Extracorporeal membrane oxygenation (ECMO) is a temporary mechanical circulatory support system used as rescue therapy in both acute lung injury (venovenous ECMO [VV-ECMO]) and refractory cardiogenic shock (venoarterial ECMO [VA-ECMO]). Its use has significantly increased over the last decade, especially during the COVID-19 pandemic [1]. Although lifesaving, it is associated with a major risk of both thrombotic and hemorrhagic complications. During ECMO, the coagulation cascade is activated by continuous contact between the blood and the procoagulant circuit’s synthetic surface. The Extracorporeal Life Support Organization recommends using unfractionated heparin (UFH) for thromboprophylaxis during ECMO [2]. However, because of its narrow therapeutic window and high inter- and intra-individual variability in response, UFH use is associated with a risk of bleeding [3]. The ECMO circuit itself also increases the risk of bleeding since it results in platelet dysfunction and acquired von Willebrand syndrome [4].
Maintaining the coagulation balance in order to prevent both bleeding and thrombotic events is challenging for clinicians. Hence, monitoring with anticoagulation dose adjustment is crucial but still poorly standardized. Various laboratory tests are available, and no consensus exists on the most appropriate anticoagulation monitoring for patients receiving ECMO therapy. The activated partial thromboplastin time (aPTT) is still widely used because of its availability and clinical familiarity. aPTT is markedly impacted by factors unrelated to heparin, such as the level of coagulation factors, including factors VIII, IX, XI, and XII, the presence of lupus anticoagulant, and inflammation which activates coagulation, especially through the FVIII pathway. Another assay, the activated clotting time, has also been shown to be poorly correlated with UFH dosage [5].
Thus, the most appropriate and commonly used assay for UFH monitoring is the chromogenic anti-Xa assay, which estimates heparin concentration by measuring the residual concentration of exogenous FXa in the sample. Although specific to the action of heparin, the anti-Xa assay does not reflect the whole clot formation process, unlike aPTT and the activated clotting time assay [6].
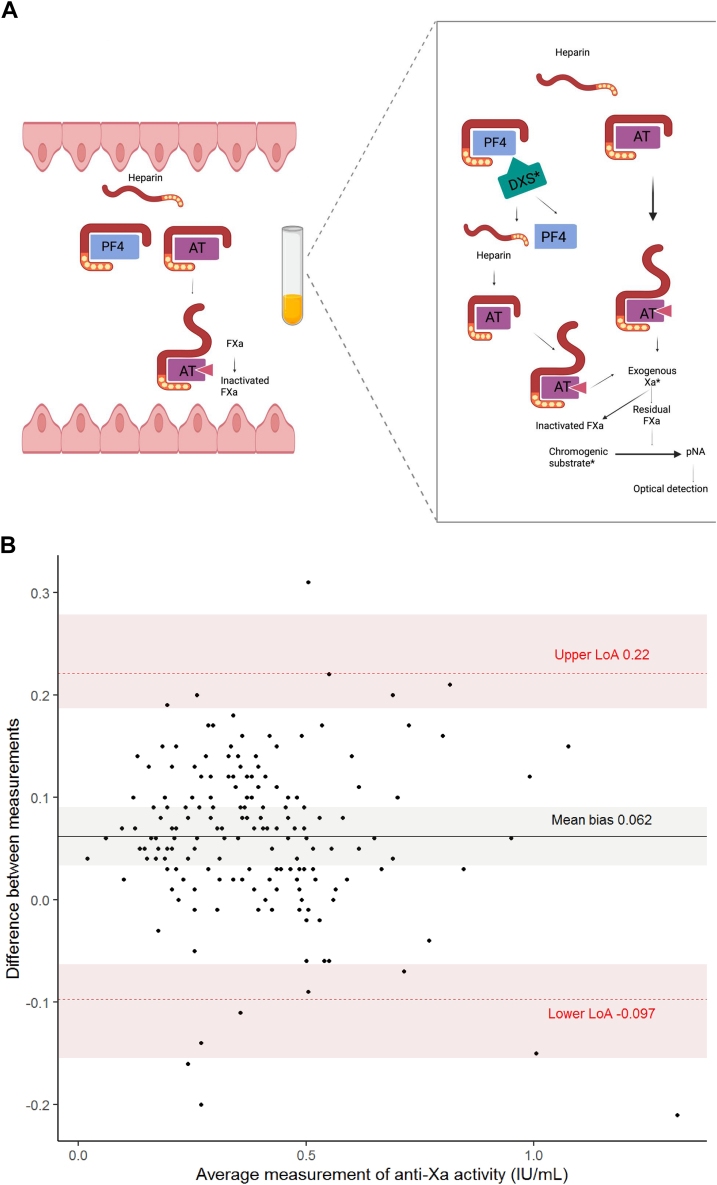
The majority of anti-Xa reagents contain dextran sulfate (DXS), a macromolecule added to prevent heparin from binding to proteins other than antithrombin, such as platelet factor 4 (PF4) in the laboratory test tubes. To exert its anticoagulant effect, UFH binds to antithrombin and enhances its activity 2000-fold. Aside from antithrombin, UFH binds nonspecifically to a wide range of plasma proteins. Among those target proteins is PF4, which binds to and neutralizes heparin with high affinity [3]. In order to achieve its anticoagulant effect, UFH has to be present in sufficient quantities to saturate PF4 and bind to antithrombin (Figure A). Hence, the amount of PF4 and other UFH-binding proteins will significantly impact the anticoagulant activity of UFH in vivo [3]. Adding DXS to an anti-Xa reagent was intended to promote interpatient agreement by releasing heparin bound to these off-target plasma proteins.
Figure.
(A) Impact of dextran sulfate on the chromogenic anti-Xa assay. (B) Bland–Altman plot.
The presence of DXS in the anti-Xa reagent could, however, induce an overestimation of heparin level in vitro that does not reflect the in vivo anticoagulant effect, as bound heparin is actually not totally functional in vivo [7]. There are relatively few studies assessing the impact of DXS on anti-Xa assays, and to the best of our knowledge, there is no published study evaluating this impact during ECMO.
The aim of our study was to compare the anti-Xa activity results measured with 2 reagents with dextran (D-anti-Xa) and without dextran (ND-anti-Xa) during ECMO. The present study was retrospectively conducted between May 2020 and November 2021 in the intensive care unit of Mulhouse General Hospital, France, and was approved by the Ethics Committee of our institution. Patients under VA- and VV-ECMO treated with UFH were monitored with both D-anti-Xa and ND-anti-Xa assays. Blood samples were collected according to the French Group of Hemostasis and Thrombosis recommendations [8]. Anti-Xa assays were performed on the ACL TOP 750 analyzer (Werfen, Instrumentation Laboratory) within 4 hours of collection. The samples were analyzed with 2 anti-Xa reagents, both from Instrumentation Laboratory, with only 1 containing DXS. The reagent without dextran contained exogenous antithrombin. The agreement between the results of anti-Xa assays with and without DXS was evaluated graphically using the Bland and Altman method [9]. The limits of agreement were calculated using the Method of Variance Estimates Recovery method. Lin’s concordance correlation coefficient (CCC) was also calculated. Statistical analysis was performed using R software and the SimplyAgree package.
Thirteen patients were enrolled, and 177 pairs of anti-Xa assays were performed. The median age of the patients was 60.5 years; 10 were men, and 3 were women.
During ECMO, 10 patients experienced bleeding, 3 of which were major. Two patients experienced venous thrombosis, while 2 experienced arterial thrombosis (Table).
Table.
Patient characteristics of the study group.
 |
ARDS, acute respiratory distress syndrome; BMI, body mass index; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; CRA, cardiorespiratory arrest; D-anti-Xa, reagent with dextran sulfate; ECMO, extracorporeal membrane oxygenation; ENT, ear, nose, and throat; IS, immunosuppression; NA, not available; NC, non-collected; ND-anti-Xa, reagent without dextran sulfate; STEMI, ST-elevation myocardial infarction; SOFA, sequential organ failure assessment; VA, venoarterial; VV, venovenous.
aAnti-Xa level: the day the bleeding or thrombotic event occurred.
The therapeutic range assigned for the anti-Xa test assay was 0.2 to 0.5 International Units (IU)/mL [10]. Forty-four discrepant pairs of anti-Xa levels were observed, representing 24.9% (44/177) of the anti-Xa tests.
Discrepant anti-Xa assay results were obtained with the 2 reagents, with and without DXS, in patients on UFH during ECMO with a median value of 0.41 IU/mL (n = 177) for the D-anti-Xa assay vs 0.32 IU/mL for the ND-anti-Xa assay with a mean bias of +0.062 IU/mL (95% CI, 0.034; 0.090; Figure B).
Agreement was poor between the 2 assays since the CCC between the ND-anti-Xa and D-anti-Xa assays was less than 0.90 (CCC = 0.8), and the limit of the Bland–Altman plot ranged from 0.097 (lower limit) to +0.22 (upper limit). However, the concordance coefficient should be interpreted with caution, as it is a measure that can be biased when repeated data are involved. The higher median of anti-Xa assays obtained with the D-anti-Xa reagent could suggest an in vitro overestimation of anticoagulation if tested with this reagent.
For 44 (24.9%) pairs of anti-Xa values, D-anti-Xa and ND-anti-Xa had different ranges. For instance, in 15 samples, D-anti-Xa values were above the upper limit of the therapeutic target reference range of 0.5 IU/mL, while the ND-anti-Xa values were within the target range. In these scenarios, if UFH dosing was monitored only with the D-anti-Xa assay, it could potentially overestimate the anti-Xa level, resulting in an underdosing of UFH and possible thrombosis. Nevertheless, we do not know which dosage method better reflects the in vivo coagulation state.
As PF4 levels are increased during ECMO [11], we can assume a potentially greater overestimation of functional heparin levels with anti-Xa reagents containing DXS in this population group. This hypothesis should be tested by conducting larger comparative studies, including critically ill patients who are not on ECMO under medical care patients.
Although Gehrie et al. [7] proposed a role for DXS in the discrepancy of anti-Xa activity results between different reagents, few additional researchers have investigated the clinical impact of the discrepant anti-Xa results. Smahi et al. [12] concluded that the STAGO reagent without DXS underestimated the anti-Xa activity. The ongoing French study (Evaluation of the Effect of Dextran Sulphate on Anti-Xa Activities Measured) should provide additional information in this regard [13].
The in vivo anticoagulation status could also be overestimated by the presence of exogenous antithrombin in the test reagent designed to improve anti-Xa measurement accuracy in patients with antithrombin deficiency. However, in cases where in vivo antithrombin is sufficient for UFH activity, reagents containing additional antithrombin could potentially overestimate in vivo anticoagulation.
The findings of this study suggest that higher anti-Xa values are obtained with D-anti-Xa compared with ND-anti-Xa reagent, even in the absence of additional antithrombin, which can potentially result in an overestimation of in vivo anticoagulation.
D-anti-Xa values were higher than ND-anti-Xa during bleeding events, but the causative role of heparin in the occurrence of bleeding and thrombosis in the study cohort of patients cannot be confirmed. The small sample size of this retrospective study limits these conclusions. Larger studies should be conducted to explore this potential overestimation of the in vivo anticoagulant heparin effect with DXS-containing reagents. It nevertheless illustrates that the results of anti-Xa should be interpreted cautiously by clinicians in situations with suspected high circulating PF4 levels, eg, during ECMO, and other clinical and biological parameters should be taken into account to evaluate the in vivo coagulation state.
Despite the efforts deployed to standardize anti-Xa assays, there are still various factors influencing the difference between results, and our study emphasizes the impact of one of them, namely the presence of DXS in anti-Xa assays.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or nonprofit sectors.
Author contributions
E.H. collected data and wrote the original draft. L.S. collected data and wrote and reviewed the manuscript. C.P. designed the methodology and was responsible for resources. I.H. conceptualized and supervised the study. All authors read and approved the final version of the paper.
Relationship Disclosure
All authors have no conflict of interest to declare.
Footnotes
Handling Editor: Dr Johnny Mahlangu.
References
- 1.Nunez J.I., Gosling A.F., O’Gara B., Kennedy K.F., Rycus P., Abrams D., et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. 2022;48:213–224. doi: 10.1007/s00134-021-06593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael A.B.V., Ryerson L.M., Ratano D., Fan E., Faraoni D., Annich G.M. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022;68:303–310. doi: 10.1097/MAT.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 3.Derbalah A., Duffull S., Newall F., Moynihan K., Al-Sallami H. Revisiting the pharmacology of unfractionated heparin. Clin Pharmacokinet. 2019;58:1015–1028. doi: 10.1007/s40262-019-00751-7. [DOI] [PubMed] [Google Scholar]
- 4.Doyle A.J., Hunt B.J. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med. 2018;5:352. doi: 10.3389/fmed.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delmas C., Jacquemin A., Vardon-Bounes F., Georges B., Guerrero F., Hernandez N., et al. Anticoagulation monitoring under ECMO support: a comparative study between the activated coagulation time and the anti-Xa activity assay. J Intensive Care Med. 2020;35:679–686. doi: 10.1177/0885066618776937. [DOI] [PubMed] [Google Scholar]
- 6.Hou X. Anticoagulation monitoring in extracorporeal membrane oxygenation. Perfusion. 2021;36:438–439. doi: 10.1177/02676591211024090. [DOI] [PubMed] [Google Scholar]
- 7.Gehrie E., Laposata M. Test of the month: the chromogenic antifactor Xa assay. Am J Hematol. 2012;87:194–196. doi: 10.1002/ajh.22222. [DOI] [PubMed] [Google Scholar]
- 8.Lecompte T., Béguin S., de Maistre E., et al. Normes acceptables d'hémostase : recommandations du groupe d'étude en hémostase et thrombose (GEHT) de la société française de biologie clinique (SFBC) 2014. https://site.geht.org/app/uploads/2016/12/Normes_acceptables_hemostase_GEHT2014.pdf sur.
- 9.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet Lond Engl. 1986;1:307–310. [PubMed] [Google Scholar]
- 10.Levy J.H., Staudinger T., Steiner M.E. How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med. 2022;48:1076–1079. doi: 10.1007/s00134-022-06723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzeffi M., Clark M., Grazioli A., Dugan C., Rector R., Dalton H., et al. Platelet factor-4 concentration in adult veno-arterial ECMO patients. Perfusion. 2021;36:688–693. doi: 10.1177/0267659120965104. [DOI] [PubMed] [Google Scholar]
- 12.Smahi M., De Pooter N., Hollestelle M.J., Toulon P. Monitoring unfractionated heparin therapy: Lack of standardization of anti-Xa activity reagents. J Thromb Haemost. 2020;18:2613–2621. doi: 10.1111/jth.14969. [DOI] [PubMed] [Google Scholar]
- 13.Rennes University Hospital Evaluation of the effect of dextran sulphate on anti-Xa activities measured in patients treated with unfractionated heparin in different indications-DEXHEP. clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT04700670 Report No.: NCT04700670.