Abstract
Background
Mucoepidermoid carcinoma of the breast is a rare special type of salivary gland-like tumor of the breast, usually displaying triple-negative phenotype. To date, only 64 cases have been reported in the English literature. Herein, we report the first case of mucoepidermoid carcinoma of the breast with human epidermal growth factor receptor 2 gene amplification.
Case presentation
A 58-year-old Caucasian woman treated with breast-conserving surgery, radiotherapy, and chemotherapy for an invasive breast carcinoma of no special type, relapsed 20 years later in the ipsilateral left breast. Histological examination of the core needle biopsy of the relapse deferred to the surgical specimen for the definitive diagnosis, because of the broad differential diagnosis. On the resected specimen we observed the presence of a poorly differentiated carcinoma with mucoepidermoid carcinoma of the breast typical features consisting of epidermoid, intermediate and mucinous cells lacking true keratinization, in keeping with the latest World Health Organization diagnostic criteria. The mucoepidermoid carcinoma of the breast was weakly estrogen receptor and androgen receptor positive and progesterone receptor negative, but exceptionally showed human epidermal growth factor receptor 2 gene amplification. Mastermind-like transcriptional coactivator 2 gene translocations were not detected by fluorescent in situ hybridization. The patient received adjuvant chemotherapy with anti-human epidermal growth factor receptor 2 therapy but no endocrine therapy. After 61 months of follow-up, no signs of local or distant recurrence were observed.
Conclusions
Mucoepidermoid carcinoma of the breast is a very rare entity. Despite being most frequently triple negative, the standard evaluation of receptor status is mandatory, as well as strict application of World Health Organization diagnostic criteria for correct patient management.
Keywords: Triple-negative breast cancer, Salivary gland-like tumors of the breast, Mucoepidermoid carcinoma of the breast, HER2, Case report
Background
Mucoepidermoid carcinoma of the breast (MEC-b) is a rare special type of breast carcinoma (BC) accounting for < 1% of all breast malignancies, and belonging to the salivary gland-like tumors. Despite being mostly classified as triple negative breast carcinoma (TNBC) it is usually considered a tumor with low-malignant potential and good prognosis [1].
According to the project Surveillance of Rare Cancers in Europe (RARECARE), rare tumors are defined as those with an incidence of < 6/100,000 per year. In 2011 the estimated cumulative incidence of all salivary gland-like tumors of the breast was 0.05/100,000 per year, with a prevalence of about 2400 new diagnoses per year in the whole of Europe [2]. A similar incidence is reported also in the USA, rendering MEC-b an exceedingly rare type of BC [3].
MEC-b is composed by a mixture of mucinous, epidermoid, and intermediate neoplastic cells arranged in solid and cystic structures. Their presence is mandatory for the diagnosis as well as the lack of true keratinization [4]. Grading of MEC-b is done either by using breast cancer criteria (in other words, Nottingham Histologic Score System) or salivary gland cancer criteria (in other words, the Armed Forces Institute of Pathology grading system) [4]. Immunohistochemistry (IHC) is useful and assists with morphology in confirming the diagnosis.
Mastermind-like transcriptional coactivator 2 (MAML2) gene translocations have been recently described in some cases, a feature shared with MEC of the salivary glands (MEC-sg) [5–8].
Herein we present a case of recurrent BC showing typical MEC morphology and demonstrating human epidermal growth factor receptor 2 (HER2) gene amplification. We also provide a review of the current literature in the view of current World Health Organization (WHO) essential criteria for diagnosis [4]. Given the reported worse prognosis of rare cancers compared with the prognosis of more common cancers [2], we aimed to improve knowledge, and provide clinical guidance for the diagnosis and treatment of such rare cases.
Case presentation
A 58-year-old Caucasian woman presented to our hospital with a self-palpated mass in the left breast.
The patient was in follow-up since 1996 for a previous BC located in the upper outer quadrant of the same breast: a grade 3 invasive breast carcinoma of no special type (IBC-NST; pT1cN0M0), hormone receptor positive (Allred score: ER 6/8 and PR 7/8) and treated by lumpectomy with axillary lymph node dissection and adjuvant chemotherapy (a-CT) (six cycles of cyclophosphamide, methotrexate, and 5-fluorouracil) followed by radiotherapy (breast 50 Gy + 16 Gy boost) without endocrine therapy. Beside the presence of breast cancer in a second degree female relative (father side) older than 55 years, no further breast- or ovary-related tumors were retained in her family. Her mother died from bladder cancer.
Clinical examination confirmed the presence of an irregular nodule localized at 3 o’clock, which by palpation measured 30 mm × 25 mm in size, free from the skin and the pectoral muscle, without lymphadenopathy. Mammography showed an irregular dense mass of 18 mm × 14 mm highly suspicious for malignancy, and ultrasounds showed a hypoechoic mass with parallel orientation, irregular contours, and heterogeneous composition (Fig. 1). On core needle biopsy a high-grade invasive BC with eosinophilic cells suspicious for squamous/epidermoid or apocrine differentiation without mucinous component was described, deferring definitive diagnosis to the surgical specimen (not shown). Standard staging with chest X-ray, abdominal ultrasound, and skeletal scintigraphy excluded the presence of distant metastasis. The patient underwent to a simple left mastectomy for a rcT1NxM0 BC.
Fig. 1.
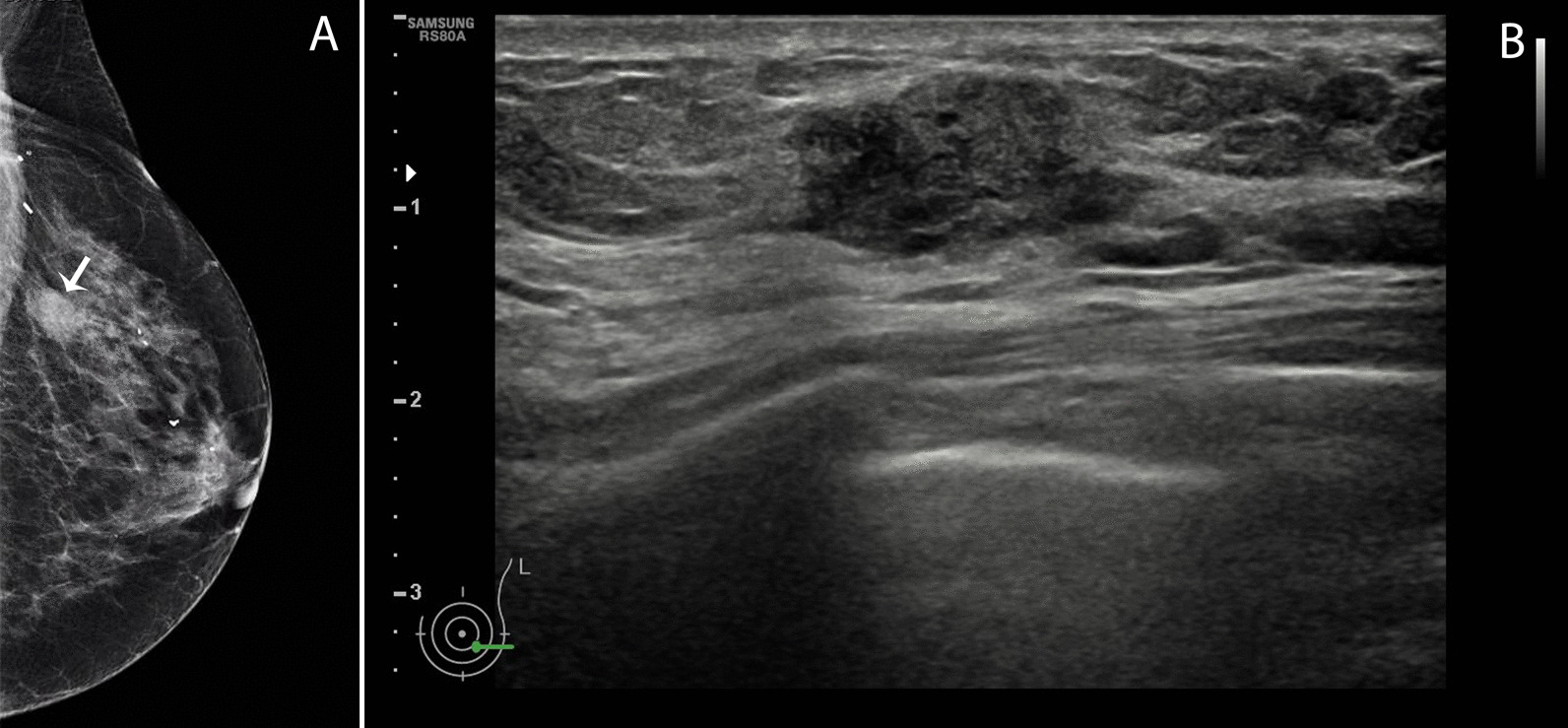
RX and ultrasound imaging of the left breast. A On mammography, the cranio-caudal (CC) prospect shows the presence of a deeply located nodular shaped dense mass at 3 o’clock with irregular borders and highly suspicious of malignancy (white arrow). Sequelae of the previous surgery are visible as well. B On ultrasound the lesion was hypoechoic showing parallel orientation, irregular contours and heterogeneous composition
Gross inspection revealed a sharply demarcated nodular and white tumor of 20 mm diameter. Microscopically, a dominant non-capsulated nodule associated with rare peripherally located lymphoid structures was observed. The tumor cells were mostly arranged in solid nests admixed with necrotic areas. A composite population including large highly pleomorphic epidermoid cells and relatively small intermediate cells with indefinite cell borders and oval-shaped nuclei in absence of mature keratinization was observed (Fig. 2A). Additionally, the presence of cribriform and microcystic structures embedded in large extracellular mucin pools, associated with columnar mucin producing epithelial cells, was noticed as well (Fig. 2B). Frequent micro-abscesses were present (Fig. 2C). We counted up to seven mitoses per mm2. Finally, a component of poorly differentiated ductal carcinoma in situ (DCIS) with MEC features was also observed.
Fig. 2.
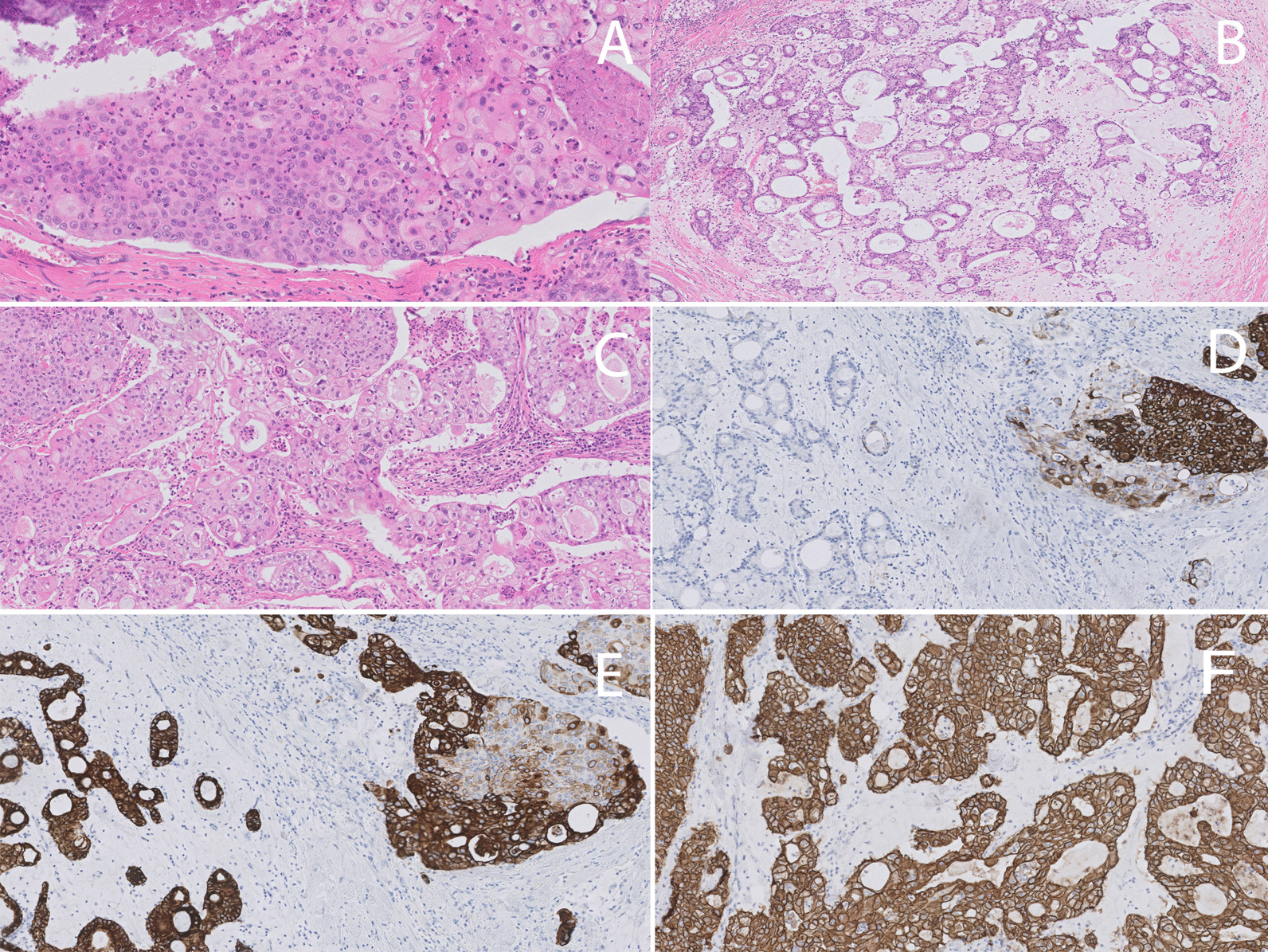
microscopic features of MEC-b [hematoxylin and eosin (H&E) and immunohistochemistry]. A The tumor cells were mostly arranged in solid nests admixed with necrotic areas (upper left and right corner). The tumor cell population was characterized by a mixture of large epidermoid cells and relatively small intermediate cells in absence of mature keratinization. B Microcystic and cribriform structures lined by tumor cells with mucinous differentiation, floating in large pools of extracellular mucine. C At high power magnification the heterogeneous tumor cell composition was clearly visible. Next to large epidermoid cells and intermediate cells, we noticed also the presence of scattered cells with clear cytoplasm and mucinous differentiation. The adjacent stroma showed moderate mixed inflammatory infiltrate characterized by high number of neutrophils. The formation of several micro-abscesses was also apparent. The typical zoning pattern described in MEC was clearly visible by sequential staining with CK 5.6 (D) and CK7 (E). The two microphotographies show a mirror picture with large epidermoid cells positive for CK5.6 but negative for CK7, and conversely the mucinous component positive for CK7 but negative for CK 5.6. F The HER2 immunostaining surprisingly showed strong and diffuse membranous staining in all tumor cells. Fluorescence in situ hybridization (FISH) analysis confirmed the amplification of the HER2 gene
By IHC the composite mixture of tumor cells was confirmed by a combination of high and low molecular weight cytokeratins (Fig. 2D, E). Areas with epidermoid differentiation showed p63 and GATA3 staining; BRST-2 was negative.
Nuclear weak AR and ER expression was observed in < 10% of the tumor cells in the mucinous component. PR was negative. HER2 showed a score of 3+ (Fig. 2F). Flurescence in situ hybridization (FISH) analysis confirmed HER2 gene amplification and showed absence of MAML2 rearrangements. The DCIS component was HER2 positive but lacked hormone receptor expression.
The revision of the IBC-NST of 1996 confirmed the absence of MEC features.
The final diagnosis of grade 3 breast MEC was proposed (rpT1Nx).
The adjuvant therapy consisted of paclitaxel (12 cycles, weekly) and trastuzumab (18 cycles, every 3 weeks). Aromatase inhibitors were not administered because of the low ER and potential unfavorable side-effect/benefit ratio. Germ-line genetic screening excluded presence of predisposing mutations for hereditary breast–ovarian cancer syndrome.
After 61 months of follow-up the patient is alive, without any sign of recurrence.
Methods
The patient provided her informed consent and clinical history and imaging were retrieved from her medical files.
IHC was performed using the following antibodies: ER (Dako, clone EP1, ready to use), PR (Dako, clone PgR1294, ready to use), AR (Dako, clone AR441, dilution 1:100), HER2 (Dako, polyclonal rabbit anti-human c-erB-2 oncoprotein, dilution 1:1000), cytokeratin 5/6 (CK5.6) (Dako, clone D5/16 B4, ready to use), cytokeratin 7 (CK7) (Dako, clone OV-T2 12/30, ready to use), transformation-related protein 63 (p63) (Dako, clone DAK-p63, ready to use), GATA binding protein 3 (GATA3) (Biomedical Care, clone L50-823, ready to use), and gross cystic disease fluid protein-15 (BRST2) (Dako, clone D6, dilution 1:300). The Dako EnVision FLEX Target Retrieval Solution High pH (50×) (Dako Omnis) was used for the antigen retrieval of all antibodies, but for BRST2 EnVision FLEX Target Retrieval Solution Low pH (50×) (Dako Omnis) was used.
FISH for HER2 [PathVysion HER-2 DNA Probe Kit (PathVysion Kit)] and MAML2 rearrangements [SPEC MAML2 Dual Color Break Apart Probe (Zytovision)/Histology FISH Accessory kit (Dako)] was performed, following vendors’ specifications.
Discussion
MEC-b is a rare subtype of TNBC that has morphomolecular features in common with MEC-sg counterpart. Breast and salivary glands are both exocrine glands derived from the embryonal ectoderm, which also explains the shared morphology with MECs from other organs. Herein we present a case of a recurrent BC with typical histopathological MEC-b features, but showing HER2 amplification.
Only 64 cases of MEC-b have been reported in English literature so far. MEC-b has been described exclusively in females aged from 29 to 86 years (average 59 years) (Table 1). Despite the predominant TNBC phenotype, low grade MEC-b are associated with good prognosis. Interestingly, BC-specific mortality and metastasis seems to occur only in high grade MEC-b, while mortality and metastasis in low- and intermediate-grade MEC-b are absent, even without a-CT [5]. These observations render the role of a-CT questionable in low-grade MEC-b. For this reason, a recent consensus statement endorses the use of tumor grading to inform clinicians about the need of a-CT in MEC-b [9]. Our case showed typical high-grade MEC-b features, using both grading systems for breast and salivary glands [4], supporting the use of a-CT.
Table 1.
Literature overview of breast MECs
| No | Authors | Year of publication | Age | Tumor dimension (cm) | Grading | Lymph node metastasis | Distant Metastasis | Type of surgery | Adjuvant therapy | Follow-up (months) | ER−PR status | HER2 status | Molecular analysis (MAML2translocation) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Patchefsky et al. [10] | 1979 | 70 | 5 | LG | NA | No | Q | NA | 10 alive | NA | NA | NA |
| 2 | 66 | 1.3 | LG | No | No | RM | NA | 94 DOR | NA | NA | NA | ||
| 3 | Kovi et al. [11] | 1981 | 46 | 11 | HG | Yes | NA | MRM | NA | NA | NA | NA | NA |
| 4 | Fisher et al. [12] | 1983 | 60 | 4 | LG | NA | No | SM | NA | 48 alive | NA | NA | NA |
| 5 | 49 | 3.7 | LG | No | No | RM | NA | 108 alive | NA | NA | NA | ||
| 6 | 57 | 2.5 | LG | No | No | MRM | NA | 120 alive | NA | NA | NA | ||
| 7 | 71 | 2 | LG | No | No | MRM | NA | 48 alive | NA | NA | NA | ||
| 8 | 65 | 2 | LG | NA | No | L | NA | 60 alive | NA | NA | NA | ||
| 9 | Ratanarapee et al. [13] | 1983 | 27 | NA | HG | Yes | Yes | NA | NA | 14 DOD | NA | NA | NA |
| 10b | Leong and Williams [14] | 1985 | 57 | 3.5 | HC | No | Yes | SM | No | 7 DOD | NA | NA | NA |
| 11 | Hastrup and Sehested [15] | 1985 | 59 | 1 | HG | No | Yes | RM | RT+CT+HT | 25 DOD | −− | NA | NA |
| 12b | Hanna and Kahn [16] | 1985 | 31 | NA | NA | Yes | No | MRM | CT | 14 alive | +− | NA | NA |
| 13b | 51 | 2 | NA | No | No | MRM | No | 8 alive | +− | NA | NA | ||
| 14b | Pettinato et al. [17] | 1989 | 72 | 7 | HG | Yes | Yes | MRM | CT | 10 DOD | NA | NA | NA |
| 15b | Luchtrath and Moll [18] | 1989 | 60 | 5 | HG | Yes | Yes | RM | NA | 30 DOD | NA | NA | NA |
| 16 | Chang et al. [19] | 1998 | 54 | 4.5 | HG | No | No | MRM | CT | 48 alive | NA | NA | NA |
| 17 | Markopoulos et al. [20] | 1998 | 40 | 2 | HG | No | No | L+ALND | NA | 60 alive | NA | NA | NA |
| 18 | Berry et al. [21] | 1998 | 51 | 3.5 | HG | No | no | MRM | NA | NA | NA | NA | NA |
| 19 | Tjalma et al. [22] | 2002 | 58 | 3.5 | HG on LG | Yes | Yes | RM | NA | 156 alive | NA | NA | NA |
| 20 | Terzi et al. [23] | 2004 | 79 | 8 | HG | Yes | No | MRM | NA | NA | NA | NA | NA |
| 21 | Di Tommaso et al. [24] | 2004 | 36 | 0.6 | HG | NA | No | Q+ALND | NA | 18 alive | NA | NA | NA |
| 22 | 55 | 1.1 | IG | Na | No | Q+ALND | NA | 3 alive | NA | NA | NA | ||
| 23 | 54 | 1.5 | LG | NA | No | Q+ALND | NA | 13 alive | NA | NA | NA | ||
| 24 | 29 | 0.8 | LG | NA | No | L | NA | 90 alive | NA | NA | NA | ||
| 25 | 80 | 0.5 | LG | NA | No | L | NA | 5 alive | NA | NA | NA | ||
| 26 | Gomez−Aracil et al. [25] | 2006 | 69 | 6 | HG | Yes | No | MRM | CT | 54 alive | +− | NA | NA |
| 27 | Horii et al. [26] | 2006 | 54 | 2.5 | LG | No | No | MRM | HT | 36 alive | +− | − | NA |
| 28b | Hornychova et al. [27] | 2007 | 30 | 8 | LG | No | No | MRM | RT+CT | 60 alive | −− | − | NA |
| 29 | 63 | 1.8 | HG | No | No | MRM | RT+CT | 18 alive | −− | − | NA | ||
| 30b | Camelo-Piragua et al. [8] | 2009 | 49 | > 5, multiple microinvasive foci with extensive in situ | IG | Yes | No | MRM | CT | 8 alive | −− | − | + (del.11q21) |
| 31b | Basbug et al. [28] | 2011 | 69 | 10 | HG | No | No | MRM | RT+CT | 12 alive | −− | − | NA |
| 32 | Turk et al. [29] | 2013 | 40 | 5.5 | NA | Yes | No | MRM | CT | 5 alive | −− | − | NA |
| 33b | Palermo et al. [30] | 2013 | 80 | 4 | HG | No | No | NA | NA | NA | −− | NA | NA |
| 34 | Fujino et al. [31] | 2016 | 71 | 1.7 | IG | No | No | SM | NA | NA | −− | − | − (RT−PCR) |
| 35 | Cheng et al. [32] | 2017 | 61 | 3 | LG | No | No | SM | No | 4 alive | ++ | − | NA |
| 36 | 66 | 1.3 | LG | No | No | SM | No | 9 alive | +− | − | NA | ||
| 37 | 49 | 1.5 | LG | No | No | MRM | No | 41 alive | −− | − | NA | ||
| 38 | 39 | 1.5 | LG | Yes | No | MRM | No | 156 alive | ++ | − | NA | ||
| 39 | Sherwell-Cabello et al. [33] | 2017 | 86 | 6 | LG | No | No | MRM | No | 3 alive | −− | − | NA |
| 40b | Burghel et al. [34]a | 2018 | 73 | < 2 | LG | No | No | L | No | 50 alive | NA | NA | NA |
| 41 | GR Bean et al. [6] | 2018 | 49 | 5 | IG | Yes | No | MRM | CT | 12 alive | −− | − | + (FISH, RT−PCR) |
| 42 | 53 | 1.6 | LG | No | No | L | RT | 16 alive | −− | − | + (FISH, NGS, RT−PCR) | ||
| 43 | Mingfei Yan et al. [5]ª | 2019 | 60 | 1.9 | LG | NA | No | L | NA | 60 alive | −− | − | + (FISH) |
| 44 | Ru-Pei Ye et al. [35] | 2020 | 42 | 2.6 | LG | NA | No | MRM | CT | 12 alive | −− | − | NA |
| 45 | Fresia Pareja et al. [7]a | 2020 | NA | NA | LG | NA | NA | NA | NA | NA | −− | − | + (FISH, RNA sequencing RT−PCR) |
| 46 | Linda Metaxa et al. [36] | 2020 | 63 | 2.1 | LG | No | No | L | No | 36 alive | + NA | NA | NA |
| 47 | Black et al. [37] | 2023 | 65 | 1.3 | LG | No | No | L | RT | 30 alive | +− | − | + (FISH, RT−PCR, NGS)c |
| 48 | He et al. [38] | 2023 | 39 | 1.2 | LG | No | No | NA | NA | 24 | −− | − | + (FISH) |
| 49 | 37 | 1.2 | LG | No | No | NA | NA | 30 | −− | − | + (FISH) | ||
| 50 | 40 | 1.5 | IG | No | No | NA | NA | 12 | −− | − | + (FISH) | ||
| 51−63b, d | Venetis et al. [39] | 2023 |
41–75 (n = 13) |
≤ 2/(n = 8) 2.1–5/(n = 2) > 5/(n = 1) NA/(n = 3) |
LG (n = 10) HG (n = 3) |
No (n = 11) Yes (n = 1) NA (n = 1) |
No (n = 10) Yes (n = 2) NA (n = 1) |
NA (n = 13) | NA (n = 13) | NA (n = 13) | +(2/13)/−(13/13) | −(13/13) |
−(10/10); (FISH) 8/13 (NGS) |
| 64 | Present case | 2023 | 58 | 2 | HG | No | No | SM | CT+TT | 61 alive | +− | + | − (FISH) |
The table summarizes the 64 cases of breast MECs so far reported in literature, with the addition of our case. Reports are listed in chronological order including available information about grading, regional and distant metastasis, therapy, follow-up, receptors status, and molecular analysis. A clear correlation between grading, distant metastasis, and deaths of disease can be observed. Relatively few data are available about molecular analysis, which was performed only in most recent cases. In italics are shown the cases that report the presence of mature squamous cells, intercellular bridges, and/or squamous pearl formation; therefore, not being fully consistent with the current diagnostic criteria of the WHO. The table has been adapted from Ru-Pei Ye et al. [35] and Murat Basbug et al. [28]
NA not available, RM radical mastectomy, MRM modified radical mastectomy, SM simple mastectomy, Q quadrantectomy, L lumpectomy, ALND axillary lymph node dissection, RT radiotherapy, CT chemotherapy, HT hormonal therapy (tamoxifen or aromatase inhibitor), TT targeted therapy, DOD died of disease, DOR died of other reasons, RT–PCR reverse transcriptase polymerase chain reaction, FISH fluorescence in situ hybridization, NGS next-generation sequencing (DNA)
aUpdated information obtained by correspondent authors via email
bCases in which presence of mature squamous cells, intercellular bridges and/or squamous pearls formation is reported
cCase 47 report CRTC3:MAML2 translocation
dVenetis et al. report presence of squamous differentiation in 4/13 cases, and absence of true keratinization in all cases of the series (n = 13); FISH was performed in 10 of the 13 cases, in 8/13 cases NGS data were also available
Furthermore, the unusual finding of HER2 amplification prompted us to combine anti-HER2 therapy with backbone a-CT. To the best of our knowledge, no HER2-positive cases of MEC-b or of other salivary gland-like tumors of the breast have been reported in the literature so far, except for one sporadic secretory carcinoma of the breast [40, 41]. On the contrary, about 5% MEC-sg may show HER2 amplification, which may relate to differentiation grade [42, 43]. Therefore, we surmise that our case might be consistent with this observation. Interestingly, about 1/6 to 1/8 of MEC-b belong to the category of the so-called ER low-positive BC, defined by ER expression in < 10% of the tumor cells [44], a feature shared also with other salivary gland-like tumors of the breast [40, 45–47]. The use of endocrine therapy in these cases is highly debated and should be individually discussed [48].
MEC-b is characterized by a mixture of epidermoid, intermediate, and mucinous neoplastic cells. Mucinous differentiation may be inconspicuous, especially in high-grade tumors. Presence of true keratinization and/or squamous pearls formation should prompt to consider another diagnosis (in other words, metaplastic carcinoma with adenosquamous pattern) [4]. To note overt keratinization is accepted in MEC-sg, perhaps explaining why in ~ 10 old MEC-b cases a mature squamous cell component is described (Table 1). As suggested here, the diagnosis of MEC-b remains extremely challenging, especially on diagnostic biopsies. Pathologists should be aware of this rare entity whenever a mixture of intermediate and large eosinophilic cells associated with mucinous differentiation is observed. Immunohistochemistry to confirm the presence of the typical “zoning pattern” is helpful [4–6].
The differential diagnosis is broad and includes apocrine carcinoma, metaplastic adenosquamous carcinoma, mucinous carcinoma, mucinous cystadenocarcinoma, and a metastatic MEC-sg. However, an in situ component should exclude the latter [4]. We excluded also the possibility of a late recurrence of the primary BC because of the lack of MEC elements, the strong hormone receptor expression in 1996, and the presence of an in situ component with MEC features in the current tumor, supporting the diagnosis of a second primary.
To date molecular analysis has been reported in 21 MEC-b, of which seven harbored CRTC1-MAML2 and one harbored CRTC3-MAML2 translocation (Table 1) [5–7, 37–39]. Remarkably, the majority of positive cases were either low or intermediate grade. Likewise in MEC-sg, MAML2 translocation seems to be the most frequent recurrent genetic alteration also in MEC-b (n = 9/21, 43% prevalence). However, we were not able to detect MAML2 translocation by FISH, which did not prevent us to confirm the diagnosis because of clear-cut morphology. Similarly Venet et al. did not detect MAML2 rearrangements in any of the 10 MEC-b tested by FISH, questioning the diagnostic value of this molecular hallmark in MEC-b. Notably, three low-grade MEC-b were not tested in their series [39]. Techniques like RT–PCR and FISH taken individually may have low sensitivity due to technical issues (for example, polymerase errors, small deletions, and so on) as compared with more sensitive techniques like RNA sequencing. Conversely, when considering our case, we may speculate a causal correlation with poor differentiation grade as suggested in MEC-sg [49].
Conclusions
MEC-b is a very rare entity. Diagnosis on small diagnostic biopsies may be challenging. Strict application of WHO criteria is desirable, as well as standard evaluation of receptor status for best patient care.
Acknowledgements
Not applicable.
Abbreviations
- a-CT
Adjuvant chemotherapy
- AR
Androgen receptor
- BC
Breast cancer
- DCIS
Ductal carcinoma in situ
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor 2
- IBC-NST
Invasive breast carcinoma of no special type
- IHC
Immunohistochemistry
- MAML2
Mastermind-like transcriptional coactivator 2
- MEC-b
Mucoepidermoid carcinoma of the breast
- MEC-sg
Mucoepidermoid carcinoma of the salivary glands
- PR
Progesterone receptor
- TNBC
Triple-negative breast carcinoma
- WHO
World health organization
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MDM, CC, and GF. The first draft of the manuscript was written by MDM and CC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
MDM was recipient of an international scholarship in the context of the ERASMUS-MUNDI as a student exchange. GF is recipient of a post-doctoral scholarship financed by the KOOR from the University Hospitals Leuven.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethical approval and consent to participate
Ethics Committee of the University Hospitals Leuven informed the authors that formal EC approval was not required for this study.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mario Della Mura and Celine Clement have contributed equally to this work.
References
- 1.Foschini MP, Morandi L, Asioli S, Giove G, Corradini AG, Eusebi V. The morphological spectrum of salivary gland type tumours of the breast. Pathology. 2017;49:215–227. doi: 10.1016/j.pathol.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Thomas A, Reis-Filho JS, Geyer CE, Jr, Wen H. Rare subtypes of triple negative breast cancer: current understanding and future directions. NPJ Breast. 2023;9:55. doi: 10.1038/s41523-023-00554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foschini MP, Geyer FC, Marchio C and Nishimura R. Mucoepidermoid carcinoma. In: WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Breast Tumours. Lyon: International Agency for Research on Cancer; 2019. pp. 149–50.
- 5.Yan M, Gilmore H, Harbhajanka A. Mucoepidermoid carcinoma of the breast With MAML2 rearrangement: a case report and literature review. Int J Surg Pathol. 2020;28:787–792. doi: 10.1177/1066896920916779. [DOI] [PubMed] [Google Scholar]
- 6.Bean GR, Krings G, Otis CN, Solomon DA, García JJ, van Zante A, Camelo-Piragua S, van Ziffle J, Chen YY. CRTC1-MAML2 fusion in mucoepidermoid carcinoma of the breast. Histopathology. 2019;74:463–473. doi: 10.1111/his.13779. [DOI] [PubMed] [Google Scholar]
- 7.Pareja F, Da Cruz PA, Gularte-Mérida R, Vahdatinia M, Li A, Geyer FC, da Silva EM, Nanjangud G, Wen HY, Varga Z, Brogi E, Rakha EA, Weigelt B, Reis-Filho JS. Pleomorphic adenomas and mucoepidermoid carcinomas of the breast are underpinned by fusion genes. NPJ Breast Cancer. 2020 doi: 10.1038/s41523-020-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camelo-Piragua SI, Habib C, Kanumuri P, Lago CE, Mason HS, Otis CN. Mucoepidermoid carcinoma of the breast shares cytogenetic abnormality with mucoepidermoid carcinoma of the salivary gland: a case report with molecular analysis and review of the literature. Hum Pathol. 2009;40:887–892. doi: 10.1016/j.humpath.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Cserni G, Quinn CM, Foschini MP, Bianchi S, Callagy G, Chmielik E, Decker T, Fend F, Kovács A, van Diest PJ, Ellis IO, Rakha E, Tot T. European Working Group for breast screening pathology: triple-negative breast cancer histological subtypes with a favourable prognosis. Cancers. 2021 doi: 10.3390/cancers13225694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patchefsky AS, Frauenhoffer CM, Krall RA, Cooper HS. Low-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med. 1979;103:196–198. [PubMed] [Google Scholar]
- 11.Kovi J, Duong HD, Leffall LS., Jr High-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med. 1981;105:612–614. [PubMed] [Google Scholar]
- 12.Fisher ER, Palekar AS, Gregorio RM, Paulson JD. Mucoepidermoid and squamous cell carcinomas of breast with reference to squamous metaplasia and giant cell tumors. Am J Surg Pathol. 1983;7:15–27. doi: 10.1097/00000478-198301000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ratanarapee S, Prinyar-Nussorn N, Chantarakul N, Pacharee P. High-grade mucoepidermoid carcinoma of the breast: a case report. J Med Assoc Thai. 1983;66:642–8. [PubMed] [Google Scholar]
- 14.Leong AS, Williams JA. Mucoepidermoid carcinoma of the breast: high grade variant. Pathology. 1985;17:516–521. doi: 10.3109/00313028509105513. [DOI] [PubMed] [Google Scholar]
- 15.Hastrup N, Sehested M. High-grade mucoepidermoid carcinoma of the breast. Histopathology. 1985;9:887–892. doi: 10.1111/j.1365-2559.1985.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanna W, Kahn HJ. Ultrastructural and immunohistochemical characteristics of mucoepidermoid carcinoma of the breast. Hum Pathol. 1985;16:941–946. doi: 10.1016/S0046-8177(85)80133-7. [DOI] [PubMed] [Google Scholar]
- 17.Pettinato G, Insabato L, De Chiara A, Manco A, Petrella G. High-grade mucoepidermoid carcinoma of the breast: fine needle aspiration cytology and clinicopathologic study of a case. Acta Cytol. 1989;33:195–200. [PubMed] [Google Scholar]
- 18.Lüchtrath H, Moll R. Mucoepidermoid mammary carcinoma: immunohistochemical and biochemical analyses of intermediate filaments. Virchows Arch A Pathol Anat Histopathol. 1989;416:105–13. doi: 10.1007/BF01606314. [DOI] [PubMed] [Google Scholar]
- 19.Chang LC, Lee N, Lee CT, Huang JS. High-grade mucoepidermoid carcinoma of the breast: case report. Changgeng Yi Xue Za Zhi. 1998;21:352–357. [PubMed] [Google Scholar]
- 20.Markopoulos C, Gogas H, Livaditou A, Floros D. Mucoepidermoid carcinoma of the breast. Eur J Gynaecol Oncol. 1998;19:291–293. [PubMed] [Google Scholar]
- 21.Berry MG, Caldwell C, Carpenter R. Mucoepidermoid carcinoma of the breast: a case report and review of the literature. Eur J Surg Oncol. 1998;24:78–80. doi: 10.1016/S0748-7983(98)80135-2. [DOI] [PubMed] [Google Scholar]
- 22.Tjalma WA, Verslegers IO, De Loecker PA, Van Marck EA. Low and high grade mucoepidermoid carcinomas of the breast. Eur J Gynaecol Oncol. 2002;23:423–425. [PubMed] [Google Scholar]
- 23.Terzi ASA, Uner A. A 79 year-old woman with a mass in the right breast. Turk J Cancer. 2004;34:38–39. [Google Scholar]
- 24.Di Tommaso L, Foschini MP, Ragazzini T, Magrini E, Fornelli A, Ellis IO, Eusebi V. Mucoepidermoid carcinoma of the breast. Virchows Arch. 2004;444:13–19. doi: 10.1007/s00428-003-0923-y. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Aracil V, Mayayo Artal E, Azua-Romeo J, Mayayo Alvira R, Azua-Blanco J, Arraiza GA. Fine needle aspiration cytology of high grade mucoepidermoid carcinoma of the breast: a case report. Acta Cytol. 2006;50:344–348. doi: 10.1159/000325967. [DOI] [PubMed] [Google Scholar]
- 26.Horii R, Akiyama F, Ikenaga M, Iwase T, Sakamoto G. Muco-epidermoid carcinoma of the breast. Pathol Int. 2006;56:549–553. doi: 10.1111/j.1440-1827.2006.02004.x. [DOI] [PubMed] [Google Scholar]
- 27.Hornychová H, Ryska A, Betlach J, Bohác R, Cízek T, Tomsová M, Obermannová R. Mucoepidermoid carcinoma of the breast. Neoplasma. 2007;54(2):168–172. [PubMed] [Google Scholar]
- 28.Basbug M, Akbulut S, Arikanoglu Z, Sogutcu N, Firat U, Kucukoner M. Mucoepidermoid carcinoma in a breast affected by burn scars: comprehensive literature review and case report. Breast Care (Basel) 2011;6:293–297. doi: 10.1159/000331316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turk E, Karagulle E, Erinanc OH, Soy EA, Moray G. Mucoepidermoid carcinoma of the breast. Breast J. 2013;19:206–208. doi: 10.1111/tbj.12080. [DOI] [PubMed] [Google Scholar]
- 30.Palermo MH, Pinto MB, Zanetti JS, Ribeiro-Silva A. Primary mucoepidermoid carcinoma of the breast: a case report with immunohistochemical analysis and comparison with salivary gland mucoepidermoid carcinomas. Pol J Pathol. 2013;64:210–215. doi: 10.5114/pjp.2013.38141. [DOI] [PubMed] [Google Scholar]
- 31.Fujino M, Mori D, Akashi M, Yamamoto H, Aibe H, Matake K, Shirahane K. Mucoepidermoid carcinoma of the breast found during treatment of lymphoma. Case Rep Oncol. 2016;9:806–881. doi: 10.1159/000452792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng M, Geng C, Tang T, Song Z. Mucoepidermoid carcinoma of the breast: four case reports and review of the literature. Medicine. 2017;96:e9385. doi: 10.1097/MD.0000000000009385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherwell-Cabello S, Maffuz-Aziz A, Ríos-Luna NP, Pozo-Romero M, López-Jiménez PV, Rodriguez-Cuevas S. Primary mucoepidermoid carcinoma of the breast. Breast J. 2017;23:753–755. doi: 10.1111/tbj.12819. [DOI] [PubMed] [Google Scholar]
- 34.Burghel GJ, Abu-Dayyeh I, Babouq N, Wallace A, Abdelnour A. Mutational screen of a panel of tumor genes in a case report of mucoepidermoid carcinoma of the breast from Jordan. Breast J. 2018;24:1102–1104. doi: 10.1111/tbj.13142. [DOI] [PubMed] [Google Scholar]
- 35.Ye RP, Liao YH, Xia T, Kuang R, Long HA, Xiao XL. Breast mucoepidermoid carcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2020;13:3192–3199. [PMC free article] [PubMed] [Google Scholar]
- 36.Metaxa L, Suaris TD, Elliott P, Exarchos G, Jones LJ, Sewedy T, Dani S, Dilks P. Primary mucoepidermoid carcinoma of the breast: a rare breast entity. Clin Oncol Res. 2020;3:2–9. [Google Scholar]
- 37.Black MA, Neumann NM, Krings G, Najjar S, Troxell ML, Wang A, et al. Genetic and immunohistochemical profiling of mammary hidradenoma and comparison to mucoepidermoid carcinoma. Mod Pathol. 2023;36:100270. doi: 10.1016/j.modpat.2023.100270. [DOI] [PubMed] [Google Scholar]
- 38.He X, You J, Chen Y, Tang H, Ran J, Guo D. Mucoepidermoid carcinoma of the breast, 3 case report and literature review. Medicine. 2023;102:e33707. doi: 10.1097/MD.0000000000033707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venetis K, Sajjadi E, Ivanova M, Andaloro S, Pessina S, Zanetti C, et al. The molecular landscape of breast mucoepidermoid carcinoma. Cancer Med. 2023;12:10725–10737. doi: 10.1002/cam4.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob JD, Hodge C, Franko J, Pezzi CM, Goldman CD, Klimberg VS. Rare breast cancer: 246 invasive secretory carcinomas from the National Cancer Data Base. J Surg Oncol. 2016;113:721–725. doi: 10.1002/jso.24241. [DOI] [PubMed] [Google Scholar]
- 41.Geyer FC, Pareja F, Weigelt B, Rakha E, Ellis IO, Schnitt SJ, Reis-Filho JS. The spectrum of triple-negative breast disease: high- and low-grade lesions. Am J Pathol. 2017;187:2139–2151. doi: 10.1016/j.ajpath.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egebjerg K, Harwood CD, Woller NC, Kristensen CA, Mau-Sørensen M. HER2 positivity in histological subtypes of salivary gland carcinoma: a systematic review and meta-analysis. Front Oncol. 2021 doi: 10.3389/fonc.2021.693394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakano T, Yamamoto H, Hashimoto K, Tamiya S, Shiratsuchi H, Nakashima T, Nishiyama K-I, Higaki Y, Komune S, Oda Y. HER2 and EGFR gene copy number alterations are predominant in high-grade salivary mucoepidermoid carcinoma irrespective of MAML2 fusion status. Histopathology. 2013;63:378–392. doi: 10.1111/his.12183. [DOI] [PubMed] [Google Scholar]
- 44.Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med. 2020;144:545–563. doi: 10.5858/arpa.2019-0904-SA. [DOI] [PubMed] [Google Scholar]
- 45.Arpino G, Clark GM, Mohsin S, Bardou VJ, Elledge RM. Adenoid cystic carcinoma of the breast: molecular markers, treatment, and clinical outcome. Cancer. 2002;94:2119–2127. doi: 10.1002/cncr.10455. [DOI] [PubMed] [Google Scholar]
- 46.Ghabach B, Anderson WF, Curtis RE, Huycke MM, Lavigne JA, Dores GA. Adenoid cystic carcinoma of the breast in the United States (1977 to 2006): a population-based cohort study. Breast Cancer Res. 2010;12:R54. doi: 10.1186/bcr2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Castillo M, Chibon F, Arnould L, Croce S, Ribeiro A, Perot G, Hostein I, Geha S, Bozon C, Garnier A, Lae M, Vincent-Salomon A, MacGrogan G. Secretory breast carcinoma. Am J Surg Pathol. 2015;39:1458–1467. doi: 10.1097/PAS.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 48.Allison KH. Prognostic and predictive parameters in breast pathology: a pathologist’s primer. Mod Pathol. 2021;2021(34):94–106. doi: 10.1038/s41379-020-00704-7. [DOI] [PubMed] [Google Scholar]
- 49.Pérez-de-Oliveira ME, Wagner VP, Araújo ALD, Martins MD, Santos-Silva AR, Bingle L, Vargas PA. Prognostic value of CRTC1-MAML2 translocation in salivary mucoepidermoid carcinoma: Systematic review and meta-analysis. J Oral Pathol Med. 2020;49:386–394. doi: 10.1111/jop.12970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


