Abstract
Serotonin is a neurotransmitter involved in the modulation of a multitude of physiological and behavioral processes. In spite of the relatively reduced number of serotonin-producing neurons present in the mammalian CNS, a complex long-range projection system provides profuse innervation to the whole brain. Heterogeneity of serotonin receptors, grouped in seven families, and their spatiotemporal expression pattern account for its widespread impact. Although neuronal communication occurs primarily at tiny gaps called synapses, wiring transmission, another mechanism based on extrasynaptic diffusion of neuroactive molecules and referred to as volume transmission, has been described. While wiring transmission is a rapid and specific one-to-one modality of communication, volume transmission is a broader and slower mode in which a single element can simultaneously act on several different targets in a one-to-many mode. Some experimental evidence regarding ultrastructural features, extrasynaptic localization of receptors and transporters, and serotonin–glia interactions collected over the past four decades supports the existence of a serotonergic system of a dual modality of neurotransmission, in which wiring and volume transmission coexist. To date, in spite of the radical difference in the two modalities, limited information is available on the way they are coordinated to mediate the specific activities in which serotonin participates. Understanding how wiring and volume transmission modalities contribute to serotonergic neurotransmission is of utmost relevance for the comprehension of serotonin functions in both physiological and pathological conditions.
Keywords: Serotonin, serotonergic fibers, volume transmission, wiring transmission, synapse, nonjunctional varicosity
Introduction
The mammalian central nervous system (CNS) has an extremely complex organization. The human brain is estimated to contain around 86.1 billion neurons and a similar number of glial cells;1 only in the neocortex the number of synapses is evaluated to be around 164 trillion,2 and in the whole adult CNS there might be over 1015 synaptic contacts.3 In light of this, synaptic communication is reasonably recognized as the principal modality through which information is processed and elaborated. The complexity of this system increases further, taking into account the high variability of the neurons that compose the CNS, each one characterized by unique combinations of morphological, neurochemical, electrophysiological, and hodological properties.
In this framework, the serotonergic system stands out due to some peculiar characteristics. Serotonin (5-hydroxytryptamine, 5-HT) producing neurons constitute a relatively small fraction of the total neurons in the CNS. In fact, it is estimated the presence of approximately 300 000 serotonergic cells in the human brain and only around 26 000 over a total of 70 million neurons in the most widely used mammalian animal model, the mouse.4−7 5-HT neurons are among the earliest generated during development, differentiating at mid-gestation along the midline of the rhombencephalon and subsequently migrating to defined areas of the brainstem where they integrate within the raphe nuclei, clustered in the B1–B9 groups.8,9 In spite of the limited number of serotonin-producing neurons, brain serotonin has been shown to be involved in the modulation of a broad range of different physiological and behavioral processes, including regulation of circadian rhythms, mood, feeding behavior, and social interaction.10−12 Moreover, prior to its activity as a neurotransmitter in the mature brain, serotonin has been shown to play a crucial role in development and plasticity as it influences processes including cell proliferation and migration, neuronal differentiation, and circuit formation.9,13−17 Accordingly, alteration of the normal serotonergic neurotransmission is associated with the emergence of psychiatric disorders that are thought to originate during development.18−21
A major factor in determining the capacity of serotonin to modulate such a vast multitude of diverse functions lies in the extensive innervation that the serotonergic neurons provide to the brain so that virtually each area receives 5-HT innervation. In fact, their projections spread throughout the CNS, from the anteriormost parts of the telencephalon to the spinal cord, forming an ascending system, responsible for the innervation of the forebrain and mostly originating from the rostral group (B5–B9) and a descending system, arising from the caudal group (B1–B4).22
Another key point concerns the multiplicity of 5-HT receptors, which are both ionotropic and metabotropic. In mammals, 14 serotonin receptors are known and they are organized in seven families (5-HT1–7), distinguished on the basis of pharmacological profiles, signal transduction pathways, and structural characteristics.23,24 In addition, the high spatiotemporal variability of their expression plays a crucial role in determining the specificity of the 5-HT effects.
The heterogeneity of 5-HT neurons represents an additional aspect to consider. In spite of being originally defined exclusively by their shared serotonergic phenotype, there is growing evidence for the existence of distinct populations of 5-HT neurons which differ in their molecular identities and functional properties, concerning traits such as gene expression, electrophysiology, connectivity, and neurochemistry.25,26
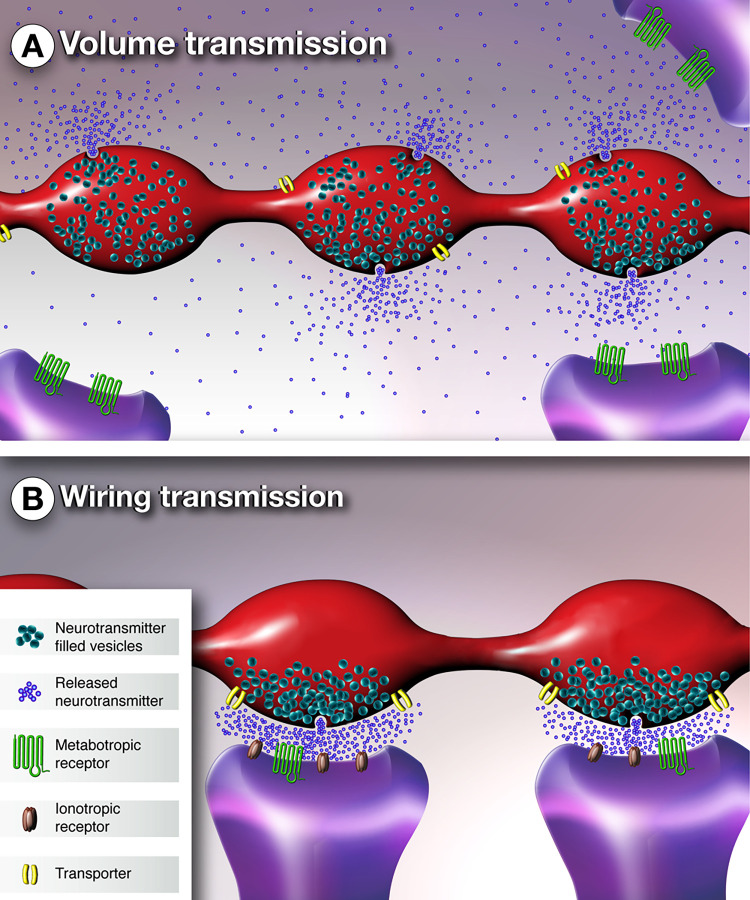
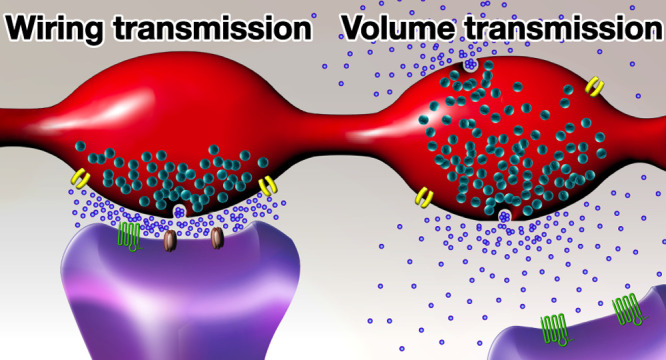
In contrast to the availability of extensive amounts of data concerning the characteristics of serotonergic transmission described so far, there is another aspect that appears to be much less explored, despite its potential relevance in determining the widespread effects of serotonin. In fact, besides the canonical synaptic exchange of information, another important modality of intercellular communication for monoaminergic systems is present. This modality, based on the diffusion of chemical signals in the extracellular space, is referred to as volume transmission (Figure 1A).27,28
Figure 1.
Schematic representation of volume and wiring transmission. (A) Simplified axonal varicosities displaying volume transmission in which the neurotransmitter is released in the extracellular space, where it diffuses and reaches metabotropic receptors located on nearby cells. (B) Simplified axonal varicosities showing wiring transmission. Each varicosity establishes a synaptic contact with specific postsynaptic element, and the neurotransmitter is released in a confined space, where it interacts with both ionotropic and metabotropic receptors.
Though it is commonly accepted that the 5-HT system relies both on the more conventional synaptic wiring transmission and on volume transmission, in the literature there is a limited body of evidence supporting the actual presence of the latter mechanism and, from a functional point of view, little is known about the role of each of the two. Starting from general considerations on the two modalities of intercellular communication in the CNS, in this review we will focus on the existing experimental evidence supporting the presence of nonsynaptic volume transmission in the serotonergic system.
Deciphering the mechanisms controlled by either wiring or volume transmission and the cascade of events that each of the two modalities promotes is critical to fully understand the action of serotonin in regulating the myriad of functions in which it is involved.
Intercellular Communication Modalities in the CNS
In the central nervous system, interneuronal communication is mainly associated with the activity of synapses. Historically, the study of synapses has had significant relevance in advancing our comprehension of how neurons communicate. In this sense, the introduction of the “neuron doctrine” marked a fundamental step toward the understanding of synaptic communication and, in general, of the functioning of the nervous system.29 This theory, proposed by Santiago Ramon y Cajal, asserted the contiguity in between neurons, in contrast to the relationship of physical continuity postulated by the “reticular theory”, which has been popular throughout the 19th century and had among its supporters Camillo Golgi. The notion of neurons as anatomically and functionally independent units implied the existence of modalities of intercellular communication relying on extracellular signals. In the same years, the term synapse has been used for the first time by Charles Sherrington,30 who derived it from the Greek verb “synaptein” (συν “with”, α̋πτειν “to touch”) in order to highlight the existence of a point of contact between neurons as physically distinct elements. However, due to limitations of light microscopy in resolving the separation between synaptic elements, the existence of synapses remained speculative until the 1950s, when advances in electron microscopy enabled one to describe finely their structure, confirming the presence of the synaptic cleft between presynaptic and postsynaptic elements.31,32
Although synapses are still considered to play a predominant role, the importance of other modalities of intercellular communication among neurons has started to be appreciated, revealing the existence of an even more complex way of action for neurotransmitters that actually might represent the ancestral modality of communication used by neurons in the early days of nervous system evolution.33 The concept of volume transmission refers to a model introduced in the 1980s,34 according to which neurochemical communication in the brain can be distinguished in two main categories, termed wiring transmission and volume transmission, acting alongside to define the activity and shape the output of neural circuits (Figure 1).
Wiring transmission (WT) can be defined as a point-to-point communication, relying on the presence of well-defined structures through which the signals are transmitted, as virtual wires connecting two specific elements (Figure 1B).28 Chemical synapses represent a prototype for this kind of communication. As the axon potential reaches the presynaptic terminal, through a Ca2+-dependent exocytotic mechanism the neurotransmitter is released in the synaptic cleft and it diffuses across few nanometers (≈20 nm) to bind and activate receptors on the postsynaptic membrane.35 Besides chemical synapses, also gap junctions, or electrical synapses, constitute an integral part of WT, which can be found in the mammalian CNS.36 The functional properties of WT are closely tied to the structural characteristics of synapses. It is a fast and highly specific communication modality in which the source of the signal and its target are in a 1:1 ratio. The presence of physically defined structures ensures relative stability in the connection between the source of the signal and the target. Thanks to those features, WT appears to be particularly well suited for prompting activation or inhibition of effector systems as well as for guaranteeing an oriented flux of information through those “hardwired” networks. Volume transmission (VT), in contrast, defines the release and diffusion of neuroactive molecules within the volume of extracellular fluids in brain parenchyma, without defined physical restraints (Figure 1A).28 The capacity of a molecule of diffusing in the extracellular space depends on its energy gradients, and it is also strongly influenced by the characteristics of the extracellular space (ECS) itself. In particular, three parameters are important in defining the migration properties of a VT signal.37 The first of them is represented by the volume fraction being the size of the ECS with respect to the volume of the whole tissue, on average estimated around 20%. The second is the tortuosity as the increase in the extension of the path that a signal has to move through, dependent on the complexity of the tridimensional structure of the ECS. Finally, the clearance that is the rate of removal of the signaling molecule from the ECS itself.
The characteristics of VT make it profoundly different from classical synaptic communication. One significant distinction can be identified in its broad reach, allowing a signal to simultaneously act on many different elements in a relatively wide area, as long as they are competent to respond. In this way there is a shift from the one-to-one relationship between the source of the message and the target that characterizes synaptic communication to a one-to-many relationship in volume transmission. Furthermore, neurons are not the only cell types involved in this kind of interaction, but also glial cells present in the CNS can be both a source and a target for those extrasynaptic signals.38,39 On the other hand, the wider spatial distance over which the signal diffuses in VT results in a slower transmission compared to WT. Those properties make the VT appropriate for long-lasting modulatory functions that do not necessarily require a quick activation or inhibition.
As volume transmission is a neurochemical modality of intercellular communication, some requirements must be met for it to occur. First, it should be possible for the neurotransmitter to reach the extracellular space, which can occur through different mechanisms such as direct extrasynaptic exocytosis, synaptic spillover, or reverse activity of transporters.40−42 As a second point, a signal-decoding system should be present outside the synapses, meaning that extrasynaptic receptors should be located on target cells.43 Moreover, since receptors have specific affinity for their ligands, it is necessary that they are reached by a sufficiently high concentration of the signaling molecule in order to be effectively activated and generate a response. Finally, the presence of a mechanism to remove the neurotransmitter from the extracellular space is required in order to interrupt the signaling, such as functional extrasynaptic transporters. Evidence regarding the fulfillment of those requirements has been collected within the CNS in relation to serotonergic neurotransmission, indirectly suggesting the presence of VT mechanisms.43−45
Ultrastructural Evidence Supporting Volume Transmission in the Serotonergic System
The introduction and the development of the concept of volume transmission are strictly linked to considerations concerning the characteristics of the central monoaminergic systems.45 In this regard, particularly significant has been the analysis of their ultrastructural features.
The central serotonergic system has been first identified and described in the mid-1960s.8 This initial characterization made use of the Falck–Hillarp method, a histochemical fluorescence technique based on the capacity of serotonin and catecholamines to react with formaldehyde forming products with defined fluorescent properties.46,47 Subsequently, the development of more sensitive and specific techniques made it possible to investigate the characteristics of 5-HT producing neurons and their projections within the CNS with a higher level of detail. Early studies employed autoradiographic techniques following tritiated 5-HT administration in vivo. Thanks to advances in immunohistochemistry, this method has then been largely replaced by immunostaining using antibodies against 5-HT itself,48 its biosynthetic enzyme tryptophan hydroxylase (TPH),49 or the serotonin transporter SERT.50
Over the years, those methods have been used to explore the ultrastructural characteristics of 5-HT innervation in several areas of different experimental models, mostly in rats, cats, and monkeys. The information collected provided insights on the intrinsic and relational morphological properties of 5-HT projections, revealing some notable features, partly shared with other monoaminergic systems.51 Serotonergic axons branch profusely and are characterized by numerous unmyelinated varicosities containing clear or dense core vesicles: those sites often lack the typical synaptic specializations or postsynaptic targets and only to a low extent seem to form proper synapses with specific neuronal targets. The presence of both junctional and nonjunctional varicosities can be considered solid evidence in support of the coexistence of WT and VT modalities of serotonin release.44 Moreover, in many regions of the CNS the frequency at which synaptic contacts are formed has been quantitatively evaluated, revealing a significant variability that suggests a differential role for WT and VT in different areas.
In some regions the asynaptic character of 5-HT innervation prevails, as it has been clearly observed for neocortex, striatum, and hippocampus. Serotonergic projections in the cerebral cortex arise from the dorsal and median raphe nuclei,48,52−54 and the fine structural characteristic of serotonergic axons has been surveyed in various regions of the cortex, by autoradiography and immunolabeling techniques and in different experimental models, such as rat, cat, and monkey.55−57 In these areas, with the exception of reports from one laboratory,58 it has been observed a lack of synaptic specializations in most of the 5-HT terminals. In particular, an assessment of the extent to which terminal-like varicosities display synaptic specializations has been performed in different regions of the adult rat cerebral cortex using 5-HT immunohistochemistry.59 The proportion of varicosities engaged in synaptic contacts (synaptic incidence) has been stereologically extrapolated to whole varicosities from thin sections, showing low values for both superficial (36%) and deep (27%) layers of the frontal cortex and for the occipital cortex (37%), while a slightly higher value (46%) has been estimated for the parietal cortex. In the striatum, as revealed by electron microscopy studies using autoradiographic labeling in rats and cats, serotonergic fibers that originate mostly from the dorsal raphe nucleus52 are to a large extent devoid of synaptic specializations.60,61 This notion has been corroborated in rats by the use of both autoradiography and 5-HT immunolabeling, which allowed estimation of the synaptic incidence to be as low as 10%.62 A comparable result (≈18%) was extrapolated from the scoring of SERT-immunolabeled varicosities obtained in the dorsolateral area of macaque putamen.63 Also the hippocampal formation, which receives a robust serotonergic innervation from both median and dorsal raphe nuclei,54,64 displays the prevalence of asynaptic terminals, the synaptic incidence being estimated to be around 12%,55 which includes the recently described synapse between serotonergic axons and primary cilia of CA1 pyramidal neurons.65
Serotonergic axons establishing synaptic contacts with specific targets are prominent in other brain regions such as the substantia nigra (SN), which receives 5-HT innervation from mesencephalic raphe nuclei.52,66−69 The SN represents one of the areas with the highest density of 5-HT innervation in the whole CNS, with the pars reticulata displaying significantly higher density as compared to pars compacta in both rat and monkey.70,71 Early ultrastructural studies demonstrated in this district the presence of synaptic contacts established by 5-HT immunoreactive fibers, mostly with dendrites.66,72 Subsequently, it has been shown a clear-cut difference in the characteristics of 5-HT innervation in the two subdivisions of SN in rat.70 In fact, in pars compacta, approximately 50% of the identified serotonergic terminal-like varicosities have been estimated to form synaptic contacts, whereas in pars reticulata, the total number of them shows synaptic membrane specializations. Those morphological characteristics suggest a predominant role for wiring transmission in SNr and a coexistence of both volume and wiring transmission in SNc.
The dorsal raphe nucleus (DRN) represents another notable region in which the morphological features of serotonergic neurons and their projections have been explored. This nucleus comprises a highly heterogeneous neuronal population, and it contains the cellular bodies of serotonergic neurons (B6–B7 groups) responsible for a large part of the 5-HT innervation to the forebrain.52,54,73 Though with a low density, the DRN of the rat is reached by 5-HT terminals that usually lack synaptic specializations74 and which could originate from other 5-HT groups, such as the caudal raphe nuclei, known to innervate the DRN.75 Moreover, early ultrastructural immunohistochemical analyses highlighted the presence of 5-HT immunoreactive small clear and dense-core vesicles in dendrites of DRN serotonergic neurons.76−78 In cats, some of those dendrites were observed to be involved in the formation of dendrodendritic synapses, while others lacked synaptic specializations, appearing as suitable sites for a possible extrasynaptic release.77 The existence of mechanisms of dendritic and also somatic 5-HT release is further sustained by direct ex vivo evidence. In particular, vesicular serotonin release from dendrites of DRN neurons has been demonstrated through a combination of three-photon microscopy and electron microscopy in rat living brain slices.79 In addition, perinuclear clusters of serotonergic vesicles have been identified in rat dorsal raphe sections and it has been shown a mechanism of nonsynaptic somatic release by potassium ions induced depolarization.80 This somatodendritic mechanism of serotonin release in the extracellular space, as well as the release from serotonergic terminals in the DRN, could be hypothesized to play a role in the mechanism of autoinhibition of 5-HT neurons’ firing, likely mediated by somatodendritic 5-HT1A autoreceptors.81
On the whole, although the available ultrastructural data concerning serotonergic innervation have been collected over several decades by different research groups, using different approaches and experimental models, the results obtained converge in supporting the dualism of the 5-HT system and a role for both wiring and volume modalities in mediating serotonergic neurotransmission.
5-HT Receptors in Nonsynaptic Transmission
As previously mentioned, serotonin acts on its targets through multiple receptors.
In this regard, a first consideration relevant to the dualism of the serotonergic system concerns the types of receptors on which serotonin exerts its action. In fact, among the seven families of 5-HT receptors, only 5-HT3 receptors are ligand-gated cation channels,82 which mediate a fast postsynaptic excitatory response, with the other six all comprehending G-protein-coupled receptors.83 While ionotropic receptors are certainly suitable for rapid and specific synaptic communication, it is interesting to note that the general properties of metabotropic receptors, such as higher affinity for the ligand, slower response, and broader effects, fit better for the modulatory role of the neurotransmitter and are consistent with the properties of VT.
Among the notions that led to the definition of VT concept, a strong contribution has also been given by the observation of relative mismatches between the localization of neurotransmitters and their respective receptors in determined brain areas.84,85 This is the case for 5-HT2A receptors, as it has been observed in a double-labeling immunohistochemistry study,86 in which the relationship between 5-HT fibers and 5-HT2A immunoreactive targets has been evaluated at light microscopic level in the rat forebrain. Discrepancies have been found in the reciprocal localizations and density of those elements in the basal forebrain, as well as in cortical regions and in the hippocampus, suggesting that serotonin, possibly released by nonjunctional varicosities, reaches the 5-HT2A receptors expressed by the targets through diffusion in the extracellular space. This hypothesis gains further support from the demonstration of the extrasynaptic localization of 5-HT2A receptors on dendritic shafts of pyramidal and local circuit neurons in the rat prefrontal cortex (PFC).87 Indeed, the extrasynaptic localization, reported also for other 5-HT receptors, such as high affinity 5-HT1 receptors, is an aspect in line with the existence of serotonergic volume transmission in the CNS. For instance, the localization of 5-HT1A receptors in sites devoid of synaptic specializations has been demonstrated on somata and dendrites of neurons in the dorsal raphe nucleus as well as in the hippocampal formation,88,89 two regions where nonsynaptic serotonergic varicosities presence is predominant. 5-HT1B receptors have also been localized extrasynaptically, in association with unmyelinated preterminal axons in substantia nigra and globus pallidus, and it has been proposed a possible role for them in the modulation of axonal impulse conduction.89,90
Concerning the activity of extrasynaptic 5-HT receptors, a crucial issue to address is also whether they are exposed to a sufficiently high concentration of the neurotransmitter. Measurements of serotonin extracellular concentration in brain tissue are in line with the functionality of extrasynaptically located 5-HT receptors.91 In order to monitor dynamic changes in neurotransmitter concentration, compatible with events of release and reuptake, it is possible to exploit the fast-scan cyclic voltammetry (FSCV), a chemical-selective electroanalytical technique with high temporal resolution.92 Given that the dimensions of carbon fiber microelectrodes are consistently larger than the synaptic cleft, it is assumed that FSCV detects extrasynaptic concentrations of the neurotransmitter as it diffuses in the extracellular space following release. By the use of this technique in rat brain slices, following stimulation, the presence of extracellular serotonin has been detected in the dorsal raphe nucleus and in pars reticulata of the substantia nigra.93,94 According to the voltammetric measurements, serotonin could diffuse around 20 μm in the extracellular space, meaning that it can interact with many different extrasynaptic elements. Moreover, its concentration, evaluated after single stimulation pulses, closely matches the affinity of 5-HT1 receptors, which not only are highly expressed in both regions but, as mentioned above, have also proven to have an extrasynaptic localization.88−90 From the same study94 emerged an apparently contradictory result. In fact, the presence of extracellular serotonin, possibly implicated in volume transmission, has been detected in the substantia nigra pars reticulata, where ultrastructural analyses have revealed that all of the terminal-like serotonergic varicosities establish synaptic contacts with postsynaptic partners. This evidence suggests that the presence of extrasynaptic serotonin is due to a mechanism of spillover from the synaptic cleft, therefore prompting the existence of VT in brain districts structurally arranged for WT.
In summary, it can be assumed that although in some brain districts the ultrastructural features hint at a prevalence of one neurotransmission modality over the other, the two modalities may actually both coexist, displaying a higher variability than previously hypothesized.
Serotonin–Glia Interplay via Volume Transmission
The likely presence of VT in brain regions structurally arranged for WT has a major impact on the interplay between serotonin and glial cells. In fact, in the CNS serotonin receptors are not expressed exclusively by neurons. Different functional 5-HT receptors are known to be expressed by distinct populations of glial cells, such as astrocytes95 and microglia,96,97 that do not establish synaptic contacts with 5-HT axons. Regardless of the existence of direct contacts with serotonergic fibers, there is strong evidence that glial cells respond to serotonin signaling to modulate important physiological and behavioral functions.
Serotonergic modulation has significant implications on the activity of astrocytes within the CNS. For instance, serotonin 1A receptors present on them promote the release of the neurotrophic factor S100β, involved in several cellular processes, such as cell cycle regulation and differentiation.98,99 In a mouse model for Parkinson’s disease, the activation of serotonin 1A receptors present on astrocytes has been reported to promote their proliferation and to upregulate antioxidative molecules, with a neuroprotectant effect.100 Another interesting effect of serotonin modulation has been found in a subset of hippocampal astrocytes expressing 5-HT4 receptors, whose activation is related to modifications of synaptic glutamate release.101 Serotonergic neurotransmission, through unspecified 5-HT receptors, has also been shown to positively modulate the astrocyte–neuron lactate shuttle, an important mechanism for the regulation of cerebral energy metabolism.102 In addition, 5-HT modulation of astrocytes has been correlated also to other effects, for example, in sleep103 and depression.104−106 Furthermore, besides being responsive to serotonergic signaling thanks to the expression of 5-HT receptors, astrocytes are also capable of uptaking extracellular serotonin107,108 thanks to the expression of the transporter SERT109,110 and other monoamine transporters, such as the organic cation transporter 3 (OCT3).111 Recently, a newly identified epigenetic role for serotonin uptake has been described.112 In particular, neuronal activity, through the increase of OCT3 expression and histone serotonylation in olfactory bulb astrocytes, has been demonstrated to enhance the levels of astrocytic GABA biosynthesis and release, with effects on olfactory processing and behavior.
Concerning microglia, which expresses 5-HT2B receptors, there is compelling evidence supporting the importance of serotonergic modulation through its activation during rodent brain development.113 In this regard, selective inactivation of 5-HT2B receptor in microglia early in life has been correlated to prolonged neuroinflammation and increased sick behavior in adult, supposedly due to defects in microglia maturation or alteration in its developmental interactions with nearby neurons.114 Its ablation during a critical postnatal developmental period has also been shown to determine the alteration of neuronal circuits and behavioral effects in the adult, impairing sociability and flexibility.115 The influence of serotonin on microglia activation directly links 5-HT to the regulation of neuroinflammation and events occurring in neurodegenerative diseases such as ALS and Parkinson’s disease or following neuronal damage.114,116−119
As previously mentioned, glial cells do not receive synaptic contacts from serotonergic fibers. Consequently, in light of the modalities of neurochemical interaction between glial cells and neurons, it is reasonable to expect that those glial-expressed 5-HT receptors are activated through VT by extrasynaptic serotonin originating from nonjunctional varicosities or synaptic cleft spillover.
On the whole, the presence of 5-HTRs on glial cells represents strong evidence for the existence of serotonergic volume transmission in the CNS. Moreover, although future experiments are mandatory to associate specific roles to either WT or VT, the activity of serotonin on glial cells clearly demonstrates not only the existence but also the relevance of a dual modality of serotonergic neurotransmission.
Role of Serotonin Transporter in Nonsynaptic Transmission
In the perspective of the wide-ranging effects of serotonin due to the presence of volume transmission, a final critical aspect to consider concerns the removal of the neurotransmitter from the extracellular space. The energy-dependent mechanism of neurotransmitter reuptake mediated by the high-affinity serotonin transporter SERT is crucial for the regulation of serotonergic neurotransmission.120 In addition to SERT, there are also other less specific transporters with lower affinity, such as organic cation transporters (OCTs), which are broadly expressed in the brain and can participate in the clearance of serotonin.121
As for other monoamine transporters, such as norepinephrine and dopamine transporters,122−124 SERT has been directly localized via immunocytochemical EM studies on the axolemma at extrasynaptic sites in different districts of the CNS.110,125−127 Specifically, functional 5-HT transporter has been identified in perisynaptic areas and on intervaricosity axonal segments devoid of synaptic specializations in rat dorsal raphe, corpus callosum, medial forebrain bundle, cingulum bundle, and cingulate cortex.127 This extrasynaptic localization of SERT is indicative of the fact that this important part of the regulation of serotonergic signaling takes place outside of synaptic contacts, in accordance with the voltammetrical measurements.94 Those data are consistent with the role of SERT in the clearance of the extracellular space from serotonin molecules involved in volume transmission.
The relevance of SERT-mediated uptake of extrasynaptic serotonin already emerges during early brain development, as perinatal exposure to selective serotonin reuptake inhibitors (SSRIs) is related to the emergence of paradoxical depressive-like and anxiety-like behaviors in the adult life.128−131 While in the adult brain SERT is mostly expressed by 5-HT producing neurons, during development it displays an early and dynamic expression in nonserotonergic neurons,132,133 as revealed by the use of SERT-Cre mouse model.134 This spatiotemporal pattern of SERT expression is likely required for a fine regulation of serotonin extracellular content. In order to address this hypothesis, the functional significance of transient SERT expression during postnatal development has been specifically investigated.135 Results confirmed that SERT is required to maintain the serotonin homeostasis for the proper formation of descending prefrontal circuits targeting DRN, which are involved in stress response. Specifically, it has been shown that transient presence SERT in PFC pyramidal neurons is required to prevent excessive activation of 5-HT7 receptors in the same district.136
Overall, the activity of transiently expressed SERT transporter in nonserotonergic neurons during critical developmental periods appears as a plausible modality through which extrasynaptic morphogenetic gradients of serotonin can be shaped, in order to control to what extent volume transmission regulates the formation of connections in the wired brain.
Conclusions, Open Questions, and Future Perspectives
Since the discovery of central 5-HT neurons, compelling evidence regarding the dualism of serotonergic transmission has been collected at several different levels, building a complex framework in which serotonin appears to exert its activity through both wiring and volume transmission.
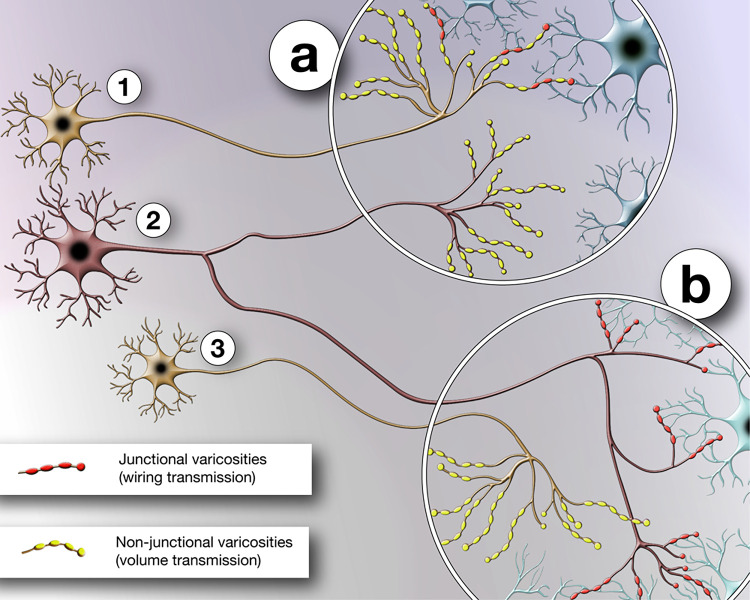
While in the serotonergic system both a rapid and precise way of communication and a broader and slower modality coexist, their specific role in the regulation of serotonin-mediated developmental, physiological, and behavioral effects has yet to be clarified and several questions are still open. As discussed above, in several brain areas, the anatomical data suggest the prevalence of either wiring or volume transmission, based on the relational characteristics of the serotonergic innervation. However, due to the extraordinarily high complexity of the serotonergic wiring, it remains to elucidate whether along a single axonal filament a promiscuous serotonin release modality is present. In such a scenario, as distinct brain areas may receive serotonergic axons from the same neuron,137,138 it is even harder to establish whether the single serotonergic neuron uses the same modality of neurotransmission in different areas (Figure 2). It therefore emerges as a matter of future research whether serotonergic neurons are predetermined for volume transmission, wiring transmission, or both modalities. If, as it seems, volume transmission plays a relevant role in serotonergic signaling, this dualism could have important consequences on aspects implicated not only with the physiological activity of this system but also with its pathological dysfunctions as well as with therapeutic interventions. For instance, in light of a sizable extrasynaptic localization of SERT, it is tempting to speculate that the inhibition of serotonin reuptake via SSRIs administration or the use of abuse drugs such as MDMA/Ecstasy could elevate the extrasynaptic serotonin content, therefore ascribing, at least in part, to volume transmission signaling the therapeutic effect or damaging effects, respectively.139 Therefore, as this dualism can have a strong impact on serotonergic neurotransmission, in the perspective of further understanding the role of serotonin in the CNS, it is mandatory to widen our knowledge regarding this aspect by applying new technologies and molecular approaches. In this sense, a possible approach could be to exploit the characteristics of retrograde tracers based on viral vectors, such as the rabies virus, which have the capacity to selectively transduce neurons at the presynaptic terminal.140 The use of such a tool to trace the serotonergic neurons would specifically allow drawing a map of the serotonergic neurons displaying wiring transmission. The combination of retrograde tracers with the capacity to label fibers irrespective of the presence of synaptic specializations, such as retrobeads,141 rAAV,142 or pseudotyped rabies virus,143 would allow us to take a step forward in understanding, for instance, whether serotonergic neurons projecting to a specific brain region use in that district both modalities or only/predominantly the volume transmission.
Figure 2.
Schematic representation of the hypothetical distribution of the two modalities of neurotransmission. In the scheme three serotonergic neurons are represented with neuron 1 projecting to target area (a) and displaying both wiring transmission and volume transmission, neuron 2 sending projections to target areas (a) and (b) where it displays volume transmission and wiring transmission modality, respectively, and neuron 3 projecting to target area (b) showing exclusively volume transmission.
Acknowledgments
We are grateful to Prof. Kjell Fuxe and members of the Massimo Pasqualetti lab for their critical reading of the manuscript, valuable discussions, and suggestions.
This work was supported by the EU H2020 MSCA ITN Project “Serotonin and Beyond” (Grant N 953327), the Next Generation EU—National Recovery and Resilience Plan, and Ministry of University and Research (Grant n ECS 00000017 “Tuscany Health Ecosystem—THE”, Spoke 8), MIT-Italy seed funds support, and AFM-Telethon Grant 23771 to M.P.
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Chemical Neurosciencevirtual special issue “Serotonin Research 2023”.
References
- Azevedo F. A. C.; Carvalho L. R. B.; Grinberg L. T.; Farfel J. M.; Ferretti R. E. L.; Leite R. E. P.; Filho W. J.; Lent R.; Herculano-Houzel S. Equal Numbers of Neuronal and Nonneuronal Cells Make the Human Brain an Isometrically Scaled-up Primate Brain. J. Comp. Neurol. 2009, 513 (5), 532–541. 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- DeFelipe J.; Alonso-Nanclares L.; Arellano J. I. Microstructure of the Neocortex: Comparative Aspects. J. Neurocytol. 2002, 31 (3–5), 299–316. 10.1023/A:1024130211265. [DOI] [PubMed] [Google Scholar]
- Silbereis J. C.; Pochareddy S.; Zhu Y.; Li M.; Sestan N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89 (2), 248–268. 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg J. R.; Adan R. A. H.; Alenina N.; Asiminas A.; Bader M.; Beckers T.; Begg D. P.; Blokland A.; Burger M. E.; Van Dijk G.; Eisel U. L. M.; Elgersma Y.; Englitz B.; Fernandez-Ruiz A.; Fitzsimons C. P.; Van Dam A.-M.; Gass P.; Grandjean J.; Havekes R.; Henckens M. J. A. G.; Herden C.; Hut R. A.; Jarrett W.; Jeffrey K.; Jezova D.; Kalsbeek A.; Kamermans M.; Kas M. J.; Kasri N. N.; Kiliaan A. J.; Kolk S. M.; Korosi A.; Korte S. M.; Kozicz T.; Kushner S. A.; Leech K.; Lesch K.-P.; Lesscher H.; Lucassen P. J.; Luthi A.; Ma L.; Mallien A. S.; Meerlo P.; Mejias J. F.; Meye F. J.; Mitchell A. S.; Mul J. D.; Olcese U.; González A. O.; Olivier J. D. A.; Pasqualetti M.; Pennartz C. M. A.; Popik P.; Prickaerts J.; De La Prida L. M.; Ribeiro S.; Roozendaal B.; Rossato J. I.; Salari A.-A.; Schoemaker R. G.; Smit A. B.; Vanderschuren L. J. M. J.; Takeuchi T.; Van Der Veen R.; Smidt M. P.; Vyazovskiy V. V.; Wiesmann M.; Wierenga C. J.; Williams B.; Willuhn I.; Wöhr M.; Wolvekamp M.; Van Der Zee E. A.; Genzel L. The Continued Need for Animals to Advance Brain Research. Neuron 2021, 109 (15), 2374–2379. 10.1016/j.neuron.2021.07.015. [DOI] [PubMed] [Google Scholar]
- Baker K. G.; Halliday G. M.; Törk I. Cytoarchitecture of the Human Dorsal Raphe Nucleus: HUMAN DORSAL RAPHE. J. Comp. Neurol. 1990, 301 (2), 147–161. 10.1002/cne.903010202. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S.; Ribeiro P.; Campos L.; Valotta Da Silva A.; Torres L. B.; Catania K. C.; Kaas J. H. Updated Neuronal Scaling Rules for the Brains of Glires (Rodents/Lagomorphs). Brain. Behav. Evol. 2011, 78 (4), 302–314. 10.1159/000330825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimura K.; Takeuchi Y.; Fujiwara K.; Tominaga M.; Yoshioka H.; Sawada T. Quantitative Analysis of the Distribution of Serotonin-Immunoreactive Cell Bodies in the Mouse Brain. Neurosci. Lett. 1988, 91 (3), 265–270. 10.1016/0304-3940(88)90691-X. [DOI] [PubMed] [Google Scholar]
- Dahlstroem A.; Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol. Scand. Suppl. 1964, SUPPL 232, 1–55. [PubMed] [Google Scholar]
- Gaspar P.; Cases O.; Maroteaux L. The Developmental Role of Serotonin: News from Mouse Molecular Genetics. Nat. Rev. Neurosci. 2003, 4 (12), 1002–1012. 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Lucki I. The Spectrum of Behaviors Influenced by Serotonin. Biol. Psychiatry 1998, 44 (3), 151–162. 10.1016/S0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Morin L. P. Serotonin and the Regulation of Mammalian Circadian Rhythmicity. Ann. Med. 1999, 31 (1), 12–33. 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]
- Ruhé H. G.; Mason N. S.; Schene A. H. Mood Is Indirectly Related to Serotonin, Norepinephrine and Dopamine Levels in Humans: A Meta-Analysis of Monoamine Depletion Studies. Mol. Psychiatry 2007, 12 (4), 331–359. 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Klempin F.; Beis D.; Mosienko V.; Kempermann G.; Bader M.; Alenina N. Serotonin Is Required for Exercise-Induced Adult Hippocampal Neurogenesis. J. Neurosci. 2013, 33 (19), 8270–8275. 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliarini S.; Pacini G.; Pelosi B.; Lunardi G.; Pasqualetti M. Lack of Brain Serotonin Affects Postnatal Development and Serotonergic Neuronal Circuitry Formation. Mol. Psychiatry 2013, 18 (10), 1106–1118. 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- Murthy S.; Niquille M.; Hurni N.; Limoni G.; Frazer S.; Chameau P.; Van Hooft J. A.; Vitalis T.; Dayer A. Serotonin Receptor 3A Controls Interneuron Migration into the Neocortex. Nat. Commun. 2014, 5 (1), 5524. 10.1038/ncomms6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzi S.; Maddaloni G.; Pratelli M.; Pasqualetti M. Fluoxetine Induces Morphological Rearrangements of Serotonergic Fibers in the Hippocampus. ACS Chem. Neurosci. 2019, 10 (7), 3218–3224. 10.1021/acschemneuro.8b00655. [DOI] [PubMed] [Google Scholar]
- Pratelli M.; Migliarini S.; Pelosi B.; Napolitano F.; Usiello A.; Pasqualetti M. Perturbation of Serotonin Homeostasis during Adulthood Affects Serotonergic Neuronal Circuitry. eNeuro 2017, 4 (2), ENEURO.0376-16.2017. 10.1523/ENEURO.0376-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer A. Serotonin-Related Pathways and Developmental Plasticity: Relevance for Psychiatric Disorders. Dialogues Clin. Neurosci. 2014, 16 (1), 29–41. 10.31887/DCNS.2014.16.1/adayer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaloni G.; Migliarini S.; Napolitano F.; Giorgi A.; Nazzi S.; Biasci D.; De Felice A.; Gritti M.; Cavaccini A.; Galbusera A.; Franceschi S.; Lessi F.; Ferla M. L.; Aretini P.; Mazzanti C. M.; Tonini R.; Gozzi A.; Usiello A.; Pasqualetti M. Serotonin Depletion Causes Valproate-Responsive Manic-like Condition and Increased Hippocampal Neuroplasticity That Are Reversed by Stress. Sci. Rep. 2018, 8 (1), 11847. 10.1038/s41598-018-30291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli M.; Pasqualetti M. Serotonergic Neurotransmission Manipulation for the Understanding of Brain Development and Function: Learning from Tph2 Genetic Models. Biochimie 2019, 161, 3–14. 10.1016/j.biochi.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia P. M. Serotonin and Brain Development: Role in Human Developmental Diseases. Brain Res. Bull. 2001, 56 (5), 479–485. 10.1016/S0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L.; Azmitia E. C. Structure and Function of the Brain Serotonin System. Physiol. Rev. 1992, 72 (1), 165–229. 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Hannon J.; Hoyer D. Molecular Biology of 5-HT Receptors. Behav. Brain Res. 2008, 195 (1), 198–213. 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Sharp T.; Barnes N. M. Central 5-HT Receptors and Their Function; Present and Future. Neuropharmacology 2020, 177, 108155 10.1016/j.neuropharm.2020.108155. [DOI] [PubMed] [Google Scholar]
- Calizo L. H.; Akanwa A.; Ma X.; Pan Y.; Lemos J. C.; Craige C.; Heemstra L. A.; Beck S. G. Raphe Serotonin Neurons Are Not Homogenous: Electrophysiological, Morphological and Neurochemical Evidence. Neuropharmacology 2011, 61 (3), 524–543. 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty B. W.; Sturrock N.; Escobedo Lozoya Y.; Chang Y.; Senft R. A.; Lyon K. A.; Alekseyenko O. V.; Dymecki S. M. A Single-Cell Transcriptomic and Anatomic Atlas of Mouse Dorsal Raphe Pet1 Neurons. eLife 2020, 9, e55523 10.7554/eLife.55523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati L. F.; Zoli M.; Strömberg I.; Fuxe K. Intercellular Communication in the Brain: Wiring versus Volume Transmission. Neuroscience 1995, 69 (3), 711–726. 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- Agnati L. F.; Guidolin D.; Guescini M.; Genedani S.; Fuxe K. Understanding Wiring and Volume Transmission. Brain Res. Rev. 2010, 64 (1), 137–159. 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- López-Muñoz F.; Boya J.; Alamo C. Neuron Theory, the Cornerstone of Neuroscience, on the Centenary of the Nobel Prize Award to Santiago Ramón y Cajal. Brain Res. Bull. 2006, 70 (4–6), 391–405. 10.1016/j.brainresbull.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Foster M.A Textbook of Physiology. Part Three: The Central Nervous System, 7th ed.; Macmillan and Co. Ltd.: London, 1897; Vol. III. [Google Scholar]
- De Robertis E. D. P.; Bennett H. S. Some Features of the Submicroscopic Morphology of Synapses in Frog and Earthworm. J. Cell Biol. 1955, 1 (1), 47–58. 10.1083/jcb.1.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay S. L. Synapses in the Central Nervous System. J. Cell Biol. 1956, 2 (4), 193–202. 10.1083/jcb.2.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz L. L.; Romanova D. Y.; Kohn A. B. Neural versus Alternative Integrative Systems: Molecular Insights into Origins of Neurotransmitters. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376 (1821), 20190762 10.1098/rstb.2019.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati L. F.; Fuxe K.; Zoli M.; Ozini I.; Toffano G.; Ferraguti F. A Correlation Analysis of the Regional Distribution of Central Enkephalin and Beta-Endorphin Immunoreactive Terminals and of Opiate Receptors in Adult and Old Male Rats. Evidence for the Existence of Two Main Types of Communication in the Central Nervous System: The Volume Transmission and the Wiring Transmission. Acta Physiol. Scand. 1986, 128 (2), 201–207. 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The Cell Biology of Synapse Formation. J. Cell Biol. 2021, 220 (7), e202103052 10.1083/jcb.202103052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R. Gap Junction Wiring: A ‘New’ Principle in Cell-to-Cell Communication in the Nervous System?. Brain Res. Rev. 1998, 26 (2), 176–183. 10.1016/S0165-0173(97)00031-3. [DOI] [PubMed] [Google Scholar]
- Syková E.; Nicholson C. Diffusion in Brain Extracellular Space. Physiol. Rev. 2008, 88 (4), 1277–1340. 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A.; Carmignoto G.; Haydon P. G.; Oliet S. H. R.; Robitaille R.; Volterra A. Gliotransmitters Travel in Time and Space. Neuron 2014, 81 (4), 728–739. 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock J. M.; Kettenmann H. Neurotransmitter Receptors on Microglia. Trends Neurosci. 2007, 30 (10), 527–535. 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barbour B.; Häusser M. Intersynaptic Diffusion of Neurotransmitter. Trends Neurosci. 1997, 20 (9), 377–384. 10.1016/S0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- De-Miguel F. F.; Leon-Pinzon C.; Torres-Platas S. G.; del-Pozo V.; Hernández-Mendoza G. A.; Aguirre-Olivas D.; Méndez B.; Moore S.; Sánchez-Sugía C.; García-Aguilera M. A.; Martínez-Valencia A.; Ramírez-Santiago G.; Rubí J. M. Extrasynaptic Communication. Front. Mol. Neurosci. 2021, 14, 638858 10.3389/fnmol.2021.638858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson G. B.; Wu Y. Dynamic Equilibrium of Neurotransmitter Transporters: Not Just for Reuptake Anymore. J. Neurophysiol. 2003, 90 (3), 1363–1374. 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Vizi E.; Fekete A.; Karoly R.; Mike A. Non-Synaptic Receptors and Transporters Involved in Brain Functions and Targets of Drug Treatment: Non-Synaptic Receptors Involved in Brain Functions. Br. J. Pharmacol. 2010, 160 (4), 785–809. 10.1111/j.1476-5381.2009.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L.; Mechawar N.. Ultrastructural Evidence for Diffuse Transmission by Monoamine and Acetylcholine Neurons of the Central Nervous System. In Progress in Brain Research; Elsevier, 2000; Vol. 125, pp 27–47, 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Dahlström A. B.; Jonsson G.; Marcellino D.; Guescini M.; Dam M.; Manger P.; Agnati L. The Discovery of Central Monoamine Neurons Gave Volume Transmission to the Wired Brain. Prog. Neurobiol. 2010, 90 (2), 82–100. 10.1016/j.pneurobio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Hökfelt T. Early Attempts to Visualize Cortical Monoamine Nerve Terminals. Brain Res. 2016, 1645, 8–11. 10.1016/j.brainres.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Falck B.; Hillarp N.-Å.; Thieme G.; Torp A. Fluorescence of Catecholamines and Related Compounds Condensed with Formaldehyde. J. Histochem. Cytochem. 1962, 10 (3), 348–354. 10.1177/10.3.348. [DOI] [Google Scholar]
- Steinbusch H. W. M. Distribution of Serotonin-Immunoreactivity in the Central Nervous System of the Rat—Cell Bodies and Terminals. Neuroscience 1981, 6 (4), 557–618. 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Joh T. H.; Shikimi T.; Pickel V. M.; Reis D. J. Brain Tryptophan Hydroxylase: Purification of, Production of Antibodies to, and Cellular and Ultrastructural Localization in Serotonergic Neurons of Rat Midbrain. Proc. Natl. Acad. Sci. U. S. A. 1975, 72 (9), 3575–3579. 10.1073/pnas.72.9.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F. C.; Xu Y.; Bledsoe S.; Lin R.; Kelley M. R. Serotonin Transporter Antibodies: Production, Characterization, and Localization in the Brain. Mol. Brain Res. 1996, 43 (1–2), 267–278. 10.1016/S0169-328X(96)00209-4. [DOI] [PubMed] [Google Scholar]
- Maley B. E.; Engle M. G.; Humphreys S.; Vascik D. A.; Howes K. A.; Newton B. W.; Elde R. P. Monoamine Synaptic Structure and Localization in the Central Nervous System. J. Electron Microsc. Technol. 1990, 15 (1), 20–33. 10.1002/jemt.1060150104. [DOI] [PubMed] [Google Scholar]
- Azmitia E. C.; Segal M. An Autoradiographic Analysis of the Differential Ascending Projections of the Dorsal and Median Raphe Nuclei in the Rat. J. Comp. Neurol. 1978, 179 (3), 641–667. 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Lidov H. G. W.; Grzanna R.; Molliver M. E. The Serotonin Innervation of the Cerebral Cortex in the Rat—an Immunohistochemical Analysis. Neuroscience 1980, 5 (2), 207–227. 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Muzerelle A.; Scotto-Lomassese S.; Bernard J. F.; Soiza-Reilly M.; Gaspar P. Conditional Anterograde Tracing Reveals Distinct Targeting of Individual Serotonin Cell Groups (B5–B9) to the Forebrain and Brainstem. Brain Struct. Funct. 2016, 221 (1), 535–561. 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Z.; Ehret M.; Maitre M.; Hamel E. Ultrastructural Analysis of Tryptophan Hydroxylase Immunoreactive Nerve Terminals in the Rat Cerebral Cortex and Hippocampus: Their Associations with Local Blood Vessels. Neuroscience 1995, 66 (3), 555–569. 10.1016/0306-4522(94)00625-F. [DOI] [PubMed] [Google Scholar]
- DeFelipe J.; Jones E. G. A Light and Electron Microscopic Study of Serotonin-Immunoreactive Fibers and Terminals in the Monkey Sensory-Motor Cortex. Exp. Brain Res. 1988, 71 (1), 171. 10.1007/BF00247532. [DOI] [PubMed] [Google Scholar]
- Descarries L.; Alain B.; Watkins K. C. Serotonin Nerve Terminals in Adult Rat Neocortex. Brain Res. 1975, 100 (3), 563–588. 10.1016/0006-8993(75)90158-4. [DOI] [PubMed] [Google Scholar]
- Parnavelas J. G.; Papadopoulos G. C. The Monoaminergic Innervation of the Cerebral Cortex Is Not Diffuse and Nonspecific. Trends Neurosci. 1989, 12 (9), 315–319. 10.1016/0166-2236(89)90037-4. [DOI] [PubMed] [Google Scholar]
- Séguéla P.; Watkins K. C.; Descarries L. Ultrastructural Relationships of Serotonin Axon Terminals in the Cerebral Cortex of the Adult Rat: 5-HT AXON TERMINALS IN CEREBRAL CORTEX. J. Comp. Neurol. 1989, 289 (1), 129–142. 10.1002/cne.902890111. [DOI] [PubMed] [Google Scholar]
- Arluison M.; De La Manche I. S. High-Resolution Radioautographic Study of the Serotonin Innervation of the Rat Corpus Striatum after Intraventricular Administration of [3H]5-Hydroxytryptamine. Neuroscience 1980, 5 (2), 229–240. 10.1016/0306-4522(80)90100-1. [DOI] [PubMed] [Google Scholar]
- Calas A.; Besson M. J.; Gaughy C.; Alonso G.; Glowinski J.; Cheramy A. Radioautographic Study of in Vivo Incorporation of3H-Monoamines in the Cat Caudate Nucleus: Identification of Serotoninergic Fibers. Brain Res. 1976, 118 (1), 1–13. 10.1016/0006-8993(76)90837-4. [DOI] [PubMed] [Google Scholar]
- Soghomonian J.-J.; Descarries L.; Watkins K. C. Serotonin Innervation in Adult Rat Neostriatum. II. Ultrastructural Features: A Radioautographic and Immunocytochemical Study. Brain Res. 1989, 481 (1), 67–86. 10.1016/0006-8993(89)90486-1. [DOI] [PubMed] [Google Scholar]
- Gagnon D.; Gregoire L.; Di Paolo T.; Parent M. Serotonin Hyperinnervation of the Striatum with High Synaptic Incidence in Parkinsonian Monkeys. Brain Struct. Funct. 2016, 221 (7), 3675–3691. 10.1007/s00429-015-1125-5. [DOI] [PubMed] [Google Scholar]
- Moore R. Y.; Halaris A. E. Hippocampal Innervation by Serotonin Neurons of the Midbrain Raphe in the Rat. J. Comp. Neurol. 1975, 164 (2), 171–183. 10.1002/cne.901640203. [DOI] [PubMed] [Google Scholar]
- Sheu S.-H.; Upadhyayula S.; Dupuy V.; Pang S.; Deng F.; Wan J.; Walpita D.; Pasolli H. A.; Houser J.; Sanchez-Martinez S.; Brauchi S. E.; Banala S.; Freeman M.; Xu C. S.; Kirchhausen T.; Hess H. F.; Lavis L.; Li Y.; Chaumont-Dubel S.; Clapham D. E. A Serotonergic Axon-Cilium Synapse Drives Nuclear Signaling to Alter Chromatin Accessibility. Cell 2022, 185 (18), 3390–3407. 10.1016/j.cell.2022.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaja N.; Doucet G.; Bolam J. P. Ultrastructure and Synaptic Targets of the Raphe-Nigral Projection in the Rat. Neuroscience 1993, 55 (2), 417–427. 10.1016/0306-4522(93)90510-M. [DOI] [PubMed] [Google Scholar]
- Lavoie B.; Parent A. Immunohistochemical Study of the Serotoninergic Innervation of the Basal Ganglia in the Squirrel Monkey. J. Comp. Neurol. 1990, 299 (1), 1–16. 10.1002/cne.902990102. [DOI] [PubMed] [Google Scholar]
- Maddaloni G.; Bertero A.; Pratelli M.; Barsotti N.; Boonstra A.; Giorgi A.; Migliarini S.; Pasqualetti M. Development of Serotonergic Fibers in the Post-Natal Mouse Brain. Front. Cell. Neurosci. 2017, 11, 202. 10.3389/fncel.2017.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A.; Descarries L.; Beaudet A. Organization of Ascending Serotonin Systems in the Adult Rat Brain. A Radioautographic Study after Intraventricular Administration of [3h]5-Hydroxytryptamine. Neuroscience 1981, 6 (2), 115–138. 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Moukhles H.; Bosler O.; Bolam J. P.; Vallée A.; Umbriaco D.; Geffard M.; Doucet G. Quantitative and Morphometric Data Indicate Precise Cellular Interactions between Serotonin Terminals and Postsynaptic Targets in Rat Substantia Nigra. Neuroscience 1997, 76 (4), 1159–1171. 10.1016/S0306-4522(96)00452-6. [DOI] [PubMed] [Google Scholar]
- Parent M.; Wallman M.-J.; Gagnon D.; Parent A. Serotonin Innervation of Basal Ganglia in Monkeys and Humans. J. Chem. Neuroanat. 2011, 41 (4), 256–265. 10.1016/j.jchemneu.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Mori S.; Matsuura T.; Takino T.; Sano Y. Light and Electron Microscopic Immunohistochemical Studies of Serotonin Nerve Fibers in the Substantia Nigra of the Rat, Cat and Monkey. Anat. Embryol. (Berl.) 1987, 176 (1), 13–18. 10.1007/BF00309747. [DOI] [PubMed] [Google Scholar]
- Vertes R. P. A PHA-L Analysis of Ascending Projections of the Dorsal Raphe Nucleus in the Rat. J. Comp. Neurol. 1991, 313 (4), 643–668. 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Descarries L.; Watkins K. C.; Garcia S.; Beaudet A. The Serotonin Neurons in Nucleus Raphe Dorsalis of Adult Rat: A Light and Electron Microscope Radioautographic Study. J. Comp. Neurol. 1982, 207 (3), 239–254. 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Bang S. J.; Jensen P.; Dymecki S. M.; Commons K. G. Projections and Interconnections of Genetically Defined Serotonin Neurons in Mice: Networks of Rhombomere-Specific Serotonin Neurons. Eur. J. Neurosci. 2012, 35 (1), 85–96. 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal G.; Ohara P. T. Vesicle-Containing Dendrites in the Nucleus Raphe Dorsalis of the Cat. A Serial Section Electron Microscopic Analysis. J. Neurocytol. 1986, 15 (6), 777–787. 10.1007/BF01625194. [DOI] [PubMed] [Google Scholar]
- Chazal G.; Ralston H. J. Serotonin-Containing Structures in the Nucleus Raphe Dorsalis of the Cat: An Ultrastructural Analysis of Dendrites, Presynaptic Dendrites, and Axon Terminals. J. Comp. Neurol. 1987, 259 (3), 317–329. 10.1002/cne.902590302. [DOI] [PubMed] [Google Scholar]
- Kapadia S. E.; De Lanerolle N. C.; LaMotte C. C. Immunocytochemical and Electron Microscopic Study of Serotonin Neuronal Organization in the Dorsal Raphe Nucleus of the Monkey. Neuroscience 1985, 15 (3), 729–746. 10.1016/0306-4522(85)90075-2. [DOI] [PubMed] [Google Scholar]
- Colgan L. A.; Cavolo S. L.; Commons K. G.; Levitan E. S. Action Potential-Independent and Pharmacologically Unique Vesicular Serotonin Release from Dendrites. J. Neurosci. 2012, 32 (45), 15737–15746. 10.1523/JNEUROSCI.0020-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushalya S. K.; Desai R.; Arumugam S.; Ghosh H.; Balaji J.; Maiti S. Three-Photon Microscopy Shows That Somatic Release Can Be a Quantitatively Significant Component of Serotonergic Neurotransmission in the Mammalian Brain. J. Neurosci. Res. 2008, 86 (15), 3469–3480. 10.1002/jnr.21794. [DOI] [PubMed] [Google Scholar]
- Andrade R.; Huereca D.; Lyons J. G.; Andrade E. M.; McGregor K. M. 5-HT 1A Receptor-Mediated Autoinhibition and the Control of Serotonergic Cell Firing. ACS Chem. Neurosci. 2015, 6 (7), 1110–1115. 10.1021/acschemneuro.5b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V.; Surprenant A.; North R. A. 5-HT3 Receptors Are Membrane Ion Channels. Nature 1989, 339 (6227), 706–709. 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Bockaert J.; Claeysen S.; Bécamel C.; Dumuis A.; Marin P. Neuronal 5-HT Metabotropic Receptors: Fine-Tuning of Their Structure, Signaling, and Roles in Synaptic Modulation. Cell Tissue Res. 2006, 326 (2), 553–572. 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between Neurotransmitter and Receptor Localizations in Brain: Observations and Implications. Neuroscience 1987, 23 (1), 1–38. 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Jansson A.; Descarries L.; Cornea-Hebert V.; Riad M.; Vergé D.; Bancila M.; Agnati L.; Fuxe K.. Transmitter-Receptor Mismatches in Central Dopamine, Serotonin and Neuropeptide Systems. Further Evidence for Volume Transmission. In The Neuronal Environment. Brain Homeostasis in Health and Disease; Humana: Totowa, NJ, 2002; pp 83–108. [Google Scholar]
- Jansson A.; Tinner B.; Bancila M.; Vergé D.; Steinbusch H. W. M.; Agnati L. F.; Fuxe K. Relationships of 5-Hydroxytryptamine Immunoreactive Terminal-like Varicosities to 5-Hydroxytryptamine-2A Receptor-Immunoreactive Neuronal Processes in the Rat Forebrain. J. Chem. Neuroanat. 2001, 22 (3), 185–203. 10.1016/S0891-0618(01)00133-8. [DOI] [PubMed] [Google Scholar]
- Miner L. A. H.; Backstrom J. R.; Sanders-Bush E.; Sesack S. R. Ultrastructural Localization of serotonin2A Receptors in the Middle Layers of the Rat Prelimbic Prefrontal Cortex. Neuroscience 2003, 116 (1), 107–117. 10.1016/S0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- Kia H. K.; Brisorgueil M.-J.; Hamon M.; Calas A.; Vergé D. Ultrastructural Localization of 5-hydroxytryptamine1A Receptors in the Rat Brain. J. Neurosci. Res. 1996, 46 (6), 697–708. . [DOI] [PubMed] [Google Scholar]
- Riad M.; Garcia S.; Watkins K. C.; Jodoin N.; Doucet É.; Langlois X.; El Mestikawy S.; Hamon M.; Descarries L. Somatodendritic Localization of 5-HT1A and Preterminal Axonal Localization of 5-HT1B Serotonin Receptors in Adult Rat Brain. J. Comp. Neurol. 2000, 417 (2), 181–194. . [DOI] [PubMed] [Google Scholar]
- Sari Y.; Lefèvre K.; Bancila M.; Quignon M.; Miquel M.-C.; Langlois X.; Hamon M.; Vergé D. Light and Electron Microscopic Immunocytochemical Visualization of 5-HT1B Receptors in the Rat Brain. Brain Res. 1997, 760 (1–2), 281–286. 10.1016/S0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- Bunin M. A.; Wightman R. M. Paracrine Neurotransmission in the CNS: Involvement of 5-HT. Trends Neurosci. 1999, 22 (9), 377–382. 10.1016/S0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- Jackson B. P.; Dietz S. M.; Wightman R. M. Fast-Scan Cyclic Voltammetry of 5-Hydroxytryptamine. Anal. Chem. 1995, 67 (6), 1115–1120. 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- Bunin M. A.; Prioleau C.; Mailman R. B.; Wightman R. M. Release and Uptake Rates of 5-Hydroxytryptamine in the Dorsal Raphe and Substantia Nigra Reticulata of the Rat Brain. J. Neurochem. 1998, 70 (3), 1077–1087. 10.1046/j.1471-4159.1998.70031077.x. [DOI] [PubMed] [Google Scholar]
- Bunin M. A.; Wightman R. M. Quantitative Evaluation of 5-Hydroxytryptamine (Serotonin) Neuronal Release and Uptake: An Investigation of Extrasynaptic Transmission. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18 (13), 4854–4860. 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L.; Schousboe I.; Hertz L.; Schousboe A. Receptor Expression in Primary Cultures of Neurons or Astrocytes. Prog. Neuropsychopharmacol. Biol. Psychiatry 1984, 8 (4–6), 521–527. 10.1016/0278-5846(84)90010-1. [DOI] [PubMed] [Google Scholar]
- Glebov K.; Löchner M.; Jabs R.; Lau T.; Merkel O.; Schloss P.; Steinhäuser C.; Walter J. Serotonin Stimulates Secretion of Exosomes from Microglia Cells: Serotonin Stimulates Microglial Exosome Release. Glia 2015, 63 (4), 626–634. 10.1002/glia.22772. [DOI] [PubMed] [Google Scholar]
- Krabbe G.; Matyash V.; Pannasch U.; Mamer L.; Boddeke H. W. G. M.; Kettenmann H. Activation of Serotonin Receptors Promotes Microglial Injury-Induced Motility but Attenuates Phagocytic Activity. Brain. Behav. Immun. 2012, 26 (3), 419–428. 10.1016/j.bbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia P. M.; Murphy R.; Azmitia E. C. Stimulation of Astroglial 5-HT1A Receptors Releases the Serotonergic Growth Factor, Protein S-100, and Alters Astroglial Morphology. Brain Res. 1990, 528 (1), 155–158. 10.1016/0006-8993(90)90210-3. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia P. M.; Clarke C.; Azmitia E. C. Localization of 5-HT1A Receptors to Astroglial Cells in Adult Rats: Implications for Neuronal-Glial Interactions and Psychoactive Drug Mechanism of Action. Synapse 1993, 14 (3), 201–205. 10.1002/syn.890140303. [DOI] [PubMed] [Google Scholar]
- Miyazaki I.; Asanuma M.; Murakami S.; Takeshima M.; Torigoe N.; Kitamura Y.; Miyoshi K. Targeting 5-HT1A Receptors in Astrocytes to Protect Dopaminergic Neurons in Parkinsonian Models. Neurobiol. Dis. 2013, 59, 244–256. 10.1016/j.nbd.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Müller F. E.; Schade S. K.; Cherkas V.; Stopper L.; Breithausen B.; Minge D.; Varbanov H.; Wahl-Schott C.; Antoniuk S.; Domingos C.; Compan V.; Kirchhoff F.; Henneberger C.; Ponimaskin E.; Zeug A. Serotonin Receptor 4 Regulates Hippocampal Astrocyte Morphology and Function. Glia 2021, 69 (4), 872–889. 10.1002/glia.23933. [DOI] [PubMed] [Google Scholar]
- Natsubori A.; Hirai S.; Kwon S.; Ono D.; Deng F.; Wan J.; Miyazawa M.; Kojima T.; Okado H.; Karashima A.; Li Y.; Tanaka K. F.; Honda M. Serotonergic Neurons Control Cortical Neuronal Intracellular Energy Dynamics by Modulating Astrocyte-Neuron Lactate Shuttle. iScience 2023, 26 (1), 105830 10.1016/j.isci.2022.105830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davla S.; Artiushin G.; Li Y.; Chitsaz D.; Li S.; Sehgal A.; Van Meyel D. J. AANAT1 Functions in Astrocytes to Regulate Sleep Homeostasis. eLife 2020, 9, e53994 10.7554/eLife.53994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duseja R.; Heir R.; Lewitus G. M.; Altimimi H. F.; Stellwagen D. Astrocytic TNFα Regulates the Behavioral Response to Antidepressants. Brain. Behav. Immun. 2015, 44, 187–194. 10.1016/j.bbi.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Xia M.; Li Z.; Li S.; Liang S.; Li X.; Chen B.; Zhang M.; Dong C.; Verkhratsky A.; Guan D.; Li B. Sleep Deprivation Selectively Down-Regulates Astrocytic 5-HT2B Receptors and Triggers Depressive-Like Behaviors via Stimulating P2X7 Receptors in Mice. Neurosci. Bull. 2020, 36 (11), 1259–1270. 10.1007/s12264-020-00524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T.; Li B.; Gu L.; Huang J.; Verkhratsky A.; Peng L. Ammonium Induced Dysfunction of 5-HT2B Receptor in Astrocytes. Neurochem. Int. 2019, 129, 104479 10.1016/j.neuint.2019.104479. [DOI] [PubMed] [Google Scholar]
- Hirst W. D.; Price G. W.; Rattray M.; Wilkin G. P. Serotonin Transporters in Adult Rat Brain Astrocytes Revealed by [3H]5-HT Uptake into Glial Plasmalemmal Vesicles. Neurochem. Int. 1998, 33 (1), 11–22. 10.1016/S0197-0186(05)80003-8. [DOI] [PubMed] [Google Scholar]
- Katz D.; Kimelberg H. Kinetics and Autoradiography of High Affinity Uptake of Serotonin by Primary Astrocyte Cultures. J. Neurosci. 1985, 5 (7), 1901–1908. 10.1523/JNEUROSCI.05-07-01901.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N.; Kiuchi Y.; Nemoto M.; Oyamada H.; Ohno M.; Funahashi H.; Shioda S.; Oguchi K. Regulation of Serotonin Transporter Gene Expression in Human Glial Cells by Growth Factors. Eur. J. Pharmacol. 2001, 417 (1–2), 69–76. 10.1016/S0014-2999(01)00906-2. [DOI] [PubMed] [Google Scholar]
- Pickel V. M.; Chan J. Ultrastructural Localization of the Serotonin Transporter in Limbic and Motor Compartments of the Nucleus Accumbens. J. Neurosci. 1999, 19 (17), 7356–7366. 10.1523/JNEUROSCI.19-17-07356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V.; Balasse L.; Callebert J.; Launay J.-M.; Giros B.; Gautron S. Altered Aminergic Neurotransmission in the Brain of Organic Cation Transporter 3-Deficient Mice. J. Neurochem. 2008, 106, 1471–1482. 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- Sardar D.; Cheng Y.-T.; Woo J.; Choi D.-J.; Lee Z.-F.; Kwon W.; Chen H.-C.; Lozzi B.; Cervantes A.; Rajendran K.; Huang T.-W.; Jain A.; Arenkiel B. R.; Maze I.; Deneen B. Induction of Astrocytic Slc22a3 Regulates Sensory Processing through Histone Serotonylation. Science 2023, 380 (6650), eade0027 10.1126/science.ade0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczak M.; Béchade C.; Gervasi N.; Irinopoulou T.; Banas S. M.; Cordier C.; Rebsam A.; Roumier A.; Maroteaux L. Serotonin Modulates Developmental Microglia via 5-HT 2B Receptors: Potential Implication during Synaptic Refinement of Retinogeniculate Projections. ACS Chem. Neurosci. 2015, 6 (7), 1219–1230. 10.1021/cn5003489. [DOI] [PubMed] [Google Scholar]
- Béchade C.; D’Andrea I.; Etienne F.; Verdonk F.; Moutkine I.; Banas S. M.; Kolodziejczak M.; Diaz S. L.; Parkhurst C. N.; Gan W. B.; Maroteaux L.; Roumier A. The Serotonin 2B Receptor Is Required in Neonatal Microglia to Limit Neuroinflammation and Sickness Behavior in Adulthood. Glia 2021, 69 (3), 638–654. 10.1002/glia.23918. [DOI] [PubMed] [Google Scholar]
- Albertini G.; D’Andrea I.; Druart M.; Béchade C.; Nieves-Rivera N.; Etienne F.; Le Magueresse C.; Rebsam A.; Heck N.; Maroteaux L.; Roumier A. Serotonin Sensing by Microglia Conditions the Proper Development of Neuronal Circuits and of Social and Adaptive Skills. Mol. Psychiatry 2023, 28, 2328. 10.1038/s41380-023-02048-5. [DOI] [PubMed] [Google Scholar]
- Arnoux A.; Ayme-Dietrich E.; Dieterle S.; Goy M.-A.; Schann S.; Frauli M.; Monassier L.; Dupuis L. Evaluation of a 5-HT2B Receptor Agonist in a Murine Model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2021, 11 (1), 23582. 10.1038/s41598-021-02900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oussini H.; Bayer H.; Scekic-Zahirovic J.; Vercruysse P.; Sinniger J.; Dirrig-Grosch S.; Dieterlé S.; Echaniz-Laguna A.; Larmet Y.; Müller K.; Weishaupt J. H.; Thal D. R.; Van Rheenen W.; Van Eijk K.; Lawson R.; Monassier L.; Maroteaux L.; Roumier A.; Wong P. C.; Van Den Berg L. H.; Ludolph A. C.; Veldink J. H.; Witting A.; Dupuis L. Serotonin 2B Receptor Slows Disease Progression and Prevents Degeneration of Spinal Cord Mononuclear Phagocytes in Amyotrophic Lateral Sclerosis. Acta Neuropathol. (Berl.) 2016, 131 (3), 465–480. 10.1007/s00401-016-1534-4. [DOI] [PubMed] [Google Scholar]
- Machado V.; Zöller T.; Attaai A.; Spittau B. Microglia-Mediated Neuroinflammation and Neurotrophic Factor-Induced Protection in the MPTP Mouse Model of Parkinson’s Disease-Lessons from Transgenic Mice. Int. J. Mol. Sci. 2016, 17 (2), 151. 10.3390/ijms17020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani N.; Everson J.; Pariante C. M.; Borsini A. Modulation of Microglial Activation by Antidepressants. J. Psychopharmacol. (Oxf.) 2022, 36 (2), 131–150. 10.1177/02698811211069110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely R. D.; Edwards R. H. Vesicular and Plasma Membrane Transporters for Neurotransmitters. Cold Spring Harb. Perspect. Biol. 2012, 4 (2), a005595–a005595. 10.1101/cshperspect.a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amphoux A.; Vialou V.; Drescher E.; Brüss M.; La Cour C. M.; Rochat C.; Millan M. J.; Giros B.; Bönisch H.; Gautron S. Differential Pharmacological in Vitro Properties of Organic Cation Transporters and Regional Distribution in Rat Brain. Neuropharmacology 2006, 50 (8), 941–952. 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hersch S. M.; Yi H.; Heilman C. J.; Edwards R. H.; Levey A. I. Subcellular Localization and Molecular Topology of the Dopamine Transporter in the Striatum and Substantia Nigra. J. Comp. Neurol. 1997, 388 (2), 211–227. . [DOI] [PubMed] [Google Scholar]
- Miner L. H.; Schroeter S.; Blakely R. D.; Sesack S. R. Ultrastructural Localization of the Norepinephrine Transporter in Superficial and Deep Layers of the Rat Prelimbic Prefrontal Cortex and Its Spatial Relationship to Probable Dopamine Terminals. J. Comp. Neurol. 2003, 466 (4), 478–494. 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Nirenberg M.; Vaughan R.; Uhl G.; Kuhar M.; Pickel V. The Dopamine Transporter Is Localized to Dendritic and Axonal Plasma Membranes of Nigrostriatal Dopaminergic Neurons. J. Neurosci. 1996, 16 (2), 436–447. 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner L. H.; Schroeter S.; Blakely R. D.; Sesack S. R. Ultrastructural Localization of the Serotonin Transporter in Superficial and Deep Layers of the Rat Prelimbic Prefrontal Cortex and Its Spatial Relationship to Dopamine Terminals. J. Comp. Neurol. 2000, 427 (2), 220–234. . [DOI] [PubMed] [Google Scholar]
- Tao-Cheng J.-H.; Zhou F. C. Differential Polarization of Serotonin Transporters in Axons versus Soma–Dendrites: An Immunogold Electron Microscopy Study. Neuroscience 1999, 94 (3), 821–830. 10.1016/S0306-4522(99)00373-5. [DOI] [PubMed] [Google Scholar]
- Zhou F. C.; Tao-Cheng J.-H.; Segu L.; Patel T.; Wang Y. Serotonin Transporters Are Located on the Axons beyond the Synaptic Junctions: Anatomical and Functional Evidence. Brain Res. 1998, 805 (1–2), 241–254. 10.1016/S0006-8993(98)00691-X. [DOI] [PubMed] [Google Scholar]
- Adjimann T. S.; Argañaraz C. V.; Soiza-Reilly M. Serotonin-Related Rodent Models of Early-Life Exposure Relevant for Neurodevelopmental Vulnerability to Psychiatric Disorders. Transl. Psychiatry 2021, 11 (1), 280. 10.1038/s41398-021-01388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge M. S.; Zhou M.; Lira A.; Hen R.; Gingrich J. A. Early-Life Blockade of the 5-HT Transporter Alters Emotional Behavior in Adult Mice. Science 2004, 306 (5697), 879–881. 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Popa D.; Léna C.; Alexandre C.; Adrien J. Lasting Syndrome of Depression Produced by Reduction in Serotonin Uptake during Postnatal Development: Evidence from Sleep, Stress, and Behavior. J. Neurosci. 2008, 28 (14), 3546–3554. 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello T. J.; Yu Q.; Goodfellow N. M.; Caffrey Cagliostro M. K.; Teissier A.; Morelli E.; Demireva E. Y.; Chemiakine A.; Rosoklija G. B.; Dwork A. J.; Lambe E. K.; Gingrich J. A.; Ansorge M. S. Postnatal Day 2 to 11 Constitutes a 5-HT-Sensitive Period Impacting Adult mPFC Function. J. Neurosci. 2014, 34 (37), 12379–12393. 10.1523/JNEUROSCI.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson S. R.; Mezey É.; Hoffman B. J. Serotonin Transporter Messenger RNA in the Developing Rat Brain: Early Expression in Serotonergic Neurons and Transient Expression in Non-Serotonergic Neurons. Neuroscience 1998, 83 (4), 1185–1201. 10.1016/S0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Zhou F. C.; Sari Y.; Zhang J. K. Expression of Serotonin Transporter Protein in Developing Rat Brain. Dev. Brain Res. 2000, 119 (1), 33–45. 10.1016/S0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]
- Narboux-Nême N.; Pavone L. M.; Avallone L.; Zhuang X.; Gaspar P. Serotonin Transporter Transgenic (SERTcre) Mouse Line Reveals Developmental Targets of Serotonin Specific Reuptake Inhibitors (SSRIs). Neuropharmacology 2008, 55 (6), 994–1005. 10.1016/j.neuropharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M.; Meye F. J.; Olusakin J.; Telley L.; Petit E.; Chen X.; Mameli M.; Jabaudon D.; Sze J.-Y.; Gaspar P. SSRIs Target Prefrontal to Raphe Circuits during Development Modulating Synaptic Connectivity and Emotional Behavior. Mol. Psychiatry 2019, 24 (5), 726–745. 10.1038/s41380-018-0260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusakin J.; Moutkine I.; Dumas S.; Ponimaskin E.; Paizanis E.; Soiza-Reilly M.; Gaspar P. Implication of 5-HT7 Receptor in Prefrontal Circuit Assembly and Detrimental Emotional Effects of SSRIs during Development. Neuropsychopharmacology 2020, 45 (13), 2267–2277. 10.1038/s41386-020-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon D.; Parent M. Distribution of VGLUT3 in Highly Collateralized Axons from the Rat Dorsal Raphe Nucleus as Revealed by Single-Neuron Reconstructions. PLoS One 2014, 9 (2), e87709 10.1371/journal.pone.0087709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele E. J.; Biswas A.; Pickel V. M. Topography of Serotonin Neurons in the Dorsal Raphe Nucleus That Send Axon Collaterals to the Rat Prefrontal Cortex and Nucleus Accumbens. Brain Res. 1993, 624 (1–2), 188–198. 10.1016/0006-8993(93)90077-Z. [DOI] [PubMed] [Google Scholar]
- Parrott A. C. Human Psychobiology of MDMA or ‘Ecstasy’: An Overview of 25 Years of Empirical Research. Hum. Psychopharmacol. Clin. Exp. 2013, 28 (4), 289–307. 10.1002/hup.2318. [DOI] [PubMed] [Google Scholar]
- Callaway E. M.; Luo L. Monosynaptic Circuit Tracing with Glycoprotein-Deleted Rabies Viruses. J. Neurosci. 2015, 35 (24), 8979–8985. 10.1523/JNEUROSCI.0409-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C.; Burkhalter A.; Dreyer W. J. Fluorescent Latex Microspheres as a Retrograde Neuronal Marker for in Vivo and in Vitro Studies of Visual Cortex. Nature 1984, 310 (5977), 498–500. 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Tervo D. G. R.; Hwang B.-Y.; Viswanathan S.; Gaj T.; Lavzin M.; Ritola K. D.; Lindo S.; Michael S.; Kuleshova E.; Ojala D.; Huang C.-C.; Gerfen C. R.; Schiller J.; Dudman J. T.; Hantman A. W.; Looger L. L.; Schaffer D. V.; Karpova A. Y. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92 (2), 372–382. 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon T. R.; Murray A. J.; Turi G. F.; Wirblich C.; Croce K. R.; Schnell M. J.; Jessell T. M.; Losonczy A. Rabies Virus CVS-N2c ΔG Strain Enhances Retrograde Synaptic Transfer and Neuronal Viability. Neuron 2016, 89 (4), 711–724. 10.1016/j.neuron.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]