Abstract
Background
Anticholinergics are medications that block the action of acetylcholine in the central or peripheral nervous system. Medications with anticholinergic properties are commonly prescribed to older adults. The cumulative anticholinergic effect of all the medications a person takes is referred to as the anticholinergic burden. A high anticholinergic burden may cause cognitive impairment in people who are otherwise cognitively healthy, or cause further cognitive decline in people with pre‐existing cognitive problems. Reducing anticholinergic burden through deprescribing interventions may help to prevent onset of cognitive impairment or slow the rate of cognitive decline.
Objectives
Primary objective
• To assess the efficacy and safety of anticholinergic medication reduction interventions for improving cognitive outcomes in cognitively healthy older adults and older adults with pre‐existing cognitive issues.
Secondary Objectives
• To compare the effectiveness of different types of reduction interventions (e.g. pharmacist‐led versus general practitioner‐led, educational versus audit and feedback) for reducing overall anticholinergic burden. • To establish optimal duration of anticholinergic reduction interventions, sustainability, and lessons learnt for upscaling • To compare results according to differing anticholinergic scales used in medication reduction intervention trials • To assess the efficacy of anticholinergic medication reduction interventions for improving other clinical outcomes, including mortality, quality of life, clinical global impression, physical function, institutionalisation, falls, cardiovascular diseases, and neurobehavioral outcomes.
Search methods
We searched CENTRAL on 22 December 2022, and we searched MEDLINE, Embase, and three other databases from inception to 1 November 2022.
Selection criteria
We included randomised controlled trials (RCTs) of interventions that aimed to reduce anticholinergic burden in older people and that investigated cognitive outcomes.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, and assessed the risk of bias of included studies. The data were not suitable for meta‐analysis, so we summarised them narratively. We used GRADE methods to rate our confidence in the review results.
Main results
We included three trials with a total of 299 participants. All three trials were conducted in a cognitively mixed population (some cognitively healthy participants, some participants with dementia). Outcomes were assessed after one to three months. One trial reported significantly improved performance on the Digit Symbol Substitution Test (DSST) in the intervention group (treatment difference 0.70, 95% confidence interval (CI) 0.11 to 1.30), although there was no difference between the groups in the proportion of participants with reduced anticholinergic burden. Two trials successfully reduced anticholinergic burden in the intervention group. Of these, one reported no significant difference between the intervention versus control in terms of their effect on cognitive performance measured by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) immediate recall (mean between‐group difference 0.54, 95% CI −0.91 to 2.05), CERAD delayed recall (mean between‐group difference −0.23, 95% CI−0.85 to 0.38), CERAD recognition (mean between‐group difference 0.77, 95% CI −0.39 to 1.94), and Mini‐Mental State Examination (mean between‐group difference 0.39, 95% CI −0.96 to 1.75). The other trial reported a significant correlation between anticholinergic burden and a test of working memory after the intervention (which suggested reducing the burden improved performance), but reported no effect on multiple other cognitive measures. In GRADE terms, the results were of very low certainty.
There were no reported between‐group differences for any other clinical outcome we investigated. It was not possible to investigate differences according to type of reduction intervention or type of anticholinergic scale, to measure the sustainability of interventions, or to establish lessons learnt for upscaling. No trials investigated safety outcomes.
Authors' conclusions
There is insufficient evidence to reach any conclusions on the effects of anticholinergic burden reduction interventions on cognitive outcomes in older adults with or without prior cognitive impairment. The evidence from RCTs was of very low certainty so cannot support or refute the hypothesis that actively reducing or stopping prescription of medications with anticholinergic properties can improve cognitive outcomes in older people. There is no evidence from RCTs that anticholinergic burden reduction interventions improve other clinical outcomes such as mortality, quality of life, clinical global impression, physical function, institutionalisation, falls, cardiovascular diseases, or neurobehavioral outcomes. Larger RCTs investigating long‐term outcomes are needed. Future RCTs should also investigate potential benefits of anticholinergic reduction interventions in cognitively healthy populations and cognitively impaired populations separately.
Keywords: Aged, Humans, Alzheimer Disease, Cardiovascular Diseases, Cholinergic Antagonists, Cholinergic Antagonists/adverse effects, Cognitive Dysfunction, Cognitive Dysfunction/prevention & control, Deprescriptions
Plain language summary
Can reducing prescriptions of anticholinergic medicines improve cognitive outcomes in older adults?
Key messages
• It is known that older people who take more medicines with an anticholinergic effect may be at greater risk of cognitive decline. • There is a lack of high‐quality evidence to show whether reducing prescriptions of anticholinergic medicines can preserve or improve cognition. Current evidence is very uncertain and very short‐term. • There is a need for large trials to investigate long‐term effects of reducing anticholinergic burden.
What are anticholinergic medicines?
Medicines can be classified by their ability to block the action of a chemical signalling system in the body called the cholinergic system. A medicine that does this is said to have anticholinergic effects and therefore is referred to as an anticholinergic medicine. Sometimes the anticholinergic effect is important for the way the medicine works, and sometimes it is an unintended side effect. A lot of common medicines have some anticholinergic effect and these can add up. The total anticholinergic effect of all the medicines someone takes is called the anticholinergic burden. An older person taking one strongly anticholinergic medicine or several mildly anticholinergic medicines may have a significant anticholinergic burden.
The cholinergic system in the brain plays an important role in cognition (thinking and remembering). There are concerns that a high anticholinergic burden may unintentionally cause or worsen cognitive problems, even speeding up the development of dementia or worsening the symptoms of people who already have dementia. Guidelines suggest that doctors should review the amount of anticholinergic medication prescribed to older people.
What did we want to find out?
In this review, we wanted to investigate interventions aimed at reducing the anticholinergic medicines prescribed to older adults. We wanted to know if these interventions were better than usual care for improving cognition and reducing diagnoses of dementia in older adults. We also wanted to know if reducing overall anticholinergic burden had any harmful effects.
What did we do?
We searched for studies that evaluated interventions to reduce anticholinergic burden compared with usual care in older adults. For the comparison to be fair, people had to be allocated randomly to the intervention or the usual care group. We included older people who had no cognitive problems and people who did, including those with dementia. We compared and summarised the results of the studies and rated our confidence in their findings, based on factors such as study methods and sizes.
What did we find?
We found three relevant trials that recruited a total of 299 older adults. All three trials included a mixture of people with and without cognitive problems. They were all short trials, measuring cognition just one to three months after the intervention. Only two trials were successful in reducing the overall anticholinergic burden of the intervention group. However, one of these trials reported that people in the intervention group did no better on cognitive tests than people who had usual care, and the other trial found that people in the intervention group had better scores in only one of several cognitive tests. No trials found that interventions to reduce anticholinergic burden led to any other improvements compared to usual care, and no trials investigated how safe the interventions were.
What are the limitations of the evidence?
Our overall confidence in the results is very low. The trials had small numbers of participants, did not study people who already had cognitive problems separately from those who did not, and had mixed success in reducing anticholinergic burden. From the available evidence, we cannot say whether interventions to reduce anticholinergic burden are safe and effective for preserving or improving cognition in older people.
How up to date is this evidence?
We searched for studies published up to 1 November 2022.
Summary of findings
Summary of findings 1. Anticholinergic burden reduction interventions compared to usual care for cognitive outcomes in older adults with and without prior cognitive impairment.
| Anticholinergic burden reduction interventions compared to usual care for cognitive outcomes in older adults with and without prior cognitive impairment | ||||
| Population: older adults with and without prior cognitive impairment Setting: nursing homes and community Intervention: anticholinergic burden reduction Comparison: usual care | ||||
| Outcome | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Cognitive decline Follow‐up: 1 month–3 months |
NA | 299 (3 RCTs)a |
⊕⊝⊝⊝ Very lowb |
Studies were too heterogeneous to pool. They examined only short‐term outcomes, and sample sizes were very small. Reported associations between anticholinergic burden reduction interventions and short‐term cognition were inconsistent: Kersten 2013 reported no significant difference between the intervention versus control in terms of their effect on cognitive performance measured by CERAD immediate recall (mean between‐group difference 0.54, 95% CI −0.91 to 2.05), CERAD delayed recall (mean between‐group difference −0.23, 95% CI−0.85 to 0.38), CERAD recognition (mean between‐group difference 0.77, 95% CI −0.39 to 1.94), and MMSE (mean between‐group difference 0.39, 95% CI −0.96 to 1.75); Tollefson 1991 reported a significant correlation between reduced anticholinergic index scores and improved cognitive scores on a digit span test within the intervention group, but provided no data; and van der Meer 2018 reported a significant improvement in cognition following intervention on the DSST scale only (treatment difference 0.70, 95% CI 0.11 to 1.30), but found no significant difference in reduction of DBI ≥ 0.5 between groups and no reduction in anticholinergic side effects, suggesting that the improvement in DSST was likely related to a concurrently reduced sedative effect. Based on this evidence, we are unable to draw any firm conclusions on the effectiveness of anticholinergic burden reduction interventions for improving cognitive outcomes. |
| Incidence of clinical dementia | NA | 0 (0) | NA | No trials investigated long‐term risk of clinical dementia. |
| Adverse effects | NA | 0 (0) | NA | No trials reported adverse events. |
| CERAD: Consortium to Establish a Registry for Alzheimer's Disease; CI: confidence interval; DBI: Drug Burden Index; DSST: Digit Symbol Substitution Test; MMSE: Mini‐Mental State Examination; NA: not applicable; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aKersten 2013; Tollefson 1991; van der Meer 2018. b Downgraded one level for imprecision (small overall sample size of included trials), one level for indirectness (mixed population types examined within trials, and the intervention in van der Meer 2018 was reducing sedatives and anticholinergics in combination), and one level for inconsistency of results.
Background
Description of the condition
Cognition (or cognitive function) is the mental process of acquiring and manipulating knowledge and understanding through experience, senses, and thought. It includes the domains of memory, language, attention, executive functioning, and visuospatial processing. Cognitive impairment is the disruption of functioning of any one of these domains. It is possible to assess cognitive function in detail using a battery of neuropsychological tests covering multiple domains, although clinicians often use brief assessment tools such as the Mini‐Mental State Examination (MMSE; Folstein 1975) or Montreal Cognitive Assessment (MoCA; Nasreddine 2005).
Dementia is a syndrome of decline in cognitive function beyond that expected from normal ageing and to an extent that interferes with usual functioning. It may affect memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgement. There are a variety of internationally accepted diagnostic criteria for dementia. The most widely used are included in the World Health Organization (WHO) International Classification of Diseases (ICD; WHO 1993), and the American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM). The most recent iteration of the DSM refers to "major neurocognitive disorder" instead of dementia (DSM 5).
The labels 'dementia' or 'major neurocognitive disorder' encompass a variety of pathologies, with specific diagnostic criteria also available for pathologically defined dementia subtypes. These include the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) criteria for dementia due to Alzheimer's disease (McKhann 1984; McKhann 2011), McKeith criteria for Lewy body dementia (McKeith 2005), Lund criteria for frontotemporal dementias (McKhann 2001), and the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) criteria for vascular dementia (Román 1993).
An individual may experience a decline in cognition that does not qualify as dementia but that is greater than would be expected as part of ageing. An objective cognitive impairment that does not significantly impact on daily activities is referred to as a mild cognitive impairment (MCI). This is a risk factor for future dementia, as one in five people with MCI may go on to develop dementia within five years (Petersen 2001).
Dementia is a major public health issue. There are currently more than 40 million people worldwide with dementia due to Alzheimer's disease (the most common subtype), and this number is projected to increase to more than 100 million by 2050 (Prince 2016). As cognitive functioning declines, people's ability to live independently also decreases. This in turn increases caregiver burden, healthcare support requirements, and institutionalisation.
Description of the intervention
Anticholinergics are medications that block the action of acetylcholine in the central or peripheral nervous system. Sometimes the anticholinergic effect is the main mechanism of action of the medication (e.g. treatments for overactive bladder), and sometimes it is an incidental effect that is not thought to be essential for the therapeutic action of the drug (e.g. some antidepressants). Many medications that are commonly prescribed to older adults are anticholinergic to a greater or lesser extent. Observational evidence has shown a consistent association between use of anticholinergic medications and development of cognitive decline or dementia in cognitively healthy older adults (Taylor‐Rowan 2021). Moreover, there is evidence that anticholinergics may increase risk of poor outcomes, such as mortality, in older adults with pre‐existing cognitive problems (Taylor‐Rowan 2022a). The cumulative anticholinergic effect of all the medications a person takes is referred to as the anticholinergic burden. Dementia guidelines now recommend reviewing anticholinergic burden in older adults, and there is increasing interest in interventions that seek to reduce prescriptions of anticholinergic medications to improve cognitive and clinical outcomes in this population (NICE 2023). Anticholinergic reduction interventions seek to reduce a person's anticholinergic burden by deprescribing commonly used anticholinergic medications. Deprescribing can include partial or complete removal of anticholinergic burden, depending on the types of anticholinergic medications a person is taking.
How the intervention might work
Previous studies have demonstrated that anticholinergic burden can be effectively reduced (Nakham 2020). There are a number of different methods to reduce anticholinergic medications, including audit, audit and feedback, education, and expert prescriber approaches (involving people with the skills and knowledge to make decisions on prescriptions, including pharmacists and other non‐medical prescribers). Methods for identifying potentially inappropriate medications also differ between studies: some employ clinical interviews and check medication appropriateness against STOPP (Screening Tool of Older Persons' Prescriptions)/START (Screening Tool to Alert to Right Treatment) criteria, whereas others rely on note‐based medication reviews. Moreover, the type of professional employed to lead the drug reduction intervention typically varies: pharmacists are the most commonly appointed professionals, but some studies have used general practitioners (GPs) or secondary care physicians. (Nakham 2020).
There are various mechanisms by which anticholinergics could interfere with cognitive outcomes. Anticholinergics block the binding of acetylcholine to cholinergic receptors in the brain and the peripheral nervous system. Acetylcholine is a neurotransmitter that plays a major role in numerous functions of the nervous system. In the brain, these include learning and memory. Experts hypothesise that anticholinergic drugs impair short‐ and long‐term cognition by disrupting the cholinergic system, with possible involvement of inflammatory or vascular pathways (Sanghavi 2022; Singh 2013).
There is also evidence to suggest that anticholinergics may increase the risk of specific types of cognitive impairment or dementia. For instance, the cholinergic hypothesis proposes that the pathology and cognitive deterioration seen in Alzheimer's disease may be significantly influenced by a disruption of cholinergic neurotransmission (Francis 1999); hence, prolonged use of anticholinergics may be more likely to induce Alzheimer's disease. Anticholinergics have also been associated with cerebral vascular dysregulation (Marzoughi 2021). This means the risk of vascular dementia may be particularly heightened by the use of anticholinergics.
Anticholinergics may also exacerbate issues in established disease. The current strategy to treat Alzheimer's disease is based on restoration of cholinergic function (Hampel 2018). This is primarily achieved via cholinesterase inhibitors; however, people on cholinesterase inhibitors often take anticholinergics concurrently, which is antagonistic to cholinergic restoration treatments (Carnahan 2004). Moreover, heightened risk of cardiovascular issues such as stroke after use of anticholinergics may indirectly increase rates of cognitive deterioration in people with pre‐existing dementia (Tan 2018).
Evidence from interventions that promote cholinergic function suggests disease‐modifying effects may be possible, including reductions in the degree of long‐term cortical thinning and hippocampal atrophy (Hampel 2018). Reducing the prescription of anticholinergics could therefore reduce the risk of long‐term cognitive problems in older adults, or reduce the rate of cognitive decline in older adults with pre‐existing neurodegenerative diseases.
Measures of anticholinergic burden
A variety of methods exist for measuring anticholinergic burden, and there is no consensus on which measure provides the most accurate and clinically useful information to guide anticholinergic burden reduction. Generally, anticholinergic burden measures assign a score to certain individual medications before a cumulative total based on all prescribed medications is calculated. Although these measures should be similar, overlap is limited because they include differing medications and assign differing scores to these medications. Methodologies for developing scales vary considerably. Where some are designed to measure both central and peripheral anticholinergic effects, others focus on serum radioreceptor anticholinergic activity assay or muscarinic receptor affinity measurements and may only capture peripheral anticholinergic effects. Consequently, variation in the anticholinergic measurement scale used to help reduce anticholinergic medications may lead to differing impacts on clinical outcomes (Hanlon 2020). Therefore, any intervention review should analyse the results for each individual scale as well as creating summary estimates for all anticholinergic burden measures coalesced. Evaluation at the individual scale level can provide clinically applicable information on the ability of respective anticholinergic burden scales to successfully guide reduction of prescribed anticholinergic medications and improve clinical outcomes, while a coalesced estimate will provide greater statistical power and precision.
Why it is important to do this review
This review is intended to serve as a companion to two published Cochrane Prognostic Factor Reviews on anticholinergic burden and risk of cognitive decline or dementia (Taylor‐Rowan 2021; Taylor‐Rowan 2022a). As several studies have reported associations between anticholinergic burden and cognitive decline (Taylor‐Rowan 2021), it follows that interventions aimed at reducing anticholinergic burden may reduce future risk of cognitive decline or dementia. However, it is currently unclear if the anticholinergic properties of these medications are the mechanism behind the apparent association. Our previous reviews highlighted the considerable risk of confounding and prodromal bias that exists within the observational literature. In this review, we aimed to evaluate the interventional evidence for reducing anticholinergic burden and the subsequent impact of these interventions on cognition and other related clinical outcomes.
Objectives
Primary objective
To assess the efficacy and safety of anticholinergic medication reduction interventions for improving cognitive outcomes in cognitively healthy older adults and older adults with pre‐existing cognitive issues
Secondary objectives
To compare the effectiveness of different types of reduction interventions (e.g. pharmacist‐led versus general practitioner‐led, educational versus audit and feedback) for reducing overall anticholinergic burden
To establish optimal duration of anticholinergic reduction interventions, sustainability, and lessons learnt for upscaling
To compare results according to differing anticholinergic scales used in medication reduction intervention trials
To assess the efficacy of anticholinergic medication reduction interventions for improving other clinical outcomes, including mortality, quality of life, clinical global impression, physical function, institutionalisation, falls, cardiovascular diseases, and neurobehavioral outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) in this review. As we are interested in the decline of overall anticholinergic burden, we did not include studies that sought to reduce a single specific anticholinergic drug. We excluded all non‐randomised intervention studies.
Types of participants
We included studies that recruited older adults (with a sample population mean age of 50 years or older). We included studies that recruited cognitively healthy people or people with pre‐existing cognitive problems. We included studies conducted in specific subgroups, such as people with Parkinson's disease, schizophrenia, or stroke, provided the studies met our other inclusion criteria.
We included studies conducted in all healthcare settings. Studies conducted in differing settings (e.g. care home versus primary care) may differ in important population demographics (e.g. mean age, dementia severity, clinical or lifestyle factors) that could alter the strength of the association between anticholinergic burden reduction and cognitive outcomes.
Types of interventions
We included interventions to reduce prescription of anticholinergic medications (to reduce anticholinergic burden). This could involve complete cessation of a certain drug or drugs, or a reduction of dose, frequency, or number of drugs. We imposed no restrictions on the duration of the intervention. We accepted any recognised anticholinergic burden measurement scale.
The control arm of the studies was no intervention intended to reduce anticholinergic medication (treatment as usual).
Types of outcome measures
Primary outcomes
Cognitive decline (i.e. change in cognitive function measured with a validated multi‐domain instrument or neuropsychological test battery or composite derived from scores in two or more cognitive domains). We did not include outcomes of change on a single cognitive domain (e.g. memory only) or delirium outcomes.
Incidence of clinical dementia diagnosed according to DSM or ICD criteria
Adverse effects of anticholinergic deprescribing interventions
Secondary outcomes
Change in anticholinergic burden
Clinical global impression (CGI)
Neuropsychiatric disturbances
Mortality
Functional impairment
Falls
Cardiovascular diseases
Quality of life
Institutionalisation
Proportion of people remaining on reduced anticholinergic medications
We did not run a specific search for our secondary outcomes.
Timing of outcome measurement
We imposed no restrictions on timing of outcome assessments.
Search methods for identification of studies
We adopted a search strategy that combined our topic of interest (anticholinergic burden) with the outcome of interest (cognitive decline or dementia).
Electronic searches
We searched the following databases (Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 12) in the Cochrane Library (searched 22 December 2022)
MEDLINE OvidSP (1946 to 1 November 2022)
Embase OvidSP (1974 to 1 November 2022)
PsycINFO OvidSP (1806 to 1 November 2022)
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1950 to 1 November 2022)
ISI Web of Science Core Collection (1928 to 1 November 2022)
We applied no language restrictions.
Searching other resources
We supplemented our search by checking the reference lists of relevant reviews and studies.
Data collection and analysis
Selection of studies
We used Covidence systematic review software to identify relevant studies. The review group Information Specialist performed a 'first pass' screen to remove clearly irrelevant titles.
Two review authors (MT, AA) independently screened the titles and abstracts of the remaining records, then the full‐text articles of all potentially eligible studies. In cases of disagreement, a third review author (TQ) acted as arbiter and made the final decision on study inclusion/exclusion.
Data extraction and management
Two review authors (MT, AA) independently extracted the data to a piloted form based on the CDPL (Cochrane Developmental, Psychosocial and Learning Problems) RCT‐only template (Appendix 2). We contacted study authors for missing data where required. We selected two studies to trial our data extraction form (Kersten 2012; Van der Meer 2018).
For each outcome of interest, we extracted odds ratios (ORs), hazard ratios (HRs), and standardised mean differences (SMDs), where available.
We also evaluated quality of reporting of interventions in each study using the Template for Intervention Description and Replication (TIDieR) checklist (Hoffmann 2014).
We planned the following comparisons.
Reduction in total anticholinergic burden versus no intervention (treatment as usual)
Cessation of anticholinergic medications versus no intervention (treatment as usual)
Assessment of risk of bias in included studies
We were interested in the effect of assignment to intervention. Two review authors (MT, AA) independently used the Cochrane risk of bias tool (RoB 2) to assess risk of bias of primary outcome results reported within each study across the following domains (Sterne 2019).
Risk of bias arising from the randomisation process
Risk of bias due to deviations from the intended interventions
Risk of bias due to missing outcome data
Risk of bias in measurement of the outcome
Risk of bias in selection of the reported result
We followed RoB 2 signalling questions supported by bespoke anchoring statements for each category to suit our review topic, based on consensus within the review author team (Appendix 3).
We judged each domain as low, some concerns, or high risk of bias. In cases of uncertainty, we contacted study authors for clarification, where possible. Overall risk of bias ratings were based on the highest risk of bias rating reported within a study domain.
Measures of treatment effect
We planned to evaluate dichotomous outcomes, such as incident dementia, by calculating RRs and 95% confidence intervals (CIs).
We anticipated that studies would measure most continuous outcomes on different cognitive scales, so we planned to use SMDs for the estimated effect. We also planned to use mean differences (MDs) to estimate effects for outcomes measured on a single scale. When interpreting the magnitude of SMD effects, we planned to follow guidance set out in the Cochrane Handbook for Systematic Reviews of Interventions (0.2 or less represents a small effect, 0.5 to 0.79 a moderate effect, and 0.8 or greater a large effect; Higgins 2022).
Unit of analysis issues
We anticipated that some eligible studies would use cluster randomisation. However, we identified no cluster‐RCTs.
Dealing with missing data
We contacted study authors to obtain missing data where necessary. We recorded imputation methods used by study authors, wherever these were provided, and considered these as possible subjects for sensitivity analyses.
Assessment of heterogeneity
We considered between‐study heterogeneity in participants, methods, and outcomes when deciding whether meta‐analysis was feasible. We planned to assess statistical heterogeneity with the I2 statistic, using the following rough guide to interpret the results (Higgins 2019).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
Assessment of reporting biases
We assessed intra‐study reporting bias as a part of our risk of bias assessment. We viewed all study protocols and compared planned analyses with reported analyses to identify inconsistencies. There were insufficient relevant studies (fewer than 10) to investigate publication bias by creating funnel plots and visually inspecting for asymmetry. However, where our search identified protocols of unpublished studies, we contacted study authors to determine their status.
Data synthesis
We planned to evaluate comparative risk of cognitive decline or dementia between the intervention and control arms and evaluate intervention success in reducing overall anticholinergic burden. Where possible, we planned to pool summary estimates for intervention effectiveness at the level of individual scales and, as an exploratory analysis, pool summary estimates across all scales. We would have calculated MDs for single‐scale analyses and SMDs for across‐scale analyses involving linear data. We planned to use a fixed‐effect or random‐effects approach depending on the level of heterogeneity between studies, using Comprehensive Meta‐Analysis software to conduct all meta‐analyses (Comprehensive Meta‐Analysis Version 3). However, it was not possible to pool summary estimates of intervention effects. We evaluated comparative risk of cognitive decline or dementia between the intervention and control arms narratively. We also evaluated intervention success in reducing overall anticholinergic burden when interpreting effects of anticholinergic burden reduction interventions on cognitive outcomes.
Subgroup analysis and investigation of heterogeneity
It was not possible to investigate any preplanned subgroup analyses (see Taylor‐Rowan 2022b).
Sensitivity analysis
As meta‐analysis was not possible, we did not perform any pre‐planned sensitivity analyses (see Taylor‐Rowan 2022b).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to evaluate our overall confidence in the results. Two review authors (MT, AA) independently rated the GRADE evidence, and a third review author (TQ) arbitrated any disagreements.
We considered the following domains when assessing the certainty of the evidence.
Risk of bias: we used RoB 2 to evaluate the overall risk of bias of included studies. Our GRADE judgement was based on the overall judgement: when most included studies (more than 50%) had an overall high risk of bias, we considered this a very serious limitation, and we downgraded the certainty of the evidence by two levels.
Inconsistency of results: we downgraded the certainty of the evidence when the effect of anticholinergic reduction interventions on cognition was heterogeneous (i.e. estimates varied across studies with regard to showing beneficial or detrimental effects, and their CIs showed minimal or no overlap); when the P value was low for the test of the null hypothesis that all studies in a meta‐analysis have the same underlying magnitude of effect; if the I2 value was greater than 50%, representing substantial heterogeneity (Higgins 2003).
Indirectness of evidence: we downgraded the certainty of the evidence where the investigation did not fully match with our broader review question. Specifically, if the intervention reduced more than just the anticholinergic burden (such as also reducing sedatives via the Drug Burden Index (DBI) scale) we downgraded for indirectness.
Imprecision of results: we downgraded when there were insufficient numbers to meet the optimal information size in the meta‐analysis (i.e. if the number of participants was less than the number of participants generated by a conventional sample size calculation for a single adequately powered study), or when the CIs did not exclude important benefit or important harm.
Publication bias: we downgraded when there was evidence of publication bias from a funnel plot or if there were registered protocols of unpublished studies that were not still ongoing.
Dose effect: we upgraded evidence when there was evidence that larger reductions in anticholinergic burden were linearly associated with better cognitive scores.
We used GRADEpro software to conduct the GRADE evaluation (GRADEpro GDT). We created a summary of findings table to present the results of the comparison anticholinergic reduction intervention versus usual care for our primary outcomes (cognitive decline, incidence of clinical dementia, and adverse effects).
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The search identified 19,169 citations. After deduplication and an initial screen by the Cochrane Information Specialist, 1864 citations remained. Two review authors (MT and AA) eliminated a further 1830 records during the title and abstract screen. They then reviewed 34 full‐text articles and included three studies (Figure 1).
1.
Flow diagram illustrating the study selection process.
Included studies
We included three multicentre RCTs involving a total of 299 participants (Kersten 2013; Tollefson 1991; van der Meer 2018). Kersten 2013 was conducted in Norway, Tollefson 1991 in the USA, and van der Meer 2018 in the Netherlands. Sample sizes ranged from 34 to 164 older adults. Mean ages ranged from 75 to 84 years, and 68.9% of participants were female.
All included trials aimed to reduce overall anticholinergic burden scores. van der Meer 2018 aimed to reduce DBI scores by 0.5 points or more (Hilmer 2007); this DBI reduction was inclusive of both anticholinergic and sedative medications. Tollefson 1991 aimed to reduce the anticholinergic index score by at least 25%, and Kersten 2013 did not specify a reduction target. All three trials employed a combined substitution or cessation approach to reducing anticholinergic burden. In two trials, pharmacists led anticholinergic burden reduction in collaboration with GPs (Kersten 2013; van der Meer 2018). Tollefson 1991 did not describe the method of implementing the intervention, although it appears to have been conducted by the primary physician. Kersten 2013 used a modified Anticholinergic Drug Scale (ADS) to measure anticholinergic burden (Carnahan 2006), van der Meer 2018 used the DBI scale to measure combined anticholinergic and sedative burden, and Tollefson 1991 used an anticholinergic index. All three trials reported severity of baseline anticholinergic burden. Baseline anticholinergic scores were median ADS of 4 in Kersten 2013, median DBI of 3 in Tollefson 1991, and mean anticholinergic index value of 4.0 to 4.3 in van der Meer 2018. Duration of anticholinergic use prior to the intervention was two weeks in Tollefson 1991 and three or more months in van der Meer 2018. Kersten 2013 did not report duration of anticholinergic use before the intervention. Only Kersten 2013 provided details of the most commonly reduced anticholinergic drug. Duration of follow‐up ranged from one month to three months.
All trials had a mixed population of cognitively impaired and cognitively healthy people, and all excluded people with severe dementia. Kersten 2013 and Tollefson 1991 recruited people in nursing homes, and van der Meer 2018 recruited people in the community. No trial reported the proportion of cognitively healthy participants. Kersten 2013 reported that 31% did not have dementia; no other trial reported proportions with or without dementia. Kersten 2013 reported the severity of pre‐existing dementia, while Tollefson 1991 and van der Meer 2018 reported baseline severity of cognitive impairment for the whole sample. No trials reported the types of dementia in their study population.
Only Kersten 2013 reported comorbidities; Tollefson 1991 measured comorbidities (according to the report) but provided no details. Only van der Meer 2018 reported the baseline number of medications.
Excluded studies
We excluded 29 studies during the full‐text review (Characteristics of excluded studies). Reasons for exclusion were ineligible study design (17 studies), ineligible outcomes (2 studies), ineligible intervention (9 studies), and ineligible population (1 study).
Risk of bias in included studies
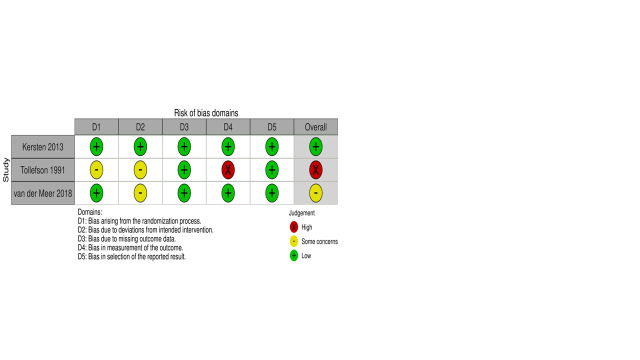
We rated the overall risk of bias for cognitive outcomes as high in Tollefson 1991 and low in Kersten 2013. There were some concerns regarding risk of bias for cognitive outcomes in van der Meer 2018. Figure 2 summarises the results of the risk of bias assessment. Details of each RoB 2 domain rating are listed below. Specific responses to each signalling question can be seen at figshare.
2.
Risk of bias regarding reported effect of assignment to anticholinergic reduction intervention of cognitive outcomes
Risk of bias arising from the randomisation process
All studies had a poor description of how randomisation was achieved and how allocation was concealed. We rated Kersten 2013 and van der Meer 2018 at low risk of bias because allocation concealment did not appear to impact on the reported cognitive outcome. Tollefson 1991 provided insufficient information on how randomisation or concealment of allocation was achieved; we considered there were some concerns, as we could not determine if the reported cognitive outcome was impacted by the randomisation or concealment process.
Bias due to deviations from the intended interventions (effect of assignment to intervention)
All trials were single‐blind as necessitated by the intervention type. No trials employed an intention‐to‐treat approach to analyse the effect of assignment to anticholinergic reduction on cognitive performance. We considered this form of bias was likely to favour the intervention. As Kersten 2013 had non‐significant findings, we judged it at low risk of bias in this domain despite lack of blinding. Tollefson 1991 and van der Meer 2018 found a significant effect of the intervention on cognitive performance; we considered there were some concerns because the reported effect of the intervention on cognitive performance may have differed if assignment to intervention had been analysed.
Missing outcome data
We judged all three trials at low risk of bias for missing outcome data. Tollefson 1991 and van der Meer 2018 reported very minimal loss to follow‐up and missing outcome data. Kersten 2013 had substantial loss‐to follow‐up/missing data, but dropout rates were comparable across groups. Hence, we considered missing data were unlikely to have impacted the reported effects of anticholinergic reduction on cognitive outcomes.
Risk of bias in the measure of the outcome
We rated Tollefson 1991 at high risk of bias because it did not report blinding of assessors conducting cognitive outcome assessments to intervention/control group status, and rater knowledge can influence cognitive assessment scores. The other two trials were at low risk of bias due to adequate blinding of assessors and appropriate methods of outcome measurement.
Risk of bias in the selection of the reported result
We considered all three trials at low risk of bias. Kersten 2013 and van der Meer 2018 demonstrated analyses consistent with preregistered study protocols. Tollefson 1991 had no study protocol, but methodological details were consistent with reported results, so we did not consider the reported outcomes to be selective or influenced by researcher bias.
Effects of interventions
See: Table 1
The trials were too diverse to conduct meta‐analysis for any primary or secondary outcomes, so we described all results narratively.
Primary outcomes
Cognitive decline
All three trials assessed short‐term cognitive change following the interventions. Kersten 2013 conducted cognitive tests at four and eight weeks after baseline, Tollefson 1991 measured cognition after one month, and van der Meer 2018 after three months.
All three trials used multiple cognitive assessment measurement tools to evaluate cognitive changes: Kersten 2013 used the MMSE and the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List; Tollefson 1991 used the Buschke Selective Reminding Test, the Brief Cognitive Rating Scale (BCRS), the Wechsler Memory Scale (WMS), the Letter Cancellation Test (LCT), and the Global Deterioration Scale; and van der Meer 2018 used the Seven‐Minute Screen (7MS), the Trail Making Test (TMT) A & B, and the Digit Symbol Substitution Test (DSST).
van der Meer 2018 reported a significant improvement in cognition on the DSST scale only (treatment difference of 0.70, 95% CI 0.11 to 1.30). However, there was no significant difference between the groups in the proportion of participants with reduction of DBI by 0.5 or more points, and there were no reported reductions in anticholinergic side effects. This discrepancy suggests that the improvement in cognition was likely related to a concurrently reduced sedative effect.
Tollefson 1991 reported a significant correlation between declining anticholinergic burden and improved scores on the WMS forward digit span test, but not the other reported cognitive scales. The study report provided no data for these effects.
Kersten 2013 reported no significant difference between the intervention versus control in terms of their effect on cognitive performance measured by CERAD immediate recall (mean between‐group difference 0.54, 95% CI −0.91 to 2.05), CERAD delayed recall (mean between‐group difference −0.23, 95% CI−0.85 to 0.38), CERAD recognition (mean between‐group difference 0.77, 95% CI −0.39 to 1.94), and MMSE (mean between‐group difference 0.39, 95% CI −0.96 to 1.75).
Incidence of clinical dementia
No trials investigated the long‐term risk of incident dementia.
Adverse effects of anticholinergic deprescribing interventions
No trials investigated adverse effects of anticholinergic deprescribing interventions; however, Tollefson 1991 reported two withdrawals from the intervention group due to participant deterioration following initial drug change.
Certainty of the evidence (GRADE)
The overall certainty of evidence for our primary outcomes was very low. We downgraded the certainty of the evidence for imprecision due to the small overall sample size of included trials, for inconsistency of trial results, and for indirectness due to the cognitively mixed populations in all three trials and the nature of the intervention in van der Meer 2018 (reducing sedatives and anticholinergics in combination).
Secondary outcomes
Change in anticholinergic burden
Kersten 2013 and Tollefson 1991 reported that the intervention achieved a significant reduction in anticholinergic burden relative to the control group. Kersten 2013 reported a decline in mean ADS scores from 4 to 2 in the intervention group versus no change in the control group. Tollefson 1991 reported that 70% of the intervention group achieved a decrease in anticholinergic index values, compared to just 8% of the control group. van der Meer 2018 found no significant difference between the intervention and control groups in reduction of anticholinergic burden, reporting that 17.3% of the intervention group participants achieved the target reduction of DBI compared to 15.9% of the control group participants (OR 1.04, 95% CI 0.47 to 2.64).
Clinical global impression
No trials investigated CGI outcomes.
Neuropsychiatric disturbances
No trials investigated neuropsychiatric disturbance outcomes.
Mortality
van der Meer 2018 assessed the number of participants who died (reported by relatives). One participant died in each group; there was no significant difference in mortality between the groups (1.2% in the intervention group versus 1.3% in the control group; P = 0.732).
Functional impairment
van der Meer 2018 assessed activities of daily living via the Groningen Activity Restriction Scale (GARS) and reported no difference between the intervention and control group in the proportion of participants with the best scores (OR 1.73, 95% CI 0.62 to 4.84). Tollefson 1991 assessed dependency via the Psychogeriatric Dependency Rating Scale (PGDRS) but did not report a significant correlation between anticholinergic burden index scores and PGDRS scores following the intervention.
Falls
van der Meer 2018 assessed risk of falls using the Up and Go test and the total number of fall incidents. There was no significant difference between the intervention and control groups in the proportion of participants with the best Up and Go test results (OR 1.37, 95% CI 0.60 to 3.14) or in the total number of fall incidents (15 participants (19.5%) in the intervention group vs 18 participants (30.5%) in the control group; P = 0.100).
Cardiovascular diseases
No trials investigated cardiovascular disease outcomes.
Quality of life
van der Meer 2018 assessed quality of life via the EuroQol Five‐Dimension Three‐Level questionnaire (EQ‐5D‐3L) and observed no significant difference between the intervention and control groups in the proportion of participants with the best scores (OR 1.43, 95% CI 0.51 to 4.03).
Institutionalisation
van der Meer 2018 assessed unplanned hospital admission via participant self‐report. There was no difference in unplanned hospital admission between the intervention and control groups (9 (11.7%) in the intervention group vs 3 (5.1%) in the control group; P = 0.149).
Proportion of people remaining on reduced anticholinergic medications
No trials investigated the proportion of people who remained on reduced anticholinergic medications.
Secondary objectives
There were insufficient data to explore our other prestated secondary objectives (comparing the effectiveness of different types of reduction interventions, establishing optimal duration of anticholinergic reduction interventions, and comparing results according to differing scales used to assess anticholinergic burden within the trials).
Discussion
Summary of main results
There is no strong evidence from RCTs that reducing anticholinergic burden benefits short‐term cognitive outcomes in either cognitively healthy older adults or in older adults with pre‐existing cognitive impairment. Moreover, while some interventions were successful in reducing anticholinergic burden, the included trials provided no evidence that the reduced burden improved other short‐term clinical outcomes.
No included trials investigated safety outcomes. This is a major oversight due to concomitant risk associated with stopping or changing medication that has been prescribed to treat an existing health condition, and there is evidence from at least one trial that anticholinergic intervention may have provoked adverse health reactions (Tollefson 1991).
Overall completeness and applicability of evidence
All included trials were conducted in a mixed population of cognitively healthy and cognitively impaired people, and two of the three trials were conducted in nursing homes. The mean age in each trial was high (from 75 years to 84 years), and more than two‐thirds of participants were female.
One trial was conducted more than 30 years before our search for this review, and commonly prescribed medications may be very different now; this limits the generalisability of our findings (Tollefson 1991).
The trials examined all cognitive outcomes over a very short time frame, ranging from one to three months. No trials evaluated any long‐term outcomes or performed subgroup analyses to examine possible differences in intervention effectiveness for people with varying degrees of baseline cognitive health or with different types of dementia. Moreover, several relevant clinical outcomes were missing from the analyses, including neuropsychiatric disturbances, global clinical impression, and cardiovascular diseases.
Quality of the evidence
The overall certainty of evidence from existing RCTs is very low. Sample sizes across the three trials were small, results on cognitive outcomes were inconsistent, one trial was at overall high risk of bias, and no trial examined the possibility that existing anticholinergic burden may have a differential impact on cognitive or clinical outcomes depending on the pre‐existing cognitive health of the population. Moreover, one trial reduced sedative medications and anticholinergic medications simultaneously, so could not directly attribute any change in cognitive or clinical outcomes to reduced anticholinergic burden.
Potential biases in the review process
Our review focused on RCTs that assessed cognition. While we evaluated other reported clinical outcomes, we only searched for trials that evaluated cognitive outcomes. Therefore, we may have missed some RCTs that provide evidence for benefits of anticholinergic reduction on clinical outcomes other than cognitive measures.
We were unable to investigate our planned secondary objectives due to lack of data. In particular, we were unable to perform separate analyses for anticholinergic deprescribing benefits/risks in cognitively healthy populations and populations with pre‐existing cognitive difficulties, as we had originally envisioned (see Taylor‐Rowan 2022b). It is possible that anticholinergic deprescribing interventions may have differential benefits/risks in these respective populations, for both long‐ and short‐term cognitive or other clinical outcomes. The mixed populations of the trials may significantly confound study results; hence, the lack of clear benefits/risks of anticholinergic deprescribing interventions reported within the included trials should be interpreted with caution.
Agreements and disagreements with other studies or reviews
Our findings are consistent with previous reviews in certain aspects: in line with Nakham 2020, we found that anticholinergic burden interventions can successfully reduce anticholinergic medication use in older adults; and like Shawaqfeh 2022, our review highlighted a lack of trials investigating anticholinergic reduction RCTs in dementia‐specific populations.
Most trials were conducted in a mixed nursing home population despite minimal evidence from observational studies suggesting an elevated risk of poor outcomes in this population/setting compared to cognitively healthy community‐based populations (Taylor‐Rowan 2021). The longest follow‐up in the included RCTs was one to three months despite substantial observational evidence to suggest anticholinergic effects on long‐term cognitive and other clinical outcomes (Taylor‐Rowan 2021; Taylor‐Rowan 2022a). In addition, no studies employed the Anticholinergic Cognitive Burden (ACB) scale, although it has the largest evidence base for predicting cognitive outcomes (Boustani 2008).
Two ongoing trials appear to be more in line with observational evidence for investigating potential benefits of anticholinergic deprescribing interventions on cognitive outcomes (Characteristics of ongoing studies). Each trial is being conducted exclusively in primary care older adult populations without prior dementia (although one trial is including only people most 'at risk' of dementia) and is investigating longer‐term outcomes at 12 to 24 months, with a combined target sample size of around 1000 participants. The addition of these trials to the evidence base will help to clarify whether anticholinergic deprescribing interventions can benefit cognitive outcomes in people without pre‐existing dementia.
Authors' conclusions
Implications for practice.
There is insufficient evidence from RCTs to support or refute the hypothesis that actively reducing or stopping prescription of medications with anticholinergic properties can improve cognitive outcomes in older people. However, in view of the evidence from observational studies of associations between higher anticholinergic burden and a variety of adverse clinical outcomes, it is appropriate for clinicians to exercise caution when prescribing medications with anticholinergic properties to older adults and to minimise such prescriptions where possible.
Implications for research.
There is a pressing need for RCTs to investigate the effects of reducing anticholinergic burden on long‐term cognitive outcomes in cognitively healthy and cognitively impaired populations. Trials should examine these populations separately, because the intervention may have differential effects on the onset of new cognitive impairment and the progression of existing impairments. It is also crucial to include a formal assessment of safety outcomes and measure the sustainability of long‐term anticholinergic burden reduction.
Standards of design and reporting must improve. Dementia type and severity, relevant baseline covariates, duration of exposure, and types of anticholinergic drugs reduced should be considered in trial design and analysis.
There are ongoing larger trials powered specifically to investigate long‐term cognitive outcomes, but more will be required.
Lastly, future trials should be guided by the extensive observational evidence base to identify appropriate populations who may benefit most from interventions to reduce anticholinergic burden.
History
Protocol first published: Issue 12, 2022
Acknowledgements
We would like to thank peer reviewers Roy Soiza and Edwin Tan and consumer reviewer Cathie Hofstetter for their comments and feedback. We would also like to thank Julia Turner, Cochrane Central Production Service for copyediting and feedback.
Appendices
Appendix 1. Sources and search strategy
| Source | Search strategy |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE (OvidSP) from 1946 |
1. cholinergic antag*.ti,ab. 2. anticholinergic*.ti,ab. 3. anti‐cholinergic*.ti,ab. 4. cholinergic Antagonists/tu 5. Cholinergic Antagonists/ae 6. AAS.ti,ab. 7. ACB.ti,ab. 8. ADS.ti,ab. 9. DAPs.ti,ab. 10. ARS.ti,ab. 11. DBI‐ACh.ti,ab. 12. SAMS.ti,ab. 13. ("chew* score" or "chew* list").ti,ab. 14. ("han's score" or "han score").ti,ab. 15. or/1‐14 16. Cognition/ 17. Cognition Disorders/ 18. Dementia/ 19. cognit*.ti,ab. 20. dement*.ti,ab. 21. alzheimer*.ti,ab. 22. "lewy bod*".ti,ab. 23. FTLD.ti,ab. 24. PDD.ti,ab. 25. "executive function*".ti,ab. 26. Attention/ 27. (speed adj2 processing).ti,ab. 28. memory.ti,ab. 29. Memory Disorders/ 30. "episodic memory".ti,ab. 31. Memory, Episodic/ 32. MCI.ti,ab. 33. Mild Cognitive Impairment/ 34. (nMCI or aMCI or mMCI or MCIa).ti,ab. 35. AAMI.ti,ab. 36. ACMI.ti,ab. 37. ARCD.ti,ab. 38. CIND.ti,ab. 39. VCI.ti,ab. 40. VAD.ti,ab. 41. major neurocognitive disorder*.ti,ab. 42. minor neurocognitive disorder*.ti,ab. 43. neurocognitive dysfunction.ti,ab. 44. Neurocognitive Disorders/ 45. or/16‐44 46. 15 and 45 |
| Embase (OvidSP) from 1974 | 1. cholinergic antag*.ti,ab. 2. anticholinergic*.ti,ab. 3. anti‐cholinergic*.ti,ab. 4. *cholinergic receptor blocking agent/ 5. AAS.ti,ab. 6. ACB.ti,ab. 7. ADS.ti,ab. 8. DAPs.ti,ab. 9. ARS.ti,ab. 10. DBI‐ACh.ti,ab. 11. SAMS.ti,ab. 12. ("chew* score" or "chew* list").ti,ab. 13. ("han’s score" or "han score").ti,ab. 14. or/1‐13 15. Cognition/ 16. Cognition Disorders/ 17. Dementia/ 18. cognit*.ti,ab. 19. dement*.ti,ab. 20. alzheimer*.ti,ab. 21. "lewy bod*".ti,ab. 22. FTLD.ti,ab. 23. PDD.ti,ab. 24. "executive function*".ti,ab. 25. Attention/ 26. (speed adj2 processing).ti,ab. 27. memory.ti,ab. 28. Memory Disorders/ 29. "episodic memory".ti,ab. 30. Memory, Episodic/ 31. MCI.ti,ab. 31. MCI.ti,ab. 32. Mild Cognitive Impairment/ 33. (nMCI or aMCI or mMCI or MCIa).ti,ab. 34. AAMI.ti,ab. 35. ACMI.ti,ab. 36. ARCD.ti,ab. 37. CIND.ti,ab. 38. VCI.ti,ab. 39. VAD.ti,ab. 40. major neurocognitive disorder*.ti,ab. 41. minor neurocognitive disorder*.ti,ab. 42. neurocognitive dysfunction.ti,ab. 43. Neurocognitive Disorders/ 44. or/15‐43 45. 14 and 44 |
| PsycINFO (OvidSP) from 1806 | 1. cholinergic antag*.ti,ab. 2. anticholinergic*.ti,ab. 3. anti‐cholinergic*.ti,ab. 4. exp Cholinergic Receptors/ 5. AAS.ti,ab. 6. ACB.ti,ab. 7. ADS.ti,ab. 8. DAPs.ti,ab. 9. ARS.ti,ab. 10. DBI‐ACh.ti,ab. 11. SAMS.ti,ab. 12. ("chew* score" or "chew* list").ti,ab. 13. ("han's score" or "han score").ti,ab. 14. or/1‐13 15. exp Cognition/ 16. exp Dementia/ 17. cognit*.ti,ab. 18. dement*.ti,ab. 19. alzheimer*.ti,ab. 20. "lewy bod*".ti,ab. 21. FTLD.ti,ab. 22. PDD.ti,ab. 23. "executive function*".ti,ab. 24. exp Attention/ 25. (speed adj2 processing).ti,ab. 26. memory.ti,ab. 27. exp Memory Disorders/ 28. "episodic memory".ti,ab. 29. exp Episodic Memory/ 30. exp Cognitive Impairment/ 31. MCI.ti,ab. 32. exp Cognitive Assessment/ 33. (nMCI or aMCI or mMCI or MCIa).ti,ab. 34. AAMI.ti,ab. 35. ACMI.ti,ab. 36. ARCD.ti,ab. 37. CIND.ti,ab. 38. VCI.ti,ab. 39. VAD.ti,ab. 40. major neurocognitive disorder*.ti,ab. 41. minor neurocognitive disorder*.ti,ab. 42. neurocognitive dysfunction.ti,ab. 43. exp Neurocognitive Disorders/ 44. or/15‐43 45. 14 and 44 |
| CINAHL (EBSCOhost) | S1 TX cholinergic antag* S2 TX anticholinergic* S3 TX anti‐cholinergic* S4 (MH "Cholinergic Antagonists+") S5 TX AAS S6 TX ACB S7 TX ADS S8 TX DAPs S9 TX ARS S10 TX DBI‐ACh S11 TX SAMS S12 TX "chew* score" or "chew* list" S13 TX "han's score" or "han score" S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15 (MH "Cognition+") S16 (MH "Cognition Disorders+") S17 (MH "Dementia+") S18 TX cognit* S19 TX dement* S20 TX alzheimer* S21 TX "lewy bod*" S22 TX FTLD S23 TX PDD S24 TX "executive function*" S25 (MH "Attention") S26 TX speed AND processing S27 TX memory S28 (MH "Memory Disorders") S29 TX "episodic memory" S30 (MH "Memory Disorders") OR (MH "Memory") S31 TX MCI S32 "Mild Cognitive Impairment" S33 TX nMCI or aMCI or mMCI or MCIa S34 TX AAMI S35 TX ACMI S36 TX ARCD S37 TX CIND S38 TX VCI S39 TX VAD S40 TX major neurocognitive disorder* S41 TX minor neurocognitive disorder* S42 TX neurocognitive dysfunction S43 "Neurocognitive Disorders" S44 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 S45 S14 AND S44 |
| Web of Science core collection | TOPIC: ("cholinergic antag*" OR anticholinergic* OR "anti‐cholinergic*" OR AAS OR ACB OR ADS OR DAPs OR ARS OR "DBI‐ACh" OR SAMS OR "chew* score" OR "chew* list" OR "hands score" OR "hans score" OR "han score") AND TOPIC: (cognit* OR dement* OR alzheimer* OR "lewy bod*" OR FTLD OR PDD OR "executive function*" OR attention OR memory OR MCI OR "major neurocognitive disorder*" OR "minor neurocognitive disorder*") Timespan: All years. Indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC. |
Appendix 2. Completed data extraction forms
| Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial (Kersten 2013) | Description | Location in text |
| Population description (from which study participants are drawn; Age restricted?) |
Long‐term nursing home residents with ADS score of 3 or greater. | |
| Setting (care home, community etc) | 22 Nursing homes in Norway | |
| Inclusion criteria | Long‐term nursing home residents with an ADS score ≥ 3. For patients with reduced capacity to assent, their closest relative provided assumed consent. | |
| Exclusion criteria | People with blindness, deafness, delirium, or severe dementia | |
| Sample size | 101 | |
| Method of recruitment of participants (e.g. phone, mail, clinic patients) | Recruited within nursing homes. A local caregiver was consulted regarding the patients’ physical and mental eligibility to participate in the study, before inclusion. | |
| Type of intervention (education versus audit and feedback, etc.; who led? i.e. GP versus pharmacist, etc.) | Pharmacist initiated reduction of anticholinergic drugs. | |
| Details of intervention (e.g. scale used to identify drugs; degree of decline in anticholinergic burden; duration of intervention; specific drugs reduced; mode of delivery) | Modified ADS scale (some drugs were modified according to Chew list). "The intervention was based on a multidisciplinary drug review within 3 days after the baseline assessment. For patients randomized to the intervention group, the clinical pharmacist performed drug reviews guided by the ADS score model to advice the respective nursing home physician whether to discontinue or replace an anticholinergic drug with a drug alternative with less or no AA. When drug alternatives were unavailable, reduction in dosage was attempted to reduce the anticholinergic burden, but dose reductions did not affect the patients’ overall ADS score" |
|
| Duration of anticholinergic drug use before intervention (if reported) | Not reported | |
| Informed consent obtained | Yes, or proxy consent. | |
| Total no. randomised (or total pop. at start of study for non‐RCTs) | 101 (complete case analysis = 67 total) |
|
| Clusters (if applicable, no., type, no. people per cluster) | "To account for possible differences in drug prescription practices between the centers, we conducted a stratified randomization where each nursing home represented one stratum. An independent research coordinator randomly allocated eligible patients within each stratum in a 1:1 manner to control or intervention group. The size of the strata varied from 2 to 15 patients with a median of 4." | |
| Degree of cross‐over (i.e. change from control to intervention group despite allocation) |
None reported (though doesn't appear to have been specifically measured) | |
| Baseline imbalances | % without dementia appears slightly imbalanced: Intervention no dementia = 40.4% Control no dementia = 20% Intervention mild dementia = 36.2% Control mild dementia = 47.5% Intervention moderate dementia = 23.4% Control moderate dementia = 32.5% |
|
| Duration of follow‐up | 8 weeks | |
| Withdrawals and exclusions (if not provided below by outcome) | 33 lost to follow‐up | |
| Age | 85 | |
| Sex | 75%–83% women | |
| Race/ethnicity | Not reported | |
| Severity of illness |
No dementia
Intervention = 40%; control = 20% Mild dementia Intervention = 36%; control = 47.5% Moderate dementia Intervention = 23.4% control = 32.5% |
|
| Co‐morbidities | Median Charlson comorbidity score = 3 intervention; 4 control. | |
| Other relevant socio demographics | Median ADS score of 4 at baseline. Most frequently used anticholinergic drug was furosemide. Hydroxyzine (antihistamine) most commonly used drug with ADS score of 3 |
|
| Subgroups measured | NA | |
| Subgroups reported | NA | |
| Outcome measure(s) |
Cognitive outcome The primary outcome was the patients' immediate free recall of words, from the Consortium to Establish a Registry for Alzheimer’s Disease 10‐wordlist. Delayed recall and recognition was secondary outcome. Global MMSE was additional outcome measure. Safety outcome None reported. Size of reduced burden reported ADS score reduced from 4 to 2 in intervention group; unchanged in control group. No other relevant outcomes measured. |
|
| Outcome data (N event/N total for intervention and control; OR/HR, etc.) | See Table 3. | |
| Notes: | ||
| The relationship of serum anticholinergic activity to mental status performance in an elderly nursing home population (Tollefson 1991) | Description | Location in text |
| Population description (from which study participants are drawn; Age restricted?) |
Age ≥ 65 years High proportion of population had psychiatric disturbances: Psychiatric diagnoses by SCID were as follows: schizophrenia, n = 13; major affective, n = 10; no psychiatric diagnosis, n = 9; substance abuse (inactive), n = 1; generalized anxiety, n = 1. |
|
| Setting (care home, community etc) | Nursing home residents (country not directly stated but probably USA) |
|
| Inclusion criteria | Nursing home residents aged ≥ 65 years who had received ≥ 1 anticholinergic drug for the previous 2 weeks. | |
| Exclusion criteria | Exclusionary criteria: 1) acute medical or psychiatric illness with potential short‐term reversible effects on cognition, e.g. pneumonia; 2) PRN‐scheduled drugs; 3) presence of non‐anticholinergic drugs that might also adversely affect cognition, e.g., digoxin, opioids, benzodiazepines, and centrally active adrenergic blockers; 4) dementia or delirium with a Global Deterioration Scale Score > 6 (the patients were excluded to facilitate demonstration of an intervention effect and to ensure ability to complete tests); 5) visual acuity, hearing, comprehension, or motor problems severe enough to prevent the study subject from completing psychometric tests; or 6) inability of patients to provide informed consent | |
| Sample size | 34 | |
| Method of recruitment of participants (e.g. phone, mail, clinic patients) | Recruited within nursing home | |
| Type of intervention (education versus audit and feedback, etc.; who led? i.e. GP versus pharmacist, etc.) | Medication review (computerised record review). Not clear who led—seems like primary physician led in response to computerised indication for change) | |
| Details of intervention (e.g. scale used to identify drugs; degree of decline in anticholinergic burden; duration of intervention; specific drugs reduced; mode of delivery) | Estimated anticholinergic index used to measure burden. Intervention group subjects were required to have a medication change made (in cooperation with the patients' primary physician) to reduce their calculated anticholinergic index by at least 25% (atropine equivalents) from baseline. Where possible, the anticholinergic agent(s) was discontinued; if not feasible, a less‐anticholinergic drug was substituted. |
|
| Duration of anticholinergic drug use before intervention (if reported) | 2 weeks. | |
| Informed consent obtained | Yes | |
| Total no. randomised (or total pop. at start of study for non‐RCTs) | 34 | |
| Clusters (if applicable, no., type, no. people per cluster) | NA | |
| Degree of cross‐over (i.e. change from control to intervention group despite allocation) |
Not reported. Extent of decline in control group vs intervention group suggests minimal to none. | |
| Baseline imbalances | Not described, but "There were no significant differences in education or other demographics between the two study groups." | |
| Duration of follow‐up | 1 month | |
| Withdrawals and exclusions (if not provided below by outcome) | None stated. | |
| Age | Mean = 79 ± 9.7 | |
| Sex | 26 female; 8 male | |
| Race/ethnicity | Not given | |
| Severity of illness | Not given | |
| Co‐morbidities | Measured but details not given | |
| Other relevant socio demographics | Details not given | |
| Subgroups measured | NA | |
| Subgroups reported | NA | |
| Outcome measure(s) |
Change in anticholinergic burden outcome
Anticholinergic index values Cognitive outcomes The Buschke Selective Reminding Test, Mini‐Mental State Examination (MMSE), Brief Cognitive Rating Scale (BCRS), Wechsler Memory Scale (WMS) (Forms I and II), Letter Cancellation Test (LCT), Psychogeriatric Dependency Rating Scale (PGDRS), Global Deterioration Scale, Saskatoon Delirium Checklist (SDC), and the Symptom, Sign, Side Effect Checklist (SSSE) (NIMH Treatment of Depression Collaborative Research Protocol). Safety outcomes Not measured, but "two patients' physicians declined participation because of patient deterioration after initial drug change)." |
|
| Outcome data (N event/N total for intervention and control; OR/HR, etc.) | See Table 3 | |
| Notes: | ||
| Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist‐led medication review: a randomised controlled trial (van der Meer 2018) | Description | Location in text |
| Population description (from which study participants are drawn; Age restricted?) |
Patients aged ≥ 65 years, living independently, using ≥ 5 medications for ≥ 3 months, including ≥ 1 psycholeptic or psychoanaleptic medication (Anatomic Therapeutic Classification (ATC) code N05 or N06) and with a DBI ≥ 1 | |
| Setting (care home, community etc) | Community (Netherlands) | |
| Inclusion criteria | Community‐based patients who were aged ≥ 65 years, living independently, using ≥ 5 medications for ≥ 3 months, including ≥ 1 psycholeptic or psychoanaleptic medication (Anatomic Therapeutic Classification (ATC) code N05 or N06) and with a DBI ≥ 1 | |
| Exclusion criteria | Exclusion criteria were limited. Life expectancy (< 3 months), non‐Dutch language speaker or advanced dementia. Patients who had received a medication review within the past 9 months before the study period and patients who needed a medication review urgently were also excluded. |
|
| Sample size | 164 | |
| Method of recruitment of participants (e.g. phone, mail, clinic patients) | Patients approached by pharmacist (within pharmacy) | |
| Type of intervention (education versus audit and feedback, etc.; who led? i.e. GP versus pharmacist, etc.) | Pharmacist led (in communication with GP). Medication review. Medication review consisted of 5 steps. Step 1 was a face‐to‐face consultation between the pharmacist and patient to discuss medication use. Second, the pharmacist undertook a pharmacotherapeutic medication review, identified potential pharmacotherapeutic problems taking into account the patient's medical records, including latest recorded episodes and lab values. Accordingly, the pharmacist drafted written recommendations for medication optimisation to discuss with the patients' GP. Third, a multidisciplinary meeting between pharmacist and GP was held. At this meeting, the potential medication problems of the patient were discussed and draft of a pharmacotherapeutic action plan was decided. Fourth is a discussion of the draft pharmacotherapeutic action plan between patient and pharmacist and/or GP. The patients' expectations and wishes were key elements in the decision‐making process and were included in the final action plan. Fifth, a follow‐up of the final pharmacotherapeutic action plan was undertaken. |
|
| Details of intervention (e.g. scale used to identify drugs; degree of decline in anticholinergic burden; duration of intervention; specific drugs reduced; mode of delivery) | DBI scale used to measure; aimed to achieve 0.5‐point reduction on DBI. Mean DBI score at baseline = 3 for both groups |
|
| Duration of anticholinergic drug use before intervention (if reported) | 3 months or more | |
| Informed consent obtained | Yes | |
| Total no. randomised (or total pop. at start of study for non‐RCTs) | 164 (80 in intervention; 84 in control) | |
| Clusters (if applicable, no., type, no. people per cluster) | In each pharmacy, patients willing to participate were then matched in pairs by gender, age, DBI, and number of medications. | |
| Degree of cross‐over (i.e. change from control to intervention group despite allocation) |
Not reported but is a possibility based on the design of the study (as stated in the paper) | |
| Baseline imbalances | Participants in the control arm used slightly more medicines at baseline (9.3 (SD 3.2) to 8.4 (SD 2.4)), and more control patients were living with a partner (53.6%–44%). | |
| Duration of follow‐up | 3 months | |
| Withdrawals and exclusions (if not provided below by outcome) | Larger proportion of attrition in intervention group compared to control group. (10 out of 75 (13%) did not receive the intervention). Only 2 lost to follow‐up in control arm. | |
| Age | 75.7 (SD 6.9) intervention; 76.6 (SD 6.7) control | |
| Sex | Majority were female (respectively 69.3% intervention and 72.0% control) | |
| Race/ethnicity | Not reported. | |
| Severity of illness | Not reported | |
| Co‐morbidities | Not reported but mean no. of medications = 8.4 (SD 2.4) intervention, 9.3 (SD 3.2) control | |
| Other relevant socio demographics | Details on pre‐existing cognitive status not reported for respective groups. | |
| Subgroups measured | NA | |
| Subgroups reported | NA | |
| Outcome measure(s) |
Change in anticholinergic use
Difference in proportion of patients having a decrease of DBI≥0.5 Cognitive outcomes Seven Minute Screen (7MS), the Trail Making Test (TMT) A & B, and Digit Symbol Substitution Test (DSST) Risk of falls Up and Go test. Number of fall incidents. Activities of daily living & Quality of life Groningen Activity Restriction Scale (GARS) Quality of life was measured by the Euroqol‐5 Dimension‐3 Level questionnaire, including visual analogue scale. Safety outcomes None reported. Mortality Via relative reporting. Hospital admission (institutionalisation) Via patient/relative reporting. |
|
| Outcome data (N event/N total for intervention and control; OR/HR, etc.) | See Table 3 | |
| Notes: | ||
Appendix 3. Anchoring statements for risk of bias assessment
We followed all signalling questions described in RoB2 to determine risk of bias. Assessment of signalling questions was supported by the following guidance to assist judgements made for respective ratings within each domain.
Signalling questions 1.1: we assessed the allocation sequence used. If it was possible for the next allocation to be predicted, we assigned a high risk of bias.
Signalling question 1.3: we evaluated the balance of prognostic factors in each group. If groups were unbalanced beyond what would be expected by chance, we assigned a high or 'some concerns' risk of bias judgement, depending upon the degree of imbalance.
Signalling question 2.1‐2.5: we expected blinding to intervention arm to be challenging for most trials, since it would usually be necessary to the trial's design for medical practitioners to know who is in the intervention group to reduce anticholinergic medication, and would be challenging to blind participants to medication reduction/cessation. Where this was the case, we scored RoB for this domain as 'some concerns' if there was evidence that this led to deviations from the intended intervention and had any impact upon the outcome. If deviation was large, we assigned a high risk of bias judgement.
Signalling question 3.1‐3.4: we applied the following rule of thumb to evaluate extent of missing data: 5% missing data was considered small; > 20% was considered large. Judgement of risk of bias considered both the proportion of missing data in respective groups and the methods used to deal with missing data (i.e. imputation versus complete data analysis only). If missing data were considerable or deemed to be a product of important study features that could systematically influence the outcome assessed, we assigned a high risk of bias judgement.
Signalling questions 4.3‐4.5: we assigned a high risk of bias judgement if no blinding to outcome assessment was performed for any subjective outcome assessments, such as self‐report questionnaires, or if aspects of cognitive assessments required assessor interpretation (e.g. clock draw).
Signalling questions 5.1‐5.3: we evaluated study protocols to establish risk of bias in outcome reporting. If any study outcomes were omitted, we rated the trial at high risk of bias. If no study protocol was provided, we assigned a 'some concerns' judgement, unless methodological details reported within the study were fully consistent with reported study results.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kersten 2013.
| Study characteristics | |
| Methods | Participants randomly assigned to intervention or control group via stratified randomisation process. Each nursing home represented 1 stratum. An independent research co‐ordinator randomly allocated participants (1:1) within each stratum. The size of the stratum varied from 2–15 participants, with a median of 4. |
| Participants |
Sample size: 101 Setting: 22 nursing homes in Norway Time frame for trial recruitment: 11 months (2008–2009) Inclusion criteria
Exclusion criteria
Baseline characteristics
|
| Interventions | Intervention: pharmacist‐led anticholinergic reduction intervention (in collaboration with GPs). Multidisciplinary drug review conducted within 3 days of baseline assessment. Drug reviews were guided by the ADS score to advise nursing home physicians about whether to discontinue or replace an anticholinergic drug with an alternative. Anticholinergic drugs were discontinued or replaced with a drug alternative with less or no anticholinergic activity. When drug alternatives were unavailable, reduction in dosage was attempted to reduce the anticholinergic burden. Control: usual care. For ethical reasons, similar drug reviews were conducted for the control group after the last follow‐up. |
| Outcomes |
Follow‐up: 4 and 8 weeks |
| Notes | |
Tollefson 1991.
| Study characteristics | |
| Methods | Participants randomly assigned to intervention or control. No details of randomisation procedure in trial report. |
| Participants |
Sample size: 34 Setting: 3 nursing homes in the USA Time frame for trial recruitment: not reported Inclusion criteria
Exclusion criteria
Baseline characteristics
|
| Interventions | Intervention: not clear how intervention was implemented. May have been primary physician‐led. Intervention group were required to have a medication change made in co‐operation with their primary physician. Anticholinergic index was reduced by at least 25% in intervention group. Medications were discontinued or, if not possible, substituted with a less anticholinergic drug. Control: usual care |
| Outcomes |
Follow‐up: 1 month |
| Notes | |
van der Meer 2018.
| Study characteristics | |
| Methods | Participants randomly assigned to intervention or control group. Randomisation process was conducted by the principal investigator, who was not involved in recruitment or data collection. |
| Participants |
Sample size: 164 Setting: 15 community pharmacies in Northern Netherlands Time frame for trial recruitment: December 2014–October 2015 Inclusion criteria
Exclusion criteria
Baseline characteristics
|
| Interventions | Intervention: anticholinergic burden reduction aimed at achieving 0.5‐point reduction in DBI via medication 'optimisation'. Pharmacist‐led medication review (in communication with GP). A 5‐step medication review took place within days after the baseline measurement, as follows.
Control: usual care |
| Outcomes |
Follow‐up: 3 months |
| Notes | |
ADS: Anticholinergic Drug Scale; ATC: Anatomic Therapeutic Classification; CCI: Charlson Comorbidity Index; CERAD: Consortium to Establish a Registry for Alzheimer's Disease; DBI: drug burden index; GP: general practitioner; MMSE: Mini‐Mental State Examination; PRN: pro re nata (as needed).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ailabouni 2017 | Ineligible study design. |
| Anda 2021 | Ineligible intervention. |
| Buckley 2021 | Ineligible study design. |
| Campbell 2019 | Ineligible outcome. |
| Cooper 1992 | Ineligible intervention. |
| Desmarais 2014 | Ineligible study design. |
| Drimer 2004 | Ineligible study design. |
| Forns 2021 | Ineligible study design. |
| Geller 2012 | Ineligible study design. |
| Griebling 2020 | Ineligible intervention. |
| Gustafsson 2018 | Ineligible intervention. |
| High 2020 | Ineligible intervention. |
| Jaidi 2018 | Ineligible study design. |
| Jaidi 2019 | Ineligible study design. |
| Karatas 2010 | Ineligible study design. |
| Lim 2020 | Ineligible intervention. |
| Lupu 2017 | Ineligible study design. |
| Lupu 2021 | Ineligible study design. |
| McEvoy 1987 | Ineligible study design. |
| Moga 2017 | Ineligible outcome. |
| Molloy 1989 | Ineligible study design. |
| Oken 1994 | Ineligible study design. |
| Potter 2016 | Ineligible intervention. |
| Roughead 2022 | Ineligible intervention. |
| Sathienluckana 2018 | Ineligible population (age). |
| Sunderland 1987 | Ineligible study design. |
| Veselinovic 2015 | Ineligible study design. |
| Wouters 2017 | Ineligible intervention. |
| Yeh 2013 | Ineligible study design. |
Characteristics of ongoing studies [ordered by study ID]
Abebe 2021.
| Study name | Reducing anticholinergic medication exposure among older adults using consumer technology: protocol for a randomized clinical trial |
| Methods | Randomised clinical trial to evaluate the effectiveness of a patient‐facing mobile application (Brain Safe app) compared to an attention control medication list app for reducing anticholinergic exposure among community‐dwelling older adults |
| Participants | Aged ≥ 60 years and above, currently using ≥ 1 prescribed strong anticholinergic, and receiving primary care |
| Interventions | Participants will have the Brain Safe app (intervention arm) or attention control medication list app (control arm) loaded onto a smartphone (study provided or personal device). All participants will be followed for 12 months and will have data collected at baseline, at 6 months, and 12 months. |
| Outcomes | Primary outcome: anticholinergic exposure measured as total standard daily dose (TSDD) computed from medication prescription electronic records Secondary outcomes: cognitive function and health‐related quality of life |
| Starting date | 16 October 2019 |
| Contact information | cvallej@regenstrief.org |
| Notes |
Campbell 2022.
| Study name | Reducing risk of dementia through deprescribing (R2D2) |
| Methods | Cluster‐randomised trial, with randomisation at the level of physicians. Physicians agreeing to participate in the trial will be randomised to intervention or usual care in blocks of 2 or 4. Physician randomisation status will determine participants' study group. |
| Participants | Primary care older adults with subjective cognitive decline or who make ≥ 1 error on a cognitive screening test, but do not have dementia, and are currently using a strong anticholinergic medication |
| Interventions | Intervention: deprescribing of target anticholinergics Control: usual care |
| Outcomes |
|
| Starting date | 20 July 2020 |
| Contact information | campbenl@iu.edu |
| Notes |
Differences between protocol and review
See Taylor‐Rowan 2022b (review protocol).
We additionally searched Cochrane CENTRAL to ensure we identified all relevant clinical trials.
We were unable to perform any planned meta‐analyses or subgroup analyses or to investigate heterogeneity using the I2 statistic owing to the scarcity and variability of the literature. We modified the Methods section on this basis.
We altered the text describing our risk of bias process to more clearly demonstrate how this was applied in the context of the RoB2 tool. We also altered the text to clarify that risk of bias was conducted at study outcome level for each domain.
Contributions of authors
MT drafted the manuscript. AN‐S conducted the search. MT and AA performed study screening, data extraction, risk of bias assessment, and GRADE evaluation. All authors contributed to interpretation of results and writing.
Sources of support
Internal sources
No sources of support provided
External sources
-
NIHR, UK
This review was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Declarations of interest
MT: none AA: none AN‐S: none PM: none CS: none JM: none TQ: none
New
References
References to studies included in this review
Kersten 2013 {published data only}
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. Journals of Gerontology: MEDICAL SCIENCES 2013;68(3):271-8. [DOI: 10.1093/gerona/gls176] [DOI] [PubMed] [Google Scholar]
Tollefson 1991 {published data only}
- Tollefson GD, Montague-Clouse J, Lancaster SP. The relationship of serum anticholinergic activity to mental status performance in an elderly nursing home population. The Journal of Neuropsychiatry and Clinical Neurosciences 1991;3:314-9. [DOI] [PubMed] [Google Scholar]
van der Meer 2018 {published data only}
- Van der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open 2018;8:e019042. [DOI: 10.1136/ bmjopen-2017-019042] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ailabouni 2017 {published data only}
- Ailabouni N, Mangin D, Nishtala PS. Deprescribing anticholinergic and sedative medicines: protocol for a feasibility trial (DEFEAT-polypharmacy) in residential aged care facilities. BMJ Open 2017;7(4):e013800. [DOI: 10.1136/bmjopen-2016-013800] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anda 2021 {published data only}
- Anda L, Johnsen E, Kroken RA, Joa I, Rettenbacher M, Løberg EM. Cognitive change and antipsychotic medications: results from a pragmatic rater-blind RCT. Schizophrenia Research: Cognition 2021;26:100204. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buckley 2021 {published data only}
- Buckley E, Jonsson A, Flood Z, Lavelle M, O'Sullivan N, et al. Potentially inappropriate medication use and mortality in patients with cognitive impairment. Age and Ageing 2021;50(Suppl 2):2013-2020. [DOI: 10.1093/ageing/afab118.09] [DOI] [PubMed] [Google Scholar]
Campbell 2019 {published data only}
- Campbell NL, Perkins AJ, Khan BA, Gao S, Farber MO, Khan S, et al. Deprescribing in the Pharmacologic Management of Delirium: a Randomized Trial in the Intensive Care Unit. Journal of the American Geriatrics Society 2019;67(4):695-702. [DOI: 10.1111/jgs.15751] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1992 {published data only}
- Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson's disease. A follow-up study of untreated patients. Brain 1992;115:1701-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Desmarais 2014 {published data only}
- Desmarais JE, Beauclair L, Annable L, Bélanger MC, Kolivakis TT, Margolese HC. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Therapeutic Advances in Pharmacology 2014;4(6):257-67. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drimer 2004 {published data only}
- Drimer T, Shahal B, Barak Y. Effects of discontinuation of long-term anticholinergic treatment in elderly schizophrenia patients. International Clinical Psychopharmacology 2004;19(1):27-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Forns 2021 {published data only}
- Forns J, Aguado J, Rivero-Ferrer E, Plana E, Dickerman B, De Albeniz XG. The effect of the anticholinergic properties of antidepressants on the incidence of dementia: a target trial emulation. Pharmacoepidemiology and Drug Safety 2021;Conference: 37th International Conference on Pharmacoepidemiology and Therapeutic Risk Management:84. [Google Scholar]
Geller 2012 {published data only}
- Geller EJ, Crane AK, Wells EC, Robinson BL, Jannelli ML, Khandelwal CM, et al. Effect of Anticholinergic Use for the Treatment of Overactive Bladder on Cognitive Function in Post-Menopausal Women. Clinical Drug Investigation 2012;32(10):697-705. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griebling 2020 {published data only}
- Griebling TL, Campbell NL, Mangel J, Staskin D, Herschorn S, Elsouda D, et al. Effect of mirabegron on cognitive function in elderly patients with overactive bladder: moCA results from a phase 4 randomized, placebo-controlled study (PILLAR). BMC Geriatrics 2020;20(1):109. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustafsson 2018 {published data only}
- Gustafsson M, Sjolander M, Pfister B, Schneede J, Lovheim H. Effects of pharmacists' interventions on inappropriate drug use and drug-related readmissions in people with dementia-a secondary analysis of a randomized controlled trial. Pharmacy 2018;6(1):7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
High 2020 {published data only}
- High RA, Danford JM, Shi ZY, Karmonik C, Kuehl TJ, Bird ET, et al. Protocol for a multicenter randomized, double blind, controlled pilot trial of higher neural function in overactive bladder patients after anticholinergic, beta-3 adrenergic agonist, or placebo. Contemporary Clinical Trials Communication 2020;20:100690. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaidi 2018 {published data only}
- Jaidi Y, Nonnonhou V, Kanagaratnam L, Bertholon LA, Badr S, Noel V, et al. Reduction of the Anticholinergic Burden Makes It Possible to Decrease Behavioral and Psychological Symptoms of Dementia. American Journal of Geriatric Psychiatry 2018;26(3):280-8. [DOI: 10.1016/j.jagp.2017.08.005] [DOI] [PubMed] [Google Scholar]
Jaidi 2019 {published data only}
- Jaïdi Y, Guilloteau A, Nonnonhou V, Bertholon LA, Badr S, Morrone I, et al. Threshold for a reduction in anticholinergic burden to decrease behavioral and psychological symptoms of dementia. Journal of the American Medical Directors Association 2019;20(2):159-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karatas 2010 {published data only}
- Karatas GK, Gunendi Z. Do anticholinergics affect reaction time? A possible impact on the course of rehabilitation. NeuroRehabilitation 2010;27(2):141-5. [DOI: 10.3233/NRE20100590] [DOI] [PubMed] [Google Scholar]
Lim 2020 {published data only}
- Lim R, Bereznicki L, Corlis M, Kalisch Ellett LM, Kang AC, Merlin T, et al. Reducing medicine-induced deterioration and adverse reactions (ReMInDAR) trial: study protocol for a randomised controlled trial in residential aged-care facilities assessing frailty as the primary outcome. BMJ Open 2020;10(4):e032851. [DOI: 10.1136/bmjopen-2019-032851] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lupu 2017 {published data only}
- Lupu AM, Clinebell K, Gannon JM, Ellison JC, Chengappa KNR. Reducing anticholinergic medication burden in patients with psychotic or bipolar disorders. Journal of Clinical Psychiatry 2017;78(9):e1270-5. [DOI] [PubMed] [Google Scholar]
Lupu 2021 {published data only}
- Lupu AM, MacCamy KL, Gannon JM, Brar JS, Chengappa KN. Less is more: deprescribing anticholinergic medications in persons with severe mental illness. Annals of Clinical Psychiatry 2021;33(2):80-92. [DOI: 10.12788/acp.0019] [DOI] [PubMed] [Google Scholar]
McEvoy 1987 {published data only}
- McEvoy JP. A double-blind crossover comparison of antiparkinson drug therapy: amantadine versus anticholinergics in 90 normal volunteers, with an emphasis on differential effects on memory function. Journal of Clinical Psychiatry 1987;48:20-3. [PubMed] [Google Scholar]
Moga 2017 {published data only}
- Moga DC, Abner EL, Rigsby DN, Eckmann L, Huffmyer M, Murphy RR, et al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimer's Research & Therapy 2017;9(1):36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molloy 1989 {published data only}
- Molloy DW, Brooymans M. Anticholinergic medications and cognitive function in the elderly. Journal of Clinical and Experimental Gerontology 1989;10:89-98. [Google Scholar]
Oken 1994 {published data only}
- Oken BS, Kishiyama SS, Kaye JA, Howieson DB. Attention deficit in Alzheimer's disease is not simulated by an anticholinergic/antihistaminergic drug and is distinct from deficits in healthy aging. Neurology 1994;44(4):657-62. [DOI] [PubMed] [Google Scholar]
Potter 2016 {published data only}
- Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLOS One 2016;11(3):e0149984. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roughead 2022 {published data only}
- Roughead EE, Pratt NL, Parfitt G, Rowett D, Kalisch-Ellett LM, Bereznicki L, et al. Effect of an ongoing pharmacist service to reduce medicine-induced deterioration and adverse reactions in aged-care facilities (nursing homes): a multicentre, randomised controlled trial (the ReMInDAR trial). Age and Ageing 2022;51(4):1-9. [DOI: 10.1093/ageing/afac092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sathienluckana 2018 {published data only}
- Sathienluckana T, Unaharassamee W, Suthisisang C, Suanchang O, Suansanae T. Anticholinergic discontinuation and cognitive functions in patients with schizophrenia: a pharmacist-physician collaboration in the outpatient department. Integrated Pharmacy Research and Practice 2018;7:161-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sunderland 1987 {published data only}
- Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. Archives of General Psychiatry 1987;44(5):418-26. [DOI: 10.1001/archpsyc.1987.01800170032006] [DOI] [PubMed] [Google Scholar]
Veselinovic 2015 {published data only}
- Veselinovic T, Vernaleken I, Janouschek H, Kellermann T, Paulzen M, Cumming P, et al. Effects of anticholinergic challenge on psychopathology and cognition in drug-free patients with schizophrenia and healthy volunteers. Psychopharmacology 2015;232(9):1607-17. [DOI: 10.1007/s00213-014-3794-9] [DOI] [PubMed] [Google Scholar]
Wouters 2017 {published data only}
- Wouters H, Scheper J, Koning H, Brouwer C, Twisk JW, Meer H, et al. Discontinuing inappropriate medication use in nursing home residents: a cluster randomized controlled trial. Annals of Internal Medicine 2017;167(9):609-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeh 2013 {published data only}
- Yeh YC, Liu CL, Peng LN, Lin MH, Chen LK. Potential benefits of reducing medication-related anticholinergic burden for demented older adults: a prospective cohort study. Geriatrics & Gerontology International 2013;13:694-700. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Abebe 2021 {published data only}
- Abebe E, Campbell NL, Clark DO, Tu W, Hill JR, Harrington AB, et al. Reducing anticholinergic medication exposure among older adults using consumer technology: protocol for a randomized clinical trial. Research in Social & Administrative Pharmacy 2021;17(5):986-92. [DOI: 10.1016/j.sapharm.2020.10.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2022 {published data only}
- Campbell NL, Holden R, Gao S, Unverzagt F, Boustani M. R2D2: a cluster-randomized trial of deprescribing to support brain health. Epidemiology (Cambridge, Mass.) 2022;70(Suppl 1):S193. [Google Scholar]
Additional references
Boustani 2008
- Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2008;4(3):311-20. [DOI: 10.2217/1745509X.4.3.311] [DOI] [Google Scholar]
Carnahan 2004
- Carnahan RM, Lund BC, Perry PJ, Chrischilles EA. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? Journal of the American Geriatriatics Society 2004;52(12):2082-7. [DOI: 10.1111/j.1532-5415.2004.52563.x] [DOI] [PubMed] [Google Scholar]
Carnahan 2006
- Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. Journal of Clinical Pharmacology 2006;46(12):1481-6. [DOI: 10.1177/0091270006292126] [DOI] [PubMed] [Google Scholar]
Comprehensive Meta‐Analysis Version 3 [Computer program]
- Comprehensive Meta-Analysis Version 3. Borenstein M, Hedges L, Higgins J, Rothstein H Biostat. Englewood: NJ, 2013.
Covidence [Computer program]
- Covidence. Version accessed prior to November 2023. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
DSM 5
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189-98. [DOI] [PubMed] [Google Scholar]
Francis 1999
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. Journal of Neurology, Neurosurgery and Psychiatry 1999;66:137-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hampel 2018
- Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain 2018;141(7):1917-33. [DOI: 10.1093/brain/awy132] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanlon 2020
- Hanlon P, Quinn TJ, Gallacher KI, Myint PK, Jani BD, Nicholl BI, et al. Assessing risks of polypharmacy involving medications with anticholinergic properties. Annals of Family Medicine 2020;18(2):148-55. [DOI: 10.1370/afm.2501] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6. [DOI] [PMC free article] [PubMed]
Higgins 2022
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3 2022.
Hilmer 2007
- Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Archives of Internal Medicine 2007;167(8):781-7. [DOI: 10.1001/archinte.167.8.781] [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1678. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
Kersten 2012
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2013;68(3):271-8. [DOI: 10.1093/gerona/gls176] [DOI] [PubMed] [Google Scholar]
Marzoughi 2021
- Marzoughi S, Banerjee A, Jutzeler CR, Prado MA, Rosner J, Cragg JJ, et al. Tardive neurotoxicity of anticholinergic drugs: a review. Journal of Neurochemistry 2021;158(6):1334-44. [DOI: 10.1111/jnc.15244] [DOI] [PubMed] [Google Scholar]
McKeith 2005
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863-72. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939-44. [DOI] [PubMed] [Google Scholar]
McKhann 2001
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Archives of Neurology 2001;58:1803-9. [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's & Dementia 2011;7:263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakham 2020
- Nakham A, Myint PK, Bond CM, Newlands R, Loke YK, Cruickshank M. Interventions to reduce anticholinergic burden in adults aged 65 and older: a systematic review. Journal of the American Medical Directors Association 2020;21(2):172-80. [DOI: 10.1016/j.jamda.2019.06.001] [DOI] [PubMed] [Google Scholar]
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society 2005;53:695-9. [DOI] [PubMed] [Google Scholar]
NICE 2023
- National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers. NICE, 2023. Available at www.nice.org.uk/guidance/ng97. [PubMed]
Petersen 2001
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133-42. [DOI] [PubMed] [Google Scholar]
Prince 2016
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. Improving healthcare for people living with dementia: coverage, quality and costs now and in the future. ADI, 2016. www.alzint.org/resource/world-alzheimer-report-2016/ (accessed prior to 27 April 2021).
Román 1993
- Román GC, Tatemichi TK, Erkinjutti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250-60. [DOI] [PubMed] [Google Scholar]
Sanghavi 2022
- Sanghavi R, Pana TA, Mamayusupova H, Maidment I, Fox C, Boekholdt SM, et al. Higher anticholinergic burden from medications is associated with significant increase in markers of inflammation in the EPIC-Norfolk prospective population-based cohort study. British Journal of Clinical Pharmacology 2022;88(7):3297-306. [DOI: 10.1111/bcp.15261] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shawaqfeh 2022
- Shawaqfeh B, Hughes CM, McGuinness B, Barry HE. A systematic review of interventions to reduce anticholinergic burden in older people with dementia in primary care. International Journal of Geriatric Psychiatry 2022;37(6):Epub ahead of print. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2013
- Singh S, Loke YK, Enright P, Furberg CD. Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax 2013;68(1):114-6. [DOI: 10.1136/thoraxjnl-2011-201275] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Tan 2018
- Tan EC, Eriksdotter M, Garcia-Ptacek S, Fastbom J, Johnell K. Anticholinergic burden and risk of stroke and death in people with different types of dementia. Journal of Alzheimer's Disease 2018;65(2):589-96. [DOI: 10.3233/JAD-180353] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor‐Rowan 2021
- Taylor-Rowan M, Edwards S, Noel-Storr AH, McCleery J, Myint PK, Soiza R, et al. Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013540. [DOI: 10.1002/14651858.CD013540.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor‐Rowan 2022a
- Taylor-Rowan M, Kraia O, Kolliopoulou C, Cross AJ, Stewart C, Myint PK, et al. Anticholinergic burden for prediction of cognitive decline or neuropsychiatric symptoms in older adults with mild cognitive impairment or dementia. Cochrane Database of Systematic Reviews 2022, Issue 8. Art. No: CD015196. [DOI: 10.1002/14651858.CD015196.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Meer 2018
- Van der Meer HG, Wouter H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open 2018;8:e019042. [DOI: 10.1136/bmjopen-2017-019042] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. International Classification of Diseases (ICD) (who.int). 7th edition. World Health Organization, 1993. [Google Scholar]
References to other published versions of this review
Taylor‐Rowan 2022b
- Taylor-Rowan M, Alharthi AA, Noel-Storr AH, Myint PK, Stewart C, McCleery J, et al. Anticholinergic deprescribing interventions for reducing risk of cognitive decline or dementia in older adults with and without prior cognitive impairment. Cochrane Database of Systematic Reviews 2022, Issue 12. Art. No: CD015405. [DOI: 10.1002/14651858.CD015405] [DOI] [PMC free article] [PubMed] [Google Scholar]