Abstract
Background
Fatigue is a common symptom of long COVID syndrome. Compared to male survivors, females have a higher incidence of post-COVID fatigue. Therefore, long-term follow-up is necessary to understand which groups of females are more vulnerable to post-COVID fatigue.
Methods
This is a nested case–control study of female COVID-19 survivors who were discharged from two designated hospitals in Wuhan, China in 2020, and received 2-year follow-up from March 1 to April 6, 2022. All patients completed the Checklist Individual Strength-subscale subjective fatigue (CIS-fatigue), a chronic obstructive pulmonary disease (COPD) assessment test (CAT), and the Hospital Anxiety and Depression Scale (HADS; including the HADS-Anxiety [HADS-A] and the HADS-Depression [HADS-D]). Individuals with CIS-fatigue scores of 27 or higher were classified as cases. The risk factors for fatigue was analysed with multivariable logistic regression analysis.
Results
A total of 899 female COVID-19 survivors were enrolled for analysis, including 47 cases and 852 controls. Compared with controls, cases had higher CAT, HADS-A and HADS-D scores, and showed a higher prevalence of symptoms, including anxiety (cases vs. controls, 44.7% vs. 4.0%, p < 0.001), chest tightness (21.2% vs. 2.3%, p < 0.001), dyspnoea (19.1% vs. 0.8%, p < 0.001) and so on. In multivariable logistic regression analysis, age (OR, 1.03; 95% CI, 1.01–1.06; p = 0.02) and cerebrovascular disease (OR, 11.32; 95% CI, 2.87–43.00; p < 0.001) were risk factors for fatigue. Fatigue had a statistically significant moderate correlation with depression (r = 0.44, p < 0.001), but not with CAT ≥ 10.
Conclusion
Female COVID-19 patients who had cerebrovascular disease and older age have higher risk of fatigue. Patients with fatigue have higher CAT scores, and are more likely to have concurrent depression.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-17382-0.
Keywords: COVID-19, Fatigue, Female, HADS, SARS-CoV-2
Introduction
Coronavirus Disease 2019 (COVID-19) has spread worldwide, with over 767 million confirmed cases as of 19 June 2023. Clinical manifestations include fever, cough, dyspnoea, myalgia, fatigue, normal or decreased leukocyte counts, and radiographic evidence of pneumonia [1]. Therapeutic options for COVID-19 remain limited, with some available in the market showing lower efficacy in real-world settings [2]. As patients recover from the acute phase, persistent, prolonged, and often debilitating sequelae are increasingly recognised in convalescent individuals, named ‘post-COVID-19 syndrome’ or ‘long COVID’ [3–5]. Long COVID was defined by WHO as symptoms in those with SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 and last for at least 2 months and cannot be explained by other diagnoses [6].
Fatigue has been consistently reported as one of the most significant symptoms of long COVID [7]. Previous studies have reported that 20–60% of patients suffer from post-COVID-fatigue [8, 9]. Although all symptoms continued to improve during the follow-up visit, recent studies have shown that fatigue is the most common symptom, regardless of the recovery time [10, 11]. According to a study by Cao et al. [10], sex, education, and preexisting comorbidities were risk factors in patients with post-COVID-19 fatigue who were discharged from hospital. Mazurkiewicz et al. included 303 non-hospitalised patients with COVID-19 and found that females more often suffered from persistent fatigue [12]. Of note, more females suffer from fatigue than males, and current evidence supports that female sex is a risk factor for post-COVID symptoms, including fatigue, anxiety, and depression [13]. However, few studies have explained the risk factors that contribute to the development of fatigue among female and few have quantitatively revealed the correlation between fatigue and mood disorders (including anxiety and depression). Therefore, further clinical analyses focusing on females are necessary to demonstrate which groups of females are more vulnerable to post-COVID fatigue up to 2 years after illness onset. Therefore, this study aimed to investigate the risk factors for fatigue in female COVID-19 survivors and the correlation between fatigue and mood disorders.
Methods
Study design and cohort
This study employed a nested case–control design using a multicentred prospective study cohort of COVID-19 patients who were discharged from Huoshenshan Hospital and Taikang Tongji Hospital (both in Wuhan, China) between February 12 and April 10, 2020. One-year and 2-year follow up (from March 1 to April 6, 2022) visits were performed on this cohort to investigate the long-term symptom burden of COVID-19, which have been reported in our previous studies [14]. The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for nested case–control studies and was approved by the Ethics Committee of the Daping Hospital, an affiliated hospital of Army Medical University (No. 202153). Informed consent was obtained from all participants or their legal representatives before the survey. All the methods were carried out in accordance with relevant guidelines and regulations from Declaration of Helsinki.
Case and control definition
The inclusion criterion was that female patients at 2 year follow up who had completed the Checklist Individual Strength–subscale subjective fatigue (CIS-fatigue), a chronic obstructive pulmonary disease (COPD) assessment test (CAT), and the Hospital Anxiety and Depression Scale (HADS; including the HADS-Anxiety [HADS-A] and the HADS-Depression [HADS-D]). The exclusion criteria included (1) those who declined to participate, (2) those unable to be contacted and (3) those deceased. CIS-fatigue, a standardized questionnaire with high internal consistency and test–retest reliability, is used to assess fatigue symptoms. The questionnaire consists of 8 items scored on a 7-point Likert scale. Total Scores range from 8 to 56 points, and a higher score indicates more clinical symptoms of general fatigue. Individuals with CIS-fatigue scores of 27 or higher were classified as cases, while those with scores below 27 were classified as controls.
Procedures and data acquisition
All female patients were contacted in the order of their discharge date documented in their medical records, and were interviewed via telephone by trained physicians. Various questionnaires were used, including a self-reported symptom table, CIS-fatigue [15, 16], CAT, and HADS (including HADS-A and HADS-D) (Additional file 1). CAT, initially designed to assess symptom burden of patients with COPD [17], was also capable to be applied to assess symptom burden of COVID-19 survivors [18]. HADS was used to measure mood symptoms of anxiety and depression [19]. Each subscale consists of 7 questions with a 4-point Likert scale (0–3). The scores of at least 8 indicates the presence of symptoms of anxiety or depression [20]. The self-reported symptom questionnaire included sweating, chest tightness, anxiety, myalgia, palpitation, cough, chest pain, dizziness, expectoration, dyspnea, headache, edema, taste change, smell reduction, sore throat, anorexia, diarrhea, hemoptysis, nausea, chill, vomiting, rhinobyon, short of breath, abdominal pain, hearing loss, alopecia, joint, and back pain. In this article, “symptoms-2y” was short for the number of symptoms reported in at 2-year follow-up.
Clinical data of patients during hospitalization were retrieved from electronic medical records, including demographic characteristics (self-report age and sex), clinical characteristics (self-reported comorbidities), and clinical treatments (ICU admission, oxygen therapy, and mechanical ventilation). In this article, "co-burden" was short for the number of self-reported comorbidities.
Disease severity was defined by World Health Organization guideline for COVID-19. Severe pneumonia refers to fever or suspected respiratory infection, plus one of the following: respiratory rate greater than 30 breaths per minute, severe respiratory distress, or oxygen saturation as measured by pulse oximetry (SpO2) less than or equal to 93% on room air [21]. We double-entered and validated all data using EpiData software version 3.1 (EpiData Association).
Statistical analysis
Continuous variables were presented as median (IQR), followed by Mann–Whitney U test, and categorical variables were presented as absolute values along with percentages, followed by the Pearson χ2 test or Fisher exact test when appropriate.
To identify factors associated with the risk of occurrence of fatigue defined by CIS-fatigue, univariable logistic regression analysis was used to identify potential risk factors with p < 0.10, and then it was adjusted by a stepwise (forward likelihood ratio) selection process in multivariable logistic regression model. All the scores of scales were subjected to Spearman correlation. Each test was 2-sided, p < 0.05 was considered significant, and correlation coefficient > 0.6 was considered highly associated. Data were analysed with SPSS statistical package version 26.0 for Windows (IBM SPSS Statistics) and R statistical software version 4.1.1 (R Project for Statistical Computing).
Results
Patient characteristics
After 2 years discharged from the hospital, 1864 patients were successfully followed up including 938 female patients. And 899 female patients were enrolled in the study for current analysis, except 39 participants who didn’t finish all the scales. Among them, 47 (5.2%) were classified as cases based on the score of CIS-fatigue (Fig. 1 and Additional file 2), while the remaining 852 were controls. The median (IQR) age of enrolled patients was 59 (50–68), and the median (IQR) length of hospital stay was 14 (8–20) days. During hospitalization, 17 patients (1.9%) were admitted to the ICU, and 627 patients (69.7%) received oxygen therapy (Table 1).
Fig. 1.
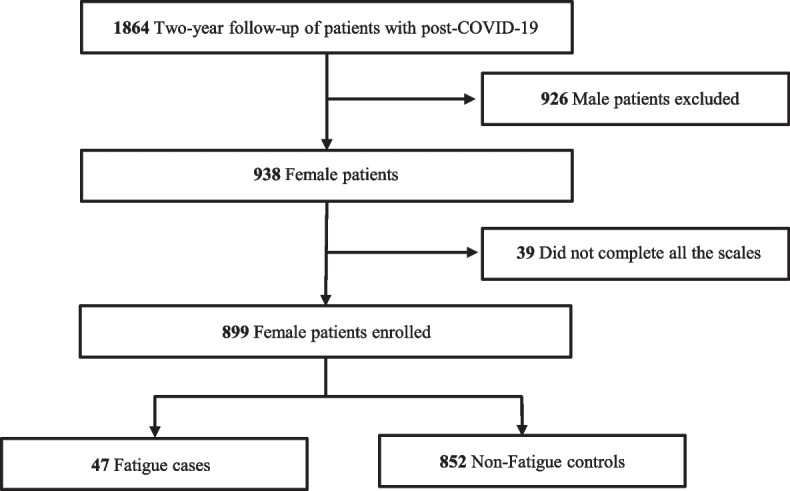
Study flowchart
Table1.
Characteristics of enrolled patients
| Patients, n (%) | P-value | |||
|---|---|---|---|---|
| Characteristic | Enrolled, N = 899a | Cases, N = 47a | Controls, N = 852a | Cases vs Controls |
| Aged | 59 (50, 68) | 66 (57, 71) | 58 (50, 67) | < 0.001‖ |
| disease severityb | 0.33 | |||
| Nonsevere | 675 (75.0%) | 32 (68.0%) | 643 (75.5%) | |
| severe | 224 (24.9%) | 15 (31.9%) | 209 (24.5%) | |
| Length of hospital stayd | 14 (8, 20) | 15 (10, 20) | 14 (8, 20) | 0.62 |
| ICU admissionc | 17 (1.9%) | 0 (0.0%) | 17 (2.0%) | > 0.99 |
| Oxygen therapyb | 627 (69.7%) | 38 (80.9%) | 589 (69.1%) | 0.12 |
| Mechanical ventilationc | 5 (0.6%) | 0 (0.0%) | 5 (0.6%) | > 0.99 |
| Hypertensionb | 247 (27.4%) | 20 (42.6%) | 227 (26.6%) | 0.027‖ |
| Diabetesb | 117 (13.0%) | 12 (25.5%) | 105 (12.3%) | 0.017‖ |
| Cardiovascular diseasec | 75 (8.3%) | 5 (10.6%) | 70 (8.2%) | 0.58 |
| Chronic liver diseasec | 26 (2.9%) | 3 (6.4%) | 23 (2.7%) | 0.15 |
| Cerebrovascular diseasec | 11 (1.2%) | 5 (10.6%) | 6 (0.7%) | < 0.001‖ |
| Chronic kidney diseasec | 19 (2.1%) | 2 (4.3%) | 17 (2.0%) | 0.26 |
| Tumorc | 15 (1.7%) | 1 (2.1%) | 14 (1.6%) | 0.56 |
| Tracheitisc | 9 (1.0%) | 2 (4.3%) | 7 (0.8%) | 0.048 |
| COPDc | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | > 0.99 |
| coBurdend | < 0.001‖ | |||
| 1 | 203 (22.6%) | 13 (27.7%) | 190 (22.3%) | |
| 2 | 103 (11.5%) | 9 (19.1%) | 94 (11.0%) | |
| 3 | 34 (3.8%) | 5 (10.6%) | 29 (3.4%) | |
| 4 | 1 (0.1%) | 1 (2.1%) | 0 (0.0%) | |
| 5 | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | |
COPD chronic obstructive pulmonary disease
‖significant at α = 0.05
aMedian (IQR); Frequency (%)
bPearson's Chi-squared test
cFisher's exact test
dWilcoxon rank sum test
The median (IQR) age of cases was 66 (57–71), while the median (IQR) age of controls was 58 (50–67). Compared with controls, cases suffered from higher prevalence of hypertension (cases vs controls, 42.6% vs 26.6%, p = 0.027), diabetes (cases vs controls, 25.5% vs 12.3%, p = 0.017), cerebrovascular disease (cases vs controls, 10.6% vs 0.7%, p < 0.001) and tracheitis (cases vs controls, 4.3% vs 0.8%, p = 0.048). However, no significant differences were found in terms of disease severity, the length of hospital stay, and the percentage of other diseases (eg. cardiovascular disease, chronic liver disease, chronic kidney disease or COPD) (Table 1).
Characteristics of long COVID syndrome at 2-year follow-up
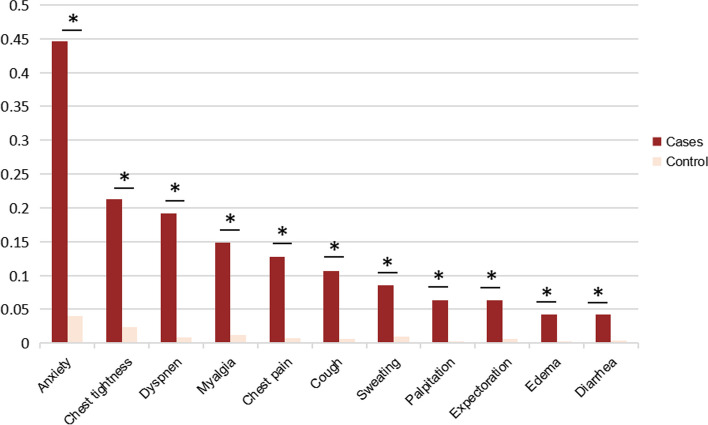
The most common post-COVID symptoms in cases were anxiety (44.7%), chest tightness (21.2%) and dyspnea (19.1%) while in controls were anxiety (4.0%), joint and back pain (3.3%) and chest tightness (2.3%) (Additional file 3). The prevalence of several symptoms was significantly higher in cases compared to controls, such as sweating, chest tightness, anxiety, myalgia, palpitation, cough, chest pain, expectoration, dyspnea, edema and diarrhea (Fig. 2). Besides, those symptoms were subjected to Spearman correlation. In cases, chest pain was highly associated with chest tightness (r = 0.74, p < 0.001) while expectoration was highly associated with cough (r = 0.76, p < 0.001) (Additional file 4).
Fig. 2.
The percentage of symptom burden of cases and controls. *significant at α = 0.05 level
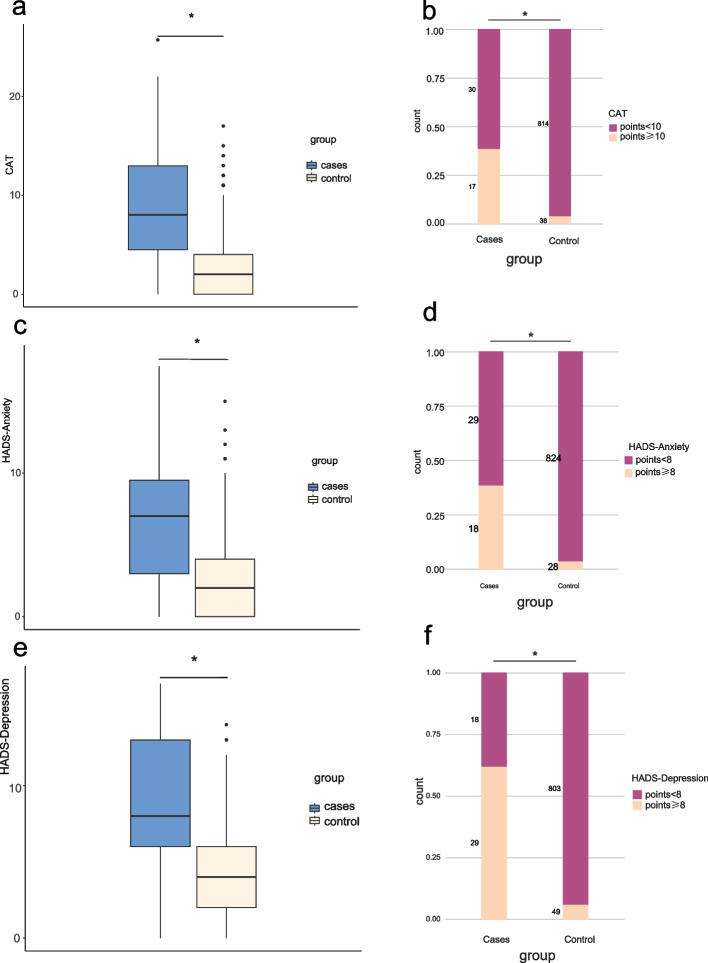
CAT scores at 2-year follow-up
Of the enrolled patients, 55 (6.1%) were grouped as CAT ≥ 10, while 844 (93.8%) were CAT < 10 (Additional file 5). Case group had higher CAT scores than control group (median [IQR], cases vs controls, 8 [4.5–13] vs 2 [0–4]; p < 0.001) (Fig. 3a). Among the 47 cases, 17 (36.1%) patients were scored as CAT ≥ 10, and the rate was higher than controls (38[4.5%]; p < 0.001) (Fig. 3b).
Fig. 3.
Total scores of CAT, HADS-Anxiety and HADS-Depression in cases and controls. A Total CAT score. B Percentage of patients with CAT score ≥ 10 and < 10; C Total HADS-Anxiety score. D Percentage of patients with HADS-A score ≥ 8 and < 8; E Total HADS-Depression score. F Percentage of patients with HADS-D score ≥ 8 and < 8. *significant at α = 0.05 level
HADS-anxiety and HADS-depression scores at 2-year follow-up
A total of 46 (5.1%) patients were grouped as having anxiety (Additional file 6). Cases had higher HADS-anxiety scores than controls (median [IQR], 7 [3–9.5] vs 2 [0–4]; p < 0.001) (Fig. 3c). Among cases, 18 (38.3%) were with anxiety, and the rate was higher than that of control subjects (28 [3.3%]; p < 0.001) (Fig. 3d).
The overall incidence of depression in survivors was 8.6% (Additional file 7). Cases had higher HADS-depression scores than controls (median [IQR], 8 [6,–13] vs 4 [2,–6]; p < 0.001) (Fig. 3e). The rate of depression in cases was 61.7%, which was higher than in controls (49 [5.8%]; p < 0.001) (Fig. 3f).
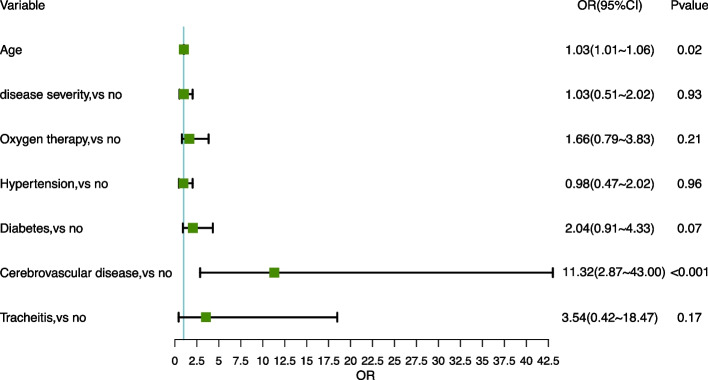
The risk factors of fatigue at 2-year follow-up
Compared with non-fatigue individuals, age, disease severity, oxygen therapy, hypertension, diabetes, cerebrovascular disease, tracheitis were associated with fatigue under univariable analysis. ln multivariable analysis, age (OR, 1.03; 95%CI, 1.01–1.06; p = 0.02) and cerebrovascular disease (OR, 11.32; 95%CI, 2.87–43.00; p < 0.001) were the risk factors of fatigue (Fig. 4). Statistically significant moderate correlations were found between CIS ≥ 27 and HADS-D ≥ 8 (r = 0.44, p < 0.001) (Fig. 5).
Fig. 4.
Logistic regression models to evaluate the risk factors for fatigue
Fig. 5.
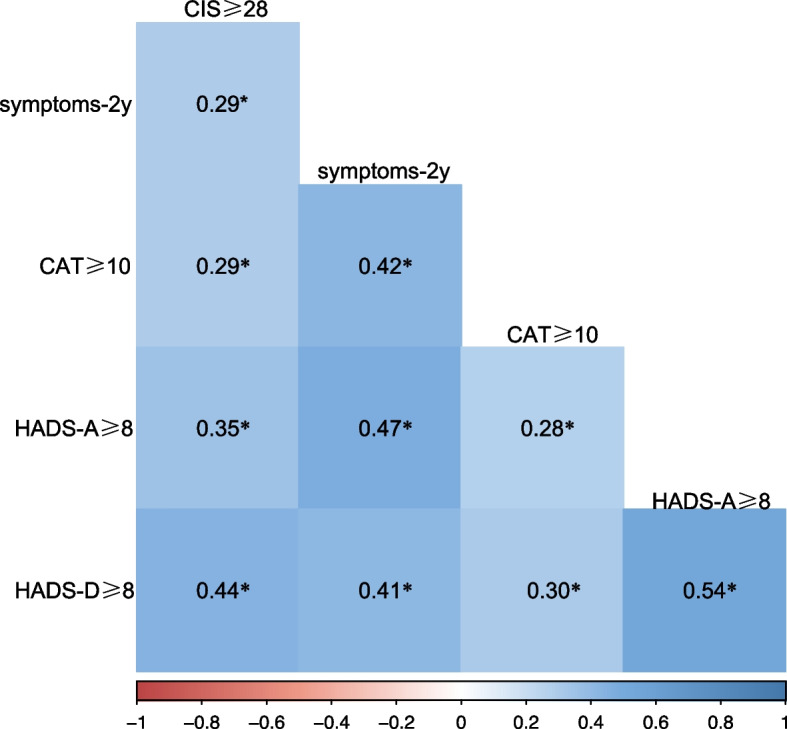
Spearman's rank correlation matrix and correlation significances of relevant variables. *significant at α = 0.05 level
Discussion
This nested case–control study focused on the incidence and risk factors of post-COVID-19 fatigue among female survivors. A significant proportion (5.2%) of survivors still experienced fatigue 2 years after discharge. Compared to controls, those with fatigue demonstrated higher scores on the CAT, HADS-anxiety, and HADS-depression. Age and cerebrovascular disease are risk factors for fatigue. Additionally, there were significant moderate correlations between CIS ≥ 27 and HADS-D ≥ 8. Collectively, these findings add to the current knowledge on post-COVID-19 fatigue, especially among female survivors.
Symptoms contributing to long COVID included fatigue, brain fog, dizziness, palpitations, loss of or change in smell or taste, chronic cough, and chest pain [22]. The underlying pathogenesis of long COVID may conclude three aspects, including immune dysregulation, persistent inflammation, and dysfunction of the endothelium [23]. IL-6, produced by abnormal immunity, may cause pulmonary fibrosis, vascular disease, and psychological disorders [24, 25], even at fatigue onset [26]. Sustained inflammation in the central-peripheral nervous system, which contributes to oxidative stress and autoimmunity, may cause neurocognitive disorders and chronic fatigue [27]. Endothelial dysfunction may lead to inflammation, which is a critical driver of pulmonary vascular diseases and other enduring complications [28].
Fatigue has long been a concern shared by many people, since it is a condition that is not only widely seen after recovery from numerous diseases [29, 30] but is also a typical symptom of patients who have been infected with coronaviruses such as SARS-CoV-1 and MERS [31]. 40.3% of survivors suffered from SARS reported fatigue four years later [32].
For SARS-CoV-2, which is also a coronavirus, fatigue was found to be a common symptom from the outset, with 69% of patients reporting persistent fatigue for nearly 2 months after discharge [33]. Moreover, fatigue is more common in female patients than male. Tracking female patients would help investigate the risk factors for post-COVID-19 fatigue, offer new insights into the sequelae of coronavirus infection, and deepen the understanding of fatigue itself [34]. Promisingly, effective therapies such as pulmonary rehabilitation, exercise training, education, and behavioural changes [5], can improve fatigue in COVID-19 survivors [35].
In the current study, we found that depression, rather than anxiety, was correlated with the onset of fatigue. This may be because, unlike anxiety, the development of depression and fatigue share common pathophysiological mechanisms of immune-mediated injury and neuroinflammation, with their causal relationship already being verified [36]. Age, as an important risk factor for adverse health outcomes (including disease severity, mortality, and severity of sequelae) of COVID-19, is the risk factor for the onset of fatigue in females [37]. There is also evidence that older patients are more likely to develop post-COVID fatigue [38]. Co-occurring cerebrovascular diseases are also risk factors for fatigue. Patients admitted with stroke during the COVID-19 pandemic had a significantly higher probability of death [39], which is one of the risk factors for the severity of COVID-19 [40]. In our study, cerebrovascular diseases also had an impact on the long-term outcomes of COVID-19. Furthermore, post-stroke fatigue is one of the most common complications of stroke. In different regions of the world, female sex is a risk factor for post-stroke fatigue [41], indicating that there may be special connections between female sex and the occurrence of fatigue. What's more, patients with cerebrovascular diseases are more likely to experience chronic fatigue [42].
This study has some limitations. First, the sample size of the study is limited. Because the enrolled patients were less than half of the eligible population discharged from hospital, the potential risk for fatigue may be underestimated. Furthermore, the study assessed physical fatigue but not chronic fatigue syndrome (CFS). CFS is a clinically defined condition characterised by severe disabling fatigue and a combination of symptoms [43]. In previous reports, many symptoms of COVID-19 were similar to those of CFS, thus some researchers have used the CIS-fatigue to evaluate fatigue in patients [16, 44]. Thirdly, patients included were infected with wild-type strain and received hospital admission. The constantly emerging coronavirus variants and the tendcy of non-hospitalized therapy may lead to different health outcomes and risk factors with our findings.
Conclusion
This study found in female COVID-19 patients, cerebrovascular disease and older age could contribute to a higher risk of fatigue. Patients with fatigue have higher CAT scores, and are more likely to have concurrent depression. More importantly, our findings improved the understanding of the possible causes of fatigue in female survivors in order to develop effective strategies for prevention.
Supplementary Information
Additional file 1. Covid-19 Survivors two-year Clinical Sequelae Follow-up Questionnaire. Scores Distribution on CIS-fatigue. Symptoms of Long Covid Syndrome. Spearman's rank correlation matrix and correlation significances of 11 self-reported symptoms. Scores Distribution on CAT. Scores Distribution on HADS-A. Scores Distribution on HADS-D.
Acknowledgements
We acknowledge all patients who participated in this study and their families. We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- CIS-fatigue
The Checklist Individual Strength-subscale subjective fatigue
- COPD
Chronic obstructive pulmonary disease
- CAT
A chronic obstructive pulmonary disease (COPD) assessment test
- HADS
The Hospital Anxiety and Depression Scale
- HADS-A
The HADS-Anxiety
- HADS-D
The HADS-Depression
- symptoms-2y
The number of symptoms reported in at 2-year follow-up
- co-burden
The number of self-reported comorbidities
- CFS
Chronic fatigue syndrome
Authors’ contributions
LL, JJ, YH and GC conceived of and designed the study. LL, YY, CX, YW, XY, XM drafted the paper. YY, CX, YD, LC, CH, NN, HT and AZ collected and verified the data. YY, CX did the analysis. All authors had full access to the data in the study, critically revised the manuscript for important intellectual content and agreed to submit the final version for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the National Natural Science Foundation of China (82272908), the National Natural Science Foundation of Chongqing (2023NSCQ-JQX0190), the Joint Scientific Research Project of the Chongqing Health Commission and the Science and Technology Commission (2020FYYX213), the Outstanding Youth Science Fund of Chongqing (cstc2020jcyjjqX0014), Chongqing Talent Fund (CQYC202005003), and Open Project Program of the State Key Laboratory of Trauma, Burn and Combined Injury (SKLYQ202102). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
Restrictions apply to the availability of these data and so they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for nested case–control studies and was approved by the Ethics Committee of the Daping Hospital, an affiliated hospital of Army Medical University (No. 202153). Informed consent was obtained from all participants or their legal representatives before the survey. All the methods were carried out in accordance with relevant guidelines and regulations from Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yidan Ye, Chuyue Xiong and Yang Dai contributed equally to this work.
Contributor Information
Ji Jiang, Email: 1023599733@qq.com.
Li Li, Email: dpyyhxlili@tmmu.edu.cn.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharun K, Tiwari R, Yatoo MI, et al. A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19. Narra J. 2022;2(3):e92. doi: 10.52225/narra.v2i3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballouz T, Menges D, Anagnostopoulos A, et al. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study. BMJ. 2023;31(381):e074425. doi: 10.1136/bmj-2022-074425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Ramirez DC, Normand K, Zhaoyun Y, Torres-Castro R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines. 2021;9(8):900. doi: 10.3390/biomedicines9080900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaweethai T, Jolley SE, Karlson EW, et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA. 2023;25:e238823. doi: 10.1001/jama.2023.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joli J, Buck P, Zipfel S, Stengel A. Post-COVID-19 fatigue: A systematic review. Front Psychiatry. 2022;13:947973. doi: 10.3389/fpsyt.2022.947973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Resp Med. 2022;10(9):863–876. doi: 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatib S, Sabobeh T, Habib A, et al. Post-COVID-19 fatigue as a major health problem: a cross-sectional study from Missouri, USA. Ir J Med Sci (1971 -) 2022; 192, 699–705 (2023). [DOI] [PMC free article] [PubMed]
- 12.Mazurkiewicz I, Chatys-Bogacka Z, Slowik J, et al. Fatigue after COVID-19 in non-hospitalized patients according to sex. Brain Behav. 2023;13(2):e2849. doi: 10.1002/brb3.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-de-Las-Peñas C, Martín-Guerrero JD, Pellicer-Valero ÓJ, et al. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J Clin Med. 2022;11(2):413. doi: 10.3390/jcm11020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Hou C, Shen Y, et al. Two-Year Health Outcomes in Hospitalized COVID-19 Survivors in China. JAMA Netw Open. 2022;5(9):e2231790. doi: 10.1001/jamanetworkopen.2022.31790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38(5):383–392. doi: 10.1016/0022-3999(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 16.Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical Outcomes Among Patients With 1-Year Survival Following Intensive Care Unit Treatment for COVID-19. JAMA-J Am Med ASSOC. 2022;327(6):559–565. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline LN. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 18.Daynes E, Gerlis C, Briggs-Price S, Jones P, Singh SJ. COPD assessment test for the evaluation of COVID-19 symptoms. Thorax. 2021;76(2):185–187. doi: 10.1136/thoraxjnl-2020-215916. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-de-Las-Peñas C, Rodríguez-Jiménez J, Palacios-Ceña M, et al. Psychometric Properties of the Hospital Anxiety and Depression Scale (HADS) in Previously Hospitalized COVID-19 Patients. Int J Environ Res Public Health. 2022;19(15):9273. doi: 10.3390/ijerph19159273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiat Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Aman AT, Wibawa T, Kosasih H, et al. Etiologies of severe acute respiratory infection (SARI) and misdiagnosis of influenza in Indonesia, 2013–2016. Influenza Other Resp. 2021;15(1):34–44. doi: 10.1111/irv.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahriani M, Ilmawan M, Fajar JK, et al. Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis - A systematic review and meta-analysis. Narra J. 2021;1(2):e36. doi: 10.52225/narraj.v1i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanjari M, Late S, Sharma R, et al. Long-term pulmonary and extra-pulmonary consequences of COVID-19: A comprehensive review of current evidence and future perspectives. Narra J. 2023;3(2):e156. doi: 10.52225/narra.v3i2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durstenfeld MS, Peluso MJ, Kelly JD, et al. Role of antibodies, inflammatory markers, and echocardiographic findings in postacute cardiopulmonary symptoms after SARS-CoV-2 infection. JCI Insight. 2022;7(10):e157053. doi: 10.1172/jci.insight.157053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jankord R, Turk JR, Schadt JC, et al. Sex difference in link between interleukin-6 and stress. Endocrinology. 2007;148(8):3758–3764. doi: 10.1210/en.2006-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. 2022;54(1):1473–1487. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41(04):302–7. 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PMC free article] [PubMed]
- 28.Manjaly ZM, Harrison NA, Critchley HD, et al. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(6):642–651. doi: 10.1136/jnnp-2018-320050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Repping-Wuts H, Fransen J, van Achterberg T, Bleijenberg G, van Riel P. Persistent severe fatigue in patients with rheumatoid arthritis. J Clin Nurs. 2007;16(11c):377–383. doi: 10.1111/j.1365-2702.2007.02082.x. [DOI] [PubMed] [Google Scholar]
- 30.Zedlitz AMEE, Visser-Meily AJMA, Schepers VP, Geurts ACH, Fasotti L. Patients with Severe Poststroke Fatigue Show a Psychosocial Profile Comparable to Patients with Other Chronic Disease: Implications for Diagnosis and Treatment. ISRN Neurol. 2011;2011:1–8. doi: 10.5402/2011/627081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn SH, Kim JL, Kim JR, et al. Association between chronic fatigue syndrome and suicidality among survivors of Middle East respiratory syndrome over a 2-year follow-up period. J Psychiatr Res. 2021;137:1–6. doi: 10.1016/j.jpsychires.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 33.Mandal S, Barnett J, Brill SE, et al. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verveen A, Müller F, Lloyd A, et al. A research agenda for post-COVID-19 fatigue. J Psychosom Res. 2022;154:110726. doi: 10.1016/j.jpsychores.2022.110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed I, Mustafaoglu R, Yeldan I, Yasaci Z, Erhan B. Effect of Pulmonary Rehabilitation Approaches on Dyspnea, Exercise Capacity, Fatigue, Lung Functions, and Quality of Life in Patients With COVID-19: A Systematic Review and Meta-analysis. Arch Phys Med Rehabil. 2022;103(10):2051–2062. doi: 10.1016/j.apmr.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazza MG, Palladini M, Villa G, De Lorenzo R, Rovere Querini P, Benedetti F. Prevalence, trajectory over time, and risk factor of post-COVID-19 fatigue. J Psychiatr Res. 2022;155:112–119. doi: 10.1016/j.jpsychires.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. 2014;31(5):562–575. doi: 10.1177/1049909113494748. [DOI] [PubMed] [Google Scholar]
- 39.Katsanos AH, Palaiodimou L, Zand R, et al. Changes in Stroke Hospital Care During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Stroke. 2021;52(11):3651–3660. doi: 10.1161/STROKEAHA.121.034601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Wu B, Yang J, Lei X, Shen W. Cardio-Cerebrovascular Disease is Associated With Severity and Mortality of COVID-19: A Systematic Review and Meta-Analysis. Biol Res Nurs. 2021;23(2):258–269. doi: 10.1177/1099800420951984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Cheng S, Zhang Z, Wang C, Wang A, Zhu W. Related risk factors associated with post-stroke fatigue: a systematic review and meta-analysis. Neurol Sci. 2021;42(4):1463–1471. doi: 10.1007/s10072-020-04633-w. [DOI] [PubMed] [Google Scholar]
- 42.Van Engelen BG, Kalkman JS, Schillings ML, Van Der Werf SP, Bleijenberg G, Zwarts MJ. Fatigue in neuromuscular disease. Ned Tijdschr Geneeskd. 2004;148(27):1336–1341. [PubMed] [Google Scholar]
- 43.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 44.Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. 2021;268:113426. doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Covid-19 Survivors two-year Clinical Sequelae Follow-up Questionnaire. Scores Distribution on CIS-fatigue. Symptoms of Long Covid Syndrome. Spearman's rank correlation matrix and correlation significances of 11 self-reported symptoms. Scores Distribution on CAT. Scores Distribution on HADS-A. Scores Distribution on HADS-D.
Data Availability Statement
Restrictions apply to the availability of these data and so they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution.