Abstract
This study was designed to examine interlimb asymmetries in responding to unpredictable changes in inertial loads, which have implications for our understanding of the neural mechanisms underlying handedness. Subjects made repetitive single joint speed constrained 20° elbow flexion movements, while the arm was supported on a horizontal, frictionless, air-jet system. On random trials, a 2-kg mass was attached to the arm splint prior to the “go” signal. Subjects were not given explicit information about the mass prior to movement nor were they able to view their limb or the mass. Accordingly, muscle activity, recorded prior to peak tangential finger acceleration, was the same for loaded and baseline trials. After this point, substantial changes in muscle activity occurred. In both limbs, the load compensation response was associated with a reduction in extensor muscle activity, resulting in a prolonged flexion phase of motion. For the nondominant arm, this resulted in effective load compensation, such that no differences in final position accuracy occurred between loaded and baseline trials. However, the dominant arm response also included a considerable increase in flexor muscle activity. This substantially prolonged the flexor acceleration phase of motion, relative to that of the nondominant arm. As a result, the dominant arm overcompensated the effects of the load, producing a large and systematic overshoot of final position. These results indicate more effective load compensation responses for the nondominant arm; supporting a specialized role of the nondominant arm/hemisphere system in sensory feedback mediated error correction mechanisms. The results also suggest that specialization of the dominant arm system for controlling limb and task dynamics is specifically related to feedforward control mechanisms.
INTRODUCTION
The explanation for hemispheric asymmetries defining handedness has been proposed by two hypotheses. The Open/Closed-loop hypothesis has emerged from studies comparing dominant and nondominant arm performance in healthy adults (Annett et al. 1979; Carson 1993; Elliott et al. 2001) and has been supported by studies examining the ipsilesional arm in unilaterally brain damaged patients (Haaland and Delaney 1981; Haaland and Harrington 1989a,b, 1996). The term, “closed-loop” refers to mechanisms that are mediated by sensory feedback, whereas “open-loop” refers to mechanisms, which for any given movement, are not affected by sensory feedback. However, open-loop mechanisms may be influenced through the feedforward use of sensory information obtained during previous movements. Many studies examining the open/closed loop hypothesis in healthy adults have focused on asymmetries in the use of visual feedback. However, a number of studies that have specifically examined manual asymmetries under varied feedback conditions have failed to reveal a dependence on visual feedback (Carson et al. 1990, 1992, 1993; Roy and Elliot 1986). An alternative hypothesis of motor lateralization has recently been proposed by Sainburg (2002): the dynamic dominance hypothesis suggests that the dominant hemisphere/limb system is specialized for controlling limb and task dynamics, whereas the nondominant arm system appears specialized for controlling limb position, or posture. The dynamic dominance and the open/closed loop hypotheses of motor lateralization can be considered, to some degree, to be convergent. This is because previous research has indicated that control of intersegmental dynamics is largely dependent on anticipatory control mechanisms, which, for any given trial, are mediated by “open-loop” mechanisms (Sainburg et al. 1995, 1999; Schneider et al. 1989). Previous research has also revealed a possible superiority of nondominant arm control for final position accuracy, when visual feedback is not available during movement (Bagesteiro and Sainburg 2002; Sainburg 2002; Sainburg and Wang 2002). Because accuracy in final position is largely related to events occurring in the latter phase of motion, when feedback mechanisms become available, these findings may be associated with an advantage for somatosensory based, closed-loop control mechanisms.
The dynamic dominance hypothesis emerged from studies examining intersegmental coordination during multijoint reaching tasks. We have shown that, during horizontal plane reaching in a variety of directions, dominant arm movements of the same amplitude and speed are made with a fraction of the joint torque and integrated EMG of nondominant arm movements (Bagesteiro and Sainburg 2002; Sainburg 2002; Sainburg and Kalakanis 2000). Because the dominant arm controller appears to account better for the interaction torques imposed on a given segment by motion of adjacent segments, hand path curvatures are independent of these interaction torques. In contrast, nondominant hand path curvatures appear to be enslaved to interaction torques, presumably due to inadequate predictions of such interactions during movement preparation. Interestingly, the nondominant arm has consistently shown slightly better final position accuracy, suggesting non-dominant arm superiority for feedback mediated regulation of final limb posture. Nevertheless, the role of feedforward and feedback processes in limb coordination asymmetries remains poorly understood.
Previous studies have examined the effects of sensory feedback on asymmetries in limb performance by using Fitts tapping paradigms (Fitts 1966, 1992; Fitts and Radford 1966), in which performance on rapid, low precision aiming is thought to reflect predominantly open-loop mechanisms. By contrast, high precision, slower movements, are associated with closed-loop mechanisms. Some of these studies have demonstrated dominant arm advantages for the “closed-loop” tasks (Elliott et al. 1994, 1995; Flowers 1975; Roy et al. 1994; Todor and Cisneros 1985), but others have failed to reveal such advantages (Carson et al. 1990, 1992; Roy and Elliott 1986). In fact, some authors have proposed a nondominant arm/hemisphere advantage for open-loop mechanisms, based on advantages in reaction time tasks (Carson et al. 1990; Elliott et al. 1993). Regardless of such conflicts, studies examining deficits after unilateral brain damage in the arm ipsilateral to the lesion have provided substantial support for the open/closed-loop hypothesis of hemispheric control (Haaland and Delaney 1981; Haaland and Harrington 1989a,b 1996; Harrington and Haaland 1997; Prestopnik et al. 2002, 2003; Wyke 1967). Various studies (Haaland and Harrington 1989b, 1996; Harrington and Haaland 1997; Prestopnik et al. 2003) have shown that dominant hemisphere lesions produce deficits in the initial, ballistic component of rapid aimed movements. By contrast, in tasks with higher precision requirements, patients with nondominant lesions show deficits in the final decelerative phase of motion, thought to reflect feedback mediated processes (Haaland and Delaney 1981; Haaland and Harrington 1989a; Prestopnik et al. 2002, 2003; Winstein and Pohl 1995). However, in a recent attempt to test hemispheric contributions of visually mediated error correction mechanisms, Haaland et al. (Harrington and Haaland 1997; Prestopnik et al. 2003) provided evidence that the closed-loop advantage of the nondominant hemisphere does not vary with visual feedback conditions. This leaves open the question of whether somatosensory based feedback mechanisms show asymmetries between the dominant and nondominant limb/hemisphere systems.
In previous studies, compensations for unexpected loads applied during rapid targeted movements occurred approximately 100 ms following movement onset (Forget and Lamarre 1987; Gottlieb 1993, 1996; Latash 1994; Pfann et al. 1998; Shapiro et al. 2002). These responses are thought to be implemented through segmental circuits. We now examined interlimb differences in such load compensation responses. Subjects made repeated single joint elbow flexion movements with both a speed constraint and a spatial target. On random trials, a 2-kg mass was rigidly attached to the forearm splint. Movements were performed in a virtual reality environment, in which hand position was only available to position the limb at the start position, and vision of the arm, hand, or mass condition was never available. The unpredictability of the mass condition allowed us to investigate feedback based correction mechanisms. We were able to test interlimb advantages and/or asymmetries in load compensation, thus extending our understanding of the control mechanisms that define handedness.
METHODS
Subjects
Twelve neurologically intact right-handed adults (6 males and 6 females) aged 21–33 yr old, performed fast point-to-point single joint elbow flexion movements. Only right-handers were recruited; handedness was determined using a 12-item version of the Edinburgh inventory (Oldfield 1971). The subjects gave informed consent prior to participation, which was approved by the Office of Regulatory Compliance of the Penn State University. Each subject performed two 80-trial experimental sessions (one with each arm). Six subjects performed with their left arm first (LR group) and the other six with their right arm first (RL group).
Experimental Setup
Figure 1 illustrates the experimental setup. Subjects sat facing a projection screen with either the right or left arm supported over a horizontal table top, positioned just below shoulder height (adjusted to subjects’ comfort), by an air-jet system, which reduces the effects of gravity and friction. A cursor representing finger position, a start circle, and a target were projected on a horizontal back-projection screen positioned above the arm. A mirror, positioned parallel and below this screen, reflected the visual display, so as to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display assured that this projection was veridical. All joints distal to the elbow were immobilized using an adjustable brace. This virtual reality environment assured that subjects had no visual feedback of their arm, or the mass condition during an experimental session. Movements of the trunk and scapula were restricted using a butterfly-shaped chest restraint. Position and orientation of the segments proximal and distal of the elbow joint were sampled using a Flock of birds (FoB) (Ascension-Technology) magnetic six-degree-of-freedom (6-DOF) movement recording system. A single sensor was attached to the upper arm segment via an adjustable plastic cuff, while another sensor was fixed to the air sled where the forearm was fitted. The sensors were positioned approximately at the center of each arm segment. The position of the following three bony landmarks was digitized using a stylus that was rigidly attached to a FoB sensor: 1) index finger tip; 2) the lateral epicondyle of the humerus; and 3) the acromion, directly posterior to the acromio-clavicular joint. These positions relative to the sensors attached to each arm segment thus remained constant throughout the experimental session. As sensor data were received from the FoB, the position of these landmarks was computed by our custom software yielding the three-dimensional (3D) position of the index finger tip. Because the table surface defined our X-Y plane, perpendicular axis displacement was constant. We, thus used the recorded X-Y coordinates of the finger tip to project a cursor onto the screen. Screen redrawing occurred fast enough to maintain the cursor centered on the fingertip throughout the sampled arm movements. During the experiment, the light was turned off, such that subjects were unable to view their arm. A vertical rod was mounted on the transverse piece of the air-sled allowing the attachment of a 2-kg mass placed 20 cm lateral to the forearm. The air-sled arrangement prevented somatosensory information about the mass through gravitational or frictional effects. In addition, subjects’ view of the arm and mass was blocked prior to the session. Mechanical cues about the mass were limited by providing sham mass trials in which any signal related to the mass application was reproduced by the experimenter.
FIG. 1.
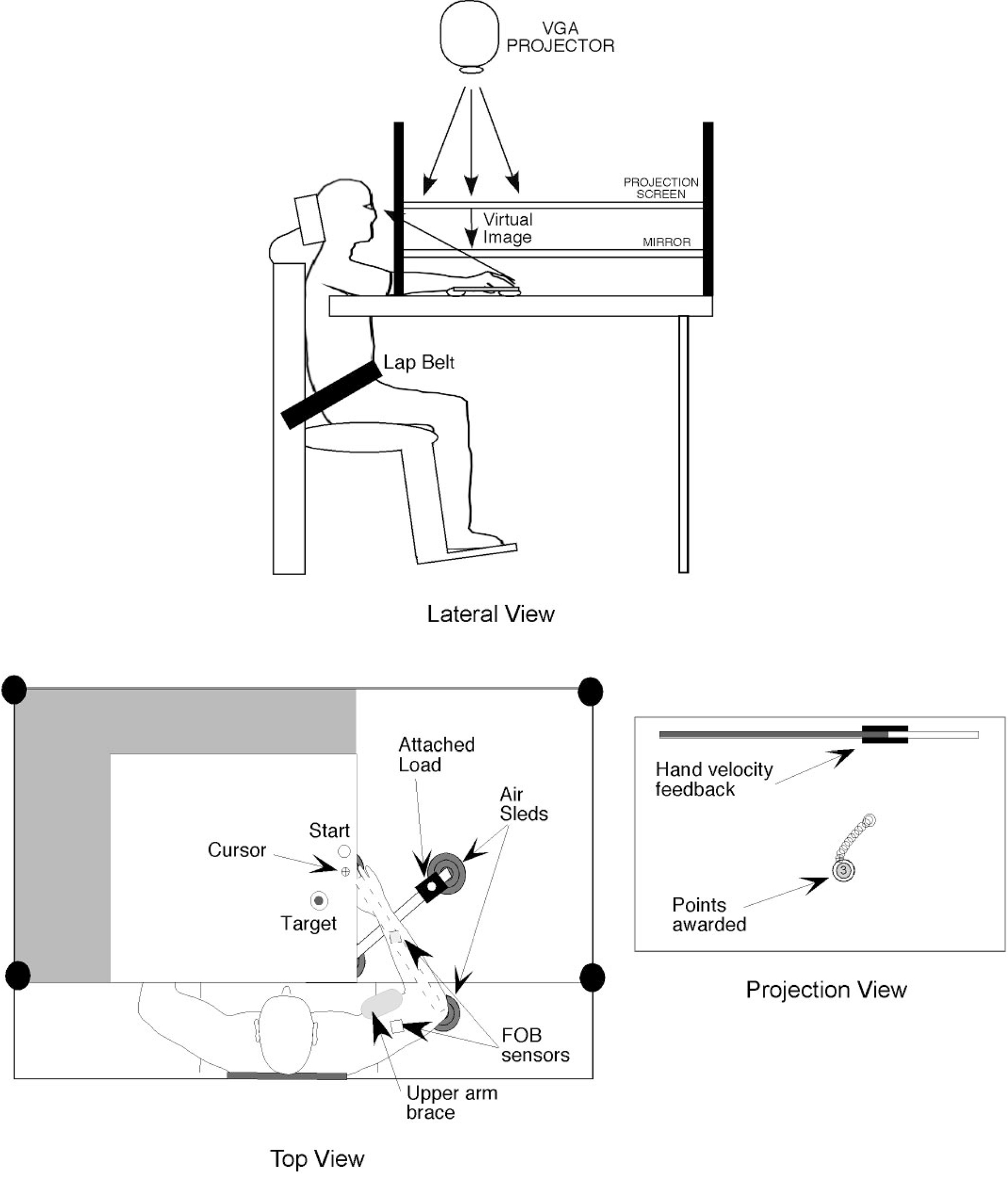
Experimental setup.
Digital data were collected at 103 Hz using a Macintosh computer, which controlled the sensors through separated serial ports, and were stored on disk for further analysis. Custom computer algorithms for experiment control and data analysis were written in REAL BASIC (REAL Software), C, and IgorPro (Wavemetric). EMG activity was recorded from four representative muscles of the elbow joint: biceps brachii, brachioradialis (elbow flexors), long head of triceps brachii, and anconeus (elbow extensors). EMG was recorded with active, bipolar stainless steel surface electrodes (Liberty Mutual MY0111) with a band-pass of 45–550 Hz. The electrode contacts were 3 mm diameter and spaced 13 mm apart. The EMG signals were digitized at 1 kHz using a Macintosh computer equipped with an A/D board (National Instruments PCI-MIO-16XE-50). The EMG signals were full-wave rectified and bin integrated every 10 ms, thereby allowing direct comparison with the kinematic data.
Experimental task
Throughout the experiment, the index finger position was displayed in real-time as a screen cursor. The shoulder-elbow angle (angle formed between upper arm and forearm) established the start and end locations, which were 120° and 100°, respectively. The upper arm was immobilized by a brace. Movements of the arm were thus restricted to the elbow joint. Subjects were to hold the cursor within the starting circle for 2 s to initiate each trial. They were instructed to move the finger to the target using a single, uncorrected motion in response to an audiovisual “go” signal. In addition, subjects were to match their hand velocity within a 1.1- to 1.4-m/s range. A hand velocity gauge (see Fig. 1, screen view) was displayed at the top of the screen, informing the subject of peak hand velocity after each trial. Feedback of the fingertip position (cursor display) was given to allow subjects to position the hand in the start circle and then was removed at the go-signal. At the final position, subjects were given knowledge of results and points were awarded for accuracy. Final position errors of <1 cm were awarded 10 points, errors between 1 and 2 cm were awarded 3 points, and errors between 2 and 3 cm were awarded 1 point. Points were displayed following each trial. At the beginning of the experimental session, the scoring system was explained to the subjects. Each subject performed 80 single joint movements with each arm. The first 30 trials were given as practice to familiarize with the task and velocity match; the following 50 trials were then randomly perturbed five times by adding a 2-kg mass rigidly attached to the forearm splint. Subjects had no knowledge of the occurrence of load addition prior to movement.
Kinematic data
The 3D position of the index finger and elbow were calculated from sensor position and orientation data. Then, joint angle was calculated from this data. All kinematic data were low-pass filtered at 8 Hz (3rd order, dual-pass Butterworth), and differentiated to yield angular velocity and acceleration values. Each trial usually started with the hand at zero velocity, but small oscillations of the hand sometimes occurred within the start circle. In this case, the onset of movement was defined by the last minimum (below 5% maximum tangential velocity) prior to the maximum in the index finger’s tangential velocity profile. Movement termination was defined as the first minimum (below 5% maximum tangential hand velocity) following the peak in tangential hand velocity. Elbow displacement and velocity were calculated from joint angle data. Movement accuracy was measured by calculating the final position error, which was calculated as the distance between the index finger location at movement end and the center of the target.
Electromyographic data
The electrode position was determined according to the maximum recorded EMG activity during isolated flexor or extensor movements. The electrodes were highly selective and minimized crosstalk. The latter was evidenced by the lack of synchronous activity in different electrodes, the voluntary activation of separate muscles in isolation, and the presence of a silent period in the EMG activity of one group of muscles associated with a burst of activity in the antagonist muscles. All signals were recorded from 300 ms before to 1 s after the trigger signal. The digitized EMG data were synchronized to peak tangential finger acceleration. The integrated EMG data were normalized to percent of maximum EMG at each muscle within subjects. The maximum EMG was found using a computer algorithm to locate the highest integrated EMG magnitude for each muscle within the experimental session. The integrated value over the interval was defined as maximum EMG and provided a standard for comparison of agonist and antagonist EMG within a subject and session. We quantified the EMG impulse (integral over 100 ms) for two intervals, 100 ms prior to peak tangential finger acceleration and 100 ms following this point.
Statistical analysis
The individual measures used in this paper were analyzed in separate ANOVAs to test for statistical significance, with the criterion level set at P < 0.05. To assess load compensation, interlimb differences were quantified in the following way: 1) two-way ANOVA analyses to test for significant differences within limbs; and 2) one-way ANOVA tests to compute difference from baseline performance using five-trial averaged profiles for each individual measure (see Fig. 3, B, D, and F). Baseline trials were taken as the trials prior to perturbation.
FIG. 3.
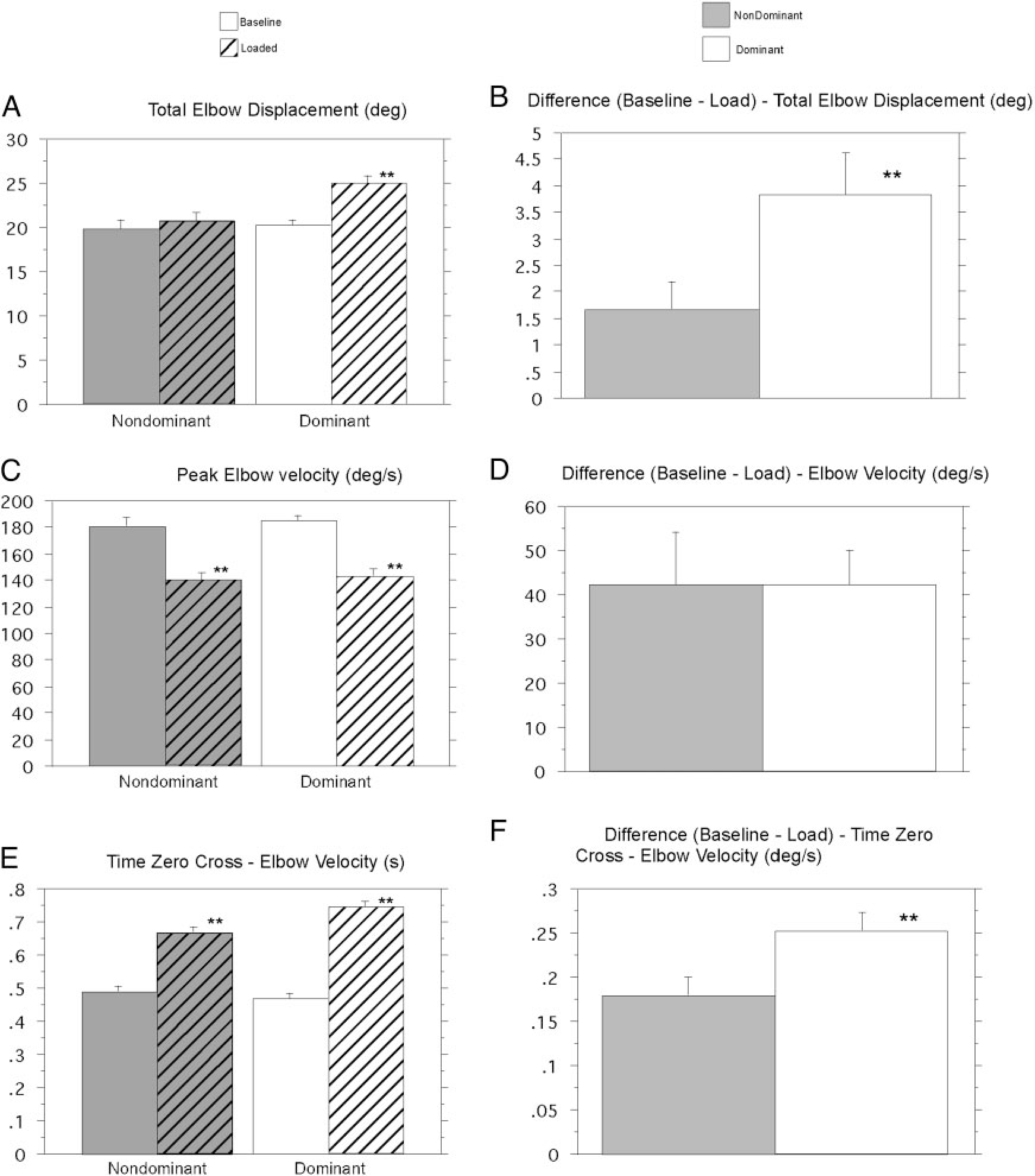
Kinematic comparisons for dominant and nondominant arm groups across subjects and conditions. A: total elbow displacement. B: difference between conditions: total elbow displacement. C: peak elbow velocity. D: difference between conditions: peak elbow velocity. E: time 0 crossing—elbow velocity. F: difference between conditions: time 0 crossing—elbow velocity. **Results from statistical analysis are significant.
RESULTS
Kinematics analysis
We first examined interlimb differences in responses to the load, within subjects, by employing a two-way ANOVA, using hand and experimental session order as factors. Session order refers to whether the data were obtained in the first or second acquisition session. Table 1 shows the results of this analysis for final position error. A main effect occurred for hand, such that nondominant arm trials were always significantly more accurate than dominant arm trials. However, a main effect also occurred for session, such that, for a given hand, the second session was always more accurate than the first session. This reflects some degree of adaptation to the mass application. Because this adaptation occurred between sessions, it represents interlimb transfer of task learning, which has previously been well documented for visuomotor adaptation (Sainburg and Wang 2002; Wang and Sainburg 2003), for catching balls of various weights (Morton et al. 2001), and for adaptation to novel Curl-Fields imposed by a robot manipulandum (Criscimagna-Hemminger et al. 2003). These studies have indicated that interlimb transfer is asymmetric, and that the direction of this asymmetry is task specific. In agreement with these findings, a significant interaction occurred between the hand and session factors in our current study. Thus differences between nondominant and dominant arm accuracy, during loaded trials, were affected by prior opposite arm performance. To minimize the effects of prior opposite arm exposure to the task, we restrict further analysis to a between group design, in which the first session performed with the dominant arm (RL group) is compared with the first session performed with the nondominant arm (LR group). Accordingly, further data analysis was subjected to two-way ANOVA, where hand (dominant, non-dominant) was used as a between-group factor; load condition (baseline, loaded) was used as a within group factor.
TABLE 1.
ANOVA: measure of movement accuracy: final position error
| Factor | DOF | F value | P value |
|---|---|---|---|
|
| |||
| Hand | 1 | 88.600 | <0.0001 |
| Session | 1 | 7.419 | 0.0086 |
| Hand × Session | 1 | 4.020 | 0.0498 |
Figure 2 compares averaged elbow displacement (Fig. 2A) and velocity profiles (Fig. 2B) from baseline and loaded trials from dominant arm group subjects (left column) and nondominant arm group subjects (right column). The effect of the load in reducing elbow acceleration is reflected by the reduced slope of the velocity profiles of loaded trials during the first 100 ms of movement, which is emphasized by the time-expanded insets of Fig. 2B. This reduction in movement speed was reflected by a distinct reduction in slope of the displacement profiles of loaded compared with unloaded trials in Fig. 2A (total elbow displacement is shown as measured at movement end). The velocity profiles for loaded trials show reduced peak amplitude (Fig. 2B), but appear similar in shape to that of unloaded trials through the peak in velocity. However, in the extensor acceleration (flexor deceleration) phase of motion, the loaded trials show a distinct prolongation of the flexor velocity phase of motion, such that the return to zero velocity is delayed beyond that of baseline trials. This corrective increase in flexor velocity duration is depicted as the time between the arrows in Fig. 2B, indicating the zero crossing of baseline and loaded trials, respectively. This prolongation of flexor motion appears to compensate the unexpected increase in inertia, allowing the elbow to flex near, or beyond that of baseline motion.
FIG. 2.
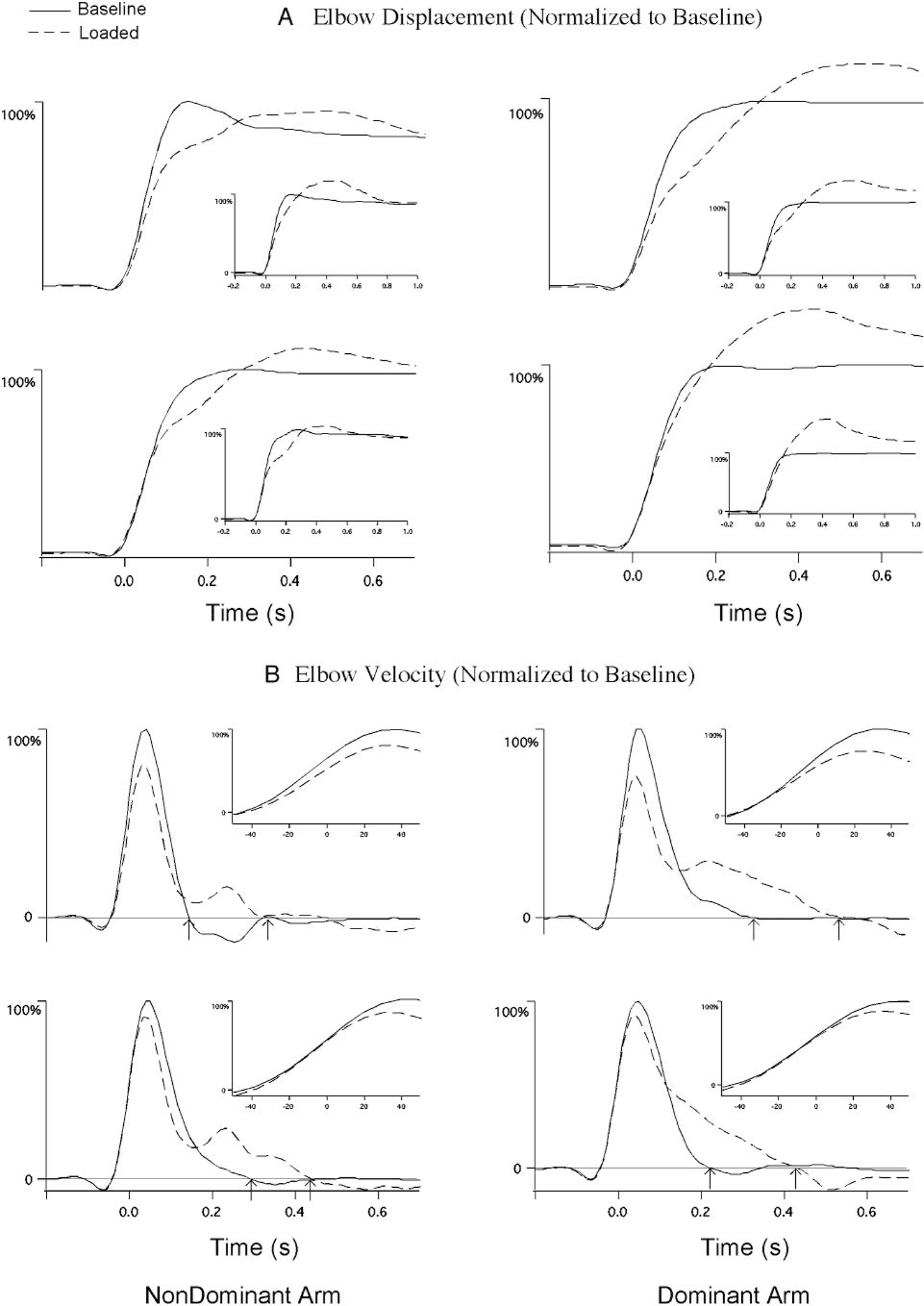
Four representative profiles from the nondominant (left column) and dominant (right column) arm groups. The insets depict sample trials that contributed to the averaged profiles. Data were synchronized to peak tangential finger acceleration and averaged across all 5 loaded trials and the 5 baseline trials that directly preceded each loaded trial. A: averaged elbow displacement. B: averaged elbow velocity. Data normalized to the average amplitude of the baseline trials. Loaded trials (– – –); baseline trials (——).
Whereas both dominant and nondominant arms show a distinct corrective increase in flexor velocity, the correction is more effective in achieving baseline final position for the nondominant arm. In contrast, dominant arm flexor velocity is more prolonged, resulting in overshoot of baseline final position. This is evident by the fact that the nondominant arm displacement profiles of Fig. 2A converge at the end of movement, whereas those for the dominant arm do not.
Figure 3 demonstrates that these findings were consistent across all subjects and trials. Under baseline conditions, both dominant and nondominant arm groups made movements of similar amplitude (Fig. 3A) and peak velocity (Fig. 3C). Measures of baseline elbow displacement and velocity were not significantly different for dominant and nondominant arm groups. Table 2 indicates a main effect for load in reducing elbow velocity, but no difference in this effect between the hands and interaction between hand and load conditions. This indicates that the initial effect of the load in reducing peak velocity was the same for both arms (Fig. 3D, P = 0.9898). This is most obvious in Fig. 3C (P < 0.0001), which shows the mean difference in peak velocities between baseline and loaded trials, for each arm. However, following peak velocity, the response to the load was implemented differently by dominant and nondominant arms. This is reflected in Tables 3 and 4, which show main effects of our two-way ANOVA for total elbow displacement and for the time of the cross zero in elbow velocity. Both analyses indicate main effects for load, as well as, an interaction between the hand and load factors. For the nondominant arm, the loaded trials showed similar accuracy to that of baseline trials (P = 0.5688; Fig. 3A, left). However, for the dominant arm, the loaded trials showed substantial overshoot in final position, relative to that of baseline (P < 0.0001; Fig. 3A, right). As a result, the difference in displacement between loaded and baseline trials, shown in Fig. 2A, was almost three times greater for the dominant arm (P = 0.0495; Fig. 3B).
TABLE 2.
ANOVA: peak elbow velocity
| Factor | DOF | F value | P value |
|---|---|---|---|
|
| |||
| Hand | 1 | 0.587 | 0.4453 |
| Load | 1 | 60.134 | <0.0001 |
| Hand × Load | 1 | 0.033 | 0.8553 |
TABLE 3.
ANOVA: total elbow displacement
| Factor | DOF | F value | P value |
|---|---|---|---|
|
| |||
| Hand | 1 | 7.055 | 0.0090 |
| Load | 1 | 9.383 | 0.0027 |
| Hand × Load | 1 | 4.668 | 0.0328 |
TABLE 4.
ANOVA: time zero cross: elbow velocity
| Factor | DOF | F value | P value |
|---|---|---|---|
|
| |||
| Hand | 1 | 2.504 | 0.1163 |
| Load | 1 | 174.839 | <0.0001 |
| Hand × Load | 1 | 8.590 | 0.0041 |
As depicted by the bar plots of Fig. 3E, the zero crossing of elbow velocity, from flexion to extension, was significantly delayed, relative to that of baseline, for both groups (dominant and nondominant P < 0.0001). Figure 3F (P < 0.0329) shows the average difference in this zero crossing between loaded and baseline trials. This elbow velocity zero crossing was substantially more prolonged for the dominant arm, which resulted in the overshoot discussed above.
Electromyographic analysis
We next examined the electromyographic activity underlying the interlimb differences in kinematic responses to load described above. For averaging, we synchronized the normalized EMG profiles to the peak in tangential hand acceleration (Amax). This critical value occurred, on average, 50 ms (SE = 3.5 ms) following movement onset.
NONDOMINANT ARM.
All four muscles showed no significant difference in activity for both loaded and baseline trials, prior to Amax (0 on x axis). Following Amax (within 100 ms), both flexors (biceps and brachioradialis) show similar activities for baseline and loaded trials. However, both extensor muscles (triceps and anconeus) show a distinct reduction in burst amplitude. It is this reduction in extensor activity that produced the prolonged flexor velocity, corresponding to the load compensation, described above.
DOMINANT ARM.
Prior to Amax, little difference between loaded and baseline trials was shown by the dominant arm muscle activity. Following Amax, a slight reduction in extensor muscle activity was evident. However, in contrast to the nondominant arm, a substantial increase in flexor muscle activity (biceps and brachioradialis) was reflected by an early second agonist burst in the EMG of loaded trials, as displayed in Fig. 4B. This resulted in the substantial prolongation of the flexor velocity phase of motion that corresponded to the overshoot of the dominant arm during loaded trials.
FIG. 4.
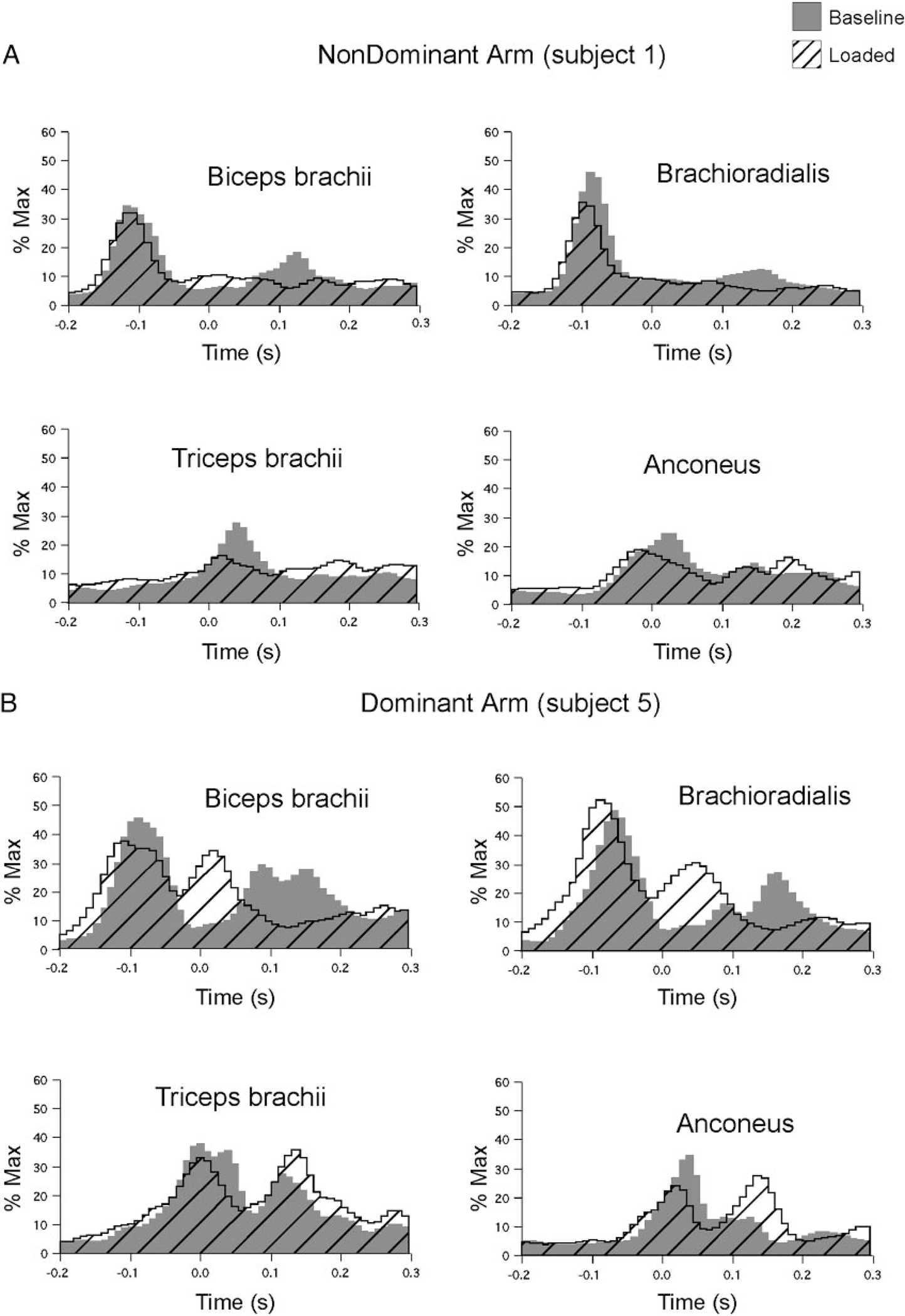
Two representative profiles of EMG recordings for biceps brachii, brachioradialis, long head of the triceps brachii, and anconeus. A: averaged nondominant arm muscle activity. B: averaged dominant arm muscle activity. Gray pattern: baseline trials; cross-hatched pattern: loaded trials.
The plots of Fig. 4, A and B, were characteristic of all subjects’ performance, as quantified in Fig. 5. EMG impulse (area under the curve) is shown for the 100 ms preceding, and following Amax, for nondominant arm and dominant arm groups. For all four muscles and both arms, no significant difference in muscle activity occurred for the period prior to Amax. This finding is consistent with the unpredictability of the load conditions. Following Amax, the dominant arm showed a significant increase in biceps EMG impulse (P = 0.0240; Fig. 5A, right), representing a near 60% increase in muscle activation. This corresponds to the distinct bursts shown in Fig. 4B. Whereas the nondominant arm showed an increase in flexor muscle activity, these increases were not significant. In terms of extensor activity, both arms showed a trend to decrease triceps activity following Amax, although this was not significant. Consistent with the examples of Fig. 4, A and B, all loaded trials showed a substantial decrease in anconeus EMG impulse following Amax compared with baseline trials (nondominant arm: P = 0.0494, Fig. 5D; dominant arm: P = 0.0302, Fig. 5D). For the nondominant arm, this alone gave rise to the load compensating increase in flexor velocity described above. For the dominant arm, this decrease in extensor activity combined with the large increase in biceps activity produced excessive flexor acceleration and resulted in the overshoot of flexor displacement quantified in Fig. 3B.
FIG. 5.
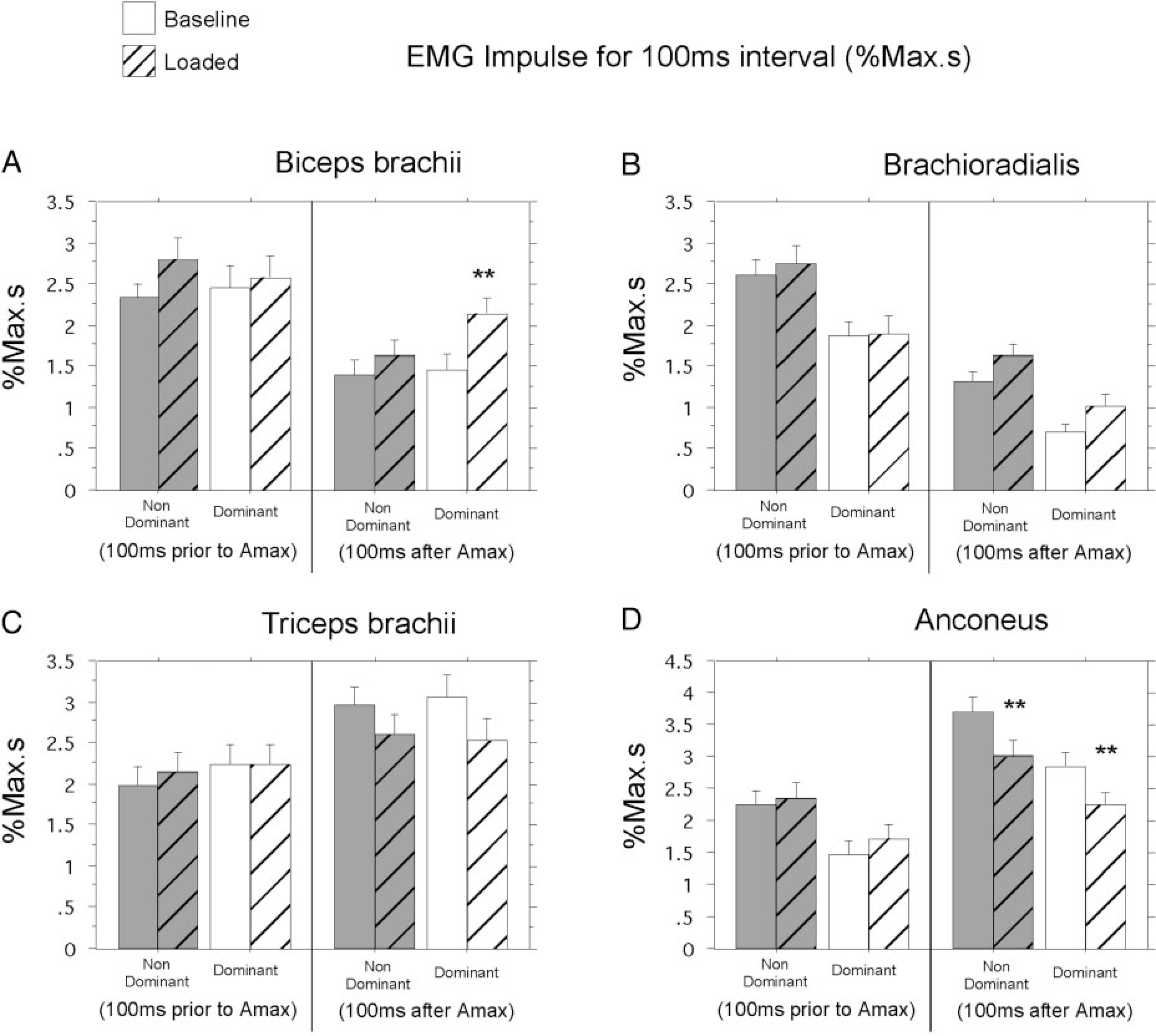
EMG impulse comparisons for dominant (white) and nondominant (gray) arm groups. A: biceps brachii. B: brachioradialis. C: long head of the triceps brachii. D: anconeus. No pattern: baseline trials; cross-hatched pattern: loaded trials. **Results from statistical analysis are significant.
DISCUSSION
This study examined interlimb differences in load compensation responses, measured through kinematic and EMG recordings during horizontal plane single-joint elbow flexion. The results indicate that interlimb differences in control are not limited to predictive mechanisms, but include proprioceptive feedback mediated responses. Subjects made repetitive 20° elbow flexion movements that were targeted in both velocity and amplitude. On random trials, a 2-kg mass was attached to the arm splint prior to the “go” signal. The unpredictability of mass occurrence was facilitated by having the arm and the mass supported on a horizontally oriented air-sled system. This removed somatosensory information about the mass through gravitational or frictional effects. In addition, subjects’ view of the arm and mass was blocked prior to the session. Thus the presence or absence of the load was unpredictable. Accordingly, muscle activity recorded prior to peak tangential finger acceleration showed no significant difference between loaded and baseline trials. However, substantial changes in EMG occurred during the 100 ms following peak tangential finger acceleration (Amax). These changes included a reduction in extensor (antagonist) muscle activity in both limbs, and a substantial increase in flexor (agonist) muscle activity, only in the dominant limb. The net effect was to prolong the flexor velocity phase of motion, which compensated the effects of the load. This compensation was effective for making accurate movements for the nondominant arm. However, the large increase in flexor muscle activity shown by the dominant arm overcompensated the effects of the load, producing a large and systematic overshoot of final position. These results indicate more effective load compensation responses for the nondominant arm.
Asymmetry in load compensation responses
Our findings indicate rapid responses to unanticipated loading of elbow flexion movements, which occurred between 50 ms (mean timing of Amax) and 150 ms following movement onset. Such responses are consistent with previous studies of load compensation during rapid voluntary movements. Rapid, single joint movements are typically produced by a characteristic triphasic EMG pattern (Forget and Lamarre 1987; Gottlieb 1996; Pfann et al. 1998; Shapiro et al. 2002). Sensory feedback, such as that imposed by unexpected loading, has been shown to affect the duration of the first agonist burst and the onset and amplitude of the antagonist burst (Forget and Lamarre 1987; Gottlieb 1996; Latash 1994; Shapiro et al. 2002). In those studies, load compensation responses were implemented at short latency through increases in agonist activity and decreases in antagonist activity that emerged near the end of the first agonist burst. Because the effect of the load must be detected through a threshold sensory event, such as a velocity error (Shapiro et al. 2002), such response latencies are consistent with segmental mediated responses. We expect that the responses recorded in the current study fall into the same category. Whereas, we did not specifically measure response latency, changes in EMG were quantified between 50 and 150 ms following movement onset. The fact that these responses were substantially different for the two limbs suggests that segmental circuits are regulated differently for dominant and nondominant arms.
Nondominant arm specialization for closed loop processes
The idea that the nondominant arm is specialized for feedback mediated error correction mechanisms has previously been proposed, yet the effects of sensory feedback on dominant and nondominant arm performance have yielded conflicting results (Carson et al. 1990, 1992; Elliott et al. 1994, 1995; Flowers 1975; Roy and Elliott 1986; Roy et al. 1994; Todor and Cisneros 1985). Many studies have attempted to differentiate dominant and nondominant control according to open or closed loop processes, usually through control of visual feedback conditions. However, little evidence has emerged to support the idea that the left and right arm controllers show differences in the efficiency with which visual feedback is utilized (Carson et al. 1990, 1992, 1993; Roy and Elliot 1986, 1989). Whereas a number of authors have proposed a dominant arm/hemisphere advantage for movement planning, initiation, or sequencing (Annett et al. 1979; Carson et al. 1995; Todor and Kyprie 1980; Todor and Smiley-Oyen 1987), others have suggested a nondominant arm/hemisphere advantage for movement preparation based on left hand advantages in reaction time (Carson et al. 1990; Elliott et al. 1993). Thus the idea of differentiating dominant and nondominant arm control by open-loop or closed-loop control mechanisms alone remains controversial.
Studies in unilateral brain damaged patients have provided consistent and strong evidence to support an open/closed-loop hypothesis of hemispheric control. Haaland and colleagues (Haaland and Delaney 1981; Haaland and Harrington 1989a,b, 1996; Harrington and Haaland 1997; Prestopnik et al. 2002, 2003; Wyke 1967) have used perceptual motor tasks, which require rapid reciprocal tapping between two targets that vary in size and/or target distance to examine movement deficits in the ipsilesional arm of stroke patients. These experiments have used horizontal movement in the ipsilesional hemispace with the ipsilesional arm in the stroke patients (e.g., right hemispace and arm for patients with right hemisphere damage) to rule out the confounding effects of motor weakness, visual field cuts, and visual neglect. Dominant hemisphere lesions produced deficits in the initial, ballistic component of reaching, but not in the secondary slower component (Haaland and Harrington 1989b, 1996; Harrington and Haaland 1997; Prestopnik et al. 2003). Patients with nondominant hemisphere lesions showed no deficits in this task. However, when the precision requirements of the task were increased, patients with nondominant lesions showed deficits in final position accuracy (Haaland and Delaney 1981; Haaland and Harrington 1989a; Prestopnik et al. 2002, 2003). These results suggested that the dominant hemisphere is important for controlling rapid, ballistic movements that are more dependent on planning (open-loop), and the nondominant hemisphere is more important for the slower movement component that is more responsible for response modification (closed-loop). Consistent with these findings, Winstein and Pohl (1995) showed that nondominant lesions produced slowing of the deceleration phase of motion, whereas, dominant lesions produced slowing of the initial, acceleration phase of motion. Our current results extend these findings to indicate that nondominant advantages for closed-loop control are, at least partially, mediated through somatosensory feedback based mechanisms.
Our findings provide evidence for nondominant arm advantages in somatosensory based load compensation responses. We demonstrate a nondominant arm advantage that appears consistent with the idea that segmental servo-mechanisms are better adapted for regulation of limb position. This may explain previous findings showing nondominant arm advantages in final position accuracy (Bagesteiro and Sainburg 2002; Sainburg and Kalakanis 2000; Sainburg and Wang 2002; Wang and Sainburg 2003). However, nondominant arm advantages for load compensation appears at odds with our previous hypothesis that the dominant arm/hemisphere system is best adapted for control of limb dynamics. The current findings, thus provide a convergence between the dynamic dominance and the open/closed-loop hypotheses of hemispheric specialization: closed-loop specialization of the nondominant arm/hemisphere system includes somatosensory mediated mechanisms that serve to control final limb position. Moreover, we now suggest that open-loop specialization of the dominant limb/hemisphere system is limited to feedforward specification of task dynamics (Sainburg 2002). Further research is necessary to examine whether the nondominant arm advantages in the current study are limited to load compensation, or whether they also occur in response to other somatosensory stimuli.
DISCLOSURES
This research was supported by National Institute of Child Health and Human Development Grant R01HD-39311.
We thank Dr. Kathy Haaland for many insightful and scholarly interactions that contributed to the considerations of hemispheric dominance discussed in this paper.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Annett J, Annett M, Hudson PT, and Turner A. The control of movement in the preferred and non-preferred hands. Q J Exp Psychol 31(Pt 4): 641–652, 1979. [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB and Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88: 2408–2421, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RG. Manual asymmetries: old problems and new directions. Hum Mov Sci 12: 479–506, 1993. [Google Scholar]
- Carson RG, Chua R, Elliott D, and Goodman D. The contribution of vision to asymmetries in manual aiming. Neuropsychologia 28: 1215–1220, 1990. [DOI] [PubMed] [Google Scholar]
- Carson RG, Chua R, Goodman D, Byblow WD, and Elliott D. The preparation of aiming movements. Brain Cogn. 28: 133–154, 1995. [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, Chua R, and Elliott D. Asymmetries in the regulation of visually guided aiming. J Motor Behav 25: 21–32, 1993. [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, and Elliott D. Asymmetries in the discrete and pseudocontinuous regulation of visually guided reaching. Brain Cogn 18: 169–191, 1992. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, and Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003. [DOI] [PubMed] [Google Scholar]
- Elliott D, Chua R, and Pollock BJ. The influence of intermittent vision on manual aiming. Acta Psychol (Amst) 85: 1–13, 1994. [DOI] [PubMed] [Google Scholar]
- Elliott D, Helsen WF, and Chua R. A century later: Woodworth’s (1899) two-component model of goal-directed aiming. Psychol Bull 127: 342–357, 2001. [DOI] [PubMed] [Google Scholar]
- Elliott D, Lyons J, Chua R, Goodman D, and Carson RG. The influence of target perturbation on manual aiming asymmetries in right-handers. Cortex 31: 685–697, 1995. [DOI] [PubMed] [Google Scholar]
- Elliott D, Roy EA, Goodman D, Chua R, Carson RG, and Maraj BKV. Asymmetries in the preparation and control of manual aiming movements. Can J Exp Psychol 47: 570–589, 1993. [Google Scholar]
- Fitts PM. Cognitive aspects of information processing. 3. Set for speed versus accuracy. J Exp Psychol 71: 849–857, 1966. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. 1954. J Exp Psychol Gen 121: 262–269, 1992. [DOI] [PubMed] [Google Scholar]
- Fitts PM and Radford BK. Information capacity of discrete motor responses under different cognitive sets. J Exp Psychol 71: 475–482, 1966. [DOI] [PubMed] [Google Scholar]
- Flowers K. Handedness and controlled movement. Br J Psychol 66: 39–52, 1975. [DOI] [PubMed] [Google Scholar]
- Forget R and Lamarre Y. Rapid elbow flexion in the absence of proprioceptive and cutaneous feedback. Hum Neurobiol 6: 27–37, 1987. [PubMed] [Google Scholar]
- Gottlieb GL. Computational model of the simplest motor program. J Motor Behav 25: 153–161, 1993. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. On the voluntary movement of compliant (inertial-viscoelastic) loads by parcellated control mechanisms. J Neurophysiol 76: 3207–3229, 1996. [DOI] [PubMed] [Google Scholar]
- Haaland KY and Delaney HD. Motor deficits after left or right hemisphere damage due to stroke or tumor. Neuropsychologia 19: 17–27, 1981. [DOI] [PubMed] [Google Scholar]
- Haaland KY and Harrington D. The role of the hemispheres in closed loop movements. Brain Cogn 9: 158–180, 1989a. [DOI] [PubMed] [Google Scholar]
- Haaland KY and Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia 27: 961–969, 1989b. [DOI] [PubMed] [Google Scholar]
- Haaland KY and Harrington DL. Hemispheric asymmetry of movement. Curr Opin Neurobiol 6: 796–800, 1996. [DOI] [PubMed] [Google Scholar]
- Harrington DL and Haaland KY. Apraxia: The Neuropsychology of Action. edited by Gonzalez Rothi LJ and Heilman KM. East Sussex, UK: Psychology Press, 1997, chapt. 9, p. 111–147. [Google Scholar]
- Latash ML. Control of fast elbow movement: a study of electromyographic patterns during movements against unexpectedly decreased inertial load. Exp Brain Res 98: 145–152, 1994. [DOI] [PubMed] [Google Scholar]
- Morton SM, Lang CE, and Bastian AJ. Inter- and intra-limb generalization of adaptation during catching. Exp Brain Res 141: 438–445, 2001. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Pfann KD, Hoffman DS, Gottlieb GL, Strick PL, and Corcos DM. Common principles underlying the control of rapid, single degree-of-freedom movements at different joints. Exp Brain Res 118: 35–51, 1998. [DOI] [PubMed] [Google Scholar]
- Prestopnik JL, Haaland KY, Knight RT, and Lee RR. Hemispheric dominance in the parietal lobe for open and closed loop movement. J Int Neuropsychol Soc 9: 1–2, 2003.12570353 [Google Scholar]
- Prestopnik JL, Haaland KY, Lee RR, and Knight RT. Hemispheric dominance in open and closed loop movements. Soc Neurosci Abstr 30: 1–2, 2002. [Google Scholar]
- Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, and Marsden CD. Manual motor performance in a deafferented man. Brain 105: 515–542, 1982. [DOI] [PubMed] [Google Scholar]
- Roy EA and Elliott D. Manual asymmetries in visually directed aiming. Can J Psychol. 40: 109–121, 1986. [DOI] [PubMed] [Google Scholar]
- Roy EA and Elliott D. Manual asymmetries in aimed movements. Q J Exp Psychol 41A:501–516, 1989. [Google Scholar]
- Roy EA, Kalbfleisch L, and Elliott D. Kinematic analyses of manual asymmetries in visual aiming movements. Brain Cogn 24: 289–295, 1994. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghez C, and Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81: 1040–1056, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H, and Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol 73: 820–835, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL and Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83: 2661–2675, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL and Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145: 437–447, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Zernicke RF, Schmidt RA, and Hart TJ. Changes in limb dynamics during the practice of rapid arm movements. J Biomech 22:805–817, 1989. [DOI] [PubMed] [Google Scholar]
- Shapiro MB, Gottlieb GL, Moore CG, and Corcos DM. Electromyographic responses to an unexpected load in fast voluntary movements: descending regulation of segmental reflexes. J Neurophysiol 88: 1059–1063, 2002. [DOI] [PubMed] [Google Scholar]
- Todor JI and Cisneros J. Accommodation to increased accuracy demands by right and left hands. J Motor Behav 17: 355–372, 1985. [DOI] [PubMed] [Google Scholar]
- Todor JI and Kyprie PM. Hand differences in the rate and variability of rapid tapping. J Motor Behav 12: 57–62, 1980. [DOI] [PubMed] [Google Scholar]
- Todor JI and Smiley-Oyen AL. Force modulation as a source of hand differences in rapid finger tapping. Acta Psychol 65: 65–73, 1987. [Google Scholar]
- Wang J and Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ and Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 105: 163–174, 1995. [DOI] [PubMed] [Google Scholar]
- Wyke M. Effect of brain lesions on the rapidity of arm movement. Neurology 17: 1113–1120, 1967. [DOI] [PubMed] [Google Scholar]


