Abstract
Promoting the recovery of neurological function in patients with traumatic spinal cord injury (TSCI) remains challenging. The balance between astrocyte-mediated neurotrophic and pro-inflammatory responses is critical for TSCI repair. Recently, the utilization of nanomaterials has been considerably explored in immunological reconstructive techniques that specifically target astrocyte-mediated inflammation, yielding positive outcomes. In this review, we aim to condense the present knowledge regarding the astrocyte-mediated inflammation following TSCI. We then review the various categories of nanomaterials utilized in the management of astrocyte-mediated inflammation in TSCI and conclude by summarizing their functions and advantages to offer novel insights for the advancement of effective clinical strategies targeting TSCI.
Keywords: Spinal cord injury, Astrocyte, Inflammation, Nanomaterials, Treatment
Graphical abstract
1. Introduction
Millions of individuals worldwide are afflicted by TSCI, a condition that can result in enduring dysfunction and paralysis [1]. Numerous variables can impede recovery following TSCI, including the progressive neuronal death associated with hostile inflammatory responses [2]. The prevailing consensus is that the inflammatory response is initiated by the activation of macrophages/microglia at the site of damage. These activated cells release pro-inflammatory cytokines that subsequently facilitate the infiltration and functionality of other immune-related cells [[3], [4], [5]]. Nevertheless, the involvement of the immune system after TSCI is immensely intricate, wherein, specifically, astrocytes assume crucial functions in the inflammation of the central nervous system (CNS) [6,7]. One aspect to consider is that activated astrocytes possess inherent neurotoxic properties by releasing chemokines, including chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 10 (CXCL10), and interleukin (IL)-6 [[8], [9], [10]]. The activity of these chemokines is mediated by the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), Janus kinase/signal transducer, and activator of transcription 3 (JAK/STAT3), or mitogen-activated protein kinase (MAPK) pathway. These pathways are crucial in the context of inflammation following TSCI [6,9,11]. Nonetheless, astrocytes possess the dual capability to impede the entry of peripheral immune cells into the spinal cord and concurrently instigating inflammation by enlisting peripheral inflammatory cells [6,8,11,12]. Hence, effectively regulating these varied astrocyte reactions is regarded as a pivotal option for the treatment of TSCI.
The present therapeutic approaches aimed at improving the outcomes of TSCI encompass surgical interventions for decompression and stabilization of the spinal cord, as well as the management of spasticity and provision of rehabilitative care [13]. Various pathways have been proposed to enhance recovery in preclinical investigations. Additionally, a range of treatment strategies are being examined to alleviate secondary damage and optimize regeneration after TSCI [14,15]. Nevertheless, numerous studies have demonstrated a lack of significant effectiveness when these interventions were tested in clinical trials [[16], [17], [18]]. Alternatively, immunological reconstruction strategies based on nanomaterials for targeting astrocyte-mediated inflammation have recently been widely explored, demonstrating good potential for clinical application [19,20]. Nanomaterials exhibit several favorable features: 1) codelivery of anti-inflammatory drugs combined with spinal cord-specific targeting, 2) the extension of the duration of bioactive cargo molecules in the bloodstream by preventing their breakdown through enzymatic processes, and 3) synergistic therapeutic effects of the functional groups of the nanomaterial itself and the encapsulated drugs [21].
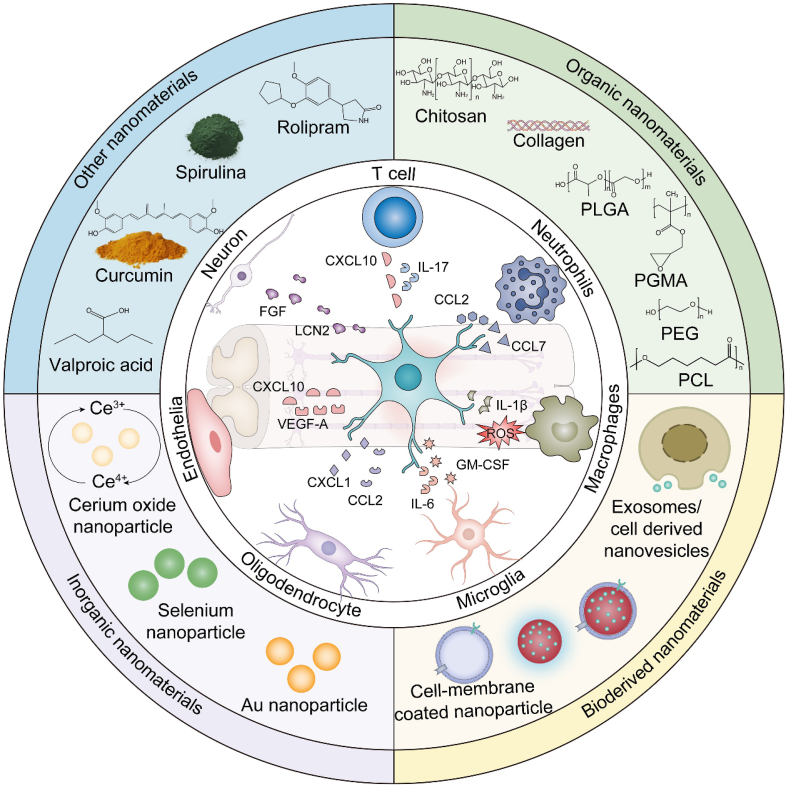
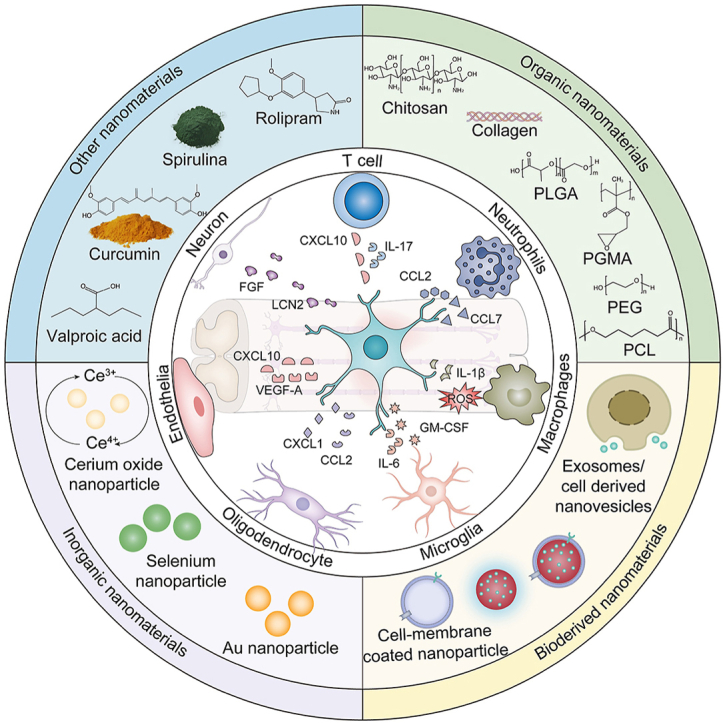
This review focuses on the roles of nanomaterials in moderating astrocyte-mediated inflammation upon TSCI. First, we present a comprehensive summary of the important roles played by astrocytes in TSCI-associated inflammation. In the following section, we proceed to elucidate the present utilization of functional nanomaterials in the regulation of astrocyte-mediated inflammation. These nanomaterials are classified into distinct categories based on their source composition, namely organic nanomaterials, bioderived nanomaterials, inorganic nanomaterials, and other nanomaterials (Fig. 1). Finally, the prospects and obstacles associated with the utilization of nanomaterials as a therapeutic approach for TSCI are presented. This review aims to furnish a comprehensive summary of the current advancements in research regarding the application of nanomaterials in the setting of astrocyte-mediated inflammation in TSCI. Our intention is to facilitate a deeper comprehension of the existing research landscape, while also proposing innovative avenues for investigating the underlying pathogenic mechanisms and developing clinical interventions.
Fig. 1.
Schematic illustration of the classification and structure of nanomaterials for modulating astrocyte-mediated inflammation in traumatic spinal cord injury.
2. A brief overview of TSCI
The field of spinal cord injury (SCI) is classified into two main categories based on the etiology: traumatic and non-traumatic. The characteristics of non-traumatic SCI have been highlighted in several recent reports [[22], [23], [24]]. Non-traumatic etiologies include benign and malignant tumors, vascular disorders, infectious diseases, and inflammatory conditions [22,23]. The incidence of non-traumatic SCI is higher than that of TSCI in several developed countries, suggesting that a more comprehensive understanding of non-traumatic SCI is required to better plan health services that can meet the anticipated future demand for non-traumatic SCI prevention and the rehabilitation of affected individuals [24]. Alternatively, in the present review, we focus on TSCI. TSCIs are usually attributed to an external physical impingement, such as sports injuries, maneuver injuries, falls, and violence [[25], [26], [27]]. TSCI is most commonly observed in the general population at the cervical spine level, accounting for approximately 60 % of cases. Thoracic level injuries account for approximately 32 % of cases, while lumbosacral level injuries account for approximately 9 % of cases [25]. Accordingly, TSCI typically leads to permanent motor- and sensory dysfunction, with autonomic as well as neurogenic bladder functions being disrupted [25]. The incidence of TSCI varies worldwide (8–246 cases per million individuals annually) [28,29]. In industrialized nations, the prevalence of TSCI is comparatively higher in North America, with a reported incidence of 39 cases per million inhabitants. By contrast, Australia and western Europe exhibit lower rates, with incidences of 16 and 15 cases per million individuals, respectively [25]. Of the approximately 768,473 new cases of SCI that occur worldwide annually, approximately 5000 new cases of TSCI are reported each year in Japan, whereas 12,500–17,000 new cases occur annually in the USA [21,29,30]. The range of estimates for acute in-hospital mortality is between 4 % and 17 %. Following hospital discharge, the yearly mortality rates continue to be consistently high. Within the first year after injury, 3.8 % of patients experience mortality. In the second year, the mortality rate decreases to 1.6 %, and for each subsequent year, it remains at 1.2 % [25]. The financial burden associated with the provision of care for individuals with TSCI is substantial, with estimated expenses ranging from $1.1 to $4.7 million per patient during their lifespan [25,31]. Therefore, TSCI imposes a significant strain on both the patient's family and society as a whole [25].
The pathophysiological progression of TSCI is characterized by primary injury and secondary injury cascades [32]. The primary injury occurs immediately following the mechanical breakdown and dislocation of the vertebral column, leading to the compression or complete rupture of the spinal cord [26,32]. The ensuing and prolonged secondary injury cascade results in further damage to the spinal cord and subsequent impairment of neurological function. In particular, the inflammatory response plays a key role among the multitude of physiopathological processes that occur following the secondary injury cascade, determining secondary pathophysiological changes and persistent neurological dysfunction [3,33,34]. Microglial cells and astrocytes are activated by several inflammatory agents, as well as adenosine triphosphate (ATP), DNA, and potassium [2,35,36], which infiltrate the injury site together with other inflammatory cells, such as activated macrophages, polymorphonuclear cells, and lymphocytes. Collectively, they propagate the inflammatory response and play a central role in the continuous death of neurons and oligodendrocytes [2,25,37]. Inflammatory cells have the capacity to remove myelin debris at the site of injury [38]. However, importantly, these cells can also exacerbate damage to the spinal cord by releasing cytotoxic by-products, such as free radicals (e.g., O2−, hydrogen peroxide, and peroxynitrite) and nitric oxide [39]. Reactive oxygen species (ROS) are responsible for inducing lipid peroxidation, DNA oxidative damage. These processes subsequently lead to necrotic and apoptotic cell death, thereby exacerbating the hostile microenvironment [[40], [41], [42]].
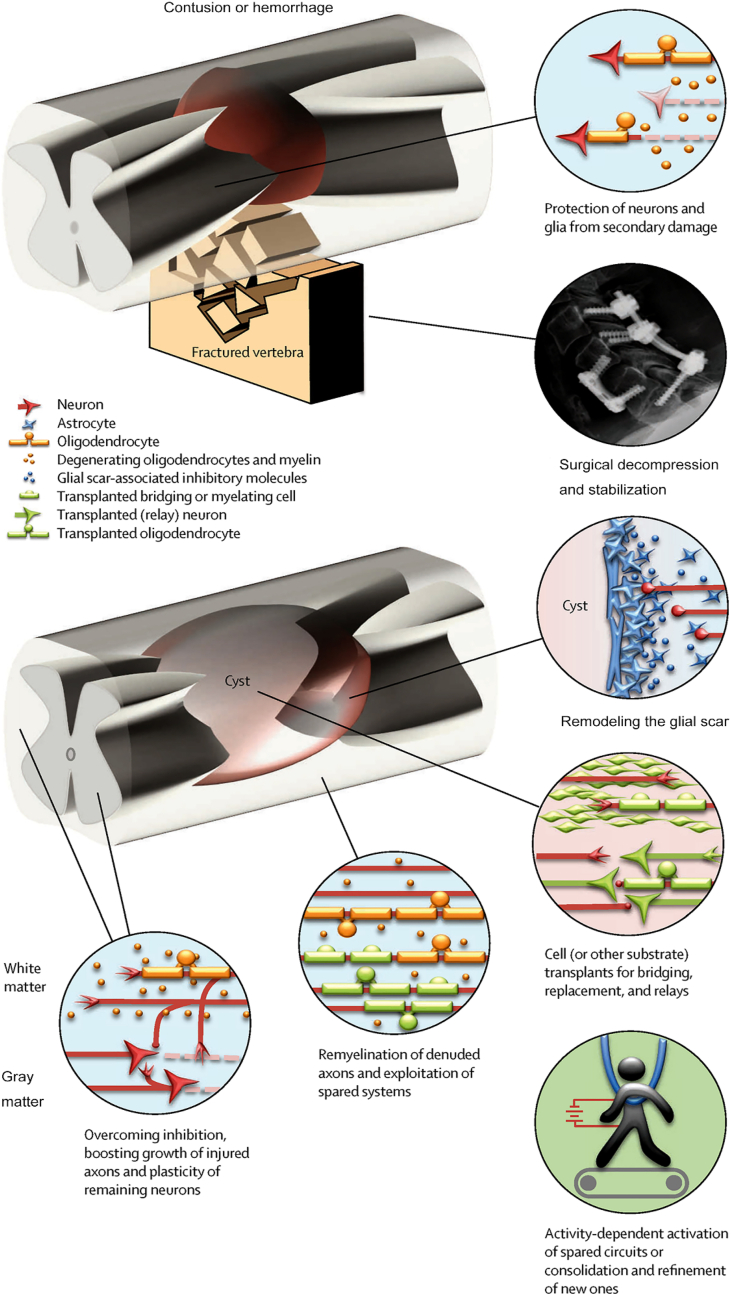
The concept of “time is spine” has become central to the management of any patient with TSCI [43,44]. Historically, numerous steps have been undertaken to restrict the damage: drugs targeting inflammation or excitotoxicity, surgical decompression, therapeutic hypothermia, pharmacological or cellular treatment, and rehabilitation and training (Fig. 2) [45]. Specifically, the use of corticosteroid methylprednisolone is an established clinical practice for the treatment of TSCI, and it may be promptly administered to patients who have suffered a severe injury and lack alternative therapeutic alternatives [46,47]. However, the efficacy of this treatment approach remains a subject of debate owing to its tendency to yield only modest therapeutic outcomes. Moreover, this approach is associated with a horde of possible consequences, including gastrointestinal hemorrhage, pneumonia, sepsis, pulmonary embolism [25,46]. Hence, it can be argued that the efficacy of methylprednisolone is limited, and there is a possibility of adverse consequences. Despite being legally condemned as a standard of care in most countries, this medication continues to be employed in clinical practice [25]. As a result, the discourse surrounding the indispensability and most advantageous scheduling of spinal surgery subsequent to TSCI has proven to be a challenging matter to settle. In the recent decades, a notable transition in the care of TSCI has been seen from a conservative approach to a more surgically aggressive one. This movement has been facilitated by advancements in surgical technique and armamentarium. Consequently, the assessment of risks and benefits associated with decompressing the injured spinal cord has been reevaluated, leading to a growing preference for early surgical intervention [45]. The implementation of hypothermia subsequent to neurotrauma is scientifically valid. Hypothermia effectively diminishes the pace of many biological responses and processes, specifically addressing numerous mechanisms associated with secondary damage, such as excitotoxicity, neuroinflammation, necrosis, and free radical generation [48]. Nevertheless, the effectiveness of systemic hypothermia is constrained by various criteria, such as the correlation between the temperature at the site of injury and the core body temperature, as well as the initiation and pace at which safe and technically feasible systemic cooling may be accomplished [49]. The therapeutic strategies for addressing chronic TSCI encompass various approaches, such as altering the glial scar, resolving the formed scar, bridging the gap resulting from cyst formation, transplanting cells into the lesion, remyelinating spared axons, regenerating injured axons, reprogramming endogenous cells, and promoting sprouting of surviving axons [45,50]. However, the results of these treatments remain unsatisfactory in both preclinical and clinical applications.
Fig. 2.
Schematic illustration of the strategies to halt the progression of injury and facilitate the repair of the spinal cord. Surgical decompression and pharmacological interventions are employed with the objective of alleviating secondary injury. The therapeutic strategies for addressing chronic TSCI encompass several approaches, such as the alteration of the glial scar, remyelination of undamaged axons, plasticity of surviving neurons, and rehabilitation and training. Copyright 2014 Lancet Neurology [45].
3. Overview of the inflammatory response upon TSCI
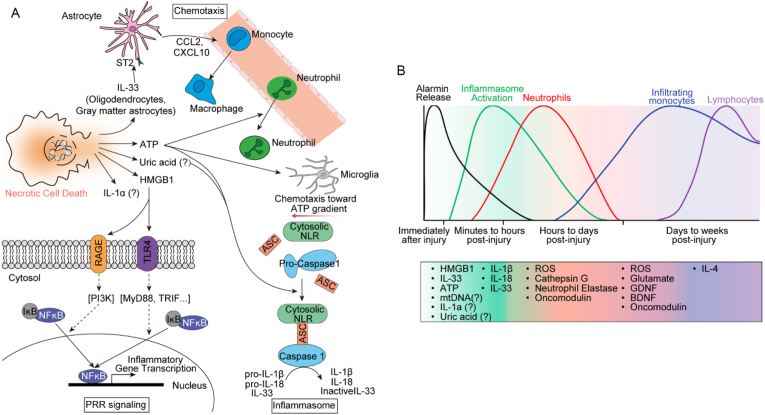
A distinct inflammatory response has been documented following TSCI [1,51]. Specifically, the immunological microenvironment is significantly influenced by microglia, macrophages, astrocytes, neutrophils, lymphocytes, the secretion of several cytokines, and the degradation of the extracellular matrix [46]. Primary injury to the spinal cord results in the necrotic death of resident neurons, glia, and stromal cells and the rapid release of alarmins (such as IL-33, high mobility group box 1 [HMGB1], and ATP) in the extracellular space (Fig. 3A) [[52], [53], [54]]. Localized cells from injured sites can produce chemokines, which recruit neutrophils, macrophages, and cytoplasmic pattern recognition receptors. These cells coordinate and promote inflammasome formation and activation through a variety of triggers. Moreover, the generation of pro-inflammatory cytokines IL-1β and IL-8, which is dependent on inflammasome activity, plays a crucial role in the development of neuroinflammation (Fig. 3A) [53]. This leads to a well-known series of events, including the mobilization of peripheral immune cells, such as neutrophils, monocytes, and lymphocytes, to the location of the injury, followed by their subsequent activation. The inflammatory cells are spatiotemporally dynamic in the presence of various type of inflammatory factors. Neutrophils arrive a few hours after injury, whereas monocytes begin to infiltrate and persist within the first day. Lymphocytes arrive days to weeks after the injury (Fig. 3B). The process of immune activation is often precisely regulated owing to its essential function. Inadequate immunological responses can lead to severe harm to the spinal cord, while the failure to initiate an appropriate immune response when required can have catastrophic outcomes.
Fig. 3.
Schematic illustration of the triggers and dynamic processes in inflammatory cells. (A) Necrotic cell death results in the extracellular release of alarmins, which further exacerbate the inflammatory response. (B) The kinetics of the molecular and cellular immune responses to damage, focusing on neutrophils, monocytes, macrophages, and lymphocytes. Pattern recognition receptors: PRRs; interleukin: IL; adenosine triphosphate: ATP; brain-derived neurotrophic factor: BDNF; reactive oxygen species: ROS; glial cell line-derived neurotrophic factor: GDNF; high-mobility group protein B1: HMGB1. Copyright 2015 Neuron [53].
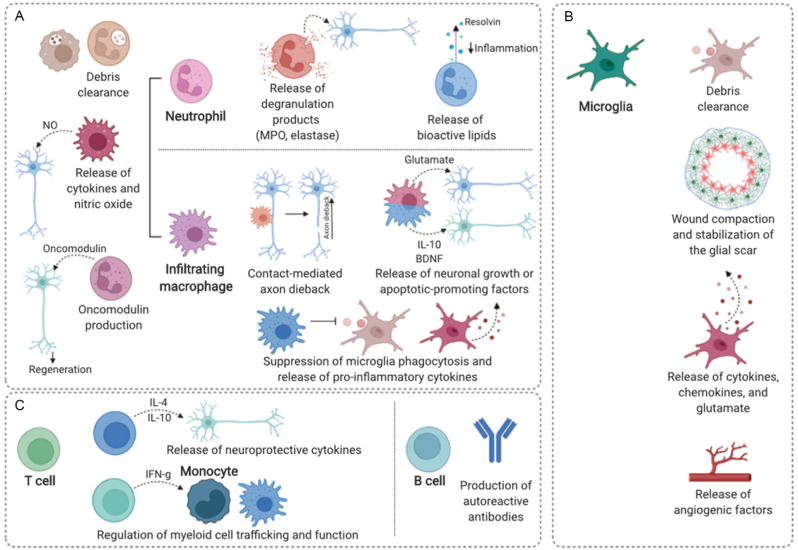
Under the alarmin-released microenvironment, neutrophils are the initial neurotrophic factor cells to be recruited to the site of TSCI. This recruitment occurs within 4 h of injury and reaches its peak between 6 and 12 h (Fig. 3, Fig. 4A) [1,53]. Neutrophils are responsible for the secretion of several substances, including tumor necrosis factor (TNF), ROS, antimicrobial proteins, matrix metalloproteases, elastases, and neutrophil extracellular traps. These secretions promote neuronal cytotoxicity and vascular leakage [55]. Subsequently, the primary influx of cellular entities that migrate to the site of injury consists predominantly of macrophages (Fig. 4A). The involvement of these macrophages has been associated with both advantageous and disadvantageous effects as their removal has been shown to enhance or hinder the process of functional recovery following TSCI, respectively. The recruitment of monocytes/macrophages with various phenotypes in their original location could potentially result in adverse consequences, such as the activation of pro-inflammatory M1 macrophages [1,56], exacerbating inflammatory response and tissue loss via inducible nitric oxide synthase (iNOS), ROS, and pro-inflammatory cytokines, or in beneficial effects, such as the activation of M2 macrophages [1,57], which support neuroprotection and regeneration in different animal models [[58], [59], [60]]. Furthermore, microglia, the immune cells residing in the spinal cord parenchyma, may have a significant impact on the harsh microenvironment [61]. This is because of their activation shortly after injury, which leads to them exhibiting comparable characteristics as infiltrating macrophages (Fig. 4B) [37]. Bellver-Landete et al. [62] observed a 67 % decrease in microglia at the lesion epicenter on day 1 post injury in mice; however, a four-fold increase in microglia was observed on day 4, which further increased in the first 2 weeks following TSCI. Nevertheless, recent research has demonstrated that microglia restrict the wound, stabilize the glial scar following injury, and remove cellular debris (Fig. 4B) [37]. Notably, macrophages possess remarkable flexibility, resulting in their similarity to activated microglia. Hence, it is imperative to advance the development of more refined and cell-specific methodologies to investigate the precise function of these immune cells in the context of TSCI and effectively utilize their therapeutic capabilities. Furthermore, the absence of agreement concerning the overarching function of macrophages/microglia in the damaged spinal cord indicates that they constitute a profoundly diverse population that necessitates further systematic investigation. Lymphocytes have also been shown to migrate and remain present at the site of damage, as depicted in Fig. 4C. Nevertheless, the precise function of these cells remains complex, as their existence in the spinal cord has been hypothesized to indicate autoimmunity and the continuation of tissue injury, but the specific mechanism is now being examined [63,64]. Furthermore, accumulating evidence supports the notion that astrocytes play vital roles in inflammatory process [[65], [66], [67]]. These astrocytes not only secrete inflammatory factors but also produce large amounts of chemokines, which further aggravate the inflammatory process (Section 4).
Fig. 4.
Schematic illustration of the diverse functions performed by immune cells following TSCI. (A) Infiltrating myeloid cells, such as neutrophils and macrophages. (B) Various roles of microglia in response to SCI. (C) Roles of T and B cells. Copyright 2022 Seminars in Immunology [1].
4. Astrocytes play an important role in TSCI-associated inflammation
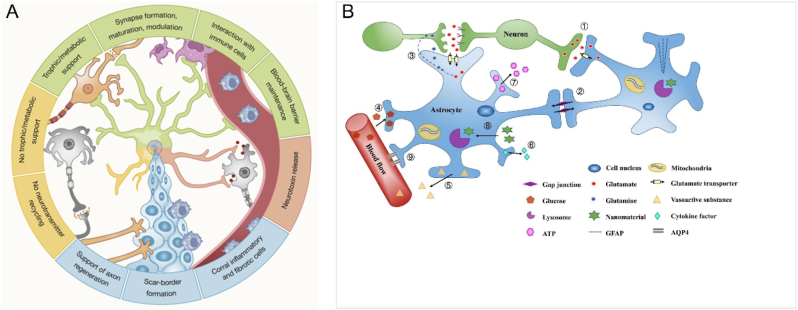
Astrocytes originate from neural progenitors. These cells, which are present in large quantities, play a crucial role in several actions that are vital for the functioning of the spinal cord [68]. Astrocytes in the spinal cord function in a manner similar to those in the brain (Fig. 5A), performing many important and complex functions. Specifically, these cells play an active role in the establishment and upkeep of the blood–spinal cord barrier (BSCB) [6,69]. They also release neurotrophic factors to govern the development of synapses and the differentiation and survival of neurons [6,70]. Additionally, they maintain the balance of extracellular fluids, ions, and neurotransmitters [6,68]. Furthermore, they supply energy sources to neurons, modulate local blood flow, control the drainage of interstitial fluid, and regulate the functioning of neural circuits, as well as neurological processes and behavior (Fig. 5B) [68,70]. The BSCB is important in maintaining the homeostasis of the spinal cord. Consequently, BSCB may emerge as a prominent target for therapeutic interventions for TSCI in the future.
Fig. 5.
Schematic illustration of the relationships between astrocytes and various cell types inside the spinal cord, as well as an overview of the functional role of the spinal cord. (A) Astrocytes maintain close associations with multiple other cell types inside the spinal cord. Reprinted with permission from Ref. [67]. (B) The primary physiological role of astrocytes. Copyright 2021 Acta Biomaterialia [92].
As our understanding of astrocyte functions deepens, an expanding corpus of evidence indicates that reactive astrocytes play a critical role in the innate immune response of the spinal cord [8,71]. Astrocytes exhibit a wide range of receptors for pathogen-related chemical patterns as well as damage- or danger-associated molecular patterns, which are responsible for initiating innate immune responses [68]. Notably, Toll-like receptors (TLRs), including TLR4, are among the receptors expressed by astrocytes. Notably, TLR4 is occasionally mistakenly linked solely with microglia [68]. Astrocytes exhibiting the A1 phenotype exhibit neurotoxic properties because of the upregulation of several genes linked to synaptic and neuronal degeneration, indicating that the A1 phenotype exerts detrimental pro-inflammatory effects [72]. By contrast, reactive A2 astrocytes demonstrate protective effects through the upregulation of neurotrophic factors that facilitate the survival and regeneration of neurons [20]. The various pathways of activation play an invaluable part in the development of TSCI since they possess both advantages and disadvantages. Hence, the precise modulation of astrocyte activation to provide a conducive microenvironment for mitigating secondary injury is of paramount importance in the context of TSCI rehabilitation [46].
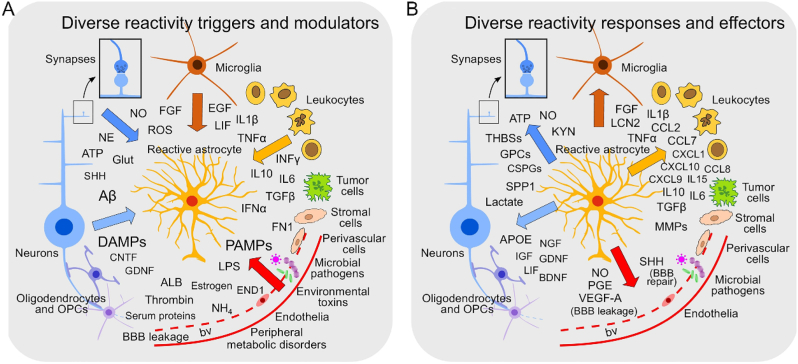
Astrocyte reactivity can be triggered by a diverse array of chemical compounds derived from multiple sources. These sources include all cell types present in the spinal cord tissue, as well as microbial infections, circulating immune cells, serum proteins, peripheral metabolic disorders, and environmental toxins (Fig. 6A) [6,8,67,73]. Astrocytes have the ability to induce inflammation in response to external stimuli that are not cell autonomous. This inflammatory response is facilitated through the flow of chemical signals and direct contacts between astrocytes and other cells of the innate immune system. This process encompasses the recruitment of peripheral inflammatory cells and the activation of microglia residing in the spinal cord, as well as different perivascular cells and leukocytes present in the bloodstream. The aforementioned interactions subsequently impact the cellular states of peripheral nerve cells and other glia, thereby initiating a detrimental loop and finally resulting in heightened and unregulated inflammation (Fig. 6B) [68].
Fig. 6.
Schematic illustration of the representation of the diverse triggers and molecular effectors in astrocytes. (A) Astrocyte reactivity can be elicited by a broad range of molecules from diverse sources. (B) Reactive astrocytes can produce a diverse array of effector molecules. Copyright 2020 Trends in Immunology [68].
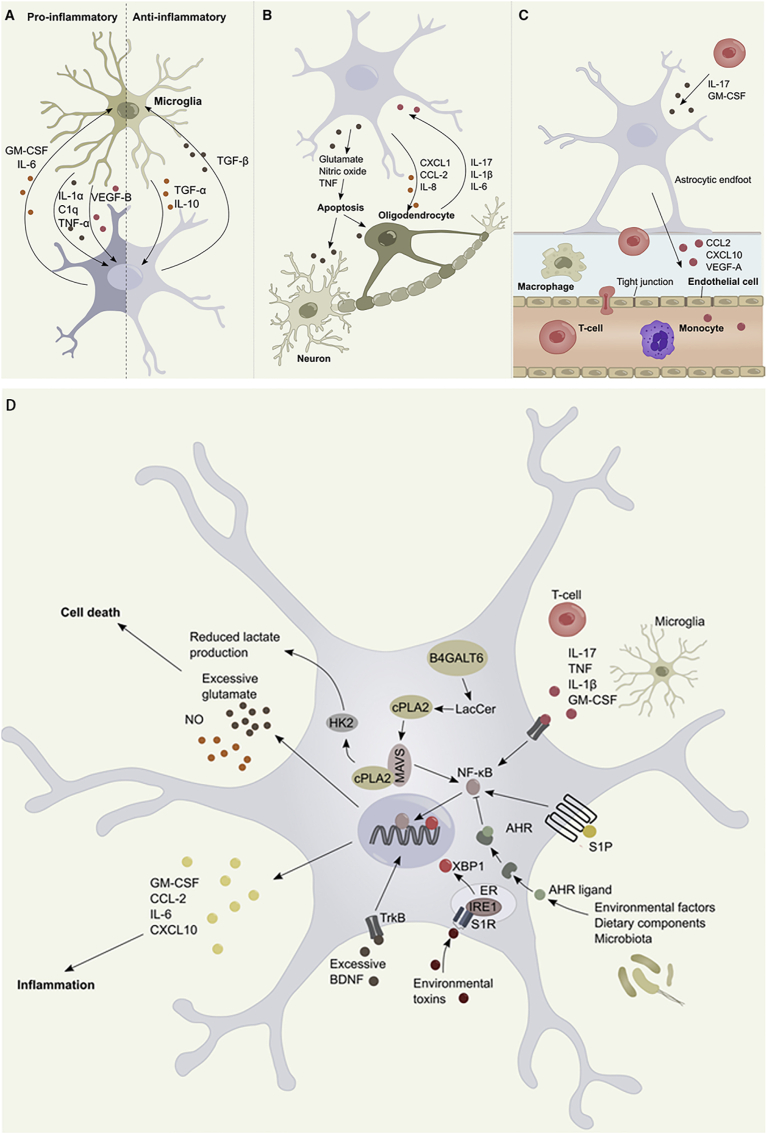
The interaction between astrocytes and cells presents in or entering CNS is of paramount importance in modulating the inflammatory responses. Inflammation is facilitated by the bilateral interaction between astrocytes and microglia, which involves the release of different cytokines and inflammatory mediators (Fig. 7A) [67]. Astrocytes facilitate the pro-inflammatory functions of microglia by releasing various cytokines, including IL-1, IL-6, TNF-α, granulocyte-macrophage colony stimulating factor, and interferon-γ-induced protein 10 [74]. This process involves the activation of relevant signaling pathways such as NF-κB and TLRs, as well as the modulation of microglial activation and regulation. These immune cascades initiated by astrocytes ultimately lead to the development of neurotoxicity. Following TSCI, astrocytes that have been activated release CCL2. This chemokine acts on microglia by utilizing the small extracellular vesicle route. Upon binding to the chemokine receptor 2, microglial activation is promoted [73]. Subsequently, the activated microglia secrete IL-1β, which interacts with neuronal cells, thereby exacerbating their apoptosis [8]. Furthermore, the release of ATP by astrocytes activated through apoptosis triggers inflammatory reactions and stimulates the formation of NLR family pyrin domain-containing 2 (NLRP2) inflammasomes [75]. This inflammasome is responsible for the cleavage of pro-caspase-1 and the subsequent secretion of caspase-1, which, in turn, initiates the creation of mature IL-1β and caspase-1 forms [75]. Astrocyte-derived extracellular vesicles have the potential to function as cellular transmitters of inflammatory signals. They can facilitate the spread and amplification of the neuroinflammatory response that is initiated by a stimulus through TLR4 activation [76]. Subsequently, the activated astrocytes release regulatory factors that have a negative effect on the microglia, reducing the immune response. Additionally, astrocytes can generate TGF-β in reaction to IL-10, produced from microglia, which, in turn, regulates the expression of pro-inflammatory genes in microglia [77]. Astrocytes have been found to exhibit the presence of the dopamine D2 receptor, which regulates innate immunity and inflammasome activity. In experimental autoimmune encephalomyelitis, the inhibition of this receptor shows beneficial effect [78,79]. In addition, the study conducted by Liddelow et al. [72] revealed that the release of IL-1α, TNF-α, and complement component 1q by microglia triggers a transcriptional reaction in astrocytes. This response is characterized by the generation of a neurotoxic factor that has not yet been identified, a decrease in phagocytic activity, and a reduction in the expression of neurotrophic factors.
Fig. 7.
Schematic illustration of astrocyte signaling within the framework of inflammation and astrocyte interaction. (A) The production of cytokines facilitates bidirectional communication between astrocytes and microglia. (B) Bidirectional communication between astrocytes and oligodendrocytes is facilitated by the production of cytokines. (C) The interactions between astrocytes and endothelial cells play a crucial role in modulating blood–spinal cord barrier (BSCB) permeability, hence facilitating the influx of leukocytes. (D) NF-κB controls multiple aspects of astrocyte-mediated inflammation responses. Copyright 2020 Neuron [6].
Furthermore, the reciprocal exchange of information between astrocytes and oligodendrocytes is of utmost importance in the inflammatory process. This is because oligodendrocytes possess a diverse range of receptors that are capable of responding to inflammatory signals released by astrocytes, and vice versa (Fig. 7B). Research indicates that oligodendrocytes may influence inflammation through mechanisms that extend beyond their role in myelination. As an illustration, the study conducted by Falcao et al. [80] revealed that oligodendrocytes play a role in phagocytosis, antigen presentation, and the stimulation of memory and effector CD4+ T cells. Oligodendrocytes are capable of secreting pro-inflammatory cytokines, including IL-1β, CCL2, IL-17, and IL-6. These cytokines can stimulate NF-κB activation and promote pro-inflammatory actions of astrocytes [81]. Furthermore, oligodendrocytes play a role in inflammation by disrupting the integrity of the blood–brain barrier, which, in turn, decreases the integrity of tight junctions [82].
Additionally, the involvement of astrocyte foot processes in the development of the glia limitans, in conjunction with capillary endothelial cells, pericytes, and the basal lamina, collectively establishes the BSCB, which serves as a physical barrier separating the spinal cord from the peripheral blood circulation (Fig. 7C). The involvement of vascular endothelial growth factor-A (VEGF-A) derived from astrocytes is crucial in the maintenance of the BSCB. It is involved in a signaling pathway that ultimately leads to an elevation in BSCB permeability [73]. The study conducted by Argaw et al. [83] provided evidence that the synthesis of VEGF-A in astrocytes is increased in response to IL-1β, a cytokine that is synthesized by activated microglia in the context of neuroinflammation. VEGF-A downregulates the expression of tight junction proteins claudin-5 and occludin in endothelial cells through the endothelial NOS pathway. This process ultimately leads to the disruption of tight junctions and compromises the integrity of the BSCB [83]. Astrocytes are also responsible for the production of substances that enhance the integrity of the BSCB in the presence of inflammatory circumstances. One instance where astrocytes contribute to the integrity of the BSCB is through the synthesis of sonic hedgehog [84]. Furthermore, activated astrocytes release CCL2 and CXCL10 to regulate the recruitment of perivascular leukocytes, monocytes, macrophages, and T cells into the spinal cord [10,85].
Astrocyte-mediated neuroinflammation is initiated and modulated by several signaling pathways, such as the JAK/STAT3, NF-κB, calcineurin, and MAPK pathways [9,11,71]. The signaling pathways of astrocytes exhibit a tendency to converge on shared downstream transcriptional regulators in conditions of inflammation. An essential step in the astrocytes mediated-inflammation is the generation of the NF-κB heterodimer (Fig. 7D). The nuclear translocation of NF-κB in astrocytes is initiated by pro-inflammatory stimuli, including TNF-α, IL-1β, IL-17, ROS, phagocytosed myelin, TLR activation, sphingolipids such as sphingosine 1-phosphate and lactosylceramide, and other factors that are associated with inflammation in TSCI [6,67,86]. In addition, the ligand-activated transcription factor aryl hydrocarbon receptor of astrocytes, whose activity is modulated by small molecules of cellular and commensal flora metabolism, also induces NF-κB nuclear translocation (Fig. 7D) [87,88]. Specifically, the TGFβ pathway inhibits NF-κB activation and nuclear translocation [71]. The STAT3 pathway plays an important role in astrocytes, wherein the activation of the STAT3 pathway damages repair and decreases cell survival [71,89]. The activation of the STAT3 pathway has consistently been reported in reactive astrocytes following acute damage. By contrast, the exacerbation of neonatal white matter injury and adult contusive SCI is observed when STAT3 is conditionally deleted in astrocytes [90,91].
5. Nanomaterials as therapeutic agents to modulate astrocyte-mediated inflammation
Concomitant with the understanding that astrocytes, as well as microglia, constitute the major players in the TSCI immune response [92], promising results have been obtained via strategies that induce a change in the inflammatory microenvironment at and around the lesion site [56]. Currently, a significant number of strategies focus exclusively on addressing one particular aspect of TSCI. Nevertheless, considering the complex and multidimensional nature of TSCI, it is reasonable to anticipate that combining several strategies will yield greater effectiveness [93]. Nanomaterials play a crucial role in combinatorial approaches since they possess the capability to provide localized drug delivery over an extended duration and assist in the regulation of astrocytes to optimize benefits. Nevertheless, most studies involving the use of nanomaterials to mediate inflammation in TSCI have focused on microglia, with few in-depth studies on the inflammatory pathways involving astrocytes. In the subsequent sub-sections, we provide a concise overview of the benefits and constraints associated with commonly employed approaches for facilitating recuperation post injury. Additionally, we emphasize novel scientific investigations demonstrating the augmented efficacy of combinatorial strategies for modulating astrocytes through the utilization of nanomaterials. When assessing the selection and configuration of nanomaterials for the purpose of modulating astrocytes after TSCI, one must consider certain overarching factors such as biodegradability, biocompatibility, cytocompatibility, physical qualities, and topographical cues [93].
5.1. Organic nanomaterials
Organic nanomaterials exhibit distinct characteristics compared to inorganic nanomaterials, primarily in terms of enhanced biodegradability and biocompatibility. Organic nanomaterials are predominantly composed of polymers that possess diverse components and characteristics: natural polymers, such as chitosan (Cs) [94,95], and collagen, and synthetic polymers, such as polycaprolactone (PCL), poly (lactide-co-glycolide) (PLG), poly (D, l-lactide co-glycolide) (PLGA) [96], poly-l-lactic acid, poly (glycidyl methacrylate) (PGMA) [97], and poly (ethylene glycol) (PEG) (Fig. 1) [98]. Active and targeted transport capabilities can be conferred upon natural or synthetic polymers by including specific functional groups. This feature is particularly significant in the context of targeting astrocyte-mediated inflammation. In addition, organic NPs can be loaded with anti-inflammatory drugs (e.g., rolipram [20,98], spirulina [99], and valproic acid [94]) to limit the inflammatory responses of astrocytes.
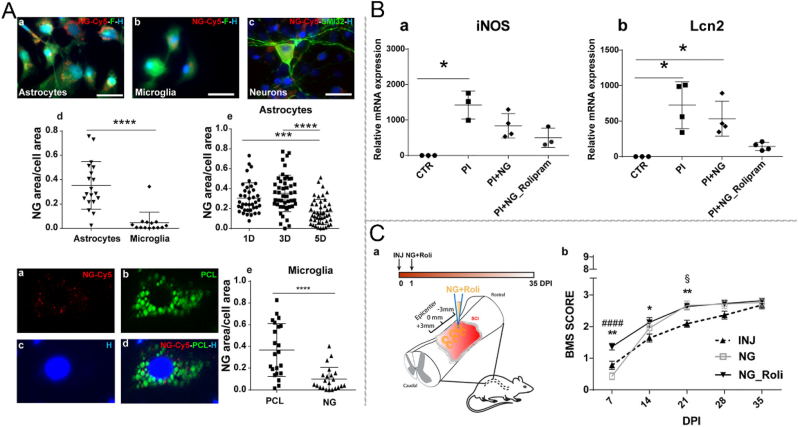
Ren et al. [19] fabricated PLGA nanoparticles (NPs) for sustained local delivery of flavopiridol. They found that these NPs effectively reduced the production of inflammatory factors by astrocytes and restricted glial scarring. As a result, behavioral performance in rats was improved. Vismara et al. [20] synthesized a nanogel using PEG and polyethylene-imine (PEI) coated with primary amines and loaded with rolipram. The application of this nanogel may mitigate the inflammatory response in A1 astrocytes, resulting in reduced iNOS and lipocalin 2 (Lcn2) production. Consequently, this reduction reverses the harmful impact of pro-inflammatory astrocytes on motor neurons in vitro. These findings demonstrate the superiority of the nanogel approach compared to the standard methods of administering anti-inflammatory therapy (Fig. 8) [20]. Furthermore, the nanogel demonstrated selective internalization within activated astrocytes in both mouse and human models. Papa et al. [98] reported that NH2 or Cy5 functionalized on the surface of PEG- and PEI-based nanogel markedly increased the nanogel uptake of astrocytes.
Fig. 8.
Schematic illustration of PEG–PEI nanogel (NG), which is selectively internalized in the activated astrocytes and limits the inflammatory response. (A) Following 24-h exposure, a substantial quantity of PEG-PEI NG is observed within the cytoplasm of astrocytes. (B) PEG-PEI NG has been seen to decrease the expression of inducible nitric oxide synthase (iNOS) and lipocalin 2 (Lcn2) in activated astrocytes. (C) Early administration of NG loaded with rolipram demonstrated enhanced locomotor ability in mice with TSCI. Copyright 2020 ACS Nano [20].
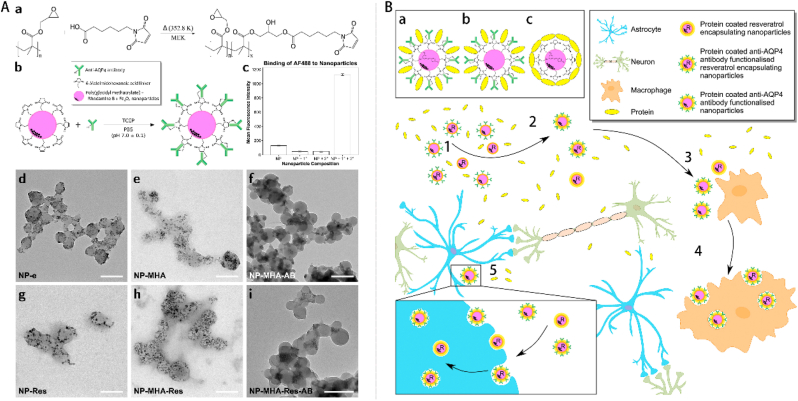
Gao et al. [100] synthesized a unique type of polypyrrole nanowires (PpyNWs) that exhibited vertical alignment and electromagnetic sensitivity. These nanowires were further doped with dexamethasone (DEX) and subjected to an external electromagnetic field twice a day for a duration of 1 week. This controlled release of DEX from the polymer reduced astrocyte-mediated inflammation. Lozic et al. [97] synthesized PGMA–6-maleimidohexanoic acid (MHA)–rhodamine-B (RhB) NPs functionalized with an anti-AQP4 antibody and encapsulated resveratrol (PGMA–MHA–RhB–RA NPs), targeting astrocytes (Fig. 9A). The results showed that the size of NPs was 150 nm, as determined using dynamic light scattering (Fig. 9A). In addition, these NPs played a vital role in ameliorating secondary injury by reducing oxidative damage to DNA and AQP4 immunoreactivity (Fig. 9B).
Fig. 9.
Schematic illustration of the process involved in the synthesis and characterization of nanoparticles. (A) The process involves the synthesis of nanoparticles followed by their interaction with naturally occurring proteins, leading to the creation of a protein corona surrounding the nanoparticles. (B) Three NP formulations were evaluated in an in vivo setting. Copyright 2016 Biomaterials [97].
PCL and PLGA are biocompatible and beneficial for traumatic brain injury as they can alleviate astrocytic activation [101]. In particular, the topography of PCL nanofibers promotes adhesion and downregulates GFAP expression, thereby leading to reduced astrocyte activity [102]. Galactose functionalization of PCL nanofibers can maintain an attenuated inflammatory profile of astrocytes over an extended culture period [103]. Moreover, valproic acid-labeled chitosan (VA-CNs) NPs that inhibit the production of IL-1β, IL-6, and TNF-α by reactive astrocytes in rats were constructed, and they represent a novel and promising therapeutic strategy for SCI [94]. Poly (lactide-co-glycolide)-graft-polyethylenimine (PgP) delivery of small interfering RNA (siRNA) targeting RhoA (siRhoA) reduced astrogliosis and inflammation in rats [104]. siRNA-loaded PLGA NPs significantly inhibited PLK4 expression in rats, together with PCNA expression [96]. We speculate that the inhibition of Plk4 might inhibit the proliferation of astrocytes and decrease the astrocyte-mediated inflammatory response, thereby promoting the functional recovery of SCI [96]. In conclusion, the process of nanopatterning polydimethylsiloxane demonstrates a reduction in the inflammatory response of astrocytes by the downregulation of gene expression associated with GFAP, TNF-α, and IL-1 [105].
5.2. Bioderived nanomaterials
Despite the potential for organic nanomaterials to be customized to suit the specific microenvironment of live organisms in terms of their components and capabilities, there are still unresolved concerns over their immunogenicity when they are used over extended periods of time. Bioderived nanomaterials derived from living organic cells, including exosomes and membrane-coated NPs with highly non-immunogenic properties, can be used to prevent immune monitoring during systemic administration [106]. Exosomes with an average diameter of 50–200 nm contain an array of membrane-associated, high-order oligomeric proteins (e.g., CD63/CD81/CD9, heat shock protein 60, and actin-interacting protein 1/ALG-2-interacting protein X), nucleic acids (e.g., DNA, mRNA, and noncoding RNA), lipids (e.g., cholesterol, phosphatidylserine, and phosphatidylinositol), and metabolites (lactate, glutamate, acetate, stearate, palmitate, and amino acids) [106,107]. Encapsulated biomolecules play a crucial role in enhancing intercellular communication activities, consequently facilitating the fusion of cell membranes, improving the efficiency of delivery and transfection, and replenishing nutrients in areas that have been damaged [107,108]. The utilization of cell membrane-based nanotechnology has come to be as a promising strategy for enhancing the delivery of pharmaceuticals. This technique combines the biomimetic properties of cell membranes with the functional adaptability of nanoparticles [109]. Cell membranes are commonly isolated from various types of cells, including red blood cells, white blood cells, stem cells, platelets, macrophages, and lymphocytes. Membrane-coated NPs, thus, constitute biomimetic NPs with the ability to prolong their circulation times and achieve specific and sensitive targeting [106].
Bone marrow stem cells (BMSCs) are frequently employed as stem cell lines in the generation of exosomes and for therapeutic applications in the treatment of TSCI. In their study, Liu et al. [110] discovered that exosomes derived from BMSCs has the ability to impede scar formation by suppressing the activation of neurotoxic A1 astrocytes. Wang et al. [111] conducted further investigations into the mechanism underlying the inhibition of A1 astrocyte activation by exosomes derived from bone marrow mesenchymal stem cells (MSC-Exo) in the context of TSCI. Their findings revealed that the inhibitory impact was associated with the downregulation of the phosphorylated NF-κB P65 subunit. The study found that MSC-Exo had a significant impact on SCI-induced A1 astrocytes, most likely by inhibiting the nuclear translocation of NF-κB p65. This resulted in a decrease in the lesion area and a reduction in the expression of TNF-α, IL-1α, and IL-1β. Additionally, MSC-Exo increased the expression of myelin basic protein, synaptophysin (Syn), and neuronal nuclei. These effects led to an improvement in Basso, Beattie, and Bresnahan scores, indicating the anti-inflammatory and neuroprotective properties of MSC-Exo in the context of TSCI [111].
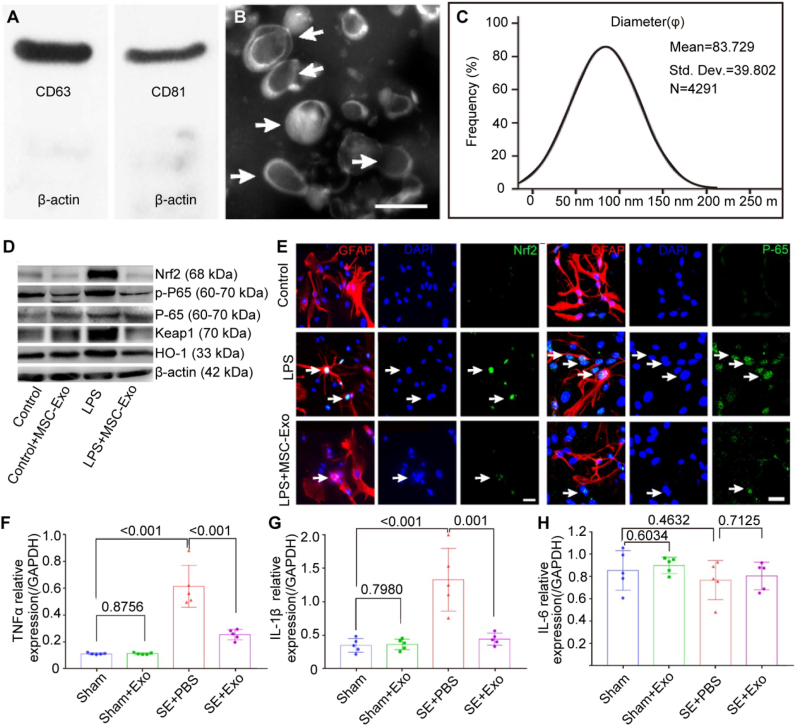
Xian et al. [112] successfully isolated MSC-Exo by employing an ultra-centrifugation procedure, which allowed for the identification of classical exosomal markers, including CD63 and CD81 (Fig. 10A). The MSC-Exo showed a typical “rim of a cup” morphology with a diameter ranging from 30 to 150 nm (Fig. 10B and C). Furthermore, their findings indicated that MSC-Exo effectively mitigated the adverse effects of inflammation on astrocyte morphology. Additionally, it was observed that the NF-E2–related factor 2 (Nrf2)–NF-κB signaling pathway played a key role in modulating astrocyte activation in mice (Fig. 10D–H). The data presented in this study indicated that MSC-Exo exhibited considerable potential as a nanotherapeutic agent in the management of neurological disorders characterized by astrocyte abnormalities.
Fig. 10.
Schematic illustration of the characterization of mesenchymal stem cell-derived extracellular vesicles (MSC-Exo) and their impact on the suppression of inflammation. (A) Western blot analysis was used to assess the protein expression in exosomes derived from the umbilical cord. (B) A representative scanning electron microscopy image is presented, depicting the morphology of MSC-Exo (indicated by arrowheads). (C) The size distribution of isolated MSC-Exo is shown. (D) The protein expression of Nrf2-NF-κB signaling in primary cultured astrocytes was assessed using western blotting. (E) Representative photos depicting the nuclear translocation of Nrf2 (shown by a white arrow) and P-65 (also indicated by a white arrow) in astrocytes triggered by LPS. (F–H) Quantitative polymerase chain reaction (qPCR) to determine the RNA levels of TNF-α (F), IL-1β(G), and IL-6 (H) in the hippocampus tissues of the experimental groups. The expression levels were measured relative to those of the reference gene Gapdh. Copyright 2019 Theranostics [112].
In recent years, exosomes and cell-derived nanovesicles have emerged as viable options for medication delivery. In their study, Jiang et al. [113] employed exosomes generated from neurons as carriers for miR-124–3p. The artificially created NPs hindered the activation of A1 astrocytes by modulating the PI3K/AKT/NF-κB signaling pathway, ultimately facilitating the restoration of normal function after TSCI in rats. Furthermore, the authors stated that the exosomes produced from neurons enhanced functional behavioral recovery by inhibiting the activation of M1 microglia and A1 astrocytes, both in vivo and in vitro [113]. The enrichment of miR-124–3p in neuron-derived exosomes was validated using a miRNA array. Subsequently, MYH9 was identified as a downstream target of miR-124–3p [113].
5.3. Inorganic nanomaterials
Inorganic nanomaterials often consist of biocompatible and inert metals, which confer stable properties and reduced dimensions to the nanomaterials. Inorganic nanomaterials have demonstrated significant efficacy and display a range of impacts in various biological applications. Cerium dioxide (CeO2) and selenium (Se) [95] have been used to modulate astrocyte-mediated inflammation in SCI. In particular, CeO2 (or nanoceria) NPs have drawn increased research attention in the field of nanomedicine as a potential anti-inflammatory and anti-oxidative biomaterial. Moderate nanoceria treatment could inhibit astrocyte-activation-related NF-κB expression and enhance Nrf2 expression in vivo and in vitro, suggesting that nanoceria could inhibit inflammation and nerve injury [114]. In addition, application of the NF-κB inhibitor pyrollidine dithiocarbamate (PDTC) to primary astrocytes demonstrated that the Nrf2 pathway could be upregulated in a dose-dependent manner by nanoceria [114]. A controlled drug delivery system containing selenium NPs may also protect the nerve cells in SCI through an anti-inflammatory effect [95]. Moreover, according to recent studies, selenium nanoparticles promoted A2 astrocyte polarization in brain models under ischemia/reoxygenation [115,116]. The selenium nanomaterials stimulate phosphoinositide 3-kinase (PI3K) activity, which confers protective effects [115,116]. Thus, selenium nanomaterials may have a similar role in TSCI. However, the primary obstacle that hinders the utilization of these nanomaterials is their clearance from the human body, as repeated administration might lead to toxicity through the accumulation of these nanomaterials. To tackle this issue, a nanostructured material has been created by integrating the advantageous electrochemical characteristics of iridium oxide (IrOx) and carbon nanotubes with the properties of poly (3,4-ethylenedioxythiophene) [117]. The results of this study indicate that this material exhibits a reduced inflammatory response in astrocytes when exposed to lipopolysaccharide, as evidenced by the decreased expression of Nos2 and Cox2 mRNA [117].
5.4. Other nanomaterials
Rolipram [20], spirulina [99], and valproic acid [94] have been applied to limit the inflammatory response of astrocytes. Resveratrol is a naturally occurring polyphenol found in grapes, berries, peanuts, and wine [75]. Resveratrol has been shown to efficiently impede the activation of the NF-κB signaling pathway through the suppression of the inhibitor of κB kinase (Iκκ) activity [75]. Resveratrol impedes the creation of the NLRP2 inflammasome in astrocytes by diminishing ATP generation and ROS production [75]. Ren et al. [19] found a significant reduction in the synthesis of inflammatory factors, including TNF-α, IL-1β, and IL-6, by astrocytes upon treatment with flavopiridol NPs. Additionally, the expression of IL-10 was found to be increased. In addition to their reactive properties, astrocytes can also produce inflammatory chemicals, including Lcn2. This secretion of Lcn2 by reactive astrocytes contributes to the perpetuation of their reactivity and has negative effects on other cells within the spinal cord [118]. Smith et al. [118] demonstrated the feasibility of utilizing the packaging RNA (pRNA)-derived three-way junction (3 W J) motif as a platform for delivering siRNAs. This approach, known as RNA nanotherapeutics, was employed to effectively downregulate the expression of GFAP, Vim, and Lcn2 in astrocytes, thereby impeding the progression of inflammation in these normally inactive cells. In turn, the study conducted by Zou et al. revealed that the utilization of tetrahedral framework nucleic acid nanomaterials effectively induces a shift in cellular polarization, specifically from the proinflammatory A1 phenotype to the neuroprotective A2 phenotype [119]. Furthermore, it has been observed that tetrahedral framework nucleic acid nanomaterials downregulate the TLRs/NF-κB signaling pathway, suggesting that this could be a potential mechanism by which nanomaterials protect astrocytes during stroke [119]. These nanomaterials may possess a comparable function in the context of spinal cord damage. In addition, another study demonstrated that the utilization of chitosan-alginate-sodium tripolyphosphate (CS-ALG-STPP) NPs significantly improved the duration of action and the amount of curcumin that can be absorbed by the body. Furthermore, the study observed a reduction in the degree of inflammation and activation of glial cells in a model of localized demyelination caused by lysolecithin (LPC) following treatment with curcumin-loaded CS-ALG-STPP NPs [120]. Recently, researchers successfully created and enhanced chitosan-coated rosmarinic acid nanoemulsions. These nanoemulsions have demonstrated notable anti-inflammatory properties in the context of lipopolysaccharide-induced inflammation in astrocytes [121].
6. Conclusions and prospects
Reactive astrocytes are crucial in the innate immune response to TSCI and in attracting and restricting inflammation. Accordingly, strategies to manipulate astrocyte reactivity in TSCI have attracted increasing research interest. The available body of evidence indicates that the reactivity of astrocytes cannot be categorized into a limited number of generalized programs that are either positive or negative to the host [68]. In addition, similar to microglia, astrocytes exhibit spatio-temporal dynamics following TSCI [122]. Several studies have demonstrated that reactive astrocytes exhibit a wide range of nuanced alterations that are particular to the condition and setting, both in the acute and chronic stages of inflammation associated with TSCI [66,123,124]. This emphasizes the increasing necessity to develop a comprehensive understanding of the alterations in distinct astrocyte functions that occur in the process of spatio-temporal dynamics in TSCI. Hence, further investigations are necessary to determine the means by which the restorative and therapeutic capabilities of astrocytes can be enhanced, while concurrently mitigating the detrimental effects of excessive inflammation that exacerbates tissue injury.
The field of nanomaterial-based immunotherapy has undergone significant advancements in recent decades, demonstrating significant potential for implementation in clinical settings. Through the continuous advancement of manufacturing techniques and the implementation of sophisticated design concepts, nanotechnology has been effectively utilized in the field of immunological regulation, enabling the management of numerous diseases [125,126]. Nevertheless, nanomaterials to modulate astrocyte-mediated inflammation remain relatively under researched. In recent years, there has been a growing recognition of the significant physiological roles played by astrocytes. Consequently, investigating the intricate interplay between nanomaterials and astrocytes has emerged as a novel area of exploration within the fields of nanomedicine and neurology [92]. A more thorough understanding of the positive and negatives aspects of each nanomaterial will facilitate their appropriate utilization and allow their development as effective therapeutic modalities for astrocyte-mediated inflammation. Organic NPs, which exhibit the greatest potential among nanomaterials for clinical translation in the short term, constitute a hotspot in nanomaterial research. Hence, it is quite probable that these nanomaterials will be able to accomplish biological engineering conversion within a short timeframe. Subsequent investigations ought to prioritize the investigation and refinement of active targeting techniques in the realm of nanomaterial design, with the aim of augmenting medication accumulation specifically at the site of the lesion. Moreover, codelivery of anti-inflammatory drugs or micromolecules in nanosized carriers enhances the efficacies of nanomaterials. However, limitations regarding the rapid, precise, and reproducible organic production of NPs, as well as their biocompatibility and safety still hinder clinical translation [127]. Alternatively, owing to the well-known toxicity of inorganic nanomaterials, their practical application in biomedicine remains elusive. Nevertheless, the technological advancements in the manufacture of inorganic NPs have reached a higher level of maturity compared to the production of organic NPs. This has resulted in a greater degree of uniformity and control in the manufacturing of each batch of inorganic NPs. Furthermore, despite several studies demonstrating the biocompatibility of inorganic NPs with brain cells in laboratory settings [20,128,129], concerns persist regarding their safety when used in living organisms.
The primary concern in utilizing immunomodulatory NPs is ensuring safety. Given the intimate interaction between NPs and the immune system, it becomes necessary to establish a standardized approach for evaluating their potential immunotoxicity. As an illustration, the process of NPs binding to serum proteins can result in the formation of a protein corona surrounding the NP surface [130,131]. Additional toxicities, such as challenges in the elimination process from the human body, should be duly considered prior to the implementation of clinical interventions. Hence, it is imperative to conduct a thorough study and assessment of the local and systemic toxicity of nanomaterials toward healthy organs and tissues. The utilization of nanomaterials that have been functionalized with effective targeting ligands and/or stimuli-sensitive capabilities can contribute to the further enhancement of safety. This is achieved by enabling targeted distribution and regulated responsive release of anti-inflammatory medicines or immunomodulators.
In general, the investigation of innovative immunotherapies by the effective manipulation of nanomaterials presents an attractive and promising area of research. Although numerous obstacles remain, profound improvements in clinical efficacy and benefits for preventing or ameliorating the detrimental effects of SCI are expected in the future.
CRediT authorship contribution statement
Wenqi Luo: Conceptualization, Funding acquisition, Writing - original draft, Writing - review & editing. Yueying Li: Conceptualization, Writing - original draft, Writing - review & editing. Chunyu Xiang: Writing - review & editing. Toshimi Aizawa: Writing - review & editing. Renrui Niu: Conceptualization, Writing - review & editing. Yiming Wang: Writing - review & editing. Jianhui Zhao: Conceptualization, Supervision, Writing - review & editing. Zeping Liu: Writing - review & editing. Chaoyuan Li: Writing - review & editing. Wanguo Liu: Supervision, Writing - review & editing. Rui Gu: Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 82271411, 51803072), the Department of Finance of Jilin Province (grant numbers 2022SCZ25, 2022SCZ10, 2021SCZ07), Jilin Provincial Science and Technology Program (grant number YDZJ202201ZYTS038), Youth Support Programmed Project of China Japan Union Hospital of Jilin University (grant number 2022qnpy11), and the China Japan Union Hospital of Jilin University Project (grant number XHQMX20233).
Contributor Information
Jianhui Zhao, Email: JianhuiZhao@jlu.edu.cn.
Wanguo Liu, Email: liuwanguo6016@jlu.edu.cn.
Rui Gu, Email: gurui@jlu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Salvador A.F.M., Kipnis J. Immune response after central nervous system injury. Semin. Immunol. 2022;59 doi: 10.1016/j.smim.2022.101629. [DOI] [PubMed] [Google Scholar]
- 2.David S., Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 3.Lin F., Liu Y., Luo W., Liu S., Wang Y., Gu R., Liu W., Xiao C. Minocycline-loaded poly(alpha-lipoic acid)-methylprednisolone prodrug nanoparticles for the combined anti-inflammatory treatment of spinal cord injury. Int. J. Nanomed. 2022;17:91–104. doi: 10.2147/IJN.S344491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran A.P., Warren P.M., Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papa S., Caron I., Erba E., Panini N., De Paola M., Mariani A., Colombo C., Ferrari R., Pozzer D., Zanier E.R., Pischiutta F., Lucchetti J., Bassi A., Valentini G., Simonutti G., Rossi F., Moscatelli D., Forloni G., Veglianese P. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 2016;75:13–24. doi: 10.1016/j.biomaterials.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Linnerbauer M., Wheeler M.A., Quintana F.J. Astrocyte crosstalk in CNS inflammation. Neuron. 2020;108:608–622. doi: 10.1016/j.neuron.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenhalgh A.D., David S., Bennett F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020;21:139–152. doi: 10.1038/s41583-020-0263-9. [DOI] [PubMed] [Google Scholar]
- 8.Rong Y., Ji C., Wang Z., Ge X., Wang J., Ye W., Tang P., Jiang D., Fan J., Yin G., Liu W., Cai W. Small extracellular vesicles encapsulating CCL2 from activated astrocytes induce microglial activation and neuronal apoptosis after traumatic spinal cord injury. J. Neuroinflammation. 2021;18:196. doi: 10.1186/s12974-021-02268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liddelow S.A., Barres B.A. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Mills Ko E., Ma J.H., Guo F., Miers L., Lee E., Bannerman P., Burns T., Ko D., Sohn J., Soulika A.M., Pleasure D. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J. Neuroinflammation. 2014;11:105. doi: 10.1186/1742-2094-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovannoni F., Quintana F.J. The role of astrocytes in CNS inflammation. Trends Immunol. 2020;41:805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon H.S., Koh S.H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes, Transl. Neurodegeneration. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva N.A., Sousa N., Reis R.L., Salgado A.J. From basics to clinical: a comprehensive review on spinal cord injury. Prog. Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Ropper A.E., Ropper A.H. Acute spinal cord compression. N. Engl. J. Med. 2017;376:1358–1369. doi: 10.1056/NEJMra1516539. [DOI] [PubMed] [Google Scholar]
- 15.Ahuja C.S., Nori S., Tetreault L., Wilson J., Kwon B., Harrop J., Choi D., Fehlings M.G. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80 doi: 10.1093/neuros/nyw080. S9–S22. [DOI] [PubMed] [Google Scholar]
- 16.Fehlings M.G., Badhiwala J.H., Ahn H., Farhadi H.F., Shaffrey C.I., Nassr A., Mummaneni P., Arnold P.M., Jacobs W.B., Riew K.D., Kelly M., Brodke D.S., Vaccaro A.R., Hilibrand A.S., Wilson J., Harrop J.S., Yoon S.T., Kim K.D., Fourney D.R., Santaguida C., Massicotte E.M., Kopjar B. Safety and efficacy of riluzole in patients undergoing decompressive surgery for degenerative cervical myelopathy (CSM-Protect): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Neurol. 2021;20:98–106. doi: 10.1016/S1474-4422(20)30407-5. [DOI] [PubMed] [Google Scholar]
- 17.Koda M., Hanaoka H., Fujii Y., Hanawa M., Kawasaki Y., Ozawa Y., Fujiwara T., Furuya T., et al. Randomized trial of granulocyte colony-stimulating factor for spinal cord injury. Brain. 2021;144:789–799. doi: 10.1093/brain/awaa466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard G., Kannan R., Liu J., Wang W., Lam T.K.T., Wang X., Adamson C., Hackett C., Schwab J.M., Liu C., Leslie D.P., Chen D., Marino R., Zafonte R., Flanders A., Block G., Smith E., Strittmatter S.M. Soluble Nogo-Receptor-Fc decoy (AXER-204) in patients with chronic cervical spinal cord injury in the USA: a first-in-human and randomised clinical trial. Lancet Neurol. 2023;22:672–684. doi: 10.1016/S1474-4422(23)00215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren H., Han M., Zhou J., Zheng Z.F., Lu P., Wang J.J., Wang J.Q., Mao Q.J., Gao J.Q., Ouyang H.W. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials. 2014;35:6585–6594. doi: 10.1016/j.biomaterials.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Vismara I., Papa S., Veneruso V., Mauri E., Mariani A., De Paola M., Affatato R., Rossetti A., Sponchioni M., Moscatelli D., Sacchetti A., Rossi F., Forloni G., Veglianese P. Selective modulation of A1 astrocytes by drug-loaded nano-structured gel in spinal cord injury. ACS Nano. 2020;14:360–371. doi: 10.1021/acsnano.9b05579. [DOI] [PubMed] [Google Scholar]
- 21.Song Y.H., Agrawal N.K., Griffin J.M., Schmidt C.E. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv. Drug Deliv. Rev. 2019;148:38–59. doi: 10.1016/j.addr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.New P.W. A narrative review of pediatric nontraumatic spinal cord dysfunction. Top. Spinal Cord Inj. Rehabil. 2019;25:112–120. doi: 10.1310/sci2502-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pataraia A., Crevenna R. Challenges in rehabilitation of patients with nontraumatic spinal cord dysfunction due to tumors: a narrative review, Wien, Klin. Woche. 2019;131:608–613. doi: 10.1007/s00508-019-1528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.New P.W., Guilcher S.J.T., Jaglal S.B., Biering-Sørensen F., Noonan V.K., Ho C. Trends, challenges, and opportunities regarding research in non-traumatic spinal cord dysfunction. Top. Spinal Cord Inj. Rehabil. 2017;23:313–323. doi: 10.1310/sci2304-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., Fehlings M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 26.David G., Mohammadi S., Martin A.R., Cohen-Adad J., Weiskopf N., Thompson A., Freund P. Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat. Rev. Neurol. 2019;15:718–731. doi: 10.1038/s41582-019-0270-5. [DOI] [PubMed] [Google Scholar]
- 27.Ge L., Arul K., Ikpeze T., Baldwin A., Nickels J.L., Mesfin A. Traumatic and nontraumatic spinal cord injuries. World Neurosurg. 2018;111:e142. doi: 10.1016/j.wneu.2017.12.008. –e148. [DOI] [PubMed] [Google Scholar]
- 28.Schwab J.M., Maas A.I.R., Hsieh J.T.C., Curt A. Raising awareness for spinal cord injury research. Lancet Neurol. 2018;17:581–582. doi: 10.1016/S1474-4422(18)30206-0. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R., Lim J., Mekary R.A., Rattani A., Dewan M.C., Sharif S.Y., Osorio-Fonseca E., Park K.B. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–e363. doi: 10.1016/j.wneu.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Jazayeri S.B., Beygi S., Shokraneh F., Hagen E.M., Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur. Spine J. 2015;24:905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 31.Aboyans V., Abraha H.N., Acharya D., Adamu A.A., Adebayo O.M., Adeoye A.M., Adsuar J.C., et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leister I., Haider T., Mattiassich G., Kramer J.L.K., Linde L.D., Pajalic A., Grassner L., Altendorfer B., Resch H., Aschauer-Wallner S., Aigner L. Biomarkers in traumatic spinal cord injury-technical and clinical considerations: a systematic review. Neurorehabilitation Neural Repair. 2020;34:95–110. doi: 10.1177/1545968319899920. [DOI] [PubMed] [Google Scholar]
- 33.Dusart I., Schwab M.E. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur. J. Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 34.Yao X.Q., Liu Z.Y., Chen J.Y., Huang Z.C., Liu J.H., Sun B.H., Zhu Q.A., Ding R.T., Chen J.T. Proteomics and bioinformatics reveal insights into neuroinflammation in the acute to subacute phases in rat models of spinal cord contusion injury. Faseb. J. 2021;35 doi: 10.1096/fj.202100081RR. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Zhang F., Xu H., Yang H., Shao M., Xu S., Lyu F. TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin. Transl. Med. 2022;12:e894. doi: 10.1002/ctm2.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Li N., Zhu L., Lin Y., Cheng H. The microglial activation profile and associated factors after experimental spinal cord injury in rats. Neuropsychiatric Dis. Treat. 2018;14:2401–2413. doi: 10.2147/NDT.S169940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellenbrand D.J., Quinn C.M., Piper Z.J., Morehouse C.N., Fixel J.A., Hanna A.S. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflammation. 2021;18:284. doi: 10.1186/s12974-021-02337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu W.S., Tian D.S., Guo Z.B., Fang J., Zhang Q., Yu Z.Y., Xie M.J., Zhang H.Q., Lü J.G., Wang W. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J. Neuroinflammation. 2012;9:178. doi: 10.1186/1742-2094-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saijo K., Glass C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 40.Lundgren C.A.K., Sjöstrand D., Biner O., Bennett M., Rudling A., Johansson A.L., Brzezinski P., Carlsson J., von Ballmoos C., Högbom M. Scavenging of superoxide by a membrane-bound superoxide oxidase. Nat. Chem. Biol. 2018;14:788–793. doi: 10.1038/s41589-018-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park M.W., Cha H.W., Kim J., Kim J.H., Yang H., Yoon S., Boonpraman N., Yi S.S., Yoo I.D., Moon J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi H., Jin L., Li J., Liang K., Li X., Ye Z., Zhu X., Oliveira J.M., Reis R.L., Mao Z., Wu M. Mesoporous polydopamine nanoparticles for sustained release of rapamycin and reactive oxygen species scavenging to synergistically accelerate neurogenesis after spinal cord injury. J. Mater. Chem. B. 2022;10:6351–6359. doi: 10.1039/d2tb00841f. [DOI] [PubMed] [Google Scholar]
- 43.Maas A.I.R., Peul W., Thomé C. Surgical decompression in acute spinal cord injury: earlier is better. Lancet Neurol. 2021;20:84–86. doi: 10.1016/S1474-4422(20)30478-6. [DOI] [PubMed] [Google Scholar]
- 44.Badhiwala J.H., Wilson J.R., Witiw C.D., Harrop J.S., Vaccaro A.R., Aarabi B., Grossman R.G., Geisler F.H., Fehlings M.G. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20:117–126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 45.Ramer L.M., Ramer M.S., Bradbury E.J. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 2014;13:1241–1256. doi: 10.1016/S1474-4422(14)70144-9. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., Xia Y., Li B., Li Y., Fu C. Reverse adverse immune microenvironments by biomaterials enhance the repair of spinal cord injury. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.812340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo W., Wang Y., Lin F., Liu Y., Gu R., Liu W., Xiao C. Selenium-doped carbon quantum dots efficiently ameliorate secondary spinal cord injury via scavenging reactive oxygen species. Int. J. Nanomed. 2020;15:10113–10125. doi: 10.2147/IJN.S282985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batchelor P.E., Kerr N.F., Gatt A.M., Aleksoska E., Cox S.F., Ghasem-Zadeh A., Wills T.E., Howells D.W. Hypothermia prior to decompression: buying time for treatment of acute spinal cord injury. J. Neurotrauma. 2010;27:1357–1368. doi: 10.1089/neu.2010.1360. [DOI] [PubMed] [Google Scholar]
- 49.Vedantam A., Levi A.D. Hypothermia for acute spinal cord injury. Neurosurg. Clin. 2021;32:377–387. doi: 10.1016/j.nec.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Clifford T., Finkel Z., Rodriguez B., Joseph A., Cai L. Current advancements in spinal cord injury research-glial scar formation and neural regeneration. Cells. 2023;12:853. doi: 10.3390/cells12060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prüss H., Tedeschi A., Thiriot A., Lynch L., Loughhead S.M., Stutte S., Mazo I.B., Kopp M.A., Brommer B., Blex C., Geurtz L.C., Liebscher T., Niedeggen A., Dirnagl U., Bradke F., Volz M.S., DeVivo M.J., Chen Y., von Andrian U.H., Schwab J.M. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 2017;20:1549–1559. doi: 10.1038/nn.4643. [DOI] [PubMed] [Google Scholar]
- 52.Vainchtein I.D., Chin G., Cho F.S., Kelley K.W., Miller J.G., Chien E.C., Liddelow S.A., Nguyen P.T., Nakao-Inoue H., Dorman L.C., Akil O., Joshita S., Barres B.A., Paz J.T., Molofsky A.B., Molofsky A.V. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359:1269–1273. doi: 10.1126/science.aal3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gadani S.P., Walsh J.T., Lukens J.R., Kipnis J. Dealing with danger in the CNS: the response of the immune system to injury. Neuron. 2015;87:47–62. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gadani S.P., Walsh J.T., Smirnov I., Zheng J., Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Chan C.C.M. Inflammation: beneficial or detrimental after spinal cord injury? Recent Pat. CNS Drug Discov. 2008;3:189–199. doi: 10.2174/157488908786242434. [DOI] [PubMed] [Google Scholar]
- 56.Ren H., Chen X., Tian M., Zhou J., Ouyang H., Zhang Z. Regulation of inflammatory cytokines for spinal cord injury repair through local delivery of therapeutic agents. Adv. Sci. 2018;5 doi: 10.1002/advs.201800529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu X., Leak R.K., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng H., Liu N., Yang Y.Y., Xing H.Y., Liu X.X., Li F., La G.Y., Huang M.J., Zhou M.W. Lentivirus-mediated downregulation of α-synuclein reduces neuroinflammation and promotes functional recovery in rats with spinal cord injury. J. Neuroinflammation. 2019;16:283. doi: 10.1186/s12974-019-1658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen H., Xu B., Yang C., Xue W., You Z., Wu X., Ma D., Shao D., Leong K., Dai J. A DAMP-scavenging, IL-10-releasing hydrogel promotes neural regeneration and motor function recovery after spinal cord injury. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121279. [DOI] [PubMed] [Google Scholar]
- 60.Ma D., Shen H., Chen F., Liu W., Zhao Y., Xiao Z., Wu X., Chen B., Lu J., Shao D., Dai J. Inflammatory microenvironment-responsive nanomaterials promote spinal cord injury repair by targeting IRF5. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202201319. [DOI] [PubMed] [Google Scholar]
- 61.Hashemizadeh S., Pourkhodadad S., Hosseindoost S., Pejman S., Kamarehei M., Badripour A., Omidi A., Pestehei S.K., Seifalian A.M., Hadjighassem M. Ac-SDKP peptide improves functional recovery following spinal cord injury in a preclinical model. Neuropeptides. 2022;92 doi: 10.1016/j.npep.2022.102228. [DOI] [PubMed] [Google Scholar]
- 62.Bellver-Landete V., Bretheau F., Mailhot B., Vallières N., Lessard M., Janelle M.E., Vernoux N., Tremblay M.È., Fuehrmann T., Shoichet M.S., Lacroix S. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019;10:518. doi: 10.1038/s41467-019-08446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schnell L., Schneider R., Berman M.A., Perry V.H., Schwab M.E. Lymphocyte recruitment following spinal cord injury in mice is altered by prior viral exposure. Eur. J. Neurosci. 1997;9:1000–1007. doi: 10.1111/j.1460-9568.1997.tb01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monahan R., Stein A., Gibbs K., Bank M., Bloom O. Circulating T cell subsets are altered in individuals with chronic spinal cord injury. Immunol. Res. 2015;63:3–10. doi: 10.1007/s12026-015-8698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwon A.H.K., Liddelow S.A. Astrocytes have a license to kill inflammatory T cells. Immunity. 2021;54:614–616. doi: 10.1016/j.immuni.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Hasel P., Rose I.V.L., Sadick J.S., Kim R.D., Liddelow S.A. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 2021;24:1475–1487. doi: 10.1038/s41593-021-00905-6. [DOI] [PubMed] [Google Scholar]
- 67.Han R.T., Kim R.D., Molofsky A.V., Liddelow S.A. Astrocyte-immune cell interactions in physiology and pathology. Immunity. 2021;54:211–224. doi: 10.1016/j.immuni.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Sofroniew M.V. Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol. 2020;41:758–770. doi: 10.1016/j.it.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blume C., Geiger M.F., Brandenburg L.O., Müller M., Mainz V., Kalder J., Albanna W., Clusmann H., Mueller C.A. Patients with degenerative cervical myelopathy have signs of blood spinal cord barrier disruption, and its magnitude correlates with myelopathy severity: a prospective comparative cohort study. Eur. Spine J. 2020;29:986–993. doi: 10.1007/s00586-020-06298-7. [DOI] [PubMed] [Google Scholar]
- 70.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colombo E., Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sofroniew M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wheeler M.A., Jaronen M., Covacu R., Zandee S.E.J., Scalisi G., Rothhammer V., Tjon E.C., Chao C.C., Kenison J.E., Blain M., Rao V.T.S., Hewson P., Barroso A., Gutiérrez-Vázquez C., Prat A., Antel J.P., Hauser R., Quintana F.J. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell. 2019;176:581–596.e18. doi: 10.1016/j.cell.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan R., Zhang Y., Botchway B.O.A., Liu X. Resveratrol can attenuate astrocyte activation to treat spinal cord injury by inhibiting inflammatory responses. Mol. Neurobiol. 2021;58:5799–5813. doi: 10.1007/s12035-021-02509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ibáñez F., Montesinos J., Ureña-Peralta J.R., Guerri C., Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J. Neuroinflammation. 2019;16:136. doi: 10.1186/s12974-019-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norden D.M., Fenn A.M., Dugan A., Godbout J.P. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu S.Z., Wu Y., Guo Y.S., Liang P.Z., Yin S., Yin Y.Q., Zhang X.L., Liu Y.F., Wang H.Y., Xiao Y.C., Liang X.M., Zhou J.W. Inhibition of astrocytic DRD2 suppresses CNS inflammation in an animal model of multiple sclerosis. J. Exp. Med. 2022;219 doi: 10.1084/jem.20210998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu J., Hu Z., Han X., Wang D., Jiang Q., Ding J., Xiao M., Wang C., Lu M., Hu G. Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of β-arrestin2 and NLRP3. Cell Death Differ. 2018;25:2037–2049. doi: 10.1038/s41418-018-0127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Falcão A.M., van Bruggen D., Marques S., Meijer M., Jäkel S., Agirre E., Samudyata E.M., Floriddia E.M., Vanichkina D.P., Ffrench-Constant C., Williams A.O., Guerreiro-Cacais A.O., Castelo-Branco G. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018;24:1837–1844. doi: 10.1038/s41591-018-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moyon S., Dubessy A.L., Aigrot M.S., Trotter M., Huang J.K., Dauphinot L., Potier M.C., Kerninon C., Melik Parsadaniantz S.M., Franklin R.J., Lubetzki C. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J. Neurosci. 2015;35:4–20. doi: 10.1523/JNEUROSCI.0849-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niu J., Tsai H.H., Hoi K.K., Huang N., Yu G., Kim K., Baranzini S.E., Xiao L., Chan J.R., Fancy S.P.J. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019;22:709–718. doi: 10.1038/s41593-019-0369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N., Mahase S., Dutta D.J., Seto J., Kramer E.G., Ferrara N., Sofroniew M.V., John G.R. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alvarez J.I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P.J., Terouz S., Sabbagh M., Wosik K., Bourbonnière L., Bernard M., van Horssen J., de Vries H.E., Charron F., Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 85.Paul D., Ge S., Lemire Y., Jellison E.R., Serwanski D.R., Ruddle N.H., Pachter J.S. Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J. Neuroinflammation. 2014;11:10. doi: 10.1186/1742-2094-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rothhammer V., Kenison J.E., Tjon E., Takenaka M.C., de Lima K.A., Borucki D.M., Chao C.C., Wilz A., Blain M., Healy L., Antel J., Quintana F.J. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc. Natl. Acad. Sci. U.S.A. 2017;114:2012–2017. doi: 10.1073/pnas.1615413114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rothhammer V., Mascanfroni I.D., Bunse L., Takenaka M.C., Kenison J.E., Mayo L., Chao C.C., Patel B., Yan R., Blain M., Alvarez J.I., Kébir H., Anandasabapathy N., Izquierdo G., Jung S., Obholzer N., Pochet N., Clish C.B., Prinz M., Prat A., Antel J., Quintana F.J. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016;22:586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rothhammer V., Kenison J.E., Li Z., Tjon E., Takenaka M.C., Chao C.C., Alves de Lima K., Borucki D.M., Kaye J., Quintana F.J. Aryl hydrocarbon receptor activation in astrocytes by laquinimod ameliorates autoimmune inflammation in the CNS, neurology(R) Neuroimmunol. Neuroinflammation. 2021;8:e946. doi: 10.1212/NXI.0000000000000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X., Li M., Tian L., Chen J., Liu R., Ning B. Reactive astrogliosis: implications in spinal cord injury progression and therapy. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/9494352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrmann J.E., Imura T., Song B., Qi J., Ao Y., Nguyen T.K., Korsak R.A., Takeda K., Akira S., Sofroniew M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okada S., Nakamura M., Katoh H., Miyao T., Shimazaki T., Ishii K., Yamane J., Yoshimura A., Iwamoto Y., Toyama Y., Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 92.Dai D., He L., Chen Y., Zhang C. Astrocyte responses to nanomaterials: functional changes, pathological changes and potential applications. Acta Biomater. 2021;122:66–81. doi: 10.1016/j.actbio.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 93.Führmann T., Anandakumaran P.N., Shoichet M.S. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv. Healthcare Mater. 2017;6 doi: 10.1002/adhm.201601130. [DOI] [PubMed] [Google Scholar]
- 94.Wang D., Wang K., Liu Z., Wang Z., Wu H. Valproic acid-labeled chitosan nanoparticles promote recovery of neuronal injury after spinal cord injury. Aging (N Y) 2020;12:8953–8967. doi: 10.18632/aging.103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Javdani M., Ghorbani R., Hashemnia M. Histopathological evaluation of spinal cord with experimental traumatic injury following implantation of a controlled released drug delivery system of chitosan hydrogel loaded with selenium nanoparticle. Biol. Trace Elem. Res. 2021;199:2677–2686. doi: 10.1007/s12011-020-02395-2. [DOI] [PubMed] [Google Scholar]
- 96.Gu Y., Zhang R., Jiang B., Xu X., Guan J.J., Jiang X.J., Zhou Y., Zhou Y.L., Chen X. Repair of spinal cord injury by inhibition of PLK4 expression through local delivery of siRNA-loaded nanoparticles. J. Mol. Neurosci. 2022;72:544–554. doi: 10.1007/s12031-021-01871-1. [DOI] [PubMed] [Google Scholar]
- 97.Lozić I., Hartz R.V., Bartlett C.A., Shaw J.A., Archer M., Naidu P.S., Smith N.M., Dunlop S.A., Iyer K.S., Kilburn M.R., Fitzgerald M. Enabling dual cellular destinations of polymeric nanoparticles for treatment following partial injury to the central nervous system. Biomaterials. 2016;74:200–216. doi: 10.1016/j.biomaterials.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Papa S., Veneruso V., Mauri E., Cremonesi G., Mingaj X., Mariani A., De Paola M., Rossetti A., Sacchetti A., Rossi F., Forloni G., Veglianese P. Functionalized nanogel for treating activated astrocytes in spinal cord injury. J. Contr. Release. 2021;330:218–228. doi: 10.1016/j.jconrel.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Min S.K., Kim C.R., Jung S.M., Shin H.S. Astrocyte behavior and GFAP expression on Spirulina extract-incorporated PCL nanofiber. J. Biomed. Mater. Res. A. 2013;101:3467–3473. doi: 10.1002/jbm.a.34654. [DOI] [PubMed] [Google Scholar]
- 100.Gao W., Borgens R.B. Remote-controlled eradication of astrogliosis in spinal cord injury via electromagnetically-induced dexamethasone release from “smart” nanowires. J. Contr. Release. 2015;211:22–27. doi: 10.1016/j.jconrel.2015.05.266. [DOI] [PubMed] [Google Scholar]
- 101.Wong D.Y., Hollister S.J., Krebsbach P.H., Nosrat C. Poly(epsilon-caprolactone) and poly (L-lactic-co-glycolic acid) degradable polymer sponges attenuate astrocyte response and lesion growth in acute traumatic brain injury. Tissue Eng. 2007;13:2515–2523. doi: 10.1089/ten.2006.0440. [DOI] [PubMed] [Google Scholar]
- 102.Min S.K., Kim S.H., Kim C.R., Paik S.M., Jung S.M., Shin H.S. Effect of topography of an electrospun nanofiber on modulation of activity of primary rat astrocytes. Neurosci. Lett. 2013;534:80–84. doi: 10.1016/j.neulet.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 103.Maclean F.L., Lau C.L., Ozergun S., O'Shea R.D., Cederfur C., Wang J., Healy K.E., Walker F.R., Tomas D., Horne M.K., Beart P.M., Nisbet D.R. Galactose-functionalised PCL nanofibre scaffolds to attenuate inflammatory action of astrocytes in vitro and in vivo. J. Mater. Chem. B. 2017;5:4073–4083. doi: 10.1039/c7tb00651a. [DOI] [PubMed] [Google Scholar]
- 104.Macks C., Jeong D., Lee J.S. Local delivery of RhoA siRNA by PgP nanocarrier reduces inflammatory response and improves neuronal cell survival in a rat TBI model. Nanomedicine. 2021;32 doi: 10.1016/j.nano.2020.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ereifej E.S., Cheng M.M., Mao G., VandeVord P.J. Examining the inflammatory response to nanopatterned polydimethylsiloxane using organotypic brain slice methods. J. Neurosci. Methods. 2013;217:17–25. doi: 10.1016/j.jneumeth.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 106.Gong W., Zhang T., Che M., Wang Y., He C., Liu L., Lv Z., Xiao C., Wang H., Zhang S. Recent advances in nanomaterials for the treatment of spinal cord injury. Mater. Today Bio. 2023;18 doi: 10.1016/j.mtbio.2022.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dutta D., Khan N., Wu J., Jay S.M. Extracellular vesicles as an emerging frontier in spinal cord injury pathobiology and therapy. Trends Neurosci. 2021;44:492–506. doi: 10.1016/j.tins.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan H., Shao D., Lao Y.H., Li M., Hu H., Leong K.W. Engineering cell membrane-based nanotherapeutics to target inflammation. Adv. Sci. 2019;6 doi: 10.1002/advs.201900605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu W., Wang Y., Gong F., Rong Y., Luo Y., Tang P., Zhou Z., Zhou Z., Xu T., Jiang T., Yang S., Yin G., Chen J., Fan J., Cai W. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J. Neurotrauma. 2019;36:469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 111.Wang L., Pei S., Han L., Guo B., Li Y., Duan R., Yao Y., Xue B., Chen X., Jia Y. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell. Physiol. Biochem. 2018;50:1535–1559. doi: 10.1159/000494652. [DOI] [PubMed] [Google Scholar]
- 112.Xian P., Hei Y., Wang R., Wang T., Yang J., Li J., Di Z., Liu Z., Baskys A., Liu W., Wu S., Long Q. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9:5956–5975. doi: 10.7150/thno.33872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang D., Gong F., Ge X., Lv C., Huang C., Feng S., Zhou Z., Rong Y., Wang J., Ji C., Chen J., Zhao W., Fan J., Liu W., Cai W. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020;18:105. doi: 10.1186/s12951-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu M.X., Zhu Y.F., Chang H.F., Liang Y. Nanoceria restrains PM2.5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf2 deficient mice. Free Radic. Biol. Med. 2016;99:259–272. doi: 10.1016/j.freeradbiomed.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 115.Varlamova E.G., Plotnikov E.Y., Baimler I.V., Gudkov S.V., Turovsky E.A. Pilot study of cytoprotective mechanisms of selenium nanorods (SeNrs) under ischemia-like conditions on cortical astrocytes. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241512217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varlamova E.G., Turovsky E.A., Babenko V.A., Plotnikov E.Y. The mechanisms underlying the protective action of selenium nanoparticles against ischemia/reoxygenation are mediated by the activation of the Ca2+ signaling system of astrocytes and reactive astrogliosis. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222312825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lichtenstein M.P., Carretero N.M., Pérez E., Pulido-Salgado M., Moral-Vico J., Solà C., Casañ-Pastor N., Suñol C. Biosafety assessment of conducting nanostructured materials by using co-cultures of neurons and astrocytes. Neurotoxicology. 2018;68:115–125. doi: 10.1016/j.neuro.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Smith J.A., Braga A., Verheyen J., Basilico S., Bandiera S., Alfaro-Cervello C., Peruzzotti-Jametti L., Shu D., Haque F., Guo P., Pluchino S. RNA nanotherapeutics for the amelioration of astroglial reactivity. Mol. Ther. Nucleic Acids. 2018;10:103–121. doi: 10.1016/j.omtn.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou M., Zhang T., Zhang X., Zhang M., Gao S., Zhang T., Li S., Cai X., Li J., Lin Y. Effect of tetrahedral framework nucleic acids on neurological recovery via ameliorating apoptosis and regulating the activation and polarization of astrocytes in ischemic stroke. ACS Appl. Mater. Interfaces. 2022;14:37478–37492. doi: 10.1021/acsami.2c10364. [DOI] [PubMed] [Google Scholar]
- 120.Naeimi R., Safarpour F., Hashemian M., Tashakorian H., Ahmadian S.R., Ashrafpour M., Ghasemi-Kasman M. Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neurosci. Lett. 2018;674:1–10. doi: 10.1016/j.neulet.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 121.Fachel F.N.S., Dal Prá M., Azambuja J.H., Endres M., Bassani V.L., Koester L.S., Henriques A.T., Barschak A.G., Teixeira H.F., Braganhol E. Glioprotective effect of chitosan-coated rosmarinic acid nanoemulsions against lipopolysaccharide-induced inflammation and oxidative stress in rat astrocyte primary cultures. Cell. Mol. Neurobiol. 2020;40:123–139. doi: 10.1007/s10571-019-00727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gong L., Gu Y., Han X., Luan C., Liu C., Wang X., Sun Y., Zheng M., Fang M., Yang S., Xu L., Sun H., Yu B., Gu X., Zhou S. Spatiotemporal dynamics of the molecular expression pattern and intercellular interactions in the glial scar response to spinal cord injury. Neurosci. Bull. 2023;39:213–244. doi: 10.1007/s12264-022-00897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang P., Ye Y. Filamentous recombinant human Tau activates primary astrocytes via an integrin receptor complex. Nat. Commun. 2021;12:95. doi: 10.1038/s41467-020-20322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patil V., Bohara R., Kanala V.K., McMahon S., Pandit A. Models and approaches to comprehend and address glial inflammation following spinal cord injury. Drug Discov. Today. 2023;28 doi: 10.1016/j.drudis.2023.103722. [DOI] [PubMed] [Google Scholar]
- 125.Ahamad N., Kar A., Mehta S., Dewani M., Ravichandran V., Bhardwaj P., Sharma S., Banerjee R. Immunomodulatory nanosystems for treating inflammatory diseases. Biomaterials. 2021;274 doi: 10.1016/j.biomaterials.2021.120875. [DOI] [PubMed] [Google Scholar]
- 126.Feng X., Xu W., Li Z., Song W., Ding J., Chen X. Immunomodulatory nanosystems. Adv. Sci. 2019;6 doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao C.G., Martins P.N. Nanotechnology applications in transplantation medicine. Transplantation. 2020;104:682–693. doi: 10.1097/TP.0000000000003032. [DOI] [PubMed] [Google Scholar]
- 128.Wang X.J., Shu G.F., Xu X.L., Peng C.H., Lu C.Y., Cheng X.Y., Luo X.C., Li J., Qi J., Kang X.Q., Jin F.Y., Chen M.J., Ying X.Y., You J., Du Y.Z., Ji J.S. Combinational protective therapy for spinal cord injury medicated by sialic acid-driven and polyethylene glycol based micelles. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119326. [DOI] [PubMed] [Google Scholar]
- 129.Wang X.J., Peng C.H., Zhang S., Xu X.L., Shu G.F., Qi J., Zhu Y.F., Xu D.M., Kang X.Q., Lu K.J., Jin F.Y., Yu R.S., Ying X.Y., You J., Du Y.Z., Ji J.S. Polysialic-acid-based micelles promote neural regeneration in spinal cord injury therapy. Nano Lett. 2019;19:829–838. doi: 10.1021/acs.nanolett.8b04020. [DOI] [PubMed] [Google Scholar]
- 130.Dalhaimer P., Florey B., Isaac S. Interactions of apolipoproteins with lipid-based nanoparticles. ACS Nano. 2023 doi: 10.1021/acsnano.2c10790. [DOI] [PubMed] [Google Scholar]
- 131.de Macedo E.F., Santos N.S., Nascimento L.S., Mathey R., Brenet S., de Moura M.S., Hou Y., Tada D.B. Interaction between nanoparticles, membranes and proteins: a surface plasmon resonance study. Int. J. Mol. Sci. 2022;24:591. doi: 10.3390/ijms24010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.