Abstract
Background
Heavy menstrual bleeding (HMB), self-reported by 37% of adolescents, can be the first sign of a bleeding disorder (BD) during adolescence. The Dutch general practitioner (GP) guideline demands laboratory diagnostics and referral for patients at risk for a BD. How often adolescents consult the GP for HMB and which diagnostic and management strategies are used are unknown.
Objectives
This study aims to estimate the incidence of HMB in adolescents in primary care and to identify diagnostic and management practices for HMB, considering the HMB GP guideline.
Methods
Retrospective analyses of a GP network database containing over 200 Dutch GPs were performed. Adolescents aged 10 to 21 years, with a new diagnosis of HMB between 2010 and 2020, and a 6-month follow-up were eligible. The incidence rate and diagnostic and therapeutic strategy data were extracted.
Results
We identified 1879 new diagnoses of HMB in adolescents. The average incidence rate was 7.91 per 1000 person-years. No diagnostic studies were performed in 67%. Laboratory studies were mainly restricted to hemoglobin levels (31%). Full coagulation screening occurred in 1.3%, and ferritin levels in 10%. Medication was prescribed in 65%; mostly hormonal treatment (56%) and/or nonsteroidal antiinflammatory drugs (NSAIDs) (18%). The referral rate was higher after >2 follow-up visits (6.7%) vs after 1 GP visit for HMB (1.6%; Odds ratio: 8.8; 95% CI: 5.1-15), mostly to gynecologists (>85%).
Conclusion
According to this GP database study, few adolescents visit their GP with HMB despite its high self-reported incidence. Most adolescents were prescribed hormonal contraception without further diagnostics. Referral was rare and mostly occurred after multiple follow-up visits.
Keywords: adolescent, diagnostic tests, heavy menstrual bleeding, primary health care, referral and consultation, von Willebrand disease
Essentials
-
•
General practitioner (GP) management of heavy menstrual bleeding (HMB) at the ages of 10 to 21 years is unknown.
-
•
We analyzed diagnostics, referrals, and treatment of HMB within a Dutch GP network of 200 GPs.
-
•
Of 8/1000 person-year GP HMB visits, 52% were treated without diagnostics.
-
•
Only 1% received full coagulation studies and referral occurred rarely in 3% of the adolescents.
1. Introduction
Heavy menstrual bleeding (HMB) is a common symptom in adolescent girls. HMB is self-reported by 37% of 16-year-old school girls, and about 13% of them receive treatment for heavy periods [1]. HMB can reduce quality of life; impair social, sport, and school participation; and trigger serious health problems such as iron deficiency anemia [[2], [3], [4]]. Nonetheless, many girls and women affected by HMB do not consult their general practitioner (GP) [5]. In the Netherlands, a retrospective cohort study showed a mean incidence of women consulting their GP for HMB of 9.3 per 1000 person-years between 2004 and 2013 [6]. There are no studies reporting the incidence and management of HMB in adolescents in current general practice.
Underlying bleeding disorders (BD), such as von Willebrand disease (VWD), coagulation factor deficiencies, and platelet disorders are an important etiology of HMB. The 2021 guideline on VWD diagnosis states that the diagnosis of VWD can be made in individuals with von Willebrand factor activity (VWF) levels <30 IU/dL and in individuals with VWF activity levels <50 IU/dL with an abnormal bleeding assessment (assessed by a bleeding assessment tool [BAT], such as the International Society of Thrombosis and Haemostasis-bleeding assessment tool [ISTH-BAT]) [7]. In primary care settings, they recommend the usage of a validated BAT as an initial screening tool to identify a person at risk for a BD, who requires additional blood testing [7]. Up to 33% of adolescents with HMB referred to second or third-line care have an underlying inherited BD, which has important implications for their current and future bleeding management [[8], [9], [10]]. However, in primary care, this often remains unrecognized, resulting in diagnostic delay, inappropriate treatment, and risk for future bleeding complications, even with mild BDs [11]. Causes of this discrepancy between the reported incidence of BDs in adolescents with HMB and underdiagnoses in primary care need further exploration.
The Dutch National Guideline “Vaginal blood loss” for General Practitioners recommends the pictorial bleeding assessment chart (PBAC) to quantify menstrual blood loss. In case of HMB, assessment of an underlying BD is advised, especially when there are anamnestic clues indicative for a BD, such as the onset of HMB at menarche and/or a positive family history for BDs. Diagnostics can consist of clinical laboratory investigation and/or referral to a hematologist. Coagulation testing is only advised if a BD is suspected and is restricted to activated partial thromboplastin time (aPTT), prothrombin time (PT), and platelet count [12].
Although onset of HMB at menarche is not rarely the first sign of a BD, its diagnosis is usually not made until adulthood [8,10]. The extent to which this diagnostic delay is rooted in general practice is currently unknown. Lack of patient and health care provider awareness and education and quick onset of HMB treatment without diagnostic procedures are expected to be causes of undiagnosed BDs in adolescents with HMB [10].
The aim of this study is to investigate the incidence of adolescents visiting their GP with HMB complaints in a large cohort of general practices in the Netherlands. Furthermore, we aim to identify diagnostic practices in adolescents who present with HMB in a first-line setting and to analyze their HMB diagnostic and therapeutic management.
2. Methods
2.1. Design and setting
This is a multicenter retrospective cohort study using the database of Julius GP Network (JHN) Utrecht, the Netherlands [13]. The JHN holds 70 general practices with over 200 GPs, who currently collaborate in research and education, and encompasses the municipality of Utrecht. It is estimated that between 250,000 and 400,000 patients were registered at practices in the JHN during the study period. It contains data derived from electronic registration of daily patient care in participating primary care practices consisting of patients from urban and (semi)rural regions, comparable with the general Dutch population, including gender, age (mean and distribution), and ethnicity [13]. Diagnoses and prescriptions are recorded according to the International Classification of Primary Care (ICPC) and Anatomical Therapeutic Chemical classification, respectively [12].
This study was approved by the Medical Ethics Committee of the University Medical Center Utrecht, Utrecht, the Netherlands, on August 6, 2021 (21/553), and funded by the Child Health Boost grant from the Wilhelmina Children's Hospital, Utrecht, the Netherlands.
2.2. Participants
All adolescent women aged 10 to 21 years, registered in the JHN database between January 2010 and December 2020, were included in the study. We identified the population at risk for a BD by selecting patients who visited the GP with HMB, received the diagnostic ICPC code X.06 (menorrhagia), and had a minimum follow-up of 6 months. There were no exclusion criteria. Anonymized data about demographics, medical characteristics, diagnostics, and therapy were used for data analyses. Ethnicity was not recorded in the JHN database. We will elaborate on this in the discussion.
2.3. Incidence rates
The incidence rate of HMB was calculated per year, using the number of adolescents with a first consultation for HMB (ICPC code X.06) and the number of person-years of the population at risk. The JHN is a dynamic population, and therefore, the incidence of HMB per person-years was calculated from the number of days each patient was registered in the cohort in a specific year, taking into account the date of patient entry in the database, date of event (first consultation with HMB), and the end of the study period or deregistration from the JHN registry.
2.4. Diagnostic and therapeutic practices
We recorded data regarding relevant comorbidities including anemia and BDs, as well as medication prescriptions prior to the date of the ICPC code HMB. After the HMB diagnosis, data on laboratory testing (complete blood count, iron panel, and coagulation screening), imaging studies (transvaginal ultrasound), use of medication (analgesics and contraceptives), and new prescriptions (hormonal treatment, iron supplementation, pain medication, and tranexamic acid) were recorded. We also recorded data on “over-the-counter medication” advised by the GP if it was recorded in the patient file. Furthermore, we extracted the number of follow-up consultations, new ICPC codes on anemia, BDs, intrauterine devices (IUDs), and second-/third-line referrals after the diagnosis HMB. An overview of the included ICPC codes can be found in the Supplementary Table.
2.5. Statistical analysis
Statistical analyses were performed in IBM SPSS Statistics 26.0 for Windows. We used descriptive statistics to report means with SD, median laboratory results with IQR, and absolute count with percentages.
To calculate odds ratios for predictors of referral, we have used univariate binary logistic regression with the following determinants: whether diagnostic testing was performed, prescription of ≥2 hormonal subscriptions, and ≥3 consultations. We have used univariate binary logistic regression to calculate the OR for receiving (any) treatment for HMB with the variable “diagnostic testing performed” as determinant.
The JHN database is built by linking the computerized medical files of GPs (source data) to the central database through patient specific code numbers. It was assumed, but not verified, that data entered by the GPs were correct. Pairwise deletion was used in case of missing values.
3. Results
3.1. Population at risk and incidence rate
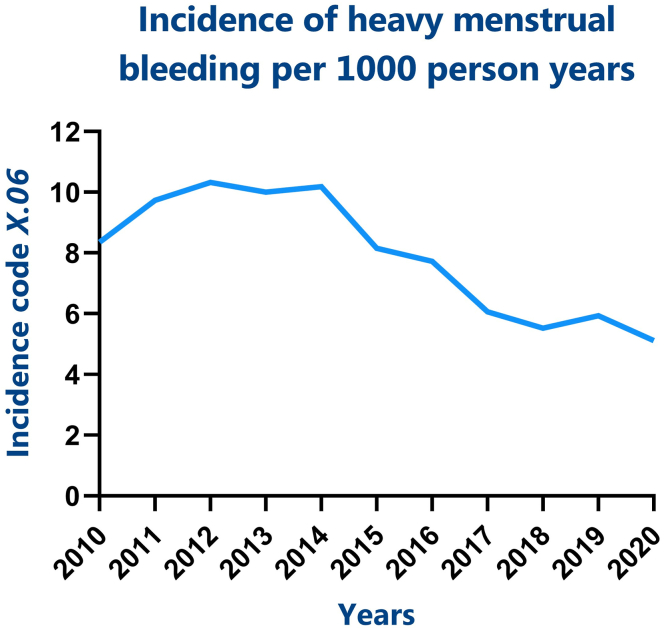
Between 2010 and 2020, a total of 253,895 persons were registered in the JHN database. Within this cohort, 1879 adolescents aged 10 to 21 years had visited their GP with HMB for the first time and had a minimum follow-up of 6 months. The average incidence rate of HMB was 7.91 per 1000 person-years (Figure 1).
Figure 1.
Incidence rate of HMB in adolescents. Code X.06 is the International Classification of Primary Care code for heavy menstrual bleeding. HMB, heavy menstrual bleeding.
3.2. Characteristics of adolescents visiting their GP with HMB
The characteristics of the study population are summarized in Table 1. The mean age at presentation was 16.1 (SD: 2.9) years. Few adolescents (n = 212, 11.3%) used contraceptives before presentation with HMB. Of the adolescents who had prior GP contact with the ICPC code IUD (n = 41, 2.2%), only 4 had an active IUD prescription within the past 3 months (hormonal IUD: n = 3; copper IUD: n = 1). A very small proportion of the adolescents presenting with HMB used comedication that could possibly affect their menstruation (n = 42, 2.2%, excluding contraceptives). Few adolescents were known to have anemia, mostly iron deficiency anemia, prior to consultation with HMB (n = 76, 4.0%).
Table 1.
Baseline characteristics of adolescents with heavy menstrual bleeding.
| Characteristics | N (%) |
|---|---|
| Number of adolescents with HMB | 1879 (100) |
| Age at first HMB consulta | 16.1 (2.9) |
| Prior use of medicationb | 42 (2.2) |
| Anticoagulants | 10 (0.5) |
| SSRI | 26 (1.4) |
| Oral prednisone | 6 (0.3) |
| Prior use of contraceptive medication | 212 (11.3) |
| Combined oral contraceptive | 117 (6.2) |
| Other combined contraceptive | 5 (0.3) |
| Progesteronec | 41 (2.2) |
| Copper IUD | 2 (0.1) |
| Hormonal IUD | 6 (0.3) |
| Prior contact with GP for IUD | 41 (2.2) |
| Comorbidity prior to HMB diagnosis | 85 (4.5) |
| Anaemiae | 76 (4.0) |
| Iron deficiency anaemia | 66 (3.5) |
| Bleeding disorderd | 9 (0.5) |
| Endometriosis | 0 (0) |
GP, general practitioner; HMB, heavy menstrual bleeding; ITP, immune thrombocytopenia; IUD, intrauterine device; SSRI, selective serotonin reuptake inhibitor.
Mean (SD).
Prescriptions during the last 3 months prior to presentation with HMB.
Oral (n = 18), intramuscular (n = 16), and subcutaneous (n = 7).
Bleeding disorder unspecified (n = 8) and ITP (n = 1).
Number of missing variables: n = 1.
3.3. Diagnostic procedures in adolescents visiting their GP with HMB
We identified the incidence and type of diagnostic testing in the patients presenting with HMB. Diagnostic measures such as the PBAC, a tool to quantify menstrual blood loss and quality of life over a month, could not be verified in the electronic medical files since this tool is not coded in the ICPC system. Furthermore, it was not recorded whether and how often GPs asked for general bleeding symptoms and/or a family bleeding history. In 67% of HMB presentations, no diagnostic testing was performed at all after a HMB consultation (Figure 2). If performed, laboratory testing was mainly restricted to measuring hemoglobin and mean corpuscular volume (MCV) levels (Table 2). A full coagulation screen, which should include an aPTT, PT, and platelet count according to the GP guideline, was performed in 1.3% (n = 25) of the population at risk. Ferritin levels were tested in 10.1% (n = 190) of the adolescents. Serum iron levels (Fe3+) were tested in 2.9% (n = 54). Iron deficiency was found in 2/3 of them (median ferritin: 14 μg/L; IQR: 8-25), of whom 80% had a normal MCV (n = 98/123; median: 85 fL; IQR: 80-88). Transvaginal or abdominal ultrasounds were also rarely performed (n = 39, 2.1%). Adolescents who underwent diagnostic testing in general were more likely to receive medical treatment for HMB (OR: 2.3; 95% CI: 1.8-2.8; Figure 2).
Figure 2.
Patterns of diagnosis and management. Flowchart of number of patient (with percentages) from the population at risk that received diagnostic testing and treatment. ∗, percentage of the population that did/did not receive diagnostics. N, number; HMB, heavy menstrual bleeding.
Table 2.
Diagnostics of n = 1879 adolescents with HMB.
| Diagnostics | N (%) | % Abnormal test result | Test result ↓ LLN or ↑ ULN |
|---|---|---|---|
| Laboratory testing | 597 (31.8) | ||
| Hemoglobin | 590 (31.4) | 17.3 | ↓ |
| MCV | 534 (28.4) | 10.3 | ↓ |
| Platelet count | 352 (18.7) | 0.1 | ↓ |
| Ferritin | 190 (10.1) | 65.6 | ↓ |
| Serum iron (Fe3+) | 54 (2.9) | 7.4 | ↓ |
| Transferrin | 15 (0.8) | 33.3 | ↑ |
| aPTT | 26 (1.4) | 7.7 | ↑ |
| PT | 25 (1.3) | 8.0 | ↑ |
| Imaging studies | 39 (2.1) | ||
| Transvaginal echo | 5 (0.3) | Not reported | |
| Abdominal echo | 34 (1.8) | Not reported |
Values are reported as absolute numbers (percentages). ↑ indicates value above ULN, and ↓ indicates value below LLN.
aPTT,activated partial thromboplastin time; HMB, heavy menstrual bleeding ; LLN, lower limit of normal; MCV, mean corpuscular volume; N, number; PT, prothrombin time; ULN, upper limit of normal.
3.4. Therapeutic management and referral of adolescents with HMB in primary care
In 35% of the adolescents presenting with HMB, no medication was prescribed/advised by the GP. In the 65% of adolescents who were advised to take medication, hormonal treatment was most frequently prescribed (56%, Table 3), which mostly consisted of a combined oral contraceptive pill (87%, n = 897/1035). Tranexamic acid was prescribed in 2.8% of adolescents, of whom a slight majority in combination with hormones (n = 34/54, 63%). Pain medication was advised and/or prescribed in 19% of the adolescents, almost exclusively NSAIDs (97%, most often naproxen), which was combined with hormonal treatment in 64% of them (n = 217/340). A combination of ≥2 different treatments was prescribed in 16% of the adolescents with HMB (Table 3).
Table 3.
Management of n = 1879 adolescents with HMB.
| Management strategy | N (%) |
|---|---|
| Hormonal treatment | 1050 (55.9) |
| Combined oral contraceptive | 897 (47.7) |
| Other combined contraceptive | 21 (1.1) |
| Progesterone | 210 (11.2) |
| Estrogena | 8 (0.4) |
| Hormonal IUD | 49 (2.6) |
| Copper IUDb | 3 (0.2) |
| Analgesics | 349 (18.6) |
| Paracetamolc,d | 7 (2.0) |
| NSAIDsc | 340 (97.4) |
| Unspecifiedc | 2 (0.5) |
| Tranexamic acid | 54 (2.9) |
| Iron supplements | 135 (7.2) |
| Combined treatment | 301 (16.0) |
| Hormonal and tranexamic acide | 36 (12.0) |
| Hormonal and iron supplementse | 88 (29.2) |
| Hormonal and NSAIDse | 217 (72.1) |
| Referral to second-/third-line care | 55 (2.9) |
| Pediatricianf | 2 (3.6) |
| Gynecologistf | 47 (85.5) |
| Unspecifiedf | 6 (11.0) |
IUD, intrauterine device; NSAID, nonsteroid anti-inflammatory drug.
Combined with: Mirena IUD (n = 1), oral contraceptive microgynon 30/50 (n = 3), oral progesterone (n = 1), and nothing (n = 3).
Combined with NSAIDs (n = 3) and oral hormonal contraceptives (n = 1).
Absolute number and percentage of analgesics.
Paracetamol is the same as acetaminophen.
Absolute number and percentage of combined treatment.
Absolute number and percentage of total referrals.
Regarding follow-up, 798 adolescents (42%) had at least 1 follow-up contact for HMB within 10 years after the first visit to their GP with HMB, mostly once (47%) or twice (22%, Table 4). Almost a third of the adolescents who revisited their GP for HMB did so repeatedly (31%, >2 times; n = 248/798). Referral to second-/third-line specialists occurred in 55 adolescents (2.9%, Table 3) with 85% of the referrals being referred to a gynecologist (n = 47/55, Table 3). The adolescents who revisited their GP with HMB >2 times were the ones most likely to be referred (OR: 8.8; 95% CI: 5.1-15; Table 4). Performance of diagnostic testing was also associated with referral (OR: 3.7; 95% CI: 2.2-6.6). More than 2 different hormonal prescriptions by the GP tended to be associated with being referred (n = 2/19 vs 53/1860; OR: 4.0; 95% CI: 0.9-18).
Table 4.
Patterns of follow-up and referral.
| Characteristics | N (%) |
|---|---|
| Follow-up consultation: yes | 798 (42.5) |
| 1 follow-up consultation | 372 (46.6) |
| Referred | 6 (1.6) |
| 2 follow-up consultations | 178 (22.3) |
| Referred | 12 (6.7) |
| ≥3 follow-up consultations | 248 (31.1) |
| Referred | 30 (12.1) |
| Follow-up consultation: no | 1081 (57.5) |
| Referred | 7 (0.6) |
N, number.
The number of follow-up consultations and number of patients referred per follow-up consultation.
4. Discussion
In this multicenter retrospective cohort study, we found an average HMB incidence rate in adolescents (aged 10-21 years) of 7.91 per 1000 person-years, out of 253,895 registered persons between 2010 and 2020. In total, 1879 adolescents visited their GP with HMB for the first time during this period, with a minimum follow-up of 6 months. In this population at risk for an underlying BD, coagulation studies were very rarely performed and referral occurred infrequently. If referral took place, it was mostly to a gynecologist. The majority of adolescents with HMB received hormonal treatment and/or NSAIDs without further diagnostic testing and follow-up consultations.
We found a relatively low incidence rate of HMB in our study population as compared with literature [1]. This may be explained by incorrect documentation of the ICPC coding system. For example, patients could have received the diagnostic code “fatigue” or “iron deficiency,” while this was related to HMB without the correct corresponding ICPC code. In addition, the incidence we have found may be an underestimation because the ICPC coding system only records pathophysiological parameters, making it impossible to collect data based on physiological parameters such as menarche. We could therefore only perform analysis of women and girls of the ages of 10 to 21 years, which include adolescents who may not have reached menarche yet and, consequently, do not exhibit HMB symptoms, underestimating the population at risk. Furthermore, it is likely that adolescents with HMB do not visit a physician due to shame, fear, or the belief that their menstruation is normal [[14], [15], [16]]. BDs often affect whole families, resulting in normalizing abnormal blood loss [9,[17], [18], [19]]. It was not possible to collect data on ethnicity from the JHN database. This can be a limitation since literature has shown that the incidence of HMB in women of Hispanic ethnicity is higher than that in women of non-Hispanic ethnicity [9], possibly leading to an underestimation of HMB incidence. However, most people living in Utrecht are non-Hispanic and of Dutch ethnicity, and therefore, we believe that this underestimation would be negligible.
A decline in the incidence rate over the years may be noticed (Figure 1). This could be a nonsignificant “by incidence” finding, for example, less GP visits during the COVID-19 pandemic, or it may be explained by the relatively young population covered by the JHN, with an increasing proportion of children over time that have not reached menarche. We believe that the population studied is representative in the Netherlands since we calculated the incidence per 1000 person-years. There may be regional differences as, for example, Utrecht has a younger population and is more densely populated than the northern regions of the Netherlands resulting in even lower incidence rates at more remote areas [6].
The Dutch guideline for GPs advises to check hemoglobin levels if a patient has HMB and only advises aPTT, PT, and platelet count when a BD is suspected. We found that full coagulation studies (without VWF testing) were only performed in 1.3%. The Dutch GP guideline does not advise GPs to determine VWF activity levels, a diagnostic parameter for VWD in combination with increased bleeding [7]. VWD cannot be discarded with normal aPTT and PT results. Determining ferritin levels is also not advised in the guideline. Studies have shown that adolescents with low serum ferritin experience symptoms like fatigue and concentration problems, while still having normal hemoglobin levels and MCV [[20], [21], [22]]. We found that iron deficiency could be present in patients with normal MCV levels in the majority of cases with low ferritin levels. Our study shows that the Dutch GP guideline for vaginal blood loss has limited advices for BD diagnostics in women with HMB. Full iron studies, or at least ferritin levels, aPTT, PT, and platelet function analysis should be included in the diagnostic workup, as well as VWF activity levels based on adequate bleeding assessment including a family history for BD [7,12,23].
We found that >50% of the population at risk was prescribed (hormonal) contraceptives. While hormonal contraceptives can improve HMB symptoms, it does not protect women with a BD during future hemostatic challenges. We therefore believe that, even though HMB symptoms can be improved with hormones, a high index of suspicion for an underlying BD should result in more diagnostic testing and referrals. The GP prescribed progesterone-only hormonal contraception to n = 210 adolescents. Progesterone-only contraceptives have a lower risk for thrombosis [24]. The reason for prescribing progesterone-only contraception instead of a combined hormonal contraception is unknown, since the JHN database does not register family history or the reason for a specific drug choice.
As recommended by the 2021 ASH/ISTH/NHF VWD guideline, the International Society on Thrombosis and Haemostasis-bleeding assessment tool (ISTH-BAT) could aid GPs in identifying a person at risk for a BD who requires additional diagnostic testing, such as in subjects with von Willebrand activity levels <50 IU/dL [7]. The ISTH-BAT has been validated in children and adults, where a score of ≥3 and ≥5 in children and adult women, respectively, is suspected for an underlying BD [25]. The diagnostic advantage of the ISTH-BAT in adolescents with HMB has been established by Jain et al. [17], but has not been validated (A. de Vaan, 2023 [unpublished data]). Future research could focus on the possible use of the ISTH-BAT, or the self-BAT, in adolescents with HMB as a diagnostic tool. We are currently investigating the practicability and feasibility of the self-BAT in adolescents and children between the ages of 10 and 21 years visiting primary care with HMB complaints. The results are expected within 2 years.
5. Strengths and Limitations
This study gives a retrospective overview of the incidence of HMB in adolescents within a large cohort of patients in primary care over a 10-year period. Detailed information on the diagnostic workup and treatment management is presented. These data, combined with existing literature, show pitfalls in the current first-line diagnostic workup strategy for patients with HMB who are at risk for an underlying BD. Limitations of this study are the limited information available on the frequency and quality of the bleeding assessment by the GP, either by a BAT, detailed bleeding history, or the use of the PBAC. Data collection was also dependent on the ICPC coding system. If a patient’s complaint is registered under a specific ICPC code, this code is often not changed to the eventual diagnoses, leading to an underestimation of the population at risk for a BD.
6. Conclusion
Overall, we found a relatively low incidence rate of adolescents visiting the GP with HMB compared with the self-reported incidence in existing literature. The majority of adolescents were prescribed oral contraception without diagnostic workup or follow-up. While the current GP guideline only advices minor laboratory testing, GPs do not tend to adhere to this. More awareness should be raised on the prevalence of bleeding disorders, the impact of HMB on a woman’s life, and the risk for an underlying BD. Both physicians and adolescents should be educated on normal menstruation patterns and the risk for an underlying BD. The current diagnostic pathway for GPs needs to be improved and future studies should focus on finding the best diagnostic pathway to avoid physical harm by setting a wrong diagnosis and under/overtreatment.
Acknowledgments
The authors thank G. Geersing for his input in the design of data collection.
Funding
This study was funded by an unrestricted grant from the Child Health program, Wilhelmina Children’s Hospital, Utrecht, The Netherlands.
Ethics statement
This study was approved by the Medical Ethics Committee of the University Medical Center Utrecht, Utrecht, the Netherlands, on August 6, 2021 (21/553).
Author contributions
S.J. van ‘t K. designed the study protocol, performed data extraction and data analysis, and wrote the first draft of the manuscript. A. de V. checked the data analysis and wrote the final manuscript. K.P.M.vG. designed the study protocol, performed data analysis and critically reviewed the manuscript. L.P., J.vL., N.vR.vK., E.T.E., W.vG., A.H., R.E.G.S., and K.F. critically reviewed the manuscript.
Relationship Disclosure
K.vG. received an unrestricted grant from the Child Health program of the Wilhelmina Children’s Hospital, Utrecht, The Netherlands, to perform this study, and she received an unrestricted research grant from Octapharma for work outside of this study. R.S. has received speaker’s fees and/or research grants from Bayer, CSL Behring, Hemab, Novartis, Novo Nordisk, Octapharma, Roche, Sobi, and Takeda, paid to the institution. K.F. received unrestricted research grants from Baxter/Shire, Sobi/Biogen, Pfizer, and Novo Nordisk; received consultancy fees paid to the institution from Bayer, Baxter/Shire, Sobi/Biogen, CSL Behring, Freeline, Novo Nordisk, Pfizer, and Roche; received speaker’s fee paid to the institution from Bayer, Baxter/Shire, Sobi/Biogen, CSL Behring, Octapharma, Pfizer, and Novo Nordisk; and received travel support from Sobi. S.van ‘tK., A.deV., L.P., J.vL., N.vR.vK., A.H., E.E., and W.vG. have no competing interests to disclose.
Footnotes
Handling Editor: Dr Bethany Samuelson Bannow
Supporting Information
References
- 1.Friberg B., Kristin Örnö A., Lindgren A., Lethagen S. Bleeding disorders among young women: a population-based prevalence study. Acta Obstet Gynecol Scand. 2006;85:200–206. doi: 10.1080/00016340500342912. [DOI] [PubMed] [Google Scholar]
- 2.Nur Azurah A.G., Sanci L., Moore E., Grover S. The quality of life of adolescents with menstrual problems. J Pediatr Adolesc Gynecol. 2013;26:102–108. doi: 10.1016/j.jpag.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Noone D., Skouw-Rasmussen N., Lavin M., van Galen K.P.M., Kadir R.A. Barriers and challenges faced by women with congenital bleeding disorders in Europe: results of a patient survey conducted by the European Hemophilia Consortium. Haemophilia. 2019;25:468–474. doi: 10.1111/hae.13722. [DOI] [PubMed] [Google Scholar]
- 4.Sidonio R.F., Haley K.M., Fallaize D. Impact of diagnosis of von Willebrand disease on patient outcomes: analysis of medical insurance claims data. Haemophilia. 2017;23:743–749. doi: 10.1111/hae.13292. [DOI] [PubMed] [Google Scholar]
- 5.Fraser I.S., Mansour D., Breymann C., Hoffman C., Mezzacasa A., Petraglia F. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey. Int J Gynaecol Obstet. 2015;128:196–200. doi: 10.1016/j.ijgo.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 6.van den Brink M.J., Saaltink A.L., Groenhof F., Kollen B.J., Berger M.Y., Lisman-van Leeuwen Y., et al. Incidence and treatment of heavy menstrual bleeding in general practice. Fam Pract. 2017;34:673–678. doi: 10.1093/fampra/cmx050. [DOI] [PubMed] [Google Scholar]
- 7.James P.D., Connell N.T., Ameer B., Di Paola J., Eikenboom J., Giraud N., et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5:280–300. doi: 10.1182/bloodadvances.2020003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson A.E., Vesely S.K., Koch T., Campbell J., O’Brien S.H. Patterns of von Willebrand disease screening in girls and adolescents with heavy menstrual bleeding. Obstet Gynecol. 2018;131:1121–1129. doi: 10.1097/AOG.0000000000002620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zia A., Jain S., Kouides P., Zhang S., Gao A., Salas N., et al. Bleeding disorders in adolescents with heavy menstrual bleeding in a multicenter prospective US cohort. Haematologica. 2020;105:1969–1976. doi: 10.3324/haematol.2019.225656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivaths L.V., Zhang Q.C., Byams V.R., Dietrich J.E., James A.H., Kouides P.A., et al. Differences in bleeding phenotype and provider interventions in postmenarchal adolescents when compared to adult women with bleeding disorders and heavy menstrual bleeding. Haemophilia. 2018;24:63–69. doi: 10.1111/hae.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivaths L., Minard C.G., O’Brien S.H., Wheeler A.P., Mullins E., Sharma M., et al. The spectrum and severity of bleeding in adolescents with low von Willebrand factor-associated heavy menstrual bleeding. Blood Adv. 2020;4:3209–3216. doi: 10.1182/bloodadvances.2020002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijer L., Bruinsma A., Pameijer A., Hehenkamp W., Janssen C., de Vries C., et al. NHG Standaard ‘Vaginaal Bloedverlies’ Vaginaal bloedverlies | NHG-Richtlijnen. [Webpage] http://richtlijnen.nhg.org/standaarden/vaginaal-bloedverlies [accessed November 11, 2022].
- 13.Smeets H.M., Kortekaas M.F., Rutten F.H., Bots M.L., van der Kraan W., Daggelders G., et al. Routine primary care data for scientific research, quality of care programs and educational purposes: the Julius General Practitioners’ Network (JGPN) BMC Health Serv Res. 2018;18:735. doi: 10.1186/s12913-018-3528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra-Mouli V., Patel S.V. Mapping the knowledge and understanding of menarche, menstrual hygiene and menstrual health among adolescent girls in low- and middle-income countries. Reprod Health. 2017;14:30. doi: 10.1186/s12978-017-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borjigen A., Huang C., Liu M., Lu J., Peng H., Sapkota C., et al. Status and factors of menstrual knowledge, attitudes, behaviors and their correlation with psychological stress in adolescent girls. J Pediatr Adolesc Gynecol. 2019;32:584–589. doi: 10.1016/j.jpag.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Miiro G., Rutakumwa R., Nakiyingi-Miiro J., Nakuya K., Musoke S., Namakula J., et al. Menstrual health and school absenteeism among adolescent girls in Uganda (MENISCUS): a feasibility study. BMC Womens Health. 2018;18:4. doi: 10.1186/s12905-017-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S., Zhang S., Acosta M., Malone K., Kouides P., Zia A. Prospective evaluation of ISTH-BAT as a predictor of bleeding disorder in adolescents presenting with heavy menstrual bleeding in a multidisciplinary hematology clinic. J Thromb Haemost. 2020;18:2542–2550. doi: 10.1111/jth.14997. [DOI] [PubMed] [Google Scholar]
- 18.McLintock C. Women with bleeding disorders: clinical and psychological issues. Haemophilia. 2018;24:22–28. doi: 10.1111/hae.13501. [DOI] [PubMed] [Google Scholar]
- 19.Matteson K.A., Clark M.A. Questioning our questions: do frequently asked questions adequately cover the aspects of women’s lives most affected by abnormal uterine bleeding? Opinions of women with abnormal uterine bleeding participating in focus group discussions. Women Health. 2010;50:195–211. doi: 10.1080/03630241003705037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soppi E.T. Iron deficiency without anemia – a clinical challenge. Clin Case Rep. 2018;6:1082. doi: 10.1002/ccr3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houston B.L., Hurrie D., Graham J., Perija B., Rimmer E., Rabbani R., et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirza F.G., Abdul-Kadir R., Breymann C., Fraser I.S., Taher A. Impact and management of iron deficiency and iron deficiency anemia in women’s health. Expert Rev Hematol. 2018;11:727–736. doi: 10.1080/17474086.2018.1502081. [DOI] [PubMed] [Google Scholar]
- 23.Favaloro E.J. The utility of the PFA-100 in the identification of von Willebrand disease: a concise review. Semin Thromb Hemost. 2006;32:537–545. doi: 10.1055/s-2006-947869. [DOI] [PubMed] [Google Scholar]
- 24.LaVasseur C., Neukam S., Kartika T., Samuelson Bannow B., Shatzel J., DeLoughery T.G. Hormonal therapies and venous thrombosis: considerations for prevention and management. Res Pract Thromb Haemost. 2022;6 doi: 10.1002/rth2.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elbatarny M., Mollah S., Grabell J., Bae S., Deforest M., Tuttle A., et al. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20:831–835. doi: 10.1111/hae.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.