Abstract

One of the important factors that determine the photoluminescence (PL) properties of gold nanoclusters pertain to the surface. In this study, four Au52(SR)32 nanoclusters that feature a series of aromatic thiolate ligands (–SR) with different bulkiness at the para-position are synthesized and investigated. The near-infrared (NIR) photoluminescence (peaks at 900–940 nm) quantum yield (QY) is largely enhanced with a decrease in the ligand’s para-bulkiness. Specifically, the Au52(SR)32 capped with the least bulky p-methylbenzenethiolate (p-MBT) exhibits the highest PLQY (18.3% at room temperature in non-degassed dichloromethane), while Au52 with the bulkiest tert-butylbenzenethiolate (TBBT) only gives 3.8%. The large enhancement of QY with fewer methyl groups on the ligands implies a nonradiative decay via the multiphonon process mediated by C–H bonds. Furthermore, single-crystal X-ray diffraction (SCXRD) comparison of Au52(p-MBT)32 and Au52(TBBT)32 reveals that fewer methyl groups at the para-position lead to a stronger interligand π···π stacking on the Au52 core, thus restricting ligand vibrations and rotations. The emission nature is identified to be phosphorescence and thermally activated delayed fluorescence (TADF) based on the PL lifetime, 3O2 quenching, and temperature-dependent PL and absorption studies. The 1O2 generation efficiencies for the four Au52(SR)32 NCs follow the same trend as the observed PL performance. Overall, the highly NIR-luminescent Au52(p-MBT)32 nanocluster and the revealed mechanisms are expected to find future applications.
Introduction
Quantum-sized, atomically precise gold nanoclusters (NCs) exhibit intriguing photoluminescence (PL) owing to their nonmetallic nature and discrete electronic energy levels.1 The small gaps of such NCs (<1.5 eV) give rise to PL in the near-infrared (NIR) region, which holds potential in deep tissue bioimaging, sensing, photonics, and anticounterfeiting applications.1−6 However, highly luminescent Au NCs in the NIR region are still rare because the deactivation of the excited state tends to be dominated by nonradiative relaxation according to the energy gap law, resulting in low quantum yields in the NIR region (QYs of Au NCs often below a few %).7
To understand the origin of the deactivation of excited states in NIR-luminescent Au NCs, structural information is critically important. Crystallographic analysis revealed that thiolate-protected Aun(SR)m NCs typically possess a core–shell structure, in which the inner Au(0) atoms form a stable core and the oligomeric –SR–[Au(I)–SR–]x surface motifs (x = 1, 2, 3, etc.) form the shell.7 By adopting different synthetic methods, the core (or kernel) of Au NCs can be tailored and a rich library of structures such as the tetrahedron, cuboctahedron, and icosahedron has been constructed.8−13 Such diversity provides rich opportunities for tailoring the PL properties.2,14
The QY can be enhanced by increasing the radiative decay (its rate constant kr) and/or decreasing the nonradiative decay (its rate constant knr); note: QY = kr/(kr + knr). Doping is quite effective for enhancing the PL of NCs.15−19 Typically, introducing heterometal atoms into Au NCs does not change the parent structure.15,16 For example, Hirai et al. showed that doping Rh, Pt, and Ir into the Au13 superatom enhanced the QY from 11 to 46, 60, and 66%, respectively, whereas the QY of PdAu12 is comparable to that of Au13.17 The phenomenon was attributed to the larger energy gap of Rh@Au12, Pt@Au12, and Ir@Au12 than Pd@Au12 and Au13.17 Song et al. found that doping a gold atom into a cubic Cu14 cage gave a QY of 71%, much higher than that of the halide-centered Cu14 cage (QY ∼40%), which was attributed to the spin–orbit coupling effect from heavy gold atom.20
Without modifying the energy gap by size control or doping, suppression of nonradiative relaxation for Au NCs can be achieved by several strategies. For example, the body-centered cubic-structured Au38S2(SR)20 features locking gold atoms and the Au4S4 ring in between the units of the core, which gives a 15% QY at 900 nm emission.21 The nonradiative relaxation can be reduced by surface rigidification or the introduction of rigid ligands. Lee et al. obtained highly luminescent Au22(SG)18 by surface rigidification.22 A typical series of rigid ligands are the diphosphines, which show a decrease in structural flexibility with the chain length shortening, e.g., (Ph2)PCH2CH2P(Ph2) vs (Ph2)PCH2P(Ph2); hence, an enhancement of QY for the NCs.16 By changing the dimeric Au-SR motifs to trimeric and monomeric types while keeping the same kernel and ligand, Wu et al. found that the PLQY of face-centered cubic-structured Au28(SR)20 shows a 7-fold increment, which suggests that the staples can change the rigidity of the whole NC and thus suppress nonradiative relaxation.23 Ligands with high electron-donating capability can also enhance the QY by electron density transfer from ligands to the kernel.24 In recent work, Zhong et al. demonstrated that the QY of Au10 NCs can be drastically improved from <0.3 to 59.6% and even up to 90.3% by self-assembling two additional ligands via a layer-by-layer organization.25 It was found that the external noncovalent interactions such as hydrogen bonding and electrostatic forces play important roles in suppressing low-frequency vibrations of the kernel.25 Li et al. observed that the suppression of surface and kernel vibrations can be achieved by embedding Au23(SR)16– NCs in polymer matrices and also by applying cryogenic conditions, which showed a large enhancement in QY from 3 to 40 and 70%, respectively.26
Motivated by these works, we aimed to evaluate the effect of the R group on reducing the nonradiative relaxation for medium-sized Au NCs protected by thiolate (–SR) because such sizes are rarely investigated in PL studies. To this end, we choose the Au52(SR)32 NC protected by aromatic ligands as a target because Au52 is a medium-sized system and the electronic and geometric structures are less affected by the change of substitution group on the –SR ligand and the energy gap of Au52 is in the NIR region7 but is not too small for efficient emission.
In this work, Au52(SR)32 NCs protected by different aromatic thiolate ligands, p-MBT (R = –C6H4–CH3), 4-EBT (R = –C6H4–CH2CH3), IPBT (R = –C6H4–CH(CH3)2), and TBBT (R = –C6H4-tBu), are synthesized and their PL properties are investigated. Interestingly, the QY of Au52(SR)32 at room temperature in dichloromethane solution largely increases from 3.8 to 18.3% by reducing the number of methyl groups on the ligand’s para-position (Au52(TBBT)32 < Au52(IPBT)32 < Au52(4-EBT)32 < Au52(p-MBT)32). This behavior is also observed at the single-particle level by using total internal reflection fluorescence (TIRF) microscopy.27 The higher QY of Au52(p-MBT)32 than those of other Au52 counterparts (14.7% for Au52(4-EBT)32 and 11.1% for Au52(IPBT)32) is rare among the NIR emitters according to the energy gap law.28 We further reveal that the r.t. PL comprises phosphorescence and thermally activated delayed fluorescence. The effect of the ligand on the nonradiative relaxation is investigated by temperature-dependent PL and absorption measurements, facilitated by single-crystal X-ray diffraction (SCXRD) analysis. The observed R-dependent optical behavior suggests that the nonradiative relaxation can be suppressed by two routes: (1) restriction of vibrations and rotations of ligands by forming an ensemble through π···π interactions between the adjacent phenyl groups on the NC and (2) reducing the high-frequency vibrational quenching from the C–H bonds. Moreover, we find that Au52 with a less bulky substituent on the ligand endows a better capability for singlet oxygen (1O2) generation.
Results and Discussions
Synthesis of Four Au52(SR)32 NCs with Different R Groups
The details of the synthesis are described in the Supporting Information. Briefly, the synthesis of Au52(p-MBT)32 was performed under ambient conditions by the reduction of Au(I)-p-MBT polymers by using NaBH4. The crude product obtained after 1 h was further purified by preparative thin-layer chromatography (PTLC), which gave a yield of 7.5% (Au atom basis). A size-focusing step at an elevated temperature (70 °C) was required for the synthesis of Au52(IPBT)32. Initially, the reduction of Au(I)-IPBT polymers generated a polydisperse crude product, which was then subjected to excess IPBT etching at 70 °C for 5 h. Finally, PTLC was used to separate the target NC, which gave a yield of 2.7% (Au atom basis). The direct reduction of Au(I)-4-EBT polymers by NaBH4 could only produce a trace amount of Au52(4-EBT)32; therefore, a ligand-exchange-induced size/structure transformation (LEIST) method was carried out on Au52(PET)32 (a previously reported Au NC with a different kernel and ultraviolet–visible (UV–vis) absorption spectral profile, PET = –SCH2CH2Ph). First, Au52(PET)32 was prepared by the previous method.29 Then, Au52(PET)32 was subjected to etching with excess 4-EBT at 70 °C. The target NC was isolated by PTLC, with a yield of 20.4% (Au52(PET)32 basis). The synthesis of Au52(TBBT)32 followed a previous report30 with slight modifications.
Photophysical Studies of Au52(SR)32 NCs at Ambient Conditions
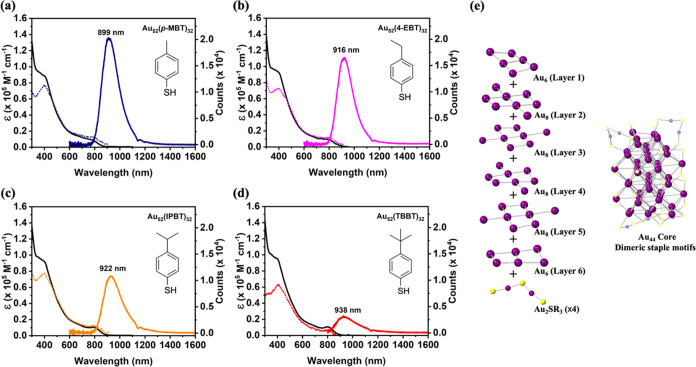
The UV–vis absorption and PL properties of the four Au52(SR)32 NCs in dilute dichloromethane (DCM) solutions were investigated under ambient conditions (Figure 1). For all of the Au52 NCs (Figure 1a–d), the absorption band at ∼400 nm mainly consists of transitions from surface ligands and Au atoms in the staple motifs,30 while the long-wavelength peak at 800 nm (its onset at 890 nm, i.e., HOMO–LUMO gap, Eg) is primarily due to the transitions in the kernels.30,31 The identical spectra indicate that these four NCs should have the same kernel and staple motifs as the previously reported layer-by-layer arrangement for the Au52(TBBT)32,31 which is shown in Figure 1e. The four Au52(SR)32 NCs exhibited a PL peak around 900–938 nm (1.32–1.39 eV) upon photoexcitation at 470 nm (2.64 eV). The agreement between absorption and excitation profiles (Figure 1a–d, dashed lines) indicates that the luminophores are the photoexcited Au52(SR)32 NCs, rather than any impurity, and that the PL originates from the Eg gap in the kernel. As the number of methyl groups in the para-position of the ligand’s phenyl group increases, the Stokes shift (SS) slightly increases from 0.172 to 0.199, 0.216, and 0.231 eV. This monotonic red-shift of the emission peak reflects a slight increment in the electron-donating ability of the substituent and can be ascribed to the ligand-induced change in the core’s electronic structure upon photoexcitation.32 Previous works on Au25(SR)18– and Au22(C≡CR)18 found that the SS increase was associated with the increase of electron-donating ability of the ligands.33−35 Similar ligand-dependent trends on the SS are also found in our current work from the optical spectra of Au52(SR)32 after an exchange with different aromatic ligands: Au52(p-MBT)32-x(4-MOBT)x (SS = 0.220 eV) and Au52(p-MBT)32–x(4-FBT)x (SS = 0.161 eV) (Figures S1 and S2 in the SI), where, 4-MOBT stands for 4-methoxybenzenethiolate and 4-FBT for 4-fluorobenzenethiolate.
Figure 1.
(a–d) Optical absorption spectra (black lines; molar absorption coefficients shown on the left y-axes), PL spectra (solid colored lines; photon numbers on the right y-axis), and normalized excitation spectra (dotted lines; right y-axis) of Au52(SR)32 with different ligands in DCM under ambient conditions. Slit width for PL and excitation measurements: 4 nm. (Note: the dip on the PL spectra at 1150 nm is due to the solvent reabsorption. The PL spectra were collected at 0.1 OD concentration at 470 nm excitation.) (e) Layer-by-layer arrangement of the kernel of Au52(SR)32 and the surface Au2(SR)3 staple motifs. Color code: purple = gold; yellow = sulfur; R groups are not shown for clarity.
The PLQYs for Au52(p-MBT)32, Au52(4-EBT)32, Au52(IPBT)32, and Au52(TBBT)32 in diluted DCM solutions were determined by the absolute method (using an integrating sphere) under ambient conditions and are listed in Table 1. Notably, with fewer methyl groups on the para-position of the ligand, the QY of Au52 shows a monotonic increase and reaches 18.3% for Au52(p-MBT)32. The average PL lifetime, which was determined by the time-correlated single photon counting technique, also exhibits an R-dependent trend (Figure 2a) similar to that of QY. Given the small variation in the PL peak positions and the similar radiative rates (kr ∼ 3 × 105 s–1, see Table 1) among the four Au52(SR)32 NCs, we conclude that the electronic structures in both the ground state and the excited state should not be much affected by the R groups.36 Therefore, the prolonged PL lifetime with fewer substituents on the ligand is indicative of the suppression of the nonradiative relaxation for the excited NCs, which was observed previously in the studies of Ag29 NCs.37−39 The measured ln(knr) values for Au52(SR)32 are between 14 and 16, which are close to the previous studies using nanosecond transient absorption spectroscopy.7,40 The comparison of their radiative rate constant (kr) and nonradiative rate constant (knr) suggests that the enhancement in QY with fewer substituents is mainly associated with the suppression of nonradiative relaxation, rather than a faster radiative relaxation (Figure 2b). It should be noted that introducing fluorine at the para-position significantly decreases the QY, while the methoxy-substituted counterpart has a minor effect on the QY (Figures S1 and S2), suggesting that the electron-rich ligand favors a higher QY, which is similar to the [Au25(SR)18]q NCs (q represents the charge).23 However, the QY is inversely proportional to the electron-donating capability of the ligands for the series Au52(p-MBT)32, Au52(4-EBT)32, Au52(IPBT)32, and Au52(TBBT)32, suggesting that the electronic effect at the para-position is counteracted by a much stronger effect of the ligand, which is identified to be the electron–vibration coupling (vide infra). The peak position and lifetime are barely changed under different solvent conditions and before/after ligand exchange, indicating that charge transfer24,38 is not a dominant factor for their PL properties (Figures S1–S3 in the SI).
Table 1. Photophysical Data for the Four Au52(SR)32 NCs in DCM Solution under Ambient Conditionsa.
Figure 2.
(a) PL decay profiles under ambient conditions in DCM. (b) Plot of radiative decay rate constant (blue symbol) and nonradiative decay rate constant (red symbol) of Au52(SR)32 protected by different ligands. (c) PL spectra of Au52(p-MBT)32 in tetrachloroethene under helium atmosphere and O2 atmosphere, respectively (excitation: 470 nm). (d) The corresponding PL decay profiles for helium and O2 atmospheres.
We further tested the PL sensitivity of Au52(SR)32 (dissolved in tetrachloroethene) to O2 (its ground state is a triplet, 3O2). After the solution was purged with O2, we found that the PL intensity was quenched to 51.4, 64.1, 65.8, and 84.6% of the initial PL intensity for Au52(p-MBT)32, Au52(IPBT)32, and Au52(TBBT)32, respectively (Figures 2c and S4). The O2-saturated solutions exhibited a distinctive emission peak from singlet oxygen (1O2, an excited state of 3O2) at 1274 nm and the average PL lifetime of Au52(SR)32 was slightly decreased (Figure 2d), suggesting that the triplet–singlet energy transfer occurred between Au52(SR)32 and oxygen. The as-confirmed triplet state population of Au52(SR)32 implies that its emission can be phosphorescence and/or thermally activated delayed fluorescence (TADF)41−43 because both types of PL are associated with the population in the triplet excited state (T1). Since the variation in the ligand series is small (i.e., merely the number of CH3 groups), the emission mechanism should be the same for the four Au52(SR)32 NCs. In the tetrachloroethene solution of Au52(p-MBT)32, its average PL lifetime (τave) is 700 ns (component τ1 = 187.0 ns (19.8%) and τ2 = 827.8 ns (80.2%)) under O2 and increases to 807 ns (component τ1 = 196.8 ns (14.8%) and τ2 = 915.4 ns (85.2%)) under the helium atmosphere. The longer lifetime in tetrachloroethene (τave= 700 ns) than that in DCM (τave = 554 ns) indicates a weaker interaction of solvent dipoles with the Au52 excited-state dipole due to the less polar nature of tetrachloroethene and hence a longer PL lifetime.
When the four Au52(SR)32 NCs were each embedded in a poly(methyl methacrylate) (PMMA) film, the QYs (Table 2) for Au52(p-MBT)32, Au52(4-EBT)32, Au52(IPBT)32, and Au52(TBBT)32 were largely increased to 1.85, 3.2, 2.4, and 5.8 times the solution QY, respectively (comparing Tables 1 and 2). Meanwhile, the average PL lifetimes were increased to 808, 768, 423, and 409 ns, respectively (Figure S5). These changes result from the suppression of ligand-induced nonradiative relaxation, which is reflected in the large decrease in knr (Table 2) compared to the solution state.
Table 2. Photophysical Data for Au52(SR)32 NCs Embedded in PMMA Films.
| polymer film | Au52(p-MBT)32 | Au52(4-EBT)32 | Au52(IPBT)32 | Au52(TBBT)32 |
|---|---|---|---|---|
| ΦPL (%) | 33.9 | 32.2 | 26.6 | 22.1 |
| τ ave (ns) | 808 | 768 | 423 | 409 |
| kr (s–1) | 4.2 × 105 | 4.2 × 105 | 2.5 × 105 | 4.5 × 105 |
| knr (s–1) | 8.2 × 105 | 8.8 × 105 | 1.7 × 106 | 1.6 × 106 |
Insights into the PL Mechanism of Au52(SR)32
The four Au52 NCs feature the same core and staple motifs, as indicated by the similar UV–vis absorption profiles and also confirmed by the determined Au52(p-MBT)32 X-ray structure (vide infra). We rationalize that the enhancement in QY for the p-MBT-protected Au52 NC may originate from two mechanisms: (1) the nonradiative relaxation via the coupling between the excited electron and the high-frequency C–H vibrations (∼3000 cm–1)44,45 should be suppressed in Au52(p-MBT)32 compared to other Au52(SR)32 NCs with more −CH3 groups; and (2) the orientation of phenyl rings on Au52 with less bulky ligands (i.e., fewer methyl groups at the para-position) may lead to stronger interligand π···π stacking interaction on the core, which facilitates the suppression of intramolecular vibrational and rotational motions.46,47 For mechanism 1, the photoexcited electron can relax via a multiphonon process (Eg ∼ 1.38 eV requires nearly four phonons of C–H vibrations); the fewer −CH3 groups in Au52(p-MBT)32 reduces the amplitude of the multiphonon decay, hence, a higher QY. For mechanism 2, the relatively large {100} facets of Au52 may result in stronger intracluster ligand π···π interactions, which suppress nonradiative relaxation. To confirm the rationale, we grew single crystals of Au52(p-MBT)32 by the vapor-diffusion of acetonitrile into a concentrated toluene solution of Au52(p-MBT)32. The SCXRD analysis revealed that the core structure of Au52(p-MBT)32 was the same as that of the previously reported Au52(TBBT)32 (see the overlaid cores in Figure 3a and also Figure S6 for a comparison of bond lengths). Despite the same core structure, distinct differences in the packing of surface ligands can be seen; specifically, the average distance between adjacent benzene rings is shorter in Au52(p-MBT)32 than in Au52(TBBT)32 (Figure 3b,c), i.e., 4.794 vs 5.481 Å, hence, more strongly restricting the ligand motions in Au52(p-MBT)32. Therefore, the different orientations of the ligands on Au52 NCs and ligand spacings should be responsible for their QY difference48 in addition to the different amplitudes of multiphonon nonradiative decay. Meanwhile, the trends of PL intensity and lifetime are consistent in the DCM solution and in the solid form (PMMA films), which supports the existence of the interligand π···π stacking interactions in the solution.
Figure 3.
(a) Overlay of Au52(p-MBT)32 and Au52(TBBT)32 SCXRD structures captures the orientation differences of carbon tails. The exceptional differences in four ligands for the two Au52 are shown in bold style. The side views of Au52(p-MBT)32 (b) and Au52(TBBT)32 (c). Color code: purple and magenta = gold; yellow and orange = sulfur; red = carbon on TBBT; light blue = carbon on p-MBT.
Temperature-Dependent PL of Au52(p-MBT)32
To understand the PL nature, temperature-dependent photophysical investigations on PL spectra and lifetimes were further carried out from 298 to 80 K (see Au52(p-MBT)32 in Figure 4a,b and the other three NCs in Figures S7–S9). Of note, in order to form clear “glass” at cryogenic temperatures for PL measurements, 2-methyl-THF was used to dissolve the NCs at room temperature, followed by temperature-dependent measurements. The temperature-dependent kr and knr values for Au52(p-MBT)32 are extracted and plotted in Figure 4c. Upon reduction in temperature, the PL peak became sharper due to the weakening of electron–vibration coupling. Note: the lattice expansion contribution is minor in the NCs.49 The enhancement of PL with decreasing temperature is due to the suppression of nonradiative relaxation, which is indicated by the prolonged lifetime and the drop in the knr value (Figure 4b,c). Interestingly, the PL peak position exhibits an anomaly between 200 and 120 K (nearly a plateau, Figure 4a, inset) rather than the normal blue-shift with decreasing temperature. However, below 120 K, the monotonic blue-shift of the peak position reappears. Similarly, the PLQY first increases and reaches 82% at ∼180 K but then decreases over 160–120 K and finally increases again (Figure 4d, similar for other ligand cases). These abnormal trends were observed in all four Au52(SR)32 NCs and indicate that TADF is involved,42 rather than sole phosphorescence, because, if the phosphorescence is the sole emission, it would monotonically increase and sharpen with decreasing temperature and would also monotonically blue-shift, rather than exhibiting the observed anomalies.
Figure 4.
(a) Temperature-dependent PL spectra of Au52(p-MBT)32 in 2-methyl-THF under a He atmosphere. (b) PL decay profiles at selected temperatures. (c) Plot of radiative decay rate constants (blue symbols) and nonradiative decay rate constants (red symbols) from 80 to 298 K. (d) Temperature-dependent PL quantum yields of Au52(p-MBT vs TBBT, similar for other ligands) in solution (note: “glass” formation at low temperatures). (e) Temperature-dependent emission lifetime of Au52(p-MBT)32 and the fitting of data by eq 2. (f) fwhm of the PL spectra as a function of temperature for Au52(p-MBT)32. The red line is the fitting result shown in eq 3.
The TADF process50,51 is governed by the reverse intersystem crossing (RISC) rate, kRISC, and can be estimated by the Arrhenius equation43,52
| 1 |
where kB is the Boltzmann constant, T is the temperature, and ΔEA is the activation energy to reach the crossing seam between the S1 and T1 potential energy surfaces. Solvent reorganization energy is assumed to be insignificant for ΔEA, so the ΔEA value can be estimated by the singlet–triplet energy separation ΔES-T.53 Below 200 K, the RISC process started to be suppressed in the Au52(SR)32 NCs, and the emission mainly arises from the triplet state (i.e., phosphorescence). As expected for the TADF mechanism, the material should exhibit a small singlet–triplet separation ΔES-T, which is confirmed by fitting the temperature-dependent lifetime with the Boltzmann-type model54 (eq 2) (Figures 4e and S10). The extracted parameters are listed in Table 3. As shown in Table 3, the fitted ΔES-T are 49, 59, 59, and 83 meV for Au52(p-MBT)32, Au52(4-EBT)32, Au52(IPBT)32, and Au52(TBBT)32, respectively. These values are lower in energy than the PL spectral blue-shift (from 899 to 838 nm, or ∼100 meV blue-shift) during the temperature decrease, suggesting that a change in the emission proportion (TADF/phosphorescence) occurred in a narrow temperature range. The temperature-dependent PL line width (full-width at half-maximum, fwhm) of Au52(p-MBT)32 is further extracted and plotted in Figure 4f, which presents a nearly linear relationship to the temperature (from 80 to 280 K), suggesting a weak electron–phonon interaction represented by eq 3,55 in which Γ0 is the PL line width at 0 K, γac and γLO are the coupling coefficients of the excited electron with acoustic phonon (ac) and longitudinal optical (LO) phonon, respectively, and ELO represents the average energy of the LO phonon. Data fitting to eq 3 gives the fitted parameters (Figure 4f, inset). It is worth noting that the coupling of optical phonons with the electron in Au52(p-MBT)32 (γLO = 111 meV) is significantly less than that in the classic Au25(PET)18– NC (γLO = 423 meV), which explains the higher QY of Au52(p-MBT)32 than that of Au25(SR)18– (QY ∼ 1%).54
 |
2 |
| 3 |
Table 3. Results from Fitting of Temperature-Dependent PL Lifetimes for the Four Au52(SR)32 NCs.
| NCs | Au52(p-MBT)32 | Au52(4-EBT)32 | Au52(IPBT)32 | Au52(TBBT)32 |
|---|---|---|---|---|
| ΔES-T, meV | 49 | 59 | 59 | 83 |
| intrinsic τ(S1), ns | 21 | 97 | 78 | 24 |
| intrinsic τ(T1), μs | 11 | 9.5 | 8.9 | 7 |
| QY at 180 K, ΦPL | 82% | 77% | 70% | 66% |
Temperature-Dependent Optical Absorption of Au52 NCs
To further understand the electron–vibration coupling in Au52 NCs, we further carried out temperature-dependent optical absorption measurements on the four Au52 NCs from r.t. down to 80 K. As shown in Figure S11, the absorption spectra of all four Au52 NCs exhibit a distinct blue-shift for the HOMO–LUMO transition (peak ∼1.55 eV at room temperature) and an increase in the oscillator strength with decreasing temperature. Such trends are caused by the suppression of phonon populations at low temperatures and are consistent with the previous observations in Au25 and Au38.49,56 Here, we apply a modified Bose–Einstein single oscillator model (eq 4) that was developed by O’Donnell and Chen to describe the absorption peak dependence on temperature57
| 4 |
In this model, all of the vibrational modes that contribute to the electron–phonon coupling of the specific electronic transition are simplified as a single oscillator,58 i.e., ⟨ℏω⟩ as the average energy of all vibrational modes, ⟨C⟩ represents the electron–phonon coupling strength, and E(0) is the electronic transition gap at 0 K. Since the emissions of the four Au52 NCs follow Kasha’s rule (i.e., PL from the lowest excited state only), we focus on the temperature-dependent evolution of Eg, and the absorption maxima at ∼1.6 eV is used as the value of Eg. The temperature-dependent Eg values of the four Au52 NCs are extracted from Figure S11 and plotted in Figure 5, in which the solid lines represent the fitting by eq 4, and the extracted parameters are given in Table 4.
Figure 5.
Temperature-dependent trends and fitting results of the absorption maxima for the four Au52(SR)32 NCs (indicated in panels (a–d)).
Table 4. Results from Fitting of Temperature-Dependent HOMO–LUMO Transition in the Four Au52(SR)32 NCs (Note: 1 meV = 8 cm–1).
| E(0)/eV | ⟨C⟩ | ⟨ℏω⟩/meV | |
|---|---|---|---|
| Au52(p-MBT)32 | 1.653 | 2.6 | 16 |
| Au52(4-EBT)32 | 1.634 | 2.9 | 19 |
| Au52(IPBT)32 | 1.611 | 3.4 | 26 |
| Au52(TBBT)32 | 1.628 | 3.8 | 32 |
Interestingly, the extracted average energy of vibrational modes that are coupled to the HOMO–LUMO transition presents a gradual decrease as the number of −CH3 groups at the para-position of the ligand decreases. Such a phenomenon indicates lower energy vibrational modes coupled to the HOMO–LUMO transition when the number of methyl groups decreases. The 260 cm–1 (32 meV) vibrational mode in Au52(TBBT)32 suggests that the Au2(TBBT)3 staple motifs are largely involved in the nonradiative energy dissipation, while the 130 cm–1 (16 meV) vibrational mode in Au52(p-MBT)32 indicates that the core vibrations (typically <200 cm–1) are the main contributor to the nonradiative relaxation. Therefore, the gradual decrease of the average energy of vibrational modes from Au52(TBBT)32 to Au52(p-MBT)32, as well as the decreasing coupling strength ⟨C⟩, explains that the suppression of surface vibrations is the main reason for the decrease in nonradiative relaxation. With significantly suppressed surface vibrations, Au52(p-MBT)32 gives rise to the highest QY among the series and is also the highest QY reported for Aun(SR)m in the NIR range, albeit near unity QY in the visible range24 has been reported by Zhong et al.
Singlet Oxygen Generation Capability and Single-Cluster PL
1,3-Diphenylisobenzofuran (DPBF) is a common probe used for sensing 1O2 in an organic solvent, which absorbs strongly at 412 nm. The photosensitized 1O2 readily reacts with DPBF to form 1,2-dibenzoylbenzene (DBB) and bleaches the absorption band at 412 nm (Figure 6a–d).59,60 The time-dependence of the absorption intensity change at 412 nm for the oxygen-saturated DMF solution containing an equal concentration of various Au52(SR)32 (all at 8 μM) and DPBF (10 μM) under irradiation (500 nm) are plotted in Figure 6e. To evaluate the net effect from Au52(SR)32, the blank solution in the absence of Au52(SR)32 (Figure S12) was subtracted from the plots in the presence of Au52(SR)32. For all four Au52(SR)32 NCs, the 412 nm band of DPBF gradually decreased over 120 min. Of note, the absorption band at 800 nm for Au52(SR)32 NCs was not bleached upon irradiation for 120 min, indicating that their structures remained intact during the process. The observed trend for the 1O2 generation rate in Figure 6e is consistent with the QY trend of the four Au52(SR)32 NCs, suggesting that the Au52 protected by less steric ligand has a higher efficiency in generating 1O2 because a less bulky substituent at the para-position allows more efficient interaction between the metal core and 3O2 in the solvent.
Figure 6.
Absorption spectra of DPBF (60 μg/mL) in DMF solutions of Au52(SR)32 NCs (concentration is adjusted to the same optical density at 470 nm): (a) Au52(p-MBT)32, (b) Au52(4-EBT)32, (c) Au52(IPBT)32, and (d) Au52(TBBT)32. (e) Plot of the 412 nm peak intensity change (%) against the irradiation time, where I0 is the initial intensity. (f) Time trajectory study of the four Au52(SR)32 NCs using TIRF.
The four Au52 NCs were further studied at the single-particle level using total internal reflection fluorescence (TIRF) microscopy27 under a 488 nm continuous laser irradiation in air. The order of the single NC’s PL intensity (Figure 6f) is the same as the ensemble (i.e., solution) PL order, indicating that the QYs of Au52(SR)32 from ensemble measurements do not involve any aggregation-induced emission (AIE) effect.61 In addition, the emission was relatively stable on the timescale of the image capture (100’s of ms), which is advantageous for their applications in imaging.
Conclusions
In summary, we have investigated the effect of the para-structure of the –SR ligand on the PL performance of Au52(SR)32 NCs and offered mechanistic insights. By reducing the para-bulkiness of the ligands, the nonradiative pathway can be effectively suppressed, thereby enhancing the PLQY. Among them, Au52(p-MBT)32 shows a PLQY of 18.3% in a non-degassed DCM solvent at room temperature, which is rare for NIR emitters. The present result reveals two factors for suppressing the nonradiative pathways: (1) reduction of the ligand’s motions via π···π stacking interaction between the adjacent phenyl rings and (2) suppression of high-frequency vibrations from the C–H oscillators. This work has also elucidated the PL nature in Au52(SR)32, which involves phosphorescence and TADF; the latter is manifested in the abnormal spectral shift and a zigzag change in PL intensity with decreasing temperature. Finally, the ligand effect on the 1O2 generation efficiency increases with the decrease of the para-bulkiness for the ligands. The obtained mechanisms and highly NIR-luminescent nanoclusters will promote the design of NIR emitters and the development of their applications in photonics, bioimaging, solar energy conversion, and many other fields.
Acknowledgments
R.J. acknowledges the financial support from the NSF (DMR-1808675).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c09846.
Experimental part includes the synthesis, PL analysis, cryogenic optical absorption, PL, and TIRF analysis; PL spectra of Au52(p-MBT)32 in different solvents (PL intensities: Tol.∼DCM > THF > DMF); blank DPBF solution in DMF in the absence of gold nanoclusters under irradiation with λ= 500 nm; cryogenic optical absorption spectra at different temperature; plots of emission lifetime collected at 470 nm excitation against temperature for the cases of 4-EBT, IPBT and TBBT; sample and crystal data for Au52(S-p-MeC6H4)32; X-ray structure (PDF)
Accession Codes
CCDC 2295328 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
§ Y.W., Z.L., and C.G. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Li Y.; Zhou M.; Song Y.; Higaki T.; Wang H.; Jin R. Double-Helical Assembly of Heterodimeric Nanoclusters into Supercrystals. Nature 2021, 594, 380–384. 10.1038/s41586-021-03564-6. [DOI] [PubMed] [Google Scholar]
- Yang G.; Pan X.; Feng W.; Yao Q.; Jiang F.; Du F.; Zhou X.; Xie J.; Yuan X. Engineering Au44 Nanoclusters for NIR-II Luminescence Imaging-Guided Photoactivatable Cancer Immunotherapy. ACS Nano 2023, 17, 15605–15614. 10.1021/acsnano.3c02370. [DOI] [PubMed] [Google Scholar]
- Liu J.; Yu M.; Zhou C.; Yang S.; Ning X.; Zheng J. Passive Tumor Targeting of Renal-Clearable Luminescent Gold Nanoparticles: Long Tumor Retention and Fast Normal Tissue Clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.; Zheng K.; Xie J. Engineering Ultrasmall Water-Soluble Gold and Silver Nanoclusters for Biomedical Applications. Chem. Commun. 2014, 50, 5143–5155. 10.1039/C3CC47512C. [DOI] [PubMed] [Google Scholar]
- Lin C.-A. J.; Lee C.-H.; Hsieh J.-T.; Wang H.-H.; Li J. K.; Shen J.-L.; Chan W.-H.; Yeh H.-I.; Chang W. H. Synthesis of Fluorescent Metallic Nanoclusters toward Biomedical Application: Recent Progress and Present Challenges. J. Med. Biol. Eng. 2009, 29, 276–283. [Google Scholar]
- Zhang Y.; Chu W.; Foroushani A. D.; Wang H.; Li D.; Liu J.; Barrow C. J.; Wang X.; Yang W. New Gold Nanostructures for Sensor Applications: A Review. Materials 2014, 7, 5169–5201. 10.3390/ma7075169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; Higaki T.; Li Y.; Zeng C.; Li Q.; Sfeir M. Y.; Jin R. Three-Stage Evolution from Nonscalable to Scalable Optical Properties of Thiolate-Protected Gold Nanoclusters. J. Am. Chem. Soc. 2019, 141, 19754–19764. 10.1021/jacs.9b09066. [DOI] [PubMed] [Google Scholar]
- Li Y.; Song Y.; Zhang X.; Liu T.; Xu T.; Wang H.; Jiang D.; Jin R. Atomically Precise Au42 Nanorods with Longitudinal Excitons for an Intense Photothermal Effect. J. Am. Chem. Soc. 2022, 144, 12381–12389. 10.1021/jacs.2c03948. [DOI] [PubMed] [Google Scholar]
- Zhuang S.; Chen D.; Ng W.-P.; Liu D.; Liu L.-J.; Sun M.-Y.; Nawaz T.; Wu X.; Zhang Y.; Li Z.; Huang Y.-L.; Yang J.; Yang J.; He J. Phosphinous Acid–Phosphinito Tetra-Icosahedral Au52 Nanoclusters for Electrocatalytic Oxygen Reduction. JACS Au 2022, 2, 2617–2626. 10.1021/jacsau.2c00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.; Gan Z.; Gu W.; You Q.; Zhao Y.; Zha J.; Li J.; Deng H.; Yan N.; Wu Z. Synthesizing Photoluminescent Au28(SCH2Ph-tBu)22 Nanoclusters with Structural Features by Using a Combined Method. Angew. Chem. 2021, 133, 18076–18080. 10.1002/ange.202105530. [DOI] [PubMed] [Google Scholar]
- He L.; Yuan J.; Xia N.; Liao L.; Liu X.; Gan Z.; Wang C.; Yang J.; Wu Z. Kernel Tuning and Nonuniform Influenceon Optical and Electrochemical Gaps of Bimetal Nanoclusters. J. Am. Chem. Soc. 2018, 140, 3487–3490. 10.1021/jacs.7b12083. [DOI] [PubMed] [Google Scholar]
- Tan Y.; Lv Y.; Xu L.; Li Q.; Chai J.; Yang S.; Yu H.; Zhu M. Cd Atom Goes into the Interior of Cluster Induced by Directional Consecutive Assembly of Tetrahedral Units on an Icosahedron Kernel. J. Am. Chem. Soc. 2023, 145, 4238–4245. 10.1021/jacs.2c13075. [DOI] [PubMed] [Google Scholar]
- Lei Z.; Li J.-J.; Nan Z.-A.; Jiang Z.-G.; Wang Q.-M. Cluster from Cluster: A Quantitative Approach to Magic Gold Nanoclusters [Au25(SR)18]−. Angew. Chem., Int. Ed. 2021, 60, 14415–14419. 10.1002/anie.202103290. [DOI] [PubMed] [Google Scholar]
- Li Q.; Zhou D.; Chai J.; So W. Y.; Cai T.; Li M.; Peteanu L. A.; Chen O.; Cotlet M.; Gu W. X.; et al. Structural Distortion and Electron Redistribution in Dual-Emitting Gold Nanoclusters. Nat. Commun. 2020, 11, 2897 10.1038/s41467-020-16686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Meng X.; Das A.; Li T.; Song Y.; Cao T.; Zhu X.; Zhu M.; Jin R. A 200-Fold Quantum Yield Boost in the Photoluminescence of Silver-Doped AgxAu25-x Nanoclusters: The 13th Silver Atom Matters. Angew. Chem., Int. Ed. 2014, 53, 2376–2380. 10.1002/anie.201307480. [DOI] [PubMed] [Google Scholar]
- Takano S.; Hirai H.; Nakashima T.; Iwasa T.; Taketsugu T.; Tsukuda T. Photoluminescence of Doped Superatoms M@Au12 (M= Ru, Rh, Ir) Homoleptically Capped by (Ph2)PCH2P(Ph2): Efficient Room-Temperature Phosphorescence from Ru@Au12. J. Am. Chem. Soc. 2021, 143, 10560–10564. 10.1021/jacs.1c05019. [DOI] [PubMed] [Google Scholar]
- Hirai H.; Takano S.; Nakashima T.; Iwasa T.; Taketsugu T.; Tsukuda T. Doping-Mediated Energy-Level Engineering of M@Au12 Superatoms (M= Pd, Pt, Rh, Ir) for Efficient Photoluminescence and Photocatalysis. Angew. Chem., Int. Ed. 2022, 61, e202207290. [DOI] [PubMed] [Google Scholar]
- Hossain S.; Niihori Y.; Nair L. V.; Kumar B.; Kurashige W.; Negishi Y. Alloy Clusters: Precise Synthesis and Mixing Effects. Acc. Chem. Res. 2018, 51, 3114–3124. 10.1021/acs.accounts.8b00453. [DOI] [PubMed] [Google Scholar]
- Soldan G.; Aljuhani M. A.; Bootharaju M. S.; AbdulHalim L. G.; Parida M. R.; Emwas A. H.; Mohammed O. F.; Bakr O. M. Gold Doping of Silver Nanoclusters: A 26-Fold Enhancement in the Luminescence Quantum Yield. Angew. Chem. 2016, 128, 5843–5847. 10.1002/ange.201600267. [DOI] [PubMed] [Google Scholar]
- Song Y.; Li Y.; Zhou M.; Liu X.; Li H.; Wang H.; Shen Y.; Zhu M.; Jin R. Ultrabright Au@Cu14 Nanoclusters: 71.3% Phosphorescence Quantum Yield in Non-Degassed Solution at Room Temperature. Sci. Adv. 2021, 7, eabd2091 10.1126/sciadv.abd2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Zeman C. J. IV; Schatz G. C.; Gu X. W. Source of Bright Near-Infrared Luminescence in Gold Nanoclusters. ACS Nano 2021, 15, 16095–16105. 10.1021/acsnano.1c04759. [DOI] [PubMed] [Google Scholar]
- Pyo K.; Thanthirige V. D.; Yoon S. Y.; Ramakrishna G.; Lee D. Enhanced Luminescence of Au22(SG)18 Nanoclusters via Rational Surface Engineering. Nanoscale 2016, 8, 20008–20016. 10.1039/C6NR07660B. [DOI] [PubMed] [Google Scholar]
- Xia N.; Yuan J.; Liao L.; Zhang W.; Li J.; Deng H.; Yang J.; Wu Z. Structural Oscillation Revealed in Gold Nanoparticles. J. Am. Chem. Soc. 2020, 142, 12140–12145. 10.1021/jacs.0c02117. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Jin R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. 10.1021/nl101225f. [DOI] [PubMed] [Google Scholar]
- Zhong Y.; Zhang J.; Li T.; Xu W.; Yao Q.; Lu M.; Bai X.; Wu Z.; Xie J.; Zhang Y. Suppression of Kernel Vibrations by Layer-by-Layer Ligand Engineering Boosts Photoluminescence Efficiency of Gold Nanoclusters. Nat. Commun. 2023, 14, 658 10.1038/s41467-023-36387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Zhou M.; So W. Y.; Huang J.; Li M.; Kauffman D. R.; Cotlet M.; Higaki T.; Peteanu L. A.; Shao Z. A Mono-Cuboctahedral Series of Gold Nanoclusters: Photoluminescence Origin, Large Enhancement, Wide Tunability, and Structure–Property Correlation. J. Am. Chem. Soc. 2019, 141, 5314–5325. 10.1021/jacs.8b13558. [DOI] [PubMed] [Google Scholar]
- Hu J.; Zhang C.-y. Simple and Accurate Quantification of Quantum Yield at the Single-Molecule/Particle Level. Anal. Chem. 2013, 85, 2000–2004. 10.1021/ac3036487. [DOI] [PubMed] [Google Scholar]
- Bixon M.; Jortner J.; Cortes J.; Heitele H.; Michel-Beyerle M. Energy Gap Law for Nonradiative and Radiative Charge Transfer in Isolated and in Solvated Supermolecules. J. Phys. Chem. A 1994, 98, 7289–7299. 10.1021/j100081a010. [DOI] [Google Scholar]
- Zhuang S.; Liao L.; Li M.-B.; Yao C.; Zhao Y.; Dong H.; Li J.; Deng H.; Li L.; Wu Z. The FCC Structure Isomerization in Gold Nanoclusters. Nanoscale 2017, 9, 14809–14813. 10.1039/C7NR05239A. [DOI] [PubMed] [Google Scholar]
- Zeng C.; Chen Y.; Liu C.; Nobusada K.; Rosi N. L.; Jin R. Gold Tetrahedra Coil up: Kekulé-like and Double Helical Superstructures. Sci. Adv. 2015, 1, e1500425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C.; Chen Y.; Iida K.; Nobusada K.; Kirschbaum K.; Lambright K. J.; Jin R. Gold Quantum Boxes: On the Periodicities and the Quantum Confinement in the Au28, Au36, Au44, and Au52 Magic Series. J. Am. Chem. Soc. 2016, 138, 3950–3953. 10.1021/jacs.5b12747. [DOI] [PubMed] [Google Scholar]
- Cirri A.; Hernández H. M.; Kmiotek C.; Johnson C. J. Systematically Tuning the Electronic Structure of Gold Nanoclusters through Ligand Derivatization. Angew. Chem., Int. Ed. 2019, 58, 13818–13822. 10.1002/anie.201907586. [DOI] [PubMed] [Google Scholar]
- Weerawardene K. D. M.; Aikens C. M. Theoretical Insights into the Origin of Photoluminescence of Au25(SR)18– Nanoparticles. J. Am. Chem. Soc. 2016, 138, 11202–11210. 10.1021/jacs.6b05293. [DOI] [PubMed] [Google Scholar]
- Ito S.; Takano S.; Tsukuda T. Alkynyl-Protected Au22(C≡CR)18 Clusters Featuring New Interfacial Motifs and R-Dependent Photoluminescence. J. Phys. Chem. Lett. 2019, 10, 6892–6896. 10.1021/acs.jpclett.9b02920. [DOI] [PubMed] [Google Scholar]
- Shibu E. S.; Pradeep T. Photoluminescence and Temperature-Dependent Emission Studies of Au25 Clusters in the Solid State. Int. J. Nanosci. 2009, 08, 223–226. 10.1142/S0219581X09005669. [DOI] [Google Scholar]
- Weerawardene K. D. M.; Aikens C. M. Origin of Photoluminescence of Ag25(SR)18– Nanoparticles: Ligand and Doping Effect. J. Phys. Chem. C 2018, 122, 2440–2447. 10.1021/acs.jpcc.7b11706. [DOI] [Google Scholar]
- Niihori Y.; Takahashi N.; Mitsui M. Photophysical and Thermodynamic Properties of Ag29(BDT)12(TPP)x (x= 0–4) Clusters in Secondary Ligand Binding–Dissociation Equilibria Unraveled by Photoluminescence Analysis. J. Phys. Chem. C 2020, 124, 5880–5886. 10.1021/acs.jpcc.9b11928. [DOI] [Google Scholar]
- Zeng Y.; Havenridge S.; Gharib M.; Baksi A.; Weerawardene K. D. M.; Ziefuß A. R.; Strelow C.; Rehbock C.; Mews A.; Barcikowski S.; et al. Impact of Ligands on Structural and Optical Properties of Ag29 Nanoclusters. J. Am. Chem. Soc. 2021, 143, 9405–9414. 10.1021/jacs.1c01799. [DOI] [PubMed] [Google Scholar]
- Khatun E.; Ghosh A.; Chakraborty P.; Singh P.; Bodiuzzaman M.; Ganesan P.; Nataranjan G.; Ghosh J.; Pal S. K.; Pradeep T. A Thirty-Fold Photoluminescence Enhancement Induced by Secondary Ligands in Monolayer Protected Silver Clusters. Nanoscale 2018, 10, 20033–20042. 10.1039/C8NR05989F. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Zeng C.; Sfeir M. Y.; Cotlet M.; Iida K.; Nobusada K.; Jin R. Evolution of Excited-State Dynamics in Periodic Au28, Au36, Au44, and Au52 Nanoclusters. J. Phys. Chem. Lett. 2017, 8, 4023–4030. 10.1021/acs.jpclett.7b01597. [DOI] [PubMed] [Google Scholar]
- Serevičius T.; Skaisgiris R.; Kreiza G.; Dodonova J.; Kazlauskas K.; Orentas E.; Tumkevicius S.; Jursenas S. TADF Parameters in the Solid State: An Easy Way to Draw Wrong Conclusions. J. Phys. Chem. A 2021, 125, 1637–1641. 10.1021/acs.jpca.0c10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.-S.; Luan X.; Su H.-F.; Li J.-J.; Yuan S.-F.; Lei Z.; Pei Y.; Wang Q.-M. Structure Determination of Alkynyl-Protected Gold Nanocluster Au22(tBuC≡C)18 and Its Thermochromic Luminescence. Angew. Chem., Int. Ed. 2020, 59, 2309–2312. 10.1002/anie.201912984. [DOI] [PubMed] [Google Scholar]
- Berberan-Santos M. N.; Garcia J. M. Unusually Strong Delayed Fluorescence of C70. J. Am. Chem. Soc. 1996, 118, 9391–9394. 10.1021/ja961782s. [DOI] [Google Scholar]
- Sun X.; James T. D.; Anslyn E. V. Arresting “Loose Bolt” Internal Conversion from – B(OH)2 Groups is the Mechanism for Emission Turn-on in Ortho-Aminomethylphenylboronic Acid-Based Saccharide Sensors. J. Am. Chem. Soc. 2018, 140, 2348–2354. 10.1021/jacs.7b12877. [DOI] [PubMed] [Google Scholar]
- Wada A.; Zhang Q.; Yasuda T.; Takasu I.; Enomoto S.; Adachi C. Efficient Luminescence from a Copper (I) Complex Doped in Organic Light-Emitting Diodes by Suppressing C–H Vibrational Quenching. Chem. Commun. 2012, 48, 5340–5342. 10.1039/c2cc31509b. [DOI] [PubMed] [Google Scholar]
- Wang S.; Tang L.; Cai B.; Yin Z.; Li Y.; Xiong L.; Kang X.; Xuan J.; Pei Y.; Zhu M. Ligand Modification of Au25 Nanoclusters for Near-Infrared Photocatalytic Oxidative Functionalization. J. Am. Chem. Soc. 2022, 144, 3787–3792. 10.1021/jacs.2c01570. [DOI] [PubMed] [Google Scholar]
- Schwans J. P.; Sunden F.; Lassila J. K.; Gonzalez A.; Tsai Y.; Herschlag D. Use of Anion–Aromatic Interactions to Position the General Base in the Ketosteroid Isomerase Active Site. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 11308–11313. 10.1073/pnas.1206710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo K.; Chakraborty I. Ligand Effects on the Photoluminescence of Atomically Precise Silver Nanoclusters. Nanoscale 2023, 15, 3120–3129. 10.1039/D2NR06619J. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Li Y.; Kahng E.; Xue S.; Du X.; Li S.; Jin R. Tailoring the Electron–Phonon Interaction in Au25(SR)18 Nanoclusters via Ligand Engineering and Insight into Luminescence. ACS Nano 2022, 16, 18448–18458. 10.1021/acsnano.2c06586. [DOI] [PubMed] [Google Scholar]
- Schinabeck A.; Leitl M. J.; Yersin H. Dinuclear Cu (I) Complex with Combined Bright TADF and Phosphorescence. Zero-Field Splitting and Spin–Lattice Relaxation Effects of the Triplet State. J. Phys. Chem. Lett. 2018, 9, 2848–2856. 10.1021/acs.jpclett.8b00957. [DOI] [PubMed] [Google Scholar]
- Yuan Z.-R.; Wang Z.; Han B.-L.; Zhang C.-K.; Zhang S.-S.; Zhu Z.-Y.; Yu J.-H.; Li T.-D.; Li Y.-Z.; Tung C.-H.; Sun D. Ag22 Nanoclusters with Thermally Activated Delayed Fluorescence Protected by Ag/Cyanurate/Phosphine Metallamacrocyclic Monolayers through In-Situ Ligand Transesterification. Angew. Chem., Int. Ed. 2022, 61, e202211628 10.1002/anie.202211628. [DOI] [PubMed] [Google Scholar]
- Aizawa N.; Harabuchi Y.; Maeda S.; Pu Y.-J. Kinetic Prediction of Reverse Intersystem Crossing in Organic Donor–Acceptor Molecules. Nat. Commun. 2020, 11, 3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H.; Nakanotani H.; Adachi C. Highly Efficient Thermally Activated Delayed Fluorescence with Slow Reverse Intersystem Crossing. Chem. Lett. 2019, 48, 126–129. 10.1246/cl.180813. [DOI] [Google Scholar]
- Deaton J. C.; Switalski S. C.; Kondakov D. Y.; Young R. H.; Pawlik T. D.; Giesen D. J.; Harkins S. B.; Miller A. J.; Mickenberg S. F.; Peters J. C. E-Type Delayed Fluorescence of a Phosphine-Supported Cu2(μ-NAr2)2 Diamond Core: Harvesting Singlet and Triplet Excitons in OLEDs. J. Am. Chem. Soc. 2010, 132, 9499–9508. 10.1021/ja1004575. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Zhou M.; Luo L.; Wang Y.; Kahng E.; Jin R. Elucidating the Near-Infrared Photoluminescence Mechanism of Homometal and Doped M25(SR)18 Nanoclusters. J. Am. Chem. Soc. 2023, 145, 19969–19981. 10.1021/jacs.3c06543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas M. S.; Bairu S.; Qian H.; Sinn E.; Jin R.; Ramakrishna G. Temperature-Dependent Optical Absorption Properties of Monolayer-Protected Au25 and Au38 Clusters. J. Phys. Chem. Lett. 2011, 2, 2752–2758. 10.1021/jz2012897. [DOI] [Google Scholar]
- O’Donnell K.; Chen X. Temperature Dependence of Semiconductor Band Gaps. Appl. Phys. Lett. 1991, 58, 2924–2926. 10.1063/1.104723. [DOI] [Google Scholar]
- Liu Z.; Li Y.; Shin W.; Jin R. Observation of Core Phonon in Electron–Phonon Coupling in Au25 Nanoclusters. J. Phys. Chem. Lett. 2021, 12, 1690–1695. 10.1021/acs.jpclett.1c00050. [DOI] [PubMed] [Google Scholar]
- Kawasaki H.; Kumar S.; Li G.; Zeng C.; Kauffman D. R.; Yoshimoto J.; Iwasaki Y.; Jin R. Generation of Singlet Oxygen by Photoexcited Au25(SR)18 Clusters. Chem. Mater. 2014, 26, 2777–2788. 10.1021/cm500260z. [DOI] [Google Scholar]
- Li Z.; Liu C.; Abroshan H.; Kauffman D. R.; Li G. Au38S2(SAdm)20 Photocatalyst for One-Step Selective Aerobic Oxidations. ACS Catal. 2017, 7, 3368–3374. 10.1021/acscatal.7b00239. [DOI] [Google Scholar]
- Wu Z.; Yao Q.; Zang S.; Xie J. Aggregation-Induced Emission in Luminescent Metal Nanoclusters. Natl. Sci. Rev. 2021, 8, nwaa208 10.1093/nsr/nwaa208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.