Abstract
A unique class of chlorate-resistant mutants of Escherichia coli which produced formate hydrogenlyase and nitrate reductase activities only when grown in medium with limiting amounts of sulfur compounds was isolated. These mutants failed to produce the two molybdoenzyme activities when cultured in rich medium or glucose-minimal medium. The mutations in these mutants were localized in the moeA gene. Mutant strains with polar mutations in moeA which are also moeB did not produce active molybdoenzymes in any of the media tested. moeA mutants with a second mutation in either cysDNCJI or cysH gene lost the ability to produce active molybdoenzyme even when grown in medium limiting in sulfur compounds. The CysDNCJIH proteins along with CysG catalyze the conversion of sulfate to sulfide. Addition of sulfide to the growth medium of moeA cys double mutants suppressed the MoeA− phenotype. These results suggest that in the absence of MoeA protein, the sulfide produced by the sulfate activation/reduction pathway combines with molybdate in the production of activated molybdenum. Since hydrogen sulfide is known to interact with molybdate in the production of thiomolybdate, it is possible that the MoeA-catalyzed activated molybdenum is a form of thiomolybdenum species which is used in the synthesis of molybdenum cofactor from Mo-free molybdopterin.
Molybdoenzymes play essential metabolic roles in most organisms from bacteria to plants and animals (34). All molybdoenzymes other than dinitrogenase contain molybdenum cofactor, which consists of a unique molybdopterin (MPT) complexed with molybdenum (1, 12, 23, 31, 34). In Escherichia coli, the biologically active form of the cofactor in molybdoenzymes is MPT guanine dinucleotide (MGD) (5, 22, 23). Synthesis of this cofactor in an active form requires transport of molybdate into the cell, activation of molybdate, synthesis of the MPT moiety, and incorporation of molybdate into MPT. Although molybdate transport and the various steps in the organic part of MGD biosynthesis are well characterized (17, 24, 33; see references 10, 22, and 23 for reviews), very little is known about the activation and incorporation of molybdenum into the cofactor (22).
Mutants which are defective in molybdate metabolism can be isolated as chlorate-resistant mutants (8, 9). A large fraction of these mutants are pleiotropic for all molybdoenzyme activities in the cell, and these comprise the three genetic loci involved in MGD synthesis, moa, mob, and moeB (see references 10, 22, 29, and 31 for reviews). The mod gene products comprise the molybdate transport system through which molybdate is transported into the cell and the Mod− phenotype can be suppressed by increasing molybdate concentration in the medium. The mog mutants which produced formate hydrogenlyase (FHL) activity containing the molybdoenzyme formate dehydrogenase-H (FDH-H) but not nitrate reductase activity was proposed to be defective in molybdochelatase (13, 32). This molybdochelatase is apparently required for production of active nitrate reductase and not for FDH-H.
The moe operon codes for two proteins, and only the physiological role of the second gene product, MoeB protein, is known. The MoeB protein activates MPT synthase, which catalyzes the conversion of MPT precursor (precursor Z) to MPT by introducing the needed sulfur to which Mo is coordinated in the molybdenum cofactor (20, 22). The MoeB protein, MPT synthase sulfurylase, is the known S donor in the activation of MPT synthase. The physiological role of MoeA protein coded by the first gene in the two member moe operon is not known. Mutants which are defective in moeA (chlE [29]) produced about 6% of the wild-type levels of MPT (12), although no molybdoenzyme activity was found in these moeA mutants. Since the MoeB protein acts as an S donor in MPT synthesis, it is possible that the first gene product, MoeA protein, also has a similar role in linking S metabolism and Mo metabolism in the cell.
During our analysis of molybdate transport-defective mutants, we identified a subgroup of chlorate-resistant mutants with a unique phenotype. Mutations in this class of mutants were mapped in the moeA gene at 18.6 min on the E. coli chromosome (3, 18). The MoeA− phenotype was suppressed when the growth medium was supplemented with sulfide. In this report, we present the physiological and genetic characteristics of E. coli moeA mutants and propose a role for the MoeA protein in the activation of molybdenum by sulfurylation.
(This work was presented at the International Symposium on Nitrogen Assimilation: Molecular and Genetic Aspects, 3 to 9 May 1997, Tampa, Fla.)
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains, which are derivatives of E. coli K-12, and plasmids used in this study are presented in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Strains | ||
| BW545 | Δ(lacU)169 rpsL | Laboratory collection |
| VJS1779 | RK4353 moe-251::Tn10d(Tc) | V. Stewart |
| VJS1783 | RK4353 moe-255::Tn10d(Tc) | V. Stewart |
| C26 | gal-26 chlE5 (moeA5) supE42 | CGSC 4459 |
| JT1 | cysN-Km | CGSC 7057 |
| JM73 | cysJ90 | CGSC 5748 |
| SE1581 | BW545 moeA101 | This study |
| SE1588 | BW545 moeA103 | This study |
| SE1905 | SE1588 zbh::Tn10 | This study |
| SE1595 | BW545 modC118 | 25 |
| SE2147 | AH69 zbh::Tn10 | This study |
| AH1 | SE1588 cysN-Km | SE1588 × P1 (JT1) |
| AH8 | JM73 moeA103 zbh::Tn10 | JM73 × P1 (SE1905) |
| AH10 | JT1 moeA103 zbh::Tn10 | JT1 × P1 (SE1905) |
| AH30 | moeB101-Km | This study |
| AH69 | moeA113 ΔzbiK-Km | This study |
| AH131 | BW545 cysN-Km | BW545 × P1 (JT1) |
| AH134 | AH131 moeA113 | AH131 × P1 (SE2147) |
| Plasmids | ||
| pACYC184 | Tcr Cmr cloning vector | 6 |
| pBR322 | Amr Tcr cloning vector | 4 |
| pMAK705 | rep(Ts) Cmr | 11 |
| pUC19 | AmrlacZ′ cloning vector | 35 |
| pUC4K | Kmr Amr | Pharmacia |
| pFGH1 | pACYC184 based, moeA+B+ | Laboratory collection |
| pAH1 | pUC19 based, moeA+B+ | This study |
| pAH2 | pUC19 based, moeA+B+ | This study |
| pAH3-1 | pUC19 based, (moeB-Km) Amr | This study |
| pAH6 | pUC19 based, ΔmoeA moeB+ | This study |
| pAH11 | pMAK705 based, (moeB-Km) rep(Ts) Cmr | This study |
| pAH20 | pUC19 based, moeA+ ΔmoeB | This study |
| pAH43 | pUC19 based, moeA113 moeB+ | This study |
| pAH44 | pAH43 zbiK-Km | This study |
| pAH55 | pMAK705 based, moeA113 moeB+ zbiK-Km | This study |
Media and growth conditions.
L broth (LB) was used as rich medium for growth of organisms under aerobic conditions, and LB with glucose (0.3%; LBG) was used as rich medium for anaerobic growth. LBG was supplemented with formate (15 mM; LBGF), sodium molybdate (1 mM; LBG-Mo), or sodium nitrate (20 mM; LBGN), as needed. The composition of glucose-minimal medium was reported previously (16). Limiting-sulfur medium (LSM) (25) had the following composition: Na2HPO4, 14.89 g; KH2PO4, 6.09 g; NaCl, 0.5 g; NH4Cl, 1.0 g; MgCl2, 0.2 g; Trypticase Peptone (BBL), 1.0 g; yeast extract (BBL), 0.5 g; glucose, 10 g; and deionized water, 1 liter. In all experiments described in this study, both the glucose-minimal medium and LSM were supplemented with 0.1 mM molybdate. Bacterial cultures were grown under anaerobic conditions as previously described (25, 27).
Genetic and molecular biological experiments were performed as described previously (17, 25). DNA was sequenced by using custom primers based on DNA sequence, using the Sanger dideoxy procedure (26) and Sequenase 2.0 (Amersham, Arlington Heights, Ill.). DNA sequence was manipulated with the Genetics Computer Group software (7) or Genepro (Riverside Scientific, Seattle, Wash.).
Isolation of chlorate-resistant mutants.
Chlorate-resistant mutants of strain BW545 were isolated as described previously (17). A culture in the mid-exponential phase of growth was serially diluted and spread on L agar supplemented with potassium chlorate (2 mg ml−1). These plates were incubated under anaerobic conditions at 37°C for 3 days. Small chlorate-resistant colonies were transferred to L agar by replica plating and incubated aerobically at 37°C for 16 h. Chlorate-resistant mutants were tested for the ability to produce dihydrogen when cultured in LBG-Mo and LSM-Mo. Chlorate-resistant mutants which were FHL positive only when grown in LSM with molybdate were used in this study.
Enzyme assay.
FHL and nitrate reductase activities of cultures in the late exponential phase of growth were determined by procedures described previously (16, 25). All FHL assays were carried out with whole cells instead of crude extracts to avoid oxygen inactivation of the enzyme. Sodium nitrate (20 mM) was included in all media used for culturing cells for determination of nitrate reductase activity.
Construction of moe plasmids.
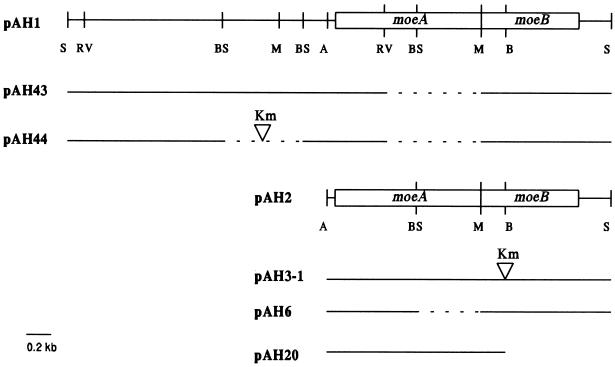
A 4.4-kb SphI fragment which contained the entire moeAB operon including the upstream regulatory region was removed from a larger plasmid, pFGH1, and cloned into plasmid vector pUC19 at the SphI site (plasmid pAH1 [Fig. 1]). DNA coding for the moeAB operon without the upstream regulatory sequences was removed from plasmid pFGH1 as a 2.3-kb AvaI-SphI fragment and cloned into plasmid vector pUC19 at the AvaI-SphI sites (plasmid pAH2 [Fig. 1]). Plasmid pAH6, which carries a deletion of moeA DNA and is still MoeB+, was constructed by removing a BssHII-MluI fragment within the moeA gene in plasmid pAH2 and self-ligating the large DNA fragment (Fig. 1). Plasmid pAH20 lacks the 574-bp BstEII-SphI fragment in plasmid pAH2 (Fig. 1) and produced only MoeA+ protein.
FIG. 1.
Restriction maps of the moe plasmids used in this study. Solid lines represent E. coli chromosomal DNA present in the plasmid; dashed lines indicate regions of chromosomal DNA that were deleted in the construction of the plasmid. Vector DNA is not presented. Each inverted triangle shows the position of insertion of a Kmr gene. A, AvaI; B, BstEII; BS, BssHII; M, MluI; RV, EcoRV; S, SphI.
Construction of moeA deletion strain.
Strain AH69, which carries an internal deletion of moeA and a kanamycin resistance (Kmr) gene cartridge which did not affect production of MoeB+ activity (moeA113), was constructed by first deleting the EcoRV-MluI fragment within the moeA gene in plasmid pAH1 (Fig. 1). The MluI site was modified with DNA polymerase I (Klenow fragment) before self-ligation in the construction of plasmid pAH43. A 661-bp BssHII fragment located 260 bp upstream of the moeAB operon (Fig. 1) was removed from plasmid pAH43 for insertion of Kmr gene from plasmid pUC4K (Pharmacia Biotech, Piscataway, N.J.). After the ends of the large fragment were filled in, a 1.3-kb HincII fragment containing Kmr gene was ligated to produce plasmid pAH44. The entire SphI fragment containing moe operon and the upstream Kmr gene (zbiK-Km) was removed from plasmid pAH44 and ligated into the SphI site in plasmid pMAK705 (11) (yielding plasmid pAH55) for mobilization into the E. coli chromosome. The moeA113 allele from plasmid pAH55 was recombined into the chromosome of strain BW545, creating strain AH69. Strain AH69 is MoeA− and MoeB+.
Deletion of moeA DNA in strain AH69 was confirmed by PCR amplification of chromosomal DNA from strain AH69 and its parent, strain BW545. Two primers flanking the moeA gene (primer 1, ATATGGCATGTAAAGGCAGG; primer 2, CCTGATCGCTGAGTTCCGCC) were synthesized (Genosys, Woodlands, Tex.) and used to amplify the moeA DNA. An expected PCR product of 1.3 kb was detected when the chromosomal DNA from strain BW545 served as the template. A fragment of this size was absent when the chromosomal DNA from moeA deletion strain AH69 was used as the template. Instead of an expected PCR product of 0.6 kb, DNA corresponding to 0.4 kb was seen after agarose gel electrophoresis. The moeA113 and zbiK-Km mutations in strain AH69 cotransduced (phage P1) with each other at a frequency close to 100% and with gal and mod genes at about 20%. The moeA113 mutation was also complemented by plasmid pAH20 (see Results). These results are in agreement with the fact that strain AH69 carries a deletion in the moeA gene.
Construction of moeB-Km strain.
To construct a moeB-Km strain, a HincII fragment containing the Kmr gene from plasmid pUC4K was ligated into the BstEII site in the moeB gene in plasmid pAH2, which had been previously modified by DNA polymerase I (Klenow fragment), yielding plasmid pAH3-1. A SphI-SacI (from vector DNA) fragment containing the entire chromosomal DNA insert from plasmid pAH3-1 was removed and ligated into the SphI-SacI sites of plasmid pMAK705 (11), resulting in plasmid pAH11. Strain BW545 was transformed with plasmid pAH11, and the moeB-Km mutation was transferred to the chromosomal DNA by homologous recombination as previously described (11), generating strain AH30. The moeB-Km mutation cotransduced (phage P1) with gal and mod mutations at a frequency of 20%, and the mutation was complemented by plasmid pAH6, containing only the moeB+ gene. The observed phenotype of strain AH30, genetic characteristics, and complementation profile are in agreement with the reported phenotype of the moeB mutation (20, 22).
Materials.
Biochemicals were purchased from Sigma Chemical Co. Other organic and inorganic chemicals were from Fisher Scientific and were analytical grade. Restriction endonuclease and DNA-modifying enzymes were purchased from Promega (Madison, Wis.) and New England Biolabs (Beverly, Mass.).
RESULTS AND DISCUSSION
During the course of our studies on molybdate transport, we isolated several spontaneous chlorate-resistant mutants of E. coli and separated them into three classes based on the ability to produce FHL activity when grown in different media. Class I mutants are defective in molybdate transport (mod) and produced active molybdoenzymes when grown in medium supplemented with molybdate. Class II mutants are pleiotropic and failed to produce FHL activity in any of the media tested. These mutants are apparently defective in moa, mob, or moeB and were not further characterized. These two classes of mutants were previously described (12, 22, 31).
This selection and screen also yielded a third class of mutants. These mutants are conditional mutants and produced FHL and nitrate reductase activities only when cultured in LSM. Two such mutants, strains SE1581 and SE1588, did not produce FHL activity and produced very low levels of nitrate reductase activity when grown in rich medium (Table 2). Changing the medium from LBG to glucose-minimal medium, even with 0.1 mM molybdate, did not alter the FHL− phenotype of the mutants (SE1581 and SE1588). When cultured in LSM, parent strain (BW545) produced about 30% of the FHL activity obtained with strain BW545 grown in LBG-Mo or glucose-minimal medium with molybdate. However, the nitrate reductase activity of the parent, strain BW545, was not significantly influenced by the growth medium. Both mutant strains, SE1581 and SE1588, produced molybdoenzymes when grown in LSM, but the levels of activity were only about 30 to 40% of that of the parent, strain BW545, grown in the same medium. Production of the two molybdoenzyme activities by the two mutants (strains SE1581 and SE1588) still required molybdate in LSM (data not presented). Increasing the concentrations of molybdate in the medium to 1 mM or higher did not fully restore the ability of these two strains to produce FHL or nitrate reductase activities in LSM. In contrast, mod mutant strain SE1595 responded to molybdate in both LBG and LSM (Table 2).
TABLE 2.
FHL and nitrate reductase activities of class III chlorate-resistant mutants
| Strain | Relevant genotype | Enzyme activity (nmol min−1 mg of cell protein−1)
|
||||||
|---|---|---|---|---|---|---|---|---|
| FHL
|
Nitrate reductase
|
|||||||
| LBG | LBG-Mo | Glucose-minimal | LSM | LBGN | LBGN-Mo | LSMN | ||
| BW545 | Wild type | 230 | 200 | 210 | 60 | 560 | 650 | 530 |
| SE1581 | <1 | <1 | <1 | 20 | 15 | 20 | 225 | |
| SE1588 | <1 | <1 | 2 | 20 | 20 | 30 | 200 | |
| SE1595 | modC | <1 | 160 | NDa | 40 | 25 | 620 | 370 |
ND, not determined.
Since the LSM was previously observed to be limiting in sulfur (16, 25) and strains SE1581 and SE1588 produced FHL and nitrate reductase activities when cultured in LSM, the two mutants were grown in a glucose-minimal medium with 0.1 mM sulfate or with 10 μg of cystine ml−1 as the sole S source. The glucose-minimal medium with a reduced level of sulfur also failed to support production of FHL activity. When nitrate (30 mM) was also included in these media, growth of strains SE1581 and SE1588 was significantly affected, although the parent, strain BW545, grew to acceptable levels. These results show that the suppression of the FHL− phenotype of strains SE1581 and SE1588 required growth in LSM. It is possible that cystine or sulfate even at 0.1 mM repressed the production of some key component required for molybdoenzyme activity in these mutants. It is possible that depletion of sulfur, which could allow production of this unique component, failed to support production of FHL activity, a membrane-bound complex, because of eventual starvation for sulfur.
Strains SE1581 and SE1588 reverted to the wild-type phenotype at a frequency of about 10−8, suggesting that the observed phenotype is due to a single mutation. In a typical chlorate-resistant population, about 25% of the cells were class III mutants. Since the phenotype of the class III mutants differs from those of other previously described chlorate-resistant mutants, the mutation in these mutants was localized in the E. coli chromosome.
Class III mutants are defective in moeA.
A recombinant plasmid carrying E. coli chromosomal DNA which can complement the mutation in strain SE1588 was obtained after transformation with DNA from an E. coli gene bank in plasmid vector pACYC184 and selection using glycerol-nitrate medium without molybdate supplementation. E. coli chromosomal DNA present in the plasmid was sequenced, and based on the DNA sequence, the complementing DNA was identified as moe DNA. A plasmid containing the moeAB DNA (plasmid pAH2) complemented the mutation in all class III mutants. Although the mutation in class III mutants was complemented by moe+ DNA, the observed phenotype of these mutants is different from the previously reported phenotype of moeA and moeB mutants (molybdoenzyme defective in all growth media [18, 20, 22]). Because of the unique nature of the phenotype of strain SE1588 (Table 2), the location of the mutation within the moe operon was first determined by complementation analysis. For these experiments, two plasmids producing only the MoeA+ (plasmid pAH20) or MoeB+ (plasmid pAH6) activity were constructed (Fig. 1). Plasmid pAH6 carries a deletion in moeA (moeA113) which still allowed production of MoeB+ activity.
In the presence of plasmid pAH2 (moeAB), strains SE1581 and SE1588 regained the ability to produce FHL and nitrate reductase activities when cultured in LBG medium (Table 3). Plasmid pAH20, which contains only an intact moeA+ gene, also complemented the mutation in strains SE1581 and SE1588 but not in moeB mutant strain AH30. However, the level of FHL activity produced by strains SE1581(pAH20) and SE1588(pAH20) was lower than the amount produced by the parent strain with or without the plasmid (less than 40%) as well as the amount produced by the mutant strains with plasmid pAH2. Similar results were also obtained with other moeA mutants (data not presented). This lower level of FHL activity produced by strains SE1581(pAH20) and SE1588(pAH20) is apparently due to overproduction of MoeA protein from the plasmid without a corresponding increase in MoeB. The reason for this is unclear. Plasmid pAH6, which contains only moeB+ DNA, complemented the moeB101 mutation in strain AH30 and not the mutation in strain SE1588. The levels of nitrate reductase activity in the plasmid-containing mutant strains SE1581 and SE1588 were about 70 to 85% of the parent levels and were not significantly altered by the moe genotype of the plasmid. These results suggest that the mutation in the class III chlorate-resistant mutants, such as SE1581 and SE1588, is located in the moeA gene. The moeA mutation in these strains was also cotransducible by phage P1 with the mod operon as well as with the gal operon. The cotransduction frequency between the moeA mutation and gal was about 20%. This finding is in agreement with the known locations of these two operons on the E. coli chromosome map (17 min for the gal-mod region and 18.6 min for the moe operon [3]).
TABLE 3.
Complementation of the mutation in class III mutants by various plasmidsa
| Strain | Relevant genotype | Enzyme activity (nmol min−1 mg of cell protein−1)
|
||||||
|---|---|---|---|---|---|---|---|---|
| FHL
|
Nitrate reductase
|
|||||||
| No plasmid | pAH2 | pAH20 | pAH6 | No plasmid | pAH2 | pAH20 | ||
| BW545 | Wild type | 230 | 220 | 220 | 170 | 650 | 730 | 620 |
| SE1581 | <1 | 160 | 90 | NDb | 40 | 500 | 510 | |
| SE1588 | <1 | 180 | 80 | <1 | 40 | 610 | 450 | |
| AH30 | moeB101-Km | <1 | 150 | <1 | 135 | 40 | 600 | 40 |
Cultures were grown in LBG medium for FHL activity and LBGN medium for nitrate reductase activity.
ND, not determined.
Based on these results, moeA mutants can be defined as having a molybdoenzyme activity-negative phenotype when grown in rich medium and a molybdoenzyme-positive phenotype when cultured in medium low in sulfur compounds or in chemically defined medium.
Mutant strains which are defective in moeA have been previously described, and these strains were reported to be defective in molybdoenzyme activity even when grown in minimal medium (32). Mutations in three of these mutants (C26, VJS1779, and VJS1783) were mapped by using plasmids pAH2, pAH6, and pAH20, carrying different regions of the moe operon (data not presented). All three mutants produced FHL activity when plasmid pAH2 (moeA+B+) was present in the cell. Plasmid pAH20 carrying only the moeA+ DNA failed to complement the moe mutations in these mutants. All three mutant strains with plasmid pAH6 (ΔmoeA moeB+) produced FHL activity only when grown in LSM, indicating that the mutation in each of these mutants is located in the moeA gene with a polar effect on moeB gene expression. In the presence of plasmid pAH6, these three mutants produced MoeB protein from the plasmid DNA, and the defect in moeA was suppressed by growth in LSM. Similar results were obtained when FHL was replaced by nitrate reductase as the assay system (data not shown).
To confirm that the observed phenotype of strains SE1581 and SE1588 is due to a mutation in the moeA gene, a ΔmoeA moeB+ mutant (moeA113; strain AH69) was constructed. The moeA113 mutation was also complemented by plasmid pAH20 and not by plasmid pAH6 (data not shown). The phenotype of strain AH69 was similar to that of strain SE1588 (Table 4).
TABLE 4.
Effect of cys mutation on production of FHL and nitrate reductase activities by moeA mutant strain SE1588
| Strain | Relevant genotype | Enzyme activity (nmol min−1 mg of cell protein−1)
|
|||
|---|---|---|---|---|---|
| FHL
|
Nitrate reductase
|
||||
| LBG | LSM | LBGN | LSMN | ||
| BW545 | Wild type | 240 | 65 | 570 | 530 |
| SE1588 | moeA103 | <1 | 35 | 50 | 320 |
| AH69 | moeA113 | <1 | 25 | 40 | 300 |
| AH131 | cysN-Km | 230 | 45 | 630 | 570 |
| AH1 | cysN-Km moeA103 | <1 | <1 | 35 | 55 |
| AH134 | moeA113 cysN-Km | <1 | <1 | 50 | 40 |
| JM73 | cysJ | 130 | 25 | 720 | 450 |
| AH8 | cysJ moeA103 | <1 | <1 | 50 | 60 |
Role of cys gene products in suppression of moeA mutation.
Our previous studies show that the mod mutants, which are defective in molybdate accumulation, are capable of utilizing the sulfate transport system coded by the cysUWA (previously cysTWA [3, 15, 25]) genes for molybdate transport. The uptake of molybdate via the sulfate transporter required cultivation of mod mutants in LSM to activate the production of sulfate transport components. Addition of cystine to LSM prevented production of molybdoenzyme activity since cystine is known to significantly reduce the level of the sulfate transporter (15, 16). Suppression of the moeA mutation by growth in LSM suggests that the cys gene products are also responsible for molybdoenzyme production in moeA mutants grown in LSM. The moeA mutants were found to be molybdate transport competent (data not presented). Double mutants with a moeA mutation and a second mutation in either cysU, cysW, or cysA still produced FHL and nitrate reductase activities when cultured in LSM, suggesting that the suppression of the moeA phenotype in LSM is not due to increased molybdate transport through the sulfate transport system. Thus, the cys gene products needed for suppression of the MoeA− phenotype must be in the cysteine biosynthetic pathway after the sulfate transport process.
Double mutants with the moeA mutation and a second mutation in any of the genes in the two operons (cysDNC and cysJIH) coding for proteins in the activation and reduction of sulfate to sulfide (15) failed to produce FHL activity in LSM. Results for two such double mutants involving one cys gene from each operon are presented in Table 4. In the construction of the double-mutant strains AH1 and AH10, the moeA103 and cysN mutations were transduced in both directions (Table 1). The moeA cysN double mutants lacked FHL and nitrate reductase activities irrespective of the growth medium. Other double mutants with point mutations in cysD or cysC and moeA103 had similar phenotypes (data not presented). A double mutant carrying cysN mutation and a deletion in the moeA gene (moeA113) (strain AH134) also failed to produce FHL and nitrate reductase activities (Table 4).
A double mutant with mutations in cysJ, a component of the second operon (cysJIH) coding for the 3′-phosphoadenosine 5′-phosphosulfate reductase and sulfite reductase (15), and moeA103 (strain AH8) also lacked FHL and nitrate reductase activities (Table 4). Spontaneous revertants of strain AH8 for Cys+ produced both molybdoenzymes when grown in LSM, suggesting that the cys and moeA mutations are the only mutations causing the observed molybdoenzyme-negative phenotype of strain AH8. Similar results were also obtained with double mutants and Cys+ revertants with cysI and cysH mutations.
The MoeA− phenotype is suppressed by sulfide.
Since all of the enzymes in the sulfate activation/reduction pathway are essential for production of FHL and nitrate reductase activities by moeA mutants grown in LSM, it is possible that the end product sulfide is necessary for the activation of Mo in the absence of MoeA protein. Apparently, the CysDNCJIH proteins and thus sulfide are not produced in the moeA mutants grown in rich medium or glucose-minimal medium due to the presence of S compounds which prevented expression of cys gene products (15) to optimum levels for suppression of the MoeA− phenotype.
When wild-type strain BW545 or cysN mutant strain AH131 was grown in rich medium or LSM, the amount of nitrate reductase activity produced by the culture was not altered by including sodium sulfide in the growth medium (Table 5). moeA mutant strain AH69 produced about 380 U (about 50% of the wild-type level) of nitrate reductase activity when grown in rich medium only when the medium was also supplemented with sodium sulfide. Similarly, the FHL activity of the mutant grown in LBG was also increased from undetectable levels to about 25 U (data not presented). Sulfide also restored the ability of the moeA cysN double mutants (strains AH1 and AH134) to produce both molybdoenzyme activities when grown in either rich medium or LSM. Addition of sulfide to glucose-minimal medium failed to suppress the FHL− phenotype of moeA mutants, probably due to the presence of sulfate in the medium. When sulfide served as the sole S source, strains SE1581 and SE1588 produced low but detectable levels of FHL activity.
TABLE 5.
Effect of sulfide on nitrate reductase activity produced by moeA mutantsa
| Strain | Relevant genotype | Nitrate reductase activity (nmol min−1 mg of cell protein−1)
|
|||
|---|---|---|---|---|---|
| LBGN | LBGN + S2− | LSMN | LSMN + S2− | ||
| BW545 | Wild type | 670 | 700 | 585 | 530 |
| AH131 | cysN-Km | 630 | 725 | 570 | 625 |
| AH69 | moeA113 | 40 | 380 | 320 | 300 |
| AH1 | moeA103 cysN-Km | 55 | 400 | 65 | 360 |
| AH134 | moeA113 cysN-Km | 50 | 280 | 40 | 275 |
Sodium molybdate concentrations were 1 mM in LBGN and 0.1 mM in LSMN. Sodium sulfide concentrations were 2 mM in LBGN and 0.2 mM in LSMN.
These results suggest that in the absence of MoeA protein in the cell (moeA mutant), sulfide, generated by the sulfate activation/reduction pathway, chemically interacts with molybdate in the production of activated molybdenum for incorporation into molybdopterin. Hydrogen sulfide is known to react with molybdate in the production of various thiomolybdate compounds (dithiomolybdate, tetrathiomolybdate, etc.), depending on the ratio of sulfide and molybdate, and this reaction is used in chemical synthesis of thiomolybdate (2). Apparently, cystine is not a sulfur donor in this process since inclusion of cystine at a concentration as high as 200 μg ml−1 in LSM or glucose-minimal medium failed to overcome the defect in the moeA cysN mutant strain AH1. Inclusion of various thiomolybdates to the culture medium of strain AH1 also did not restore the ability to produce molybdoenzymes, probably due to a lack of thiomolybdate transport. However, thiomolybdate did serve as a source of molybdate for molybdoenzyme synthesis and activity in a mod mutant (data not presented).
Putative physiological role of MoeA protein.
The results presented above suggest that the MoeA protein catalyzes the formation of activated molybdenum. It is possible that the activated molybdenum is a form of Mo-S complex (thiomolybdate?) and the MoeA protein itself or another protein provides the needed sulfur. This Mo-S complex could serve as the substrate during incorporation of molybdenum into the molybdopterin in the synthesis of molybdenum cofactor. This proposed role of MoeA as a sulfur source for the synthesis of a molybdenum-sulfur complex would be similar to the role of MoeB as a sulfur donor (MPT synthase sulfurylase) in the activation of MPT synthase (20, 22). Thus, the two proteins coded by the moe operon would catalyze similar reactions but with differing substrates.
An alternate possibility that the MoeA protein is the S donor for MoeB protein in the activation of MPT synthase small subunit cannot be ruled out. In this role, the MoeA protein would be similar to the NifS protein of Azotobacter vinelandii, which apparently provides the needed sulfur for biosynthesis of Fe-Mo cofactor by desulfuration of cysteine (36). It is possible that in the absence of MoeA protein, sulfide serves as a direct source of S for MoeB protein, although at a lower level. However, in other studies of transcription of hyc-lac and nar-lac, MoeA protein and not MoeB protein was found to be required for optimum expression of the two operons coding for molybdoenzymes, in response to molybdate (unpublished data). Such a requirement for only the MoeA protein is difficult to reconcile with a putative role of MoeA as strictly an S donor for MoeB activity in molybdopterin synthesis. Additionally, the MoeA protein and NifS sequences are not similar. Direct biochemical experiments are needed to establish the role of MoeA protein in molybdenum cofactor biosynthesis and Mo metabolism.
Recently, the crystal structure of FDH-H, a component of the FHL complex, was reported and it was shown that the FDH-H contains two MGD molecules coordinating a single molybdenum through four sulfur atoms (5). A similar structure was identified in the dimethyl sulfoxide reductase from Rhodobacter capsulatus, which also contains two MGD molecules (28). The Mo is coordinated in these molybdoenzymes by four S atoms from the two MGD molecules. The MoeA-produced thiomolybdenum species could serve as the donor of Mo in the production of active cofactor by transferring Mo from one Mo-S complex to sulfur in the MPT.
In three different eukaryotes, Drosophila melanogaster (14), rat (21), and Arabidopsis thaliana (30), the MoeA and Mog protein homologs constitute a single protein. It is believed that these MoeA-Mog proteins catalyze the same steps in Mo-MPT synthesis in these organisms also. Among the three organisms, only DNA coding for the protein from Arabidopsis was isolated by complementation of an E. coli mog mutation. However, this Arabidopsis cDNA encoding the MoeA-Mog hybrid protein failed to complement the E. coli moeA mutation (16a). In E. coli, the MoeA and Mog proteins are independent and genetically well separated (moeA, 18.7 min; mog, 0.2 min [3]). It is possible that the activated molybdenum species produced by the MoeA protein is transferred to the Mog protein as a prelude to incorporation into molybdopterin. Joshi et al. (13) proposed that the Mog protein in E. coli is a molybdochelatase which binds molybdenum prior to incorporation into MPT. This suggested role would be in agreement with the proposed model except that the Mo would be a thiomolybdenum species. Alternatively, the possibility that a MoeA-Mog complex catalyzes the synthesis of thiomolybdenum in which the MoeA protein is the sulfur donor and the thiomolybdenum is a protein-bound intermediate cannot be ruled out. However, a soluble or dissociable Mo complex can be formed, as demonstrated by the ability of the moeA mutants to produce active molybdoenzymes, such as nitrate reductase, in the presence of molybdate and sulfide in the growth medium.
Conclusion.
The results presented above show that in E. coli, the MoeA protein is essential for production of active molybdoenzymes and that this requirement for MoeA protein can be suppressed by the enzymes in the sulfate activation/reduction pathway or by supplementation of the medium with sulfide. The MoeA protein apparently catalyzes the production of a Mo-S complex which can be generated at a lower rate by direct interaction between Mo and sulfide. This complex could be a thiomolybdenum species since H2S and molybdate can yield thiomolybdate. The thiomolybdenum compound is apparently the Mo donor in the production of the Mo cofactor.
ACKNOWLEDGMENTS
We thank V. Stewart for providing strains and W. Klipp for suggesting the investigation into the role of sulfide.
This work was supported by Public Health Service grant GM48667 from the National Institutes of Health.
Footnotes
Florida Agricultural Experiment Station Journal Series no. R06089.
REFERENCES
- 1.Allen R M, Chatterjee R, Madden M S, Ludden P W, Shah V K. Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Crit Rev Biotechnol. 1994;14:225–249. doi: 10.3109/07388554409079834. [DOI] [PubMed] [Google Scholar]
- 2.Aymonino P J, Ranade A C, Dieman E, Muller A. Study of formation and relative reaction rates of different thioanions of molybdenum and tungsten. Z Anorg Allg Chem. 1969;371:300–305. [Google Scholar]
- 3.Berlyn M K B, Low K B, Rudd K E. Linkage map of Escherichia coli K-12, edition 9. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1715–1902. [Google Scholar]
- 4.Bolivar F, Roriquez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 5.Boyington C J, Gladyshev V N, Khangulov S V, Stadtman T C, Sun P D. Crystal structure of formate dehydrogenase H: catalysis involving Mo, molybdopterin, selenocysteine, and an Fe4S4 cluster. Science. 1997;275:1305–1308. doi: 10.1126/science.275.5304.1305. [DOI] [PubMed] [Google Scholar]
- 6.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubourdieu M, Andrade E, Puig J. Molybdenum and chlorate resistant mutants in Escherichia coli K-12. Biochem Biophys Res Commun. 1976;70:766–773. doi: 10.1016/0006-291x(76)90658-6. [DOI] [PubMed] [Google Scholar]
- 9.Glaser J H, DeMoss J A. Comparison of nitrate reductase mutants of Escherichia coli selected by alternative procedures. Mol Gen Genet. 1972;116:1–10. doi: 10.1007/BF00334254. [DOI] [PubMed] [Google Scholar]
- 10.Grunden A M, Shanmugam K T. Molybdate transport and regulation in bacteria. Arch Microbiol. 1997;168:345–354. doi: 10.1007/s002030050508. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinton S M, Dean D. Biogenesis of molybdenum cofactors. Crit Rev Microbiol. 1990;17:169–188. doi: 10.3109/10408419009105724. [DOI] [PubMed] [Google Scholar]
- 13.Joshi M S, Johnson J L, Rajagopalan K V. Molybdenum cofactor biosynthesis in Escherichia coli mod and mog mutants. J Bacteriol. 1996;178:4310–4312. doi: 10.1128/jb.178.14.4310-4312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamdar K P, Shelton M, Finnerty V. The drosophila molybdenum cofactor gene cinnamon is homologous to three Escherichia coli cofactor proteins and to the rat protein gephyrin. Genetics. 1994;137:791–801. doi: 10.1093/genetics/137.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kredich N M. Biosynthesis of cysteine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 514–527. [Google Scholar]
- 16.Lee J H, Wendt J C, Shanmugam K T. Identification and characterization of a new gene, molR, essential for utilization of molybdate by Escherichia coli. J Bacteriol. 1990;172:2079–2087. doi: 10.1128/jb.172.4.2079-2087.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Mandel, R. R. Personal communication.
- 17.Maupin-Furlow J A, Rosentel J K, Lee J H, Deppenmeier U, Gunsalus R P, Shanmugam K T. Genetic analysis of the modABCD operon (molybdate transport) of Escherichia coli. J Bacteriol. 1995;177:4851–4856. doi: 10.1128/jb.177.17.4851-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nohno T, Kasai Y, Saito T. Cloning and sequencing of the Escherichia coli chlEN operon involved in molybdopterin biosynthesis. J Bacteriol. 1988;170:4097–4102. doi: 10.1128/jb.170.9.4097-4102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pateman J A, Cove D J, Rever B M, Roberts D B. A common cofactor for nitrate reductase and xanthine dehydrogenase which also regulates the synthesis of nitrate reductase. Nature. 1964;201:58. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- 20.Pitterle D M, Johnson J L, Rajagopalan K V. In vitro synthesis of molybdopterin from precursor Z using purified converting factor. Role of protein-bound sulfur in formation of the dithiolene. J Biol Chem. 1993;268:13506–13509. [PubMed] [Google Scholar]
- 21.Prior P, Schmidt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J, Betz H. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan K V. Biosynthesis of the molybdenum cofactor. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 674–679. [Google Scholar]
- 23.Rajagopalan K V, Johnson J L. The pterin molybdenum cofactors. J Biol Chem. 1992;267:10199–10202. [PubMed] [Google Scholar]
- 24.Rech S, Wolin C, Gunsalus R P. Properties of periplasmic ModA molybdate-binding protein of Escherichia coli. J Biol Chem. 1996;271:2557–2562. doi: 10.1074/jbc.271.5.2557. [DOI] [PubMed] [Google Scholar]
- 25.Rosentel J K, Healy F, Maupin-Furlow J A, Lee J H, Shanmugam K T. Role of molybdate transport system(s) in the regulation of formate hydrogenlyase synthesis in Escherichia coli. J Bacteriol. 1995;177:4857–4864. doi: 10.1128/jb.177.17.4857-4864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sankar P, Shanmugam K T. Biochemical and genetic analysis of hydrogen metabolism in Escherichia coli: the hydB gene. J Bacteriol. 1988;170:5433–5439. doi: 10.1128/jb.170.12.5433-5439.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindelin H, Kisker C, Hilton J, Rajagopalan K V, Rees D C. Chrystal structure of DMSO reductase: redox-linked changes in molybdopterin coordination. Science. 1996;272:1615–1621. doi: 10.1126/science.272.5268.1615. [DOI] [PubMed] [Google Scholar]
- 29.Shanmugam K T, Stewart V, Gunsalus R P, Boxer D H, Cole J A, Chippaux M, Demoss J A, Giordano G, Lin E C C, Rajagopalan K V. Proposed nomenclature for the genes involved in molybdenum metabolism in Escherichia coli and Salmonella typhimurium. Mol Microbiol. 1992;6:3452–3454. doi: 10.1111/j.1365-2958.1992.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 30.Stallmeyer B, Nerlich A, Schiemann J, Brinkmann H, Mandel R R. Molybdenum co-factor biosynthesis: the Arabidopsis thaliana cDNA cnx1 encodes a multifunctional two-domain protein homologous to a mammalian neuroprotein, the insect protein cinnamon and three Escherichia coli proteins. Plant J. 1995;8:751–762. doi: 10.1046/j.1365-313x.1995.08050751.x. [DOI] [PubMed] [Google Scholar]
- 31.Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988;52:190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart V, MacGregor C H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982;151:788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walkenhorst H, Hemschemeier S, Eichenlaub R. Molecular analysis of the molybdate uptake operon, modABCD, of Escherichia coli and modR a regulatory gene. Microbiol Res. 1995;50:347–361. doi: 10.1016/S0944-5013(11)80016-9. [DOI] [PubMed] [Google Scholar]
- 34.Wootton J C, Nicolson R E, Cock J M, Walters D E, Burke J F, Doyle W A, Cray R C. Enzymes depending on the pterin molybdenum cofactor: sequence families, spectroscopic properties of molybdenum and possible cofactor-binding domains. Biochim Biophys Acta. 1991;1057:157–185. doi: 10.1016/s0005-2728(05)80100-8. [DOI] [PubMed] [Google Scholar]
- 35.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 36.Zheng L, White R H, Cash V L, Jack R F, Dean D R. Cysteine desulfurase activity indicates a role for NifS in metallocluster biosynthesis. Proc Natl Acad Sci USA. 1993;90:2754–2758. doi: 10.1073/pnas.90.7.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]