Abstract
Purpose of Review
Genetic or acquired lipodystrophies are characterized by selective loss of body fat along with predisposition towards metabolic complications of insulin resistance, such as diabetes mellitus, hypertriglyceridemia, hepatic steatosis, polycystic ovarian syndrome, and acanthosis nigricans. In this review, we discuss the various subtypes and when to suspect and how to diagnose lipodystrophy.
Recent Findings
The four major subtypes are autosomal recessive, congenital generalized lipodystrophy (CGL); acquired generalized lipodystrophy (AGL), mostly an autoimmune disorder; autosomal dominant or recessive familial partial lipodystrophy (FPLD); and acquired partial lipodystrophy (APL), an autoimmune disorder. Diagnosis of lipodystrophy is mainly based upon physical examination findings of loss of body fat and can be supported by body composition analysis by skinfold measurements, dual-energy x-ray absorptiometry, and whole-body magnetic resonance imaging. Confirmatory genetic testing is helpful in the proband and at-risk family members with suspected genetic lipodystrophies. The treatment is directed towards the specific comorbidities and metabolic complications, and there is no treatment to reverse body fat loss. Metreleptin should be considered as the first-line therapy for metabolic complications in patients with generalized lipodystrophy and for prevention of comorbidities in children. Metformin and insulin therapy are the best options for treating hyperglycemia and fibrates and/or fish oil for hypertriglyceridemia.
Summary
Lipodystrophy should be suspected in lean and muscular subjects presenting with diabetes mellitus, hypertriglyceridemia, non-alcoholic fatty liver disease, polycystic ovarian syndrome, or amenorrhea. Diabetologists should be aware of lipodystrophies and consider genetic varieties as an important subtype of monogenic diabetes.
Keywords: Congenital generalized lipodystrophy, CGL, Familial partial lipodystrophy, FPLD, Acquired generalized lipodystrophy, AGL, Acquired partial lipodystrophy, APL, Acanthosis nigricans, Hypertriglyceridemia
Introduction
Lipodystrophy syndromes are a heterogeneous group of genetic or acquired disorders characterized by selective loss of adipose tissue. The loss of body fat can vary from being generalized (involving nearly all the body fat depots) to partial (affecting only the limbs or upper body) or localized to small areas. Genetic lipodystrophies may manifest at birth or may present with fat loss later in life. The onset of acquired lipodystrophies usually occurs during childhood, but these disorders may also present later in life. Patients with generalized or partial lipodystrophies have a predisposition towards developing metabolic complications of insulin resistance, such as diabetes mellitus, hypertriglyceridemia, hepatic steatosis, polycystic ovarian syndrome, and acanthosis nigricans [1]. The severity of the metabolic complications is generally related to the extent of body fat loss.
While the inherited and acquired (autoimmune) lipodystrophy syndromes (excluding antiretroviral therapy–induced lipodystrophy in human immunodeficiency virus (HIV)–infected patients) are rare, with estimated prevalence of around 1.3 to 4.7 cases per million, recent data suggest that they may be more prevalent than estimated previously [2], particularly familial partial lipodystrophies (FPLD), which may be underdiagnosed. For example, in Reunion Island, a French overseas territory in the Indian Ocean, a founder variant in lamin A/C (LMNA) gene, c.1961_1962insG p.(Thr655Asnfs*49) causes familial partial lipodystrophy of the Dunnigan variety (FPLD2) with high prevalence of diabetes in heterozygotes (44%) and particularly high in homozygotes (70%)[3]. Recently, clinical prevalence of lipodystrophy was estimated to be as high as 1 in 20,000 individuals and genetic prevalence of FPLD to be ~1 in 7000 in the general population based on the electronic health record information of >1.3 million adults from the Geisinger Health System for lipodystrophy diagnostic codes in the USA [4]. However, there may be some overestimation partly due to a founder effect in the Geisinger Health System. Most interestingly, a recent genome-wide association study identified five robust clusters of T2DM loci and traits, with one being ‘lipodystrophy-like” fat distribution [5]. Thus, diabetologists are more likely to see patients with inherited and acquired lipodystrophy syndromes or “lipodystrophy-like” phenotype among patients presenting with type 2 diabetes mellitus (T2DM). Although the precise estimates for what proportion of patients with diabetes are due to lipodystrophies as well as population prevalence of lipodystrophies remains unknown, it is likely that diabetologists may be underdiagnosing lipodystrophies. With more awareness of the lipodystrophy syndromes, it is expected that in the future diabetologists will consider the diagnosis of genetic lipodystrophies as an important subtype of monogenic diabetes, just like maturity-onset diabetes of the young (MODY). In addition, these disorders can be diagnosed by lipidologists, endocrinologists, gynecologists, hepatologists, or dermatologists.
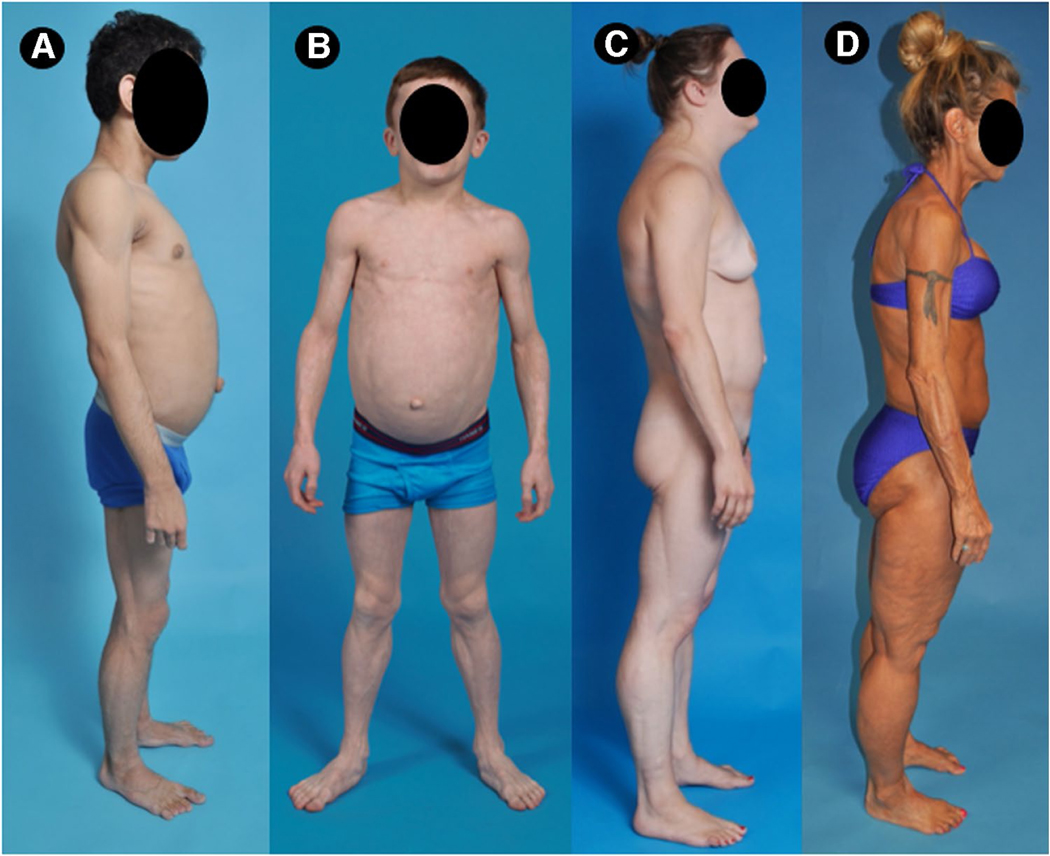
Lipodystrophies can be classified based on etiology (genetic or acquired) and the extent of fat loss (generalized or partial). This yields four major subtypes; congenital generalized lipodystrophy (CGL)[6], acquired generalized lipodystrophy (AGL) [7], familial partial lipodystrophy (FPLD) [1, 8], and acquired partial lipodystrophy (APL) [9] (Fig. 1, Table 1).
Fig. 1.
Clinical features of patients with various types of lipodystrophies. A Lateral view of 15-year-old male from Brazil with congenital generalized lipodystrophy, type 2 (CGL2) due to compound heterozygous disease-causing variants c.517_518insA, (p.Thr173Asnfs*5) and c.604C>T (p.Arg202*) in BSCL2 gene. The patient had generalized loss of subcutaneous (sc) fat with acanthosis nigricans in the axillae and neck. He had insulin-resistant diabetes mellitus, hepato-splenomegaly, and umbilical prominence. B Anterior view of an 8-year-old Caucasian male with acquired generalized lipodystrophy (AGL). He had panniculitis in lower extremities at age 3, with subsequent generalized loss sc fat. There was no acanthosis nigricans, but he had hepatomegaly and umbilical prominence. C Lateral view of 29-year-old Caucasian female with familial partial lipodystrophy of the Dunnigan variety (FPLD2) due to disease-causing heterozygous c.1444C>T (p.Arg482Trp) variant in LMNA. She had marked loss of sc fat from the extremities and from the gluteal region but had increased sc fat in the face, neck, chest, and upper back. D Lateral view of a 59-year-old Caucasian female with acquired partial lipodystrophy (Barraquer-Simons syndrome). She had marked loss of sc fat from the face, neck, upper extremities, chest, and abdomen but had increased sc fat deposition in the lower extremities
Table 1.
Classification, clinical features, and etiology of the four major subtypes of lipodystrophy
| Generalized | Partial | |||
|---|---|---|---|---|
| Genetic | Acquired | Genetic | Acquired | |
| Subtype | Congenital generalized lipodystrophy | Acquired generalized lipodystrophy | Familial partial lipodystrophy | Acquired partial lipodystrophy |
| Genes for autosomal recessive subtypes | AGPAT2, BSCL2, CAV1, CAVIN1 [10–13] | None | CIDEC, LIPE, PCYT1A [14–18] | None |
| Genes for autosomal dominant subtypes | None | None | LMNA, PPARG, AKT2, PLIN1, ADRA2A [19–24] | None |
| Pathogenesis | Inability to store triglycerides in adipose tissue | Autoimmune, panniculitis, idiopathic (perilipin 1 autoantibody) | Various different mechanisms | Autoimmune (Complement 3 nephritic factor autoantibody) |
| Lipodystrophy phenotype | Near total absence of body fat since birth or soon after | Gradual loss of subcutaneous fat from nearly entire body | Absence of fat from the limbs | Absence of fat from the upper body, including the face, neck, arms and chest |
| Metabolic complications | + | + | + | Mild or none |
Present
Inherited congenital generalized lipodystrophies (CGL) are autosomal recessive due to biallelic disease-causing variants in AGPAT2 (CGL type 1), BSCL2 (CGL type 2), CAV1 (CGL type 3), or CAVIN1 (CGL type 4) [10–13]. A similar syndrome has been reported to be due to biallelic PCYT1A and PPARG variants [14, 25]. Familial partial lipodystrophies, on the other hand, are mostly autosomal dominant with heterozygous disease-causing variants in LMNA (FPLD2), PPARG (FPLD3), AKT2 (FPLD other), PLIN1 (FPLD4), and ADRA2A (FPLD7) [19–24] and rarely due to autosomal recessive inheritance due to biallelic disease-causing variants in CIDEC (FPLD5), LIPE (FPLD6), and PCYT1A (FPLD Other)[14–18]. Besides these major categories, some of the other rare syndromes can also present with lipodystrophy. Various progeroid syndromes can have autosomal recessive (LMNA, ZMPSTE24, SPRTN, WRN, BANF1 [18, 26–29]) or dominant inheritance (LMNA, FBN1, CAV1, POLD1, KCNJ6 [30–35]), and can present with partial or generalized pattern of lipodystrophy along with progeroid features and variable metabolic complications. SHORT syndrome (short stature, hyperextensibility or inguinal hernia, ocular depression, Rieger anomaly, and teething delay) also has variable subcutaneous fat loss and metabolic features caused by heterozygous variants in PIK3R1 gene [36]. JMP syndrome (joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome) and CANDLE syndrome (chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature) are autoinflammatory syndromes caused by biallelic variants in PSMB8 gene [37]. Acquired generalized lipodystrophy is also a heterogeneous disorder which presents with panniculitis, in association with autoimmune diseases, or could be idiopathic. Recent data reveal circulating perilipin-1 autoantibody in a subset of patients with AGL [38]. Acquired partial lipodystrophy is also an autoimmune disorder and is associated with the presence of complement 3 nephritic factor autoantibody.
Common Lipodystrophy Subtypes
Congenital Generalized Lipodystrophy (CGL)
CGL is an autosomal recessive disorder (Online Mendelian inheritance in man OMIM # 269700) reported to date in approximately 500 patients with population prevalence estimated to be 1 in 10 million [1, 39–42]. Most cases are reported in consanguineous families from Brazil, Lebanon, Peru, and Scandinavia [11, 41, 43]. Essential diagnostic criteria are near-total, generalized lack of body fat with extreme muscularity from birth or soon thereafter. The characteristic body fat distribution pattern should be confirmed on physical examination using conventional anthropometry and, if possible, by whole-body magnetic resonance imaging (MRI). The affected children demonstrate an accelerated growth and advanced bone age with voracious appetite and acromegaloid features with enlarged mandible, hands, and feet [1, 44, 45]. Focal lytic lesions in the long bones such as the humerus, femur, radius, ulna, carpal, tarsal, or phalangeal bones have been reported in some patients [45–48]. Prominence of umbilicus or umbilical hernias and underlying hepatomegaly and/or splenomegaly can present in early childhood, and steatohepatitis can progress to fibrosis, cirrhosis, or end-stage liver disease in some cases which may require hepatic transplantation [1, 44, 48–50]. Acanthosis nigricans and signs of insulin resistance can appear during early childhood, and most of the patients develop diabetes during the pubertal years. Hypertriglyceridemia is prevalent in over 70% of patients and develops mostly in late childhood and adolescence and only rarely during infancy. The levels of serum leptin and adiponectin are markedly reduced in patients with CGL secondary to near-total loss of body fat [51, 52]. Severe hypoleptinemia may induce voracious appetite in patients with CGL which may further exacerbate metabolic complications. Hypertrophic cardiomyopathy, and mild mental retardation have also been reported. [53, 54]. Mild hirsutism, clitoromegaly, and irregular menstrual periods are common in females with CGL and some present with primary or secondary amenorrhea and polycystic ovaries [40, 55, 56]. Some males with CGL may have infertility due to oligospermia or abnormal sperm morphology [57, 58]. Breast development is normal, but overlying subcutaneous fat layer surrounding the mammary tissue is absent [50]. Patients with CGL3 due to CAV1 variants have been shown to have dysphagia and megaesophagus due to achalasia ([59, 60], whereas patients with CGL4 due to CAVIN1 variants are predisposed to myopathy and muscular weakness and can die due to ventricular arrhythmias and cardiomyopathy [61].
Familial Partial Lipodystrophy (FPLD)
FPLD is characterized by subcutaneous fat loss from the upper and lower extremities and variable fat loss from the trunk. So far, FPLD has 8 different genetic subtypes, and most patients have autosomal dominant inheritance, but some may have autosomal recessive inheritance. Some subtypes can have regional fat accumulation causing “buffalo” or “dorsocervical” hump, double chin, increased fat in the face, and supraclavicular region resulting in Cushingoid appearance. The most prevalent subtype is familial partial lipodystrophy-Dunnigan variety (FPLD2, OMIM 151660), which is an autosomal dominant disorder, caused by heterozygous disease-causing variants in lamin A/C (LMNA) gene. Approximately 500 patients have been reported to have FPLD2, with female predominance [1, 62, 63]. Patients have normal fat distribution at birth and during childhood, but develop gradual loss of subcutaneous fat from the upper and lower extremities usually before puberty, especially in females [1, 63]. Excess fat deposition is also noted in intraabdominal region, intermuscular fasciae, and in the pubic region in females [64]. FPLD2 is more readily recognized in women than in men because of the unusual muscular appearance of the extremities in women. There is marked phenotypic heterogeneity among children harboring FPLD2-causing LMNA mutations, and metabolic abnormalities like hypertriglyceridemia can present in adolescent years in females [63]. Affected adults, especially women, develop more severe metabolic abnormalities related to insulin resistance such as hypertriglyceridemia and diabetes mellitus [62, 65]. These metabolic derangements predispose patients to premature atherosclerosis, especially in the affected women [62, 66]. Polycystic ovarian syndrome (PCOS) and hepatic steatosis are other common features of FPLD. Some rare patients with FPLD2 also develop cardiomyopathy and conduction system defects requiring cardiac transplantation or pacemaker implantation.
Acquired Generalized Lipodystrophy (AGL)
AGL is characterized by progressive generalized loss of subcutaneous fat including the palms and soles and usually manifests in childhood. It is approximately three times more common in females than in males [7]. AGL is often associated with autoimmune diseases. Three subtypes have been reported based on etiology: the panniculitis variety (type 1), the autoimmune diseases variety (type 2), and the idiopathic variety (type 3). Type 1 (panniculitis variety) is seen in about 25% patients and usually starts with localized lipodystrophy with the panniculitis, which spreads to become generalized. Type 2 (autoimmune diseases variety ) is seen in 25% patients and is associated with juvenile dermatomyositis, Sjogren’s syndrome, chronic active hepatitis, Hashimoto’s thyroiditis, and juvenile rheumatoid arthritis. Idiopathic subtype affects nearly half of the patients and has several other associations. AGL has also been reported as a paraneoplastic manifestation of pilocytic astrocytoma in young children [67]. Recent case reports have shown temporal relationship of AGL with the anti-programmed cell death (PD) 1 therapy (pembrolizumab and nivolumab) for a metastatic melanoma [68, 69]. Metabolic complications are extremely common, and they can develop severe hepatic steatosis and fibrosis, diabetes, and hypertriglyceridemia [7, 70]. Recently, anti-perilipin 1 autoantibodies have been reported in a few patients with AGL [38]. However, whether they play a causal role or are just a marker of immune damage to adipocytes is not clear.
Acquired Partial Lipodystrophy (APL)
APL is characterized by loss of subcutaneous fat in a craniocaudal progression, initially affecting the face and then gradually progressing to the neck, shoulders, arms, and the trunk. It is approximately four times more prevalent in females than males (4:1) and usually manifests in childhood or adolescence [9]. There is excess fat accumulation in the hips, buttocks, and lower extremities. Several autoimmune diseases like systemic lupus erythematosus and dermatomyositis and various infections have been reported by these patients; however, exact etiology of fat loss has not been determined to date. Patients can have low serum complement 3 (C3) levels and circulating autoantibody called complement 3-nephritic factor which blocks degradation of the enzyme C3 convertase. About 20% of patients have membranopro-liferative glomerulonephritis (MPGN) [9]. Metabolic complications are not commonly seen; however, end-stage renal disease requiring renal transplantation has been reported.
When to Suspect Lipodystrophy
Clinicians should suspect lipodystrophy when a newborn, infant, or a young child presents with muscular appearance with failure to thrive in infancy in the absence of malnutrition or catabolic state (Table 2). If the child presents with autoantibody negative, insulin-resistant diabetes, or with acanthosis nigricans, severe hypertriglyceridemia with recurrent acute pancreatitis, hepatomegaly with and without splenomegaly, ultrasound documentation of hepatic steatosis, elevated serum transaminases, PCOS or amenorrhea in lean child/adolescent, and lipodystrophy should be strongly suspected; however, not all patients manifest them at the presentation. Many children with AGL or APL can be mis-diagnosed as having anorexia nervosa or malnutrition before a correct diagnosis is made. It should be noted that, although there is usually absence of type 1 diabetes–associated autoantibodies in these patients, there have been few case reports of type 1 diabetes in association with AGL [71, 72].
Table 2.
When to suspect lipodystrophy
| Generalized lipodystrophy | Partial lipodystrophy | |
|---|---|---|
| Family history | Positive family history in siblings only (autosomal recessive inheritance) | Positive family history in multiple generations (autosomal dominant inheritance) Positive family history in siblings only (autosomal recessive inheritance) |
| Clinical features | • Generalized Muscularity with loss of body fat | • Muscular extremities with loss of subcutaneous fat |
| • Prominent veins | • Cushingoid habitus | |
| • Voracious appetite | • Mild features in men | |
| • Acromegaloid features • Prominence of umbilicus |
• Increased fat in the face, neck, dorsocervical, perineal and intra-abdominal regions | |
| • Hepatomegaly | ||
| • Acanthosis nigricans | •Hepatomegaly | |
| • Irregular menstrual periods with polycystic ovaries | • Irregular menstrual periods with polycystic ovaries | |
| Metabolic features | • Autoantibody negative diabetes with evidence of severe insulin resistance*: high insulin requirement for metabolic control | |
| • Severe acanthosis nigricans | ||
| • Severe hypertriglyceridemia (triglycerides ≥ 500 mg/dL) and history of acute pancreatitis associated with hypertriglyceridemia | ||
| • Steatohepatitis and hepatomegaly | ||
There have been few case reports of type 1 diabetes in association with AGL
In adults, generalized lipodystrophy should be suspected if the patient has lean and muscular appearance with autoantibody negative diabetes, severe hypertriglyceridemia or its complications, non-alcoholic fatty liver disease, and PCOS or amenorrhea. Generalized lipodystrophy is usually easy to recognize; however, partial lipodystrophy can be more subtle and especially difficult to diagnose in men. Metabolic disease is out of proportion to adiposity in patients with partial lipodystrophy. In our experience, many patients with FPLD were initially suspected to have Cushing’s syndrome due to the presence of buffalo hump and round face. However, patients with FPLD had no proximal muscle weakness, facial plethora, purple striae, or bruising without trauma. Other clinical features suggestive of progeroid syndromes like beaked nose, premature greying or loss of hair, thin nose and lips, high-pitched voice, thin skin with mottled pigmentation, small mandible, dental crowding, resorption of terminal phalanges and lateral half of clavicles, and joint contractures should also raise suspicion for associated lipodystrophy. Parental consanguinity should increase the suspicion of autosomal recessive disorders. If someone in the family is diagnosed with genetic lipodystrophy, all siblings, parents, and other relatives should be screened for lipodystrophies either using clinical criteria or genetic testing.
Recent data from genome-wide association studies (GWAS) for type 2 diabetes have identified five robust clusters with one of them being “lipodystrophy-like” fat distribution (low body mass index, adiponectin, and high-density lipoprotein [HDL] cholesterol, and high triglycerides [5, 73]. These observations suggest a possibility that subtle lipodystrophy phenotype may be prevalent among some patients with type 2 diabetes. Some of these individuals may have lipodystrophy which was unrecognized on phenotyping. Clinicians should therefore perform careful physical examination in all individuals presenting with type 2 diabetes to look for signs of lipodystrophy Table 2.
Confirming the Diagnosis of Lipodystrophy
In patients with clinical suspicion of lipodystrophy based on their history, physical examination, and clinical and metabolic features, diagnosis can be further supported by body composition analysis by skinfold measurements, dual-energy x-ray absorptiometry (DXA), and whole-body magnetic resonance imaging (MRI) [74, 75]. Skinfold thickness values below the 10th percentile of normal values can increase the likelihood of generalized lipodystrophy diagnosis (Table 3). Various conditions should be considered in the differential diagnosis of patients with lipodystrophies (Table 4).
Table 3.
Tenth percentile values of skinfold thickness at various sites in adults and children [75]
Table 4.
Differential diagnosis for lipodystrophies
| A. Generalized lipodystrophies |
| 1. Conditions presenting with severe weight loss: |
| a. Malnutrition |
| b. Anorexia nervosa |
| c. Thyrotoxicosis |
| d. Adrenocortical insufficiency |
| e. Cancer cachexia |
| f. HIV-associated wasting |
| g. Chronic infections. |
| 2. Insulin receptor mutations: Rabson-Mendenhall syndrome and Donohue syndrome |
| 3. Severe uncontrolled T1DM or T2DM with marked weight loss due to extreme hyperglycemia |
| 4. Acromegaly |
| 5. Pancreatic diabetes due to exocrine and endocrine pancreatic insufficiency |
| B. Partial Lipodystrophies: |
| 1. Cushing’s syndrome |
| 2. Truncal obesity |
| 3. Multiple symmetric lipomatosis |
Since partial lipodystrophy can have variable fat distribution, diagnosis can be challenging. Our data reveal that in children, DXA-derived lower limb fat (%) and triceps skinfold thickness below 1st %ile of normal females age 8–18 years from the National Health and Nutrition Examination Survey (NHANES) are sensitive predictors of FPLD2 [63]. We also showed that there is marked loss of subcutaneous fat from the extremities much before the pubertal age in female children with FPLD2 [63]. We have further shown that in adult females; DXA-derived lower limb fat (%) has the best specificity (0.995) and sensitivity (1.0) compared with the upper limb fat, truncal fat, the ratio of lower limb to truncal fat, and triceps skinfold thickness for the diagnosis of FPLD2 [79]. The 1st percentile values for lower limb fat (%) among the adult female NHANES participants range from 25.8 to 29.3% for various age groups; thus, adult females with lower limb fat of <25% and metabolic complications should be further evaluated for diagnosis of FPLD2 [79]. Unfortunately, due to small sample size of boys and adult males with FPLD2, the diagnostic value of anthropometric parameters for them has not been defined (59,76).
Confirmatory genetic testing is helpful in suspected inherited lipodystrophies and should be considered in the proband and at-risk family members. However, there is strong evidence for additional loci for genetic lipodystrophies, and current knowledge is evolving gradually; hence, negative genotyping does not rule out a genetic condition. In patients with acquired lipodystrophy, serum complement levels and autoantibodies can support the diagnosis.
Screening for Comorbidities
The prevalence of macro-vascular complications of diabetes in patients with lipodystrophy has not been systematically studied. Atherosclerotic vascular complications such as coronary heart disease are uncommon in patients with generalized lipodystrophy; however, their prevalence is higher in females with familial partial lipodystrophy [42].
Proteinuria and nephropathy are frequent findings in patients with lipodystrophy. Estimated prevalence for macroalbuminuria (≥300 mg albumin/d) is about 35–60% in generalized lipodystrophy and 21% in partial lipodystrophy [80, 81]. Glomerular hypertrophy, mesangial expansion, podocyte injury, diabetic nephropathy, focal segmental glomerulosclerosis, and membranoproliferative glomerulonephritis have been reported in patients with lipodystrophy, and interestingly, only a small minority of patients with lipodystrophy have biopsy consistent with diabetic nephropathy [80, 82].
Different subtypes have variable onset of metabolic complications, and all patients with generalized lipodystrophies and familial partial lipodystrophies should be screened for comorbidities. Patients with APL are at low risk for metabolic complications, but MPGN and end-stage renal disease have been reported, and screening is required for these.
Diabetes mellitus: Fasting plasma glucose, hemoglobin A1c, and, if needed, oral glucose tolerance test should be conducted at diagnosis and annually.
Hypertriglyceridemia: Fasting lipid panel (triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol) should be obtained at diagnosis and annually if asymptomatic, and more frequently with occurrence of xanthomas or pancreatitis.
Steatohepatitis: Transaminases (alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT)) should be measured at diagnosis and then annually. Liver ultrasound and elastography should be performed at diagnosis, then as clinically indicated.
Kidney disease: Urine protein should be measured at diagnosis and then annually using 24-h urine collection or urine protein to creatinine ratio.
PCOS/secondary amenorrhea: Gonadotropins, total and free testosterone and pelvic ultrasonography should be performed as clinically indicated.
Management
Since most patients with lipodystrophies are predisposed to developing diabetes mellitus and hypertriglyceridemia, their risk of atherosclerotic vascular disease is already high. Therefore, they should avoid smoking and if they have hypertension; it should be well-controlled to reduce their risk of atherosclerosis. Hypertriglyceridemia also predisposes patients with lipodystrophies to the risk of acute pancreatitis, and therefore, all patients should avoid alcohol consumption which can exacerbate hypertriglyceridemia and women should avoid any form of estrogen therapy, which can elevate serum triglyceride concentrations. Avoidance of alcohol intake is also recommended to prevent any further damage to the liver as patients with lipodystrophies already have increased prevalence of non-alcoholic fatty liver disease.
Patients with lipodystrophies should be encouraged to do more exercise and avoid weight gain to mitigate metabolic complications except for patients with FPLD2 and cardiomyopathy, and those with CGL, type 4, who are predisposed to catecholaminergic polymorphic ventricular tachycardia.
The treatment is directed towards the specific comorbidities and metabolic complications, and there is no treatment to reverse body fat loss. Most patients need a balanced macronutrient diet with 50–60% carbohydrate, 20–30% fat, and approximately 20% protein diet; however; patients with chylomicronemia require extremely low-fat diet with < 15% energy from dietary fat [75]. Physical activity and exercise can help with metabolic complications; however, strenuous activity should be avoided in patients with FPLD2 who have concomitant cardiomyopathy or for those with CGL4 who are predisposed to catecholaminergic polymorphic ventricular tachycardia and sudden death [75]. Metreleptin replacement therapy (with diet) is the first-line treatment for metabolic complications in generalized lipodystrophy and may be considered for prevention of comorbidities in children [75, 83, 84]. Patients with partial lipodystrophy have less consistent response to metreleptin therapy [85], but studies have shown good response in partial lipodystrophy patients with severe metabolic derangements or low serum leptin levels [86]. Metreleptin is approved only for generalized lipodystrophies in the USA; however, it has been approved for all subtypes of lipodystrophy in Japan and in Europe. Metformin and insulin therapy are usually needed for patients with diabetes; and concentrated insulin should be considered in patients requiring large doses. Thiazolidinediones has been shown to improve metabolic complications in some patients with partial lipodystrophy [87]; however, they can cause unwanted excess fat deposition in non-lipodystrophic regions [88]. Because of increased prevalence of hypertriglyceridemia in patients with lipodystrophies and predisposition to develop acute pancreatitis, fibrates are the first choice of therapy and in some patients may be combined with low dose statins. In addition, fish oil can be used for patients with persistent hypertriglyceridemia despite fibrate therapy. Niacin should be avoided for risk of insulin resistance and deterioration of glycemic control and bile acid sequestrants can exacerbate hypertriglyceridemia and should not be used [75]. Statins, fibrates, and ezetimibe should be avoided in patients with CGL4 and others with myopathy. Estrogen therapy either as contraceptive, for treatment of PCOS, or as hormone replacement therapy should be avoided as any form of estrogen has the potential to exacerbate hypertriglyceridemia and cause acute pancreatitis in patients with lipodystrophies. While progesterone containing contraceptives may not cause hypertriglyceridemia, they have the potential to cause weight gain and insulin resistance due to their glucocorticoid receptor transactivation activity.
Only limited anecdotal data are available regarding the use of glucagon-like peptide 1 receptor (GLP-1R) agonists in patients with lipodystrophies. GLP-1R agonist, liraglutide, in combination with metformin stabilized blood glucose for a duration of 4 years in a female with FPLD2 [89]. Therapy with exenatide and liraglutide has been reported to improve glycemic control and result in weight loss in 49-year-old and 60-year-old females, respectively, with clinical diagnosis of partial lipodystrophy [90]. Only a few patients with lipodystrophies have been reported to have undergone bariatric surgery. Two patients had pathogenic LMNA variant and 2 had pathogenic PLIN1 variant, whereas 4 patients had no causal variants in LMNA or PPARG genes (most likely FPLD1) [91–96]. Most of these patients with FPLD had improvement in metabolic parameters in addition to weight loss with roux-en-Y gastric bypass. A recent study reveals anti-perilipin 1 autoantibody to be specifically enriched in a subset of patients with AGL, particularly those with panniculitis and other features of autoimmunity [97].
Acknowledgments
We thank Tea Huseinbegovic, B.S., for help with the illustration.
Funding
A.G. and N.P are supported by the National Institutes of Health grant R01-DK105448 and A.G. is also supported by the Southwestern Medical Foundation.
Footnotes
Declarations
Conflict of Interest N.P. have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication. A.G. consults for Amryt Pharma PLC and Regeneron and has received grant support from Amryt Pharma PLC, Regeneron, Quintiles, Akcea Pharmaceuticals, and Intercept Pharmaceuticals. A.G. is coholder of a patent for “use of leptin for treating human lipoatrophy and a method of determining predisposition to said treatment” but receives no financial compensation.
References
- 1.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–34. [DOI] [PubMed] [Google Scholar]
- 2.Chiquette E, et al. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre P, et al. Metabolic and cardiac phenotype characterization in 37 atypical Dunnigan patients with nonfarnesylated mutated prelamin A. Am Heart J. 2015;169(4):587–93. [DOI] [PubMed] [Google Scholar]
- 4.Gonzaga-Jauregui C, et al. Clinical and Molecular prevalence of lipodystrophy in an unascertained large clinical care cohort. Diabetes. 2020;69(2):249–58. [DOI] [PubMed] [Google Scholar]
- 5.Udler MS, et al. Type 2 diabetes genetic loci informed by multitrait associations point to disease mechanisms and subtypes: A soft clustering analysis. PLoS Med. 2018;15(9):e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patni N, Garg A. Congenital generalized lipodystrophies-new insights into metabolic dysfunction. Nat Rev Endocrinol. 2015;11(9):522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore). 2003;82(2):129–46. [DOI] [PubMed] [Google Scholar]
- 8.Dunnigan MG, et al. Familial lipoatrophic diabetes with dominant transmission. A new syndrome Q J Med. 1974;43(169):33–48. [PubMed] [Google Scholar]
- 9.Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore). 2004;83(1):18–34. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal AK, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31(1):21–3. [DOI] [PubMed] [Google Scholar]
- 11.Magre J, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28(4):365–70. [DOI] [PubMed] [Google Scholar]
- 12.Kim CA, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93(4):1129–34. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi YK, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119(9):2623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne F, et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci U S A. 2014;111(24):8901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio-Cabezas O, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1(5):280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert JS, et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N Engl J Med. 2014;370(24):2307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farhan SM, et al. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can J Cardiol. 2014;30(12):1649–54. [DOI] [PubMed] [Google Scholar]
- 18.Donadille B, et al. Partial lipodystrophy with severe insulin resistance and adult progeria Werner syndrome. Orphanet J Rare Dis. 2013;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst KL, et al. Kobberling type of familial partial lipodystrophy: an underrecognized syndrome. Diabetes Care. 2003;26(6):1819–24. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9(1):109–12. [DOI] [PubMed] [Google Scholar]
- 21.Shackleton S, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24(2):153–6. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87(1):408–11. [DOI] [PubMed] [Google Scholar]
- 23.George S, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304(5675):1325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandotra S, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364(8):740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyment DA, et al. Biallelic mutations at PPARG cause a congenital, generalized lipodystrophy similar to the Berardinelli-Seip syndrome. Eur J Med Genet. 2014;57(9):524–6. [DOI] [PubMed] [Google Scholar]
- 26.Novelli G, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet. 2002;71(2):426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal AK, et al. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12(16):1995–2001. [DOI] [PubMed] [Google Scholar]
- 28.Lessel D, et al. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet. 2014;46(11):1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabanillas R, et al. Nestor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. Am J Med Genet A. 2011;155A(11):2617–25. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson M, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Sandre-Giovannoli A, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300(5628):2055. [DOI] [PubMed] [Google Scholar]
- 32.Graul-Neumann LM, et al. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3’ terminus of the FBN1-gene. Am J Med Genet A. 2010;152A(11):2749–55. [DOI] [PubMed] [Google Scholar]
- 33.Garg A, et al. Whole exome sequencing identifies de novo heterozygous CAV1 mutations associated with a novel neonatal onset lipodystrophy syndrome. Am J Med Genet A. 2015;167A(8):1796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weedon MN, et al. An in-frame deletion at the polymerase active site of POLD1 causes a multisystem disorder with lipodystrophy. Nat Genet. 2013;45(8):947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masotti A, et al. Keppen-Lubinsky syndrome is caused by mutations in the inwardly rectifying K+ channel encoded by KCNJ6. Am J Hum Genet. 2015;96(2):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thauvin-Robinet C, et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet. 2013;93(1):141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal AK, et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87(6):866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corvillo F, et al. Autoantibodies Against Perilipin 1 as a Cause of Acquired Generalized Lipodystrophy. Front Immunol. 2018;9:2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg A. Lipodystrophies. Am J Med. 2000;108(2):143–52. [DOI] [PubMed] [Google Scholar]
- 40.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59(1):16–23. [DOI] [PubMed] [Google Scholar]
- 42.Hussain I, Patni N, Garg A. Lipodystrophies, dyslipidaemias and atherosclerotic cardiovascular disease. Pathology. 2019;51(2):202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pardini VC, et al. Leptin levels, beta-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatrophic diabetes. J Clin Endocrinol Metab. 1998;83:503–8. [DOI] [PubMed] [Google Scholar]
- 44.Seip M, Trygstad O. Generalized lipodystrophy, congenital and acquired (lipoatrophy). Acta Paediatr Suppl. 1996;413:2–28. [DOI] [PubMed] [Google Scholar]
- 45.Westvik J. Radiological features in generalized lipodystrophy. Acta Paediatr Suppl. 1996;413:44–51. [DOI] [PubMed] [Google Scholar]
- 46.Brunzell JD, Shankle SW, Bethune JE. Congenital generalized lipodystrophy accompanied by cystic angiomatosis. Ann Intern Med. 1968;69(3):501–16. [DOI] [PubMed] [Google Scholar]
- 47.Fleckenstein JL, et al. The skeleton in congenital, generalized lipodystrophy: evaluation using whole-body radiographic surveys, magnetic resonance imaging and technetium-99m bone scintigraphy. Skelet Radiol. 1992;21(6):381–6. [DOI] [PubMed] [Google Scholar]
- 48.Chandalia M, et al. Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1995;80(10):3077–81. [DOI] [PubMed] [Google Scholar]
- 49.Anonymous. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 1–1975. N Engl J Med. 1975;292(1):35–41. [DOI] [PubMed] [Google Scholar]
- 50.Garg A, et al. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1992;75(2):358–61. [DOI] [PubMed] [Google Scholar]
- 51.Haque WA, et al. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395–8. [DOI] [PubMed] [Google Scholar]
- 52.Antuna-Puente B, et al. Higher adiponectin levels in patients with Berardinelli-Seip congenital lipodystrophy due to seipin as compared with 1-acylglycerol-3-phosphate-o-acyltransferase-2 deficiency. J Clin Endocrinol Metab. 2010;95(3):1463–8. [DOI] [PubMed] [Google Scholar]
- 53.Van Maldergem L, et al. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet. 2002;39(10):722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal AK, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840–7. [DOI] [PubMed] [Google Scholar]
- 55.Upreti V, et al. An unusual cause of delayed puberty: Berardinelli- Seip syndrome. J Pediatr Endocrinol Metab. 2012;25(11–12):1157–60. [DOI] [PubMed] [Google Scholar]
- 56.Maguire M, et al. Pregnancy in a woman with congenital generalized lipodystrophy: leptin’s vital role in reproduction. Obstet Gynecol. 2012;119(2 Pt 2):452–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang M, et al. Lack of testicular seipin causes teratozoospermia syndrome in men. Proc Natl Acad Sci U S A. 2014;111(19):7054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebihara C, et al. Seipin is necessary for normal brain development and spermatogenesis in addition to adipogenesis. Hum Mol Genet. 2015;24(15):4238–49. [DOI] [PubMed] [Google Scholar]
- 59.Karhan AN, et al. Biallelic CAV1 null variants induce congenital generalized lipodystrophy with achalasia. Eur J Endocrinol. 2021;185(6):841–54. [DOI] [PubMed] [Google Scholar]
- 60.Cao H, et al. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis. 2008;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patni N, Hegele RA, Garg A. Caveolar dysfunction and lipodystrophies. Eur J Endocrinol. 2022;186(3):C1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776–82. [DOI] [PubMed] [Google Scholar]
- 63.Patni N, et al. Regional body fat changes and metabolic complications in children with Dunnigan lipodystrophy-causing LMNA Variants. J Clin Endocrinol Metab. 2018;104(4):1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 1999;84(1):170–4. [DOI] [PubMed] [Google Scholar]
- 65.Haque WA, et al. Risk factors for diabetes in familial partial lipodystrophy, Dunnigan variety. Diabetes Care. 2003;26(5):1350–5. [DOI] [PubMed] [Google Scholar]
- 66.Hegele RA. Premature atherosclerosis associated with monogenic insulin resistance. Circulation. 2001;103(18):2225–9. [DOI] [PubMed] [Google Scholar]
- 67.Patni N, et al. A novel syndrome of generalized lipodystrophy associated with pilocytic astrocytoma. J Clin Endocrinol Metab. 2015;100(10):3603–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haddad N, et al. Acquired generalized lipodystrophy under immune checkpoint inhibition. Br J Dermatol. 2020;182(2):477–80. [DOI] [PubMed] [Google Scholar]
- 69.Jehl A, et al. Acquired generalized lipodystrophy: a new cause of anti-PD-1 immune-related diabetes. Diabetes Care. 2019;42(10):2008–10. [DOI] [PubMed] [Google Scholar]
- 70.Savage DB, et al. Complement abnormalities in acquired lipodystrophy revisited. J Clin Endocrinol Metab. 2009;94(1):10–6. [DOI] [PubMed] [Google Scholar]
- 71.Park JY, et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar R, et al. Acquired generalised lipodystrophy and type 1 diabetes mellitus in a child: a rare and implacable association. BMJ Case Rep. 2018;2018:bcr-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srinivasan S, et al. A polygenic lipodystrophy genetic risk score characterizes risk independent of BMI in the diabetes prevention program. J Endocr Soc. 2019;3(9):1663–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Handelsman Y, et al. The clinical approach to the detection of lipodystrophy - an AACE consensus statement. Endocr Pract. 2013;19(1):107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown RJ, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. [DOI] [PubMed] [Google Scholar]
- 77.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12(3):175–81. [PubMed] [Google Scholar]
- 78.Dezenberg CV, et al. Predicting body composition from anthropometry in pre-adolescent children. Int J Obes Relat Metab Disord. 1999;23(3):253–9. [DOI] [PubMed] [Google Scholar]
- 79.Vasandani C, et al. Diagnostic value of anthropometric measurements for familial partial lipodystrophy, Dunnigan Variety. J Clin Endocrinol Metab. 2020;105(7)2132–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Javor ED, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89(7):3199–207. [DOI] [PubMed] [Google Scholar]
- 81.Akinci B, et al. Renal complications of lipodystrophy: a closer look at the natural history of kidney disease. Clin Endocrinol. 2018;89(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Musso C, et al. Spectrum of renal diseases associated with extreme forms of insulin resistance. Clin J Am Soc Nephrol. 2006;1(4):616–22. [DOI] [PubMed] [Google Scholar]
- 83.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–8. [DOI] [PubMed] [Google Scholar]
- 84.Beltrand J, et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics. 2007;120(2):e291–6. [DOI] [PubMed] [Google Scholar]
- 85.Simha V, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab. 2012;97(3):785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diker-Cohen T, et al. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luedtke A, et al. Thiazolidinedione response in familial lipodystrophy patients with LMNA mutations: a case series. Horm Metab Res. 2012;44(4):306–11. [DOI] [PubMed] [Google Scholar]
- 88.Simha V, Rao S, Garg A. Prolonged thiazolidinedione therapy does not reverse fat loss in patients with familial partial lipodystrophy, Dunnigan variety. Diabetes Obes Metab. 2008;10(12):1275–6. [DOI] [PubMed] [Google Scholar]
- 89.Banning F, et al. Insulin secretory defect in familial partial lipodystrophy Type 2 and successful long-term treatment with a glucagon-like peptide 1 receptor agonist. Diabet Med. 2017;34(12):1792–4. [DOI] [PubMed] [Google Scholar]
- 90.Oliveira J, et al. Glucagon-like peptide-1 analogues - an efficient therapeutic option for the severe insulin resistance of lipodystrophic syndromes: two case reports. J Med Case Rep. 2017;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melvin A, et al. Roux-en-Y gastric bypass surgery in the management of familial partial lipodystrophy type 1. J Clin Endocrinol Metab. 2017;102(10):3616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grundfest-Broniatowski S, et al. Successful treatment of an unusual case of FPLD2: The role of Roux-en-Y gastric bypass-case report and literature review. J Gastrointest Surg. 2017;21(4):739–43. [DOI] [PubMed] [Google Scholar]
- 93.Kozusko K, et al. Clinical and molecular characterization of a novel PLIN1 frameshift mutation identified in patients with familial partial lipodystrophy. Diabetes. 2015;64(1):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciudin A, et al. Successful treatment for the Dunnigan-type familial partial lipodystrophy with Roux-en-Y gastric bypass. Clin Endocrinol. 2011;75(3):403–4. [DOI] [PubMed] [Google Scholar]
- 95.McGrath NM, Krishna G. Gastric bypass for insulin resistance due to lipodystrophy. Obes Surg. 2006;16(11):1542–4. [DOI] [PubMed] [Google Scholar]
- 96.Utzschneider KM, Trence DL. Effectiveness of gastric bypass surgery in a patient with familial partial lipodystrophy. Diabetes Care. 2006;29(6):1380–2. [DOI] [PubMed] [Google Scholar]
- 97.Mandel-Brehm C, et al. Autoantibodies to perilipin-1 define a subset of acquired generalized lipodystrophy. Diabetes. 2022; db211172 (online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]