Abstract
Background
Aquatic exercise (AE) is becoming ever more popular as a physical therapy, while it is unclear what precise improvements it will produce and how effective it will be in comparison with other non-surgical therapies. The study aimed to assess whether AE positively impacts chronic musculoskeletal disorder patients in terms of pain, physical function, and quality of life.
Methods
PRISMA guidelines were followed, and our study protocol was published online at PROSPERO under registration number CRD42023417411. We searched PubMed, Embase, Web of Science, and Cochrane library databases for English-language articles published before April 11, 2023, including studies from all relevant randomized controlled trials (RCTs). After screening, we ultimately included 32 RCTs with a total of 2,200 participants. We also performed subgroup analyses for all included studies. This meta-analysis calculated standardized mean difference (SMD) with 95% confidence interval (CI), and the variance was estimated using a random-effects model. The quality of the included studies was assessed by using the Cochrane collaborative "risk of bias" assessment tool (version 2.0). Thus ensuring that the literature included is of high quality.
Results
This meta-analysis included 32 trials with 2,200 participants; these patients were all between the ages of 38–80. The study showed that compared to the no exercise (NE) group, patients in the AE group experienced a remarkable reduction in pain (SMD: -0.64, P < 0.001), a significant increase in physical function (SMD: 0.62, P < 0.001), and a statistically significant improvement in quality of life (SMD: −0.64, P < 0.001). When compared to land-based exercise (LE), AE significantly relieves patients' pain (SMD: −0.35, P = 0.03).
Conclusions
This is the first systematic review and meta-analysis to study whether AE could improve chronic musculoskeletal disorders. The evidence suggests that AE benefits pain, physical function, and quality of life in adults with chronic musculoskeletal conditions compared to NE. Furthermore, when compared to LE, AE continues to provide a better improvement in patient pain. More long-term clinical trials are needed to confirm AE's positive effects and improvement mechanisms and the more existential advantages compared to LE.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-023-04417-w.
Keywords: Water exercise, Musculoskeletal pain, Sports medicine, Rehabilitation medicine
Introduction
Chronic musculoskeletal disorders are the second leading cause of disability, with approximately 1.71 billion people worldwide suffering from the disease to date [1]. Per the 11th revision of the International Classification of Diseases and Related Health Problems [2], the definition of chronic musculoskeletal disorder is a persistent disorder lasting more than 3 months and is typically characterized by chronic pain and functional disability [3]. Patients often show stiffness and pain in joints and muscles, injury, and inflammation in related parts of the body [4]. In severe cases, complications, such as hypertension and depression, make patients' survival less efficient and greatly limited, as well as imposing a heavy burden on society and the economy [1]. For this reason, the therapy of chronic musculoskeletal disorders has always been the focus of attention of patients, the clinical and scientific community, and society.
In the traditional conservative treatment of chronic musculoskeletal disorders, drugs, injections, or electroshock therapy are often costly [5] and the results are not particularly gratifying to patients [6]. Fortunately, aquatic exercise (AE) may bring new treatment options for patients. AE refers to water-based therapy or training [7]. In the last 20 years, AE has become increasingly popular as an emerging physical therapy for patients and physicians alike [8]. It has the unique advantage of being less costly while meeting the corresponding psychosocial needs and reducing the patient's feelings of helplessness and isolation. At the same time, since the patients involved are mainly middle-aged and elderly [9], a special group with a higher risk of falling, and the buoyancy generated by water can reduce the possibility of injury to the participants. Moreover, as buoyancy reduces the pressure of gravity on muscles and joints, it is also more suitable for special groups such as obese, postmenopausal women, and injured athletes [10].
In recent years, a large number of clinical trials have been conducted on the topic of AE, relevant research found that AE has the potential to improve the treatment of chronic musculoskeletal diseases, such as the improvement of pain and quality of life. However, to date, while some studies have described AE as an effective treatment for osteoarthritis and fibromyalgia by improving pain and quality of life, there are still studies concluding that AE is not effective [11–13]. Meanwhile, we found that these studies were limited to a specific disease [14]. Of the relevant meta-analyses currently available, the systematic review by McVeigh et al. [15] and Waller et al. [16] had the drawback of including a small number of articles due to the early years of the study. The study by Heywood et al. [17] could not evaluate the therapeutic effects of AE in a comprehensive and multifaceted manner due to the variable quality of the included articles and the limitations of the observed indicators, the research analysis by Batterham et al. [18] and Lu et al. [19] focused on only one type of disease within musculoskeletal disorders and did not summarize this general group of disorders. Besides that, Zão et al. [20] only performed a systematic review and did not perform a meta-analysis. There have been no studies using meta-analysis methods to assess whether AE could improve chronic musculoskeletal disorders. The purpose of this study is to investigate the efficacy and role of AE in the treatment of chronic musculoskeletal disorders. We also hope to provide a reference for future clinical applications.
Methods
Protocol and registration
The present systematic review and meta-analysis have been completed for registration in PROSPERO (No. CRD42023417411) and followed up the standard Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook to ensure a transparent review. The prior research question and search strategy were formulated according to the Population, Intervention, Control/Comparison, and Outcome (PICO) framework to enhance search precision and ensure extensive data extraction to be representative and unbiased. The research question was: Whether AE could improve chronic musculoskeletal disorders?
Search strategy
The authors systematically searched four databases, PubMed, Embase, Web of Science, and Cochrane Library, for articles published in English before April 11, 2023, and screened all retrieved articles based on the inclusion criteria. We mainly used the following combinations of Mesh terms for the literature search: (hydrotherapy OR aquatic therapy) AND musculoskeletal diseases AND chronic disease. In the meantime, we also screened references in relevant reviews and meta-analyses to avoid omitting qualified articles. The detailed search strategy and results can be shown in Additional file 1: Table S1.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) article type is randomized controlled trial (RCT); (2) participants had to be diagnosed with at least one musculoskeletal disorder that persisted for more than three months, with no age restriction; (3) the experimental group used AE therapy (AE interventions included any such as endurance, resistance, strength, balance, flexibility training in water, and warm-up aerobic exercise), while the control group was studied with land-based exercise (LE) therapy or no exercise (NE) (including non-activity, such as education, and meditation); and (4) the study had to measure the baseline values of each indicator for each group of subjects before the intervention and the corresponding values at the end of the intervention, with some data from studies with follow-up at the end of the intervention, were also included, measuring indicators including pain, physical function and quality of life.
The exclusion criteria were as follows: (1) articles published in languages other than English; (2) studies without a control group; (3) articles that only mentioned that the participants were in the state of being in the preoperative phase of joint replacement or had completed the relevant surgery, but did not mention the specific diagnosed disease of the participants; (4) the experimental group used other therapies, such as spa therapy and balneotherapy; (5) the experimental group engaged in AE along with LE; (6) the control group used any therapy other than LE and no sports that might have had some effect on the physical fitness of the participants; (7) full text or complete data are not available from relevant sources and the relevant data mentioned in the article is not available; and (8) duplicate published studies.
Data extraction
The two authors (JW and YC) independently followed a pre-designed table for the extraction of relevant data from the screened articles. Any problems that arose during the extraction of the data have been resolved after a thorough discussion between the authors. Data extracted by the authors from each study included article authorship, date of publication, participants' diagnosis, demographic characteristics (number of participants, gender, age), intervention characteristics (intervention-specific measures, intervention period, and frequency), baseline and post-intervention outcome data (major endings include pain, physical function, and quality of life), the types of data included were mean, standard deviation (SD), and sample size (if the study had no SD, quartiles or 95% confidence interval (CI) or standard error (SE) or standard error of the mean (SEM) were included and converted to SD by a conversion formula). In the case of multiple assessment scales for the same indicator in the included outcome data, preference was given to the scale used for the primary outcome or to the more well-known and universal scale. Table 1 represents the list of outcome measures that met the inclusion criteria.
Table 1.
Outcome measures eligible to be included in the meta-analysis
| Outcomes | Scales |
|---|---|
| Pain | VAS, SF-36, FIQ, WOMAC, KOOS, BPI, HAQ, MPQ |
| Physical function | SF-36, SF-12, FIQ, WOMAC |
| Quality of life | FIQ, KOOS, HAD, BAI, ODI, PQOL, SF-36, HAQ, AQOL, EQ-5D, WOMAC |
VAS, Visual Analog Scale; SF−36, Medical Outcomes Study 36−Item Short−Form Health Survey; FIQ, Fibromyalgia Impact Questionnaire; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; KOOS, Knee Injury and Osteoarthritis Outcome Score; BPI, Brief Pain Inventory; HAQ, Health Assessment Questionnaire; MPQ, McGill Pain Questionnaire; SF−12, Medical Outcomes Study 12−Item Short−Form Health Survey; HAD, Hospital Anxiety and Depression Scale; BAI, Beck Anxiety Inventory; ODI, Oswestry Disability Index; PQOL, Perceived Quality of Life Scale; AQoL, Arthritis Quality of Life Scale; EQ−5D, European Quality of Life−5 Dimensions Scale.
Quality assessment and risk of bias
Two reviewers (JW and YC) used the Cochrane collaborative "risk of bias" assessment tool to conduct a bias analysis of the included randomized controlled studies. It contains a total of six domains: selective bias (random sequence generation, allocation concealment), implementation bias (subject, trial personnel unblinding), measurement bias (outcome assessor unblinding), follow-up bias (incomplete outcome data), reporting bias (selective reporting of results), and other bias (other factors causing risk of bias). The risk of bias was ranked into three levels: low risk, high risk, and unknown risk. The criteria for determining each risk were those documented in the Cochrane Handbook for the Systematic Evaluation of Interventions. In the event of disagreement between two reviewers when evaluating the same study, a third reviewer (TW) was invited to conduct the evaluation.
Statistical analysis
The authors used Review Manager software (version 5.3) to analyze the effects of AE on patients with chronic musculoskeletal disorders, measuring pain, physical function, and quality of life. Pain, physical function, and quality of life were analyzed as subgroups according to the type of diseases the patients had (osteoarthritis, fibromyalgia, low back pain, and ankylosing spondylitis). Each study established at least one control group, and the effect of the intervention was assessed by comparing baseline and post-intervention values between the test and control groups. Data from each group were combined and meta-analyzed, described using standard mean difference (SMD) and 95% CI, and the authors used chi-square tests to examine heterogeneity and I2 to assess the effect of heterogeneity. Substantial heterogeneity was considered to exist if I2 ≥ 50%. The effects were considered negligible for SMD < 0.2, small for 0.2 ≤ SMD < 0.5, moderate for 0.5 ≤ SMD < 0.8, and high for SMD ≥ 0.8. Considering the existence of different studies using different scales when measuring the same indicator, a random-effects model was selected and P < 0.05 was considered statistically significant. Potential factors that lead to possible heterogeneity, i.e., disease type, were investigated by subgroup analysis. We also used the software to create funnel plots for the stability of studies and performed sensitivity analyses to identify possible sources of heterogeneity by excluding included studies on a study-by-study basis to observe changes in heterogeneity.
Results
Study selection
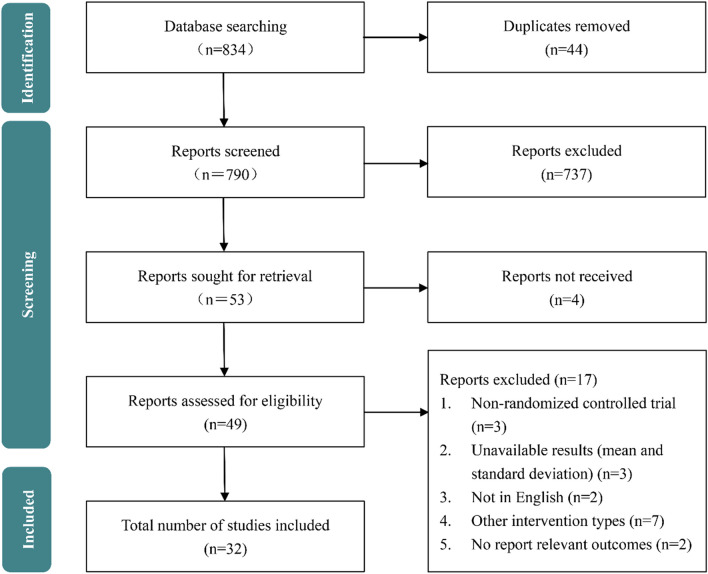
The research provided a detailed PRISMA flow chart in Fig. 1. According to our search strategy, we searched 834 articles from four databases. After removing duplicate articles and some articles that were not relevant to the subject, the remaining 53 articles were for full-text review. Then, 4 reports were not accessible and 17 studies were excluded for various reasons. Finally, 32 studies were included in this meta-analysis.
Fig. 1.
Flowchart of the selection of studies included in the meta-analysis
Study characteristics and quality assessment
This meta-analysis included 32 RCTs [9, 11–13, 21–48], relating 2,200 subjects with an approximate age of 38–80. Table 2 shows the training periods of AE ranged from 3 to 32 weeks and the training frequencies from 1 to 5 sessions per week. In terms of training time for AE, most experiments set it from 30 to 60 min. Most studies reported outcome measures of pain (93.75%), physical function (62.5%), and quality of life (68.75%). The quality assessment of RCTs is shown in Additional file 1: Fig. S1. Overall, in randomized studies, the low risk was dominant in the three key indicators. However, as some articles lacked key information on the risk of bias, these articles were flawed by an unknown bias. Additionally, these unspecified risks of bias exist mainly due to participant and personnel blinding, allocation concealment, and the blinding of outcome.
Table 2.
Characteristics of the included trial
| Author | Year of publication | Disease | Subject (age: Mean + SD) | Intervention methods | Intervention Period | Intervention time | Outcomes |
|---|---|---|---|---|---|---|---|
| Alkatan et al | 2016 | Osteoarthritis | 40 subjects | AE + LE | 12 weeks [3/week] | AE:20–45'Intervention LE:20–45'Intervention | Pain; physical function; quality of life |
| AE(N = 20) | |||||||
| LE(N = 20) | |||||||
| Andrade et al | 2018 | Fibromyalgia | 54 F AE(N = 27) 48 ± 8 NE(N = 27) 47 ± 8 | AE + NE | 16 weeks [2/week] | AE: 45'Intervention | Pain; physical function; quality of life |
| Assis et al | 2006 | Fibromyalgia | 60 F AE(N = 30) 43.43 ± 10.76 LE(N = 30) 42.17 ± 10.05 | AE + LE | 15 weeks [3/week] | AE:10'W + 40 MW + 10'Str LE:10'W + 40 MW + 10'Str | physical function; quality of life |
| Baena-Beato et al | 2014 | Low back pain | 38 subjects AE(N = 21) 50.9 ± 9.6 12F + 9 M NE(N = 17) 46.2 ± 9.8 10F + 7 M | AE + NE | 2 months [5/week] | AE:10'W + 35–45'MW + 10'str | Pain; physical function; quality of life |
| Belza et al | 2002 | Osteoarthritis | 157 subjects AE(N = 36) NE(N = 121) | AE + NE | 20 weeks [2/week] | AE: 1 h Intervention | Pain; quality of life |
| Britto et al | 2020 | Fibromyalgia | 33 F AE(N = 16) 50.25 ± 6.09 LE(N = 17) 46.18 ± 10.84 | AE + LE | 8 weeks [3/week] | AE:10'W + 40'MW + 10'Str LE:10'w + 40'MW + 10'Str | Pain; physical function; quality of life |
| Cuesta-Vargas et al | 2012 | Low back pain | 49 subjects AE(N = 25) NE(N = 24) | AE + NE | 16 weeks [3/week] | AE:30'Intervention | Pain; physical function |
| Dundar et al | 2009 | Low back pain | 65 subjects AE(N = 32) 35.3 ± 7.8 LE(N = 33) 34.8 ± 8.3 | AE + LE | 4 weeks [5/week] | AE:15'W + 40 MW + 5'Str LE:60'Intervention | Pain; physical function |
| Dundar et al | 2014 | Ankylosing spondylitis | 69 subjects AE(N = 35) 42.3 ± 11.3 5F + 30 M LE(N = 34) 43.1 ± 11.7 6F + 28 M | AE + LE | 4 weeks [5/week] | AE:15'W + 40 MW + 5'Str LE:60'Intervention | Pain; physical function |
| Evcik et al | 2008 | Fibromyalgia | 61 subjects AE(N = 31) 43.8 ± 7.7 LE(N = 30) 42.8 ± 7.6 | AE + LE | 5 weeks [3/week] | AE:20'W + 35 MW + 5'Str LE:60'Intervention | Pain |
| Fonseca et al | 2019 | Fibromyalgia | 46 F AE(N = 27) 53.78 ± 10.40 LE(N = 19) 54.47 ± 11.18 | AE + LE | 10 weeks[1/week] | AE:5'W + 45'MW + 10'Str LE:45'MW | Pain; quality of life |
| Fransen et al | 2007 | Osteoarthritis | 96 subjects AE(N = 55) 70.0 ± 6.3 40F + 15 M NE(N = 41) 69.6 ± 6.1 34F + 7 M | AE + NE | 12 weeks [2/week] | AE:60'Intervention | Pain; physical function |
| Guillemin et al | 1994 | Low back pain | 102 subjects AE(N = 50) NE(N = 52) | AE + NE | 3 weeks | AE:15'Intervention | Pain; quality of life |
| Gusi et al | 2006 | Fibromyalgia | 34 F AE(N = 17) 51 ± 10 NE(N = 17) 51 ± 9 | AE + NE | 12 weeks [3/week] | AE:10'W + 40 MW + 10'Str | Pain; quality of life |
| Hale et al | 2011 | Osteoarthritis | 35 subjects AE(N = 20) NE(N = 15) | AE + NE | 12 weeks [2/week] | AE:20'-60'Intervention | Pain; physical function; quality of life |
| Hinman et al | 2007 | Osteoarthritis | 71 subjects AE(N = 36) 63.3 ± 9.5 24F + 12 M NE(N = 35) 61.5 ± 7.8 24F + 11 M | AE + NE | 6 weeks [2/week] | AE:45'-60'Intervention | Pain; physical function; quality of life |
| Lim et al | 2010 | Osteoarthritis | 75 subjects AE(N = 26) 65.7 ± 8.9 23F + 3 M LE(N = 25) 67.7 ± 7.7 21F + 4 M NE(N = 24) 63.6 ± 5.3 21F + 3 M | AE + LE + NE | 8 weeks [3/week] | AE:5'W + 30 MW + 5'Str LE:5'W + 30 MW + 5'Str | Pain; physical function |
| Lund et al | 2008 | Osteoarthritis | 79 subjects AE(N = 27) 65 ± 12.6 22F + 5 M LE(N = 25) 68 ± 9.5 20F + 5 M NE(N = 27) 70 ± 9.9 20F + 7 M | AE + LE + NE | 8 weeks [2/week] | AE:10'W + 30'MW + 10'Str LE:10'W + 30'MW + 10'Str | Pain; quality of life |
| Mannerkorpi et al | 2009 | Fibromyalgia | 166 F AE(N = 81) 44.6 ± 9.26 NE(N = 85) 46.5 ± 8.30 | AE + NE | 20 weeks [1/week] | AE:45'Intervention | Pain; physical function; quality of life |
| McIlroy et al | 2017 | Osteoarthritis | 13 F AE(N = 7) 64.3 ± 8.7 NE(N = 6) 62.3 ± 6.6 | AE + NE | 6 weeks | AE:30'Intervention | Pain; physical function |
| Munguía-Izquierdo et al | 2008 | Fibromyalgia | 58 subjects AE(N = 34) 50 ± 7 NE(N = 24) 46 ± 8 | AE + NE | 16 weeks [3/week] | AE:10'W + 40 MW + 10'Str | Pain; physical function; quality of life |
| Munukka et al | 2016 | Osteoarthritis | 84 F AE(N = 42) 64 ± 2 NE(N = 42) 64 ± 2 | AE + NE | 16 weeks [3/week] | AE:60'Intervention | Pain; quality of life |
| Patrick et al | 2001 | Osteoarthritis | 249 subjects AE(N = 125) 65.7 109F + 16 M NE(N = 124) 66.1 106F + 18 M | AE + NE | 20 weeks [2/week] | AE:45–60'Intervention | Pain; quality of life |
| Sahin et al | 2018 | Osteoarthritis | 59 F AE(N = 30) 60.46 ± 6.82 | NE(N = 29) 58.23 ± 7.55 | AE + NE 3 weeks [5/week] | AE:45'-60'W | Quality of life |
| Silva et al | 2008 | Osteoarthritis | 64 subjects AE(N = 32) 59 ± 7.60 30F + 2 M | LE(N = 32) 59 ± 6.08 29F + 3 M | AE + LE 18 weeks [3/week] | AE:50'Intervention LE:50'Intervention | Pain |
| Sjogren et al | 1997 | Low back pain | 56 subjects AE(N = 28) LE(N = 28) | AE + LE | 6 weeks [2/week] | AE:50'Intervention LE:50'Intervention | Pain; physical function |
| Taglietti et al | 2018 | Osteoarthritis | 60 subjects AE(N = 31) 67.3 ± 5.9 8 M + 23F NE(N = 29) 68.7 ± 6.7 11 M + 18F | AE + NE | 8 weeks [2/week] | AE:60'Intervention | Pain; physical function; quality of life |
| Tomas-Carus et al | 2007 | Fibromyalgia | 34 F AE(N = 17) 51 ± 10 NE(N = 17) 51 ± 9 | AE + NE | 12 weeks [3/week] | AE: 10'W + 40'MW + 10'Str | Pain; physical function; quality of life |
| Tomas-Carus et al | 2008 | Fibromyalgia | 30 F AE(N = 15) 50.7 ± 10.6 NE(N = 15) 50.9 ± 6.7 | AE + NE | 8 months [3/week] | AE:10'w + 50'Intervention | Pain; physical function; quality of life |
| Tomas-Carus et al | 2009 | Fibromyalgia | 30 F AE(N = 15) 50.7 ± 10.6 NE(N = 15) 50.9 ± 6.7 | AE + NE | 32 weeks [3/week] | AE:10'W + 50'MW | Pain; physical function |
| Waller et al | 2017 | osteoarthritis | 87 F AE(N = 43) 63.8 ± 2.4 NE(N = 44) 63.9 ± 2.4 | AE + NE | 16 weeks [3/week] | AE: 60'Intervention | Pain; quality of life |
| Wang et al | 2010 | Osteoarthritis | 78 subjects AE(N = 26) 66.7 ± 5.6 22F + 4 M LE(N = 26) 68.3 ± 6.4 23F + 3 M NE(N = 26) 67.9 ± 5.9 22F + 4 M | AE + LE + NE | 12 weeks [3/week] | AE:5'W + 40 MW + 5'Str LE:5'W + 40 MW + 5'Str | Pain; quality of life |
Pain
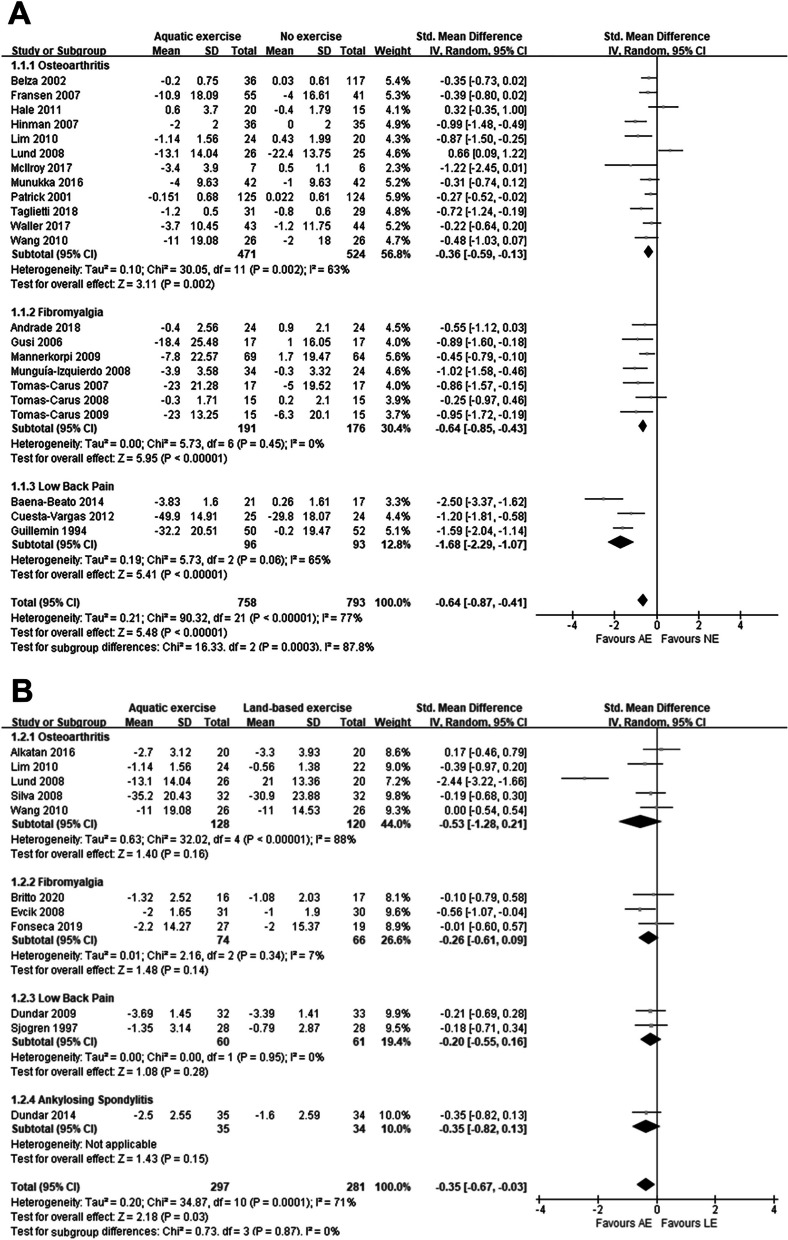
Regarding pain, thirty studies were included in the meta-analysis. Among them, twenty-two studies [9, 11–13, 21–23, 27, 29–33, 35–37, 39–41, 44–46] reported the comparison of AE and NE, eleven studies [11, 13, 25, 26, 28, 30, 34, 38, 42, 47, 48] reported the comparison of AE and LE, and only three studies [11, 13, 30] compared three interventions.
From Fig. 2A, we found that AE was effective in reducing participants' pain as compared to NE (SMD: -0.64, P < 0.001). Further subgroup analysis of the various musculoskeletal disorders classifications showed that AE had a significant therapeutic effect on osteoarthritis (SMD: −0.36, P = 0.002), fibromyalgia (SMD: -0.64, P < 0.001) and low back pain (SMD: −1.68, P < 0.001).
Fig. 2.
Forest plot of pain outcomes A AE versus NE; B AE versus LE
Also, compared to LE, we found that AE could significantly relieve patients' pain (SMD: −0.35, P = 0.03) (Fig. 2B).
Physical function
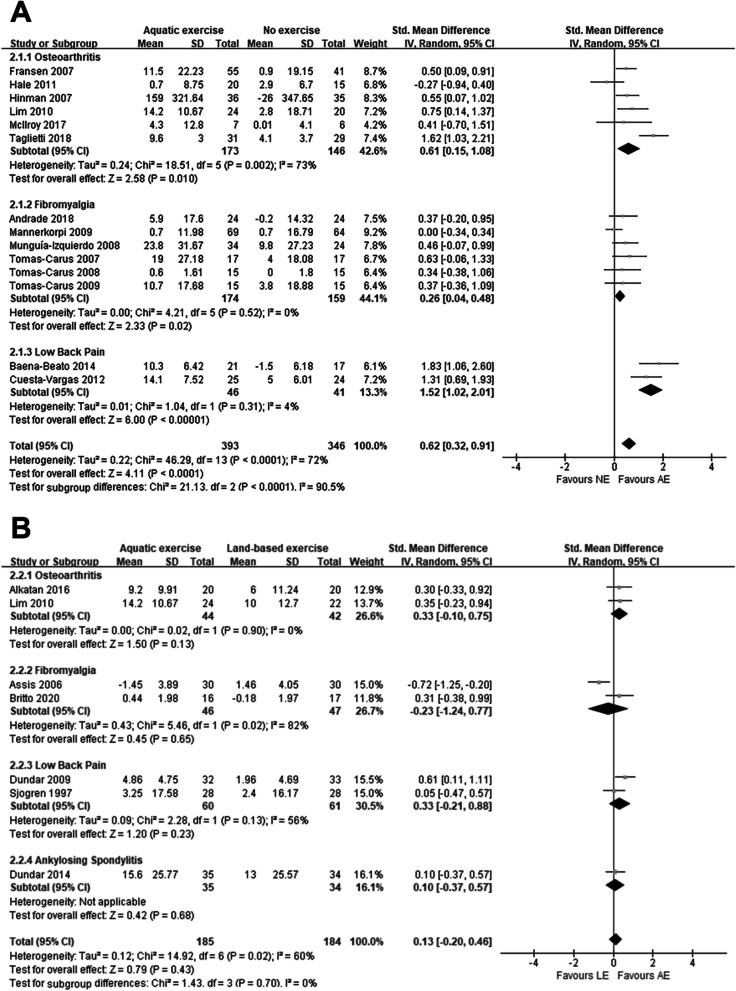
Regarding physical function, twenty studies were included in the meta-analysis. Among them, fourteen studies [12, 21–23, 29–31, 33, 37, 39–41, 44, 45] reported the comparison of AE and NE, seven studies [28, 30, 34, 42, 43, 47, 48] reported the comparison of AE and LE, and only one study [30] compared three interventions.
From Fig. 3A, we can see that the patients involved in AE had a significant improvement in physical function compared to NE (SMD: 0.62, P < 0.001). Among the various subgroups of AE compared with NE, we found significant treatment effects for osteoarthritis (SMD: 0.61, P = 0.01), fibromyalgia (SMD: 0.26, P = 0.02), and low back pain (SMD: 1.52, P < 0.001).
Fig. 3.
Forest plot of physical function outcomes A AE versus NE; B AE versus LE
Interestingly, after analyzing the treatment capacity of AE and LE, we found that for the various musculoskeletal disorders we included AE showed no effective relief, as shown in Fig. 3B. Although we can see after looking back at each of the included studies, the results of most studies showed improvement of AE on patients' physical functioning due to LE. However, the combined analyses revealed that the results were not significant. This may be attributed to the limited amount of literature included or differences in the intensity of AE versus LE interventions.
Quality of life
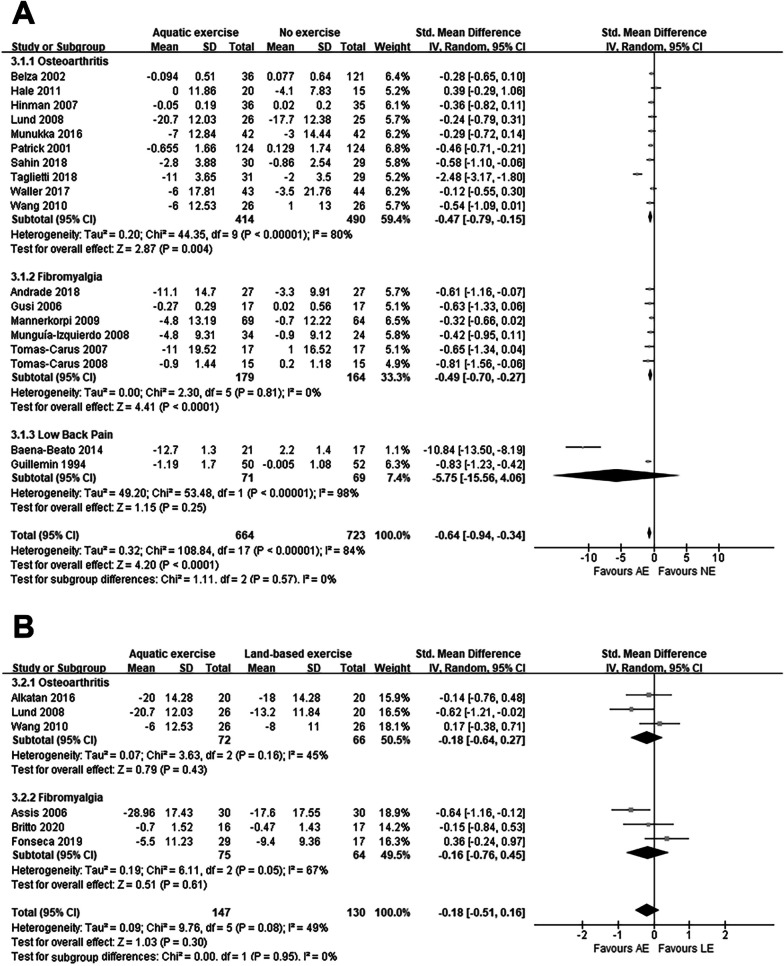
Regarding quality of life, twenty-two studies were included in the meta-analysis. Among them, eighteen studies [9, 11–13, 21–24, 27, 29, 32, 33, 35, 36, 39, 44–46] reported the comparison of AE and NE, six studies [11, 13, 26, 34, 43, 47] reported the comparison of AE and LE, and two studies [11, 13] compared three interventions.
After the comparison of AE and NE, we could see that AE improved the quality of life of the participants (SMD: -0.64, P < 0.001), and after our subgroup analysis, it was clear that almost all experiments showed an effective improvement of quality of life by AE except for low back pain. The detailed results can be seen in Fig. 4A.
Fig. 4.
Forest plot of quality of life outcomes A AE versus NE; B AE versus LE
As with the previous outcomes, there was no significant difference in the improvement in quality of life between AE and LE (Fig. 4B).
Publication bias and sensitivity analysis
To assess publication bias, the funnel plots of all outcome measures were obtained (Fig. S2-4). On visual inspection, all the funnel plots seem symmetrical with the effect estimates, and it indicated that the results are without significant publication bias. Additionally, sensitivity analyses were conducted to exclude each result, and the results proved the stability.
Discussion
This is a systematic review and meta-analysis of whether AE has a positive impact on the treatment of chronic musculoskeletal disorders, the results of the study showed that patients who performed AE showed considerable improvements in pain, physical function, and quality of life compared to those who did NE. In the meantime, AE showed a more significant improvement in the vital indicator of pain compared to patients conducting LE but did not show a remarkable advantage in terms of physical function and quality of life. Nevertheless, our review of the included literature showed that overall, the initial number of participants was lower [26] and the rate of follow-up missed during the intervention was higher [11] in the LE group compared to AE, suggesting that patient engagement is higher in AE than in LE and that the effectiveness of an intervention is based on the efficacy of the intervention itself combined with patient engagement, so that subject engagement is critical in assessing the final effect. This is because even if an intervention is very effective, it will not show much of a benefit if the patient's willingness to participate is weak.
From the authors' investigation, there have been no previous studies on whether AE can improve chronic musculoskeletal disorders, thus this is the first systematic review and meta-analysis of this disease. Meanwhile, we selected important indicators such as pain, physical function, and quality of life, which have a high assessment value for patients' activities of daily living, to ensure the scientific accuracy of the study. Moreover, the total number of patients included in this study was 2200, and the advantage of a large population base makes the study results more credible. Besides, we included and summarized multiple types of chronic musculoskeletal disorders, which makes the study results more applicable to a wider population.
Impact of AE on pain
The study used a pain assessment scale that allowed the assessors to determine whether AE had a positive impact on the improvement of pain values. The combined results showed that in a comparative analysis of AE versus NE and LE, AE was found to have a significantly better improvement in pain values than NE and LE. Also, the corresponding subgroup analysis showed that in all subgroups, the comparison between AE and NE showed a statistical difference, which can be interpreted in light of how AE affects the process of pain production. Pain is a sophisticated physiological phenomenon and as the main clinical manifestation in patients with osteoarthritis, fibromyalgia, etc., it is generated for complex reasons. In the normal population, the central nervous system balances the level of excitation and inhibition, so that no pain is generated, but in patients with chronic pain, this balance is disrupted [49]. In Baraniuk's study [50], it is known that the MEAP (Met-enk-Arg6-Phe7) in the cerebrospinal fluid of patients with fibromyalgia and low back pain compared to the normal group concentrations were significantly altered, and this suggests that altered levels of central nervous system opioid may cause or exacerbate fibromyalgia. Therefore, the consumption of opioids before and after the intervention can be used to determine the degree of pain reduction or worsening [51].
It is well established that physical exercise, a cost-effective and safe rehabilitation therapy, reduces pain levels by enhancing neurological 5-HT (5-hydroxytryptamine) neurotransmission, decreasing 5-HT transporter protein expression, and increasing 5-HT receptor expression through low-intensity aerobic training [52]. One step further, physical exercise in an aquatic environment may be more beneficial for pain relief. The pain-relieving effect of AE may stem from the combined effect of exercise, warm water, and buoyancy on thermoreceptors and mechanoreceptors [17], water pressure, water viscosity, and water temperature stimulate the senses during AE, promoting the triggering of thermoreceptors and mechanoreceptors and blocking the conduction of nociceptors (nociceptors are small-diameter nerve fiber endings that respond to the tissue environment) [53]. At the same time, the temperature and pressure of the water stimulate the skin, and while submerged in water, methionine encephalin plasma levels rise and reduce plasma levels of β-endorphin, corticotropin, and prolactin [53]. It deserves to be mentioned that the process of muscle activity produces several cytotoxic substances, the continuous accumulation of which activates sensitizes, or awakens nociceptors thereby producing pain, cytotoxic substances including histamine, serotonin, bradykinin, adrenaline, etc. [54], some research has reported increased levels of glutamate in fibromyalgia patients, which as a neurotransmitter transmitting pain stimulates the nociceptors, while the bradykinin stimulates the release of norepinephrine and prostaglandins to sensitize the nociceptors further. Hence, the improvement of pain can also be explained by the mechanism that the association of water pressure and temperature produces competing stimulation of nerve endings [55], thus being able to reduce injury from the periphery, and that hydrotherapy can also relax muscles, reduce their tension and decrease pain.
Effect of AE on physical function
In assessing this indicator of patients' physical function, the majority of studies used the SF-36 (Medical Outcomes Study 36-Item Short-Form Health Survey) scale [12, 22, 28, 29, 31, 48], while some of them chose the WOMAC (Western Ontario and McMaster University Osteoarthritis Index) and FIQ (Fibromyalgia Impact Questionnaire) scales [30, 37, 44]. Because the assessment scales are much more similar, the results are also more accurate. Physical function is significant as an important indicator to evaluate the effect of AE on patient improvement. Patients with chronic musculoskeletal disorders show low physical function in several aspects, for instance, patients with fibromyalgia syndrome are prone to fatigue and dyspnea, which may be related to changes in the respiratory system, and according to clinical observations, patients have lower respiratory muscle endurance, inspiratory muscle strength, and chest mobility [56]. It was also found that the patient's heart rate was significantly elevated, cardiovascular sympathetic nerve activity showed increased, vagal nerve activity decreased, and the regulation of the sinus node was reduced, and thus, improving the patient's physical function was most significant in improving cardiovascular and respiratory function. Several studies have demonstrated that aerobic exercise can improve neurological disorders as well as cardiopulmonary function [57] and that the aquatic environment can reduce cardiovascular stress in patients, allowing for more intense training. A study by Zamunér et al. [58] demonstrated that aquatic therapy not only increased patients' aerobic capacity but also improved cardiac autoregulation; meanwhile, it was also reported in the paper that AE increased patients' oxygen uptake at rest and during exercise, as evidence of a certain degree of improvement in cardiopulmonary function [59].
For the subgroup analysis of physical function, AE compared with NE showed statistically significant differences in all subgroups. Therefore, this result further suggests that AE has a significant improvement in physical function compared to NE in most categories of chronic musculoskeletal disorders. While the comprehensive results of AE compared to LE showed no statistical difference, this result suggests that AE and LE are similar in terms of their effectiveness in improving the physical function of patients. When comparing the intervention methods of the studies in each group, it was found that more trials provided more differences in the training programs for AE and LE. The differences are not limited to the training period and intensity, and the variations in the training program will have an impact on the final results, so more research is needed to verify the final results of AE and LE.
Impact of AE on quality of life
Quality of life is a very sophisticated metric that assesses a wide range of aspects, including patient mood, fatigue, perceived ability, and mental health, all of which can influence a patient's ability to live a normal life. Given the complexity of this index, the scales used to assess patients' quality of life varied across studies, and we, therefore, included the corresponding data by selecting scales with relatively similar assessment methods and criteria after due consideration. Quality of life is the best indicator of a patient's normal life, so the emphasis on improving the quality of life for patients is overwhelming. With AE intervention, warm water can put the patient's muscles in a relaxed state and reduce the pressure of gravity on the joints, in addition, exercise can promote the release of β-endorphin and dopamine in the patient's body [60], β-endorphin is a kind of endogenous morphine-like substance in the human body, which has a strong analgesic effect, dopamine is the most abundant catecholamine neurotransmitter in the brain, which transmits signals of excitement as well as happiness, and it plays an important role in human movement and learning. The increased release of these two substances has a calming and analgesic effect on the subjects, as well as relieving anxiety and achieving the effects of antidepressants. In the meantime, the aquatic environment provides a relaxing and comfortable atmosphere for patients, which can increase their pleasure [28], relieving their depression or irritability caused by their disease and the pain it brings. Besides, water exercise can also be considered as a kind of water immersion method, which affects some physiological responses of the body, such as changing the fluid in the cells and blood vessels, reducing edema, increasing blood flow by diastole, and increasing cardiac output, which can relieve fatigue and have psychological benefits for the participants [61].
The meta-analysis of the quality of life indicator showed a significant improvement in AE compared to NE, but no statistically significant difference when compared to LE, which may be due to the short intervention period in some of the studies resulting in similar changes in various aspects of the subjects, as a result, no significant difference could be revealed between the two programs. Meanwhile, we also found statistics showing that patients with osteoarthritis have lower physical mobility compared to the general population, and nearly 50% of patients will be reluctant to perform additional exercise training due to pain [14, 62]. So there is a problem of low patient willingness to treat and low participation rate, while the level of participation of patients in both groups will also affect the final results. Subgroup analysis showed that the low back pain subgroup showed no statistical difference in the AE versus NE comparison, and this is most likely due to the small number of literature with two articles, which makes it biased from the actual situation.
Limitations and future research
There are several limitations of this systematic review and meta-analysis. Firstly, this study only included RCTs published in English, and some high-quality articles published in languages other than English were excluded, so future meta-analyses should include these excluded high-quality articles as well as some valuable studies that may not have been published yet; secondly, because the number of included literature within some subgroups was found to be small when subgroup analysis was conducted in this study, which could lead to a large heterogeneity in some subgroup analysis and make the results of meta-analysis differ from the actual results. Thus, the number of included literature should be increased as much as possible in future studies to further improve the reliability of the study; the third is that the intervention protocols are not identical across studies, so the intervention intensity, as well as the intervention period, can vary, both of which can affect the final intervention effect; in the fourth, there is a placebo effect for aquatic therapy, and most of the literature included in this paper lacked a placebo control, so the placebo effect could not be excluded [33].
Considering the limitations mentioned above, future meta-analyses should carefully consider the period of interventions included in the paper, either too long or too short could adversely affect the final results, and the intensity of interventions needs to be kept as similar as possible between studies, in addition to further evaluation of the placebo effect of AE to determine whether it affects the results and to what extent. In other words, most of the AE therapies in the studies included in this article require the supervision of professional physiotherapists and the development of exercise programs for patients that are appropriate to their physical conditions, but there is still much space to improve the popularity of AE because of the limited space and related exercise facilities available for AE. Therefore, in the foreseeable future, more achievable and affordable AE programs should be developed for the stakeholders to increase their popularity, allowing patients to receive treatment in a safer and more comfortable environment, reducing pain, and improving their quality of life. Moreover, since AE applies to a wide range of groups and there are some differences in treatment measures and contraindications among different diseases, it is recommended that future studies should address individual differences and disease differences. For instance, AE showed significant improvement in physical function compared to NE in patients with osteoarthritis and fibromyalgia, but not in patients with low back pain. Therefore, it is essential to develop exercise programs for different patients that match their intervention objectives, physical conditions, and personal activity habits to maximize patient participation and intervention effects [63].
Given the positive relationship established in this meta-analysis that AE has a positive effect on the treatment of chronic musculoskeletal disorders compared to NE, future research is required to explore whether there is a positive effect of AE in areas other than the indicators studied here. Moreover, AE has been found to have a significant improvement in pain compared to LE in patients with chronic musculoskeletal disorders, but the efficacy has not yet been demonstrated in terms of physical function and quality of life, so more studies are needed to compare its efficacy in other aspects.
Conclusions
The results of the study showed that AE significantly improved pain, physical function, and quality of life in patients with chronic musculoskeletal disorders compared to NE; when compared to LE, AE was only found to improve pain in patients. Considering the clinical application of this rehabilitation tool, more long-term clinical trials are still needed to further confirm the positive effects and improvement mechanisms of AE and the more existential advantages compared to LE as a treatment measure.
Supplementary Information
Additional file 1: Table S1 The detailed search strategy and results. Figure S1 The quality assessment of RCTs. A Risk-of-bias item presented as percentages across RCTs for population with diseases; B Judgments about risk-of-bias item for each RCTs. + indicates low risk, ? indicates unclear risk, − indicates high risk. Figure S2 Funnel plot of pain outcomes. A AE versus NE; BAE versus LE. Figure S3 Funnel plot of physical function outcomes. A AE versus NE; B AE versus LE. Figure S4 Funnel plot of quality of life outcomes A AE versus NE; BAE versus LE.
Acknowledgements
Not applicable.
Abbreviations
- AE
Aquatic exercise
- RCT
Randomized controlled trial
- LE
Land-based exercise
- NE
No exercise
- SD
Standard deviation
- CI
Confidence interval
- SE
Standard error
- SEM
Standard error of the mean
- SMD
Standardized mean difference
- MEAP
Met-enk-Arg6-Phe7
- 5-HT
5-Hydroxytryptamine
- SF-36
Medical Outcomes Study 36-Item Short-Form Health Survey
- WOMAC
Western Ontario and Mcmaster University Osteoarthritis Index
- FIQ
Fibromyalgia Impact Questionnaire
Author contributions
TW, JW, and YC contributed to the conception and design of the study, acquisition of data, and drafting of the manuscript; JW, YC, and SD contributed significantly to the analysis and/or interpretation of data; all authors revised the manuscript critically for important intellectual content.
Funding
The authors received no funding for this work.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianyue Wang, Jiamin Wang and Yuheng Chen have equally contributed to this work
References
- 1.Kazeminasab S, et al. Neck pain: global epidemiology, trends, and risk factors. BMC Musculoskelet Disord. 2022;23:26. doi: 10.1186/s12891-021-04957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholas M, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160:28–37. doi: 10.1097/j.pain.0000000000001390. [DOI] [PubMed] [Google Scholar]
- 3.Geneen LJ, et al. Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Database of Syst Rev. 2017 doi: 10.1002/14651858.CD011279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcaraz MJ, Compañ A, Guillén MI. Extracellular vesicles from mesenchymal stem cells as novel treatments for musculoskeletal diseases. Cells. 2019 doi: 10.3390/cells9010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korthals-de Bos IB, et al. Cost effectiveness of physiotherapy, manual therapy, and general practitioner care for neck pain: economic evaluation alongside a randomised controlled trial. BMJ (Clinical research ed) 2003;326:911. doi: 10.1136/bmj.326.7395.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol. 2010;6:191–197. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- 7.Peng MS, et al. Efficacy of therapeutic aquatic exercise vs physical therapy modalities for patients with chronic low back pain: a randomized clinical trial. JAMA Netw Open. 2022;5:e2142069. doi: 10.1001/jamanetworkopen.2021.42069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faíl LB, et al. Benefits of aquatic exercise in adults with and without chronic disease-A systematic review with meta-analysis. Scand J Med Sci Sports. 2022;32:465–486. doi: 10.1111/sms.14112. [DOI] [PubMed] [Google Scholar]
- 9.Waller B, et al. Effects of high intensity resistance aquatic training on body composition and walking speed in women with mild knee osteoarthritis: a 4-month RCT with 12-month follow-up. Osteoarthritis Cartilage. 2017;25:1238–1246. doi: 10.1016/j.joca.2017.02.800. [DOI] [PubMed] [Google Scholar]
- 10.Bidonde J, et al. Aquatic exercise training for fibromyalgia. Cochrane Database of Syst Rev. 2014 doi: 10.1002/14651858.Cd011336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund H, et al. A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J Rehabil Med. 2008;40:137–144. doi: 10.2340/16501977-0134. [DOI] [PubMed] [Google Scholar]
- 12.Tomas-Carus P, et al. Aquatic training and detraining on fitness and quality of life in fibromyalgia. Med Sci Sports Exerc. 2007;39:1044–1050. doi: 10.1249/01.mss.0b0138059aec4. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, et al. Comparing the efficacy of aquatic exercises and land-based exercises for patients with knee osteoarthritis. J Clin Nurs. 2011;20:2609–2622. doi: 10.1111/j.1365-2702.2010.03675.x. [DOI] [PubMed] [Google Scholar]
- 14.Bartels EM, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD005523.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McVeigh JG, McGaughey H, Hall M, Kane P. The effectiveness of hydrotherapy in the management of fibromyalgia syndrome: a systematic review. Rheumatol Int. 2008;29:119–130. doi: 10.1007/s00296-008-0674-9. [DOI] [PubMed] [Google Scholar]
- 16.Waller B, Lambeck J, Daly D. Therapeutic aquatic exercise in the treatment of low back pain: a systematic review. Clin Rehabil. 2009;23:3–14. doi: 10.1177/0269215508097856. [DOI] [PubMed] [Google Scholar]
- 17.Heywood S, et al. Effectiveness of aquatic exercise in improving lower limb strength in musculoskeletal conditions: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98:173–186. doi: 10.1016/j.apmr.2016.08.472. [DOI] [PubMed] [Google Scholar]
- 18.Batterham SI, Heywood S, Keating JL. Systematic review and meta-analysis comparing land and aquatic exercise for people with hip or knee arthritis on function, mobility and other health outcomes. BMC Musculoskelet Disord. 2011;12:123. doi: 10.1186/1471-2474-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu M, et al. Effectiveness of aquatic exercise for treatment of knee osteoarthritis: systematic review and meta-analysis. Z Rheumatol. 2015;74:543–552. doi: 10.1007/s00393-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 20.Zão A, Cantista P. The role of land and aquatic exercise in ankylosing spondylitis: a systematic review. Rheumatol Int. 2017;37:1979–1990. doi: 10.1007/s00296-017-3829-8. [DOI] [PubMed] [Google Scholar]
- 21.Andrade CP, Zamunér AR, Forti M, Tamburús NY, Silva E. Effects of aquatic training and detraining on women with fibromyalgia: controlled randomized clinical trial. Eur J Phys Rehabil Med. 2019;55:79–88. doi: 10.23736/s1973-9087.18.05041-4. [DOI] [PubMed] [Google Scholar]
- 22.Baena-Beato P, et al. Aquatic therapy improves pain, disability, quality of life, body composition and fitness in sedentary adults with chronic low back pain. Controll Clin Trial Clin Rehab. 2014;28:350–360. doi: 10.1177/0269215513504943. [DOI] [PubMed] [Google Scholar]
- 23.Tomas-Carus P, et al. Eight months of physical training in warm water improves physical and mental health in women with fibromyalgia: a randomized controlled trial. J Rehabil Med. 2008;40:248–252. doi: 10.2340/16501977-0168. [DOI] [PubMed] [Google Scholar]
- 24.Sahin HG, Kunduracilar Z, Sonmezer E, Ayas S. Effects of two different aquatic exercise trainings on cardiopulmonary endurance and emotional status in patients with knee osteoarthritis. J Back Musculoskelet Rehabil. 2019;32:539–548. doi: 10.3233/bmr-171116. [DOI] [PubMed] [Google Scholar]
- 25.Silva LE, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88:12–21. doi: 10.2522/ptj.20060040. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca ACS, et al. Effects of aquatic physiotherapy or health education program in women with fibromyalgia: a randomized clinical trial. Physiother Theory Pract. 2021;37:620–632. doi: 10.1080/09593985.2019.1639229. [DOI] [PubMed] [Google Scholar]
- 27.Patrick DL, et al. Economic evaluation of aquatic exercise for persons with osteoarthritis. Med Care. 2001;39:413–424. doi: 10.1097/00005650-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Dundar U, Solak O, Yigit I, Evcik D, Kavuncu V. Clinical effectiveness of aquatic exercise to treat chronic low back pain: a randomized controlled trial. Spine. 2009;34:1436–1440. doi: 10.1097/BRS.0b013e3181a79618. [DOI] [PubMed] [Google Scholar]
- 29.Mannerkorpi K, Nordeman L, Ericsson A, Arndorw M. Pool exercise for patients with fibromyalgia or chronic widespread pain: a randomized controlled trial and subgroup analyses. J Rehabil Med. 2009;41:751–760. doi: 10.2340/16501977-0409. [DOI] [PubMed] [Google Scholar]
- 30.Lim JY, Tchai E, Jang SN. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: a randomized controlled trial. PM & R : J Injury, Func Rehab. 2010;2:723–731. doi: 10.1016/j.pmrj.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Tomas-Carus P, et al. Improvements of muscle strength predicted benefits in HRQOL and postural balance in women with fibromyalgia: an 8-month randomized controlled trial. Rheumatology (Oxford) 2009;48:1147–1151. doi: 10.1093/rheumatology/kep208. [DOI] [PubMed] [Google Scholar]
- 32.Belza B, Topolski T, Kinne S, Patrick DL, Ramsey SD. Does adherence make a difference? Results from a community-based aquatic exercise program. Nurs Res. 2002;51:285–291. doi: 10.1097/00006199-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32–43. doi: 10.2522/ptj.20060006. [DOI] [PubMed] [Google Scholar]
- 34.Britto A, et al. Effects of water- and land-based exercises on quality of life and physical aspects in women with fibromyalgia: a randomized clinical trial. Musculoskeletal Care. 2020;18:459–466. doi: 10.1002/msc.1481. [DOI] [PubMed] [Google Scholar]
- 35.Guillemin F, Constant F, Collin JF, Boulange M. Short and long-term effect of spa therapy in chronic low back pain. Br J Rheumatol. 1994;33:148–151. doi: 10.1093/rheumatology/33.2.148. [DOI] [PubMed] [Google Scholar]
- 36.Gusi N, Tomas-Carus P, Häkkinen A, Häkkinen K, Ortega-Alonso A. Exercise in waist-high warm water decreases pain and improves health-related quality of life and strength in the lower extremities in women with fibromyalgia. Arthritis Rheum. 2006;55:66–73. doi: 10.1002/art.21718. [DOI] [PubMed] [Google Scholar]
- 37.Fransen M, Nairn L, Winstanley J, Lam P, Edmonds J. Physical activity for osteoarthritis management: a randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Rheum. 2007;57:407–414. doi: 10.1002/art.22621. [DOI] [PubMed] [Google Scholar]
- 38.Evcik D, Yigit I, Pusak H, Kavuncu V. Effectiveness of aquatic therapy in the treatment of fibromyalgia syndrome: a randomized controlled open study. Rheumatol Int. 2008;28:885–890. doi: 10.1007/s00296-008-0538-3. [DOI] [PubMed] [Google Scholar]
- 39.Munguía-Izquierdo D, Legaz-Arrese A. Assessment of the effects of aquatic therapy on global symptomatology in patients with fibromyalgia syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:2250–2257. doi: 10.1016/j.apmr.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Cuesta-Vargas AI, et al. Deep water running and general practice in primary care for non-specific low back pain versus general practice alone: randomized controlled trial. Clin Rheumatol. 2012;31:1073–1078. doi: 10.1007/s10067-012-1977-5. [DOI] [PubMed] [Google Scholar]
- 41.McIlroy S, Sayliss L, Browning P, Bearne LM. Aquatic therapy for people with persistent knee pain: a feasibility study. Musculoskeletal Care. 2017;15:350–355. doi: 10.1002/msc.1179. [DOI] [PubMed] [Google Scholar]
- 42.Sjogren T, Long N, Storay I, Smith J. Group hydrotherapy versus group land-based treatment for chronic low back pain. Physiotherapy Res Int: J Res Clin Phys Therapy. 1997;2:212–222. doi: 10.1002/pri.107. [DOI] [PubMed] [Google Scholar]
- 43.Assis MR, et al. A randomized controlled trial of deep water running: clinical effectiveness of aquatic exercise to treat fibromyalgia. Arthritis Rheum. 2006;55:57–65. doi: 10.1002/art.21693. [DOI] [PubMed] [Google Scholar]
- 44.Hale LA, Waters D, Herbison P. A randomized controlled trial to investigate the effects of water-based exercise to improve falls risk and physical function in older adults with lower-extremity osteoarthritis. Arch Phys Med Rehabil. 2012;93:27–34. doi: 10.1016/j.apmr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Taglietti M, et al. Effectiveness of aquatic exercises compared to patient-education on health status in individuals with knee osteoarthritis: a randomized controlled trial. Clin Rehabil. 2018;32:766–776. doi: 10.1177/0269215517754240. [DOI] [PubMed] [Google Scholar]
- 46.Munukka M, et al. Efficacy of progressive aquatic resistance training for tibiofemoral cartilage in postmenopausal women with mild knee osteoarthritis: a randomised controlled trial. Osteoarthritis Cartilage. 2016;24:1708–1717. doi: 10.1016/j.joca.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Alkatan M, et al. Improved Function and Reduced Pain after Swimming and Cycling Training in Patients with Osteoarthritis. J Rheumatol. 2016;43:666–672. doi: 10.3899/jrheum.151110. [DOI] [PubMed] [Google Scholar]
- 48.Dundar U, et al. Effect of aquatic exercise on ankylosing spondylitis: a randomized controlled trial. Rheumatol Int. 2014;34:1505–1511. doi: 10.1007/s00296-014-2980-8. [DOI] [PubMed] [Google Scholar]
- 49.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129. doi: 10.1016/j.neuroscience.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004;5:48. doi: 10.1186/1471-2474-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brummett CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology. 2013;119:1434–1443. doi: 10.1097/ALN.0b013e3182a8eb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bobinski F, et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156:2595–2606. doi: 10.1097/j.pain.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reilly KA, Bird HA. Prophylactic hydrotherapy. Rheumatology (Oxford) 2001;40:4–6. doi: 10.1093/rheumatology/40.1.4. [DOI] [PubMed] [Google Scholar]
- 54.Coutaux A, Adam F, Willer JC, Le Bars D. Hyperalgesia and allodynia: peripheral mechanisms. Joint Bone Spine. 2005;72:359–371. doi: 10.1016/j.jbspin.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Melzack R, Wall PD. Pain mechanisms: a new theory. Science (New York, N.Y.) 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 56.Forti M, Zamunér AR, Andrade CP, Silva E. Lung Function, respiratory muscle strength, and thoracoabdominal mobility in women with fibromyalgia syndrome. Respir Care. 2016;61:1384–1390. doi: 10.4187/respcare.04401. [DOI] [PubMed] [Google Scholar]
- 57.Bidonde J, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.Cd012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamunér AR, et al. Effects of a hydrotherapy programme on symbolic and complexity dynamics of heart rate variability and aerobic capacity in fibromyalgia patients. Clin Exp Rheumatol. 2015;33:S73–81. [PubMed] [Google Scholar]
- 59.Andrade CP, et al. Oxygen uptake and body composition after aquatic physical training in women with fibromyalgia: a randomized controlled trial. Eur J Phys Rehabil Med. 2017;53:751–758. doi: 10.23736/s1973-9087.17.04543-9. [DOI] [PubMed] [Google Scholar]
- 60.Adam D, Ramli A, Shahar S. Effectiveness of a combined dance and relaxation intervention on reducing anxiety and depression and improving quality of life among the cognitively impaired elderly. Sultan Qaboos Univ Med J. 2016;16:e47–53. doi: 10.18295/squmj.2016.16.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcock IM, Cronin JB, Hing WA. Physiological response to water immersion: a method for sport recovery? Sports Med (Auckland, NZ) 2006;36:747–765. doi: 10.2165/00007256-200636090-00003. [DOI] [PubMed] [Google Scholar]
- 62.Gay C, Chabaud A, Guilley E, Coudeyre E. Educating patients about the benefits of physical activity and exercise for their hip and knee osteoarthritis. Systematic literature review. Ann Phys Rehab Med. 2016;59:174–183. doi: 10.1016/j.rehab.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Kim Y, Vakula MN, Waller B, Bressel E. A systematic review and meta-analysis comparing the effect of aquatic and land exercise on dynamic balance in older adults. BMC Geriatr. 2020;20:302. doi: 10.1186/s12877-020-01702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 The detailed search strategy and results. Figure S1 The quality assessment of RCTs. A Risk-of-bias item presented as percentages across RCTs for population with diseases; B Judgments about risk-of-bias item for each RCTs. + indicates low risk, ? indicates unclear risk, − indicates high risk. Figure S2 Funnel plot of pain outcomes. A AE versus NE; BAE versus LE. Figure S3 Funnel plot of physical function outcomes. A AE versus NE; B AE versus LE. Figure S4 Funnel plot of quality of life outcomes A AE versus NE; BAE versus LE.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.