Abstract
It has been shown that learning visuomotor rotations with multiple target directions, compared with a single target direction, leads to greater generalization to untrained targets within the same limb. This implies that multiple direction learning results in a more complete internal model of the visuomotor transform. It has also been documented that the extent of transfer of movement information regarding visuomotor adaptations between the limbs is limited, relative to that between different configurations of the same limb. The present study thus investigated the origin of this restriction in interlimb transfer, by comparing the effects of eight-direction and one-direction training conditions with one arm on the subsequent performance with the other arm. It was hypothesized that if multiple direction learning leads to a more complete model of the novel visuomotor transform, interlimb transfer should be enhanced relative to that following single direction training. However, if no differences are observed between single and multiple direction training conditions, this would suggest that such learning is effector dependent. We also tested the hypothesis that interlimb transfer of visuomotor adaptation is not obligatory, by examining the effects of visual rotation direction (same or oppositely directed visuomotor rotations for the two arms). All subjects first adapted to a 30° rotation, either clockwise or counterclockwise, in the visual display during reaching movements. Following this, they adapted to a 30° rotation in either the same or opposing direction with the other arm. Results showed that initial training with the non-dominant arm facilitated subsequent performance with the dominant arm in terms of initial direction control, but only under the same rotation condition. Both single and eight direction training conditions led to substantial transfer in subsequent performance with the other arm, but multiple direction training was no more beneficial than single direction training. This finding suggests that the previously reported intralimb advantages of multiple direction learning are effector specific. Our findings are discussed in the context of hierarchical models of motor control to explain the intralimb advantages of multiple direction training.
Keywords: Handedness, Visual rotation, Manual asymmetry, Hemispheric specialization, Generalization
Introduction
Numerous previous reports have shown that, when exposed to visually rotated display conditions during reaching, subjects’ hand trajectories are distorted early in adaptation (Krakauer et al. 1999; Sainburg 2002; Sainburg and Wang 2002; Tong et al. 2002; Wang and Sainburg 2003). Over repeated trials, their hand paths become similar to those of pre-exposure performance. However, when the visual rotation is again removed, the trajectories are typically distorted in the opposite direction to the previously applied rotation. These “after-effects” are thought to represent internal models of the altered mapping between visual coordinates, which are extrinsic to the body, and joint coordinates, which are intrinsic to the body (Imamizu et al. 1998; Kawato 1999; Krakauer et al. 2000; Sainburg 2002; Sainburg and Wang 2002; Wang and Sainburg 2003; Wolpert and Ghahramani 2000).
We have previously shown that such visuomotor adaptations can transfer from one arm to the other. Many other tasks have also been used to show that movement information learned with one limb transfers to affect the speed or accuracy of the same movements made with the other limb (Criscimagna-Hemminger et al. 2003; Dizio and Lackner 1995; Elliott and Roy 1981; Imamizu and Shimojo 1995; Laszlo et al. 1970; Marzi et al. 1991; Morton et al. 2001; Parlow and Kinsbourne 1989; Stoddard and Vaid 1996; Taylor and Heilman 1980; Thut et al. 1996). In most cases, transfer is not symmetric but the direction of greatest transfer, dominant to non-dominant or vice versa, appears to be task dependent. For example, we previously showed that initial training to a rotated visual display, using the non-dominant arm, facilitates the initial direction of subsequent dominantarm movements. In contrast, initial training with the dominant arm facilitates the final position, but not the initial direction of subsequent non-dominant-arm movements (Sainburg and Wang 2002). When the display rotation was oppositely directed for the two arms, initial training with neither arm affected subsequent opposite arm performance. This revealed that movement information obtained during the opposite arm training is not obligatorily transferred to interfere with subsequent performance with the other arm (Wang and Sainburg 2003). In contrast, within the same arm, adaptation to an oppositely rotated display is known to interfere with the recall of previous movement information (Krakauer et al. 2000; Tong et al. 2002). Taken together, these findings suggest that learning of a visuomotor rotation is stored in limb-specific memory, but can be subsequently accessed by the other limb’s controller. Previous research has also indicated that interlimb transfer of visuomotor rotations is limited, such that the initial trials of opposite arm performance are substantially less accurate than those of final adaptation (Sainburg and Wang 2002; Wang and Sainburg 2003). However, it is not yet known whether this limit is due to deficiencies in the stored information, or rather to restrictions in opposite arm access to that information.
Previous research, examining performance in the dominant arm, indicates that adaptation to a novel visuomotor transformation in one region of workspace generalizes to another region of workspace that has not been experienced during training (Ghahramani et al. 1996; Krakauer et al. 2000; Vetter et al. 1999). However, the degree of transfer is controversial. Vetter et al. (1999) previously showed that sensorimotor rotations learned in one region can generalize throughout the workspace. These findings indicate that the ability of the nervous system to apply the learned transform is not limited to the state space experienced during learning. However, the extent of generalization appears to depend on the range of conditions practiced during initial learning. Krakauer et al. (2000) recently showed that adaptation to a visuomotor rotation during movements made toward multiple target directions leads to generalization over a larger range of workspace than that following single target practice. Whereas the visuomotor adaptation took longer when training with multiple target directions, the range of generalized directions increased with the number of training directions. These findings suggest that the larger the range of conditions experienced during practice, the more complete is the learned sensorimotor transformation.
The purpose of this study was to determine whether interlimb transfer of visuomotor rotations is greater following multiple direction adaptation than following a single one. If more extensive practice with the opposite arm leads to greater interlimb transfer, the restriction in transfer can be attributed to inadequate model development. However, if opposite arm transfer does not improve with more complete learning, this would suggest that learning is, at least partially, effector dependent. The latter findings would be surprising because earlier studies have shown that generalization of visuomotor adaptation occurs best when movements are consistent within an extrinsic, as opposed to an intrinsic, coordinate system (Wigmore et al. 2002). This suggests that such visuomotor adaptations are not effector specific.
Methods
Subjects
The subjects were 48 neurologically intact right-handed adults (26 female 22 male), aged from 18 to 40 years. They were recruited from the university community, and were paid for their participation. Informed consent was solicited prior to participation. Right-handedness was assessed using the ten-item version of the Edinburgh inventory (Oldfield 1971).
Apparatus
Subjects sat facing a table with either the right or left arm supported over a horizontal surface, positioned just below shoulder height, by a frictionless air jet system (Fig. 1A). A start circle, target, and cursor representing the index finger position were projected on a horizontal back-projection screen positioned above the arm (Fig. 1B). A mirror, positioned parallel to and below this screen, reflected the visual display, so as to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display ensured that this projection was veridical. The start circle and the targets appeared at the same positions (i.e., center of workspace) for both arms. Position and orientation of each limb segment was sampled using a magnetic 6-degrees-of-freedom movement recording system, Flock of Birds (Ascension Technology, Burlington, VT, USA). The position of the following three bony landmarks was digitized: (1) index finger tip, (2) the lateral epicondyle of the humerus, and (3) the acromion, directly posterior to the acromio-clavicular joint. As sensor data was received from the Flock of Birds, the position of these landmarks was computed by our custom software. For more detailed information, see Sainburg and Wang (2002).
Fig. 1.
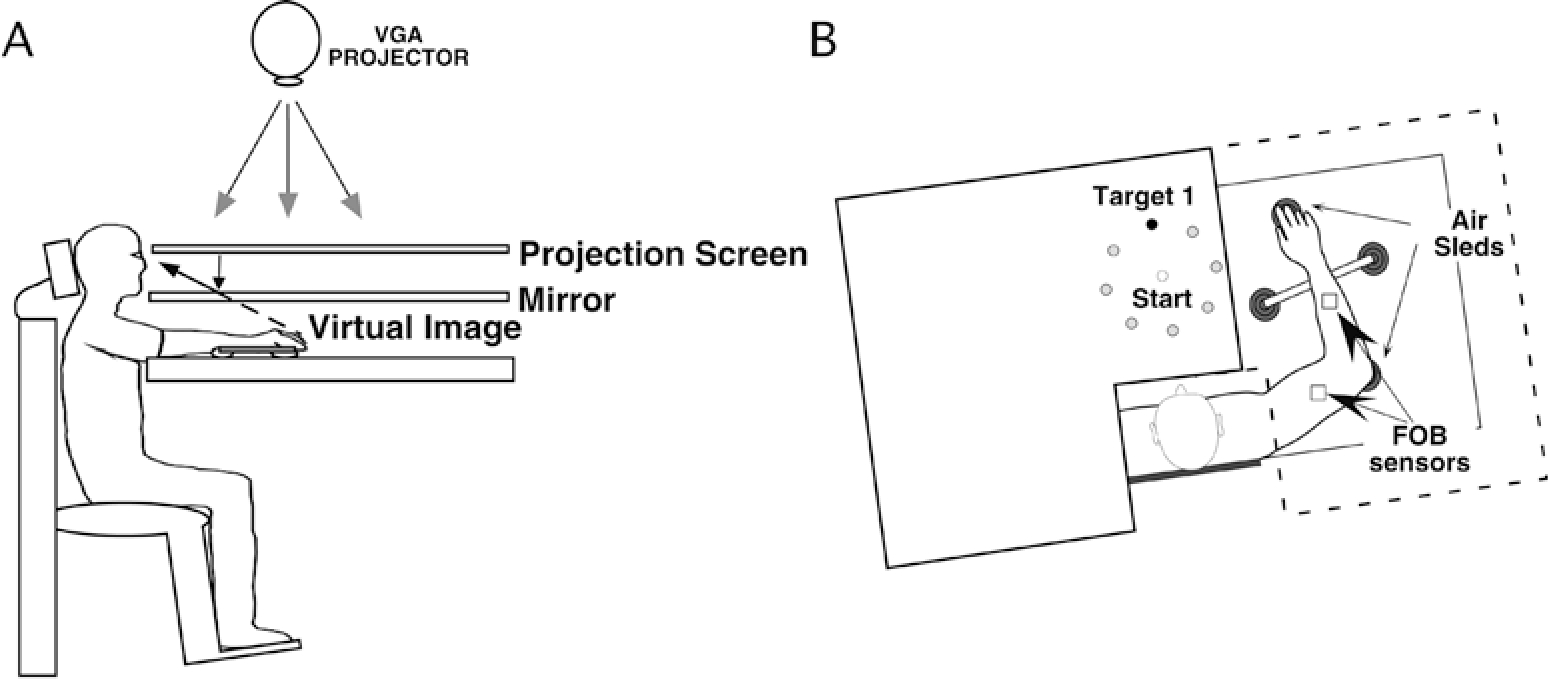
A, B Experimental setup. A Side view. Subjects were seated in a dentist-type chair with the arm supported by an air jet system that removed the effects of friction on arm movement. Targets and the cursor representing finger position were back-projected on a screen placed above the arm. A mirror placed below this screen reflected the image, such that the projection was perceived in the plane of the arm. B Top view. The positions of the Flock of Birds sensors are shown. For the eight-target training condition, a target was randomly displayed on one of the eight target locations. Black dot represents the location of the target displayed for the one-target training condition
Experimental design
The experiment consisted of two sessions: baseline (no visual rotation), and exposure (visual rotation) sessions. Subjects performed two blocks of trials in each session, with each block being performed with either the dominant or non-dominant arm. During the exposure session, the position of the cursor was rotated 30° either clockwise (CW) or counterclockwise (CCW) relative to the start circle. In this study there were three variables of main interest: the number of target directions experienced during initial exposure to visual rotations (one versus eight directions), the compatibility of rotations between the arms in direction (same versus opposing rotations), and the sequence of the arms exposed to visual rotations (dominant arm first versus non-dominant arm first). Therefore, the subjects were first allocated to one of two groups: one that experienced eight target directions (targets 1–8) during the first block of exposure session with one arm; and the other that experienced only one (target 1) of the eight directions during the same block. During the second block of exposure session with the other arm, both groups of subjects experienced only one target direction (target 1), which had been experienced by both groups during the first block. Each group was further divided into two groups: one that experienced CCW visual rotations for both arms (same rotation); and the other that experienced CCW and CW rotations for the dominant and non-dominant arms, respectively (opposing rotation). Half the subjects in each group then performed with the non-dominant arm first (LR), while the other half performed with the dominant arm first (RL). Table 1 shows the sequence of the experimental blocks for each group.
Table 1.
Experimental design. All subjects were right-handed; half performed with the dominant arm first (RL) while the other half performed with the non-dominant arm first (LR). R Right arm, L left arm, CW clockwise, CCW counterclockwise
| No. of targets trained | Rotation direction | Group | Baseline | Exposure | ||
|---|---|---|---|---|---|---|
|
| ||||||
| 8 | Same | RL (n=6) | R 0° | L 0° | R 30° CCW | L 30° CCW |
| (192 trials) | (192 trials) | (192 trials) | (64 trials) | |||
| LR (n=6) | L 0° | R 0° | L 30° CCW | R 30° CCW | ||
| (192 trials) | (192 trials) | (192 trials) | (64 trials) | |||
| Opposite | RL (n=6) | R 0° | L 0° | R 30° CCW | L 30° CW | |
| (192 trials) | (192 trials) | (192 trials) | (64 trials) | |||
| LR (n=6) | L 0° | R 0° | L 30° CW | R 30° CCW | ||
| (192 trials) | (192 trials) | (192 trials) | (64 trials) | |||
| 1 | Same | RL (n=6) | R 0° | L 0° | R 30° CCW | L 30° CCW |
| (192 trials) | (192 trials) | (64 trials) | (64 trials) | |||
| LR (n=6) | L 0° | R 0° | L 30° CCW | R 30° CCW | ||
| (192 trials) | (192 trials) | (64 trials) | (64 trials) | |||
| Opposite | RL (n=6) | R 0° | L 0° | R 30° CCW | L 30° CW | |
| (192 trials) | (192 trials) | (64 trials) | (64 trials) | |||
| LR (n=6) | L 0° | R 0° | L 30° CW | R 30° CCW | ||
| (192 trials) | (192 trials) | (64 trials) | (64 trials) | |||
Prior to movement, a target (2 cm in diameter; 13 cm away from the starting position) was displayed on the horizontal tabletop. (For the subjects in the eight-direction condition, the eight targets were presented in a pseudorandom sequence.) Subjects were instructed to move the finger from the start circle (1.5 cm in diameter) to the target using a single, rapid motion in response to an auditory “go” signal. During the movement, visual feedback was provided by a screen cursor. At the end of each trial, knowledge of results was provided in the form of a circle indicating the final location of the index finger, and by points awarded for accuracy: 1 point for accuracy <3 cm, 3 points for accuracy <2 cm, and 10 points for accuracy <1 cm. No points were given for movements that took longer than 0.250 s.
Each block of the baseline session comprised 192 trials. During the exposure session, the first block for the eight-direction group comprised 192 trials, divided into 24 cycles, with each cycle containing eight consecutive trials of all different targets, while the same block for the one-direction group comprised 64 trials, divided into eight epochs, with each epoch containing eight consecutive trials of the same target. The second block of the exposure session was identical for every group, comprising 64 trials, also divided into eight epochs. The blocks in which only one target direction was present comprised 64 trials, instead of 192, to avoid detrimental effects due to boredom and/or loss of motivation. The amount of training for the two groups was chosen, based on preliminary data, to counterbalance the total number of trials (i.e., general task experience) and the number of trials to target 1 (i.e., specific target direction experience). Our preliminary data demonstrated that adaptation to the rotation occurred well before trial 24 in the one-direction group, and well before cycle 24 for the eight-direction group. In fact, as detailed in Fig. 3 (inset), final adaptation levels were achieved by the seventh trial to this target in both groups of subjects. Each block of trials was separated by a 10-min break.
Fig. 3.
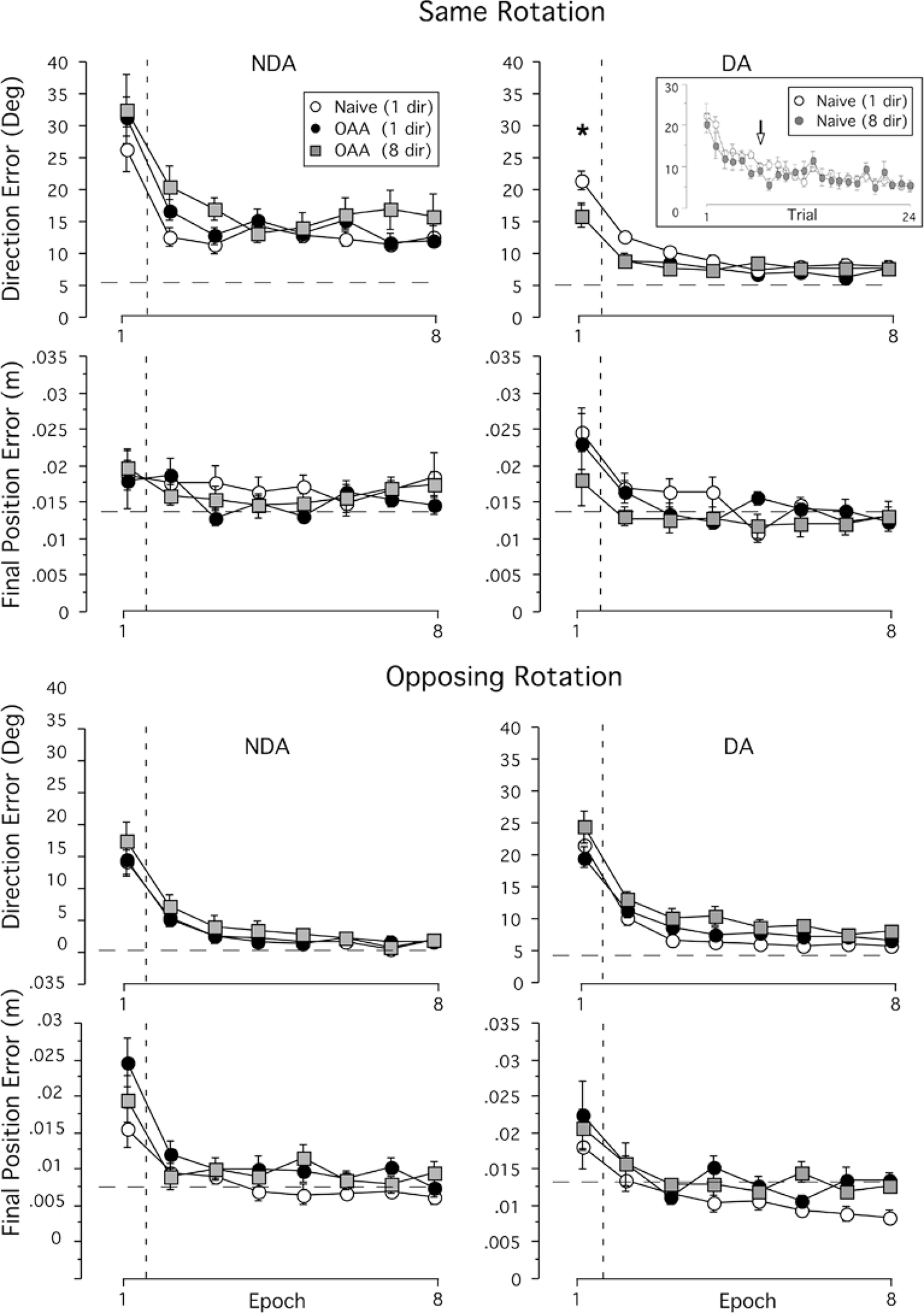
Mean performance measures of direction error at peak tangential arm velocity (rows 1 and 3) and final position error (rows 2 and 4), are shown for non-dominant (NDA, column 1) and dominant (DA, column 2) arms, for the same (upper panels) and opposing (lower panels) visual rotation conditions separately. Every data point shown on x-axis represents the average of eight consecutive trials across all subjects (mean ±SE). Performance measures for naive performance (open circles) and performance following opposite arm adaptation for the eight-direction (filled squares) and one-direction (filled circles) training groups are shown separately. Horizontal dashed lines represent the final adaptation value (the mean of the last eight cycles) during baseline performance of the naïve performance under the one-direction training condition. This final adaptation value was not significantly different from that of the baseline performance following opposite arm adaptation under either the one- or eight-direction training condition. Asterisk indicates a significant difference between naive performance and performance following opposite arm adaptation (OAA) at P<0.05. Differences between eight-direction and one-direction training groups were not statistically significant. Every data point on x-axis in the inset (top right) represents the average of each trial across all subjects (mean ±SE) during initial exposure training with the dominant arm. Performance measures for the eight-direction (filled circles) and one-direction (open circles) training groups are shown separately. Arrow indicates the first trial that is not significantly different from the mean of trials 19–24 with an α-level of 0.05
Data analysis
Two measures of performance were calculated: hand-path direction errors at peak tangential arm velocity (Vmax) and final position error. Direction error was calculated as the angular difference between the vectors defined by the target and by the hand-path position at movement start and at Vmax (absolute values were used to calculate the means for each cycle/epoch). Final position error was calculated as the two-dimensional distance between the index finger at movement termination and the center of the target.
Because the purpose of this study was to examine the effect of initial training with one arm on subsequent performance with the other arm, pair-wise comparisons using Bonferroni/Dunn analysis were made between naive performance and the performance following opposite arm adaptation for the dominant arm blocks (right-arm performances by LR and RL groups), and also for the non-dominant-arm blocks (left-arm performances by LR and RL groups). Thus, performances following opposite arm adaptation under both eight- and one-direction training conditions were compared with the naive performance of the same arm under the one-direction training condition. These pair-wise comparisons were of primary interest at the very first epoch, because previous studies have shown substantial effects of initial training on the very first trials of the subsequent testing session (Krakauer et al. 1999; Sainburg and Wang 2002; Tong et al. 2002; Wigmore et al. 2002).
Results
Mean direction and final position errors at the first epoch
Figure 2 shows typical hand-paths of representative subjects during naive performance, as well as during subsequent performance following opposite arm adaptation. In the figure, The hand-paths of the first four (1–4 of 64) and the last four (61–64 of 64) consecutive trials within the same exposure session are illustrated as black and gray lines, respectively. The first few trials of naive performance are initially directed about 30° CCW to the target, in accord with the imposed visual rotation. Following adaptation, exemplified by the last four trials, movements become relatively straight and are oriented toward the target. The facilitating effect of the experience of the same rotation (CCW) with the non-dominant arm prior to dominant-arm movement is illustrated in column 2 (top) of Fig. 2. Whereas the first two trials are similar to those of naive performance, the next two trials are already nearly as accurate as that of final adaptation. Whether non-dominant-arm adaptation was done to a single target or to eight targets, subsequent dominant-arm adaptation was similarly facilitated. Initial dominant-arm training had no apparent influence on non-dominant-arm adaptation, irrespective of whether it was done to one or to eight targets. In addition, when the direction of visual rotation was opposing for the two arms, opposite arm training did not appear to have any effect on the subsequent performance of either arm.
Fig. 2.
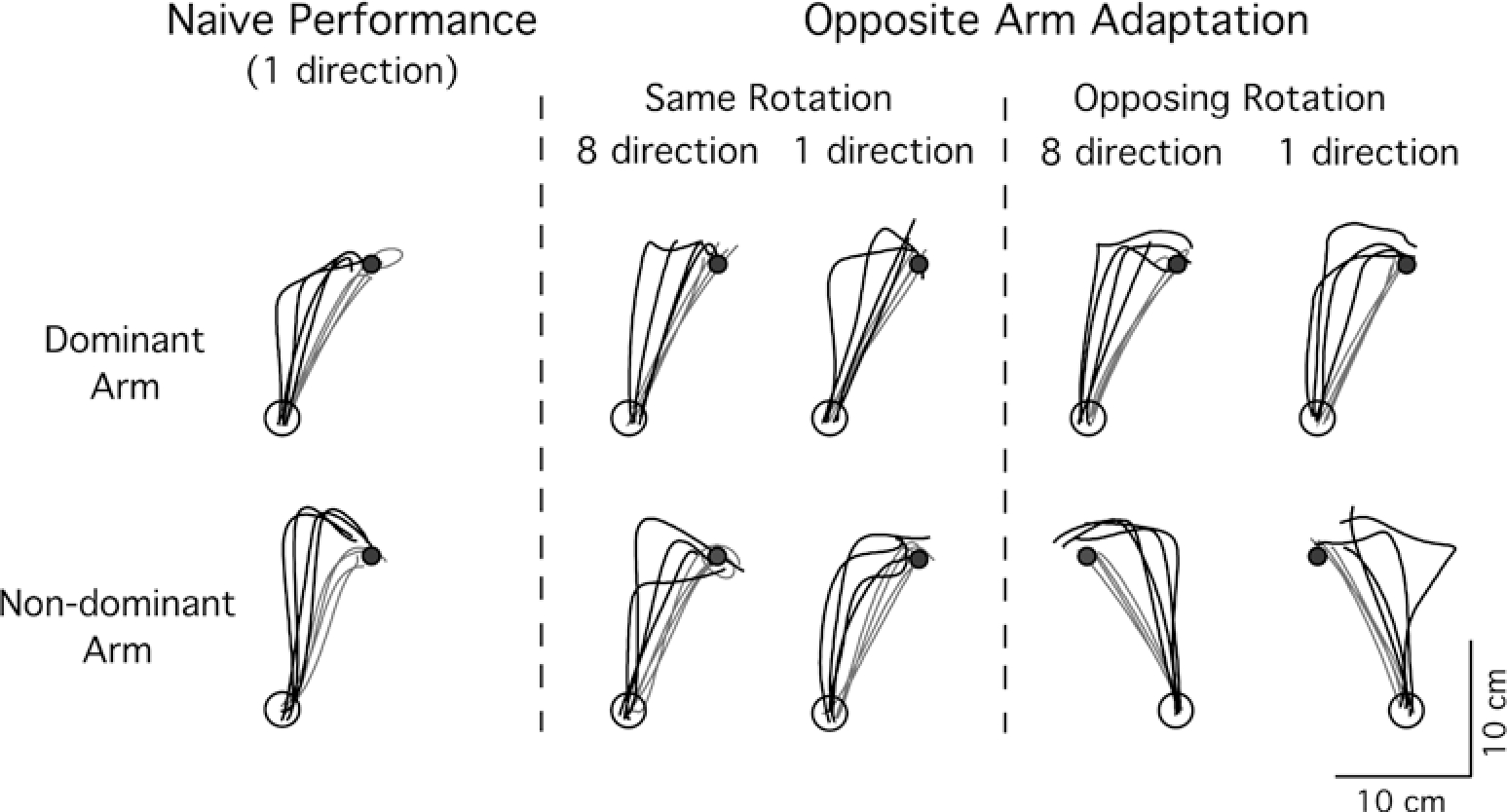
Hand-paths of representative subjects from the eightdirection and one-direction training groups. hand paths for the dominant arm are shown along the top row, whereas those for the non-dominant arm are shown below. Column 1 shows hand-paths of the first four (black lines) and the last four (gray lines) trials of naive performance by each arm during initial exposure. Column 2 shows hand-paths of the first four and the last four trials of performance following opposite arm adaptation for the eight-direction and one-direction training groups, respectively, under the same visual rotation condition; column 3 shows hand-paths of the first four and the last four trials of performance following opposite arm adaptation for the eight-direction and one-direction training groups, respectively, under the opposing visual rotation condition
We quantified these effects, in terms of initial direction error and final position accuracy, by making pair-wise comparisons between naive performance and the performance following opposite arm adaptation, for each arm and for each rotation direction separately (Fig. 3). We expected that the effects of opposite arm training should be apparent in the first epoch (eight trials) of the session (see Sainburg and Wang 2002; Wang and Sainburg 2003). Statistical analysis revealed that only the dominant arm showed a substantial effect of opposite arm training, only when the direction of rotation was the same for both arms, and only for movement direction errors (P<0.001). The inset of Fig. 3 depicts a comparison between the dominant arm, naive performance of the eight-direction group and that of the one-direction group. For this comparison, data is only shown for the movements made toward target 1 (total of 24 trials). The direction errors for target 1 were not significantly different between the two groups during initial training, and both groups reached the final adaptation level (the mean of trials 19–24) by the seventh trial, as marked by the arrow in Fig. 3. Both groups maintained that level of performance without further improvements or decrements. These results indicate that both groups showed similar levels of adaptation to target 1 during opposite arm exposure. Under opposing rotation conditions, as illustrated in Fig. 3 bottom panel, no effect of opposite arm adaptation occurred, either positive or negative. We previously showed that the lack of interference from opposite direction learning reflected selective access of opposite-arm-derived, visuomotor transform information (Wang and Sainburg 2003).
Effects of eight- and one-direction training conditions across individual trials
Figure 4 compares the effects of eight-direction and onedirection non-dominant-arm training on subsequent performance with that of the dominant arm. The mean direction errors of the dominant-arm performance for the same rotation condition are shown. This is the only condition for which interlimb transfer was demonstrated. In order to characterize the time-course of adaptation, we show the average errors for trial 1, trials 2–4, trials 13–15, and trials 22–24. Trial 1 has been segregated from other trials because we have previously shown that the effect of opposite arm adaptation on this trial is always negligible (Wang and Sainburg 2003). Direction errors shown on the y-axis of Fig. 4 are expressed as the percentage decrease in error, relative to the average error for the same trial range of dominant-arm performance under the naive conditions [i.e., 100×(error from naive performance − error from opposite arm adaptation)/error from naïve performance]. Statistical analyses showed that at trial 1, the direction errors during subsequent performance following opposite arm adaptation under either training condition were not significantly different from that of naive performance (P=0.38 and 0.29 for eight- and one-target training conditions, respectively). The facilitating effects from prior opposite arm training became apparent after the first trial. The percentage improvement in direction errors, collapsed across trials 2–4, was significant (P<0.01); and for trials 13–15, the improvement increased substantially (P<0.0001). However, by trials 22–24, performance became similar to that of baseline, reflecting adaptation to the rotated condition (P=0.68 and 0.36 for eight- and one-target training conditions, respectively). As demonstrated in Fig. 4 by the similarity in bars within each cluster of trials, direction errors never differed significantly depending on whether subjects practiced with eight- or one-direction targets (P=0.86, 0.97, 0.36, and 0.62 at trials 1, 2–4, 13–15, and 22–24, respectively).
Fig. 4.
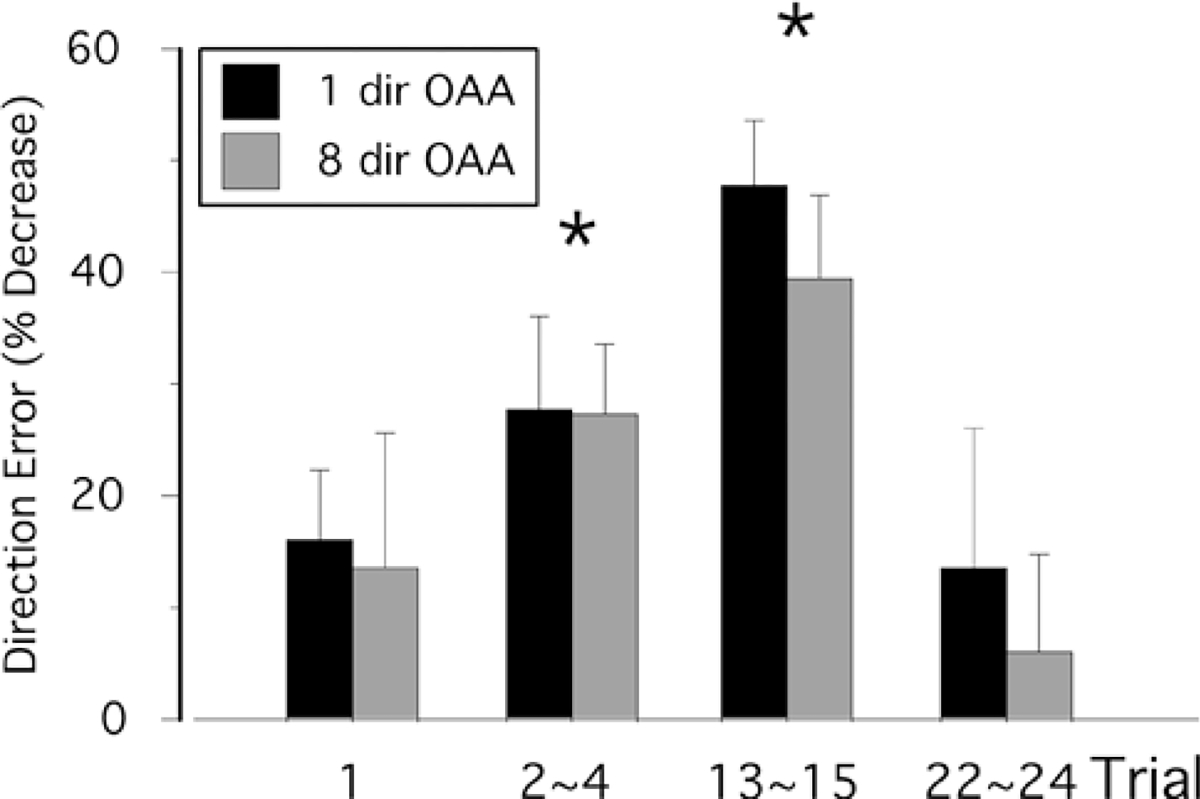
Direction errors of the dominant arm performance for selective trials under the same rotation condition. Black bars represent performance of the one-direction group, whereas gray bars represent performance of the eight-direction group. Direction errors on y-axis are expressed as the percentage of decrease in error relative to that of naive performance, with 0% indicating no decrease in error, or no improvement in performance following opposite arm adaptation (OAA), as compared to naive performance. Asterisks represent significant differences at P<0.05 between naive performance and performance following OAA. Differences between eightand one-direction training groups were not statistically significant
Discussion
Multiple direction learning does not benefit interlimb transfer
It has previously been shown that learning visuomotor rotations with multiple target directions, compared with a single target direction, leads to greater transfer to untrained targets within the same limb (Krakauer et al. 2000). This implies that multiple direction learning results in a more complete internal model of the visuomotor transform. It has also been documented that interlimb transfer of visuomotor adaptations is limited, relative to that reported for intralimb generalization (Krakauer et al. 2000; Morton et al. 2001; Sainburg and Wang 2002; Wang and Sainburg 2003). In the present study, we thus examined the origin of this restriction in interlimb transfer. We expected that if multiple direction learning leads to a more complete model of the novel visuomotor transform, interlimb transfer should be enhanced, relative to that following single-direction training. However, if no differences are observed between single- and multiple-direction training conditions, interlimb transfer must be restricted by factors other than the nature of the learned transform. We also tested whether interlimb transfer of visuomotor adaptation is obligatory. Previous data (Wang and Sainburg 2003) indicated that when the direction of rotations was the same, opposite arm training improved subsequent dominant arm adaptation. However, when the direction of rotations was opposite, no interference in subsequent performance occurred. Because the first trial, following opposite arm training, was never significantly different from naive performance, this trial appeared to be used to probe whether the opposite-arm-derived transform was applicable to current movement conditions. We further examined this hypothesis by testing interlimb transfer following the same or oppositely directed visuomotor rotations in the current study.
Our results corroborate our previous findings, indicating transfer of initial movement direction accuracy from only the non-dominant to the dominant arm (Sainburg and Wang 2002; Wang and Sainburg 2003). Also, consistent with previous reports, transfer was incomplete, such that the dominant-arm performance following opposite arm adaptation in its initial phase was substantially less accurate than that in its final phase. Both eight- and one direction training conditions led to substantial transfer in subsequent performance with the opposite arm, although interlimb transfer following multiple direction training was not different than that following single-direction training. The fact that interlimb transfer occurred at all supports the idea that visuomotor rotation learning is not limb specific. However, the lack of improvement in interlimb transfer with multiple direction training suggests that the previously reported intralimb advantages of multiple direction learning are limb, or effector, specific.
The lack of advantage of multiple direction learning may be best understood by considering a hierarchical model of motor control. At least three serially organized processes underlie visually guided reaching behavior: visuomotor transformations, trajectory specification, and dynamic transformations (Ghez et al. 1989, 1991; Imamizu et al. 1998; Jordan and Rumelhart 1992; Kawato 1999; Kawato et al. 1988, 1990; Krakauer et al. 1999; Krakauer et al. 2000; Rosenbaum and Chaiken 2001; Sainburg 2002). According to this model, visuomotor transformations occur upstream to dynamic transformations, in which the trajectory plan is transformed into dynamic properties reflecting the forces required to complete the motion. We have previously shown that the latter process is, to a large extent, limb specific (Sainburg 2002). Thus, adaptation to novel inertial dynamics shows asymmetries between the limbs, whereas adaptation to novel visuomotor rotations does not. The current finding, that multiple direction training does not benefit interlimb generalization, suggests that the advantage of such experience may not emerge until the newly learned visuomotor transform becomes directly associated with the dynamic properties of the limb to be used. This implies that the levels of the control hierarchy described above are not independent of one another. However, further research is necessary to determine whether the generalization advantages of multiple direction learning are specific to the effectors being employed, even within a single limb.
Why is information transferred incompletely from one arm to the other?
Opposite arm training improved the direction accuracy of the first epoch of dominant-arm movements by approximately 15 to 30% (see Fig. 4, trials 1 to 4). Given the current data, we cannot make a direct comparison to estimate the amount of information retained within performances in the same arm. However, data from previous studies indicate that the amount of retained information within the arm is quite large, in comparison. For example, Wigmore et al. (2002) showed, using center-out reaching tasks, that the amount of information retained over a 24-h period within the dominant arm was approximately 54% with 30° visual rotation, and 66% with 45° rotation. Morton et al. (2001) directly compared intralimb with interlimb transfer of weight information, using a ball-catching task. That study demonstrated that the extent of interlimb transfer was 58%, whereas that of intralimb transfer to different limb configurations was 100%. Such findings suggest incomplete transfer of learning between the limbs.
Logan (1988) hypothesized that the initiation and the final phases of learning involve two independent neural processes. This idea may explain the current limitation observed in interlimb transfer. According to Logan’s instance theory of automatization, the initial phase of adaptation is dominated by algorithmic learning, in which one successively improves a rule-based method of control. However, once a store of specific performance instances becomes available, retrieval of these instances competes with such algorithmic processes. Because instance retrieval is quicker than algorithmic control, this process dominates the later, asymptotic, phase of adaptation. Based on this idea, we now hypothesize that the initial phase of adaptation, following opposite arm performance, reflects the interlimb transfer of a general visuomotor algorithm. However, more accurate performance remains dependent on the development of effector-specific instances. Interlimb transfer may be incomplete because effector-specific instances are not available during early performance. This idea is consistent with the argument made by Morton et al. (2001) that interlimb generalization involves an internal representation that is not effector specific, whereas intralimb generalization involves a separate representation that is effector specific. This hypothesis might also explain why adaptation to opposing rotations causes interference with subsequent performances with the same arm (Krakauer et al. 1999; Wigmore et al. 2002), but adaptation to a given rotation with one arm does not interfere with opposite arm adaptation to an opposing rotation. Such, apparently paradoxical, findings might be explained by assuming that interference occurs due to the obligatory retrieval of effector-specific instances that are incompatible, and interference may not occur across the limbs because such effector-specific instances are not available (Wang and Sainburg 2003).
Acknowledgements
This research was supported by US National Institutes of Health grants R01HD39311 and NRSA 1-F32-NS-46239–1.
References
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R (2003) Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89:168–176 [DOI] [PubMed] [Google Scholar]
- Dizio P, Lackner JR (1995) Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol 74:1787–1792 [DOI] [PubMed] [Google Scholar]
- Elliott D, Roy EA (1981) Interlimb transfer after adaptation to visual displacement: patterns predicted from the functional closeness of limb neural control centres. Perception 10:383–389 [DOI] [PubMed] [Google Scholar]
- Ghahramani Z, Wolpert DM, Jordan MI (1996) Generalization to local remappings of the visuomotor coordinate transformation. J Neurosci 16:7085–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Hening W, Favilla M (1989) Gradual specification of response amplitude in human tracking performance. Brain Behav Evol 33:69–74 [DOI] [PubMed] [Google Scholar]
- Ghez C, Hening W, Gordon J (1991) Organization of voluntary movement. Curr Opin Neurobiol 1:664–671 [DOI] [PubMed] [Google Scholar]
- Imamizu H, Shimojo S (1995) The locus of visual-motor learning at the task or manipulator level: implications from intermanual transfer. J Exp Psychol Hum Percept Perform 21:719–733 [DOI] [PubMed] [Google Scholar]
- Imamizu H, Uno Y, Kawato M (1998) Adaptive internal model of intrinsic kinematics involved in learning an aiming task. J Exp Psychol Hum Percept Perform 24:812–829 [DOI] [PubMed] [Google Scholar]
- Jordan MI, Rumelhart DE (1992) Forward Models: supervised learning with a distal teacher. cognitive science 16:307–354 [Google Scholar]
- Kawato M (1999) Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9:718–727 [DOI] [PubMed] [Google Scholar]
- Kawato M, Isobe M, Maeda Y, Suzuki R (1988) Coordinates transformation and learning control for visually-guided voluntary movement with iteration: a Newton-like method in a function space. Biol Cybern 59:161–177 [DOI] [PubMed] [Google Scholar]
- Kawato M, Maeda Y, Uno Y, Suzuki R (1990) Trajectory formation of arm movement by cascade neural network model based on minimum torque-change criterion. Biol Cybern 62:275–288 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Ghez C (1999) Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2:1026–1031 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C (2000) Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20:8916–8924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo JI, Baguley RA, Bairstow PJ (1970) Bilateral transfer in tapping skill in the absence of peripheral information. J Mot Behav 2:261–271 [DOI] [PubMed] [Google Scholar]
- Logan GD (1988) Toward an instance theory of automatization. Psychol Rev 95:492–527 [Google Scholar]
- Marzi CA, Bisiacchi P, Nicoletti R (1991) Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia 29:1163–1177 [DOI] [PubMed] [Google Scholar]
- Morton SM, Lang CE, Bastian AJ (2001) Inter- and intra-limb generalization of adaptation during catching. Exp Brain Res 141:438–445 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M (1989) Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn 11:98–113 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Chaiken SR (2001) Frames of reference in perceptual-motor learning: evidence from a blind manual aiming task. Psychol Res 65:119–127 [DOI] [PubMed] [Google Scholar]
- Sainburg RL (2002) Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142:241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J (2002) Interlimb transfer of visuomotor rotations: Independence of direction and final position information. Exp Brain Res 145:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Vaid J (1996) Asymmetries in intermanual transfer of maze learning in right- and left-handed adults. Neuropsychologia 34:605–608 [DOI] [PubMed] [Google Scholar]
- Taylor HG, Heilman KM (1980) Left-hemisphere motor dominance in righthanders. Cortex 16:587–603 [DOI] [PubMed] [Google Scholar]
- Thut G, Cook ND, Regard M, Leenders KL, Halsband U, Landis T (1996) Intermanual transfer of proximal and distal motor engrams in humans. Expl Brain Res 108:321–327 [DOI] [PubMed] [Google Scholar]
- Tong C, Wolpert DM, Flanagan JR (2002) Kinematics and dynamics are not represented independently in motor working memory: evidence from an interference study. J Neurosci 22:1108–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Goodbody SJ, Wolpert DM (1999) Evidence for an eye-centered spherical representation of the visuomotor map. J Neurophysiol 81:935–939 [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2003) Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore V, Tong C, Flanagan JR (2002) Visuomotor rotations of varying size and direction compete for a single internal model in motor working memory. J Exp Psychol Hum Percept Perform 28:447–457 [DOI] [PubMed] [Google Scholar]
- Wolpert D, Ghahramani Z (2000) Computational principles of movement neuroscience. Nat Neurosci 3:1212–1217 [DOI] [PubMed] [Google Scholar]


