Abstract
Background
Integrative Chinese and Western medicine (ICWM) is commonly used for the treatment of ulcerative colitis (UC) in clinical practice. However, it is unclear whether the details of ICWM interventions, such as selection rationale, implementation design, and potential interactions, were adequately reported. Therefore, this study aimed to assess the quality of reporting in the ICWM interventional randomized controlled trials (RCTs) of UC and to identify the common problems if any.
Methods
Through a search of 10 international electronic databases, we identified RCTs of UC with ICWM interventions published in English or Chinese from the inception date of each database up to 16 June 2023. Literature screening was strictly conducted based on the inclusion and exclusion criteria of the Population, Concept, and Context (PCC) framework. The general characteristics of the included studies were described. The quality of reporting was assessed according to three checklists, including the CONSORT (Consolidated Standards of Reporting Trials) with 36 items (except for one item 1b about abstract), the CONSORT for Abstracts (17 items), and a self-designed ICWM-related checklist (27 items covering design rationale, intervention details, outcome assessments, and analysis). The reporting scores of RCTs published before and after 2010 were compared.
Results
A total of 1458 eligible RCTs were included. For the reporting compliance, the median score (interquartile ranges) of the CONSORT (72 score in total), the CONSORT for Abstract (34 score), and ICWM-related (54 score) items was 21 (18–25), 13 (12–15), and 18 (15–21), respectively. Although the time period comparisons showed that reporting quality of included publications improved significantly after the CONSORT 2010 issued (P < 0.01), more than 50% of items were evaluated as poor quality (reporting rate < 65%) among each checklist, especially in the CONSORT for Abstract and ICWM-specific items.
Conclusion
Although CONSORT appears to have enhanced the reporting of RCTs in UC, the quality of ICWM specifics is variable and in need of improvement. Reporting guidelines of the ICWM recommendations should be developed to improve their quality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-023-02402-2.
Keywords: Ulcerative colitis (UC), CONSORT guideline, Randomized controlled trial (RCT), Reporting quality, Integrative Chinese and Western medicine (ICWM)
Introduction
Ulcerative colitis (UC) is a global disease, the incidence and prevalence of which have increased in several regions of the world [1, 2]. As an immune-mediated chronic inflammatory bowel disease, UC is characterized by continuous and superficial inflammation of the colon. However, its etiology and pathogenesis have not yet been determined and are generally considered to be influenced by genetic, environmental, and microbial factors [3]. The treatment goal of UC is to achieve symptomatic relief, endoscopic healing, normalization of serum and fecal markers, absence of disability, restoration of quality of life, and normal growth in children [4].
Conventional treatments control symptoms through pharmacotherapy, including aminosalicylates, corticosteroids, immunomodulators, and biologics, with other general measures or surgical resection if necessary [5]. Aminosalicylates are known to be the first-line treatment option for mild to moderate UC [6], but they may cause gastrointestinal discomfort such as nausea, abdominal pain, and diarrhea [7, 8]. Corticosteroids are a kind of treatment selection for UC patients who have inadequate response to mesalazine, however, their long-term treatment is not recommended due to significant adverse effects such as an increased risk of mortality, infection, diabetes mellitus, hypertension, and osteoporosis [7, 9]. Immunomodulators and biologics are beneficial to patients with moderate to severe disease activity, corticosteroid dependence, or those at high risk of colectomy, but up to 74% of UC patients may lose response over time [10]. Limitations such as inadequate response, side effects, dependence, and drug resistance to Western medicine (WM) remain to be solved [11, 12].
Chinese medicine (CM), a medical system based on distinctive Chinese cultural theories and practices, has been increasingly introduced into the treatment of UC, especially in Asia [13]. CM treatment mainly includes Chinese herbal formula (oral or rectal), acupuncture, and moxibustion. Due to its multi-targeted mode of action, CM has unique advantages for treating inflammatory bowel diseases, including maintaining intestinal integrity, reducing inflammation, and decreasing oxidative stress, with minor side effects [14]. With the extensive studies on UC in recent years, it is confirmed that both CM and WM have unique advantages in UC management.
In China, most patients of UC preferred Integrated Chinese and Western medicine (ICWM) therapy over CM or WM alone. Several reviews supported the promising effect and few side effects of ICWM for UC [15, 16], but most of the included trials had a high risk of bias and major methodological deficiencies, including an insufficient description of the randomization process, lacking appropriate therapeutic endpoints, and missing power calculations. Although the quality of reporting in RCTs in medical sciences has been discussed, the quality of reporting in RCTs on the treatment of UC with ICWM has not yet been assessed after the publication of the CONSORT (Consolidated Standards of Reporting Trials) 2010 Statement. Furthermore, it is unclear whether the details of ICWM interventions, such as selection rationale, indications and timing of interventions, implement design and potential interactions, were adequately reported in current randomized clinical trials (RCTs). Therefore, this review aimed to assess the quality of reporting in RCTs of ICWM for UC, based on the checklists of CONSORT 2010 [17] and a self-designed ICWM-specific checklist.
Materials and methods
Eligibility criteria
This study included ICWM interventional RCTs of UC published in English or Chinese from the date of inception for each database up to 16 June 2023. We included studies on subjects given the diagnosis of UC defined by clear diagnostic criteria or references, regardless of age, gender, course of disease, and severity. The ICWM intervention is defined as the combination of CM therapies and WM treatments. Specifically, we included a wide range of CM interventions, such as herbal medicines, acupuncture, and moxibustion, while only pharmacological therapy of WM was in the intervention group. There were no limitations in the types of control groups and the assessed outcomes. Repeat publications, non-randomized or non-controlled trials, quasi-randomized controlled trials, non-ICWM interventional trials, study protocols, reviews, observational studies, case reports, abstracts, full-text reports not found, and non-human studies were excluded. Table 1 provides details on the inclusion and exclusion criteria of PCC used in this review. The conduction of this review is referred to the Chapter 11-Scoping Reviews of Joanna Briggs Institute Reviewer's Manual for Evidence Synthesis [17, 18], also with some modifications in terms of format as this review focused on the quality assessment.
Table 1.
Inclusion and exclusion criteria based on the Population, Concept, and Context (PCC) framework
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population |
Studies were included if they: • Included subjects given the diagnosis of UC defined by clear diagnostic criteria or references, regardless of age, gender, course of disease, and severity |
Focus exclusively on: • Non-human studies |
| Concept |
Studies were included if they: • Used ICWM intervention in the management of UC. The ICWM intervention is defined as the combination of CM therapies and WM treatments. Specifically, we included a wide range of CM interventions, such as herbal medicines, acupuncture, and moxibustion, while only pharmacological therapy of WM was in the intervention group. AND • No limitations in the types of control groups and the assessed outcomes |
Focus exclusively on: • Non-ICWM interventional trials. OR • Non-randomized or quasi-randomized controlled trials. OR • Non-controlled trials. OR • Observational studies. OR • Case reports. OR • Study protocols. OR • Reviews |
| Context |
Studies were included if they: • Published in English or Chinese. AND • Published on/before 16 June 2023 |
Focus exclusively on: • Abstracts or full-text reports not found. OR • Repeat publications |
Search strategy
A systematic search was conducted on 17 June 2023 for the following databases: MEDLINE < 1946 to 16 June 2023 > , Embase < 1974 to 16 June 2023 > , CENTRAL (Cochrane Central Register of Controlled Trials) < May 2023 > , Web of Science < 1900 to 16 June 2023 > , CINAHL < 1937 to 16 June 2023 > , AMED (Allied and Complementary Medicine Database) < 1985 to May 2023 > , CNKI (China National Knowledge Infrastructure) < 1979 to 16 June 2023 > , Wanfang < 1979 to 16 June 2023 > , VIP (Chinese Science and Technology Periodical Database) < 1989 to 16 June 2023 > , CBM (Chinese Biomedical Literature Database) < 1978 to 16 June 2023 > . The search terms were “ulcerative colitis”, “inflammatory bowel disease”, “randomized controlled trial”, “random”, “Chinese medicine”, “herbal”, and “drug”. The detailed search strategy is presented in Additional file 1: Appendix 1.
Study selection
Endnote 20 (Clarivate, Philadelphia, PA 19130) was used for de-duplication, title and abstract screening. After de-duplication, four review authors (JLZ, NNW, FL, and PJC) independently screened titles and abstracts of the retrieved records based on the inclusion and exclusion criteria, and another two review authors (JSD and JW) conducted a second check. Full text of potentially relevant papers were reviewed (JLZ, NNW, FL, and PJC) and double-checked (JSD and JW) for further assessment of eligibility. Differences of opinion were settled by consensus.
Data extraction
We developed a data extraction Microsoft Excel form to extract data on general characteristics of included records, including study title, publication year and language, information of corresponding author(s), types of journals, types of study design (e.g., assignment, randomization, blinding, sample size and participating centers), features of interventions, types of participant(s) and control(s), period of treatment and follow-up (if any), as well as the categories of diagnosis criteria, outcome(s) and study conclusions. We piloted the extraction form on a random sample of ten included articles and achieved consistency in data item interpretations. Then, four trained authors (JLZ, FL, NNW, and JSD) independently extracted the data, and another two review authors (XZ and JW) conducted a second check. Disagreements were resolved by discussion.
Reporting quality assessment
The reporting quality of included studies was evaluated according to a standard checklist of the CONSORT 2010 statement, of which the checklist of the CONSORT for Abstract was extracted for independent evaluation. For rating the CONSORT items, the assessment rules were referred to the CONSORT 2010 statement (including the CONSORT for Abstract) with its explanation and elaboration document which provides the definitions and rationale for each checklist item and examples of good reporting (e.g., scored as 2 points) [19, 20]. The total score of the CONSORT checklist and CONSORT for the abstract checklist was 72 and 34, respectively.
A specially designed checklist comprised of 27 items related to specific characteristics of ICWM trials (Table 2) was developed by five researchers (XZ, JL, PW, FH, and ZXB) based on an internal discussion. This list focused on the identification of critical issues in the procedure of ICWM design, implementation, and assessment, particularly in the selection rationale, details of therapy combination, and the efficacy assessment. Each item/question was scored in terms of three possibilities: “2” for “fully reported”, “1” for “partially reported”, and “0” for “not reported” or “not applicable”. The total score of the ICWM-specific checklist was 54. For rating the ICWM items, the details of scoring rules are presented in Additional file 1: Appendix 2 which includes the explanations for each question and examples of eligible reporting. The quality assessment was independently conducted by one review author and verified by another review author. A total of four authors (YHW, JSD, NNW, and WTC) participated in the quality assessment. Possible disagreements were resolved with the consultation of the third senior review authors (XZ or ZXB).
Table 2.
Questions for assessing the reporting of ICWM-specific items
| Item no | Specifics |
|---|---|
| Q1 | Whether the feature of ICWM was presented in the section of “Title” (e.g., generalized term of ICWM, or specific CM and WM interventions provided in the title)? |
| Q2 | Whether the eligibility criteria of participants include both Chinese and Western medical diagnosis in Methods of Abstract? |
| Q3 | Whether the study objectives or hypotheses were focused on the ICWM interventions in the Abstract? |
| Q4 | Whether the outcome measures included both CM and WM-related endpoints in the Abstract? |
| Q5 | Whether the effect of studied ICWM interventions was reported in the Conclusion of the Abstract? |
| Q6 | Whether the features or design of the ICWM study were reflected in Keywords? |
| Q7 | Whether the reason/rationale about ICWM intervention for the study design was reported in Background? |
| Q8 | Whether any necessity/advantage about ICWM intervention was reported in the Background? |
| Q9 | Whether the objectives or hypotheses were focused on the ICWM interventions in the Background (e.g., improve the efficacy/safety, or reduce the side effects)? |
| Q10 | Whether the eligibility criteria of participants include both Chinese and Western medical diagnosis in Methods? |
| Q11 | Whether the specific information of disease (e.g., classification of disease, treatment points, stages of diseases) of the ICWM was reported in Methods? |
| Q12 | Whether any specific criteria related ICWM in the selection of study centers? |
| Q13 | Whether the specific type/way of integration of CM and WM interventions (such as overlying, one-after-another, or add-on design) was reported in Methods? |
| Q14 | In the ICWM group, whether CM intervention(s) was reported with sufficient details to allow replication, including how and when they were administered? |
| Q15 | In the ICWM group, whether WM intervention(s) was reported with sufficient details to allow replication, including how and when they were administered? |
| Q16 | Whether the rationale for the choice of the control group(s) was provided? |
| Q17 | In the control group, whether sufficient details were reported to allow replication? |
| Q18 | Whether any description of treatment providers’ background (e.g., qualification and/or experiences in ICWM, or whether the providers conducted CM and WM separately)? |
| Q19 | Whether any measures were adopted to evaluate or improve the compliance of participants? |
| Q20 | Whether the outcome measures included both CM and WM-related endpoints in Methods? |
| Q21 | For the studies with open label, whether any reasons or explanations for such design was reported? |
| Q22 | In the control group(s), did the placebo of WM invention(s) was included? If so, whether sufficient details were provided? |
| Q23 | In the control group(s), did the placebo of CM invention(s) was included? If so, whether sufficient details were provided? |
| Q24 | In the section of Results, whether any information about the participants exposed to ICWM treatment prior to recruitment was mentioned in the baseline data? |
| Q25 | Whether any discussion about external validity of ICWM results reported, particularly in different environments? |
| Q26 | Whether interpretation and significance of studied ICWM interventions for the disease was reported in the Discussion? |
| Q27 | Whether any potential conflicts of interests were clearly reported? |
ICWM Integrative Chinese and Western medicine, CM Chinese medicine, WM Western medicine
Data analysis
As this review is focused on reporting characteristics and quality evaluation, we thereby applied frequency and percentage to present categorical variables, and mean (standard deviation) or median (interquartile range, IQR) to present continuous variables in the section of “Results”. For individual item of reporting quality, the compliance rate was calculated with the number of items acquired “2” based on the total number of included reports, which was further categorized as three levels: excellent compliance (> 90%), good compliance (between 65 and 90%), and poor compliance (< 65%). The total scores of the CONSORT, CONSORT for Abstract, and ICWM-specific checklists of RCTs published before and after 2010 were compared with Student’s t test or Mann–Whitney U test. Statistics analyses were performed using SPSS software (version 28.0). Statistical significance was defined as two-sided P value < 0.05.
Results
Literature search
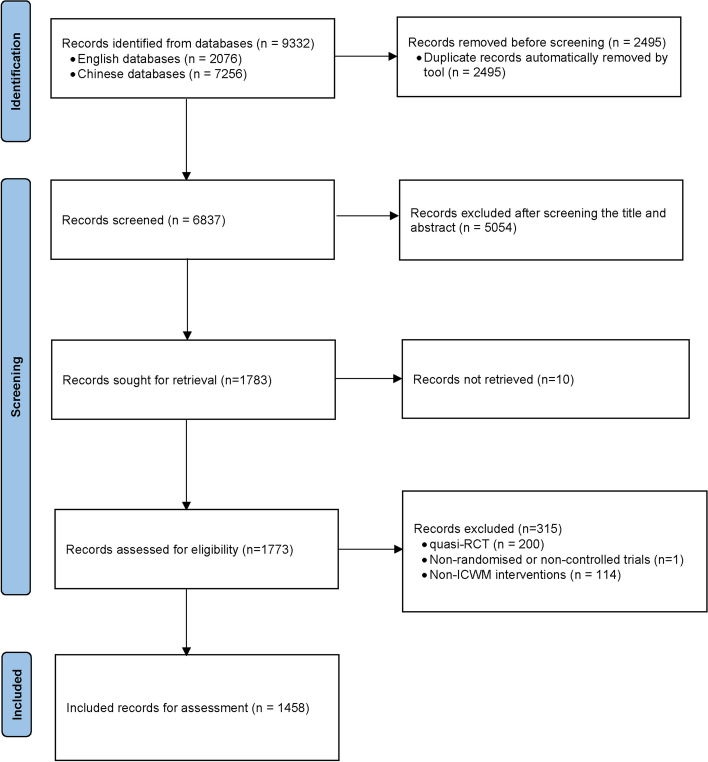
The flowchart of the selection and screening process is shown in Fig. 1. Briefly, the electronic search yielded 9332 records, after removing duplicates and screening titles and abstracts, a total of 1773 reports were identified for the full-text assessment. Finally, we included 1458 eligible RCTs for analysis, of which 1385 articles with abstracts and 73 without abstracts.
Fig. 1.
Flow chart of the literature screening and selection
Characteristics of included trials
A total of 1458 RCTs of ICWM for UC were included between January 1998 to June 2023. The number of these studies increased gradually during the first 10 years and then presented a rapid increase starting from 2009 (Fig. 2). The most common design of included studies was a single-center (97.94%), two parallel arms (94.10%), a sample size of 51–100 (70.30%), Chinese herbal formula (90.53%) as CM treatment, and intervention period within 30 days (48.49%). There were 575 (39.44%) studies that applied CM diagnosis when recruited UC participants, while only 349 (23.94%) adopted CM-related outcomes to assess the efficacy of treatments. Around 98.63% (1438) trials concluded a confirmed efficacy of ICWM for UC. However, the missing reporting is common for several critical aspects, such as 97.94% of trials did not whether to adopt blinding or not, and 71.88% of trials did not specify the studied phase(s) of UC (e.g., active, remission, or both). Details are shown in Table 3.
Fig. 2.
The number of ICWM interventional RCTs of UC publications between Jan 1998 to June 2023
Table 3.
Characteristics of included articles (n = 1458)
| Characteristics | N (%) |
|---|---|
| Study center | |
| Single center | 1428 (97.94) |
| Multicenter | 22 (1.51) |
| Not reported | 8 (0.55) |
| Journal type | |
| English journal, with impact factor 5–10 | 1 (0.07) |
| English journal, with impact factor 3–5 | 2 (0.14) |
| English journal, with impact factor < 3 | 3 (0.21) |
| Chinese core journal | 66 (4.53) |
| Chinese non-core journal | 1386 (95.06) |
| Sample size | |
| > 300 | 6 (0.41) |
| 101–300 | 295 (20.23) |
| 51–100 | 1025 (70.30) |
| ≤ 50 | 132 (9.05) |
| UC stages of trial participants | |
| Active | 368 (25.24) |
| Remission | 19 (1.30) |
| Both | 23 (1.58) |
| Not specified | 1048 (71.88) |
| Number of groups | |
| 2 | 1372 (94.10) |
| 3 | 79 (5.42) |
| 4 or above | 7 (0.48) |
| Blinding | |
| Single-blinded | 9 (0.62) |
| Double-blinded | 18 (1.23) |
| Open-label | 3 (0.21) |
| Not reported | 1428 (97.94) |
| Intervention type of CM | |
| Chinese herbal formulas | 1320 (90.53) |
| Single herbs | 28 (1.92) |
| Moxibustion | 20 (1.37) |
| Acupuncture | 12 (0.82) |
| Catgut-embedding therapy | 11 (0.75) |
| Massage | 2 (0.14) |
| Autohemotherapy at acupoint | 1 (0.07) |
| Complex interventiona | 64 (4.39) |
| Intervention period | |
| ≤ 30 days | 707 (48.49) |
| 31–60 days | 489 (33.54) |
| 61–90 days | 167 (11.45) |
| > 90 days | 27 (1.85) |
| Not reported | 70 (4.80) |
| Follow-up period | |
| ≤ 90 days | 66 (4.53) |
| 91–180 days | 109 (7.48) |
| 181–360 days | 70 (4.80) |
| > 360 days | 12 (0.82) |
| Not reported | 1201 (82.37) |
| Type of controls | |
| Integrated CM and WM | 78 (5.35) |
| Including placebo | 17 (1.17) |
| Solely WM as control | 1359 (93.21) |
| Solely CM as control | 4 (0.27) |
| Diagnostic criteria of UC | |
| Both CM and WM | 575 (39.44) |
| CM | 2 (0.14) |
| WM | 881 (60.43) |
| CM-related outcomes | |
| CM pattern score | 303 (20.78) |
| CM symptomsb | 46 (3.16) |
| Not reported | 1109 (76.06) |
| Conclusions on the efficacy of ICWM | |
| Confirmed efficacy | 1438 (98.63) |
| Beneficial | 20 (1.37) |
aDetails are shown in Additional file 1: Appendix 3
bCM symptoms included the tongue and pulse manifestations
Reporting completeness and features
The results of adherence to the CONSORT, the CONSORT for Abstract, and ICWM-specific checklist items are presented in Tables 4, 5, and 6. For the completeness of the CONSORT checklist, the median (IQR) reporting score was 21 (18–25). Specifically, the reporting quality was excellent (> 90%) in 4 items (2a, 6b 15, and 22); good (65–90%) in 5 items (4b, 5, 11b, 13a, and 16); and poor (< 65%) in 27 items (1a, 2b, 3a, 3b, 4a, 6a, 7a, 7b, 8a, 8b, 9, 10, 11a, 12a, 12b,13b, 14a, 14b,17a, 17b, 18,19, 20, 21, 23, 24, and 25). For the CONSORT for Abstract, the reporting score was 13 (12–15). The quality of reporting was excellent (> 90%) in 3 items (3, 6, and 15); good (65–90%) in 1 item (10); and poor (< 65%) in 13 items (1, 2, 4, 5, 7, 8, 9, 11, 12, 13, 14, 16, and 17).
Table 4.
Reporting assessment of included studies based on the CONSORT items (n = 1458)
| Section/topic | Item number and description | Fully reported | Partially reported | Not reported |
|---|---|---|---|---|
| Title and abstract | 1a. Identification as a randomized trial in the title | 13 (0.89) | – | 1445 (99.11) |
| 1b. Structured summary of trial design, methods, results, and conclusions | See Table 5 | |||
| Introduction | ||||
| Background | 2a. Scientific background and explanation of rationale | 1356 (93.00) | – | 102 (7.00) |
| Objectives | 2b. Specific objectives or hypotheses | 535 (36.69) | – | 923 (63.31) |
| Methods | ||||
| Trial design | 3a. Description of trial design (such as parallel, factorial) including allocation ratio | 4 (0.27) | 63 (4.32) | 1391 (95.40) |
| 3b. Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 13 (0.89) | – | 1445 (99.11) | |
| Participants | 4a. Eligibility criteria for participants | 861 (59.05) | 458 (31.41) | 139 (9.53) |
| 4b. Settings and locations where the data were collected | 1046 (71.74) | 91 (6.24) | 321 (22.02) | |
| Interventions | 5. The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 1094 (75.03) | 358 (24.55) | 6 (0.41) |
| Outcomes | 6a. Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 3 (0.21) | 596 (40.88) | 859 (58.92) |
| 6b. Any changes to trial outcomes after the trial commenced, with reasons | 1458 (100) | – | – | |
| Sample size | 7a. How sample size was determined | 5 (0.34) | 3 (0.21) | 1450 (99.45) |
| 7b. When applicable, explanation of any interim analyses and stopping guidelines | 41 (2.81) | – | 1417 (97.19) | |
| Sequence generation | 8a. Method used to generate the random allocation sequence | 522 (35.80) | 835 (57.27) | 101 (6.93) |
| 8b. Type of randomization; details of any restriction (such as blocking and block size) | 11 (0.75) | 511 (35.05) | 936 (64.20) | |
| Allocation concealment mechanism | 9. Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 16 (1.10) | 507 (34.77) | 935 (64.13) |
| Implementation | 10. Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 6 (0.41) | – | 1452 (99.59) |
| Blinding | 11a. If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how a | 1 (0.07) | 9 (0.62) | 1445 (99.31) |
| 11b. If relevant, description of the similarity of interventions b | 13 (76.47) | 4 (23.53) | – | |
| Statistical methods | 12a. Statistical methods used to compare groups for primary and secondary outcomes | 16 (1.10) | 1185 (81.28) | 257 (17.63) |
| 12b. Methods for additional analyses, such as subgroup analyses and adjusted analyses | 0 (0) | – | 1458 (100) | |
| Results | ||||
| Participant flow | 13a. For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome | 1278 (87.65) | 173 (11.87) | 7 (0.48) |
| 13b. For each group, losses and exclusions after randomization, together with reasons | 53 (3.64) | 31 (2.13) | 1374 (94.24) | |
| Recruitment | 14a. Dates defining the periods of recruitment and follow-up | 238 (16.32) | 1034 (70.92) | 186 (12.76) |
| 14b. Why the trial ended or was stopped | 0 (0) | – | 1458 (100) | |
| Baseline data | 15. A table showing baseline demographic and clinical characteristics for each group | 1456 (99.86) | – | 2 (0.14) |
| Numbers analyzed | 16. For each group, the number of participants (denominator) included in each analysis and whether the analysis was by originally assigned groups | 1279 (87.72) | – | 179 (12.28) |
| Outcomes and estimation | 17a. For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 0 (0) | 1450 (99.45) | 8 (0.55) |
| 17b. For binary outcomes, the presentation of both absolute and relative effect sizes is recommended | 0 (0) | – | 1458 (100) | |
| Ancillary analyses | 18. Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | 0 (0) | – | 1458 (100) |
| Harms | 19. All-important harms or unintended effects in each group | 550 (37.72) | 23 (1.58) | 885 (60.70) |
| Discussion | ||||
| Limitations | 20. Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 193 (13.24) | – | 1265 (86.76) |
| Generalizability | 21. Generalizability (external validity, applicability) of the trial findings | 9 (0.62) | – | 1449 (99.38) |
| Interpretation | 22. Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 1458 (100) | – | – |
| Other information | ||||
| Registration | 23. Registration number and name of trial registry | 3 (0.21) | – | 1455 (99.79) |
| Protocol | 24. Where the full trial protocol can be accessed, if available | 1 (0.07) | 1 (0.07) | 1456 (99.86) |
| Funding | 25. Sources of funding and other support (such as the supply of drugs), role of funders | 3 (0.21) | 282 (19.34) | 1173 (80.45) |
a3 studies were not calculated for open-label
b1441 studies were not calculated as it is not placebo design
Table 5.
Reporting assessment of included studies based on the CONSORT for Abstract checklist (n = 1385)
| Section/topic | Item number and description | Fully reported | Partially reported | Not reported |
|---|---|---|---|---|
| Title | 1. Identification of the study as randomized | 14 (1.01) | – | 1371 (98.99) |
| Authors | 2. Contact details for the corresponding author | 234 (16.90) | 1151 (83.10) | – |
| Trial design | 3. Description of the trial design (e.g., parallel, cluster, non-inferiority) | 1301 (93.34) | – | 84 (6.66) |
| Participants | 4. Eligibility criteria for participants and the settings where the data were collected | 314 (22.67) | 1071 (77.33) | |
| Interventions | 5.Interventions intended for each group | 893 (64.48) | 391 (28.23) | 101 (7.29) |
| Objective | 6.Specific objective or hypothesis | 1374 (99.21) | – | 11 (0.79) |
| Outcome | 7.Clearly defined primary outcome for this report | 0 (0) | 761 (54.95) | 624 (45.05) |
| Randomization | 8.How participants were allocated to interventions | 312 (22.53) | 957 (69.10) | 116 (8.38) |
| Blinding | 9.Whether or not participants, caregivers, and those assessing the outcomes were blinded to group assignment | 1 (0.07) | 12 (0.87) | 1372 (99.06) |
| Numbers randomized | 10.Number of participants randomized to each group | 1097 (79.21) | 12 (0.87) | 276 (19.93) |
| Recruiting objects | 11.Clinical Trial Status | 0 (0) | 714 (51.55) | 671 (48.45) |
| Numbers analyzed | 12.Number of participants analyzed in each group | 44 (3.18) | 6 (0.43) | 1335 (96.39) |
| Outcome | 13.For the primary outcome, a result for each group and the estimated effect size and its precision | 0 (0) | 63 (4.55) | 1322 (95.45) |
| Harms | 14.Important adverse events or side effects | 150 (10.83) | 188 (13.57) | 1047 (75.60) |
| Results | 15.Generalized interpretation of outcome | 1375 (99.28) | – | 10 (0.72) |
| Trial registration | 16.Registration number and name of trial register | 0 (0) | 1 (0.07) | 1384 (99.93) |
| Funding | 17.Source of funding | 2 (0.14) | 284 (20.51) | 1100 (79.42) |
Table 6.
Reporting assessment of included studies based on ICWM-specific items (n = 1458)
| Item number and description | Fully reported | Partially reported | Not reported |
|---|---|---|---|
| Q1. Whether the feature of ICWM was presented in the section of “Title” (e.g., generalized term of ICWM, or specific CM and WM interventions provided in the title)? | 1027 (70.44) | – | 431 (29.56) |
| Q2. Whether the eligibility criteria of participants included both Chinese and Western medical diagnosis in Methods of Abstract?a | 250 (18.05) | 1135 (81.95) | - |
| Q3. Whether the study objectives or hypotheses were focused on the ICWM interventions in Abstrac?a | 851 (61.44) | – | 534 (38.56) |
| Q4. Whether the outcome measures included both CM and WM-related endpoints in the Abstract?a | 232 (16.75) | 528 (38.12) | 625 (45.13) |
| Q5. Whether the effect of studied ICWM interventions was reported in Conclusion of Abstract?a | 929 (67.08) | – | 456 (32.92) |
| Q6. Whether the features or designs of the ICWM study reflected in Keywords? | 1040 (71.33) | 366 (25.10) | 52 (3.57) |
| Q7. Whether the reason/rationale for ICWM intervention for the study design was reported in Background? | 243 (16.67) | 457 (31.34) | 758 (51.99) |
| Q8. Whether any necessity/advantage of ICWM intervention was reported in the Background? | 114 (7.82) | – | 1344 (92.18) |
| Q9. Whether the objectives or hypotheses focused on the ICWM interventions in the Background (e.g., improve the efficacy/safety, or reduce the side effects)? | 305 (20.92) | 1153 (79.08) | |
| Q10. Whether the eligibility criteria of participants include both Chinese and Western medical diagnosis in Methods? | 339 (23.25) | 236 (16.19) | 883 (60.56) |
| Q11. Whether the specific information of disease (e.g., classification of disease, treatment points, stages of diseases) of the ICWM was reported in Methods? | 410 (28.12) | – | 1048 (71.88) |
| Q12. Whether any specific criteria related to ICWM in the selection of study centers? | 39 (2.67) | – | 1419 (97.33) |
| Q13. Whether the specific type/way of integration of CM and WM interventions (such as overlying, one-after-another, or add-on design) was reported in Methods? | 1336 (91.63) | – | 122 (8.37) |
| Q14. In the ICWM group, whether CM intervention(s) reported with sufficient details to allow replication, including how and when they were administered? | 1136 (77.91) | – | 322 (22.09) |
| Q15. In the ICWM group, whether WM intervention(s) was reported with sufficient details to allow replication, including how and when they were administered? | 1343 (92.11) | – | 115 (7.89) |
| Q16. Whether the rationale for the choice of the control group(s) was provided? | 1458 (100) | – | - |
| Q17. In the control group, whether sufficient details were reported to allow replication? | 1325 (90.88) | – | 133 (9.12) |
| Q18. Whether any description of treatment providers’ background (e.g., qualification and/or experiences in ICWM, or whether the providers conducted CM and WM separately)? | 12 (0.82) | – | 1446 (99.18) |
| Q19. Whether any measures were adopted to evaluate or improve the compliance of participants? | 4 (0.27) | – | 1454 (99.73) |
| Q20. Whether the outcome measures included both CM and WM related endpoints in Methods? | 349 (23.94) | – | 1109 (76.06) |
| Q21. For the studies with open label, whether any reasons or explanations for such design was reported?b | 0 (0) | – | 3 (100)- |
| Q22. In the control group(s), did the placebo of WM invention(s) was included? If so, whether sufficient details were provided?c | 8 (47.06) | – | 9 (52.94) |
| Q23. In the control group(s), did the placebo of CM invention(s) was included? If so, whether sufficient details were provided?c | 16 (94.12) | – | 1 (5.88) |
| Q24. In the section of Results, whether any information about the participants exposed to ICWM treatment prior to recruitment was mentioned in the baseline data? | 0 (0) | – | - |
| Q25. Whether any discussion about external validity of ICWM results was reported, particular in different environments? | 0 (0) | – | - |
| Q26. Whether interpretation and significance of studied ICWM interventions for the disease was reported in the Discussion? | 306 (20.99) | 567 (38.89) | 585 (40.12) |
| Q27. Whether any potential conflicts of interest were clearly reported? | 15 (1.03) | 36 (2.47) | 1407 (96.50) |
a73 studies were not calculated as they did not have an Abstract
b3 studies were not calculated for open-label
c1441 studies were not calculated as it is not a placebo design
The reporting score of the ICWM-specific items was 18 (15–21). Four items were “excellent” (Q13, Q15, Q16, Q17, Q23), and “good” reporting in 5 items (Q1, Q5, Q6, Q14). The remaining 18 items were reported poorly (Q2, Q3, Q4, Q7, Q8, Q9, Q10, Q11, Q12, Q18, Q19, Q20, Q21, Q22, Q24, Q25, Q26, Q27), of which 16 items showed extremely low (< 33%), particular in the rationale for ICWM design with definite objectives or hypotheses, specific timepoint/stage of integrative therapy for UC, CM-related diagnosis criteria and outcome(s), discussion about the internal and external validity of ICWM results and value of ICWM design were insufficient or not articulated in most studies. The total scores of the CONSORT, the CONSORT for Abstract, and ICWM-specific checklist items were significantly improved after 2010 (all P < 0.01; Table 7).
Table 7.
Overall reporting quality scores for included studies, by subgroup
| Year of publication(n) | For CONSORT items1 | For CONSORT for Abstract items2 | For ICWM-specific items3 |
|---|---|---|---|
| 1998–2010 (n = 300) | 17 (15–20) | 11 (9–12) | 16 (13–19) |
| 2011–2022 (n = 1158) | 23 (20–26)*,a | 14 (12–15)*,b | 19 (15–23)*,c |
| Total reports (n = 1458) | 21 (18–25) | 13 (12–15) | 18 (15–21) |
1A perfect score is 72 for the CONSORT checklist
2A perfect score is 34 for the CONSORT for Abstract checklist
3A perfect score is 54 for the ICWM-specific checklist
*Compared with 1998–2010, P < 0.01
aCompared with the group before 2010, the enhancement of CONSORT was 4.92 (4.4–5.43)
b Compared with the group before 2010, the enhancement of CONSORT for Abstract was 3.02 (2.69–3.35)
c Compared with the group before 2010, the enhancement of ICWM-specific items was 3.03 (2.48–3.59)
Discussion
ICWM has been widely applied for UC in China [16]. There have been a large number of ICWM RCTs of UC published in Chinese and English literature. This study provides a scoping review of the reporting quality of the RCTs of ICWM for UC publications between January 1998 to June 2023. Unfortunately, the reporting quality we reviewed was suboptimal and substantial improvement could be required to meet the recommendations of reporting guidelines. Based on the CONSORT checklist, the items with good reporting in the included articles cover scientific background and rationale; settings and locations where the data were collected, intervention details; baseline demographic and clinical characteristics; and interpretation consistent with results. Although subgroup analysis showed better reporting quality after the CONSORT checklist was updated in 2010, this review demonstrated that there is much room for improvement with respect to ICWM RCTs reporting.
The inadequate reporting domains of reviewed articles include title and abstract, introduction, methods, results, discussion, and other information. With respect to the title and abstract, the reporting percentage for “identification as a randomized trial in the title” of the trials was only 0.89%. Most Chinese articles did not report this item. Indexers may not classify a report as an RCT if the authors do not explicitly report this information, therefore limiting the accessibility [21]. Contact details for the corresponding author were reported in 16.9% of trials. Inadequate contact information would restrict readers from contacting trialists for additional information or clarifications of reported data [22]. Among 1385 (95%) RCTs with abstract, the reporting quality was good in the description of the trial design, objective, number of participants randomized, and results in the section of “Abstract”. However, the overall quality of abstract reporting has been unsatisfactory, presenting the inadequate reporting of participants, interventions, outcomes, randomization, blinding, recruiting objects, outcomes, harms, trial registration, and funding. Regarding introduction, there was only 1/5 studies reported specific objectives or hypotheses. The majority of included trials had poor reporting in methods, including trial design, outcomes, sample size estimation, allocation concealment, implementation, blinding, and statistical methods. In the “Results” section, losses and exclusions after randomization, periods of recruitment and follow-up, precise outcomes, and adverse events were also poorly reported. Limitations, generalizability, registration, protocol, and funding reporting had much room for improvement. There are the major reasons why the total score of the CONSORT checklist was suboptimal. Similar problems were found in the previous study examining systematic reviews of ICWM [23].
Due to the specific characteristics of ICWM interventions, the ICWM-specific checklist was designed to assess the reporting features of ICWM trials, which mainly focused on the rationale, implementation and assessment, details of therapy combination, and efficacy assessment. According to the subgroup analysis, we found that the reporting quality in ICWM-specific items was enhanced after 2010. However, there were several problems in the ICWM reporting. First of all, the rationale or reason for choosing ICWM interventions was reported in less than 20% of studies. Secondly, the diagnostic criteria and outcome assessments of both CM and WM were rarely adopted simultaneously. Additionally, limited trials had reported specific situations (e.g., treatment points, stages of diseases, types of conditions) for the applicable scope of ICWM, settings, and locations where the trials were conducted, the background of treatment providers, measures to improve compliance, reasons for study design, interpretation of ICTM interventions, and conflicts of interests. There was also no study that provided information about participants exposed to ICWM treatment prior to recruitment and external validity of ICWM. Though 98.63% of studies concluded promising efficacy of ICWM for UC, the above problems not only limit the repeatability and reproducibility of the findings, hamper other researchers from identifying gaps that need to be addressed in the design and reporting of future studies, but also mislead healthcare providers in suggesting treatment decisions for patients. Given the deficiencies of reporting identified during this review, strengthening the reporting of the CONSORT are urgently needed. Besides, an extension for ICWM specifics of the CONSORT would be worth considering to improve the current situation.
In this review, we found several problems in the characteristics of included RCTs. Firstly, only 4.95% of articles were published in relatively high-quality journals, such as the Chinese Medical Core Journals and English journals with impact factors. These journals usually provide a peer review process, to assess the validity, quality, and often the originality of articles for publication [24]. Secondly, the most common design was a single center (97.94%) and sample size of 51–100 (70.3%), indicating high-quality ICWM interventional RCTs with multicenter design and larger sample size are needed. Thirdly, the intervention period of no more than 30 days was applied in half of the studies. However, whether such a short intervention period for UC was enough to obtain maximum effectiveness is unknown. Moreover, 82.37% of studies did not report the follow-up period, therefore the long-term effectiveness of ICWM is questionable [25]. Finally, more than two-thirds (71.88%) of the trials did not report the specific stages or phases of UC. TCM treatment strategies for inflammatory bowel diseases are based on disease gradation, stage, and segmentation from multi-dimensional treatment, including etiology, symptoms, syndromes, and internal and external treatment [26]. Chinese medical syndromes of active and remission stages of UC are significantly different [27]. Omit syndrome differentiation in Chinese medicine intervention may lead to unsuccessful treatment [28].
Our review has some limitations. First, this review identified full-text RCTs of UC with ICWM interventions published up to 16 June 2023 in the targeted databases. Any records which had not been included in these databases by that cut-off period, or without available full-text, as well as the grey literature have not been included. In addition, we included only articles in English and Chinese because of language limitations. As such, we may not have captured otherwise eligible trials published in other languages. Second, we rated the items with “not applicable” reporting as “2”, which might overoptimize the total scores. Furthermore, the CONSORT checklist is not intended to construct a “quality score”. Thus, using scores based on the reporting checklists might limit the accuracy of our evaluations and simplify the actual situation. Third, methodology quality (e.g., risk of bias) assessment was not conducted because this review only focused on the reporting quality.
Conclusion
The reporting quality of ICWM RCTs for UC is suboptimal and substantial improvement is required to meet CONSORT guidelines. Consistent with previous studies [29–31], this review found improved reporting quality in reviewed RCTs after the CONSORT checklist was updated in 2010. We suggest that authors should adhere to the CONSORT 2010 statement in designing, conducting, and reporting RCTs and that researchers should consider developing a series of standard reporting items specifically relevant to ITCWM design, as an extension to the CONSORT. This might be an effective strategy for achieving the improvement needed [32].
Supplementary Information
Additional file 1: Appendix 1. Search strategy. Appendix 2. Scoring rules for ICWM-specific items. Appendix 3. Types of CM complex intervention (based on Chinese herbal formulas). Appendix 4. Included studies (n=1,458) of this review.
Acknowledgements
The authors thank all the financial support from the China Center for Evidence-Based Traditional Chinese Medicine, CCEBTM (2020YJSZX-5); Chinese Medicine Development Fund, Hong Kong, China (20B2/027A); and Health@InnoHK Initiative Fund of the Hong Kong Special Administrative Region Government (ITC RC/IHK/4/7).
Abbreviations
- CM
Chinese medicine
- CONSORT
Consolidated Standards of Reporting Trials
- ICWM
Integrated Chinese and Western medicine
- IQR
Interquartile range
- RCTs
Randomized clinical trials
- UC
Ulcerative colitis
- WM
Western medicine
Authors’ contributions
ZXB conceived the research projects and finalized the manuscript. XZ designed this study and revised the manuscript. NNW and JLZ developed and performed the search strategy. JLZ, NNW, FL, and PJC searched and screened the literature. JLZ, JSD, NNW, YHW, WTC, and JW extracted, assessed, and checked the data. JSD, JLZ, XZ, and CPC analyzed the data and drafted the manuscript. PW, JL, FH, and APL provided critical comments for this study. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the China Center for Evidence-Based Traditional Chinese Medicine, CCEBTM (2020YJSZX-5); Chinese Medicine Development Fund (No. 20B2/027A), Hong Kong, China; and Health@InnoHK Initiative Fund of the Hong Kong Special Administrative Region Government (ITC RC/IHK/4/7). The funders had no role in the design of the study, in the collection, analysis, and interpretation of data, nor the writing of the manuscript.
Availability of data and materials
The data analyzed for this study are included in the manuscript and supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors agree to publish this article.
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jialing Zhang and Jiashuai Deng contributed equally to this work.
Contributor Information
Zhaoxiang Bian, Email: bianzxiang@gmail.com.
Xuan Zhang, Email: zhangxuan@hkbu.edu.hk.
References
- 1.Molodecky NA, Soon S, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Da Silva BC, Lyra AC, Rocha R, Santana GO. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol: WJG. 2014;20(28):9458. doi: 10.3748/wjg.v20.i28.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 4.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol: WJG. 2011;17(27):3204. doi: 10.3748/wjg.v17.i27.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elhag DA, Kumar M, Saadaoui M, et al. Inflammatory bowel disease treatments and predictive biomarkers of therapeutic response. Int J Mol Sci. 2022;23(13):6966. doi: 10.3390/ijms23136966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front Med. 2021; 8: 765474. [DOI] [PMC free article] [PubMed]
- 8.Sehgal P, Colombel JF, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther. 2018;47(12):1597–1609. doi: 10.1111/apt.14688. [DOI] [PubMed] [Google Scholar]
- 9.Dorrington AM, Selinger CP, Parkes GC, Smith M, Pollok RC, Raine T. The historical role and contemporary use of corticosteroids in inflammatory bowel disease. J Crohns Colitis. 2020;14(9):1316–1329. doi: 10.1093/ecco-jcc/jjaa053. [DOI] [PubMed] [Google Scholar]
- 10.Roda G, Jharap B, Neeraj N, Colombel J-F. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1):e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sands B. Immunosuppressive drugs in ulcerative colitis: twisting facts to suit theories? Gut. 2006;55(4):437–441. doi: 10.1136/gut.2005.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin D, Mody R, Davis K, Wang CC. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn's disease. Aliment Pharmacol Ther. 2014;39(10):1143–1155. doi: 10.1111/apt.12727. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Li B-G, Su Y-H, et al. Potential activity of traditional Chinese medicine against ulcerative colitis: a review. J Ethnopharmacol. 2022;289:115084. doi: 10.1016/j.jep.2022.115084. [DOI] [PubMed] [Google Scholar]
- 14.Yuan S, Wang Q, Li J, et al. Inflammatory bowel disease: an overview of Chinese herbal medicine formula-based treatment. Chinese Medicine. 2022;17(1):1–17. doi: 10.1186/s13020-022-00633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Jiang M, Lu A. Considerations of traditional Chinese medicine as adjunct therapy in the management of ulcerative colitis. Clin Rev Allergy Immunol. 2013;44(3):274–283. doi: 10.1007/s12016-012-8328-9. [DOI] [PubMed] [Google Scholar]
- 16.Chen SM, Ji GY, Liu XR, et al. Efficacy of integrated traditional Chinese and western medicine on ulcerative colitis: a systematic review and meta-analysis. Pharmacol Res Mod Chin Med. 2022;4(1):100145.
- 17.Peters MDJ, Godfrey C, McInerney P, et al. Chapter 11: Scoping Reviews (2020 version). In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis, JBI, 2020. Available from https://synthesismanual.jbi.global. 10.46658/JBIMES-20-12.
- 18.Peters MD, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119-26. [DOI] [PubMed]
- 19.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Dickersin K, Manheimer E, Wieland S, et al. Development of the Cochrane Collaboration’s CENTRAL Register of controlled clinical trials. Eval Health Prof. 2002;25(1):38–64. doi: 10.1177/016327870202500104. [DOI] [PubMed] [Google Scholar]
- 22.Hopewell S, Clarke M, Moher D, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5(1):e20. doi: 10.1371/journal.pmed.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan FF, Xu Q, Sun Q, Zhao SJ, Wang P, Guo XR. Assessment of the reporting quality of randomized controlled trials on treatment of coronary heart disease with traditional chinese medicine from the chinese journal of integrated traditional and Western medicine: a systematic review. PLoS One. 2014;9(1):e86360. doi: 10.1371/journal.pone.0086360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly J, Sadeghieh T, Adeli K. Peer review in scientific publications: benefits, critiques, & a survival guide. Ejifcc. 2014;25(3):227. [PMC free article] [PubMed] [Google Scholar]
- 25.Ji J, Huang Y, Wang X-F, et al. Review of clinical studies of the treatment of ulcerative colitis using acupuncture and moxibustion. Gastroenterol Res Pract. 2016;2016:9248589. doi: 10.1155/2016/9248589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Luo H, Tan D, et al. A recent update on the use of Chinese medicine in the treatment of inflammatory bowel disease. Phytomedicine. 2021;92:153709. doi: 10.1016/j.phymed.2021.153709. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y-H, Cong L-L. Study on the Chinese medical syndrome distribution of ulcerative colitis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(4):450–454. [PubMed] [Google Scholar]
- 28.Liu J-P, Han M, Li X-X, et al. Prospective registration, bias risk and outcome-reporting bias in randomised clinical trials of traditional Chinese medicine: an empirical methodological study. BMJ Open. 2013;3(7):e002968. doi: 10.1136/bmjopen-2013-002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Jones A, Lepage L, Group C Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;285(15):1992–1995. doi: 10.1001/jama.285.15.1992. [DOI] [PubMed] [Google Scholar]
- 30.Plint AC, Moher D, Morrison A, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185(5):263–267. doi: 10.5694/j.1326-5377.2006.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu XT, Zhang X, Wen S, Peng L, Hong Q, Kang D. Impact of the Consolidated Standards of Reporting Trials (CONSORT) checklist on reporting of randomized clinical trials in traditional Chinese medicine. J Evid Based Med. 2015;8(4):192–208. doi: 10.1111/jebm.12173. [DOI] [PubMed] [Google Scholar]
- 32.EQUATOR Network. Guidelines for Reporting Clinical Trials on Integrated Chinese and Western Medicine. Registered on 9 Jan 2023. Accessed at: https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-clinical-trials/#INTEGRATE
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Search strategy. Appendix 2. Scoring rules for ICWM-specific items. Appendix 3. Types of CM complex intervention (based on Chinese herbal formulas). Appendix 4. Included studies (n=1,458) of this review.
Data Availability Statement
The data analyzed for this study are included in the manuscript and supplementary files.