Abstract
This Account highlights the recent contributions made by our laboratory in the development of novel strategies to synthesize fluorinated amines. These strategies allow the practitioner to efficiently access carbamoyl fluorides, thiocarbamoyl fluorides as well as trifluoromethylamines using CO2 or CS2 as benign C1 sources. In addition, a novel N(SCF3)CF3 moiety was synthesized. Noteworthy, we demonstrated that this reagent could also be used in radical- or electrophilic-based trifluoromethylthiolation reactions.
Keywords: Carbamoyl fluorides, CO2 activation, Thiocarbamoyl fluorides, Trifluoromethylamines, Deoxyfluorination, SF6 activation, Trifluoromethylthiolation, Organophotoredox catalysis
Introduction
The synthesis of fluorinated compounds has attracted much attention these last years, and today a plethora of compounds bearing a fluorine atom have found a variety of applications in life sciences.1−3 The driving force for investigating the incorporation of fluorine moieties on several classes of molecules is due to the intrinsic physiochemical properties induced by the fluorine atom including high lipophilicity, high stability, and better resistance toward oxidation.4−6 In addition to fluorine, the nitrogen atom is also ubiquitous in several valuable compounds.7 Thus, the association of both of these atoms should clearly be attractive for several research domains. In this context, we recently established a research program aiming to develop new fluorinated amine derivatives. This Account highlights the contributions made by our laboratory in the past few years.
Synthesis of Carbamoyl Fluorides, Thiocarbamoyl Fluorides, and Trifluromethylamines
The synthesis of carbamoyl fluorides has attracted much attention these last five years due to the increased stability of such a motif in comparison to other halogenated analogues.8−14 However, historical synthetic strategies were mainly based on the use of toxic difluorophosgene derivatives.15−17 More friendly alternatives were since developed relying on the in situ generation of difluorophosgene from a trifluoromethoxy anion20−23 or the use of PDFA24 as a difluorocarbene source in the presence of an oxidant (Scheme 1). It should also be mentioned that other starting materials were used for the synthesis of carbamoyl fluorides including hydroxylamine derivatives.25−28
Scheme 1. Current Syntheses of Carbamoyl Fluorides Starting with Amines.
Regarding our contribution for the synthesis of carbamoyl fluorides, we thought about intercepting the well-established reactivity of amines with atmospheric pressure of CO2 yielding to carbamates. We wondered if deoxyfluorination reagents could react with carbamates to afford the desired carbamoyl fluorides. Thus, we could obtain these value-added products using the inexpensive, safe, and abundant greenhouse gas CO2 as a C1 source. Pleasingly, upon subjecting the amine to atmospheric pressure of CO2 in the presence of DAST and DMAP as an external base, very good to excellent yields of the desired products 2a–2f were obtained (Scheme 2).18 It is worth noting that other deoxyfluorination reagents, including XtalFluor-E, XtalFluor-M and Fluolead, were competent under the reaction conditions, albeit with lower reaction efficiency. Furthermore, labeled 13CO2 could also be successfully used under our reaction conditions and the developed method allows for the direct formation of 13C labeled carbamoyl fluorides.
Scheme 2. Synthesis of Carbamoyl Fluorides through Deoxyfluorination of CO2.
With the idea of making the synthesis of carbamoyl fluorides greener and even more friendly, we then turned our attention to the development of more benign deoxyfluorination reagents. Indeed, we ambitioned to replace DAST with a more sustainable reagent. We identified the valorization of SF6, the most potent greenhouse gas,27 as an attractive strategy. Indeed, given its high stability,28 SF6 is extensively applied as an insulating and arc-quenching gas in circuit breakers and electrical switchgears,29 but its use as a reagent is currently limited. Recent studies reported the activation and the use of this gas as deoxyfluorination reagent.30−35 We showed recently that the activation of atmospheric pressure of SF6 could be achieved by using commercially available tetrakis(dimethylamino)ethylene (TDAE) as a two-electron donor upon blue LED irradiation. We succeeded to isolate an ion pair complex TDAE-SF5–F as a solid (Scheme 3).19
Scheme 3. Synthesis of TDAE-SF5–F Reagent.
With the solid in hand, we assume that such a reagent could serve as a safe source of SF4, a strong deoxyfluorination reagent. In this context, we investigated its usefulness in the synthesis of carbamoyl fluorides under our previously reported CO2 protocol to generate carbamoyl fluorides and valorize two greenhouse gases in the synthetic process. Herein, three equivalents of our SF5-based reagent in the presence of DMAP in MeCN provided the formation of the desired carbamoyl fluorides 2b, 2c, and 2e–g in moderate to excellent isolated yields (Scheme 4).19
Scheme 4. Synthesis of Carbamoyl Fluorides through Deoxyfluorination of CO2 with TDAE-SF5–F.
In parallel to carbamoyl fluorides, thiocarbamoyl fluorides are also attractive class of compounds.17,36 The synthesis of these compounds has gained much attention these last years by using amine derivatives as starting materials and several complementary approaches have been developed.37 It has been demonstrated that nucleophilic trifluoromethylthiolation reagents could be used in conjunction with amines to access the desired thiocarbamoyl fluorides (Scheme 5).38,40,44 Herein, the nucleophilic trifluoromethylthiolating reagent generates thiodifluorophosgene and fluoride anion, and the amine subsequently reacts with thiodifluorophosgene to generate the desired product.39 Other approaches consisted in the in situ generation of thiodifluorophosgene by mixing the Langlois reagent or CF3SO2Cl with triphenylphosphine (Scheme 5)40−42,45−49 Finally, the use of the Ruppert-Prakash reagent or any precursor of difluorocarbene in combination with elementary sulfur were also used for the synthesis of carbamoyl fluorides (Scheme 5).40,43
Scheme 5. Current Syntheses of Thiocarbamoyl Fluorides Starting with Amines.
The strategy we developed consisted of using CS2 instead of CO2 to translate our recently reported concept for the synthesis of carbamoyl fluoride to access the desired thiocarbamoyl fluorides (Scheme 6).37 Subjecting amines to CS2 in the presence of DAST and DIPEA in DCM at room temperature allowed the formation of the desired thiocarbamoyl compounds 3a–f in moderate to excellent isolated yields.
Scheme 6. Synthesis of Carbamoyl Fluorides through Fluorinative Desulfurization of CS2 with DAST.
It has been shown that thiocarbamoyl fluorides have interesting reactivity and can be exploited for direct access to trifluoromethyl amines through a desulfurinative fluorination process. Indeed, inspired by Schoenebeck’s procedure,38 we subjected the thiocarbamoyl fluorides to an excess of silver(I) fluoride in MeCN at 50 °C to afford the desired trifluoromethylamines 4a–c in high yields (Scheme 7).37 One of the major advantages of our procedure is the ease of purification, as the desired products were obtained after simple filtration through a Celite-pad. It should be mentioned that some of these trifluoromethylamines are highly water sensitive, and thus degradation of the trifluoromethylamines is often observed upon aqueous workups.
Scheme 7. Synthesis of Trifluoromethylamines from Thiocarbamoyl Fluorides.
As a greener alternative, we have also been able in this case to replace DAST by our SF6-derivated reagent TDAE-SF5–F for the synthesis of thiocarbamoyl fluorides.19 It turns out that 3 equivalents of our deoxyfluorination reagent in conjunction with Et3N allows the formation of the targeted compounds 3a–c and 3g–i in good to excellent yields. The reaction was performed in DCM at room temperature for 2 h (Scheme 8).
Scheme 8. Synthesis of Thiocarbamoyl Fluorides through Fluorinative Desulfurization of CS2 with TDAE-SF5–F.
Synthesis of Novel N(SCF3)CF3 Compounds and Their Applications
The stability of trifluoromethylamines has been investigated by researchers from AstraZeneca.50 In their study, the authors demonstrated that several trifluoromethylamines were water sensitive. However, the study also highlighted that other classes of amines including trifluoromethylamides, trifluoromethylsulfonamides and bis(trifluoromethyl)amines derivatives offer greater stability.49 Thus, we were eager to develop new trifluoromethylated amine motifs that could be stable under aqueous conditions and to assess their potential applications in drug discovery. Herein, we identified N(SCF3)CF3 as a viable and promising motif based on the properties of both the SCF3 and CF3 groups. From a retrosynthetic standpoint, we envisioned in situ generation of trifluoromethylamino nucleophiles by reacting isothiocyanate derivatives with silver(I) fluoride following a procedure reported by the Schoenebeck group. This intermediate would then react with an electrophilic SCF3 reagent to afford the targeted product. This hypothesis was tested by using Munavalli’s electrophilic trifluoromethylthiolating reagent. Pleasingly, under the developed conditions, several aliphatic as well as aromatic N(SCF3)CF3 groups were synthesized in moderate to very good yields (Scheme 9). However, aniline derivatives with electron withdrawing groups were not tolerated under our reaction conditions.
Scheme 9. Synthesis of N(SCF3)CF3 Using Munavalli’s Reagent.
To address this limitation, we investigated other SCF3 electrophilic sources and were able to successfully couple electron-deficient anilines by reacting the nucleophilic trifluoromethylamines with the (SCF3)2 dimer using a two-chamber reactor. Indeed, the use of Langlois’s reagent (CF3SO2Na) with Ph2PCl allows the formation of the electrophilic dimer in chamber 2 (C2). Given the gaseous nature of this dimer at room temperature, it directly condenses into chamber 1 (C1) and reacts with the formed nucleophilic trifluoromethylamine anion. With this technology, we were able to convert several electron-deficient isothiocayanate derivatives to their corresponding N(SCF3)CF3 analogues in moderate to excellent yields (Scheme 10). Interestingly, we demonstrated that the protocol was scalable with 3.74 g of p-Ph–C6H4N(SCF3)CF3 obtained starting from a 20 mmol scale.
Scheme 10. Synthesis of N(SCF3)CF3 Using (SCF3)2 Dimer.
Afterward, we studied the overall stability of this new motif by subjecting p-Ph–C6H4N(SCF3)CF3, 6m, to various media. Although this compound demonstrated excellent stability in aqueous and other physiological media, it turns out that the degradation of this product was observed under basic conditions. Under this line, we wanted to evaluate the ability of this compound to be used as a shelf-stable reagent for the incorporation of fluorinated motifs.
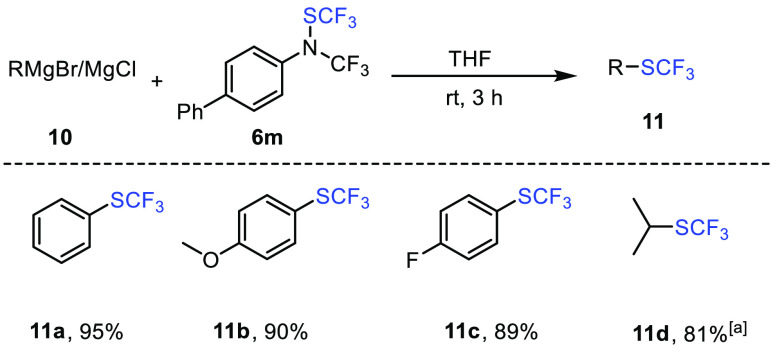
We first reacted compound 6m with nucleophilic Grignard reagents. To our delight, the desired trifluoromethylthiolated products could be obtained in excellent yields with several aromatic and aliphatic Grignard starting materials at room temperature in THF (Scheme 11).51
Scheme 11. Trifluoromethylthiolation of Grignard with Reagent 6m.
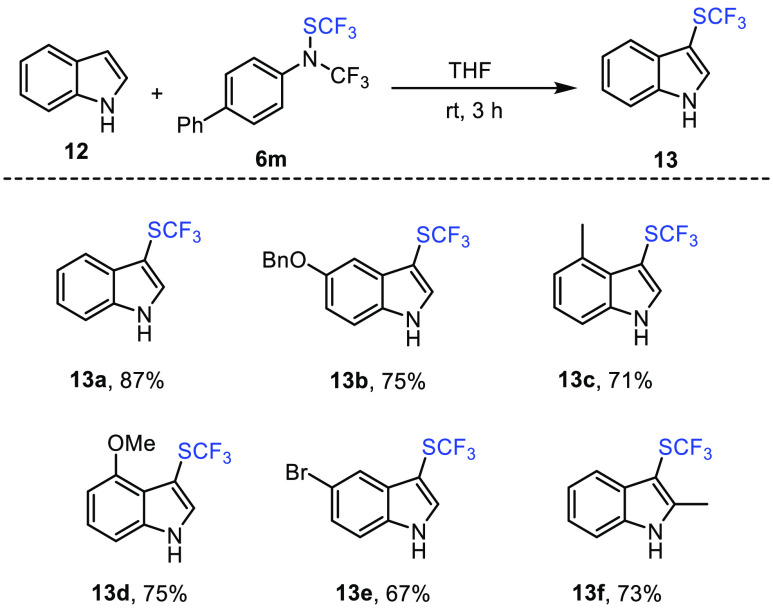
Afterward, we were eager to test the reactivity of this new shelf-stable trifluoromethylthiolating reagent with other nucleophiles. We were able to demonstrate that indole derivatives could be used as nucleophilic partners for the synthesis of trifluoromethylthiolated compounds in very good to excellent yields. The reaction was performed in DMF at 90 °C in the presence of catalytic amount of NaCl (5 mol %) for 24 h (Scheme 12).52
Scheme 12. Trifluoromethylthiolation of Indole Derivatives with Reagent 6m.
Then, we turned our attention to study the usefulness of reagent 6m in the trifluoromethylthiolation of styrenes under photochemical conditions. Vinyl-SCF3 compounds were successfully synthesized using PC1 (4CzIPN) as organophotocalyst under blue LED irradiation (Scheme 13).52 The use of stoichiometric amount of sodium bromide as an activator is mandatory as already reported by Hopkinson, Glorius and co-workers.53
Scheme 13. Synthesis of vinyl-SCF3 under organophotocatalyzed conditions with reagent 6m.
Moreover, by using the same organophotocatalyst we were able to synthesize aroyl-SCF3 derivatives starting from the corresponding aldehydes.52 The key to success is the use of a catalytic amount of sodium benzoate as a HAT catalyst. The desired aroyl-SCF3 were obtained in very good to excellent yields (Scheme 14).
Scheme 14. Synthesis of Aroyl-SCF3 under Organophotocatalyzed Conditions with Reagent 6m.
In conclusion, we have been able to design new routes to access carbamoyl fluorides, thiocarbamoyl fluorides, as well as trifluoromethylamines by activating small molecules including CO2 and CS2 and starting with widely available amine derivatives. While initial methods were based on the use of commercially available DAST as a deoxyfluorination reagent, we have been able to propose an alternative by developing a new stable deoxyfluorination reagent through the activation of the most potent greenhouse gas, SF6. Moreover, the synthesis of new N(SCF3)CF3) motifs has been developed in our laboratory by using two complementary approaches that are based on the in situ generation of the nucleophilic trifluoromethyl anion. While the first one uses Munavalli’s reagent as an electrophilic source of SCF3 to trap the CF3 anion, the second makes use of a two-chamber reactor for the safe generation of the electrophilic (SCF3)2 dimer. Finally, we demonstrated that p-Ph–C6H4N(SCF3)CF3 could be used as a shelf-stable reagent for trifluoromethylthiolation reactions. Future directions in our laboratory are dedicated to the development of other classes of fluorinated amines.
Acknowledgments
Financial support from the CNRS, the University Lyon 1, the Agence Nationale de la Recherche (grant to A.T., ANR-JCJC-2020-CDI-DEOX) and la Région Auvergne-Rhône-Alpes (GES-MEDOC, grant to A.T.) are gratefully acknowledged. Y.Y. thanks the CSC (China Scholarship Council) for a doctoral fellowship.
Data Availability Statement
The data underlying this study are available in the published article.
Author Contributions
CRediT: Yi Yang investigation, writing-original draft; Alexis Taponard investigation, writing-original draft; Julien C. Vantourout supervision; Anis Tlili supervision.
The authors declare no competing financial interest.
References
- Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe G., Leroux F., Eds.; Elsevier Science: London, 2018; pp 459–518. [Google Scholar]
- Kirsch P. In Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2013. [Google Scholar]
- O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]
- Leo A.; Hansch C.; Elkins D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. 10.1021/cr60274a001. [DOI] [Google Scholar]
- Linclau B.; Wang Z.; Compain G.; Paumelle V.; Fontenelle C. Q.; Wells N.; Weymouth-Wilson A. Investigating the Influence of (Deoxy)fluorination on the Lipophilicity of Non-UV-Active Fluorinated Alkanols and Carbohydrates by a New log P Determination Method. Angew. Chem. Int., Ed. 2016, 55, 674–678. 10.1002/anie.201509460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y.; Kamisaki H.; Yanada R.; Takemoto Y. Palladium-Catalyzed Intramolecular Cyanoamidation of Alkynyl and Alkenyl Cyanoformamides. Org. Lett. 2006, 8, 2711–2713. 10.1021/ol060733+. [DOI] [PubMed] [Google Scholar]
- Hande S. M.; Nakajima M.; Kamisaki H.; Tsukano C.; Takemoto Y. Flexible Strategy for Syntheses of Spirooxindoles using Palladium-Catalyzed Carbosilylation and Sakurai-Type Cyclization. Org. Lett. 2011, 13, 1828–1831. 10.1021/ol2003447. [DOI] [PubMed] [Google Scholar]
- Eastwood M. S.; Douglas C. J. Synthesis of the Madangamine Alkaloid Core by a C-C Bond Activation Cascade. Org. Lett. 2019, 21, 6149–6154. 10.1021/acs.orglett.9b02331. [DOI] [PubMed] [Google Scholar]
- Wu X.; Tang Z.; Zhang C.; Wang C.; Wu L.; Qu J.; Chen Y. Pd-Catalyzed Regiodivergent Synthesis of Diverse Oxindoles Enabled by the Versatile Heck Reaction of Carbamoyl Chlorides. Org. Lett. 2020, 22, 3915–3921. 10.1021/acs.orglett.0c01197. [DOI] [PubMed] [Google Scholar]
- Fan P.; Lan Y.; Zhang C.; Wang C. Nickel/Photo-Cocatalyzed Asymmetric Acyl-Carbamoylation of Alkenes. J. Am. Chem. Soc. 2020, 142, 2180–2186. 10.1021/jacs.9b12554. [DOI] [PubMed] [Google Scholar]
- Liu L.; Ishida N.; Murakami M. Atom- and Step-Economical Pathway to Chiral Benzobicyclo-[2.2.2]octenones through Carbon-Carbon Bond Cleavage. Angew. Chem., Int. Ed. 2012, 51, 2485–2488. 10.1002/anie.201108446. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Dong G. (4 + 1) vs (4 + 2): Catalytic Intramolecular Coupling between Cyclobutanones and Trisubstituted Allenes via C-C Activation. J. Am. Chem. Soc. 2015, 137, 13715–13721. 10.1021/jacs.5b09799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichette Drapeau M.; Tlili A. Modern synthesis of carbamoyl fluorides. Tetrahedron Lett. 2020, 61, 152539 10.1016/j.tetlet.2020.152539. [DOI] [Google Scholar]
- Quan H.; Zhang N.; Zhou X.; Qian H.; Sekiya A. Syntheses of isocyanates via amines and carbonyl fluoride. J. Fluorine Chem. 2015, 176, 26–30. 10.1016/j.jfluchem.2015.05.007. [DOI] [Google Scholar]
- Liu L.; Gu Y.-C.; Zhang C.-P. Recent Advances in the Synthesis and Transformation of Carbamoyl Fluorides, Fluoroformates, and Their Analogues. Chem. Rec. 2023, e202300071 10.1002/tcr.202300071. [DOI] [PubMed] [Google Scholar]
- Onida K.; Tlili A. Direct Synthesis of Carbamoyl Fluorides by CO2 Deoxyfluorination. Angew. Chem., Int. Ed. 2019, 58, 12545–12548. 10.1002/anie.201907354. [DOI] [PubMed] [Google Scholar]
- Taponard A.; Jarrosson T.; Khrouz L.; Médebielle M.; Broggi J.; Tlili A. Metal-Free SF6 Activation: A New SF5-Based Reagent Enables Deoxyfluorination and Pentafluorosulfanylation Reactions. Angew. Chem., Int. Ed. 2022, 61, e202204623 10.1002/anie.202204623. [DOI] [PubMed] [Google Scholar]
- Song H.-X.; Han Z.-Z.; Zhang C.-P. Concise and Additive-Free Click Reactions between Amines and CF3SO3CF3. Chem.—Eur. J. 2019, 25, 10907–10912. 10.1002/chem.201901865. [DOI] [PubMed] [Google Scholar]
- Turksoy A.; Scattolin T.; Bouayad Gervais S.; Schoenebeck F. Facile Access to AgOCF3 and Its New Applications as a Reservoir for OCF2 for the Direct Synthesis of N–CF3, Aryl or Alkyl Carbamoyl Fluorides. Chem.—Eur. J. 2020, 26, 2183–2186. 10.1002/chem.202000116. [DOI] [PubMed] [Google Scholar]
- Bonnefoy C.; Chefdeville E.; Tourvieille C.; Panossian A.; Hanquet G.; Leroux F.; Toulgoat F.; Billard T. Chem.—Eur. J. 2022, 28, e202201589 10.1002/chem.202201589. [DOI] [PubMed] [Google Scholar]
- Petzold D.; Nitschke P.; Brandl F.; Scheidler V.; Dick B.; Gschwind R. M.; König B. Visible-Light-Mediated Liberation and In Situ Conversion of Fluorophosgene. Chem.—Eur. J. 2019, 25, 361–366. 10.1002/chem.201804603. [DOI] [PubMed] [Google Scholar]
- Cadwallader D.; Tiburcio R.; Cieszynski G. A.; Le C. M. Synthesis of Carbamoyl Fluorides Using a Difluorophosgene Surrogate Derived from Difluorocarbene and Pyridine N-Oxides. J. Org. Chem. 2022, 87, 11457–11468. 10.1021/acs.joc.2c01017. [DOI] [PubMed] [Google Scholar]
- Baars H.; Engel J.; Mertens L.; Meister D.; Bolm C. The Reactivity of Difluorocarbene with Hydroxylamines: Synthesis of Carbamoyl Fluorides. Adv. Synth. Catal. 2016, 358, 2293–2299. 10.1002/adsc.201600308. [DOI] [Google Scholar]
- Song J. W.; Lim H. N. Synthesis of Carbamoyl Fluorides via a Selective Fluorinative Beckmann Fragmentation. Org. Lett. 2021, 23, 5394–5399. 10.1021/acs.orglett.1c01721. [DOI] [PubMed] [Google Scholar]
- Lindley A. A.; McCulloch A. Regulating to reduce emissions of fluorinated greenhouse gases. J. Fluorine. Chem. 2005, 126, 1457–1462. 10.1016/j.jfluchem.2005.09.011. [DOI] [Google Scholar]
- Seppelt K. Molecular Hexafluorides. Chem. Rev. 2015, 115, 1296–1306. 10.1021/cr5001783. [DOI] [PubMed] [Google Scholar]
- Okubo H.; Beroual A. Recent trend and future perspectives in electrical insulation techniques in relation to sulfur hexafluoride (SF6) substitutes for high voltage electric power equipment. IEEE Electr. Insul. Mag. 2011, 27, 34–42. 10.1109/MEI.2011.5739421. [DOI] [Google Scholar]
- McTeague T. A.; Jamison T. F. Photoredox Activation of SF6 for Fluorination. Angew. Chem., Int. Ed. 2016, 55, 15072–15075. 10.1002/anie.201608792. [DOI] [PubMed] [Google Scholar]
- Rueping M.; Nikolaienko P.; Lebedev Y.; Adams A. Metal-free reduction of the greenhouse gas sulfur hexafluoride, formation of SF5 containing ion pairs and the application in fluorinations. Green Chem. 2017, 19, 2571–2575. 10.1039/C7GC00877E. [DOI] [Google Scholar]
- Berg C.; Braun T.; Ahrens M.; Wittwer P.; Herrmann R. Activation of SF6 at Platinum Complexes: Formation of SF3 Derivatives and Their Application in Deoxyfluorination Reactions. Angew. Chem., Int. Ed. 2017, 56, 4300–4304. 10.1002/anie.201612417. [DOI] [PubMed] [Google Scholar]
- Tomar P.; Braun T.; Kemnitz E. Photochemical activation of SF6 by N-heterocyclic carbenes to provide a deoxyfluorinating reagent. Chem. Commun. 2018, 54, 9753–9756. 10.1039/C8CC05494K. [DOI] [PubMed] [Google Scholar]
- Kim S.; Khomutnyk Y.; Bannykh A.; Nagorny P. Synthesis of Glycosyl Fluorides by Photochemical Fluorination with Sulfur(VI) Hexafluoride. Org. Lett. 2021, 23, 190–194. 10.1021/acs.orglett.0c03915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Nagorny P. Electrochemical Synthesis of Glycosyl Fluorides Using Sulfur(VI) Hexafluoride as the Fluorinating Agent. Org. Lett. 2022, 24, 2294–2298. 10.1021/acs.orglett.2c00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crousse B. Recent Advances in the Syntheses of N-CF3 Scaffolds up to Their Valorization. Chem. Rec. 2023, e202300011 10.1002/tcr.202300011. [DOI] [PubMed] [Google Scholar]
- Onida K.; Vanoye L.; Tlili A. Direct Synthesis of Thiocarbamoyl Fluorides and Trifluoromethylamines Through Fluorinative Desulfurization. Eur. J. Org. Chem. 2019, 2019, 6106–6109. 10.1002/ejoc.201901113. [DOI] [Google Scholar]
- Scattolin T.; Deckers K.; Schoenebeck F. Efficient Synthesis of Trifluoromethyl Amines through a Formal Umpolung Strategy from the Bench-Stable Precursor (Me4N)SCF3. Angew. Chem., Int. Ed. 2017, 56, 221–224. 10.1002/anie.201609480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattolin T.; Pu M.; Schoenebeck F. Investigation of (Me4N)SCF3 as a Stable, Solid and Safe Reservoir for S = CF2 as a Surrogate for Thiophosgene. Chem.—Eur. J. 2018, 24, 567–571. 10.1002/chem.201705240. [DOI] [PubMed] [Google Scholar]
- Zhen L.; Fan H.; Wang X.; Jiang L. Synthesis of Thiocarbamoyl Fluorides and Isothiocyanates Using CF3SiMe3 and Elemental Sulfur or AgSCF3 and KBr with Amines. Org. Lett. 2019, 21, 2106–2110. 10.1021/acs.orglett.9b00383. [DOI] [PubMed] [Google Scholar]
- Liang S.; Wei J.; Jiang L.; Liu J.; Mumtaz Y.; Yi W. One-pot synthesis of trifluoromethyl amines and perfluoroalkyl amines with CF3SO2Na and RfSO2Na. Chem. Commun. 2019, 55, 8536–8539. 10.1039/C9CC03282G. [DOI] [PubMed] [Google Scholar]
- Wei J.; Liang S.; Jiang L.; Yi W. Synthesis of Thiocarbamoyl Fluorides and Isothiocyanates Using Amines with CF3SO2Cl. J. Org. Chem. 2020, 85, 12374–12381. 10.1021/acs.joc.0c01634. [DOI] [PubMed] [Google Scholar]
- Yu J.; Lin J.-H.; Xiao J.-C. Reaction of Thiocarbonyl Fluoride Generated from Difluorocarbene with Amines. Angew. Chem., Int. Ed. 2017, 56, 16669–16673. 10.1002/anie.201710186. [DOI] [PubMed] [Google Scholar]
- Tyrra W. Die Desulfonierung–Fluorierung von Thiuramdisulfiden, [R2NC(S)S]2 und Silberdithiocarbamaten, Ag[SC(S)NR2] (R = CH3, CH3CH2, C6H5CH2), mit Silber(I)fluorid, AgF — ein einfacher Zugang zu Diorgano(trifluormethyl)aminen, R2NCF3, und Thiocarbamoylfluoriden, R2NC(S)F. J. Fluorine. Chem. 2001, 109, 189–194. 10.1016/S0022-1139(01)00391-8. [DOI] [Google Scholar]
- Yang Y.; Xu L.; Yu S.; Liu X.; Zhang Y.; Vicic D. A. Triphenylphosphine-Mediated Deoxygenative Reduction of CF3SO2Na and Its Application for Trifluoromethylthiolation of Aryl Iodides. Chem.—Eur. J. 2016, 22, 858–863. 10.1002/chem.201504790. [DOI] [PubMed] [Google Scholar]
- Xu X.-H.; Matsuzaki K.; Shibata N. Synthetic Methods for Compounds Having CF3–S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731–764. 10.1021/cr500193b. [DOI] [PubMed] [Google Scholar]
- Liao Y.-Y.; Deng J.-C.; Ke Y.-P.; Zhong X.-L.; Xu L.; Tang R.-Y.; Zheng W. Isothiocyanation of amines using the Langlois reagent. Chem. Commun. 2017, 53, 6073–6076. 10.1039/C7CC02373A. [DOI] [PubMed] [Google Scholar]
- Liang S.; Wei J.; Jiang L.; Liu J.; Mumtaz Y.; Yi W. One-pot synthesis of trifluoromethyl amines and perfluoroalkyl amines with CF3SO2Na and RfSO2Na. Chem. Commun. 2019, 55, 8536–8539. 10.1039/C9CC03282G. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Saffon-Merceron N.; Vantourout J. C.; Tlili A. Novel N(SCF3)(CF3)-amines: synthesis, scalability and stability. Chem. Sci. 2023, 14, 3893–3898. 10.1039/D2SC06542H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiesser S.; Chepliaka H.; Kollback J.; Quennesson T.; Czechtizky W.; Cox R. J. Trifluoromethyl Amines and Azoles: An Underexplored Functional Group in the Medicinal Chemist’s Toolbox. J. Med. Chem. 2020, 63, 13076–13089. 10.1021/acs.jmedchem.0c01457. [DOI] [PubMed] [Google Scholar]
- These results are part of Yi Yang PhD thesis; Yang Y.Synthesis of novel fluorinated building blocks. Ph.D. Thesis, Université Claude Bernard Lyon 1, 2023. [Google Scholar]
- Yang Y.; Tang R.; Abid S.; Khrouz L.; Vantourout J. C.; Tlili A. Organophotocatalyzed Synthesis of Vinyl-SCF3 and Benzoyl-SCF3 Using a New Shelf-Stable PhPh-N-(SCF3)(CF3) Reagent. Eur. J. Org. Chem. 2023, 39, e202300619 10.1002/ejoc.202300619. [DOI] [Google Scholar]
- Honeker R.; Garza-Sanchez R. A.; Hopkinson M. N.; Glorius F. Visible-Light-Promoted Trifluoromethylthiolation of Styrenes by Dual Photoredox/Halide Catalysis. Chem.—Eur. J. 2016, 22, 4395–4399. 10.1002/chem.201600190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study are available in the published article.