Abstract
This review describes stress-related biological mechanisms linking interpersonal racism to life course health trajectories among African Americans. Interpersonal racism, a form of social exclusion enacted via discrimination, remains a salient issue in the lives of African Americans, and it triggers a cascade of biological processes originating as perceived social exclusion and registering as social pain. Exposure to discrimination increases sympathetic nervous system activation and upregulates the HPA axis, increasing physiological wear and tear and elevating the risks of cardiometabolic conditions. Consequently, discrimination is associated with morbidities including low birth weight, hypertension, abdominal obesity, and cardiovascular disease. Biological measures can provide important analytic tools to study the interactions between social experiences such as racial discrimination and health outcomes over the life course. We make future recommendations for the study of discrimination and health outcomes, including the integration of neuroscience, genomics, and new health technologies; interdisciplinary engagement; and the diversification of scholars engaged in biosocial inequities research.
Keywords: allostatic load, discrimination, genomics, HPA axis, racism, social exclusion
INTRODUCTION
Inequities in morbidity and mortality rates for African Americans compared to Whites are long-standing and remain striking in the United States. Although the processes from which these disparities arise are multifaceted, they are rooted in the historical legacy of racism that is woven into the fabric of life in the United States. The United States is a racialized social system of interlinked domains across layers of social organization (Reskin 2012), supported by practices and behaviors that produce a network of relations at social, political, economic, and ideological levels that together shape life chances and health inequities (Bonilla-Silva 2015). These forces act on the bodies and minds of African Americans, eroding psychic resources and wearing bodies down. We focus on a specific aspect of the United States’ racialized social system: the role of perceived interpersonal discrimination over the life course. This review emphasizes how these negative social experiences get under the skin via interactions with biological processes that support individuals’ capacities for responsivity and adaptation to stress.
Interpersonal discrimination is a mechanism of social exclusion that remains a pervasive problem in the United States (Priest & Williams 2018). In fact, in a 2017 national survey, 92% of African Americans reported that discrimination exists in the United States today, and 75% of those respondents believed that interpersonal discrimination is a significant social problem (NPR et al. 2017). This review, therefore, focuses on (a) the pathways by which health inequities emerge through interactions between the negative social experiences of interpersonal discrimination and stress biology; (b) how these interactions emerge and are understood at different periods of the life course; and (c) important new directions for future research at the intersection of the biological and the social. Biosocial processes linking discrimination to health are not exclusive to African Americans, but we use African Americans’ unique conditions in the United States as an important example. Much of what we discuss here is likely relevant to other stigmatized social and racial/ethnic groups who experience high rates of interpersonal discrimination and social exclusion (Pescosolido & Martin 2015). Moreover, the problem of racism is not unique to the United States, and other societies also present racial health inequities to which the biosocial processes reviewed here likely apply (Paradies 2006, Pascoe & Smart Richman 2009).
DEFINING DISCRIMINATION
Discrimination is the unjust or prejudicial treatment of a category of people. Minority racial status is not a prerequisite for discrimination, but it is an important dimension along which groups of people experience systemic adverse treatment (Williams & Mohammed 2009). This systematic component with respect to race in general and African Americans in particular reflects racism, that is, “the social categorization and stratification of social groups into races that devalues and disempowers groups” (Priest & Williams 2018, p. 163). Racism is commonly conceptualized across multiple levels. At the individual level, intrapersonal racism reflects individuals’ internalized attitudes and beliefs about innate superiority or inferiority. Structural racism reflects the systematic exclusion from institutions and markets, such as schools, employment, health, housing, credit, and justice (Reskin 2012), as well as the withholding of symbolic resources within social institutions and society more generally (Priest & Williams 2018).
Interpersonal racism, the focus of this review, is at a minimum a dyadic process of “discrimination between people, with varying degrees of frequency and intensity, including manifestation from racially motivated assault to verbal abuse, ostracism, and exclusion” (Priest & Williams 2018, p. 163). Such acts can reflect explicit biases (e.g., Jim Crow racism) and the aggressive acts of abuse associated with racist ideologies, as well as implicit biases (e.g., aversive or colorblind racism) of which the perpetrator may not be aware (Bonilla-Silva 2017) and that produce subtler forms of exclusion (e.g., microaggressions; Sue et al. 2007). Each of these dimensions of racism contributes to African American health inequities, and each is interdependent with the others.
SOCIOLOGY AND HEALTH BIOLOGY
Systematic and meta-analytic reviews of traditional survey-based studies demonstrate that perceived interpersonal discrimination is associated with a broad range of mental and physical health outcomes (Paradies et al. 2015, Priest et al. 2013, Pascoe & Smart Richman 2009). These studies document consistent correlations of perceived discrimination with psychological constructs such as depressive symptoms, anxiety, anger, low self-esteem, and negative well-being, as well as associated physical manifestations including poor self-rated health, low birth weight, hypertension, obesity, high blood pressure, and cardiovascular disease. Models linking discrimination to health usually posit two global pathways. The first pathway comprises physiological stress-related processes, and the second reflects behavioral patterns (e.g., alcohol and substance use, diet, sexual risk; e.g., Richman et al. 2018). This review focuses on the first set of stress biology pathways.
The pervasive adverse influences of discrimination on health point to a complex set of interactions between human biology, the human brain’s capacity for sociality, and our social interdependencies on one another. Our brains process and prepare for environmental (i.e., social) demands by monitoring, regulating, and coordinating internal systems in a process of predictive regulation termed allostasis (McEwen 1998, Sterling 2012). Allostatic mechanisms manage energy and homeostatic parameters when confronted with a stressor (McEwen 1998). From a social science perspective, these allostatic parameters—physiological states—are latent variables. Revealing these physiological indicators (i.e., biomarkers) allows researchers to peer into processes otherwise invisible to the observer and often to the participants themselves. Many biomarkers are highly sensitive, making it possible to determine health status characteristics prior to the onset of morbidity. Moreover, the latent nature of biological measures also assists in addressing reverse causality concerns (e.g., negative affect bias) through traditional survey measures that link perceived interpersonal discrimination to different facets of self-reported health (Richman et al. 2018).
Processes in our bodies are typically affected before we get sick enough to consciously recognize the change in our health condition. To the extent that stressors become chronic, the modulation of allostatic regulation can begin to tax systems over time, placing strain on those systems and wearing them out due to the accumulating allostatic load burden (see also McEwen 1998). Allostatic load refers to the wear and tear on bodily systems that occurs from frequent allostatic modulation arising from exposure to chronic stress. The ongoing strain can limit physiological adaptive capacity to stress by causing a failure to adapt to repeated stressors, by halting neuroendocrine and autonomic responses, and generally by limiting the ability to respond adequately to stressors (McEwen & Gianaros 2010). In this way, we could say that allostatic states accumulate over the life course and are, over time, converted into physiological allostatic load traits.
Biological measures, thus, hold potential for understanding how social conditions and experiences adversely affect health over the life course and at different points within it. Such measures allow researchers to peer back earlier into life before illness manifests, and to quantify the tolls exacted by socioenvironmental conditions. Together, period-specific modulation and accumulation processes are critical for characterizing the what, when, and why of health outcomes. We cannot review all the biological pathways by which racism in the United States affects health (e.g., differential exposure to environmental toxins as a result of residential segregation, etc.), and we focus instead on the subdomains of neurobiology, stress physiology, and genomic factors.
DISCRIMINATION AND HEALTH OVERVIEW
Sociologists have long recognized the pivotal role of social support and connection in human health (House et al. 1988). Durkheim’s (1951) argument that social dynamics pattern individual pathologies, of which integration and anomie are critical factors, is a staple of sociological training. Interpersonal racism and the associated exclusionary acts of discrimination, whether intentional or due to insensitivity, withhold their targets’ opportunities for inclusion and support. Targets of racism and discrimination are thereby denied the symbols of group membership and the motivating positive emotional energy that individuals derive from successful social experiences (Collins 2014). Because interpersonal discrimination is socially patterned by racism, these experiences contribute to large-scale population health inequities by regulating stress exposure risk beyond other forms of social disadvantage [e.g., socioeconomic status (SES)] that are also patterned by racism (Glass & McAtee 2006, Phelan & Link 2015).
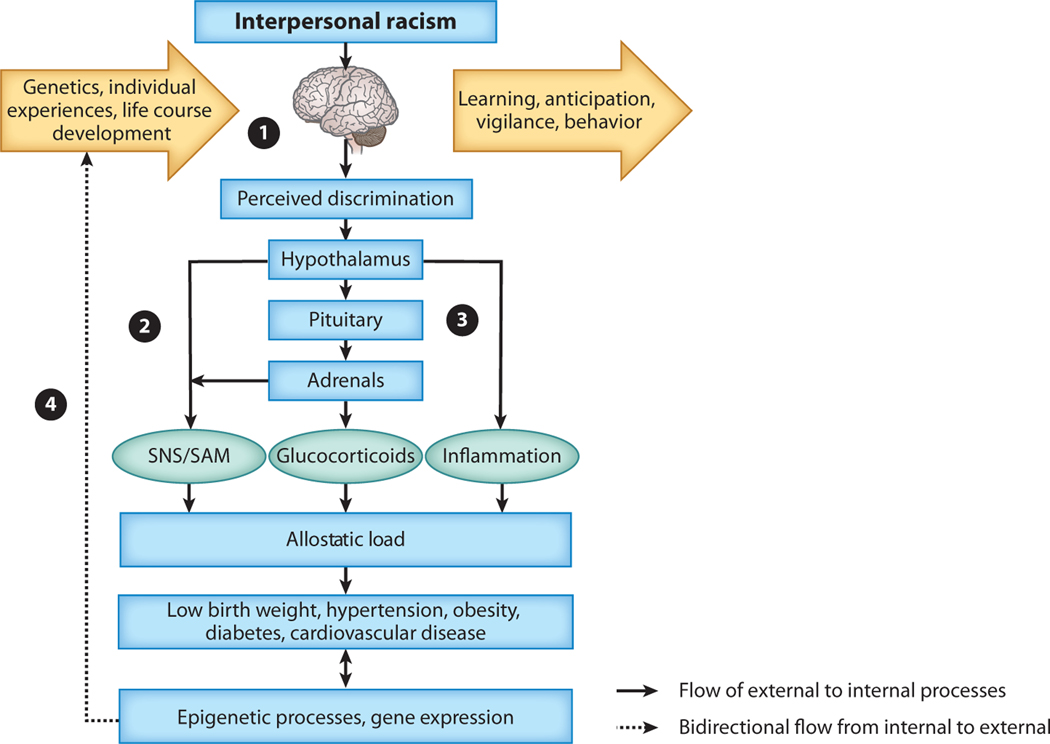
To characterize why these experiences are harmful, it is important to begin not with the body, but with the brain. For a social stressor like perceived interpersonal discrimination to get under the skin, it must first get into the mind. Figure 1 presents a conceptual model illustrating the stress-related pathways connected to interpersonal discrimination that we describe in the following sections (see also Albert et al. 2013).
Figure 1.
Visual description of the reviewed biosocial links between interpersonal discrimination and health. This figure is a visual abstract of the themes discussed in the paper and is not representative of all possible biosocial relationships. Arrow boxes indicate temporal processes, blue boxes capture a range of predictors and outcomes, and ovals indicate specific physiological response products that, over time, reduce health. In section (❶) interpersonal discrimination is identified by the brain as a stressor requiring immediate physiologic response, and also, over time, it becomes a learned process that creates anticipation and vigilance towards possible future exposures. Sympathetic nervous system arousal occurs in response to discrimination stress exposure (❷) and in concert with the upregulation of the hypothalamic-pituitary-adrenocortical axis (❸). Together, these systems initiate stress activation including sympathetic-adrenal-medullary (SAM), glucocorticoid, and inflammatory responses. When stress exposure is chronic, these responses create allostatic load, or wear and tear on the body, and increase risks for a variety of adverse health outcomes throughout the life course. Epigenetic processes and gene expression (❹) contribute to the process in a bidirectional manner. Social stress can potentially moderate gene expression and epigenetic processes over the life course and across biological systems. The temporal nature of this process is depicted in the left to right flow of Figure 1 and the epigenetic/expression feedback in Figure 1❹. Abbreviations: SAM, sympathetic-adrenal-medullary system; SNS, sympathetic nervous system.
Neural Processing of Social Threats
In everyday language, negative social experiences are referred to as painful. When we have been mistreated, we “feel hurt” by the experience (Eisenberger 2012). There is truth in these colloquialisms. The neural structures supporting the emotional component of pain (as compared to the somatic component) are shared with those supporting the experience of social pain that results from social rejection and exclusion (Eisenberger 2012). Social connection is an important facet of human survival. The mechanisms supporting protection from physical threats via physical pain may have been conserved to support the feelings of social pain experienced when social inclusion and therefore survival are threatened (Eisenberger 2013). The pathway between interpersonal discrimination, neurobiological processes, and perceived discrimination is depicted in Figure 1❶.
A number of brain regions are responsible for processing different aspects of fear and pain, including the amygdala, the dorsal anterior cingulate cortex (dACC), the anterior insula (AI), and the periaqueductal gray (PAG). These regions detect and coordinate responses to perceived threat, and along with the dorsomedial prefrontal cortex (DMPFC), they further support different aspects of social exclusion processing (Eisenberger 2013), suggesting the shared basis for physical and social pain mentioned above. These systems further interact with those that process safety in the absence of negative outcomes, including the ventromedial prefrontal cortex (VMPFC) and the posterior cingulate cortex (PCC) (Delgado et al. 2006, Schiller & Delgado 2010). Together, these different systems monitor and respond to the social environment, including threats to inclusion, and interact with key brain regions that mediate stress response systems.
These regions are embedded in large-scale intrinsic functional networks, including the salience network (somatovisceral emotional experiences), the mentalizing network (“theory of mind”), and the central executive network (cognition) (Barrett & Satpute 2013). The interactions within and among these networks allow the social environment to be processed and monitored, support socioenvironmental learning, and enable future social experiences and the anticipation of the potential threats embedded in future encounters (Eisenberger & Lieberman 2004). Consequently, whereas interpersonal discrimination can have localized harmful effects on a person via the immediate needs it presents, the threat of such experiences is also a learning process, as depicted in the outward arrow box in Figure 1❶. These encounters shape how individuals understand their experiences, form expectancies for future encounters, and therefore monitor and prepare the body (i.e., predictive regulation) for the social interactions embedded in the social environments they inhabit and pass through (i.e., vigilance; Blair & Raver 2012, Lewis et al. 2015).
These neural processes are therefore important for monitoring and recognizing discrimination as a first phase in the downstream stress-related physiological cascades that we have depicted in the descending pathways in Figure 1. Several studies now document the links between activity in the neural regions involving social exclusion–related brain areas and different aspects of the stress process. These regions monitor the environment for social feedback, including threats to social inclusion, and coordinate physiological responses (Eisenberger 2013).
Sympathetic Nervous System
At the broadest level, the nervous system consists of the central nervous system (CNS), which contains the brain and spinal cord, and the peripheral nervous system (PNS), which contains nerves and ganglia outside of the CNS. An important subdivision of the PNS is the autonomic nervous system (ANS), which controls a range of bodily functions (e.g., heart rate, respiration, digestion). In the ANS, the sympathetic nervous system (SNS) prepares the body to deal with the demands of the environment, including threats to social inclusion (Bosch et al. 2009), and it is responsible for the fight-or-flight response. A related balancing branch, the parasympathetic nervous system (PSNS), manages recovery (rest-and-digest response). The SNS and PSNS work mostly, but not entirely, in opposition to one another. SNS modulation, in particular, can be damaging to health when stress is chronic (see Figure 1❷).
CNS activity in the dACC, AI, PAG, and amygdala associated with social exclusion and inclusion processing (Eisenberger 2013) is connected to increased SNS activity (e.g., blood pressure, heart rate) (Critchley et al. 2003, McEwen & Gianaros 2010). Moreover, damage to the dACC decreases reactivity to mental stressors (Critchley et al. 2003), suggesting that the dACC mediates ANS activity. VMPFC activity during fear extinction tasks predicts decreased SNS activity (Phelps et al. 2004) and reduced cardiovascular responses during a social stressor (Wager et al. 2009), possibly due to associations with the PSNS (Hänsel & von Känel 2008). The hypothalamus mediates SNS reactivity to social exclusion threats such as perceived discrimination. The hypothalamus is a structure located in the limbic system composed of small nuclei that manage a variety of ANS functions. Relevant hypothalamus-mediated SNS activity has been referred to as the sympathoadrenal or sympathetic-adrenal-medullary (SAM) system (Cohen et al. 2007). SNS-triggered SAM activity releases the adrenal hormones epinephrine/adrenaline and norepinephrine/noradrenaline, which increase blood sugar, heart rate, and blood circulation, and reallocates energy to be utilized during the acute-phase response to a stressor.
An important consequence of this elevated SAM activity is increased wear and tear on the cardiovascular system (Brotman et al. 2007). Cardiovascular responding shows different patterns depending on whether the stressor is positive or negative. In the former case, heart rate increases and blood vessels dilate, lowering total peripheral resistance while increasing cardiac output and keeping blood pressure relatively stable. During negative stressors, by contrast, blood vessels contract, restricting blood flow for fast circulation and increasing blood pressure (Brondolo et al. 2003). This elevated blood pressure, amounting to hypertension when chronic, is particularly dangerous due to increased blood viscosity from elevated blood glucose levels and to increases in certain cholesterol particles that contribute to arterial scarring and elevated cellular inflammation, which are precursors to atherosclerosis (Sapolsky 2004). These conditions are all indications of higher allostatic load in the body and markers of accelerated stress-induced wear and tear (McEwen 1998).
HPA Axis
Sociological research has sought to understand the stress process in terms of socially patterned stressors that shape mental and physical health (Pearlin 2010). These studies have documented stress typologies and characterized key mediators and moderators in the stress process, such as social support (Pearlin 1999). The stress process model argues that the consequences of exposure to stressors such as chronic economic hardships create psychological burdens that are challenging for individuals to bear, leading to declines in health resulting from maladaptive psychological and behavioral coping (Turner 2013). More recent stress process models include attention to physiological stress reactivity, adaptation, and load via the hypothalamic-pituitary-adrenal (HPA) axis ( Jackson et al. 2010, Turner 2013), depicted in Figure 1❸.
Along with SNS/SAM processes, the HPA axis is a key mediator of stress responsivity and long-term health outcomes. The HPA axis is a complex set of interactions comprising direct effects and feedback loops among the hypothalamus, the pituitary gland, and the adrenal glands. The primary function of the HPA axis is to regulate metabolic (neuroendocrine) and immune function (McEwen & Gianaros 2010). This system is primarily responsible for metabolizing carbohydrates, fats, and proteins; for gluconeogenesis (internal production of blood glucose); and for inflammatory immune function regulation (Sapolsky 2004). The HPA axis elevates circulating hormone levels, starting with the activation of corticotropin releasing hormone (CRH), which triggers the production of adrenocorticotropic hormone (ACTH) into the bloodstream. ACTH triggers the release of glucocorticoid hormones, including cortisol. Cortisol, a stress hormone, regulates metabolic function (e.g., circulating blood glucose, fat storage, insulin response), immune response (e.g., increased inflammation for wound healing), and mood (Sapolsky 2004). Cortisol facilitates easy energy access by reducing the body’s sensitivity to insulin, the hormone responsible for regulating uptake of blood glucose for storage in cells, while increasing gluconeogenesis. In addition, cortisol promotes the breakdown of fats into fatty acids (lipolysis) and contributes to the initial inhibition of inflammatory and acute-phase immune responses to infection (McEwen 2003).
Neural sensitivity to social exclusion and reactivity to negative social experiences such as perceived interpersonal discrimination are linked directly to HPA axis activity. For example, social stress experiments show increased HPA activity in response to social evaluative threats (Bosch et al. 2009, Dickerson et al. 2009). Greater activity in the brain regions involved in processing and monitoring for threat is also implicated. Activation in the dACC during a mental stress task is associated with increased stress hormone output (Wang et al. 2005), and increased cortisol is also predicted from dACC and DMPFC activity in response to social exclusion (Dedovic et al. 2009). Although such increases are adaptive in the short term, chronic increases in circulating blood glucose, coupled with the dampened response to insulin regulated by higher cortisol levels, heighten the risk for insulin resistance, abdominal obesity, and type 2 diabetes (Black 2003). As with SNS/SAM, HPA pathways also create wear and tear on the arteries via an increase in particle-dense blood. This increased blood viscosity is thought to cause inflammation and arterial scarring, both of which are risk factors for cardiovascular disease and indicators of allostatic load (Seeman et al. 2010).
Genomics
Genetic research is perhaps the most controversial area of biologically informed sociological research. Concern that genetic research will be used to support racist agendas is a reflection of the larger societal problem of racist ideologies, racial domination, and dehumanization (Zuberi et al. 2015). In practice, genomic research on African ancestry groups lags behind studies of European ancestry groups, despite evidence that more multiethnic research is needed (Need & Goldstein 2009). This lag partly reflects the challenges of population stratification and admixture resulting from slavery, a reliance on nonrepresentative higher-SES European-ancestry samples, a genotyping technology tailored for the more genetically homogenous European ancestry, and the complex politics of race (Bentley et al. 2017, Márquez-Luna et al. 2017, Popejoy & Fullerton 2016). Consequently, Eurocentric estimates perform poorly for groups from different ancestral lineages and who experience different environments (Márquez-Luna et al. 2017, Ware et al. 2017). Multiple reviews document the current methods, results, and major concerns of genetic research for social scientists, including the lack of diversity in study populations (Duster 2015, Freese 2008, Mitchell 2018).
The a priori rejection of genetic information based on the key (and well-documented) distinction between genetic ancestry and the social attribution of race impairs our understanding of how life in a racialized social system affects health through genetically influenced biological pathways. Genetic variation, depicted in the entering arrow box in Figure 1❶, contributes to variability of all the biological systems discussed above, and therefore it influences the individual capacities underlying sensitivity to social exclusion and need for inclusion (Preller et al. 2016), emotional and physiological reactivity (Mitchell et al. 2013, Moore & Depue 2016, Pluess 2015), and individual differences in allostatic load accumulation (Brody et al. 2013). Moreover, the activity of genetic networks varies during development, modulating the organism’s responses to the environment at different life stages (Manuck & McCaffery 2014, Mitchell et al. 2013). Biosocial interactions likely occur at a much higher level of biological organization than the gene, which is likely why most genetic effects are exceptionally small and come from across the genome (Belsky et al. 2013). Because the genome evolved to set the parameters for environmental responsiveness, understanding the underlying architecture of these biological processes provides clear benefits. Further, genes may be highly informative if we consider that some of the biological levels are too dynamic or too invasive to measure, such as the neurotransmitter systems that regulate communication between nodes in the neural networks involved in monitoring and responding to social inclusion and exclusion (Preller et al. 2016).
Moreover, it is more useful to think in terms of genomics and not just genes, as we have indicated at the bottom of Figure 1. The gene is a predictor that can be linked to the environment via gene by environment interaction (GxE), but other genome features occupy different locations in causal models with environmentally dependent pathways. Gene expression is the process by which the information contained on the chromosome (DNA, epigenetics, etc.) is used to assemble functional gene products. Research indicates that social stress regulates gene expression, potentially moderating allostatic response and load accumulation. For example, social stress is associated with upregulated pro-inflammatory immune response and downregulated antiviral immune response (Slavich & Cole 2013). Loneliness is also implicated in differential expression of the genes involved in reward circuits in the CNS (Canli et al. 2017), and the social stress of loneliness is associated with differential expression of genes containing glucocorticoid receptor response elements (Cole 2013).
Gene expression is also dependent on epigenetic processes, as indicated by the dashed feedback loop in Figure 1❹. Epigenetics—for example, DNA methylation—is the study of the chromosomal alterations that influence gene activity and expression without changing the nucleotide sequence (Champagne 2018). Methylation, the process by which methyl groups attach to DNA by binding to a cytosine base, typically diminishes and can even turn off gene expression (less commonly, genes can also be turned on). Interpersonal discrimination is associated with methylation patterns (Brody et al. 2016, Saban et al. 2014), as is stress more generally (Champagne 2010, Mitchell et al. 2015). Epigenetic regulation of the genome is environmentally dependent and sensitive to social experience. Both methylation (Horvath 2013) and length of telomeres—DNA sequences at the end of the chromosome that (generally) shorten with aging and stress (Shalev et al. 2013)—appear to provide indications of the degree of cellular adversity and biological aging. Interpersonal discrimination is associated with shorter telomere length in adults (Chae et al. 2014, Lee et al. 2017) and in the placentas of newborns of women exposed to discrimination during pregnancy ( Jones et al. 2017). Further, it is well documented that all of these genomic processes (from epigenetics to gene expression) are highly developmental and change throughout the life course (Champagne 2010, Manuck & McCaffery 2014, Mitchell et al. 2015, Shalev et al. 2013).
DISCRIMINATION AND HEALTH OVER THE LIFE COURSE
The stress process model provides an important heuristic guide for understanding the consequences of socially stratified and patterned stressors for well-being (Pearlin 2010). Of particular importance is how exposure to chronic stress is regulated by ascribed characteristics of varying social value (i.e., race) and their convergence with role statuses (i.e., student, parent, employee etc.) over the life course (Pearlin 1999). Over their lives, individuals move from birth (when adverse birth outcomes shape health and developmental trajectories), to childhood (when social networks remain small and dependency on parents remains high), through adolescence (when social networks expand and youth individuate from parents), and into adulthood (with its myriad roles, demands, and dependencies). For stigmatized groups like African Americans, it is worth considering whether some periods may be more sensitive to social rejection and exclusion than others and how health deficits accumulate over that time (Colen 2011). The social exclusion of interpersonal discrimination is a moment of learning that conditions how future social interactions are experienced. It is also a moment of allostatic responding via SNS/SAM, HPA, and genomic mechanisms that, when viewed over the life course, contribute to a stress-response time series over which allostatic loads accumulate. Considering this process over the life course is important to understand the when, why, and what of physiological functioning and therefore to identify the first emergence of the biological manifestations of health disparities documented by midlife. The temporal nature of this process is depicted in the left to right flow of Figure 1 and the epigenetic/expression feedback in Figure 1❹.
Birth
African Americans experience substantially disparate birth outcomes compared to Whites. Birth disparities have not improved significantly since the Jim Crow era (Sullivan 2013), and the odds of low birth weight (<2.5 kg) and preterm birth for African Americans remain respectively 1.6 and 1.9 times larger than they are for Whites, even after controlling for a variety of factors like SES (Schempf et al. 2007). Notably, the odds of unfavorable birth outcomes among African immigrants in the United States decline substantially as time spent in the country increases, converging with those of African American women by the third generation (David & Collins 1997). African American women exposed to discrimination during pregnancy have elevated blood pressure, and their offspring have lower birth weights and higher preterm delivery risks (Hilmert et al. 2008, 2014; Slaughter-Acey et al. 2016), outcomes strongly correlated with infant mortality (Collins & David 2009, Schempf et al. 2007).
The in utero environment is a critical period shaping health risk trajectories. Exposure to stressful conditions influences the neural and physiological stress pathways of the fetus via cascading metabolic, epigenetic, and gene expression alterations (Champagne 2010, Godfrey & Barker 2001, Mitchell et al. 2015, Thayer & Kuzawa 2015). Poor birth outcomes are associated with abdominal obesity, insulin resistance, hypertension, type 2 diabetes, and cardiovascular disease (Nobili et al. 2008, Singhal et al. 2003), conditions for which African Americans are disproportionately at risk (CDC 2005, Zhang et al. 2009). Though these conditions represent health risks, they are the body’s way of preparing the offspring for the environmental stressors that may be experienced outside the womb. In this way, the environment in the womb mirrors maternal stress-related factors, preparing the child for the mother’s social environment.
For example, women who experience stress while pregnant secrete higher levels of CRH from both brain and placenta, upregulating fetal neuroendocrine and HPA axis response during key developmental periods of gestation (Collins & David 2009). Consequently, the fetus may be exposed to higher levels of stress hormones, including cortisol, which can restrict growth and elevate preterm delivery risk (Shapiro et al. 2013). When the fetus is exposed to high levels of stress hormones through the placenta, glucocorticoid (i.e., stress hormones) receptors are downregulated in the hippocampus, disrupting a key pathway modulating HPA axis activation. At the same time, receptor density in the amygdala is increased, affecting a key region involved in SNS/SAM and HPA responsivity and activation (Wadhwa 2005). These influences may emerge through epigenetic processes that prepare the offspring for life in a high-stress environment. In other words, the in utero environment prepares the offspring stress reactivity profile for stressful environmental conditions outside the womb (Kuzawa & Sweet 2009, Mitchell et al. 2015, Shalev et al. 2013).
Childhood and Adolescence
In many ways, African American children in the United States are not given the same opportunities to enjoy childhood as White youth (Goff et al. 2014). By the time they reach three or four years of age, young children of color are able to discern the members of dominant social groups and to perceive negative racial stereotypes (Averhart & Bigler 1997, Branch & Newcombe 1986). This awareness may reflect African American experiences of discrimination in the form of racial slurs and taunts, bullying and social exclusion, harassment by the police, and the disproportionate allocation of punitive treatments in school (Perry & Morris 2014, Sanders-Phillips 2009). Consequently, African American children are at risk of experiencing elevated feelings of danger, social isolation, and psychological distress (Sanders-Phillips 2009). Together, these factors work to upregulate stress response systems via brain-mediated pathways, and in combination with greater stress across life domains due to factors such as residential segregation and other features of the United States’ racial hierarchy (e.g., Massey & Denton 1993), they may exceed some individuals’ ability to cope and respond effectively (Sanders-Phillips et al. 2009).
In their systematic review, Priest and colleagues (2013) found that exposure to discrimination from birth through age 18 was linked to a range of negative mental health outcomes. Early life stress is linked to higher blood pressure, blood glucose, body mass index, and pro-inflammatory immune function in childhood and adolescence, thus elevating chronic disease risk as youth age and physiological insults accumulate (Goosby et al. 2016, Miller & Chen 2010). In children as young as 9 or 10 years old, exposure to discrimination is associated with elevated blood pressure and higher inflammatory markers (Goosby et al. 2015) as well as flatter diurnal cortisol curves (Martin et al. 2012). Moreover, a number of studies now document genomic (i.e., GxE, epigenetics, telomere length, etc.) correlates of social experiences disproportionally experienced by African American children that may modify stress-health pathways in the long term (Champagne 2018, Mitchell et al. 2017, Notterman & Mitchell 2015, Shalev et al. 2013).
Adolescence is marked by a host of interlinked physiologic and social transitions, including neural sensitivity to social exclusion (Masten et al. 2009). Motivated by the biological changes due to pubertal onset and development, youth become increasingly aware of their status in peer social hierarchies as they shift from parents to peers as their primary socializing agents (Goosby et al. 2013). They may also become aware that they inhabit highly racialized systems of oppression as they are exposed to discriminatory experiences in the expanding range of social environments their autonomy allows them to navigate (Hope et al. 2015, Morris & Perry 2016). Also during this time, adolescents may be exposed to more discrimination and become increasingly cognizant of the vicarious discrimination and microaggressions experienced by themselves, their family members, friends, peers, and others (Wickrama et al. 2017). Such experiences may add to or exacerbate their existing stress burden, increasing allostatic load earlier in life and setting the stage for morbidity and mortality inequities including obesity, hypertension, and cardiovascular disease (Goosby & Heidbrink 2013).
Studies examining African American/White differences from adolescence into adulthood suggest that the secretion of cortisol among African American adolescents is higher at bedtime and flatter during the day, indicating higher levels of stress activation (DeSantis et al. 2007). These profiles are also possible indications of reduced allostatic responding due to stress cascade modifications resulting from persistent stressor anticipation and exposure (DeSantis et al. 2007, 2015). Consequently, adolescent reports of discrimination are associated with flatter cortisol curves in adulthood (Adam et al. 2015). Importantly, diurnal cortisol curves appear to peak during days characterized by positive affect, as indicated by a steeper decline during the day (Hoyt et al. 2015). Interpersonal discrimination is also positively associated with higher allostatic load (Brody et al. 2013) and elevated pro-inflammatory cytokine markers (Brody et al. 2015), an indicator of inflammation.
Adulthood
Adulthood is generally the time when illness manifests. Earlier life course stages set up the health patterns during adulthood, altering aspects of CNS social processing, moderating health physiology and genomic mechanisms, and accumulating wear and tear. Stressors broaden and deepen with age, social roles become more complicated, and family and other interdependencies become more crucial. Given these many challenges, early adversity sets the individual baseline upon which the unique stressors of adulthood continue building.
Emerging adulthood.
Emerging adulthood (∼ages 18–29) involves various transitions or turning points, including education, employment, parenthood, and marriage, all of which can transpire in the context of interpersonal discrimination (Hope et al. 2015). Such disruptive exposures can exacerbate the existing stress burden associated with normative transitions during this period. Indeed, the African American/White allostatic load gap is already pronounced at ages 18–24 and continues to widen through middle age (Geronimus et al. 2006). In one functional magnetic resonance imaging (fMRI) study on discrimination, adults in this age range showed greater social pain–related CNS activity and reduced neural activity associated with emotion regulation in response to negative social treatment, though discrimination was associated with lower social pain but greater regulatory CNS activity (Masten et al. 2010).
Important experimental laboratory studies have demonstrated that exposure to discrimination for African American college students is linked to ANS and SNS responses. Blood pressure, an indicator of SNS activation, is positively associated with discrimination in individuals with lower Afrocentric orientation relative to college students with stronger Afrocentric orientation (Neblett & Carter 2012). Perceived discrimination among African American (but not White) college students is linked to lower heart rate variability, an indicator of SNS-PSNS modulation and a cardiovascular risk factor (Hill et al. 2017, Williams et al. 2017). These laboratory studies provide important clues regarding the physiological load accumulated by minority college students in predominantly White spaces.
As they transition into parental roles, African Americans must consider their children’s experiences with race-related stressors such as discriminatory experiences in schools and with law enforcement (Dow 2016). Though little is known about how these worries affect parents’ health, there is evidence that such conditions can lead to psychological stress and rumination (Murry et al. 2001). Indeed, according to one estimate among college-educated adults, African Americans’ allostatic load levels are 32% higher than those of comparable Whites (Howard & Sparks 2015). However, it is not clear how much of this disparity is due to the unique contributions of parenting stress, to the high probability of contacts with Whites for this group of relatively advantaged African Americans (i.e., interpersonal discrimination), and to other factors (i.e., structural or intrapersonal racism, behaviors, etc.).
Middle age through senescence.
By middle age, African American adults show numerous signs of accelerated aging. For example, as measured by telomere length, African American women aged 49 to 55 are estimated to be 7.5 years biologically older than SES-comparable White counterparts (Geronimus et al. 2010). There is recent evidence indicating that African American adults aged 51 and older who reported the highest lifetime discrimination exposures had shorter telomere lengths relative to those who reported low to moderate levels of lifetime discrimination (Lee et al. 2017). Discrimination among middle-age and older African Americans is also associated with other health-related markers predictive of chronic stress–related conditions such as diabetes, heart disease, and stroke. Discrimination is associated with higher levels of the inflammatory marker C-reactive protein (Lewis et al. 2010), elevated stress hormones (Fuller-Rowell et al. 2012), abdominal/visceral fat (Lewis et al. 2011), high blood pressure (Lewis et al. 2009), oxidative stress (Szanton et al. 2012), and coronary artery calcification (Everage et al. 2012). These outcomes are important to note given the acute differences in health outcomes between African Americans and Whites during middle age, and they likely reflect patterns of wear and tear accumulation over many years.
African American adults have persistently higher allostatic load relative to Whites until they reach age 60–65, at which point such disparities appear to reduce in magnitude, perhaps due to mortality selection (Levine & Crimmins 2014). When comparing the allostatic load levels of African American and White adults, Duru and colleagues (2012) found evidence that the disparities in diabetes- and cardiovascular-related mortality were partially explained by allostatic load, and these differences were independent of SES (Duru et al. 2012). These findings are significant given that African Americans are also more likely to experience earlier onset of age-related chronic diseases and fatal chronic conditions (Levine & Crimmins 2014). In fact, 28% of cardiovascular deaths among African Americans occur at less than 65 years of age compared to 13% for Whites, a difference that persists after controlling for SES ( Jolly et al. 2010).
It is important to recognize the intersecting life-course pathways that come to shape African American health disparities, including factors such as improved SES that may lead to additional race-related stressors. Indeed, a recent study using the 1979 National Longitudinal Survey of Youth (NLSY79) showed both that African Americans reported higher rates of discrimination as they moved up the socioeconomic ladder relative to their SES-stable counterparts and that the high rates of discrimination explained the racial disparity in health outcomes among upwardly mobile adults (Colen et al. 2018). Additionally, an important and understudied area requiring more attention is the role of death and bereavement as an extension of systemic racial inequality that interpersonal discrimination likely contributes to. African American health inequities contribute to the likelihood of experiencing the loss of multiple loved ones over the life course, which is a traumatic and acute stressor that appears to exacerbate individual and intergenerational health risks within African American families (Umberson et al. 2017).
FUTURE ISSUES
In this review we have emphasized stress-related processes modulated by experiences of interpersonal discrimination, so we now focus on promising directions within this domain. There remain a number of important avenues for continuing to illuminate the harsh inequities of life in a racialized social system. We have focused on African Americans in this review, but research on other marginalized social groups exists (though substantially smaller), and at a time of increased aggression and hostility toward people of color, immigrants, sexual minorities, and people of non-Christian faiths, more research is needed. In this final section, we discuss important developments emerging across a variety of disciplines that hold promise for better measurement of racial inequality and the dynamic, adaptive biosocial pathways through which social conditions shape health trajectories. Rather than reifying disciplinary boundaries, we see great value in incorporating biological assessments into sociological research.
Neurobiology
We began our review of biosocial mechanisms noting the importance of the CNS as the mediator of social experience and regulator of SNS/SAM and HPA axis activity. Neurosociological progress since Massey (2002) in his 2001 presidential address to the American Sociological Association introduced the limbic system and its role in cognition has been limited. That is not to say, however, that there has been no progress. Theorists have grappled with the role of social encounters in positive emotions and motivation (Collins 2014) and with the relationships between emotions and neurobiology for social organization (Turner 2007). A more recent wave of scholars is becoming involved in neuroscience research using brain imaging to answer sociological questions (Kalkhoff et al. 2016; Kiat & Cheadle 2017; Kiat et al. 2016, 2017; Melamed et al. 2017). At the same time, there is a growing interest in combining demographic population perspectives with neuroscientific frameworks (Falk et al. 2013). We have argued that because the brain is the key mediator of both experience and physiological regulation (McEwen 1998, Sterling 2012), a deeper understanding of it is necessary for broadly conceptualizing stress-related and experiential emotion-based processes. Such understandings are likely important for genetic and some genomic pathways, because many pathways that are considered sociologically relevant (e.g., 5-HTTLPR) underlie neurobiological systems. Imaging technologies may, in the future, shed light on how discrimination affects structural and functional neural network connectivity and may thereby illuminate important aspects of social and emotional processing. Currently, the neuroscience of discrimination exposure is in its infancy if compared to general experiences of social exclusion, and more work is needed to determine how these experiences influence the brain to modify the ways individuals experience and process complex social environments and interactions (Kiat et al. 2016, Masten et al. 2010). The brain is the organ that decodes and responds to social experiences and is an important new frontier for sociological researchers.
Sensor-Based Health Measurement
The knowledge base for understanding the basic biological pathways supporting health has developed rapidly and continues to do so. New assays and technologies will continue to provide greater insight into specific mechanisms within different systems as the utility of saliva, blood, and other tissues continues to expand. Whereas biological researchers seek to peer into different processes in the body, sociologists often seek more global indicators and indexes, for example when they use multiple biomarkers to estimate allostatic load. Although many of the biological underpinnings already in place have utility in sociological research, looking forward sociologists may begin considering noninvasive biosignals as compared to, or for use in conjunction with, biomarkers. Rapid technological development is quickly expanding the nature and type of tools available to researchers thanks to the tremendous diffusion of smartphones and wearable devices as technology companies seek to capitalize on these markets.
The tools of ambulatory research (Trull & Ebner-Priemer 2014), such as ecological momentary assessment, promise to more accurately characterize the nature of discriminatory experiences, their frequencies, and the ways they are experienced. Discrimination research has mostly relied on scales with item categories that provide only gross estimates of exposure rates. Cell phone frameworks are now available for passive data collection (Ferreira et al. 2015), along with applications for collecting real-time social contact networks within which individuals’ social experiences can be situated (Fournet & Barrat 2014). Wearable devices are also receiving a great deal of attention, with consumers using a range of sensors to gather data about themselves with the goal of informing their health decisions (Swan 2013). Health assessment devices currently use signals measured on the body, such as photoplethysmography (PPG) sensors for heart rate (Lu et al. 2009), changes in skin conductance due to SNS activity (Boucsein 2012), and accelerometers (Goosby et al. 2018). These approaches provide detailed information on aspects of the CNS, the PSNS, and physical activity and sleep. Taken together, this engineering-based side of biological assessment holds promise for monitoring emotional lability, activity and sleep recovery, social contacts, and high-frequency participant reports on these and other factors. The timescales of some of these processes, sometimes of the order of seconds, can be much finer than they are for traditional biomarkers, which can span minutes or years. However, these measures assess state-based allostatic processes and will need to be linked to longer-term measures of trait-based traditional biomarkers of allostatic load to clarify how dynamic dimensions of interpersonal discrimination are associated with health biology to form biosocial signals of sociological significance.
Genomics
As described above, the vast majority of work linking genetics to health outcomes has been conducted on White European-ancestry populations (Need & Goldstein 2009, Popejoy & Fullerton 2016), and in their current state many of these genetic correlates do not perform as well in non-European-ancestry populations (Ware et al. 2017). Nevertheless, as more African and other non-European genetic work is conducted, the CNS underpinnings of social exclusion sensitivity and vigilance, HPA reactivity, and behavioral or health outcomes may help detect the effects of discrimination by controlling how genes and experiences interact to regulate the risks of poor health outcomes. Discrimination may, like other social stressors, be moderated by genotype (i.e., by GxE). Finding those GxE will be challenging, and understanding the stress dynamics by which interpersonal discrimination affects health-related processes may require greater use of molecular approaches such as epigenetics and gene expression. Further, in nearly all genomic research, ancestry will continue to be an incredibly complex confounder. Ancestry is strongly correlated with genotype frequency, and it may be strongly correlated with, but not be the same as, socially ascribed race (Guo et al. 2014), which is what typically defines health inequities. Extremely careful and thoughtful research is needed to disentangle GxE factors and explain how discrimination interacts with the body to dysregulate physiological systems and increase allostatic load, thereby becoming embodied.
The relatively recent use of genomic mechanisms in stress—and especially discrimination—research has not afforded sufficient time to fully determine which measures are robust biological mechanisms and which are symptoms of health decline. For example, is telomere shortening associated with discriminatory stress resulting in higher rates of cancer, or is telomere shortening simply a summary of chronic systemic dysregulation (especially at younger ages)? Work in the next decade will likely begin by separating the extent to which these measures are acting as mechanisms or biomarkers. This distinction almost certainly varies by stressor and health outcome combination. Biosocial researchers are finally harnessing the necessary longitudinal data in non-White samples to address these types of questions with respect to the unique constellations of stressors experienced by non-European-ancestry populations (see Popejoy & Fullerton 2016), and such research is supported by a recent NIH funding opportunity for social epigenetics research on minority health and health disparities. The models resulting from this research will provide new opportunities for understanding how social and genetic factors interact to shape complex behavioral phenotypes and disease susceptibility.
Training
As biological data expand into sociology, there is a tremendous need for collaboration between biological scientists and health inequality and discrimination researchers across a range of increasingly relevant fields, such as computer science and engineering, molecular biology, immunology, and neuroscience. Biosocial problems are hard problems. Scholars in these areas will continue to explore the links between stress and health alongside their colleagues in the social sciences, and it is worth considering how this research will benefit from the inclusion of sociologists in inter-disciplinary teams. Sociological perspectives that emphasize the role of discrimination at multiple levels of social organization have much to offer because they inherently recognize that discrimination today reflects ongoing historical processes whose roots spread deep and wide within our culture. In the same way that sociologists are unfamiliar with the complexity of biological systems, biologists and health scientists tend to be reductionist with respect to social context. Furthermore, the sociologists who study different facets of discrimination themselves come from diverse backgrounds, experiences, and perspectives. In particular, just as the biological data have often been examined in White or European-ancestry samples, the vast majority of researchers in this area are White. We strongly encourage scholars of color to lend their experience, knowledge, and skills to this work, and we believe that broad and inclusive participation will help protect the future of biosocial science from the mistakes of the past. In short, biosocial work—even concerning discrimination—will continue to expand rapidly, and we argue it will be far more consequential and accurate if sociologists are significantly involved.
CONCLUSION
This review has focused on an important dimension of the United States’ racialized social system: the role of interpersonal discrimination and how this social experience becomes embodied. Through stress process mechanisms, social experiences are translated into short-term physiological states; over time, through learning processes and repeated experiences, the allostatic modulation of physiological states accumulates allostatic load and potentially modifies and is modified by genomic processes. The health inequities experienced by African Americans over the life course begin at birth, when reproductive processes program the newborn for the stressful environment experienced by the mother, whose level of physiological stress is the result of a life-long adaptation process. Although we have pointed to some promising directions for future research, other scholars would almost certainly have pointed in other directions and emphasized discrimination at other levels of social organization. The mechanisms conducive to poor health are many, and large-scale patterns of racial inequity have long been embedded in different facets of the racist social organization of the United States. However, even if we strip away the broad macro-structural patterns of inequality in the United States and focus instead on the systemic yet small-scale interpersonal interactions in which discrimination is enacted face-to-face, differential treatment via exclusionary acts has large-scale consequences for population health.
SUMMARY POINTS.
The majority of African Americans believe interpersonal discrimination is an important social issue.
Interpersonal racism is enacted through discrimination, a form of social exclusion that is processed in the brain as social pain in the same regions associated with the emotional components of physical pain.
Activity in the neural regions that process social exclusion is associated with increased SNS and HPA axis activity.
SNS and HPA activity increase the risk of cardiometabolic conditions and cardiovascular wear and tear.
Genomics can be an important analytical tool to investigate the interactions between experiences of racial inequality and life-course health outcomes.
Discrimination against African Americans is associated with adverse birth outcomes, hypertension, abdominal obesity, cardiovascular disease, and other associated morbidities over the life course.
In the future, sociologists are encouraged to integrate neuroscience, genomics, and new health technologies; to engage in interdisciplinary collaboration; and to diversify the pool of scholars engaged in biosocial health inequities research.
ACKNOWLEDGMENTS
The authors would like to thank Doug Massey and the anonymous reviewer for their helpful comments on earlier drafts.
Glossary
- Amygdala
a brain region serving several functions, including processing basic threats (including physical pain and social rejection), memory, decision making, and emotions
- Dorsal anterior cingulate cortex (dACC)
a subdivision of the anterior cingulate cortex involved in appraising social experiences, including social pain, physical pain, and reward-based learning and memory
- Anterior insula (AI)
a limbic-related cortex involved in processing norm violations, emotions, empathy, social decision making, interoceptive awareness, and pain
- Periaqueductal gray (PAG)
the primary control center for descending pain modulation
- Dorsomedial prefrontal cortex (DMPFC)
a brain region involved in creating a sense of the self and of the mental states of others (“theory of mind”) and in processing social threat and exclusion
- Ventromedial prefrontal cortex (VMPFC)
a brain region involved in social decision making and emotion regulation
- Posterior cingulate cortex (PCC)
a brain region highly interconnected with a wide range of intrinsic control networks, including the processing of emotions and memory
- Intrinsic functional networks
widespread brain regions that are functionally interconnected when processing task demands
- Salience network
network involved in detecting and filtering salient stimuli, with anatomical connections important for autonomic nervous system and hormonal modulation
- Mentalizing network
a network involved in understanding the mental states of self and others that guide or are responsible for overt behavior
- Central executive network
a network subserving basic cognitive processes such as attentional control, cognitive inhibition, inhibitory control, working memory, and cognitive flexibility
- Central nervous system (CNS)
the part of the nervous system containing the brain and the spinal cord
- Peripheral nervous system (PNS)
the nerves and ganglia outside the brain and spinal cord that relay information from the CNS to the rest of the body
- Autonomic nervous system (ANS)
a largely unconscious PNS subsystem regulating bodily functions such as heart rate, digestion, respiratory rate, pupillary response, urination, and sexual arousal
- Sympathetic nervous system (SNS)
the fight-or-flight subsystem of the ANS that accelerates the heart rate, constricts blood vessels, and raises blood pressure
- Parasympathetic nervous system (PSNS)
a subdivision of the ANS that serves to slow the heart rate, increase intestinal and glandular activity, and relax the sphincter muscles
- Sympathetic-adrenal-medullary (SAM) system
hypothalamus-mediated stress response system that controls adrenaline and noradrenaline release, upregulating the SNS and downregulating the PSNS
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, et al. 2015. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: a 20-year prospective study. Psychoneuroendocrinology 62:279–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MA, Slopen N, Williams DR. 2013. Cumulative psychological stress and cardiovascular disease risk: a focused review with consideration of black-white disparities. Curr. Cardiovasc. Risk Rep. 7:318–25 [Google Scholar]
- Averhart CJ, Bigler RS. 1997. Shades of meaning: skin tone, racial attitudes, and constructive memory in African American children. J. Exp. Child Psychol. 67:363–88 [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. 2013. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 23:361–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Caspi A. 2013. Genetics in population health science: strategies and opportunities. Am. J. Public Health 103:S73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley AR, Callier S, Rotimi CN. 2017. Diversity and inclusion in genomic research: why the uneven progress? J. Community Genet. 8:255–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. 2003. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun. 17:350–64 [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC. 2012. Child development in the context of adversity: experiential canalization of brain and behavior. Am. Psychol. 67:309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Silva E. 2015. The structure of racism in color-blind, “post-racial” America. Am. Behav. Sci. 59:1358–76 [Google Scholar]
- Bonilla-Silva E. 2017. Racism Without Racists: Color-Blind Racism and the Persistence of Racial Inequality in America. Lanham, MD: Rowman & Littlefield [Google Scholar]
- Bosch JA, De Geus EJC, Carroll D, Goedhart AD, Anane LA, et al. 2009. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom. Med. 71(8):877–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein W. 2012. Electrodermal Activity. New York: Springer [Google Scholar]
- Branch CW, Newcombe N. 1986. Racial attitude development among young black children as a function of parental attitudes: a longitudinal and cross-sectional study. Child Dev. 57:712–21 [Google Scholar]
- Brody GH, Miller GE, Yu T, Beach SRH, Chen E. 2016. Supportive family environments ameliorate the link between racial discrimination and epigenetic aging. Psychol. Sci. 27:530–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen Y-F, Kogan SM, Evans GW, et al. 2013. Cumulative socioeconomic status risk, allostatic load, and adjustment: a prospective latent profile analysis with contextual and genetic protective factors. Dev. Psychol. 49(5):913–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Miller GE, Chen E. 2015. Discrimination, racial identity, and cytokine levels among African-American adolescents. J. Adolesc. Health 56:496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo E, Rieppi R, Kelly KP, Gerin W. 2003. Perceived racism and blood pressure: a review of the literature and conceptual and methodological critique. Ann. Behav. Med. 25:55–65 [DOI] [PubMed] [Google Scholar]
- Brotman DJ, Golden SH, Wittstein IS. 2007. The cardiovascular toll of stress. Lancet 370:1089–100 [DOI] [PubMed] [Google Scholar]
- Canli T, Wen R, Wang X, Mikhailik A, Yu L, et al. 2017. Differential transcriptome expression in human nucleus accumbens as a function of loneliness. Mol. Psychiatry 22(7):1069–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Cent. Dis. Control Prev.). 2005. Racial/ethnic and socioeconomic disparities in multiple risk factors for heart disease and stroke–United States, 2003. Morb. Mortal. Wkly. Rep. 54:113–17 [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, et al. 2014. Discrimination, racial bias, and telomere length in African-American men. Am. J. Prev. Med. 46:103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. 2010. Epigenetic influence of social experiences across the lifespan. Dev. Psychobiol. 52:299–311 [DOI] [PubMed] [Google Scholar]
- Champagne FA. 2018. Social and behavioral epigenetics: evolving perspectives on nature-nurture interplay, plasticity, and inheritance. In The Palgrave Handbook of Biology and Society, ed. Meloni M, Cromby J, Fitzgerald D, Lloyd S, pp. 227–50. New York: Springer [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. 2007. Psychological stress and disease. JAMA 298:1685–87 [DOI] [PubMed] [Google Scholar]
- Cole SW. 2013. Social regulation of human gene expression: mechanisms and implications for public health. Am. J. Public Health 103:S84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen CG. 2011. Addressing racial disparities in health using life course perspectives. Du Bois Rev.: Soc. Sci. Res. Race 8:79–94 [Google Scholar]
- Colen CG, Ramey DM, Cooksey EC, Williams DR. 2018. Racial disparities in health among nonpoor African Americans and Hispanics: the role of acute and chronic discrimination. Soc. Sci. Med. 199:167–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JW Jr., David RJ. 2009. Racial disparity in low birth weight and infant mortality. Clin. Perinatol. 36:63–73 [DOI] [PubMed] [Google Scholar]
- Collins R. 2014. Interaction Ritual Chains. Princeton, NJ: Princeton Univ. Press [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, et al. 2003. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126:2139–52 [DOI] [PubMed] [Google Scholar]
- David RJ, Collins JW. 1997. Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. N. Engl. J. Med. 337:1209–14 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, et al. 2009. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 1293:49–60 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. 2006. Extending animal models of fear conditioning to humans. Biol. Psychol. 73:39–48 [DOI] [PubMed] [Google Scholar]
- Desantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. 2007. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J. Adolesc. Health 41:3–13 [DOI] [PubMed] [Google Scholar]
- Desantis AS, Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. 2015. Racial and ethnic differences in diurnal cortisol rhythms: Are they consistent over time? Psychosom. Med. 77:6–15 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny M. 2009. Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychol. Sci. 20:1237–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow DM. 2016. The deadly challenges of raising African American boys. Gend. Soc. 30:161–88 [Google Scholar]
- Durkheim E. 1951. Suicide. New York: Free Press [Google Scholar]
- Duru OK, Harawa NT, Kermah D, Norris KC. 2012. Allostatic load burden and racial disparities in mortality. J. Natl. Med. Assoc. 104:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duster T. 2015. A post-genomic surprise: the molecular reinscription of race in science, law and medicine. Br. J. Sociol 66:1–27 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. 2012. The neural bases of social pain: evidence for shared representations with physical pain. Psychosom. Med. 74:126–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. 2013. Social ties and health: a social neuroscience perspective. Curr. Opin. Neurobiol. 23:407–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. 2004. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci. 8:294–300 [DOI] [PubMed] [Google Scholar]
- Everage NJ, Gjelsvik A, Mcgarvey ST, Linkletter CD, Loucks EB. 2012. Inverse associations between perceived racism and coronary artery calcification. Ann. Epidemiol. 22:183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R, et al. 2013. What is a representative brain? Neuroscience meets population science. Proc. Natl. Acad. Sci. 110:17615–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D, Kostakos V, Dey AK. 2015. AWARE: mobile context instrumentation framework. Front. ICT 2:6 Fournet J, Barrat A. 2014. Contact patterns among high school students. PLOS ONE 9:e107878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese J. 2008. Genetics and the social science explanation of individual outcomes. Am. J. Sociol. 114:S1–35 [DOI] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Doan SN, Eccles JS. 2012. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology 37:107–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. 2006. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 96:826–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. 2010. Do US black women experience stress-related accelerated biological aging? A novel theory and first population-based test of black-white differences in telomere length. Hum. Nat. 21:19–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, Mcatee MJ. 2006. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc. Sci. Med. 62:1650–71 [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJP. 2001. Fetal programming and adult health. Public Health Nutr. 4:611–24 [DOI] [PubMed] [Google Scholar]
- Goff PA, Jackson MC, Di Leone BA, Culotta CM, Ditomasso NA. 2014. The essence of innocence: consequences of dehumanizing Black children. J. Pers. Soc. Psychol. 106:526–45 [DOI] [PubMed] [Google Scholar]
- Goosby BJ, Bellatorre A, Walsemann KM, Cheadle JE. 2013. Adolescent loneliness and health in early adult- hood. Sociol. Inq. 83:505–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Cheadle JE, McDade T. 2016. Birth weight, early life course BMI, and body size change: chains of risk to adult inflammation? Soc. Sci. Med. 148:102–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Cheadle JE, Strong-Bak W, Roth T, Nelson TD. 2018. Perceived discrimination and adolescent sleep in a community sample. Russell Sage J. Soc. Sci. 4(4):43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Heidbrink C. 2013. The transgenerational consequences of discrimination on African-American health outcomes: discrimination and health. Sociol. Compass 7:630–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Malone S, Richardson EA, Cheadle JE, Williams DT. 2015. Perceived discrimination and markers of cardiovascular risk among low-income African American youth. Am. J. Hum. Biol. 27:546–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Fu Y, Lee H, Cai T, Mullan Harris K, Li Y. 2014. Genetic bio-ancestry and social construction of racial classification in social surveys in the contemporary United States. Demography 51:141–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsel A, von Känel R. 2008. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosocial Med. 2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LK, Hoggard LS, Richmond AS, Gray DL, Williams DP, Thayer JF. 2017. Examining the association between perceived discrimination and heart rate variability in African Americans. Cult. Divers. Ethn. Minor. Psychol. 23:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmert CJ, Dominguez TP, Schetter CD, Srinivas SK, Glynn LM, et al. 2014. Lifetime racism and blood pressure changes during pregnancy: implications for fetal growth. Health Psychol. 33:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmert CJ, Schetter CD, Dominguez TP, Abdou C, Hobel CJ, et al. 2008. Stress and blood pressure during pregnancy: racial differences and associations with birthweight. Psychosom. Med. 70:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope EC, Hoggard LS, Thomas A. 2015. Emerging into adulthood in the face of racial discrimination: physiological, psychological, and sociopolitical consequences for African American youth. Transl. Issues Psychol. Sci. 1:342–51 [Google Scholar]
- Horvath S. 2013. DNA methylation age of human tissues and cell types. Genome Biol. 14(10):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Umberson D, Landis KR. 1988. Structures and processes of social support. Annu. Rev. Sociol. 14:293–318 [Google Scholar]
- Howard JT, Sparks PJ. 2015. The role of education in explaining racial/ethnic allostatic load differentials in the United States. Biodemogr. Soc. Biol. 61:18–39 [DOI] [PubMed] [Google Scholar]
- Hoyt LT, Craske MG, Mineka S, Adam EK. 2015. Positive and negative affect and arousal: cross-sectional and longitudinal associations with adolescent cortisol diurnal rhythms. Psychosom. Med. 77:392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JS, Knight KM, Rafferty JA. 2010. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am. J. Public Health 100:933–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S, Vittinghoff E, Chattopadhyay A, Bibbins-Domingo K. 2010. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. Am. J. Med. 123:811–18 [DOI] [PubMed] [Google Scholar]
- Jones CW, Gambala C, Esteves KC, Wallace M, Schlesinger R, et al. 2017. Differences in placental telomere length suggest a link between racial disparities in birth outcomes and cellular aging. Am. J. Obstet. Gynecol. 216:294.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoff W, Serpe RT, Pollock J, Miller B, Pfeiffer M. 2016. Neural processing of identity-relevant feedback. In New Directions in Identity Theory and Research, ed. Stets JE, Serpe RT, pp. 195–238. New York: Oxford Univ. Press [Google Scholar]
- Kiat JE, Cheadle JE. 2017. The impact of individuation on the bases of human empathic responding. NeuroImage 155:312–21 [DOI] [PubMed] [Google Scholar]
- Kiat JE, Straley E, Cheadle JE. 2016. Escalating risk and the moderating effect of resistance to peer influence on the P200 and feedback-related negativity. Soc. Cogn. Affect. Neurosci. 11:377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiat JE, Straley E, Cheadle JE. 2017. Why won’t they sit with me? An exploratory investigation of stereotyped cues, social exclusion, and the P3b. Soc. Neurosci. 12:612–25 [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Sweet E. 2009. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am. J. Hum. Biol. 21:2–15 [DOI] [PubMed] [Google Scholar]
- Lee DB, Kim ES, Neblett EW. 2017. The link between discrimination and telomere length in African American adults. Health Psychol. 36:458–67 [DOI] [PubMed] [Google Scholar]
- Levine ME, Crimmins EM. 2014. Evidence of accelerated aging among African Americans and its implications for mortality. Soc. Sci. Med. 118:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. 2010. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav. Immun. 24:438–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Barnes LL, Bienias JL, Lackland DT, Evans DA, Mendes De Leon CF. 2009. Perceived discrimination and blood pressure in older African American and white adults. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 64A:1002–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, Williams DR. 2015. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu. Rev. Clin. Psychol. 11:407–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Kravitz HM, Janssen I, Powell LH. 2011. Self-reported experiences of discrimination and visceral fat in middle-aged African-American and Caucasian women. Am. J. Epidemiol. 173:1223–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Yang F, Taylor JA, Stein JF. 2009. A comparison of photoplethysmography and ECG recording to analyse heart rate variability in healthy subjects. J. Med. Eng. Technol. 33:634–41 [DOI] [PubMed] [Google Scholar]
- Manuck SB, McCaffery JM. 2014. Gene-environment interaction. Annu. Rev. Psychol. 65:41–70 [DOI] [PubMed] [Google Scholar]
- Márquez-Luna C, Loh PR, Price AL. 2017. Multiethnic polygenic risk scores improve risk prediction in diverse populations. Genet. Epidemiol. 41:811–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CG, Bruce J, Fisher PA. 2012. Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: the role of parental psychosocial risk and monitoring. Horm. Behav. 61:661–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey DS. 2002. A brief history of human society: the origin and role of emotion in social life. Am. Sociol. Rev. 67:1–29 [Google Scholar]
- Massey DS, Denton NA. 1993. American Apartheid: Segregation and the Making of the Underclass. Cambridge, MA: Harvard Univ. Press [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, et al. 2009. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 4:143–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI. 2010. An fMRI investigation of attributing negative social treatment to racial discrimination. J. Cogn. Neurosci. 23:1042–51 [DOI] [PubMed] [Google Scholar]
- McEwen BS. 1998. Stress, adaptation, and disease: allostasis and allostatic load. Ann. N. Y. Acad. Sci. 840:33–44 [DOI] [PubMed] [Google Scholar]
- McEwen BS. 2003. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism 52:10–16 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. 2010. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 1186:190–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D, Kalkhoff W, Han S, Li X. 2017. The neural bases of status-based influence. Socius 3:1–10 [Google Scholar]
- Miller GE, Chen E. 2010. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol. Sci. 21:848–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. 2018. The genetics of human behavior: a hopeless opus? In The Oxford Handbook of Evolution, Biology, and Society, ed. Hopcraft RL, pp. 221–40. New York: Oxford Univ. Press [Google Scholar]
- Mitchell C, McLanahan S, Brooks-Gunn J, Garfinkel I, Hobcraft J, Notterman D. 2013. Genetic differential sensitivity to social environments: implications for research. Am. J. Public Health 103:S102–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, McLanahan S, Schneper L, Garfinkel I, Brooks-Gunn J, Notterman D. 2017. Father loss and child telomere length. Pediatrics 140(2):e20163245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Schneper LM, Notterman DA. 2015. DNA methylation, early life environment, and health outcomes. Pediatr. Res. 79:212–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SR, Depue RA. 2016. Neurobehavioral foundation of environmental reactivity. Psychol. Bull. 142:107– 64 [DOI] [PubMed] [Google Scholar]
- Morris EW, Perry BL. 2016. The punishment gap: school suspension and racial disparities in achievement. Soc. Probl. 63:68–86 [Google Scholar]
- Murry VM, Brown PA, Brody GH, Cutrona CE, Simons RL. 2001. Racial discrimination as a moderator of the links among stress, maternal psychological functioning, and family relationships. J. Marriage Fam. 63:915–26 [Google Scholar]
- Neblett EW, Carter SE. 2012. The protective role of racial identity and Afrocentric worldview in the association between racial discrimination and blood pressure. Psychosom. Med. 74:509–16 [DOI] [PubMed] [Google Scholar]
- Need AC, Goldstein DB. 2009. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 25:489–94 [DOI] [PubMed] [Google Scholar]
- Nobili V, Alisi A, Panera N, Agostoni C. 2008. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr. Endocrinol. Rev. 6:241–47 [PubMed] [Google Scholar]
- Notterman DA, Mitchell C. 2015. Epigenetics and understanding the impact of social determinants of health. Pediatr. Clin. N. Am. 62:1227–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NPR, Robert Wood Johnson Found., Harvard T.H. Chan Sch. Public Health. 2017. Discrimination in America: experiences and views of African Americans. Rep, Oct. https://www.npr.org/assets/img/2017/10/23/discriminationpoll-african-americans.pdf [Google Scholar]
- Paradies Y. 2006. A systematic review of empirical research on self-reported racism and health. Int. J. Epidemiol. 35:888–901 [DOI] [PubMed] [Google Scholar]
- Paradies Y, Ben J, Denson N, Elias A, Priest N, et al. 2015. Racism as a determinant of health: a systematic review and meta-analysis. PLOS ONE 10:e0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe EA, Smart Richman L. 2009. Perceived discrimination and health: a meta-analytic review. Psychol. Bull. 135:531–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI. 1999. The stress process revisited. In Handbook of the Sociology of Mental Health, ed. Aneshensel CS, Phelan JC, pp. 395–415. New York: Springer [Google Scholar]
- Pearlin LI. 2010. The life course and the stress process: some conceptual comparisons. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 65B:207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BL, Morris EW. 2014. Suspending progress: collateral consequences of exclusionary punishment in public schools. Am. Sociol. Rev. 79(6):1067–87 [Google Scholar]
- Pescosolido BA, Martin JK. 2015. The stigma complex. Annu. Rev. Sociol. 41:87–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JC, Link BG. 2015. Is racism a fundamental cause of inequalities in health? Annu. Rev. Sociol. 41:311–30 [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, Ledoux JE. 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43:897–905 [DOI] [PubMed] [Google Scholar]
- Pluess M. 2015. Individual differences in environmental sensitivity. Child Dev. Perspect. 9:138–43 [Google Scholar]
- Popejoy AB, Fullerton SM. 2016. Genomics is failing on diversity. Nature 538:161–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, et al. 2016. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc. Natl. Acad. Sci. 113:5119–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest N, Paradies Y, Trenerry B, Truong M, Karlsen S, Kelly Y. 2013. A systematic review of studies examining the relationship between reported racism and health and wellbeing for children and young people. Soc. Sci. Med. 95:115–27 [DOI] [PubMed] [Google Scholar]
- Priest N, Williams DR. 2018. Racial discrimination and disparities in health. In The Oxford Handbook of Stigma, Discrimination, and Health, ed. Major B, Dovidio JF, Link BG, pp. 163–82. New York: Oxford Univ. Press [Google Scholar]
- Reskin B. 2012. The race discrimination system. Annu. Rev. Sociol. 38:17–35 [Google Scholar]
- Richman LS, Pascoe EA, Lattaner M. 2018. Interpersonal discrimination and physical health. In Oxford Handbook of Stigma, Discrimination, and Health, ed. Major B, Dovidio JF, Link BG, pp. 203–18. New York: Oxford Univ. Press [Google Scholar]
- Saban KL, Mathews HL, Devon HA, Janusek LW. 2014. Epigenetics and social context: implications for disparity in cardiovascular disease. Aging Dis. 5:346–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders-Phillips K. 2009. Racial discrimination: a continuum of violence exposure for children of color. Clin. Child Fam. Psychol. Rev. 12:174–95 [DOI] [PubMed] [Google Scholar]
- Sanders-Phillips K, Settles-Reaves B, Walker D, Brownlow J. 2009. Social inequality and racial discrimination: risk factors for health disparities in children of color. Pediatrics 124:S176–86 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. 2004. Why Zebras Don’t Get Ulcers: The Acclaimed Guide to Stress, Stress-Related Diseases, and Coping. New York: Holt. 3rd ed. [Google Scholar]
- Schempf AH, Branum AM, Lukacs SL, Schoendorf KC. 2007. The contribution of preterm birth to the Black-White infant mortality gap, 1990 and 2000. Am. J. Public Health 97:1255–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. 2010. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci. 14:268–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. 2010. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann. N. Y. Acad. Sci. 1186:223–39 [DOI] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. 2013. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38:1835–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD, Fraser WD, Frasch MG, Séguin JR. 2013. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J. Perinat. Med. 41:631–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. 2003. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am. J. Clin. Nutr. 77:726–30 [DOI] [PubMed] [Google Scholar]
- Slaughter-Acey JC, Sealy-Jefferson S, Helmkamp L, Caldwell CH, Osypuk TL, et al. 2016. Racism in the form of micro aggressions and the risk of preterm birth among black women. Ann. Epidemiol. 26(1):7–13.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. 2013. The emerging field of human social genomics. Clin. Psychol. Sci. 1:331–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. 2012. Allostasis: a model of predictive regulation. Physiol. Behav. 106:5–15 [DOI] [PubMed] [Google Scholar]
- Sue DW, Capodilupo CM, Torino GC, Bucceri JM, Holder AMB, et al. 2007. Racial microaggressions in everyday life: implications for clinical practice. Am. Psychol. 62:271–86 [DOI] [PubMed] [Google Scholar]
- Sullivan S. 2013. Inheriting racist disparities in health: epigenetics and the transgenerational effects of white racism. Crit. Philos. Race 1:190–218 [Google Scholar]
- Swan M. 2013. The quantified self: fundamental disruption in big data science and biological discovery. Big Data 1:85–99 [DOI] [PubMed] [Google Scholar]
- Szanton SL, Rifkind JM, Mohanty JG, Miller ER, Thorpe RJ, et al. 2012. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int. J. Behav. Med. 19:489–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer ZM, Kuzawa CW. 2015. Ethnic discrimination predicts poor self-rated health and cortisol in pregnancy: insights from New Zealand. Soc. Sci. Med. 128:36–42 [DOI] [PubMed] [Google Scholar]
- Trull TJ, Ebner-Priemer U. 2014. The role of ambulatory assessment in psychological science. Curr. Dir. Psychol. Sci. 23:466–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JH. 2007. Human Emotions: A Sociological Theory. New York: Routledge [Google Scholar]
- Turner RJ. 2013. Understanding health disparities: the relevance of the stress process model. Soc. Ment. Health 3:170–86 [Google Scholar]
- Umberson D, Olson JS, Crosnoe R, Liu H, Pudrovska T, Donnelly R. 2017. Death of family members as an overlooked source of racial disadvantage in the United States. Proc. Natl. Acad. Sci. 114:915–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD. 2005. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology 30:724–43 [DOI] [PubMed] [Google Scholar]
- Wager TD, Van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. 2009. Brain mediators of cardiovascular responses to social threat. Part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage 47:836–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, et al. 2005. Prefusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc. Natl. Acad. Sci. 102:17804–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware EB, Schmitz LL, Faul JD, Gard A, Mitchell C, et al. 2017. Heterogeneity in polygenic scores for common human traits. bioRxiv 106062. 10.1101/106062 [DOI] [Google Scholar]
- Wickrama KAS, O’Neal KW, Holmes C. 2017. Towards a heuristic research model linking early socioeconomic adversity and youth cumulative disease risk: an integrative review. Adolesc. Res. Rev. 2(3):161–79 [Google Scholar]
- Williams DP, Pandya KD, Hill LK, Kemp AH, Way BM, et al. 2017. Rumination moderates the association between resting high-frequency heart rate variability and perceived ethnic discrimination. J. Psychophysiol. 10.1027/0269-8803/a000201 [DOI] [Google Scholar]
- Williams DR, Mohammed SA. 2009. Discrimination and racial disparities in health: evidence and needed research. J. Behav. Med. 32:20–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang Y, Huang ES. 2009. Changes in racial/ethnic disparities in the prevalence of type 2 diabetes by obesity level among US adults. Ethn. Health 14(5):439–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi T, Patterson EJ, Steward QT. 2015. Race, methodology, and social construction in the genomic era. Ann. Am. Acad. Political Soc. Sci. 661:109–27 [Google Scholar]