Key Points
Question
How does neoadjuvant chemotherapy followed by transoral robotic surgery and neck resection (NECTORS) affect longitudinal and multidimensional quality of life in patients with human papillomavirus−associated oropharyngeal squamous cell carcinoma (HPV-OPSCC)?
Findings
This multicenter cohort study found that patients with HPV-OPSCC who were treated with NECTORS experienced immediate posttreatment toxic effects that resolved in all domains by month 3 to 6 and remained stable at 24 months posttreatment.
Meaning
These findings indicate that NECTORS was an effective approach for treatment of patients with stage III or IVa HPV-OPSCC and may be preferred because it preserves quality of life in all domains.
Abstract
Importance
Efforts are underway to deintensified treatment protocols for patients with human papillomavirus virus−associated oropharyngeal squamous cell carcinoma (HPV-OPSCC) to achieve similar excellent oncologic outcomes while reducing treatment-related adverse effects. Transoral robotic surgery (TORS) as primary treatment often requires adjuvant therapy due to the high incidence of nodal metastasis. Treatment with neoadjuvant chemotherapy followed by TORS and neck dissection (NECTORS), reserving radiation therapy for salvage, yields excellent oncologic outcomes.
Objective
To assess patient-reported quality of life (QOL) and functional outcomes among patients with HPV-OPSCC who undergo NECTORS.
Design, Settings, and Participants
This was a multicenter prospective cohort study of patients with HPV-OPSCC treated with the NECTORS protocol in 2017 to 2022. Consecutive patients with stage III or IVa HPV-OPSCC treated with NECTORS in 2017 to 2022 who had completed the primary QOL questionnaire at baseline and at least once during the 24-month follow-up period were included. Ninety-four patients were eligible, and 67 were included in the analyses.
Outcome Measures
QOL questionnaires at baseline, and at month 1, 3, 6, 12, 18, and 24 posttreatment. Global score on the 30-item European Organization for Research and Treatment of Cancer Core quality of life questionnaire (EORTC QLQ-C30) was the primary outcome; the head and neck extension module (EORTC QLQ-HN35); the MD Anderson Dysphagia Inventory for dysphagia-related QOL; and the Decision Regret Scale were also used. Paired t tests assessed change between the baseline and 12- or 24-month patient-reported outcomes.
Results
Among the study population of 67 patients (median [range] age, 63 [58-67] years; 54 [80.6%] male) with HPV-OPSCC, the most frequent cancer subsites were palatine tonsil (41 [61%]) and base of tongue (26 [39%]); none required adjuvant RT. Global QOL at 24 months improved compared with baseline (mean difference, 9.49; 95% CI, 2.45 to 16.53). All EORTC QLQ-C30 functional scores returned to baseline or improved within 3 to 6 months posttreatment and remained stable at 24 months. EORTC QLQ-HN35 symptom scale scores improved or were stable at 24 months. The MD Anderson Dysphagia Inventory scores demonstrated no significant difference between baseline and month 12 for global scores (mean difference, 6.15; 95% CI, −4.18 to 16.49) and composite scores (mean difference, 2.73; 95% CI, −1.62 to 7.09). Median (range) score on the Decision Regret Scale was 5 of 100 (0-30), representing mild overall regret.
Conclusion and Relevance
The findings of this multicenter cohort study indicate that use of the NECTORS protocol is associated with excellent QOL outcomes. QOL measures returned to baseline levels or were better than baseline, which represents positive outcomes for patients with HPV-OPSCC who undergo this treatment regimen.
This multicenter cohort study evaluates quality of life associated with transoral robotic surgery and neck dissection among patients undergoing this treatment for human papillomavirus−associated oropharyngeal squamous cell carcinoma.
Introduction
There is an increasing incidence of human papillomavirus−associated oropharyngeal squamous cell carcinoma (HPV-OPSCC).1,2 Patients with this form of OPSCC have a better prognosis than those with conventional OPSCC treated with standard therapies.3,4,5 Clinical presentation differs between these 2 groups given that patients with HPV-OPSCC tend to be younger and healthier, and most (60%-70%) have positive nodal disease at the time of diagnosis.2,6,7,8,9
Standard management algorithms for newly diagnosed HPV-OPSCC cases opt for definitive concurrent chemoradiotherapy (CRT) or surgical resection with adjuvant radiation (RT) or CRT depending on pathologic risk stratification; therefore, 90% or more of these patients are treated with RT.10,11 Transoral robotic surgery (TORS) is currently practiced in the management of early OPSCC, often requiring adjuvant RT given the high nodal burden associated with this disease.10,12 When considering locoregional effects of overall treatment, TORS with adjuvant treatment is not a de-escalation. RT to the oropharynx and neck has been associated with substantial long-term and cumulative morbidity, including xerostomia, dental loss and poor oral health, dysphagia, possible gastrostomy tube dependency, and airway and/or upper esophageal stenosis.5,13,14,15 Considering that patients with HPV-OPSCC are young and have a good prognosis, they may live for many years with posttreatment toxic effects.
Many clinical trials evaluate deintensification strategies to maintain positive oncologic outcomes while minimizing adverse treatment effects on patients’ quality of life (QOL). In 2016, a new paradigm for the treatment of HPV-OPSCC was shown to be feasible by Sadeghi et al15: neoadjuvant chemotherapy followed by definitive transoral surgery and selective neck dissection (NECTORS), reserving RT for salvage. This protocol has yielded excellent oncologic outcomes.16,17,18 We hypothesized that NECTORS preserves patient-reported QOL outcomes. In the current study, we report on 2-year follow-up longitudinal QOL outcomes among patients who received the NECTORS protocol as definitive treatment.
Methods
Ethics approval for the study was obtained from the McGill University Health Centre research ethics board (MP-37-2018-3443 and MP-37-2019-4659). Written informed consent was obtained from each participant before registration and treatment. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
This prospective cohort study was conducted from January 2017 to August 2022 at the McGill University Health Centre and the Jewish General Hospital (Montreal, Canada). Eligible patients had completed the NECTORS trial treatment protocol until August 2022 and were reported as having completed longitudinal QOL questionnaires.14,16 As described in previous publications,16,17,18 eligible patients were 18 to 80 years of age and diagnosed with stage III or IVa (per American Joint Committee on Cancer’s Cancer Staging Manual, 7th edition19) treatment-naive HPV-OPSCC. Patients had no evidence of distant metastases, 5 or fewer nodal metastases on radiology findings without gross extracapsular extension (ECE; minimal radiologic ECE was not excluded), Eastern Cooperative Oncology Group performance status less than 2, and no previous malignant neoplasm in the past 5 years.
The included patients completed QOL questionnaires at designated time points: baseline (ie, pretreatment) and at month 1, 3, 6, 12, 18, and 24 posttreatment. Questionnaires were sent, collected, and managed using REDCap electronic data capture tools hosted at McGill University20,21 or completed in-person during clinic visits. Patients who were included in the QOL analysis had completed the primary QOL questionnaire at pretreatment and 1 or more questionnaires during the 24 months posttreatment.
Adverse events were recorded and graded per the Common Terminology Criteria for Adverse Events, version 5.0 (US National Cancer Institute guidelines).22 Chemotherapy-related kidney dysfunction and myelosuppression were monitored with regular laboratory testing. Kidney injury of grade 3 or higher was recorded as follows: grade 3, acute kidney injury requiring hospitalization or creatinine level 3 to 6 times the upper limit of normal; grade 4, life-threatening consequences or dialysis indicated or more than 6 times the upper limit of normal creatinine; and grade 5, death. Neutropenia of grade 3 or higher was recorded as follows: grade 3, absolute neutrophil count from 500 to 1000 µL (to calculate × 109/L, multiply by 0.001) or grade 4, less than 500 µL. Thrombocytopenia of grade 3 or higher was recorded as follows: grade 3, platelet count from 25 to 50 × 103/µL (to calculate 109/L, multiply by 1) and grade 4, less than 25 × 103/µL. Patients underwent pre- and posttreatment audiometry assessments to monitor ototoxicity, which was defined as hearing impairment on a 1, 2, 3, 4, 6, and 8 kHz audiogram or tinnitus in at least 1 ear. Four grades of ototoxicity were defined as follows: grade 1, average threshold shift of 15 to 25 dB at 2 contiguous test frequencies; grade 2, average threshold shift of greater than 25 dB at 2 contiguous test frequencies; grade 3, average threshold shift of greater than 25 dB at 3 contiguous test frequencies or if therapeutic intervention indicated; and grade 4, decrease in hearing to profound bilateral loss or nonserviceable hearing. Tinnitus was defined with 3 grades as follows: grade 1, mild symptoms with no intervention indicated; grade 2, moderate symptoms that limit instrumental activities of daily living; and grade 3, severe symptoms that limit self-care activities of daily living.
At pretreatment, all participants were presented to a multidisciplinary tumor board for therapeutic recommendations; they met with a head and neck radiation oncologist and a medical oncologist, and if eligible, were offered both standard of care (RT or CRT) and the NECTORS regimen. Standard nonoperative management consisted of 66 to 70 Gy of intensity-modulated RT to the primary site and to the involved lymph nodes or high-risk level lymph nodes, with or without concurrent high-dose cisplatin (100 mg/m2 every 3 weeks).11 Patients enrolled in NECTORS had baseline margins tattooed if the tumor extended beyond of tonsillar subunit or ipsilateral base of tongue. The neoadjuvant chemotherapy regimen in NECTORS consisted of 3 cycles of docetaxel (75 mg/m2) and cisplatin (75 mg/m2) as described previously.14,15,16 Laterality of neck dissection was guided by radiologic distribution of nodal disease and/or location of primary tumor. Primary base of tongue tumors or primary tonsil tumors with more than a 1-cm extension into the soft palate or the base of tongue underwent bilateral neck dissection independent of radiologic findings. Intraoperative elective tracheostomy was performed and kept for 14 days as per the policy for patients undergoing TORS at 1 participating institution (Jewish General Hospital). Postoperative pathology results were discussed with the tumor board to determine the need for adjuvant RT.
Patients who completed both pretreatment and 24-month QOL questionnaires were included in the paired analysis. Of the 94 patients who were treated with the NECTORS protocol from January 2017 to August 2022, 67 patients met all the inclusion criteria (insufficient QOL forms, n = 27).
The global score on the 30-item European Organization for Research and Treatment of Cancer Core quality of life questionnaire (EORTC QLQ-C30) was the primary outcome. The remaining EORTC QLQ-C30 domain and symptom scores, as well as other QOL metrics were used, including the head and neck extension module (EORTC QLQ-HN35) measuring head and neck cancer-related symptoms, and the MD Anderson Dysphagia Inventory (MDADI) to measure dysphagia-related QOL.23,24,25,26 Higher domain scores represent better QOL, while higher symptom scores represent worse QOL. A cross-sectional assessment of psychosocial stress was undertaken using the Decision Regret Scale (DRS), a score of distress due to a health care decision. Thresholds of regret scores are coded as: none, 0; mild, 1 to 25; and moderate to strong, greater than 25.27,28 All measures have been previously used and have good psychometric properties when administered to patients with head and neck cancer.26,27,29
Demographic data collected included age, sex, medical history, relationship status, occupation, and postal code. Race and ethnicity data were not collected or included in the analyses. Age and medical history determined the Charlson Comorbidity Index. Occupation was classified into high-, medium-, and low-level skills per the International Standard Classification of Occupations.30 Postal codes were used as an area-based proxy for socioeconomic status, measured by the Deprivation Index31 that was created from 2016 Canadian census data, divided into material and social deprivation indices where geographical units report a quintile score. Quintile 1 is the most privileged or least deprived, and quintile 5 is the least privileged or most deprived.
Statistical Analysis
Descriptive statistics described the study population; proportions were calculated for categorical variables and means (SD) for continuous variables. Data were presented with 95% CIs. Paired t tests were used to assess change in baseline and 2-year patient-reported outcome scores, and Analysis of Variance was used for DRS analysis. Bivariable analysis of the primary outcome and demographic variables was performed. Statistical significance was set at P < .05. Statistical analysis was performed using R, version 3.4.1 (The R Foundation for Statistical Computing).
Results
Demographic and Objective Data
The study population of 67 patients had a median (SD) age of 63 (58-67) years, with 54 (80.6%) male and 13 (19.4%) female individuals. Baseline characteristics are shown in Table 1. Forty-three patients were treated within the NECTORS trial, and 24 patients were treated with the same protocol outside of the trial. All 67 patients received neoadjuvant chemotherapy followed by TORS and neck dissection. Two patients (2.9%) did not complete the third cycle of chemotherapy due to a hypersensitivity reaction (n = 1) and recurrent panic attacks (n = 1); both had major partial response observed on imaging results after cycle 2 and on postoperative pathology. No patient received adjuvant RT or CRT. Median (IQR) follow-up was 44 months (28-57) months. Three patients (4.5%) had regional recurrence; 2 were treated with salvage CRT and the third was enrolled in the BNT113-01 trial (NCT04534205) to receive pembrolizumab. Two patients who completed salvage CRT have no evidence of further recurrence or progression at the 36-month follow-up. Primary tumor site was palatine tonsil or tonsillar fossa in 41 patients (61.2%) and base of tongue in 26 patients (38.8%). The clinical tumor (T) staging within the cohort was T1, 26 patients (38.8%); T2, 30 patients (44.8%); T3, 10 patients (14.9%); and T4a, 1 patient (1.5%). The clinical nodal (N) staging was N0, 2 patients (3.0%); N1, 16 patients (23.9%); N2a, 15 patients (22.4%); N2b, 30 patients (44.8%); and N2c, 4 patients (6.0%). A similar distribution of patients required unilateral neck dissection (36 [53.7%]) and bilateral (31 [46.3%]) neck dissection. Clear margins for the primary tumor site were achieved in all patients. There were 30 patients (44.8%) who were former smokers; 6 (9.0%) who were current smokers; and the median (IQR) pack-years in these 2 groups was 24.5 (10-35) packs. The median Charlson Comorbidity Index was 4. Patients’ occupations were stratified by skill level: 32 (47.8%) had highly skilled occupations; 22 (32.8%), medium skill; 4 (6.0%), low skill; and 9 (13.4%), unemployed or unknown occupation. The Deprivation Index scores showed that only 13 patients (19.0%) lived in areas representing the 2 most socioeconomically privileged quintiles, and 25 (37.3%) lived in areas of the top 2 privileged quintiles.
Table 1. Baseline Demographic and Treatment Characteristics of Study Patients (N = 67).
| Characteristic | Patients, No. (%) |
|---|---|
| Age, median (IQR), y | 63 (58-67) |
| Female | 13 (19.4) |
| Male | 54 (80.6) |
| Primary tumor site | |
| Tonsil or tonsillar fossa | 41 (61.2) |
| Base of tongue | 26 (38.8) |
| Clinical T stagea | |
| T1 | 26 (38.8) |
| T2 | 30 (44.8) |
| T3 | 10 (14.9) |
| T4a | 1 (1.5) |
| Clinical N stagea | |
| N0 | 2 (3.0) |
| N1 | 16 (23.9) |
| N2a | 15 (22.4) |
| N2b | 30 (44.8) |
| N2c | 4 (6.0) |
| Overall cancer stagea | |
| II | 1 (1.5) |
| III | 22 (32.8) |
| IVa | 44 (65.7) |
| Laterality of neck dissection | |
| Unilateral | 36 (53.7) |
| Bilateral | 31 (46.3) |
| Feeding tube, mean (SD), d | 7.8 (6.4) |
| Smoking status | |
| Never smoked | 31 (46.2) |
| Former smoking | 30 (44.8) |
| Current smoking | 6 (9.0) |
| Pack-years (for currently/formerly smoking), median (IQR) | 24.5 (10-35) |
| Charlson Comorbidity Index, median (range) | 4 (2-6) |
| Occupation skill levelb | |
| High | 32 (47.8) |
| Medium | 22 (32.8) |
| Low | 4 (6.0) |
| Unknown/no occupation | 9 (13.4) |
| Social deprivation index, quintilec | |
| 1 | 4 (6.0) |
| 2 | 9 (13.4) |
| 3 | 14 (20.9) |
| 4 | 15 (22.4) |
| 5 | 23 (34.3) |
| Unknown | 2 (3.0) |
| Material deprivation index, quintilec | |
| 1 | 16 (23.9) |
| 2 | 9 (13.4) |
| 3 | 18 (26.9) |
| 4 | 12 (17.9) |
| 5 | 10 (14.9) |
| Unknown | 2 (3.0) |
Cancer stages defined by the American Joint Committee on Cancer.19
Skill levels defined per the International Standard Classification of Occupations.30
Material and social deprivation indices defined by the census bureau of Canada, with quintile 1 indicating the most privileged/least deprived and quintile 5, the least privileged/most deprived.
A bivariable analysis compared EORTC global score with age, sex, relationship status, urban location, occupation skill level, deprivation index score, smoking pack-years, Charlson Comorbidity Index, tumor site, and T and N stages. Only sex correlated with EORTC values, where male patients had higher EORTC baseline global QOL values than female patients (estimate, 13.84; 95% CI, 2.91-24.77).
A nasogastric feeding tube was placed for all patients for postoperative nutritional support in hospital for a mean (SD) of 7.8 (6.4) days. No patients required a percutaneous gastrostomy tube during treatment or follow-up. Six patients had elective tracheostomy per an institutional policy for patients undergoing TORS (Jewish General Hospital). One patient had a temporary tracheostomy for 14 days after managing a postoperative tonsillar bleed. All patients were decannulated.
Grade 3 or higher adverse events are summarized with complete data in eTable 1 in Supplement 1. Chemotherapy-related adverse events included diarrhea (n = 1), a hypersensitivity reaction (n = 1), and ototoxicity (described in the next section). There was no evidence of chemotherapy-related grade 3 kidney injury or myelosuppression. Surgery-related adverse events included intraoperative hypotension delaying surgery (n = 1), keloid scar (n = 1), chyle leak (n = 2), and postoperative bleeding (n = 4).
Fifty-two patients (77.6%) completed both pretreatment and postchemotherapy audiometry. Thirty-four patients (65.4%) recorded no change in hearing. Five patients (9.6%) had mild hearing changes that did not reach ototoxicity thresholds . Eight patients (15.4%) had grade 1 ototoxic effects in at least 1 ear, and 5 patients (9.6%) had grade 3 ototoxic effects in at least 1 ear.
Quality of Life Outcomes
Findings of the EORTC QLQ-C30
The EORTC QLQ-C30 functional scores at baseline through 24 months are shown in Figure 1 and Table 2. The EORTC QLQ-C30 global score improved from baseline to 24 months (mean difference, 9.49; 95% CI, 2.45 to 16.53). Emotional functioning and social functioning improved at 24 months compared with baseline (mean difference, 11.11; 95% CI, 4.61 to 17.61; and 7.41; 95% CI, 2.28 to 12.53, respectively). Physical, role, and cognitive functioning returned to pretreatment values (mean difference, −0.37 [95% CI, −1.89 to 1.15], 6.02 [95% CI, −0.88 to 12.91], and 1.39 [−6.16 to 8.94], respectively).
Figure 1. Quality of Life Ratings on the 30-item European Organization for Research and Treatment of Cancer Core’s Quality of Life Questionnaire From Patients Treated With Neoadjuvant Chemotherapy Followed by Transoral Robotic Surgery and Neck Dissection, by Function Score.
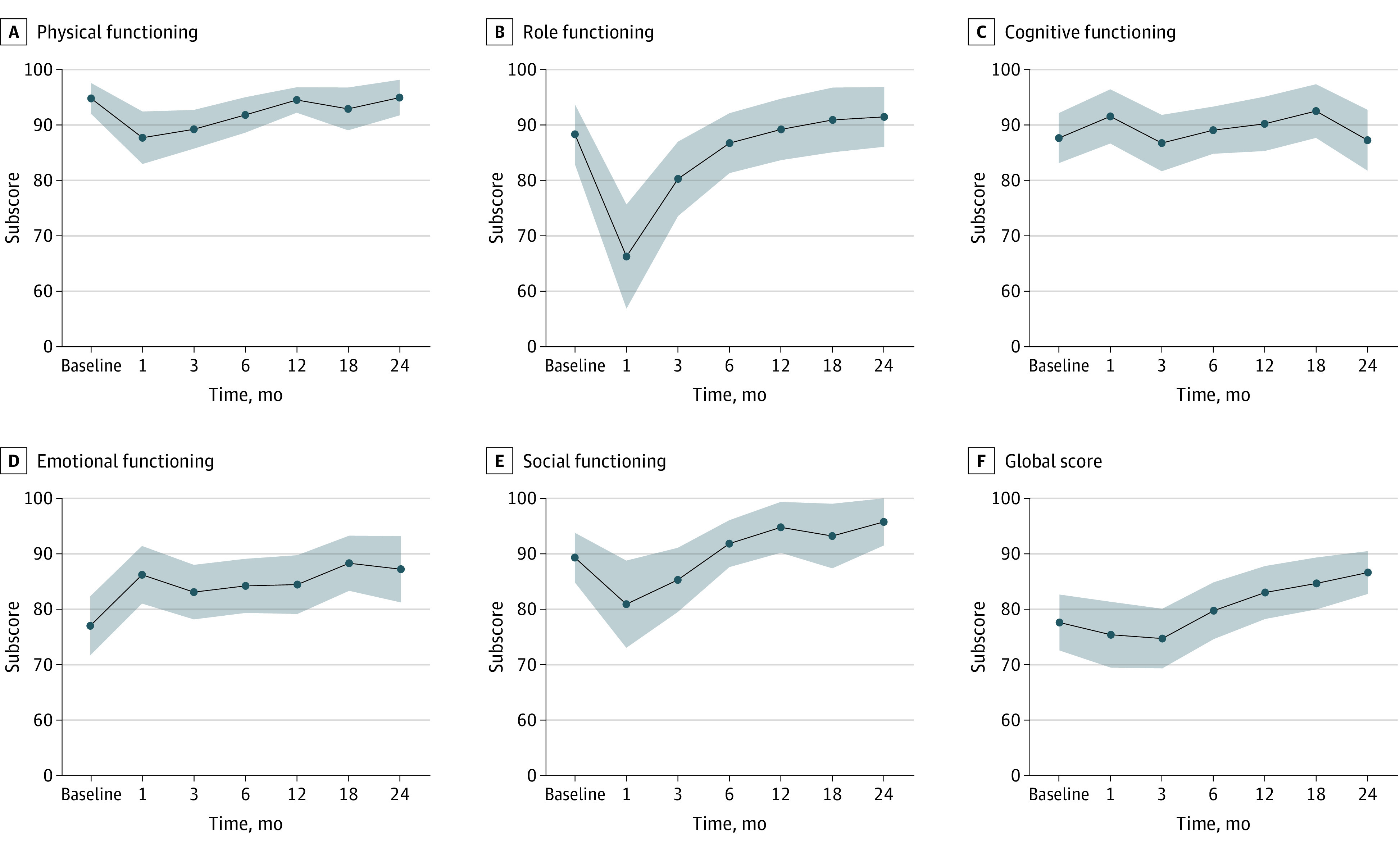
Table 2. Quality of Life Ratings Among Patients Treated With NECTORS, by Function (EORTC QLQ-C30) and Symptoms (EORTC QLQ-HN35) Scores, Over Time.
| Measure | Mean (SD) [No.] | Mean differencea | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 mo | 3 mo | 6 mo | 12 mo | 18 mo | 24 mo | |||
| Function | |||||||||
| Global score | 77.6 (20.6) [67] | 75.4 (19.0) [42] | 74.7 (20.8) [60] | 79.7 (19.4) [58] | 83.0 (17.3) [53] | 84.7 (15.3) [44] | 86.6 (11.7) [38] | 9.49 | (2.45 to 16.53) |
| Emotional | 77.0 (21.8) [67] | 86.2 (16.4) [41] | 83.1 (19.0) [60] | 84.2 (18.7) [59] | 84.4 (19.4) [54] | 88.3 (16.3) [44] | 87.2 (18.4) [39] | 11.11 | (4.61 to 17.61) |
| Social | 89.3 (18.3) [67] | 80.9 (24.9) [41] | 85.3 (22.4) [60] | 91.8 (16.2) [59] | 94.8 (16.8) [54] | 93.2 (19.1) [44] | 95.7 (13.1) [39] | 7.41 | (2.28 to 12.53) |
| Physical | 94.7 (11.3) [67] | 87.6 (14.9) [41] | 89.2 (13.5) [60] | 91.8 (12.2) [59] | 94.4 (8.4) [54] | 92.8 (12.7) [44] | 94.9 (9.9) [39] | −0.37 | (−1.89 to 1.15) |
| Role | 88.3 (22.1) [67] | 66.3 (29.7) [41] | 80.3 (25.9) [60] | 86.7 (20.7) [59] | 89.2 (20.3) [54] | 90.9 (19.2) [44] | 91.5 (16.6) [39] | 6.02 | (−0.88 to 12.91) |
| Cognitive | 87.6 (18.4) [67] | 91.5 (15.4) [41] | 86.7 (19.6) [60] | 87.8 (18.6) [59] | 90.1 (17.9) [54] | 92.4 (15.9) [44] | 87.2 (16.9) [39] | 1.39 | (−6.16 to 8.94) |
| Symptom | |||||||||
| Appetite | 11.6 (19.8) [66] | 10.3 (21.8) [39] | 16.7 (23.4) [60] | 8.62 (16.0) [58] | 8.18 (22.6) [53] | 8.51 (21.4) [47] | 5.13 (12.2) [39] | −8.11 | (0.51 to 15.70) |
| Constipation | 8.08 (18.5) [66] | 5.83 (14.9) [40) | 5.56 (12.5) [60] | 6.90 (16.2) [58] | 7.55 (14.1) [53] | 4.26 (11.2) [47] | 7.69 (14.2) [39] | 2.70 × 10-10 | (−6.41 to 6.41) |
| Diarrhea | 6.06 (15.4) [66] | 9.17 (21.3) [40] | 5.56 (17.5) [60] | 8.05 (16.9) [58] | 5.03 (17.8) [53] | 4.26 (13.2) [47] | 4.27 (13.6) [39] | −0.90 | (−5.79 to 3.99) |
| Dyspnea | 8.59 (19.7) [66] | 16.7 (20.0) [40] | 14.4 (22.4) [60] | 12.6 (19.6) [58] | 7.55 (15.5) [53] | 9.4 (17.0) [47] | 9.4 (17.0) [39] | 2.70 × 10-10 | (−6.93 to 6.93) |
| Fatigue | 22.4 (22.4) [66] | 31.9 (26.4) [40] | 27.2 (25.7) [60] | 21.6 (20.9) [58] | 15.5 (18.1) [53] | 16.8 (22.1) [47] | 13.7 (18.8) [39] | −12.01 | (−20.58 to −3.44) |
| Financial implications | 11.1 (24.5) [65] | 12.5 (20.9) [40] | 13.3 (23.1) [60] | 11.5 (21.2) [58] | 11.9 (25.4) [53] | 11.3 (23.3) [47] | 7.69 (16.2) [39] | −7.21 | (−14.20 to −0.21) |
| Nausea/vomiting | 3.54 (9.92) [66] | 3.33 (12.1) [40] | 1.94 (5.4) [60] | 2.01 (6.3) [58] | 2.83 (7.83) [53] | 2.84 (7.22) [47] | 2.99 (7.52) [39] | −3.15 | (−7.66 to 1.35) |
| Pain | 15.4 (22.1) [66] | 25.0 (26.1) [40] | 19.7 (24.1) [60] | 16.1 (20.0) [58] | 10.7 (13.9) [53] | 10.6 (19.5) [47] | 8.12 (15.7) [39] | −9.01 | (−16.60 to −1.42) |
| Sleep | 26.8 (31.1) [66] | 33.3 (31.1) [40] | 23.3 (27.0) [60] | 23.6 (25.0) [58] | 22.0 (24.4) [53] | 12.8 (22.6) [47] | 8.55 (16.6) [39] | −19.82 | (−29.78 to −9.86) |
Abbreviations: EORTC QLQ-C30, the European Organization for Research and Treatment of Cancer Core’s 30-item quality of life questionnaire; EORTC QLQ-HN35, the European Organization for Research and Treatment of Cancer Core’s Quality of Life Questionnaire, head and neck extension module; NECTORS, neoadjuvant chemotherapy followed by transoral robotic surgery and neck dissection.
From baseline to month 24, and 36 patients included in the analyses with paired data.
The EORTC QLQ-C30 symptom scores at baseline to 24 months are available in Table 2. Several EORTC QLQ-C30 symptom scores improved from baseline to 24 months, including appetite (mean difference, −8.11; 95% CI, 0.51 to 15.70), fatigue (mean difference, −12.01; 95% CI, −20.58 to −3.44), financial implications (mean difference, −7.21; 95% CI, −14.20 to −0.21), pain (mean difference, −9.01; 95% CI, −16.60 to −1.42), and sleep (mean difference, −19.82; 95% CI, −29.78 to −9.86). The other EORTC QLQ-C30 symptom scales returned to baseline in 3 to 6 months posttreatment and remained stable at 24 months (ie, constipation, diarrhea, dyspnea, and nausea/vomiting). No other EORTC QLQ-C30 symptom scores declined from baseline at 24 months.
Findings of the EORTC QLQ-HN35
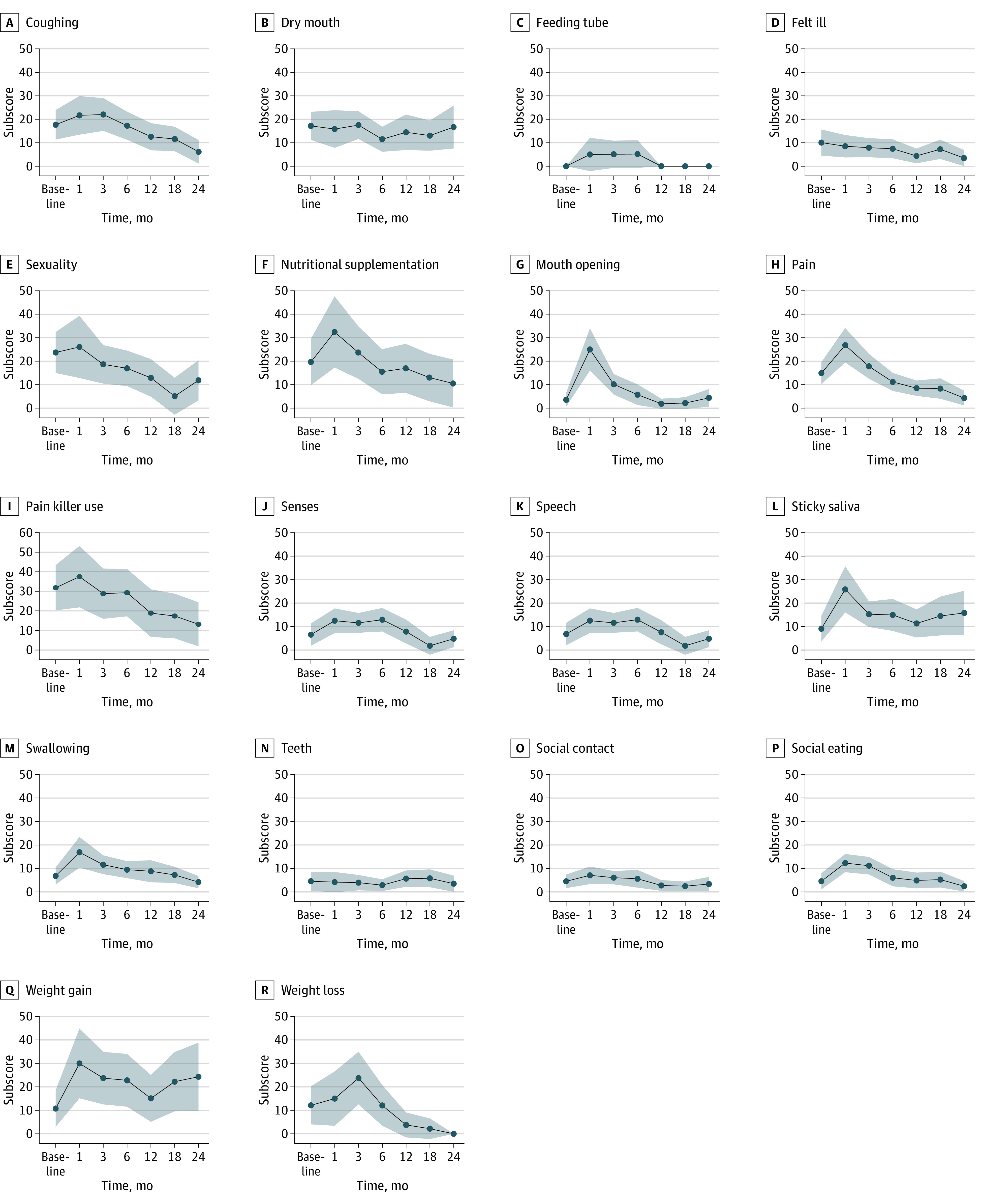
The symptom scores from the EORTC QLQ-HN35 are shown in Figure 2 and eTable 2 in Supplement 1. EORTC QLQ-HN35 symptom scales improved at 24 months, including coughing (mean difference, −11.11; 95% CI, −11.74 to −3.48), pain (mean difference, −10.61; 95% CI, −17.23 to −3.99), painkiller use (mean difference, −24.24; 95% CI, −46.01 to −2.47), and sexuality (mean difference, −15.15; 95% CI, −25.43 to −4.88). Patients experienced weight loss during treatment that remained stable at 1 month posttreatment to 24 months (mean difference, −15.15; 95% CI, −28.06 to −2.24). The remaining EORTC QLQ-HN35 symptom scales returned to baseline at 3 to 6 months posttreatment and were stable at 24 months, including dry mouth, feeling ill, nutritional supplementation, mouth opening, senses, speech, social contact, social eating, and weight gain. No other EORTC QLQ-HN35 symptoms declined from baseline to the 24-month reassessment.
Figure 2. Quality of Life Ratings per the Head and Neck Extension Module of the European Organization for Research and Treatment of Cancer Core’s Quality of Life Questionnaire From Patients Treated With Neoadjuvant Chemotherapy Followed by Transoral Robotic Surgery and Neck Dissection, by Symptom Scores.
Findings of the MDADI
MDADI scores from baseline to 1 year, including subscales, are shown in Table 3. All MDADI scores returned to baseline at month 12. Global and composite scores (mean difference, 6.15 [95% CI, −4.18 to 16.49] and 2.73 [95% CI, −1.62 to 7.09]) returned to baseline.
Table 3. Dysphagia-Associated Quality of Life Ratings on the MDADI Among Patients Treated With NECTORS, by Global and Subscale Scores.
| Measure | Mean (SD) [No.] | Mean differencea | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 mo | 3 mo | 6 mo | 12 mo | |||
| Global | 93.3 (19.2) [27] | 82.9 (24.8) [28] | 88.1 (22.4) [27] | 90.4 (22.8) [23] | 92.7 (20.7) [30] | 6.15 | (−4.18 to 16.49) |
| Emotional | 90.9 (10.8) [27] | 88.2 (12.7) [29] | 87.3 (14.2) [27] | 89.3 (15.1) [23] | 93.5 (8.9) [27] | 1.49 | (−3.80 to 6.77) |
| Functional | 92.2 (11.7) [27] | 91.4 (10.1) [29] | 92.0 (16) [27] | 91.0 (16.2) [23] | 96.4 (9.2) [24] | 5.15 | (−0.75 to 11.05) |
| Physical | 93.2 (11.6) [27] | 82.7 (13.8) [29] | 85.0 (23.6) [27] | 86.3 (20.7) [23] | 94.4 (11.6) [27] | 2.12 | (−5.20 to 9.44) |
| Composite | 92.2 (9.5) [27] | 86.8 (9.81) [29] | 87.6 (17.6) [27] | 88.5 (16.7) [23] | 94.7 (8.8) [27] | 2.73 | (−1.62 to 7.09) |
Abbreviations: MDADI, M. D. Anderson Dysphagia Inventory; NECTORS, neoadjuvant chemotherapy followed by transoral robotic surgery and neck dissection.
Baseline to month 12, and 13 patients included in the analyses with paired data.
Findings of the Decision Regret Scale
The median (range) DRS score was 5 (0-30) of 100, representing mild regret overall. Decision regret scores are plotted in eFigure in Supplement 1.
Discussion
This study identified preservation of longitudinal patient-reported QOL outcomes in patients with HPV-OPSCC treated with the NECTORS protocol. Utilization of several QOL metrics provided a multidimensional assessment of the positive results of this treatment regimen.
Description and Benefits of NECTORS
NECTORS is based on systemic escalation with neoadjuvant chemotherapy and locoregional de-escalation with minimally invasive surgery.16,17,18 Advantages include treating micrometastases, down-staging the primary tumor to allow close margins on surgical resection and reserving postoperative adjuvant RT or CRT for salvage. Margins were defined as positive or negative; negative and close margins were adequate.18 Research on adequate margin distance in HPV-OPSCC has shown that close margins (ie, >1 mm to ≤5 mm) have similar disease-free and overall survival rates than those with margins greater than 5 mm.32,33 In a prior report from our research group,18 patients treated with NECTORS with negative margins (n = 52) have been free of disease on follow-up; 2 patients with positive margins developed distant metastases to the lungs (n = 1) and local recurrence and death (n = 1). That study cohort of patients treated with this same NECTORS protocol demonstrated pathologic complete response in the primary site (72%) and neck (56%).18 Given this response, consolidation surgery and neck dissection are performed as definitive treatment, with more than 95% of patients avoiding adjuvant or salvage radiation. This is a de-escalation of locoregional treatment, unlike the up-front TORS approach, which required adjuvant RT in 89% of patients.10 Recently, Samaniego et al34 demonstrated that neoadjuvant chemotherapy induces HPV-specific T-cell immunity. This is likely contributing to the success of neoadjuvant chemotherapy approach prior to TORS, in addition to the direct cytotoxic effect of chemotherapy on the tumor. We are currently studying our study cohort to corroborate this finding. This protocol’s neoadjuvant chemotherapy regimen delivers a lower cisplatin dose than standard concurrent CRT, limiting chemotherapy’s secondary effects.
This study cohort demonstrated a locoregional recurrence rate of 3 in 67 patients, and the 2 patients who completed salvage therapy have been free of disease for 36 months. Previous cohorts treated with the NECTORS protocol demonstrated a recurrence rate of approximately 4%, and those who experienced recurrence had high survival rates with salvage therapy.16,17,18 This survival rate is in contrast to patients with OPSCC who were treated with up-front CRT, which historically has had a risk of recurrence of approximately 10% to 15%.35,36,37,38,39 Most recurrences in patients with HPV-OPSCC are distant metastatic disease (54 of 81 patients [67%]) and 5-year survival rates in these patients are extremely low, with 98% mortality.39 Currently, the patients included in this prospective cohort are in good health, with no evidence of disease; 1 patient is receiving salvage pembrolizumab. The data on this cohort must still mature to document survival and recurrence data.
Benefits for QOL
Traditional open surgery approaches can produce disfigurement, dysphagia, aspiration, and speech difficulties that are minimized with the TORS approach.40,41 Chemotherapy toxics effects include neutropenia, anemia, thrombocytopenia, ototoxicity, and others.42 Grade 3 ototoxic effects were demonstrated in less than 10% of patients. A systematic review43 reports a wide range of ototoxic effect rates in patients with head and neck cancer who receive concurrent CRT (17%-88%). Patients with head and neck cancer treated with high dose cisplatin (100 mg/m2 every 3 weeks) are more likely to experience hearing loss than those receiving weekly low-dose (40 mg/m2) cisplatin (68% vs 24%; P < .01).44 Those receiving high-dose cisplatin have ototoxic effects after a median (range) cumulative dose of 197.2 mg/m2 (39.5-511.5 mg/m2).44 Patients treated with NECTORS may have lower rates of ototoxic effects because the protocol uses a lower dose of neoadjuvant cisplatin (75 mg/m2) every 3 weeks for 3 cycles, with a cumulative dose of 225 mg/m.2 Moreover, the NECTORS protocol does not treat with up-front RT, avoiding exposure of the cochlea to RT.
RT-related secondary effects of treatment are well represented in the EORTC QLQ-HN35.14,45 In our group treated with NECTORS, all domains returned to baseline levels within 3 to 6 months posttreatment. The primary goal of oncologic treatment is still eradication of disease, and this has been previously demonstrated using the NECTORS protocol.16,17,18 However, in the present study, longitudinal patient-reported outcome measures collected through the EORTC QLQ-C30, EORTC QLQ-HN35, and MDADI questionnaires demonstrate recovery to baseline scores as well. The NECTORS treatment protocol maintains QOL after the initial expected decrease in subjective scores during and immediately after treatment.
The Decision Regret Scale assessed remorse regarding a treatment decision to offer a better understanding of the psychological distress of being diagnosed and treated for cancer and opting for a nonstandard care protocol. A cross-sectional study by Goepfert et al27 assessed decisional regret among patients who were previously treated for oropharyngeal cancer. Among 628 patients treated with CRT, decisional regret was expressed by 13.5%—within the mild range, but higher than among the NECTORS cohort. Patients who undergo CRT have low levels of regret on this assessment, but RT adverse effects are progressive, and the regret level should be reassessed at a later time point. In contrast, in the NECTORS cohort, the adverse effects profile is unlikely to progress because of the absence of adjuvant RT, and the cross-sectional assessment of the patients who underwent NECTORS is indicative of long-term sentiments.
Comparison With Previous Trials
There are limitations in the ability to compare clinical trials due to the variety of QOL patient-reported outcome metrics, measurements at different time points, and adverse effect profiles of treatment modalities. However, an important trial has been reviewed and compared with the NECTORS protocol.
The ECOG-ACRIN 3311 trial10 enrolled patients with stage III and IVa19 HPV-OPSCC, who received up-front TORS and neck dissection, and were treated with risk-based adjuvant therapy postoperatively to evaluate the oncologic effectiveness of adjuvant 50 Gy vs 60 Gy RT. Patients without worrisome features were observed (group A) and patients with poor pathological features were treated with adjuvant CRT with 66 Gy and weekly cisplatin (group D). The intermediate groups were randomized to 50 Gy (group B) or 60 Gy (group C) of adjuvant RT. This TORS-first approach required adjuvant treatment in 89% of patients despite excluding patients with matted lymph nodes, bilateral lymphadenopathy, and/or T3 stage disease. At 1-year posttreatment, patients in group A had a similar trajectory of MDADI composite scores as our study’s cohort, demonstrating a slight increase from baseline (89.1 to 94.7). The 90% of patients in groups B, C, and D who required adjuvant RT/CRT did not return to pretreatment values by 1 year and remained 9 to 15 points below their baseline. Therefore, NECTORS’ neoadjuvant chemotherapy downstages the tumor, and surgery consolidates the treatment without adjuvant RT, which has been shown to be associated with QOL outcomes similar to those of group A in the ECOG ACRIN 3311 study, ie, without any adjuvant therapy after TORS.
Limitations
This study had some limitations worth noting. The MDADI instrument was introduced late in the study, which explains why there were fewer of these completed and no 24-month findings. There was no local CRT group with adequate available data to provide a comparison; however, this cohort was directly compared with their pretreatment values. Baseline QOL values are skewed because patients complete these questionnaires after a cancer diagnosis. However, this limitation is present in many, if not all, studies and gives insight into an explanation for why select posttreatment scores can exceed baseline values. Although surgical trials have a selection bias toward including healthy patients fit for surgery, this cohort had minimal exclusion criteria, including those with matted and/or large lymphadenopathy, minimal ECE on pre-operative imaging, smokers of any smoking pack-year history, and T3 and N2c stages.
Conclusions
The findings of this multicenter cohort study indicate that neoadjuvant chemotherapy followed by TORS and neck dissection, while reserving RT for salvage, may be an approach that preserves QOL in all domains for patients with stage III and IVa HPV-OPSCC. Hence, NECTORS treatment protocol is an effective therapeutic option for HPV-OPSCC with the neoadjuvant chemotherapy allowing definitive TORS and neck dissection and avoiding adjuvant RT or CRT and associated toxic effects of RT. This is a new paradigm in management of HPV-OPSCC.
eTable 1. Treatment-related toxic effects
eTable 2. EORTC QLQ-HN35 symptom scales
eFigure. Decision regret scale
Data Sharing Statement
References
- 1.Nichols AC, Palma DA, Dhaliwal SS, et al. The epidemic of human papillomavirus and oropharyngeal cancer in a Canadian population. Curr Oncol. 2013;20(4):212-219. doi: 10.3747/co.20.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor A, Eade T, Veivers D, Gill AJ, Pang L. Human papillomavirus and oropharyngeal squamous cell carcinoma: a 12-year retrospective review in a New South Wales tertiary referral centre. Aust J Otolaryngol. 2019;2. doi: 10.21037/ajo.2019.01.01 [DOI] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anantharaman D, Muller DC, Lagiou P, et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int J Epidemiol. 2016;45(3):752-761. doi: 10.1093/ije/dyw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIlwain WR, Sood AJ, Nguyen SA, Day TA. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(5):441-447. doi: 10.1001/jamaoto.2014.141 [DOI] [PubMed] [Google Scholar]
- 8.Khalid MB, Ting P, Pai A, et al. Initial presentation of human papillomavirus-related head and neck cancer: a retrospective review. Laryngoscope. 2019;129(4):877-882. doi: 10.1002/lary.27296 [DOI] [PubMed] [Google Scholar]
- 9.Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB. The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg. 2014;151(3):375-380. doi: 10.1177/0194599814538605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris RL, Flamand Y, Weinstein GS, et al. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: an ECOG-ACRIN Cancer Research Group Trial (E3311). J Clin Oncol. 2022;40(2):138-149. doi: 10.1200/JCO.21.01752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network . Head and neck cancers (version 2). 2022. Accessed August 23, 2022. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 12.Palma DA, Prisman E, Berthelet E, et al. Assessment of toxic effects and survival in treatment de-escalation with radiotherapy vs transoral surgery for HPV-associated oropharyngeal squamous cell carcinoma: the ORATOR2 phase 2 randomized clinical trial. JAMA Oncol. 2022;8(6):1-7. doi: 10.1001/jamaoncol.2022.0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vainshtein JM, Moon DH, Feng FY, Chepeha DB, Eisbruch A, Stenmark MH. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91(5):925-933. doi: 10.1016/j.ijrobp.2014.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3)(suppl):S58-S63. doi: 10.1016/j.ijrobp.2009.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582-3589. doi: 10.1200/JCO.2007.14.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadeghi N, Mascarella MA, Khalife S, et al. Neoadjuvant chemotherapy followed by surgery for HPV-associated locoregionally advanced oropharynx cancer. Head Neck. 2020;42(8):2145-2154. doi: 10.1002/hed.26147 [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi N, Li NW, Taheri MR, Easley S, Siegel RS. Neoadjuvant chemotherapy and transoral surgery as a definitive treatment for oropharyngeal cancer: A feasible novel approach. Head Neck. 2016;38(12):1837-1846. doi: 10.1002/hed.24526 [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi N, Khalife S, Mascarella MA, et al. Pathologic response to neoadjuvant chemotherapy in HPV-associated oropharynx cancer. Head Neck. 2020;42(3):417-425. doi: 10.1002/hed.26022 [DOI] [PubMed] [Google Scholar]
- 19.American Joint Committee on Cancer . In: Edge SB, ed. AJCC Cancer Staging Manual. 7th ed. Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Accessed June 1, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
- 23.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 24.Bjordal K, de Graeff A, Fayers PM, et al. ; EORTC Quality of Life Group . A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur J Cancer. 2000;36(14):1796-1807. doi: 10.1016/S0959-8049(00)00186-6 [DOI] [PubMed] [Google Scholar]
- 25.Singer S, Arraras JI, Chie WC, et al. Performance of the EORTC questionnaire for the assessment of quality of life in head and neck cancer patients EORTC QLQ-H&N35: a methodological review. Qual Life Res. 2013;22(8):1927-1941. doi: 10.1007/s11136-012-0325-1 [DOI] [PubMed] [Google Scholar]
- 26.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the MD Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870-876. [PubMed] [Google Scholar]
- 27.Goepfert RP, Fuller CD, Gunn GB, et al. Symptom burden as a driver of decisional regret in long-term oropharyngeal carcinoma survivors. Head Neck. 2017;39(11):2151-2158. doi: 10.1002/hed.24879 [DOI] [PubMed] [Google Scholar]
- 28.Sheehan J, Sherman KA, Lam T, Boyages J. Association of information satisfaction, psychological distress and monitoring coping style with post-decision regret following breast reconstruction. Psychooncology. 2007;16(4):342-351. doi: 10.1002/pon.1067 [DOI] [PubMed] [Google Scholar]
- 29.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol. 1999;17(3):1008-1019. doi: 10.1200/JCO.1999.17.3.1008 [DOI] [PubMed] [Google Scholar]
- 30.International Labour Organization . International Standard Classification of Occupations, Skill Levels. Accessed October 31, 2023. https://ilostat.ilo.org/resources/concepts-and-definitions/classification-occupation/
- 31.Statistics Canada . The Canadian Inex of Multiple Deprivation, 2016. Accessed November 2, 2023. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5274
- 32.Lee DH, Kim GJ, Kim HB, et al. Close surgical margins in oral and oropharyngeal cancer: do they impact prognosis? Cancers (Basel). 2022;14(12):2990. doi: 10.3390/cancers14122990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holcomb AJ, Herberg M, Strohl M, et al. Impact of surgical margins on local control in patients undergoing single-modality transoral robotic surgery for HPV-related oropharyngeal squamous cell carcinoma. Head Neck. 2021;43(8):2434-2444. doi: 10.1002/hed.26708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samaniego C, Friedman J, Yang X, et al. Neoadjuvant chemotherapy enhances tumor-specific T cell immunity in patients with HPV-associated oropharyngeal cancer. Head Neck. 2023;45(9):2294-2302. doi: 10.1002/hed.27463 [DOI] [PubMed] [Google Scholar]
- 35.Contrera KJ, Smile TD, Mahomva C, et al. Locoregional and distant recurrence for HPV-associated oropharyngeal cancer using AJCC 8 staging. Oral Oncol. 2020;111:105030. doi: 10.1016/j.oraloncology.2020.105030 [DOI] [PubMed] [Google Scholar]
- 36.Garden AS, Dong L, Morrison WH, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(4):941-947. doi: 10.1016/j.ijrobp.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 37.Gleber-Netto FO, Rao X, Guo T, et al. Variations in HPV function are associated with survival in squamous cell carcinoma. JCI Insight. 2019;4(1):e124762. doi: 10.1172/jci.insight.124762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50(15):2636-2648. doi: 10.1016/j.ejca.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 39.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49(1):79-85. doi: 10.1016/j.oraloncology.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 40.Williams CE, Kinshuck AJ, Derbyshire SG, et al. Transoral laser resection versus lip-split mandibulotomy in the management of oropharyngeal squamous cell carcinoma (OPSCC): a case match study. Eur Arch Otorhinolaryngol. 2014;271(2):367-372. doi: 10.1007/s00405-013-2501-5 [DOI] [PubMed] [Google Scholar]
- 41.de Almeida JR, Byrd JK, Wu R, et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systematic review. Laryngoscope. 2014;124(9):2096-2102. doi: 10.1002/lary.24712 [DOI] [PubMed] [Google Scholar]
- 42.Goepfert H, Toth BB. Head and neck complications of systemic cancer chemotherapy. Laryngoscope. 1979;89(2 Pt 1):315-319. [DOI] [PubMed] [Google Scholar]
- 43.Theunissen EA, Bosma SC, Zuur CL, et al. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy and radiotherapy: a systematic review of the literature. Head Neck. 2015;37(2):281-292. doi: 10.1002/hed.23551 [DOI] [PubMed] [Google Scholar]
- 44.Teft WA, Winquist E, Nichols AC, et al. Predictors of cisplatin-induced ototoxicity and survival in chemoradiation treated head and neck cancer patients. Oral Oncol. 2019;89:72-78. doi: 10.1016/j.oraloncology.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 45.Choby GW, Kim J, Ling DC, et al. Transoral robotic surgery alone for oropharyngeal cancer: quality-of-life outcomes. JAMA Otolaryngol Head Neck Surg. 2015;141(6):499-504. doi: 10.1001/jamaoto.2015.0347 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Treatment-related toxic effects
eTable 2. EORTC QLQ-HN35 symptom scales
eFigure. Decision regret scale
Data Sharing Statement


