Abstract
Background:
The reinforcing properties of nicotine play a critical role in smoking and vaping. There is a need for treatments that decrease the reinforcing properties of nicotine and thereby improve smoking and vaping rates. Dopamine plays a role in the reinforcing properties of nicotine, but little is known about the role of dopamine D2-like receptors in nicotine intake and whether there are sex differences in the effects of dopaminergic drugs on nicotine intake.
Aim:
The goal of the present studies was to investigate the effects of the D1/D2-like receptor antagonist flupentixol and the D2-like receptor antagonist L-741626 on nicotine self-administration in male and female rats.
Methods:
The effects of flupentixol and L-741626 on operant responding for nicotine and food and locomotor activity in a small open field were investigated.
Results:
There were no sex differences in baseline nicotine intake. The D1/D2-like receptor antagonist flupentixol and the D2-like receptor antagonist L-741626 decreased operant responding for nicotine. Blockade of D1/D2-like receptors and blockade of D2-like receptors also decreased operant responding for food and decreased locomotor activity. Flupentixol induced a greater decrease in operant responding for food in males than females. However, in the other tests, there were no sex differences in the effects of the dopamine receptor antagonists.
Conclusions:
Blockade of D1/D2-like receptors with flupentixol and D2-like receptors with L-741626 decreases nicotine and food intake in rats of both sexes. These compounds also decrease locomotor activity which might be indicative of a sedative effect.
Keywords: Nicotine, dopamine, dopamine receptors, self-administration, reward, rats
INTRODUCTION
Tobacco addiction is a chronic brain disorder that is characterized by compulsive smoking, nicotine withdrawal, and relapse after a period of abstinence (Bruijnzeel, 2012). Smoking increases the risk for many diseases, including cancer, heart disease, and Alzheimer’s disease (Ott et al., 1998; Ambrose and Barua, 2004; Sasco et al., 2004). Nicotine is the primary psychoactive compound in tobacco smoke that mediates the pharmacological effects of tobacco products and causes addiction to these products (Stolerman and Jarvis, 1995). Recently, electronic cigarettes (e-cigs) and other Electronic Nicotine Delivery Systems (ENDS) have been developed that deliver nicotine but not the other tobacco smoke constituents. The use of e-cigs (i.e., vaping), like smoking, leads to the development of nicotine dependence (Morean et al., 2018). Tobacco use remains common, and about 19 percent of the adults in the world smoke tobacco (WHO, 2019). A 2021 Gallup poll showed that 16 percent of US adults smoked cigarettes in the past week. Among younger Americans (18–29 yrs. of age), vaping is more common than smoking (17 percent vs. 8 percent)(Gallup, 2021).
Animal models have been developed to study the reinforcing properties of nicotine (Chellian et al., 2021a). Nicotine induces conditioned place preference in rats over a wide range of doses (Le Foll and Goldberg, 2005). Furthermore, rats rapidly acquire intravenous nicotine self-administration when nicotine intake is paired with visual cues (Bruijnzeel and Markou, 2003; Corrigall and Coen, 1989; Caggiula et al., 2002). Nicotine self-administration in rats is diminished by drugs that block central but not peripheral nicotinic acetylcholine receptors (nAChRs)(Watkins et al., 1999; DeNoble and Mele, 2006). Treatment with the nAChR antagonist mecamylamine also helps people quit smoking (Rose et al., 1998). The neurotransmitter dopamine plays a critical role in the reinforcing properties of nicotine. Dopamine mediates its effect in the brain via D1-like (D1 and D5 receptors) and D2-like (D2, D3, and D4 receptors) receptors (Bourne, 2001). Clinical studies point toward lower D2 receptor availability in the striatum of smokers (Fehr et al., 2008). Lifetime smoking and nicotine dependence are associated with low ventral striatal D2 receptor availability (Okita et al., 2016). Smoking causes a downregulation of D2-like receptors in the ventral striatum (Okita et al., 2016). On a similar note, chronic nicotine administration decreases D2 receptor levels in the nucleus accumbens in rats (Janson et al., 1992). Few pharmacological studies have investigated the role of D2-like receptors in nicotine self-administration in rats. The dopamine D2-like receptor antagonists spiperone and sulpiride decrease nicotine self-administration in adult male rats (Sorge and Clarke, 2009; Corrigall and Coen, 1991). However, besides binding to D2 receptors, spiperone also binds to serotonin receptors, and sulpiride has a high affinity for both D2 and D3 receptors (Metwally et al., 1998; Chavanon et al., 2013).
L-741626 is a more recently developed selective D2-like receptor antagonist (Grundt et al., 2007). L-741626 has a higher affinity for D2 than for D3 and D4 receptors (D2 Ki 11.2 nM, D3 Ki = 163 nM, D4 Ki = 1520 nM) (Grundt et al., 2007). In a previous study with male rats, the effects of L-741626 and the D1-like receptor antagonist SCH 39166 on cocaine self-administration were investigated (Hiranita et al., 2013). Both L-741626 and SCH 39166 caused a rightward shift in the dose-effect curve, thus indicating that these drugs decrease the reinforcing properties of cocaine. Co-administration of L-741626 and SCH 39166 has additive effects and completely blocks cocaine intake. In another study, L-741626 decreased cue-induced reinstatement of nicotine seeking in male rats (Khaled et al., 2014). However, the effects of L-741626 on nicotine self-administration in rats has not been investigated.
Previous work has provided insight into the role of D2-like receptors in the reinforcing properties of nicotine, but some questions remain unanswered. There is extensive evidence for sex differences in brain D2 receptor levels, but sex differences in the effects of D2-like receptor antagonists on nicotine intake have not been investigated (Williams et al., 2021). For example, it has been reported that women have higher D2-like receptor binding potentials in the frontal cortex than men (Kaasinen et al., 2001). In addition, young female rats have higher D2 receptor expression levels in the striatum, and young male rats have higher D2 receptor expression levels in the frontal cortex (Orendain-Jaime et al., 2016). Furthermore, studies that investigated the role of D2-like receptors in nicotine intake only used male rats (Sorge and Clarke, 2009; Corrigall and Coen, 1991). The goal of the present studies was to investigate the effects of the mixed dopamine D1/D2-like receptor antagonist flupentixol and the D2-like receptor antagonist L741626 on operant responding for nicotine and food and locomotor activity in male and female rats (Kulagowski et al., 1996; Nielsen et al., 1973). It was shown that blockade of D1/D2-like receptors and D2-like receptors reduces nicotine intake, food intake, and locomotor activity in rats of both sexes.
MATERIALS AND METHODS
Animals
Adult male and female Wistar rats (males 200–250 g, females 175–225 g, 8–9 weeks of age) were purchased from Charles River (Raleigh, NC). The rats were housed with a rat of the same sex in a climate-controlled vivarium on a reversed 12 h light-dark cycle (light off at 7 AM). Food and water were available ad libitum in the home cage except for when the rats were allowed to respond for nicotine or food. During these periods, they were fed 90–95 percent of their ad lib home cage intake. The experimental protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
Drugs
(−)-Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO) and flupentixol dihydrochloride (E/Z isomers, 60:40 ratio; Tocris bioscience, Minneapolis, MN) were dissolved in sterile saline (0.9 % sodium chloride). L-741626 (Cayman chemical company, Ann Arbor, MI) was dissolved in lactic acid (Sigma-Aldrich, St. Louis, MO), mixed in distilled water, and the pH was adjusted to 4.4 with 1M NaOH. L-741626 was administered subcutaneously (SC), and flupentixol was administered intraperitoneally (IP) in a volume of 1 ml/kg body weight. Nicotine was dissolved in sterile saline, and the rats self-administered 0.03 or 0.06 mg/kg/infusion (inf) of nicotine in a volume of 0.1 ml/inf. Nicotine and L-741626 doses are expressed as base, and flupentixol doses are expressed as salt.
Experimental design
In the first experiment, the effect of flupentixol (Expt. 1A) and L-741626 (Expt. 1B) on nicotine self-administration was investigated. In the second experiment, the effect of flupentixol (Expt. 2A) and L-741626 (Expt. 2B) on operant responding for food was investigated, and in the third experiment, the effect of flupentixol (Expt. 3A) and L-741626 (Expt. 3B) on locomotor activity in the small open field was investigated. The drug treatment schedule was the same for all three experiments (See Fig. 1 for a schematic overview of the experiments). Flupentixol was administered (IP) 30 min before the behavioral tests, and L-741626 was administered (SC) 15 min before the tests. It was also determined if flupentixol affects operant responding for nicotine and food and locomotor activity 24 and 48 h after treatment and if L-741626 affects these parameters 24 h after treatment. The drug doses were administered according to a Latin square design. The highest dose of flupentixol, 1mg/kg, was not included in the Latin square design and was administered at last. There were at least 72 h between injections with flupentixol and 48 h between injections with L-741626. The doses of L-741626 and flupentixol were based on previous studies in rats (Chaperon et al., 2003; Khaled et al., 2014; Wenzel et al., 2013; Engster et al., 2015; Veeneman et al., 2011; Hiranita et al., 2013).
Figure 1. Schematic overview of the experiment.

Experiment 1: Male and female rats were trained to respond for food pellets and then they received intravenous (IV) catheters. After nine baseline nicotine self-administration sessions the effects of flupentixol (Expt. 1A) and L-741626 (Expt. 1B) on nicotine self-administration was investigated. Experiment 2: Male and female rats were trained to respond for food pellets and the effects of flupentixol (Expt. 2A) and L-741626 (Expt. 2B) on operant responding for food was investigated. Experiment 3: After the food study (experiment 2), the rats were habituated to the small open field and the effects of flupentixol (Expt. 3A) and L-741626 (Expt. 3B) on locomotor activity, rearing, and stereotypies were investigated. IVSA, intravenous self-administration.
Experiment 1: effects of flupentixol and L-741626 on nicotine self-administration
The rats were trained to respond for food pellets (45 mg, F0299, Bio-Serv, Frenchtown, NJ) in operant chambers (Med Associates, St. Albans, VT) under a fixed-ratio 1, time-out 10s (FR1-TO10s) schedule. After completing the food training sessions, the rats were implanted with a catheter in the jugular vein. The rats were allowed to self-administer nicotine for nine days (daily 1-h sessions). The rats self-administered 0.03 mg/kg/inf of nicotine under an FR1-TO10s schedule for three days and then 0.06 mg/kg/inf of nicotine under an FR1-TO60s for six days. The effect of flupentixol (0, 0.5, 0.75, and 1 mg/kg, IP) and L-741626 (0, 0.3, 1, and 3 mg/kg, SC) on nicotine intake was investigated in daily 1 h sessions (0.06 mg/kg/inf). The rats were fed 90–95 percent of their baseline food intake immediately after the nicotine self-administration sessions.
Experiment 2 and 3: effects of flupentixol and L-741626 and operant responding for food and locomotor activity
Adult male and female rats were trained to respond for food pellets (45 mg, F0299, Bio-Serv, Frenchtown, NJ) in operant chambers (Med Associates, St. Albans, VT) under an FR1-TO10s schedule. After the food training sessions, the effects of flupentixol (0, 0.5, 0.75, and 1 mg/kg, IP) and L-741626 (0, 0.3, 1, and 3 mg/kg, SC) on operant responding for food pellets was investigated in daily 20-min sessions under an FR1-TO10s schedule (Expt. 2). The rats were fed 90–95 percent of their baseline food intake in the home cage after operant responding for food. After the food study, the rats were fed ad-lib, and the effects of flupentixol and L-741626 on locomotor activity were investigated (Expt. 3). The rats were habituated to the small open field on three consecutive days (20-min sessions) and then the effects of flupentixol (0, 0.5, 0.75, and 1 mg/kg, IP) and L-741626 (0, 0.3, 1, and 3 mg/kg, SC) on locomotor activity were investigated in 20-min sessions.
Food training
Food training was conducted in the operant chambers as described before (Yamada and Bruijnzeel, 2011; Bruijnzeel et al., 2009). Responding on the active lever resulted in the delivery of a food pellet. Food delivery was paired with a cue light, which remained illuminated throughout the time-out period. Responding on the inactive lever was recorded but did not have scheduled consequences. The food training sessions were conducted for 10 days. Instrumental training started under an FR1-TO1s reinforcement schedule for 5 days (30 min session per day). After five food training sessions, the rats were singly housed and remained singly housed for the remainder of the study. On day 6, the time-out period was increased to 10 s. The rats were allowed to respond for food pellets under an FR1-TO10s schedule (20 min sessions) for 5 days. During the 10 s time-out period, both levers were retracted. The rats were fed 90–95 percent of their baseline food intake in the home cage during the food training period.
Intravenous catheter implantation and operant responding for nicotine
The catheters were implanted as described before (Chellian et al., 2021c; Chellian et al., 2021b). The rats were anesthetized with an isoflurane-oxygen vapor mixture (1–3%) and prepared with a catheter in the right jugular vein. The catheters consisted of polyurethane tubing (length 10 cm, inner diameter 0.64 mm, outer diameter 1.0 mm, model 3Fr, Instech Laboratories, Plymouth Meeting, PA). The right jugular vein was isolated, and the catheter was inserted 3 cm for males and 2.5 cm for females. The tubing was tunneled subcutaneously and connected to a vascular access button (Instech Laboratories, Plymouth Meeting, PA). The button was exteriorized through a small, 1-cm, incision between the scapulae. After the surgery, the rats were given at least seven days to recover. The rats received IV infusions of the antibiotic Gentamycin (4 mg/kg, Sigma-Aldrich, St. Louis, MO) for seven days. A sterile heparin solution (0.1 ml, 50 U/ml) was flushed through the catheter before and after administering the antibiotic or nicotine self-administration. Then 0.05 ml of a sterile heparin/glycerol lock solution (500 U/ml) was infused into the catheter. The animals received carprofen (5 mg/kg, SC) daily for 72 hours after the surgery. One day before nicotine self-administration, the rats were allowed to respond for food pellets under the FR1-TO10s schedule (20-min session). The rats were allowed to self-administer nicotine for nine daily 1 h sessions. During the first three sessions, the rats self-administered 0.03 mg/kg/inf of nicotine under an FR1-TO10s schedule. For the following six sessions the rats self-administered 0.06 mg/kg/inf of nicotine under an FR1-TO60s schedule. The 0.06 mg/kg/inf dose of nicotine is a relatively high dose and leads to high levels of nicotine intake (Shoaib and Stolerman, 1999). High doses of nicotine can induce seizures in rodents (Corrigall and Coen, 1989; Hanson, 1979; Damaj et al., 1999; Chaudhri et al., 2005). To lower the risk for seizures, the time-out period was increased to 60 s when the dose of nicotine was increased from 0.03 to 0.06 mg/kg/inf. The timeout period (10 – 60 s) does not affect total nicotine intake over a 1 h nicotine self-administration session (Corrigall and Coen, 1989). On the first day of nicotine self-administration and on the day that the rats were switched to the 0.06 mg/kg/inf dose the amount of nicotine that the rats could self-administer was limited to prevent aversive effects (i.e., seizures and negative mood state). The maximum number of infusions was set to 20 for the first nicotine self-administration session (0.03 mg/kg/inf) and to 10 for the first session in which the rats received the 0.06 mg/kg/inf dose. Active lever responding resulted in the delivery of a nicotine infusion (0.1 ml infused over a 5.6-s period). The initiation of the delivery of an infusion was paired with a cue light, which remained illuminated throughout the time-out period. Inactive lever responses were recorded but did not have scheduled consequences. Both levers were retracted during the time-out period. During the self-administration period, the rats received about 90–95 percent of their normal ad lib food intake in the home cage. The rats were fed immediately after the operant sessions.
Small open field test
The small open field test was conducted as described before (Chellian et al., 2021c; Chellian et al., 2020b; Bruijnzeel et al., 2022). The small open field test was conducted to assess locomotor activity, rearing, and stereotypies. These behaviors were measured using an automated animal activity cage system (VersaMax Animal Activity Monitoring System, AccuScan Instruments, Columbus, OH, USA). Horizontal beam breaks and total distance traveled reflect locomotor activity, and vertical beam breaks reflect rearing. The distance traveled depends on the path of the animal in the open field and is considered a better indicator of locomotor activity than horizontal beam breaks. Repeated interruptions of the same beam are a measure of stereotypies (stereotypy count)(Calma et al., 2021). The setup consisted of four animal activity cages made of clear acrylic (40 cm × 40 cm × 30 cm; L x W x H), with 16 equally spaced (2.5 cm) infrared beams across the length and width of the cage. The beams were located 2 cm above the cage floor (horizontal activity beams). An additional set of 16 infrared beams was located 14 cm above the cage floor (vertical activity beams). All beams were connected to a VersaMax analyzer, which sent information to a computer that displayed beam data through Windows-based software (VersaDat software). The small open field test was conducted in a dark room, and the cages were cleaned with a Nolvasan solution (chlorhexidine diacetate) between animals. Each rat was placed in the center of the small open field, and activity was measured for 20 min.
Statistics
Baseline nicotine intake was analyzed with a two-way ANOVA with sex as a between subjects factor and time as a within subjects factor. The main goal of this work was to determine if flupentixol and L-741626 had an effect on operant responding for nicotine and food and locomotor activity. The effects of flupentixol and L-741626 on operant responding for nicotine and food and open-field behavior were analyzed with a two-way ANOVA with sex as a between subjects factor and drug treatment as a within subjects factor. Separate ANOVA analyses were conducted for the 24 h (flupentixol and L-741626) and 48 h (flupentixol) time points to determine if there were any delayed effects of flupentixol or L-741626 treatment. For all statistical analyses, significant effects in the ANOVA were followed by Bonferroni’s posthoc tests to determine which groups differed from each other. The posthoc tests for the males and females were conducted separately if there were significant main effects of treatment and sex or treatment × sex interaction effects in the ANOVA. Furthermore, the data from the males and females were combined for the posthoc tests if there was a significant main effect of treatment in the ANOVA and there was no main effect of sex or sex × treatment interaction. P-values that were less or equal to 0.05 were considered significant. Significant main effects, interaction effects, and posthoc comparisons are reported in the Results section. Data were analyzed with SPSS Statistics version 28 and GraphPad Prism version 9.3.1.
RESULTS
Baseline nicotine self-administration before flupentixol treatment:
During the first three nicotine self-administration sessions (0.03 mg/kg/inf), nicotine intake (Fig. 2A), responding on the active lever (Fig. S1A), and responding on the inactive lever (Fig. S1A) did not change over time and nicotine intake was not affected by the sex of the rats (Nicotine intake: Time F2,26 =2.814, NS; Time x Sex 2,26=0.051, NS; Sex F1,13=2.032, NS; Active lever: Time F2,26 =1.675, NS; Time x Sex 2,26=0.013, NS; Sex F1,13=2.914, NS; Inactive lever: Time F2,26 =0.368, NS; Time x Sex 2,26=1.086, NS; Sex F1,13=1.816, NS). During the following six sessions (0.06 mg/kg/inf), nicotine intake (Fig. 2B) and responding on the active lever (Fig. S1B) increased and was not affected by the sex of the rats (Nicotine intake: Time F5,65 =3.658, P<0.01; Time x Sex 5,65=0.85, NS; Sex F1,13=0.45, NS; Active lever: Time F5,65 =2.507, P<0.05; Time x Sex 5,65=0.395, NS; Sex F1,13=1.451, NS). Responding on the inactive lever (Fig. S1B) did not change over time and was not affected by the sex of the rats (Inactive lever: Time F5,65 =0.524, NS; Time x Sex 5,65=1.077, NS; Sex F1,13=1.323, NS).
Figure 2. Baseline nicotine intake in male and female rats.

Before the drug treatments, the rats self-administered 0.03 mg/kg/inf of nicotine for three days and then 0.06 mg/kg/inf of nicotine for six days in 1 h sessions (A, B, Flupentixol; C, D, L-741626; E, Flupentixol and L-741626 groups combined). There was no significant difference in nicotine intake between the male and female rats. Flupentixol baseline group (males n=7, females, n=8); L-741626 baseline group (males n=9, females n=8); Flupentixol and L-741626 groups combined (males n=16, females n=16). Data are expressed as means ± SEM.
Baseline nicotine self-administration before L-741626 treatment:
During the first three nicotine self-administration sessions (0.03 mg/kg/inf), nicotine intake (Fig. 2C) and responding on the active lever (Fig. S1C) decreased and was not affected by the sex of the rats (Nicotine intake: Time F2,30 =8.316, P<0.01; Time x Sex 2,30=0.073, NS; Sex F1,15=0.036, NS; Active lever: Time F2,30 =5.907, P<0.01; Time x Sex 2,30=0.789, NS; Sex F1,15=0.322, NS). Responding on the inactive lever (Fig. S1C) increased over time (Days 1–3) and was not affected by the sex of the rats (Inactive lever: Time F2,30 =5.816, P<0.01; Time x Sex 2,30=2.458, NS; Sex F1,15=2.096, NS). During the following six sessions (0.06 mg/kg/inf), nicotine intake (Fig. 2D) and responding on the active lever (Fig. S1D) increased and was not affected by the sex of the rats (Nicotine intake: Time F5,75 =2.269, P<0.01; Time x Sex 5,75=1.275, NS; Sex F1,15=0.085, NS; Active lever: Time F5,75 =3.833, P<0.01; Time x Sex 5,75=0.782, NS; Sex F1,15=0.875, NS). Responding on the inactive lever (Fig. S1D) did not change over time and was not affected by the sex of the rats (Inactive lever: Time F5,75 =2.269, NS; Time x Sex 5,75=1.304, NS; Sex F1,15=0.098, NS).
Baseline combined nicotine intake before flupentixol and L-741626 treatment:
It was also determined if there was a sex difference in nicotine intake when the baseline data from the first (flupentixol) and second experiment (L-741626) were combined (before testing the dopamine antagonists) (Fig. 2E). There was a no sex difference in nicotine intake during the first three sessions when all the rats self-administered 0.03 mg/kg/inf of nicotine (Sex F1,30=1.531, NS; Time x Sex F2,60=0.006, NS). There was also no sex difference in nicotine intake during the following six sessions when the rats had access to 0.06 mg/kg/inf of nicotine (Sex F1,30=0.581, NS; Time x Sex F5,150=1.947, NS). Nicotine intake decreased during the first three sessions (0.03 mg/kg/inf; Time F2,60 =8.824, P<0.001) and increased during the following six sessions (0.06 mg/kg/inf; Time F5,150 =7.408, P<0.001). Nicotine intake changed over time from day 1 to day 9, but there was no effect of sex on nicotine intake during this 9-day period (Time F8,240 =8.749, P<0.001; Time x Sex F8,240=0.938, NS; Sex F1,30=1.013, NS).
Experiment 1A: Effect of the D1/D2-like receptor antagonist flupentixol on operant responding for nicotine
30-min post flupentixol treatment:
Nicotine intake (Fig. 3A), responding on the active lever (Fig. S2A), and responding on the inactive lever (Fig. S2D) was decreased 30-min after treatment with the D1/D2-like receptor antagonist flupentixol and there was no effect of sex (Nicotine intake: Treatment F3,39=30.702, P<0.001; Treatment x Sex F3,39=1.271, NS; Sex F1,13=0.147, NS; Active lever: Treatment F3,39=23.71, P<0.001; Treatment x Sex F3,39=0.982, NS; Sex F1,13=0.216, NS; Inactive lever: Treatment F3,39=3.305, P<0.05; Treatment x Sex F3,39=2.24, NS; Sex F1,13=1.42, NS). The posthoc test showed that 0.75 and 1 mg/kg of flupentixol decreased nicotine intake and responding on the active lever but not responding on the inactive lever in the rats (male and female data combined; Fig. S3A, S3C, S3E).
Figure 3. Effects of the D1/D2-like receptor antagonist flupentixol on nicotine and food intake and locomotor activity in male and female rats.

The effects of flupentixol on nicotine (A, 30 min; B, 24 h; C, 48 h) and food intake (D, 30 min; E, 24 h; F, 48 h) and locomotor activity (G, 30 min; H, 24 h, I, 48 h) was investigated. Treatment with flupentixol decreased nicotine intake 30-min later and nicotine intake was increased 24 h and 48 h after treatment. Flupentixol decreased food intake 30 min after treatment and this effect was greater in males than females. Flupentixol decreased locomotor activity 30 min and 24 h after treatment. Nicotine self-administration (males n=7, females, n=8), operant responding for food and small open field test (males n=8, females, n=8). Asterisks indicate significant different from rats of the same sex that received vehicle. * P<0.05, ** P<0.01, *** P<0.001. Data are expressed as means ± SEM.
24-h post flupentixol treatment:
Nicotine intake and responding on the active lever was increased 24 h after treatment with flupentixol and nicotine intake (Fig. 3B) and active lever responding (Fig. S2B) was not affected by the sex of the rats (Nicotine intake: Treatment F3,39=4.892, P<0.01; Treatment x Sex F3,39=0.364, NS; Sex F1,13=0.075, NS; Active lever: Treatment F3,39=4.743, P<0.01; Treatment x Sex F3,39=0.237, NS; Sex F1,13=0.029, NS). The posthoc test did not reveal an effects of flupentixol on nicotine intake and responding on the active lever in the rats (male and female data combined; Fig. S3B, S3D). Responding on the inactive lever (Fig. S2E) was not affected by treatment with flupentixol or the sex of the rats (Inactive lever: Treatment F3,39=0.213, NS; Treatment x Sex F3,39=0.495, NS; Sex F1,13=0.546, NS.
48-h post flupentixol treatment:
Nicotine intake (Fig. 3C) and responding on the active lever (Fig. S2C) was affected by treatment with flupentixol and the sex of the rats (Nicotine intake: Treatment F3,39=0.163, NS; Treatment x Sex F3,39=3.687, P<0.05; Sex F1,13=0.255, NS; Active lever: Treatment F3,39=0.402, NS; Treatment x Sex F3,39=5.855, P<0.01; Sex F1,13=0.311, NS). The posthoc test showed that 0.75 mg/kg of flupentixol increased responding on active lever in the males (Fig. S2C). Responding on the inactive lever (Fig. S2F) was not affected by treatment with flupentixol or the sex of the rats (Inactive lever: Treatment F3,39=2.276, NS; Treatment x Sex F3,39=1.253, NS; Sex F1,13=0.234, NS).
Experiment 1B: Effect of the D2-like receptor antagonist L-741626 on operant responding for nicotine
15-min post L-741626 treatment:
Nicotine intake (Fig. 4A) and responding on the active lever (Fig. S4A) was decreased 15-min after treatment with the D2-like receptor antagonist L-741626 and there was no effect of sex (Nicotine intake: Treatment F3,45=5.634, P<0.01; Treatment x Sex F3,45=0.171, NS; Sex F1,15=0.357, NS; Active lever: Treatment F3,45=6.661, P<0.01; Treatment x Sex F3,45=0.937, NS; Sex F1,15=0.294, NS). The posthoc test showed that 3 mg/kg of L-741626 decreased nicotine intake and responding on the active lever in the rats (male and female data combined; Fig. S5A, S5C). Treatment with L-741626 or the sex of the rats did not affect responding on the inactive lever (Fig. S4C) (Inactive lever: Treatment F3,45=1.178, NS; Treatment x Sex F3,45=2.008, NS; Sex F1,15=0.018, NS).
Figure 4. Effects of the D2-like receptor antagonist L-741626 on nicotine and food intake and locomotor activity in male and female rats.
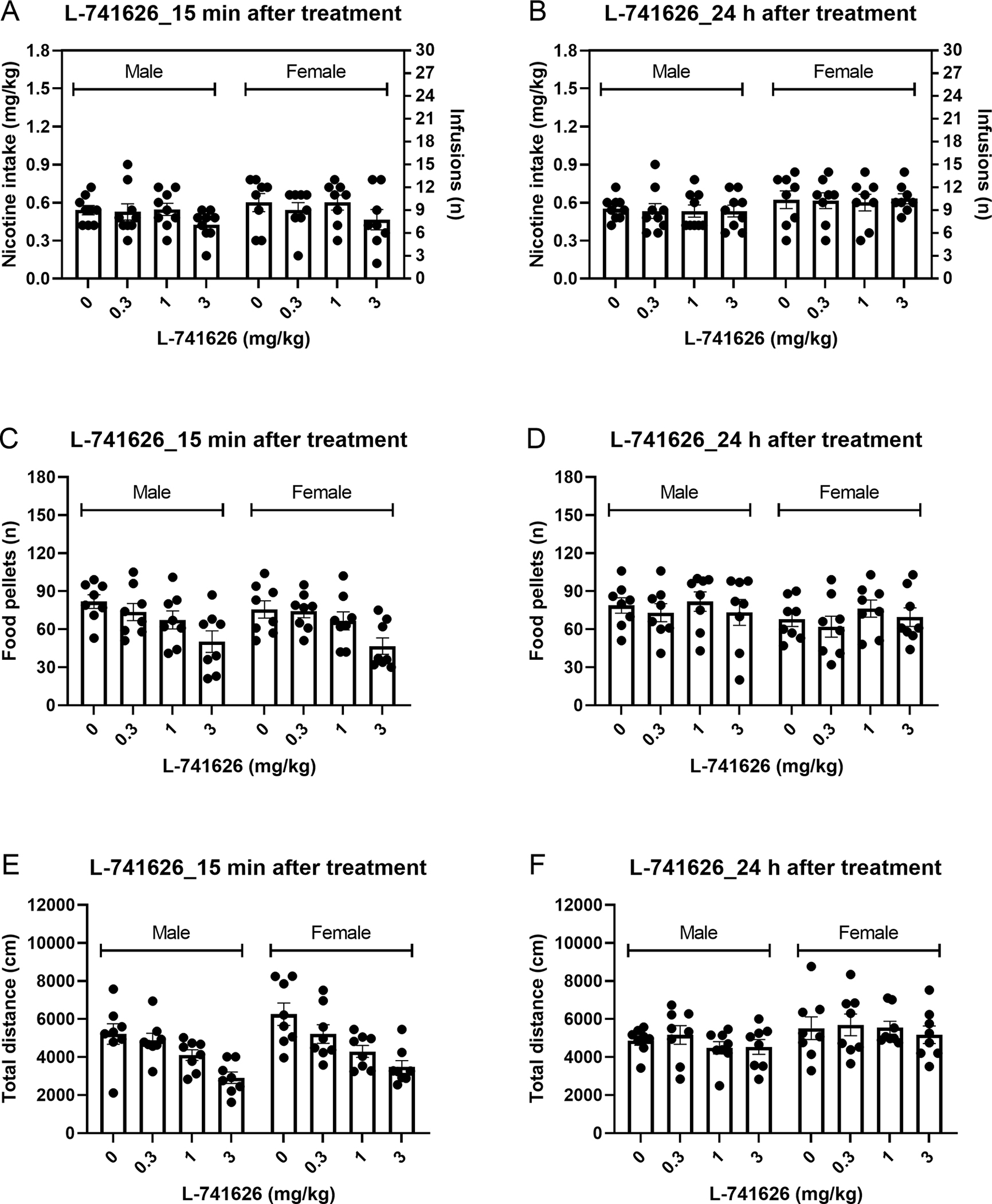
The effects of L-741626 on nicotine (A, 15 min; B, 24 h) and food intake (C, 15 min; D, 24 h) and locomotor activity (E, 15 min; F, 24 h) was investigated. Treatment with L-741626 decreased nicotine intake, food intake, and locomotor activity 15 min after treatment. Treatment with L-741626 did not affect nicotine intake, food intake, and locomotor activity at the 24 h time point. Nicotine self-administration (males n=9, females, n=8), operant responding for food, and small open field test (males n=8, females, n=8). Data are expressed as means ± SEM.
24-h post L-741626 treatment:
Treatment with L-741626 or the sex of the rats did not affect nicotine intake(Fig. 4B) (Nicotine intake: Treatment F3,45=0.126, NS; Treatment x Sex F3,45=0.07, NS; Sex F1,15=1.678, NS), responding on the active lever (Fig. S4B) (Active lever: Treatment F3,45=0.303, NS; Treatment x Sex F3,45=0.167, NS; Sex F1,15=1.783, NS), or responding on the inactive lever (Fig. S4D) (Inactive lever: Treatment F3,45=0.272, NS; Treatment x Sex F3,45=0.853, NS; Sex F1,15=0.018, NS).
Experiment 2A: Effect of the D1/D2-like receptor antagonist flupentixol on operant responding for food
30-min post flupentixol treatment:
Flupentixol decreased food intake and responding on the active lever and this effect was greater in the males than the females (Fig. 3D, S2G; Food intake: Treatment F3,42=43.045, P<0.001; Treatment x Sex F3,42=15.796, P<0.001; Sex F1,14=0.365, NS; Active lever: Treatment F3,42=43.598, P<0.001; Treatment x Sex F3,42=16.257, P<0.001; Sex F1,14=0.563, NS). The posthoc test showed that 1 mg/kg of flupentixol decreased food intake and responding on the active lever in the males (Fig 3D, S2G). Flupentixol also decreased responding on the inactive lever and this was not affected by the sex of the rats (Fig. S2J; Inactive lever: Treatment F3,42=2.823, P<0.05; Treatment x Sex F3,42=0.337, NS; Sex F1,14=0.342, NS). The posthoc test did not reveal an effects of flupentixol on inactive lever responses (male and female data combined, Fig S3F).
24-h post flupentixol treatment:
There was no effect of flupentixol or the sex of the rats on food intake, responding on the active lever, or responding on the inactive lever 24-h post treatment (Fig. 3E, S2H, S2K; Food intake: Treatment F3,42=2.004, NS; Treatment x Sex F3,42=1.338, NS; Sex F1,14=3.322, NS; Active lever: Treatment F3,42=1.849, NS; Treatment x Sex F3,42=1.745, NS; Sex F1,14=3.003, NS; Inactive lever: Treatment F3,42=1.266, NS; Treatment x Sex F3,42=0.215, NS; Sex F1,14=4.372, NS).
48-h post flupentixol treatment:
There was no effect of flupentixol or the sex of the rats on food intake, responding on the active lever, or responding on the inactive lever 48-h post treatment (Fig. 3F, S2I, S2L; Food intake: Treatment F3,42=1.061, NS; Treatment x Sex F3,42=2.535, NS; Sex F1,14=0.069, NS; Active lever: Treatment F3,42=0.487, NS; Treatment x Sex F3,42=2.9, NS; Sex F1,14=0.06, NS; Inactive lever: Treatment F3,42=0.893, NS; Treatment x Sex F3,42=0.36, NS; Sex F1,14=0.783, NS).
Experiment 2B: Effect of the D2-like receptor antagonist L-741626 on operant responding for food
15-min post L-741626 treatment:
The D2-like receptor antagonist L-741626 decreased food intake and responding on the active lever 15-min post treatment and there were no sex effects (Fig. 4C, S4E; Food intake: Treatment F3,42=12.735, P<0.001; Treatment x Sex F3,42=0.164, NS; Sex F1,14=0.127, NS; Active lever: Treatment F3,42=13.328, P<0.001; Treatment x Sex F3,42=0.197, NS; Sex F1,14=0.28, NS). The posthoc test showed that 3 mg/kg of L-741626 decreased food intake and responding on the active lever in the rats (male and female data combined; Fig S5B, S5D). Treatment with L-741626 did not affect responding on the inactive lever and the females responded more on the inactive lever than the males (Fig. S4G; Inactive lever: Treatment F3,42=0.684, NS; Treatment x Sex F3,42=1.176, NS; Sex F1,14=5.576, P<0.05).
24-h post L-741626 treatment:
There was no effect of L-741626 or the sex of the rats on food intake and responding on the active lever(Fig. 4D, S4F; Food intake: Treatment F3,42=2.112, NS; Treatment x Sex F3,42=0.31, NS; Sex F1,14=0.768, NS; Active lever: Treatment F3,42=1.803, NS; Treatment x Sex F3,42=0.544, NS; Sex F1,14=0.78, NS). Treatment with L-741626 did not affect responding on the inactive lever and the females made more inactive lever presses than the males (Fig. S4H; Inactive lever: Treatment F3,42=1.446, NS; Treatment x Sex F3,42=0.623, NS; Sex F1,14=8.911, P<0.01).
Experiment 3A: Effect of the D1/D2-like receptor antagonist flupentixol on locomotor activity
30-min post flupentixol treatment:
Flupentixol decreased the total distance traveled 30 min post-treatment and the females traveled a greater distance than the males (Fig. 3G; Total distance: Treatment F3,42=25.81, P<0.001; Treatment x Sex F3,42=0.799, NS; Sex F1,14=8.388, P<0.05). The posthoc test showed that 0.75 and 1 mg/kg of flupentixol decreased the total distance traveled in the male and female rats (Fig 3G). Treatment with flupentixol also decreased the number of horizontal beam breaks, and there was a trend towards the females having more horizontal beam breaks than the males (Fig. S6A; Horizontal activity: Treatment F3,42=35.499, P<0.001; Treatment x Sex F3,42=1.625, NS; Sex F1,14=4.535, P=0.051). The posthoc test showed that 0.75 and 1 mg/kg of flupentixol decreased the horizontal activity in the males and females (Fig S6A). Flupentixol decreased vertical beam breaks, and the females had more vertical beam breaks than the males (Fig. S6D; Vertical activity: Treatment F3,42=22.304, P<0.001; Treatment x Sex F3,42=0.95, NS; Sex F1,14=12.452, P<0.01). The posthoc test showed that 0.75 and 1 mg/kg of flupentixol decreased the vertical activity in the males and females (Fig S6D). Flupentixol also decreased stereotypies and there was no effect of sex on stereotypies (Fig. S6G; Stereotypies: Treatment F3,42=30.525, P<0.001; Treatment x Sex F3,42=1.487, NS; Sex F1,14=0.564, NS). The posthoc test showed that 0.75 and 1 mg/kg of flupentixol decreased stereotypies in the rats (male and female data combined; Fig S7A).
24-h post flupentixol treatment:
Flupentixol decreased the total distance traveled 24 h post-treatment and the females traveled a greater distance than the males (Fig. 3H; Total distance: Treatment F3,42=3.239, P<0.05; Treatment x Sex F3,42=1.228, NS; Sex F1,14=5.874, P<0.05). The posthoc test did not reveal an effects of flupentixol on the total distance traveled in the males and females (Fig. 3H). Flupentixol also decreased horizontal beam breaks, and there was no effect of sex on horizontal beam breaks (Fig. S6B; Horizontal activity: Treatment F3,42=6.069, P<0.01; Treatment x Sex F3,42=1.149, NS; Sex F1,14=1.077, NS). The posthoc test showed that 0.75 and 1 mg/kg of flupentixol decreased horizontal activity in the rats (male and female data combined; Fig. S7D). Treatment with flupentixol did not affect vertical beam breaks, and the females had more vertical beam brakes than the males (Fig. S6E; Vertical activity: Treatment F3,42=1.957, NS; Treatment x Sex F3,42=0.994, NS; Sex F1,14=16.436, P<0.01). Treatment with flupentixol decreased the number of stereotypies, and the number of stereotypies was not affected by the sex of the rats (Fig. S6H; Stereotypies: Treatment F3,42=4.881, P<0.01; Treatment x Sex F3,42=0.211, NS; Sex F1,14=0.136, NS). The posthoc test showed that 0.75 and 1 mg/kg of flupentixol decreased stereotypies in the rats (male and female data combined; Fig. S7B).
48-h post flupentixol treatment:
Flupentixol did not affect the total distance traveled or horizontal beam breaks 48 h post-treatment, and the sex of the rats did not affect the total distance traveled and horizontal beam breaks (Fig. 3I, S6C; Total distance: Treatment F3,42=0.744, NS; Treatment x Sex F3,42=1.393, NS; Sex F1,14=0.977, NS; Horizontal activity: Treatment F3,42=1.468, NS; Treatment x Sex F3,42=1.138, NS; Sex F1,14=0.003, NS). Flupentixol decreased the number of vertical beam breaks, and the females had more vertical beam breaks than the males (Fig. S6F; Vertical activity: Treatment F3,42=2.883, P<0.05; Treatment x Sex F3,42=2.588, NS; Sex F1,14=7.323, P<0.05). The posthoc test showed that 1 mg/kg of flupentixol decreased vertical beam breaks in the females (Fig. S6F). Treatment with flupentixol decreased the number of stereotypies, and the number of stereotypies was not affected by the sex of the rats (Fig. S6I; Stereotypies: Treatment F3,42=2.836, P<0.05; Treatment x Sex F3,42=0.642, NS; Sex F1,14=1.756, NS). The posthoc test showed that 1 mg/kg of flupentixol decreased stereotypies in the rats (male and female data combined; Fig. S7C).
Experiment 3B: Effect of the D2-like receptor antagonist L-741626 on locomotor activity
15-min post L-741626 treatment:
Treatment with L-741626 decreased the total distance traveled, and the total distance traveled was not affected by the sex of the rats (Fig. 4E; Treatment F3,42=33.677, P<0.001; Treatment x Sex F3,42=0.974, NS; Sex F1,14=1.171, NS). The posthoc test showed that 0.3, 1, and 3 mg/kg of L-741626 decreased the total distance traveled in the rats (male and female data combined; Fig. S9A). Treatment with L-741626 decreased horizontal beam breaks, and the females had the same number of horizontal beam breaks as the males (Fig. S8A; Treatment F3,42=30.299, P<0.001; Treatment x Sex F3,42=0.885, NS; Sex F1,14=0.077, NS). The posthoc tests show that 1 and 3 mg/kg of L-741626 decrease horizontal activity in the rats (male and female data combined; Fig. S9B). L-741626 decreased vertical beam breaks, and the females had more vertical beam breaks than the males (Fig. S8C; Treatment F3,42=31.157, P<0.001; Treatment x Sex F3,42=0.625, NS; Sex F1,14=4.861, P<0.05). The posthoc test showed that 3 mg/kg of L-741626 decreased vertical activity in the males and 0.3, 1, and 3 mg/kg of L-741626 decreased vertical activity in females (Fig. S8C). L-741626 decreased stereotypies, and the number of stereotypies was not affected by the sex of the rats (Fig. S8E; Treatment F3,42=17.367, P<0.001; Treatment x Sex F3,42=1.184, NS; Sex F1,14=2.11, NS). The posthoc tests show that 1 and 3 mg/kg of L-741626 decrease stereotypies in the rats (male and female data combined; Fig. S9C).
24-h post L-741626 treatment:
Treatment with L-741626 did not affect the total distance traveled 24 h later, and the males and the females traveled the same distance (Fig. 4F; Treatment F3,42=1.909, NS; Treatment x Sex F3,42=0.41, NS; Sex F1,14=1.767, NS). Treatment with L-741626 and the sex of the rats did not affect horizontal beam breaks (Fig. S8B; Treatment F3,42=1.284, NS; Treatment x Sex F3,42=0.955, NS; Sex F1,14=0.097, NS). Treatment with L-741626 did not affect vertical beam breaks, but the females had more vertical beam breaks than the males (Fig. S8D; Treatment F3,42=0.807, NS; Treatment x Sex F3,42=0.444, NS; Sex F1,14=6.837, P<0.05). The number of stereotypies was not affected by L-741626 or the sex of the rats (Fig. S8F; Treatment F3,42=1.161, NS; Treatment x Sex F3,42=1.516, NS; Sex F1,14=2.018, NS).
DISCUSSION
The main goal of the present study was to investigate the effects of the mixed D1/D2-like receptor antagonist flupentixol and the D2-like receptor antagonist L-741626 on the self-administration of nicotine (0.06 mg/kg/inf) in male and female rats. There was no sex difference in baseline nicotine intake or nicotine intake during the drug treatment period. The D1/D2-like receptor antagonist flupentixol and the D2-like receptor antagonist L-741626 decreased operant responding for nicotine. Blockade of the D1/D2-like receptors and D2-like receptors also decreased operant responding for food and decreased locomotor activity, rearing, and stereotypies. Flupentixol differently affected operant responding for food in the males and the females. Flupentixol induced a greater decrease in operant responding for food in the males than the females. In all other studies, the dopamine antagonists had the same effects in males and females. These findings indicate that blockade of D1/D2-like receptors and D2-like receptors decreases operant responding for nicotine and food and decreases locomotor activity, rearing, and stereotypies in the small open field.
The present study investigated sex differences in operant responding for nicotine and food and locomotor activity. The rats maintained a stable level of nicotine intake (0.06 mg/kg/inf). We did not observe a sex difference in nicotine intake during the baseline period (the 9-day period before treatment with the dopamine antagonists) or during the drug treatment period. Our laboratory has conducted numerous nicotine self-administration studies in rats of both sexes. In a recent study with a similar design (food training followed by IV nicotine intake) and nicotine dose (0.06 mg/kg/inf), we did not observe a sex difference in nicotine intake (Chellian et al., 2022). In other studies, in which the rats were trained to respond for food pellets before the self-administration of a lower dose of nicotine, 0.03 mg/kg/inf, we also did not observe a sex difference in nicotine intake (Chellian et al., 2021b; Chellian et al., 2020a). In a study in which we investigated the spontaneous acquisition of nicotine self-administration (no prior food training), the females self-administered more nicotine than the males (Chellian et al., 2021c). Overall, the outcome of our studies is in line with those of other investigators. Several investigators reported that there are no sex differences in nicotine intake after nicotine intake has been established, and the rats are tested under test schedules with low response requirements (Feltenstein et al., 2012; Donny et al., 2000; Grebenstein et al., 2013; Swalve et al., 2016). Other studies reported that female rats self-administer more nicotine than males during the acquisition phase in studies without prior food training and that visual cues have a greater effect on nicotine intake in females than males (Park et al., 2007; Donny et al., 2000; Wang et al., 2018; Chaudhri et al., 2005).
In this study, we also determined sex differences in open-field behavior. In the first experiment, the females displayed more locomotor activity and rearing than the males. In the second experiment, the females displayed more rearing than the males, but there was no sex difference in locomotor activity (i.e., distance traveled). Our previous studies showed that females display more locomotor activity and rearing in open field tests (Knight et al., 2021; Chellian et al., 2022). Sex differences in open-field behavior and other behavioral tests are relatively small, and because of the relatively small groups sizes in animal studies, many studies do not have the statistical power to conclude that there are sex differences. In the present study, we did not find a sex difference in operant responding for food pellets. This is in line with previous studies that reported that there are no sex differences in operant responding food pellets in rats (Chellian et al., 2020c; Chellian et al., 2022).
The present study showed that the D1/D2-like receptor antagonist flupentixol (intake: 0.59 mg/kg of nicotine, vehicle group; 0.48 mg/kg of nicotine, 0.75 mg/kg flupentixol; 0.16 mg/kg of nicotine, 1 mg/kg flupentixol) induced a greater decrease in nicotine intake than the D2-like receptor antagonist L-741626 (intake: 0.57 mg/kg nicotine, vehicle group; 0.57 mg/kg of nicotine, 1 mg/kg L-741626; 0.44 mg/kg of nicotine, 3 mg/kg L-741626). On a similar note, treatment with flupentixol induced a greater decrease in food intake than L-741626. This work is in line with studies that showed that blockade of D1-like receptors induces a relatively large decrease in nicotine and food intake in rats (Corrigall and Coen, 1991; Chellian et al., 2022). In contrast, treatment with the D2-like receptor antagonist spiperone (and also serotonin 5-HT1A and serotonin 5-HT2A antagonist) induces a relatively small decrease in nicotine and food intake in rats (Metwally et al., 1998; Corrigall and Coen, 1991).
In the present study, blockade of D1/D2-like receptors and blockade of D2-like receptors decreased nicotine self-administration. Several studies also provide support for a role of D1- and D2-like receptors in the reinstatement of nicotine seeking behavior. The selective D1-like receptor antagonist SCH 23390 and the selective D2-like receptor antagonists L-741626 and eticlopride decrease cue-induced reinstatement of nicotine seeking in male rats (Khaled et al., 2014; Liu et al., 2010). In our study, both flupentixol and L-741626 decreased operant responding for food. The males were more sensitive to the effects of flupentixol on food intake than females. The highest dose of flupentixol (1 mg/kg) decreased food intake in the males but not in the females. A similar trend was observed with the effect of flupentixol on nicotine intake. An intermediate dose of flupentixol (0.75 mg/kg) decreased nicotine intake in the males but not in the females. This sex difference in the effect of flupentixol on nicotine and food intake might be due to sex differences in dopamine receptor levels. Several studies have reported that D1 receptor levels are higher in the nucleus accumbens of male than female rats (Andersen et al., 1997; Hasbi et al., 2020). In previous studies, the selective D1-like receptor antagonists SCH 23390 and SCH 39166, the selective D2-antagonists eticlopride and L-741626, and the D1/D2-like receptor antagonist flupentixol also decreased operant responding for food in rats (Liu et al., 2010; Barrett et al., 2004; Thomsen et al., 2017; Corrigall and Coen, 1991; Veeneman et al., 2012; Hall et al., 2015). Taken together, these studies indicate that the dopamine D1- and D2-like receptors play a role in the reinforcing properties of nicotine and food.
The role of D1- and D2-like receptors in the rewarding effects of nicotine have also been investigated with the intracranial self-stimulation (ICSS) procedure. Nicotine increases the sensitivity to rewarding electrical stimuli in the medial forebrain bundle and lowers the ICSS brain reward thresholds (i.e., nicotine potentiates brain reward function). Blockade of D1-like receptors with SCH 23390 prevents the reward-enhancing effects of nicotine in the ICSS, but blockade of D2-like receptors with eticlopride did not prevent the reward-enhancing effects of nicotine (Harrison et al., 2002). These ICSS studies are not entirely in line with the outcome of the self-administration studies, which suggest that D1-like receptors and, to a lesser degree, D2-like receptors play a role in the reinforcing properties of nicotine. In contrast to the ICSS work, conditioned place preference studies with mice suggest that D2-like receptors play a critical role in the rewarding properties of nicotine. Repeated nicotine administration induces conditioned place preference in wild-type mice but not in D2 receptor knockout mice (Wilar et al., 2019). Furthermore, treatment with SCH 23390 or the D2-like receptor antagonist eticlopride completely prevented nicotine-induced conditioned place preference in the mice. Prior work has also investigated the role of dopamine receptors in the hyperactivity associated with nicotine treatment (O’Neill et al., 1991). It was shown that the D1-like receptor antagonist SCH 23390, the D2-like receptor antagonist raclopride, and the D1/D2-like receptor antagonist fluphenazine diminished the nicotine-induced increase in locomotor activity. These findings suggest that the effects of D2-like receptor blockade in nicotine studies depend on the test conditions. Blockade of the D2-like receptors decreases operant responding for nicotine and the reward enhancing effects of nicotine in the conditioned place preference procedure and diminishes nicotine-induced hyperactivity. In contrast, blockade of D2-like receptors with eticlopride did not prevent the reward-enhancing effects of nicotine in the ICSS procedure.
In the present study, we did not determine the brain sites that mediate the effects of the D1- and D2-like receptor antagonists. However, other studies have started to explore which brain sites mediate the effects of the D1- and D2-like receptor antagonists on the rewarding and aversive effects of nicotine. The administration of the D1-like receptor antagonist SCH 23390, but not the D2-like receptor antagonist haloperidol, into the nucleus accumbens, insular cortex, and the parietal association cortex diminished nicotine self-administration in female rats (Kutlu et al., 2013; Hall et al., 2015). The administration of the D1-like receptor antagonist SCH 23390, but not the D2-like receptor antagonist sulpiride, into the nucleus accumbens shell also prevented the acquisition of nicotine-induced conditioned place preference in rats (Spina et al., 2006). Interestingly, the administration of flupentixol into the nucleus accumbens, SCH 23390 into the nucleus accumbens core, and eticlopride into the nucleus accumbens shell switched the effects of nicotine from aversive to rewarding in a place conditioning procedure in rats (Laviolette et al., 2008). Taken together, these studies indicate that dopamine D1-like receptors in the nucleus accumbens play a critical role in the rewarding properties of nicotine. It is still largely unknown via which brain sites selective D2-like receptors antagonists mediate their effects on nicotine intake. However, studies with cocaine and food have shown that D2-like receptors in the ventral tegmental area and the nucleus accumbens play a role in reward function (Dobbs et al., 2019; Peng et al., 2021). Additional work is needed to determine the brain sites that mediate the effects of selective D2-like receptor antagonists on nicotine intake.
This study also investigated the long-term effects of treatment with flupentixol and L-741626. Flupentixol decreased operant responding for nicotine and food and locomotor activity in the small open field 30 min after treatment. Twenty-four hours after treatment with flupentixol, operant responding for nicotine was increased, operant responding for food was not affected, and locomotor activity was decreased. Furthermore, 48 h after treatment with flupentixol, operant responding for nicotine was increased in the males. The increase in operant responding for nicotine at the 24 h and 48 h time point might have been because operant responding for nicotine was decreased after treatment with flupentixol (30 min post-treatment). Rats typically increase their nicotine intake after a brief period of decreased intake or deprivation (O’Dell and Koob, 2007). Locomotor activity was also slightly but significantly decreased 24 h after flupentixol treatment. Treatment with L-741626 decreased operant responding for nicotine and food and locomotor activity in the small open field 15 min after treatment. Treatment with L-741626 did not affect operant responding for nicotine and food or locomotor activity at the 24 h time point. Therefore, in contrast to flupentixol, treatment with L-741626 does not have long-term (24 h) effects.
In conclusion, the present study shows that blockade of D1/D2-like receptors and D2-like receptors decreases operant responding for nicotine and food and locomotor activity in the small open field. The self-administration studies suggest that both D1-like and D2-like receptors contribute to the reinforcing properties of nicotine. The D1/D2-like and D2-like receptor antagonists reduced nicotine intake but also had sedative effects that may hamper their use as smoking cessation drugs.
Supplementary Material
Funding:
This work was supported by a NIDA grant (DA046411) to AB.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
CRediT authorship contribution statement
R. Chellian: Formal analysis, Investigation, Writing - Review & Editing, Visualization. A. Behnood-Rod: Investigation, Project administration. R. Wilson: Investigation, Project administration. K. Lin: Investigation. G. King: Investigation. A. Bruijnzeel: Conceptualization, Formal analysis, Writing - Original Draft, Visualization, Supervision, Project administration, Funding acquisition.
REFERENCES
- Ambrose JA and Barua RS. (2004) The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American college of cardiology 43: 1731–1737. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, et al. (1997) Sex differences in dopamine receptor overproduction and elimination. Neuroreport 8: 1495–1498. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, et al. (2004) Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 47 Suppl 1: 256–273. [DOI] [PubMed] [Google Scholar]
- Bourne JA. (2001) SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS drug reviews 7: 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. (2012) Tobacco addiction and the dysregulation of brain stress systems. Neurosci.Biobehav.Rev. 36: 1418–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Behnood-Rod A, Malphurs W, et al. (2022) Oxycodone decreases anxiety-like behavior in the elevated plus-maze test in male and female rats. Behav Pharmacol 33: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW and Markou A. (2003) Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50: 20–28. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M and Isaac S. (2009) Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol.Psychiatry 66: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, et al. (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 163: 230–237. [DOI] [PubMed] [Google Scholar]
- Calma ID, Persons AL and Napier TC. (2021) Mitochondrial function influences expression of methamphetamine-induced behavioral sensitization. Scientific Reports 11: 24529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaperon F, Tricklebank MD, Unger L, et al. (2003) Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology 44: 1047–1053. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, et al. (2005) Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180: 258–266. [DOI] [PubMed] [Google Scholar]
- Chavanon M-L, Wacker J and Stemmler G. (2013) Paradoxical dopaminergic drug effects in extraversion: dose-and time-dependent effects of sulpiride on EEG theta activity. Front Hum Neurosci 7: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Behnood-Rod A, Bruijnzeel DM, et al. (2021a) Rodent models for nicotine withdrawal. Journal of Psychopharmacology 35: 1169–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Behnood-Rod A, Wilson R, et al. (2021b) Rewarding effects of nicotine self-administration increase over time in male and female rats. Nicotine & Tobacco Research 23: 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Behnood-Rod A, Wilson R, et al. (2021c) Adolescent nicotine treatment causes robust locomotor sensitization during adolescence but impedes the spontaneous acquisition of nicotine intake in adult female Wistar rats. Pharmacology Biochemistry and Behavior 207: 173224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Behnood-Rod A, Wilson R, et al. (2020a) Adolescent nicotine and tobacco smoke exposure enhances nicotine self-administration in female rats. Neuropharmacology 176: 108243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Behnood-Rod A, Wilson R, et al. (2022) Dopamine D1-like receptor blockade and stimulation decreases operant responding for nicotine and food in male and female rats. Scientific Reports 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Behnood-Rod A, Wilson R, et al. (2020b) Exposure to smoke from high-but not low-nicotine cigarettes leads to signs of dependence in male rats and potentiates the effects of nicotine in female rats. Pharmacology Biochemistry and Behavior 196: 172998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellian R, Wilson R, Polmann M, et al. (2020c) Evaluation of sex differences in the elasticity of demand for nicotine and food in rats. Nicotine & Tobacco Research 22: 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA and Coen KM. (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99: 473–478. [DOI] [PubMed] [Google Scholar]
- Corrigall WA and Coen KM. (1991) Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 104: 171–176. [DOI] [PubMed] [Google Scholar]
- Damaj M, Glassco W, Dukat M, et al. (1999) Pharmacological characterization of nicotine-induced seizures in mice. Journal of Pharmacology and Experimental Therapeutics 291: 1284–1291. [PubMed] [Google Scholar]
- DeNoble VJ and Mele PC. (2006) Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology 184: 266–272. [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Bock R, et al. (2019) D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology 44: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, et al. (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 151: 392–405. [DOI] [PubMed] [Google Scholar]
- Engster KM, Wismar J, Kroczek AL, et al. (2015) The dopamine antagonist flupentixol does not alter ghrelin-induced food intake in rats. Neuropeptides 53: 19–27. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, et al. (2008) Association of low striatal dopamine D 2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. American Journal of Psychiatry 165: 507–514. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM and See RE. (2012) Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug & Alcohol Dependence 121: 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup. (2021) Smoking and Vaping Remain Steady and Low in U.S. Washington, D.C., https://news.gallup.com/poll/353225/smoking-vaping-remain-steady-low.aspx. [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, et al. (2013) Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav 114–115: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Husband SLJ, Luedtke RR, et al. (2007) Analogues of the dopamine D2 receptor antagonist L741, 626: Binding, function, and SAR. Bioorganic & medicinal chemistry letters 17: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Slade S, Allenby C, et al. (2015) Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology 99: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson H (1979) Nicotine Self-Administration. Cigarette smoking as a dependence process 23: 70. [Google Scholar]
- Harrison AA, Gasparini F and Markou A. (2002) Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 160: 56–66. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Rahal H, et al. (2020) Sex difference in dopamine D1-D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol Sex Differ 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Mereu M, Soto PL, et al. (2013) Self-administration of cocaine induces dopamine-independent self-administration of sigma agonists. Neuropsychopharmacology 38: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson AM, Hedlund PB, Hillefors M, et al. (1992) Chronic nicotine treatment decreases dopamine D2 agonist binding in the rat basal ganglia. Neuroreport 3: 1117–1120. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Hietala J, et al. (2001) Sex differences in extrastriatal dopamine D2-like receptors in the human brain. American Journal of Psychiatry 158: 308–311. [DOI] [PubMed] [Google Scholar]
- Khaled MA, Pushparaj A, Di Ciano P, et al. (2014) Dopamine D3 receptors in the basolateral amygdala and the lateral habenula modulate cue-induced reinstatement of nicotine seeking. Neuropsychopharmacology 39: 3049–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P, Chellian R, Wilson R, et al. (2021) Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacology Biochemistry and Behavior 204: 173168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulagowski JJ, Broughton HB, Curtis NR, et al. (1996) 3-[[4-(4-Chlorophenyl) piperazin-1-yl] methyl]-1 H-pyrrolo [2, 3-b] pyridine: An Antagonist with High Affinity and Selectivity for the Human Dopamine D4 Receptor. J Med Chem 39: 1941–1942. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Burke D, Slade S, et al. (2013) Role of insular cortex D(1) and D(2) dopamine receptors in nicotine self-administration in rats. Behav.Brain Res. 256: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lauzon NM, Bishop SF, et al. (2008) Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. J Neurosci 28: 8025–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B and Goldberg SR. (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 178: 481–492. [DOI] [PubMed] [Google Scholar]
- Liu X, Jernigen C, Gharib M, et al. (2010) Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol 21: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally KA, Dukat M, Egan CT, et al. (1998) Spiperone: influence of spiro ring substituents on 5-HT2A serotonin receptor binding. J Med Chem 41: 5084–5093. [DOI] [PubMed] [Google Scholar]
- Morean ME, Krishnan-Sarin S and O’Malley SS. (2018) Assessing nicotine dependence in adolescent e-cigarette users: the 4-item Patient-Reported Outcomes Measurement Information System (PROMIS) Nicotine Dependence Item Bank for electronic cigarettes. Drug and Alcohol Dependence 188: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen IM, Pedersen V, Nymark M, et al. (1973) The comparative pharmacology of flupenthixol and some reference neuroleptics. Acta pharmacologica et toxicologica 33: 353–362. [DOI] [PubMed] [Google Scholar]
- O’Dell LE and Koob GF. (2007) ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol.Biochem.Behav. 86: 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M, Dourish C and Iversen S. (1991) Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology 104: 343–350. [DOI] [PubMed] [Google Scholar]
- Okita K, Mandelkern MA and London ED. (2016) Cigarette Use and Striatal Dopamine D2/3 Receptors: Possible Role in the Link between Smoking and Nicotine Dependence. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendain-Jaime EN, Ortega-Ibarra JM and López-Pérez SJ. (2016) Evidence of sexual dimorphism in D1 and D2 dopaminergic receptors expression in frontal cortex and striatum of young rats. Neurochem Int 100: 62–66. [DOI] [PubMed] [Google Scholar]
- Ott A, Slooter AJ, Hofman A, et al. (1998) Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet 351: 1840–1843. [DOI] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han S-H, et al. (2007) Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacology Biochemistry and Behavior 86: 297–305. [DOI] [PubMed] [Google Scholar]
- Peng B, Xu Q, Liu J, et al. (2021) Corticosterone Attenuates Reward-Seeking Behavior and Increases Anxiety via D2 Receptor Signaling in Ventral Tegmental Area Dopamine Neurons. Journal of neuroscience 41: 1566–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM and Westman EC. (1998) Nicotine–mecamylamine treatment for smoking cessation: The role of pre-cessation therapy. Exp Clin Psychopharmacol 6: 331. [DOI] [PubMed] [Google Scholar]
- Sasco A, Secretan M and Straif K. (2004) Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung cancer 45: S3–S9. [DOI] [PubMed] [Google Scholar]
- Shoaib M and Stolerman IP. (1999) Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 143: 318–321. [DOI] [PubMed] [Google Scholar]
- Sorge RE and Clarke PB. (2009) Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther 330: 633–640. [DOI] [PubMed] [Google Scholar]
- Spina L, Fenu S, Longoni R, et al. (2006) Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacology (Berl) 184: 447–455. [DOI] [PubMed] [Google Scholar]
- Stolerman IP and Jarvis MJ. (1995) The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117: 2–10. [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR and Carroll ME. (2016) Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology (Berl) 233: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Butler P, et al. (2017) Effects of Acute and Chronic Treatments with Dopamine D(2) and D(3) Receptor Ligands on Cocaine versus Food Choice in Rats. J Pharmacol Exp Ther 362: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, et al. (2011) Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 214: 863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MM, van Ast M, Broekhoven MH, et al. (2012) Seeking-taking chain schedules of cocaine and sucrose self-administration: effects of reward size, reward omission, and α-flupenthixol. Psychopharmacology (Berl) 220: 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Han W, Chitre AS, et al. (2018) Social and anxiety-like behaviors contribute to nicotine self-administration in adolescent outbred rats. Scientific Reports 8: 18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, et al. (1999) Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol.Biochem.Behav. 62: 743–751. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Su ZI, Shelton K, et al. (2013) The dopamine antagonist cis-flupenthixol blocks the expression of the conditioned positive but not the negative effects of cocaine in rats. Pharmacol Biochem Behav 114-115: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2019) WHO. (July 26, 2019). Prevalence of global adult tobacco smoking in 2007 and 2017, by income level [Graph]. In Statista. Retrieved February 01, 2022, from https://www.statista.com/statistics/449561/worldwide-prevalence-of-tobacco-smoking-in-adults-by-gender-and-income-level/. [Google Scholar]
- Wilar G, Shinoda Y, Sasaoka T, et al. (2019) Crucial role of dopamine D2 receptor signaling in nicotine-induced conditioned place preference. Mol Neurobiol 56: 7911–7928. [DOI] [PubMed] [Google Scholar]
- Williams OO, Coppolino M, George SR, et al. (2021) Sex Differences in Dopamine Receptors and Relevance to Neuropsychiatric Disorders. Brain Sciences 11: 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H and Bruijnzeel AW. (2011) Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology 60: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


