infants born with known information on pregnancy complications, 4 (0.9%) had documented congenital anomalies. Median follow-up was 7.8 years (IQR, 4.5-12.6 years). No significant difference in disease-free survival was observed between patients with or without a pregnancy after breast cancer (adjusted hazard ratio, 0.99; 95% CI, 0.81-1.20). Patients who had a pregnancy had significantly better breast cancer–specific survival and overall survival.
Conclusions and Relevance
In this global study, 1 in 5 young BRCA carriers conceived within 10 years after breast cancer diagnosis. Pregnancy following breast cancer in BRCA carriers was not associated with decreased disease-free survival.
Trial Registration
ClinicalTrials.gov Identifier: NCT03673306
This retrospective cohort study assesses the cumulative incidence of pregnancy after cancer and disease-free survival in BRCA carriers diagnosed with invasive breast cancer at age 40 years or younger.
Introduction
A substantial proportion of young women with newly diagnosed breast cancer are interested in future fertility.1,2 More than 12% of these young patients carry a germline pathogenic variant in the BRCA1 or BRCA2 genes.3 Reproductive counseling of BRCA carriers is particularly complex considering the psychological fear of transmitting the pathogenic variant to their offspring,4 the possible negative impact of deficient BRCA function on their ovarian reserve and fertility potential,5 and the indication to undergo risk-reducing bilateral salpingo-oophorectomy at a young age due to increased risk of ovarian cancer.6 Moreover, while several studies have demonstrated the safety of conceiving following treatment completion for breast cancer, the evidence for BRCA carriers is very limited.7 Concerns exist about maternal and fetal safety of conceiving after breast cancer due to the hormone surge during pregnancy potentially increasing breast cancer (a hormone-driven tumor) recurrence risk and the possible negative fetal effects of prior exposure of women and their reproductive organs to anticancer therapies.7
We previously reported preliminary results from a study including 1252 BRCA carriers from 30 centers showing no apparent negative consequences in maternal or fetal outcomes in patients with a pregnancy after breast cancer.8 However, the overall sample size was smaller than expected per study initial statistical assumptions and limited analyses could be performed.8 Hence, concerns remain regarding feasibility and safety of pregnancy in this population.9 Thus, additional patients have been included in this larger international study, which includes the 1252 BRCA carriers in the first report,8 in order to investigate the cumulative incidence of pregnancy after breast cancer and reproductive and disease outcomes in BRCA carriers.
Methods
Study Design, Setting, and Patients
This was an international, multicenter, hospital-based, retrospective cohort study including female BRCA carriers with a history of breast cancer.8
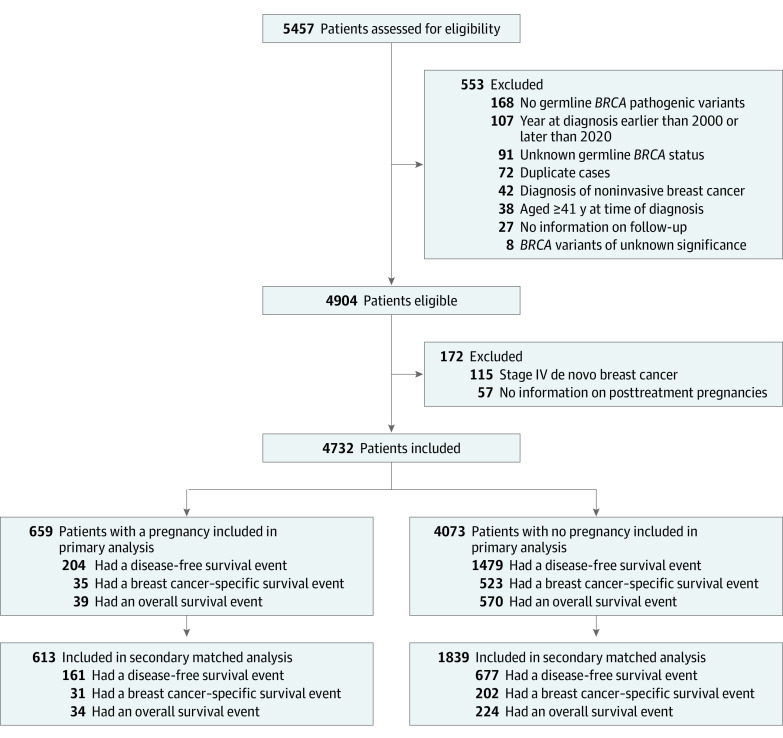
To be eligible for inclusion, female participants (sex was assigned based on medical records) had to be diagnosed at age 40 years or younger with invasive breast cancer between January 2000 and December 2020 carrying germline likely pathogenic or pathogenic variants in the BRCA1 and/or BRCA2 genes. Healthy BRCA carriers or patients with BRCA variants of unknown significance, noninvasive breast cancer, or history of other malignancies prior to breast cancer diagnosis were excluded. Patients with de novo stage IV breast cancer or lack of data on posttreatment pregnancies were also excluded. Data sets from countries with more than 1 participating center were cross-checked to exclude potential patient duplication.
Data Collection and Study Oversight
Data collected for all eligible patients included breast cancer history and treatment, type of germline BRCA pathogenic variant and risk-reducing management, recurrence data, survival, and reproductive outcomes. Diagnostic and staging workup, treatment, and follow-up were performed by each center according to clinical practice. Patients’ pregnancy status was determined based on follow-up information collected from the medical records (by patient self-report during follow-up clinic visits and/or by serial patient survey depending on the center). Information on pregnancy status was based on the first pregnancy (regardless of outcome) after breast cancer diagnosis. Patients whose initial breast cancer diagnosis occurred while pregnant, without a subsequent new pregnancy, were not considered to have become pregnant after diagnosis.
The Institut Jules Bordet (Brussels, Belgium) was the coordinating center and served as the central ethics committee. The study also received ethics approval by the local, regional, or national institutional review boards of participating centers whenever required by regulations. Written informed consent was obtained from participants before inclusion for centers with this requirement. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was followed to report this work.10
Statistical Analysis
The study protocol is available in Supplement 1. The primary objectives of this study were to determine the cumulative incidence of pregnancy after breast cancer and its prognostic impact in BRCA carriers. The primary end points were the cumulative incidence of pregnancy and disease-free survival. There were 12 secondary end points, including breast cancer–specific survival and overall survival, as well as pregnancy, fetal, and obstetric outcomes. The following parameters for fetal and obstetric outcomes were assessed: patient age at conception, time from breast cancer diagnosis to conception (ie, pregnancy interval), type of conception, number of preterm (<37 weeks) or full-term (≥37 weeks) pregnancies, live births, induced or spontaneous abortions, congenital malformations, pregnancy and/or obstetric complications, and incidence and duration of breastfeeding.
Predefined subgroup analyses according to specific BRCA gene (BRCA1 or BRCA2), hormone receptor status (positive or negative), ERBB2 status (positive or negative), exposure to chemotherapy (prior exposure or no prior exposure), and exposure to endocrine therapy (prior exposure or no prior exposure) were conducted.
Categorical variables were summarized using proportions, and the χ2 test for heterogeneity was used for comparison; continuous variables were summarized using medians and IQRs and compared using the Wilcoxon-Mann-Whitney test. The Kaplan-Meier method was used to compute the cumulative incidence of pregnancy. The median follow-up was computed using the reverse Kaplan-Meier method.11
To assess the prognostic impact of pregnancy after breast cancer, we examined the association of pregnancy status with the rate of several outcomes. A disease-free survival event was defined as the occurrence of 1 of the following invasive events: locoregional recurrence, distant metastases, new contralateral or ipsilateral invasive breast cancer, second primary malignancy, or death due to any cause. A breast cancer–specific survival event was defined as death due to breast cancer, and patients who died for reasons other than breast cancer were censored at the date of death. An overall survival event was defined as death due to any cause. Observation time of patients without a survival event of interest was censored on the date of their last contact. Rates for disease-free survival events were computed as the ratio between the total number of events and the total of the observation times. Patients who became pregnant after breast cancer contributed to the nonpregnant observation time until estimated time of conception.
To quantify the association between pregnancy and subsequent events, we used an extended Cox model with occurrence of pregnancy as a time-varying covariate. The multivariate models included as stratification factors the variables associated with survival outcomes that were differently distributed between patients who became pregnant after breast cancer and those who did not (ie, region, age, nodal status, hormone receptor status, and type of breast surgery). To avoid the exclusion of patients with missing information, we grouped into the “unknown” category patients with missing values on each covariate included in the model. No imputation or other method for handling missing data was applied.
A secondary analysis matched each patient with a pregnancy after breast cancer with 3 patients without subsequent pregnancy according to disease-free interval (defined as time from breast cancer diagnosis to conception), specific BRCA gene, hormone receptor status, nodal status, and year at diagnosis (eAppendix in Supplement 2). For this analysis, all survival outcomes were calculated from the date of conception (or a similar disease-free interval in matched patients with no pregnancy) and are presented by Kaplan-Meier plots.
All statistical analyses were 2-sided with P < .05 considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
Patients
From 78 centers worldwide, 4732 of 5457 patients were eligible for this analysis; for most of the 1252 BRCA carriers from 30 centers previously reported, the follow-up was updated.8 Among the included patients, during the observation time, 659 had at least 1 pregnancy following breast cancer and 4073 did not (Figure 1). Overall, median follow-up was 7.8 years (IQR, 4.5-12.6 years) (eTable 1 in Supplement 2). The last delivery was October 7, 2022; the last follow-up was February 20, 2023.
Figure 1. Participant Flow.
Median age at diagnosis in the overall cohort was 35 years (IQR, 31-38 years). Compared with patients with no pregnancy, those with a pregnancy after breast cancer were younger at diagnosis of breast cancer. They were also more likely to carry BRCA1 pathogenic variants, to have node-negative and hormone receptor–negative breast cancer, to undergo breast-conserving surgery, and, if diagnosed with hormone receptor–positive disease, to receive ovarian suppression treatment as part of adjuvant endocrine therapy for a shorter period (Table 1; eTable 2 in Supplement 2). During follow-up, 2443 patients (51.6%) underwent risk-reducing salpingo-oophorectomy, 279 (42.3%) of those with a pregnancy after breast cancer and 2164 (53.1%) of those with no pregnancy.
Table 1. Patient Characteristics at Diagnosis of Breast Cancer.
| Characteristics | No. (%) | ||
|---|---|---|---|
| Overall cohort (n = 4732) | Patients with a pregnancy (n = 659)a | Patients with no pregnancy (n = 4073) | |
| Region | |||
| Southern Europe | 2080 (44.0) | 303 (46.0) | 1777 (43.6) |
| Asia | 780 (16.5) | 130 (19.7) | 650 (16.0) |
| Northern Europe | 709 (15.0) | 110 (16.7) | 599 (14.7) |
| North America | 519 (11.0) | 59 (9.0) | 460 (11.3) |
| Eastern Europe | 304 (6.4) | 22 (3.3) | 282 (6.9) |
| Australia/Oceania | 193 (4.1) | 26 (3.9) | 167 (4.1) |
| Latin America/South America | 147 (3.1) | 9 (1.4) | 138 (3.4) |
| Year at diagnosis of breast cancer | |||
| 2000-2004 | 604 (12.8) | 106 (16.1) | 498 (12.2) |
| 2005-2008 | 788 (16.7) | 141 (21.4) | 647 (15.9) |
| 2009-2012 | 1005 (21.2) | 170 (25.8) | 835 (20.5) |
| 2013-2016 | 1158 (24.5) | 159 (24.1) | 999 (24.5) |
| 2017-2020 | 1177 (24.9) | 83 (12.6) | 1094 (26.9) |
| Age at diagnosis of breast cancer, y | |||
| ≤30 | 977 (20.7) | 331 (50.2) | 646 (15.9) |
| 31-35 | 1720 (36.4) | 262 (39.8) | 1458 (35.8) |
| 36-40 | 2035 (43.0) | 66 (10.0) | 1969 (48.3) |
| Median (IQR) | 35 (31-38) | 30 (28-33) | 35 (32-38) |
| Specific BRCA gene | |||
| BRCA1 | 3033 (64.1) | 483 (73.3) | 2550 (62.6) |
| BRCA2 | 1663 (35.1) | 170 (25.8) | 1493 (36.7) |
| BRCA1 and BRCA2 | 26 (0.6) | 3 (0.5) | 23 (0.6) |
| BRCA, unknown if BRCA1 or BRCA2 | 10 (0.2) | 3 (0.5) | 7 (0.2) |
| Tumor characteristics | |||
| Histology | n = 4575 | n = 634 | n = 3941 |
| Ductal carcinoma | 3921 (85.7) | 560 (88.3) | 3361 (85.3) |
| Lobular carcinoma | 135 (3.0) | 10 (1.6) | 125 (3.2) |
| Mixed ductal/lobular | 57 (1.3) | 7 (1.1) | 50 (1.3) |
| Invasive, not specified | 201 (4.4) | 24 (3.8) | 177 (4.5) |
| Otherb | 261 (5.7) | 33 (5.2) | 228 (5.8) |
| Gradec | n = 4266 | n = 605 | n = 3661 |
| G1 | 79 (1.9) | 8 (1.3) | 71 (1.9) |
| G2 | 991 (23.2) | 119 (19.7) | 872 (23.8) |
| G3 | 3196 (74.9) | 478 (79.0) | 2718 (74.2) |
| Sized | n = 4500 | n = 629 | n = 3871 |
| T1 (≤2 cm) | 1811 (40.2) | 282 (44.8) | 1529 (39.5) |
| T2 (>2 to ≤5 cm) | 2050 (45.6) | 270 (42.9) | 1780 (46.0) |
| T3 (>5 cm) to T4 | 639 (14.2) | 77 (12.2) | 562 (14.5) |
| Nodal statusd | n = 4546 | n = 638 | n = 3908 |
| N0 | 2434 (53.5) | 399 (62.5) | 2035 (52.1) |
| N1 | 1556 (34.2) | 180 (28.2) | 1376 (35.2) |
| N2 to N3 | 556 (12.2) | 59 (9.3) | 497 (12.7) |
| Hormone receptor status | n = 4655 | n = 648 | n = 4007 |
| ER and/or PR positive | 2126 (45.7) | 216 (33.3) | 1910 (47.7) |
| ER and PR negative | 2529 (54.3) | 432 (66.7) | 2097 (52.3) |
| ERBB2 status | n = 4490 | n = 625 | n = 3865 |
| Negative | 4151 (92.5) | 589 (94.2) | 3562 (92.2) |
| Positive | 339 (7.6) | 36 (5.8) | 303 (7.8) |
| Treatment | |||
| Breast surgery | n = 4635 | n = 646 | n = 3989 |
| None | 15 (0.3) | 2 (0.3) | 13 (0.3) |
| Breast-conserving surgery | 1826 (39.4) | 315 (48.8) | 1511 (37.9) |
| Mastectomy | 2794 (60.3) | 329 (50.9) | 2465 (61.8) |
| Received chemotherapy | 4319/4700 (91.9) | 611/658 (92.9) | 3708/4042 (91.7) |
| Type of chemotherapye | n = 4169 | n = 598 | n = 3571 |
| Anthracycline and taxane based | 3051 (73.2) | 414 (69.2) | 2637 (73.8) |
| Anthracycline based | 798 (19.1) | 143 (23.9) | 655 (18.3) |
| Taxane based | 188 (4.5) | 19 (3.2) | 169 (4.7) |
| Other | 132 (3.2) | 22 (3.7) | 110 (3.1) |
| Received endocrine therapyf | 1987/2098 (94.7) | 197/215 (91.6) | 1790/1883 (95.1) |
| Type of endocrine therapyg | n = 1969 | n = 196 | n = 1773 |
| Tamoxifen alone | 702 (35.7) | 64 (32.7) | 638 (36.0) |
| Tamoxifen plus LHRH agonist | 550 (27.9) | 81 (41.3) | 469 (26.5) |
| LHRH agonist alone | 43 (2.2) | 7 (3.6) | 36 (2.0) |
| Aromatase inhibitor with or without LHRH agonist | 355 (18.0) | 21 (10.7) | 334 (18.8) |
| Tamoxifen and aromatase inhibitor (with or without LHRH agonist) | 293 (14.9) | 19 (9.7) | 274 (15.5) |
| Other | 26 (1.3) | 4 (2.0) | 22 (1.2) |
| Duration of endocrine therapy, median (IQR), mo | 60 (27-60) | 48 (24-60) | 60 (28-60) |
| Unknown, No. | 507 | 40 | 467 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; LHRH, luteinizing hormone–releasing hormone.
Patients with a pregnancy included women with at least 1 pregnancy (irrespective of the outcome) any time following breast cancer diagnosis. Information on pregnancy after breast cancer was collected from medical records based on patient self-report during follow-up clinic visits and/or by serial patient survey, depending on the center.
Other histology findings included medullary (n = 87), metaplastic (n = 25), mucinous (n = 19), papillary (n = 9), micropapillary (n = 7), apocrine (n = 7), squamous cell (n = 4), tubular carcinoma (n = 4), salivary gland type (n = 3), secretory (n = 3), pleomorphic variant (n = 3), comedocarcinoma (n = 1), neuroendocrine (n = 1), adenosquamous (n = 1), cribriform (n = 1), colloid (n = 1), and unknown (n = 85).
Histologic grade was based on the degree of tumor histologic differentiation.
Tumor size and nodal status were assessed clinically for patients who received neoadjuvant systemic therapy and pathologically for those who received breast surgery as first treatment.
Calculated among patients who received chemotherapy.
Calculated among patients with hormone receptor–positive breast cancer.
Calculated among patients with hormone receptor–positive breast cancer who received endocrine therapy.
Overall, the cumulative incidence of pregnancy at 10 years was 22% (95% CI, 21%-24%) (Figure 2). In patients with hormone receptor–positive and hormone receptor–negative breast cancer, the cumulative incidence of pregnancy at 10 years was 18% (95% CI, 16%-21%) and 26% (95% CI, 24%-29%), respectively (P < .001) (Figure 2).
Figure 2. Cumulative Incidence of Pregnancy Overall and According to Hormone Receptor Status.
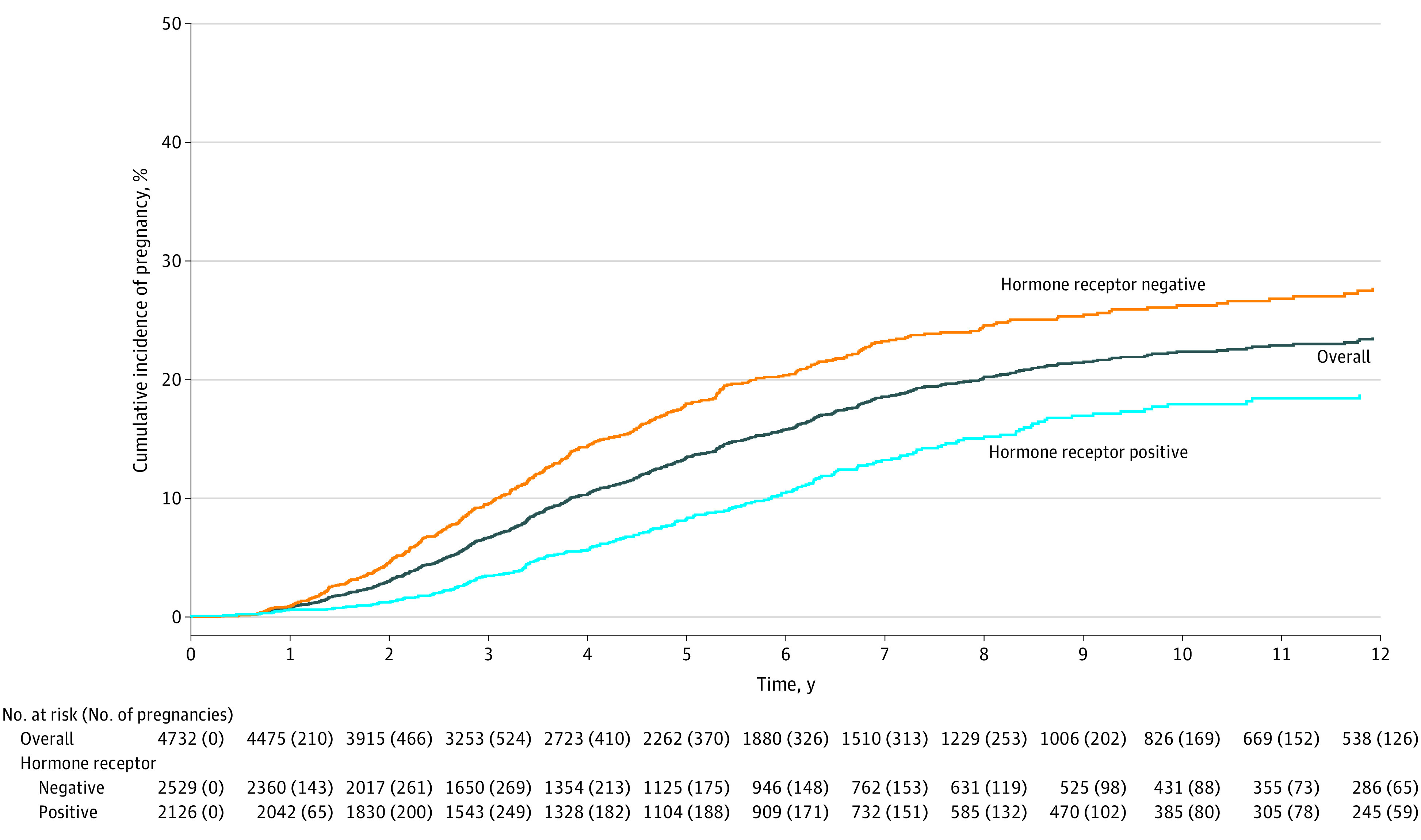
Median observation time in the overall cohort was 5.6 years (IQR, 2.9-9.4 years); in patients with hormone receptor–positive disease, 5.8 years (IQR, 3.0-9.3 years); and in patients with hormone receptor–negative disease, 5.6 years (IQR, 2.7-9.6 years).
The median age at conception was 34.7 years (IQR, 31.8-37.3 years). The median time from breast cancer diagnosis to conception was 3.5 years (IQR, 2.2-5.3 years; 27.8% of pregnancies occurred after 5 years). This interval was significantly longer in patients with hormone receptor–positive breast cancer (4.3 years [IQR, 2.8-6.3 years]; 39.8% of pregnancies occurred after 5 years) than in those with hormone receptor–negative disease (3.2 years [IQR, 2.0-4.8 years]; 22.0% of pregnancies occurred after 5 years) (P < .001). A total of 121 patients (20.8%) had a pregnancy with use of assisted reproductive technology. Of the 659 patients who had a pregnancy (Table 2; eTable 3 in Supplement 2), 45 (6.9%) and 63 (9.7%) had an induced abortion or a miscarriage, respectively. Among 517 patients (79.7%) with a completed pregnancy, 406 (91.0%) delivered at term (≥37 weeks) and 54 (10.4%) had twins. Congenital anomalies were documented in 4 of 470 infants (0.9%) born with known information on pregnancy complications.
Table 2. Pregnancy, Fetal, and Obstetric Outcomes in Patients With a Pregnancy After Breast Cancer.
| Outcomes | No. (%) (n = 659) |
|---|---|
| Age at pregnancy, median (IQR), y | 34.7 (31.8-37.3) |
| Time from diagnosis to conception, median (IQR) y | 3.5 (2.2-5.3) |
| Pregnancy interval | |
| ≤2 Years after diagnosis | 131 (19.9) |
| Between >2 and ≤5 years after diagnosis | 345 (52.4) |
| >5 Years after diagnosis | 183 (27.8) |
| Type of conception | |
| Spontaneous pregnancy | 461/582 (79.2) |
| Use of assisted reproductive technology | 121/582 (20.8) |
| Embryo transfer after oocyte/embryo cryopreservation at diagnosis of breast cancer | 48 |
| Embryo transfer following oocyte donation | 29 |
| Ovarian stimulation for IVF/ICSI/ovulation induction after anticancer treatment | 36 |
| Unknown type of assisted reproductive technology | 8 |
| Pregnancy outcome | n = 649 |
| Delivered | 517 (79.7) |
| Ongoing pregnancy | 24 (3.7) |
| Miscarriage | 63 (9.7) |
| Induced abortion | 45 (6.9) |
| No. of live births from first pregnancy after breast cancera | n = 517 |
| 1 | 463 (89.6) |
| 2 | 54 (10.4) |
| Timing of deliverya | n = 446 |
| At term (≥37 wk) | 406 (91.0) |
| Preterm (<37 wk) | 40 (9.0) |
| Complicationsa | n = 423 |
| None | 365 (86.3) |
| Pregnancy complications | 27 (6.4) |
| Delivery complications | 22 (5.2) |
| Congenital abnormalitiesb,c | 4 (0.9) |
| Fetal complicationsb,c | 3 (0.6) |
| Other complicationsc | 2 (0.5) |
| Breastfeedinga | 133/403 (33.0) |
| Duration, median (IQR), mo | 5 (2-6) |
| Unknown, No. | 50 |
Abbreviations: IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection.
Calculated from the total number of delivered pregnancies.
Calculated from the total number of infants born to patients with known information on pregnancy complications (n = 470).
Congenital abnormalities included cardiac malformation (n = 2), congenital diaphragmatic hernia (n = 1), and chromosome abnormality with karyotype 47,XXY (n = 1). Fetal complications included respiratory distress (n = 2) and neonatal icterus treated with phototherapy (n = 1). Other complications included maternal internal carotid artery aneurysm (n = 1) and kidney failure in the infant due to hypoxia (n = 1).
Disease-Free Survival
There were 1683 disease-free survival events (Figure 1). The pattern of disease-free survival events among patients with a pregnancy after breast cancer and those without a pregnancy are reported in eTable 1 in Supplement 2. The association between pregnancy and the occurrence of disease-free survival events was not statistically significant (unadjusted hazard ratio [HR], 0.97 [95% CI, 0.82-1.15], P = .74; adjusted HR, 0.99 [95% CI, 0.81-1.20], P = .90). In adjusted subgroup analyses, a statistically significant interaction between occurrence of pregnancy and the following variables was observed: specific BRCA gene (BRCA1: adjusted HR, 0.80 [95% CI, 0.63-1.01]; BRCA2: adjusted HR, 1.55 [95% CI, 1.12-2.16]; P = .007 for interaction); hormone receptor status (hormone receptor–positive: adjusted HR, 1.30 [95% CI, 0.95-1.76]; hormone receptor–negative: adjusted HR, 0.76 [95% CI, 0.60-0.95]; P = .009 for interaction); and use of endocrine therapy (no use of endocrine therapy: adjusted HR, 0.85; [95% CI, 0.67-1.08]; use of endocrine therapy: adjusted HR, 1.55 [95% CI, 1.08-2.21]; P = .01 for interaction) (Table 3; eTable 4 in Supplement 2).
Table 3. Subgroup Analyses of Disease-Free Survival in Patients Who Had a Pregnancy (vs Patients With No Pregnancy).
| Variables | No. of patients/No. of events | Univariate hazard ratio (95% CI) | P value | Multivariate hazard ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Study group | 4732/1683 | 0.97 (0.82-1.15) | .74 | 0.99 (0.81-1.20) | .90 |
| Specific BRCA gene | |||||
| BRCA1 | 3033/1101 | 0.79 (0.64-0.97) | <.001a | 0.80 (0.63-1.01) | .007a |
| BRCA2 | 1663/569 | 1.61 (1.22-2.12) | 1.55 (1.12-2.16) | ||
| BRCA1 and BRCA2 | 26/11 | 1.82 (0.33-10.1) | 4.49 (0.28-72.17) | ||
| BRCA, unknown if BRCA1 or BRCA2 | 10/2 | 1.11 (0.05-23.2) | NE | ||
| Hormone receptor status | |||||
| Positive | 2126/715 | 1.29 (0.98-1.70) | .04a | 1.30 (0.95-1.76) | .009a |
| Negative | 2529/951 | 0.82 (0.67-1.01) | 0.76 (0.60-0.95) | ||
| Unknown | 77/17 | 1.08 (0.25-4.74) | 0.28 (0.04-2.21) | ||
| ERBB2 status | |||||
| Positive | 339/111 | 0.66 (0.24-1.80) | .30a | 0.61 (0.22-1.71) | .08a |
| Negative | 4151/1471 | 1.01 (0.85-1.21) | 1.07 (0.87-1.31) | ||
| Unknown | 242/101 | 0.61 (0.30-1.26) | 0.42 (0.17-1.02) | ||
| Chemotherapy | |||||
| No chemotherapy | 381/138 | 1.06 (0.61-1.87) | .31a | 0.77 (0.39-1.52) | .47a |
| (Neo)adjuvant chemotherapy | 4319/1534 | 0.97 (0.82-1.16) | 1.00 (0.82-1.23) | ||
| Unknown | 32/11 | NE | 0.77 (0.39-1.52) | ||
| Endocrine therapy | |||||
| No endocrine therapy | 2640/998 | 0.82 (0.67-1.01) | .02a | 0.85 (0.67-1.08) | .01a |
| Endocrine therapy | 1987/659 | 1.35 (1.01-1.81) | 1.55 (1.08-2.21) | ||
| Unknown | 105/26 | 0.77 (0.18-3.23) | 0.13 (0.01-2.95) |
Abbreviation: NE, not evaluable.
P value for interaction.
Secondary Survival Outcomes
There were 558 breast cancer–specific survival events (Figure 1). The occurrence of pregnancy was associated with a lower rate of breast cancer–specific survival events (HR, 0.53 [95% CI, 0.37-0.74], P < .001; adjusted HR, 0.60 [95% CI, 0.40-0.88], P = .009). No significant interaction was observed between occurrence of pregnancy and any variable in adjusted subgroup analyses (eTable 5 in Supplement 2).
There were 609 overall survival events (Figure 1). The occurrence of pregnancy was associated with a lower rate of death due to any cause (unadjusted HR, 0.52, [95% CI, 0.38-0.72], P < .001; adjusted HR, 0.58 [95% CI, 0.40-0.85], P = .005). No significant interaction was observed between occurrence of pregnancy and any variable in adjusted subgroup analyses (eTable 6 in Supplement 2).
The eAppendix in Supplement 2 shows results from a secondary matched analysis that included 2452 patients, 613 with a pregnancy after breast cancer and 1839 matched patients with no pregnancy (eAppendix, eTables 2-9, and eFigures 1-3 in Supplement 2).
Discussion
This global study provides descriptive information on pregnancy after breast cancer in BRCA carriers from a much larger cohort than prior findings.8 For the 22% of young BRCA carriers who conceived within 10 years after breast cancer diagnosis, subsequent pregnancy was not associated with adverse maternal prognosis or fetal outcomes.
The cumulative incidence of pregnancy observed in this study is higher than previously reported in young breast cancer survivors.7 This may be due to younger patient age at time of diagnosis, an increased priority of pregnancy for the indication to undergo risk-reducing bilateral salpingo-oophorectomy during reproductive years, and the large proportion of patients not requiring adjuvant endocrine therapy (54.3% had hormone receptor–negative breast cancer). As expected,12 young women with a history of hormone receptor–positive breast cancer had lower cumulative incidence of pregnancy and longer time from diagnosis to conception than those with hormone receptor–negative disease, presumably due to use of endocrine therapy, during which pregnancy is contraindicated.13 Nevertheless, 60% of pregnancies in patients with hormone receptor–positive breast cancer occurred within 5 years of diagnosis. This percentage is expected to increase based on the reassuring early results of the POSITIVE trial showing the safety of temporary interruption of endocrine therapy to attempt pregnancy 18 to 30 months into endocrine therapy.14
Women who are BRCA carriers face unique reproductive concerns. Significant knowledge gaps and misconceptions exist among physicians in their oncofertility counseling.4 Young breast cancer survivors have reduced chances of future conception compared with the general population and young survivors of most other malignancies.7 Current guidelines recommend close monitoring of posttreatment pregnancies in adult women with a history of cancer due to a higher risk of pregnancy complications, including preterm births, in cancer survivors compared with the general population.15 The current recommendation is to wait at least 1 year following chemotherapy completion to attempt pregnancy due to a higher risk of pregnancy complications in women conceiving within 1 year following the end of cytotoxic therapy.7,16 Results from our study, in which 80.1% of pregnancies occurred more than 2 years after diagnosis, provide evidence in the specific cohort of young BRCA carriers with a rate of pregnancy complications that are in line with the expectations in a population of women with similar age and no history of breast cancer.17,18,19 The majority of information for this analysis was extracted from oncology medical records. These records are not specifically designed for recording maternal or fetal outcomes; hence, there is a potential risk of underreporting of adverse pregnancy outcomes and the data should be considered with caution.
The majority of the pregnancies occurred spontaneously (79.2%) despite receipt of prior chemotherapy in more than 90% of patients. Considering that the median age at diagnosis in patients with a pregnancy after breast cancer was 30 years, the risk of treatment-induced premature ovarian insufficiency and associated infertility can be considered relatively low in these patients.15 Nevertheless, all young women diagnosed with cancer during reproductive years should be offered the opportunity to access fertility preservation strategies before initiating systemic anticancer therapies.15,20,21 This is particularly relevant in young BRCA carriers considering their possible interest in accessing preimplantation genetic diagnosis for monogenic diseases,15 as well as the potential increased risk of chemotherapy-induced premature ovarian insufficiency compared with age-matched noncarriers.22 In addition, further treatments, including adjuvant olaparib for 1 year following cytotoxic therapy in BRCA carriers at higher risk of disease recurrence or carboplatin and pembrolizumab as neoadjuvant therapy for triple-negative breast cancer, may pose added risk to fertility, having shown possible gonadotoxicity in mouse models.23,24,25 Future studies are needed to understand these risks.26
Only limited prior maternal safety data have specifically focused on BRCA carriers conceiving after breast cancer.7 With the exception of the disease-free survival analysis with the extended Cox model showing an adjusted HR of 0.99 (95% CI, 0.81-1.20), the other analyses of breast cancer–specific survival and overall survival showed significantly better outcomes for young BRCA carriers with a pregnancy after breast cancer. The consistent findings of safety in the different models and analyzed outcomes, the large sample size with global representation, and the median follow-up of nearly 8 years support the lack of detrimental prognostic effect of pregnancy after breast cancer in BRCA carriers.
Results for most of the analyzed subgroups were consistent with those of the overall study. However, there was a significant interaction between occurrence of pregnancy and specific BRCA gene. Specifically, pregnancy appeared to be associated with lower event rates among BRCA1 carriers in all the analyses. On the contrary, among BRCA2 carriers, the analysis identified a signal for a possible association between pregnancy and adverse disease-free survival outcomes (adjusted HR, 1.55; 95% CI, 1.12-2.16). No interaction between occurrence of pregnancy and specific BRCA gene was observed for the other survival outcomes. An apparent protective association was observed in BRCA1 carriers in breast cancer–specific survival and overall survival; HRs were close to 1.00 with the 95% CI crossing unity in both directions in BRCA2 carriers. Thus, while the results appear reassuring for BRCA1 carriers, more caution is needed to counsel BRCA2 carriers. Considering that there is evidence of a potentially differential impact of reproductive factors on breast cancer risk and outcomes according to the specific BRCA gene,27,28,29,30 our data highlight the need to pursue further research efforts in this area. A possible impact of hormone receptor status cannot be excluded considering that BRCA1 and BRCA2 carriers tend to more often develop hormone receptor–negative and hormone receptor–positive breast cancers, respectively. Several prior studies suggested that pregnancy after breast cancer is associated with improved outcomes in patients with a history of hormone receptor–negative disease and may have no effect in those with hormone receptor–positive tumors.7 Our study has also shown an interaction between occurrence of pregnancy and hormone receptor status (and, subsequently, use of endocrine therapy) for the disease-free survival end point only. Our finding that the 95% CI crossed unity suggests no detrimental impact of pregnancy in the group of patients with hormone receptor–positive breast cancer. This is in line with the evidence from other studies addressing specifically the question on the safety of pregnancy following history of hormone receptor–positive breast cancer.31 In our study, more than half of these patients had a pregnancy within the first 5 years, and timing of pregnancy after breast cancer did not appear to affect the results. Long-term follow-up of the POSITIVE trial,14 which included a small group of BRCA carriers, will provide further evidence in this regard.
Interpretation of these results should note that the treatment landscape of early breast cancer has evolved substantially over the past 20 years. This is particularly relevant for premenopausal women with hormone receptor–positive breast cancer; currently, these patients more frequently receive a recommendation to have ovarian function suppression as part of adjuvant endocrine therapy.21 Moreover, in some health systems, patients with high risk of disease recurrence may receive adjuvant abemaciclib for 2 years in addition to endocrine therapy or adjuvant olaparib for 1 year following standard chemotherapy to further reduce the risk of recurrence.21 Therefore, the lack of clear detriment seen in survival outcomes in this study, including among patients with hormone receptor–positive disease (the majority of them being BRCA2 carriers), should be considered in the context of patient risk of recurrence and the better available treatment today.
Limitations
This study has some limitations. First, the retrospective observational design of the study and the choice of exposure variable limit the ability to draw causal conclusions. Second, information on pregnancies after breast cancer as well as pregnancy, fetal, and obstetric outcomes was extracted mainly from oncology medical records that were not set up specifically for these outcomes or serial patient survey, and was based on patient self-report; this may be particularly true for peripartum and neonatal complications. Hence, a potential risk of underreporting cannot be excluded. Third, some information on pregnancy outcomes was missing or not recorded (eg, reasons for induced abortion); data on pregnancy desire, contraceptive use, or potential use of restaging imaging studies before attempting pregnancy were not collected. Fourth, the study included data from 78 centers worldwide with different health care systems; patients were treated over a period of 20 years, during which the treatment of early breast cancer has improved, particularly for hormone receptor–positive disease. Patients diagnosed toward the end of the period for study inclusion had less observation time to conceive as well as to evaluate outcomes and recurrences. Finally, despite all attempts to account for the potential confounding in this type of analyses, it cannot be excluded that patients at higher risk of disease recurrence were counseled differently and/or that healthier women without impending subclinical recurrence were more able to become pregnant.
Conclusions
This study showed that more than 1 in 5 young BRCA carriers became pregnant following diagnosis of early breast cancer and that disease-free survival was comparable with those who did not become pregnant. Our results can inform counseling of young BRCA carriers interested in conceiving following breast cancer diagnosis.
Study Protocol
List of abbreviations
eAppendix. Supplementary methods and results - secondary matched analysis
eTable 1. Pattern of invasive disease-free survival events
eTable 2. Patient characteristics at diagnosis of breast cancer among patients included in the secondary matched analysis
eTable 3. Pregnancy, fetal, and obstetric outcomes in patients with a pregnancy after breast cancer included in the secondary matched analysis
eTable 4. Subgroup analyses of disease-free survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 5. Subgroup analyses of breast cancer-specific survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 6. Subgroup analyses of overall survival (patients with a pregnancy vs. patients with no pregnancy group)
eTable 7. Impact of interval between diagnosis and pregnancy, outcome of pregnancy, and breastfeeding status on disease-free survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 8. Impact of the interval between diagnosis and pregnancy, outcome of pregnancy, and breastfeeding status on breast cancer-specific survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 9. Impact of the interval between diagnosis and pregnancy, outcome of pregnancy, and breastfeeding status on overall survival (patients with a pregnancy vs. patients with no pregnancy)
eFigure 1. Prognostic impact of pregnancy after breast cancer in young BRCA carriers in the secondary matched analysis: disease-free survival
eFigure 2. Prognostic impact of pregnancy after breast cancer in young BRCA carriers in the secondary matched analysis: breast cancer-specific survival
eFigure 3. Prognostic impact of pregnancy after breast cancer in young BRCA carriers in the secondary matched analysis: overall survival
Nonauthor Collaborators. BRCA BCY Collaboration
Data Sharing Statement
References
- 1.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32(11):1151-1156. doi: 10.1200/JCO.2013.52.8877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangiardi-Veltin M, Sebbag C, Rousset-Jablonski C, et al. ; Seintinelles Research Network . Pregnancy, fertility concerns and fertility preservation procedures in a national study of French breast cancer survivors. Reprod Biomed Online. 2022;44(6):1031-1044. doi: 10.1016/j.rbmo.2021.12.019 [DOI] [PubMed] [Google Scholar]
- 3.Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180. doi: 10.1016/S1470-2045(17)30891-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine E, Knoll MA, Maslow BL. Fertility considerations for reproductive-aged carriers of deleterious BRCA mutations: a call for early intervention. JCO Oncol Pract. 2022;18(3):165-168. doi: 10.1200/OP.21.00389 [DOI] [PubMed] [Google Scholar]
- 5.Turan V, Lambertini M, Lee DY, et al. Association of germline BRCA pathogenic variants with diminished ovarian reserve: a meta-analysis of individual patient-level data. J Clin Oncol. 2021;39(18):2016-2024. doi: 10.1200/JCO.20.02880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sessa C, Balmaña J, Bober SL, et al. ; ESMO Guidelines Committee . Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO clinical practice guideline. Ann Oncol. 2023;34(1):33-47. doi: 10.1016/j.annonc.2022.10.004 [DOI] [PubMed] [Google Scholar]
- 7.Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2021;39(29):3293-3305. doi: 10.1200/JCO.21.00535 [DOI] [PubMed] [Google Scholar]
- 8.Lambertini M, Ameye L, Hamy AS, et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J Clin Oncol. 2020;38(26):3012-3023. doi: 10.1200/JCO.19.02399 [DOI] [PubMed] [Google Scholar]
- 9.Buonomo B, Massarotti C, Dellino M, et al. Reproductive issues in carriers of germline pathogenic variants in the BRCA1/2 genes: an expert meeting. BMC Med. 2021;19(1):205. doi: 10.1186/s12916-021-02081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 11.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343-346. doi: 10.1016/0197-2456(96)00075-X [DOI] [PubMed] [Google Scholar]
- 12.Shandley LM, Spencer JB, Fothergill A, et al. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril. 2017;107(1):243-252. doi: 10.1016/j.fertnstert.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonomo B, Brunello A, Noli S, et al. Tamoxifen exposure during pregnancy: a systematic review and three more cases. Breast Care (Basel). 2020;15(2):148-156. doi: 10.1159/000501473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partridge AH, Niman SM, Ruggeri M, et al. ; International Breast Cancer Study Group; POSITIVE Trial Collaborators . Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388(18):1645-1656. doi: 10.1056/NEJMoa2212856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambertini M, Peccatori FA, Demeestere I, et al. ; ESMO Guidelines Committee . Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2020;31(12):1664-1678. doi: 10.1016/j.annonc.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 16.Hartnett KP, Mertens AC, Kramer MR, et al. Pregnancy after cancer: does timing of conception affect infant health? Cancer. 2018;124(22):4401-4407. doi: 10.1002/cncr.31732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2022. NCHS Data Brief. 2023;(477):1-8. [PubMed] [Google Scholar]
- 18.Goetzinger KR, Shanks AL, Odibo AO, Macones GA, Cahill AG. Advanced maternal age and the risk of major congenital anomalies. Am J Perinatol. 2017;34(3):217-222. doi: 10.1055/s-0036-1585410 [DOI] [PubMed] [Google Scholar]
- 19.Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:l869. doi: 10.1136/bmj.l869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994-2001. doi: 10.1200/JCO.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 21.Paluch-Shimon S, Cardoso F, Partridge AH, et al. ESO-ESMO Fifth International Consensus guidelines for breast cancer in young women (BCY5). Ann Oncol. 2022;33(11):1097-1118. doi: 10.1016/j.annonc.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 22.Oktay KH, Bedoschi G, Goldfarb SB, et al. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency. Fertil Steril. 2020;113(6):1251-1260. doi: 10.1016/j.fertnstert.2020.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winship AL, Griffiths M, Lliberos Requesens C, Sarma U, Phillips KA, Hutt KJ. The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: implications for fertility preservation. Hum Reprod. 2020;35(8):1864-1874. doi: 10.1093/humrep/deaa128 [DOI] [PubMed] [Google Scholar]
- 24.Ntemou E, Vidal PD, Alexandri C, Van den Steen G, Lambertini M, Demeestere I. Ovarian toxicity of carboplatin and paclitaxel in mouse carriers of mutation in BRIP1 tumor suppressor gene. Sci Rep. 2022;12(1):1658. doi: 10.1038/s41598-022-05357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winship AL, Alesi LR, Sant S, et al. Checkpoint inhibitor immunotherapy diminishes oocyte number and quality in mice. Nat Cancer. 2022;3(8):1-13. doi: 10.1038/s43018-022-00413-x [DOI] [PubMed] [Google Scholar]
- 26.Cui W, Rocconi RP, Thota R, et al. Measuring ovarian toxicity in clinical trials: an American Society of Clinical Oncology research statement. Lancet Oncol. 2023;24(10):e415-e423. doi: 10.1016/S1470-2045(23)00390-X [DOI] [PubMed] [Google Scholar]
- 27.Tryggvadottir L, Olafsdottir EJ, Gudlaugsdottir S, et al. BRCA2 mutation carriers, reproductive factors and breast cancer risk. Breast Cancer Res. 2003;5(5):R121-R128. doi: 10.1186/bcr619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cullinane CA, Lubinski J, Neuhausen SL, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer. 2005;117(6):988-991. doi: 10.1002/ijc.21273 [DOI] [PubMed] [Google Scholar]
- 29.Pan H, He Z, Ling L, et al. Reproductive factors and breast cancer risk among BRCA1 or BRCA2 mutation carriers: results from ten studies. Cancer Epidemiol. 2014;38(1):1-8. doi: 10.1016/j.canep.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju091. doi: 10.1093/jnci/dju091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arecco L, Blondeaux E, Bruzzone M, et al. Safety of pregnancy after breast cancer in young women with hormone receptor-positive disease: a systematic review and meta-analysis. ESMO Open. 2023;8(6):102031. doi: 10.1016/j.esmoop.2023.102031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
List of abbreviations
eAppendix. Supplementary methods and results - secondary matched analysis
eTable 1. Pattern of invasive disease-free survival events
eTable 2. Patient characteristics at diagnosis of breast cancer among patients included in the secondary matched analysis
eTable 3. Pregnancy, fetal, and obstetric outcomes in patients with a pregnancy after breast cancer included in the secondary matched analysis
eTable 4. Subgroup analyses of disease-free survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 5. Subgroup analyses of breast cancer-specific survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 6. Subgroup analyses of overall survival (patients with a pregnancy vs. patients with no pregnancy group)
eTable 7. Impact of interval between diagnosis and pregnancy, outcome of pregnancy, and breastfeeding status on disease-free survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 8. Impact of the interval between diagnosis and pregnancy, outcome of pregnancy, and breastfeeding status on breast cancer-specific survival (patients with a pregnancy vs. patients with no pregnancy)
eTable 9. Impact of the interval between diagnosis and pregnancy, outcome of pregnancy, and breastfeeding status on overall survival (patients with a pregnancy vs. patients with no pregnancy)
eFigure 1. Prognostic impact of pregnancy after breast cancer in young BRCA carriers in the secondary matched analysis: disease-free survival
eFigure 2. Prognostic impact of pregnancy after breast cancer in young BRCA carriers in the secondary matched analysis: breast cancer-specific survival
eFigure 3. Prognostic impact of pregnancy after breast cancer in young BRCA carriers in the secondary matched analysis: overall survival
Nonauthor Collaborators. BRCA BCY Collaboration
Data Sharing Statement