Abstract
Therapies utilised in breast cancer management have been found to induce or worsen the genitourinary symptoms of menopause (GSM), a group of physical symptoms associated with the systemic loss of estrogen. These symptoms are often undertreated due to concerns surrounding cancer recurrence, especially when considering treatments with possible pro-estrogenic effects. As breast cancer prognosis continues to improve, clinicians are increasingly focussing on managing these symptoms amongst survivors. This systematic review primarily aimed to determine the risk of breast cancer recurrence amongst survivors using vaginal hormones and selective estrogen receptor modulator therapies recommended for use in GSM in the United Kingdom amongst currently published randomised clinical trials (RCTs). The secondary aim was to determine whether these RCTs demonstrated a significant rise in serum estrogen levels following the use of these therapies. A literature search revealed three RCTs suitable for assessment, two evaluating vaginal estrogen and one evaluating vaginal DHEA treatment. Our review determined that amongst published RCTs, no studies have aimed to assess for breast cancer recurrence; however among the studies observing for serious adverse effects of vaginal estrogen preparations, none have reported an increased incidence. Furthermore, these studies did not report a persistent or significant increase in serum estrogen levels following the use of vaginal estrogen products and low concentration (3.25 mg/day) DHEA gel. Larger RCTs studying commonly used vaginal preparations and selective estrogen receptor modulator treatments for GSM over longer follow-up periods will be vital to better assess the risk of breast cancer recurrence in survivors receiving these treatments.
Keywords: Menopause, breast cancer, estrogen, vasomotor symptoms, urogenitalatrophy, hormone replacement therapy
Introduction
Breast cancer continues to be one of the most diagnosed cancers in the UK with over 55,000 women diagnosed each year between 2016 and 2018. 1 In recent years, a decline in mortality rates has been seen amongst women diagnosed with breast cancer given improvements in diagnosis and management. However, the use of certain treatment modalities including chemotherapy agents and adjuvant endocrine therapies such as aromatase inhibitors (AIs) to manage breast cancer has been found to induce or worsen genitourinary symptoms of menopause (GSM) in both pre- and postmenopausal women.2–4
The GSM are a group of physical changes and symptoms, including vulvovaginal dryness, burning, irritation, dyspareunia, and urinary symptoms of urgency, dysuria or recurrent urinary tract infection (UTI) which are associated with the systemic loss of estrogen. 5 Studies have shown that these symptoms have a negative impact on quality of life in breast cancer survivors due to a variety of issues including low self-esteem, body image and reduced sexual function.6–8 As the prognosis of breast cancer improves for women, it is vital that a greater focus is maintained on optimising patient’s quality of life and managing the long-term side effects of cancer treatment as part of the aftercare for survivors. 9
Hormonal estrogen treatment is the current gold standard in managing GSM in healthy postmenopausal women; however, the use of systemic estrogens in breast cancer survivors has been determined to be unsafe due to an increased risk of disease recurrence.10,11
Vaginal hormonal estrogen remains the first-line hormonal therapy in the United Kingdom for breast cancer survivors experiencing genitourinary symptoms of menopause.12,13 Studies have shown that vaginal estrogen effectively relieves the GSM and significantly improves vaginal mucosal health and vaginal pH in postmenopausal women. 5 Furthermore in healthy postmenopausal women, the Women's Health Initiative observational study determined that the risk of breast cancer was not increased following the use of vaginal estrogens. 14 Studies determining this risk of cancer recurrence following vaginal estrogen therapy amongst breast cancer survivors however remain conflicting.15,16
Other more recently approved vaginal hormonal therapies include dehydroepiandrosterone (DHEA), an androgen precursor which partially transforms into estrogen through aromatisation in the vaginal wall. 17 This therapy has been found to effectively manage symptoms of GSM within clinical trials in healthy postmenopausal women and that the strictly local effect of Prasterone correlated with the lack of significant drug-related adverse events. 18 Ospemifene is yet another treatment approved for the management of moderate-to-severe GSM in healthy postmenopausal women and is a selective estrogen receptor modulator (SERM) that acts by binding to estrogen receptors in hormone-responsive tissues. 19 It has a pro-estrogenic effect on the vaginal tissues but an anti-estrogenic effect at the level of the breast tissue. 19 Meta-analyses in healthy postmenopausal women have indicated that Ospemifene significantly improves symptoms associated with postmenopausal vulvovaginal symptoms compared to placebo and additionally has a good safety profile.20,21 However, due to lack of sufficient safety data, both DHEA and Ospemifene are currently not recommended for treatment of GSM in breast cancer survivors.
Whilst there is a clarity on the efficacy and safety of vaginal hormones and selective estrogen receptor modulating therapies in the management of GSM in women without a history of breast pathology, there remain reservations amongst doctors and women about the safety of these treatments in women with a history of breast cancer and the potential risk of breast cancer recurrence particularly with treatments containing estrogen or having a pro-estrogenic effect. The WISDOM study conducted in the United States, surveying 644 clinicians, determined that only 34% of obstetricians and gynaecologists and only 17% of primary care clinicians felt comfortable prescribing vaginal estrogen therapies for vulval and vaginal symptoms to postmenopausal women with a personal history of breast cancer due to concerns about estrogen exposure. 22
In order to bring more clarity to this clinical question, this systematic review aims to primarily assess the safety of vaginal hormones and selective estrogen receptor modulators for the management of GSM with regards to the risk of cancer recurrence amongst breast cancer survivors amongst current published randomised clinical trials (RCTs), particularly those therapies or preparations currently licensed and recommended for use in postmenopausal women in the United Kingdom. The secondary aim of this review was to determine whether these identified RCTs demonstrated any significant rise in serum estrogen levels noted following the use of vaginal treatments in women having these therapies.
Materials and methods
Study selection
This review aimed to assess current medical literature to determine the risk of breast cancer recurrence in breast cancer survivors using vaginal hormonal therapies or oral selective estrogen receptor modulator treatment to treat symptoms associated with GSM.
Inclusion criteria for selecting the studies were as follows: i) studies recruiting women (age >18) who have been diagnosed with and treated for breast cancer; ii) randomised clinical trials (RCTs) including vaginal hormones or selective estrogen receptor modulators currently recommended for the management of GSM in healthy postmenopausal women under guidelines in the United Kingdom including vaginal estrogens, vaginal DHEA and oral Ospemifene within at least one intervention arm of the trial; iii) completed studies with fully published results; and iv) publications in English. RCTs were selected for evaluation within this study as this is the gold-standard methodology for assessing true cause and effect relationships between a therapeutic intervention and any potential outcomes unlike observational or non-randomised studies. 23 RCTs only evaluating vaginal testosterone cream were not assessed in this study as this is not currently recommended for use in the UK for the management of GSM symptoms in postmenopausal women. 24
Literature search
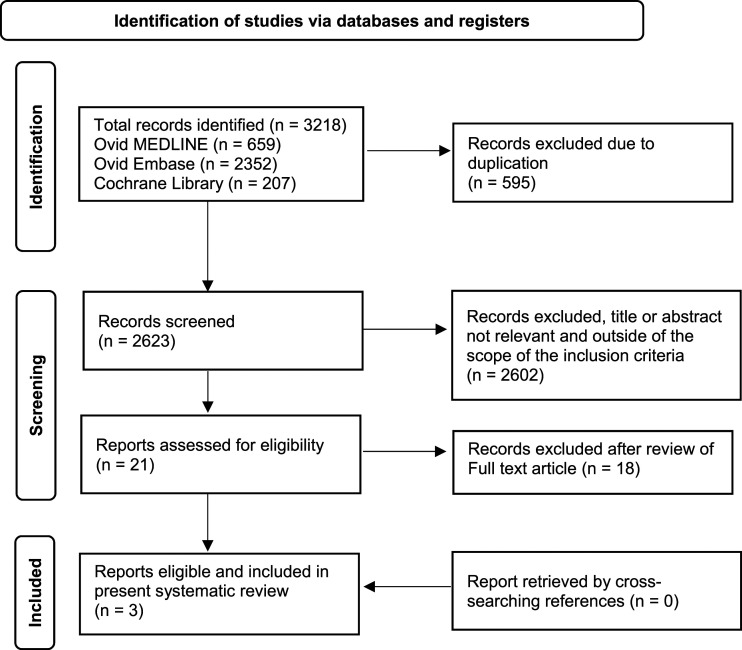
A systematic literature review was conducted by the authors searching the following databases: Cochrane Library (1996–December 2022), Ovid MEDLINE (1946–December 2022) and Ovid Embase (1974–December 2022). This was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) as outlined in Figure 1. 25 Searches were conducted using relevant MeSH terms and keywords related to ‘breast cancer’, ‘menopause’, ‘climacteric’, ‘genitourinary system’, ‘genitourinary syndrome of menopause’, ‘vaginal atrophy’, ‘vaginal hormonal therapy’, ‘estrogen’, ‘Ospemifene’ and ‘DHEA’. In addition to electronic database searching, bibliographies from relevant publications were cross searched for any further relevant studies. Identified citations were imported into EndNote and duplicates removed. Only the most updated results of a same study were selected. References of included studies were cross searched in order to identify any additional studies. The search was re-run prior to submission on 16th December 2022, and no new studies were identified.
Figure 1.
PRISMA flow chart summarising the process of identifying eligible randomised clinical studies. 25
Data extraction
Data collected from each publication were as follows: name of the first author, year of the most updated publication, interventional treatment(s), comparator treatment, treatment regimens, total number of randomised breast cancer survivors, time points at which the study participants were assessed, recorded serum estrogen levels and any recorded episodes of breast cancer recurrence. A thorough analyses and assessment of all included reports were performed independently by both authors, and any disagreement was solved among the authors.
Quality assessment
The studies included within this review were qualitatively assessed using the latest version of the CONSORT 2010 25-item checklist. 26 Evidence of each article entirely fulfilling a single checklist item scored one point and those without complete evidence scored zero for that item. 27 As such, each article scores a minimum of 0 and a maximum of 37. Each article has been divided into three categories based on the score: high-quality (article score >24.67), moderate-quality (12.34< study score <24.67) and poor-quality (study score <12.34). The quality of the articles was assessed by one author (IH), and any disagreements were settled by consulting the additional author (VST). Table 1 presents the results of this assessment.
Table 1.
Quality assessment scores of each article according to the CONSORT checklist (2010). 26
Results
Electronic searches of the included databases using the medical terms cited above produced 3218 records. After removing duplicates, 2623 publications remained. Of these, 2602 records were excluded following assessment of the title or abstract and 18 on assessment of the full-text due to not fulfilling the inclusion criteria in whole (Figure 1). A total of 3 RCTs were found and analysed, with none retrieved by cross searching references. The final 3 articles deemed eligible for inclusion within this systematic review included 1 trial comparing vaginal ET versus placebo, 1 trial comparing vaginal ET with topical testosterone and 1 trial comparing different dosages of DHEA gel with a vaginal moisturiser.28–30 Interventions included in this review were vaginal estrogen in the form of creams and rings and DHEA in the form of a gel. No studies that fit our inclusion criteria included Ospemifene as a treatment intervention. Overall, 577 breast cancer survivors in total were included within these studies, with 90 randomised to receive vaginal estrogen products and 295 randomised to receive vaginal DHEA gel (Table 2).
Table 2.
RCTs assessing the safety of vaginal and oral selective estrogen receptor modulator therapies for treatment of genitourinary syndrome of menopause in breast cancer survivors.
| Author and year | Total number of breast cancer survivors as study participants | Intervention regimen (n) | Comparator regimen (n) | Patient Characteristics | Assessment points | Serum estrogen levels | Recurrence of breast cancer |
|---|---|---|---|---|---|---|---|
| Melisko et al, 2016 30 | 76 | Estring 2 mg silicone ring, inserted once a day for 90 days (n = 40) | 0.5 g 1% intravaginal testosterone (IVT), 0.5 g a day for 2 weeks, 3 times a week from weeks 3 to 10 (n = 36) | Treated with an aromatase inhibitor for at least 30 days | Assessed at baseline, 4 and 12 weeks | Mean values | No recurrence noted |
| Estring ring at baseline 26.8 pg/mL | |||||||
| IVT at baseline 9.0 pg/mL | |||||||
| A transient elevation in estradiol was observed in 12% of patients’ using IVT (range, 11–113 pg/mL. Persistent elevation in estradiol observed in and additional 12% of patients' using IVT (range, 16–45 pg/mL) | |||||||
| A transient elevation in estradiol was observed in 11% of patients’ using the Estring ring (range, 11–29 pg/mL). No persistent elevation in estradiol was seen in any patients' using the Estring vaginal ring | |||||||
| Barton et al, 2018 28 | 440 | i) DHEA 3.25 mg/d moisturiser gel of 12 weeks (n = 147). ii)DHEA 6.5 mg/d moisturiser gel for 12 weeks (n = 149) | Control plain moisturiser, 1 syringe of gel daily every night for 12 weeks (n = 147) | If patients on endocrine therapy (tamoxifen or aromatase inhibitors), they must have been receiving the treatment for 2 months prior to the start of the trial without planned changes | Assessed at baseline and at 12 weeks following daily application | Change in mean concentration of estradiol from baseline levels | No recurrence noted |
| Plain moisturiser – 0.2 ± 2.5 pg/mL, 3.25 mcg/dL DHEA 0.9 ± 5.0, 6.5 mcg/dL DHEA - 0.6 ± 1.9 | |||||||
| Serum estradiol was significantly increased from baseline at 12 weeks in those on 6.5 mg/day DHEA but not those on 3.25 mg/day and not in those on aromatase inhibitors | |||||||
| Hirschberg et al, 2020 29 | 61 | 0.005% (50 ug/g) Estriol vaginal gel, 1 g a day for 3 weeks, 2 g a week during weeks 4–12 (n = 50) | Non-hormonal moisturising gel, 1 g a day for 3 weeks, 2 g a week during weeks 4–12 (n = 11) | Hormone receptor positive and any HER2 status, treated with an aromatase inhibitor for at least 6 months | Assessed 2 weeks prior to the start of treatment, at baseline and at weeks 1, 3, 8 and 12 of treatment and 30 +/− 5 days after the last dose | Median estriol levels | No recurrence noted |
| Estriol gel at baseline 0.5 pg/mL, week 1 3.9 pg/mL, week 3 1.9 pg/mL, week 8 0.5 pg/mL and week 12 0.5 pg/mL | |||||||
| Moisturising gel at baseline 0.5 pg/mL, week 1 0.5 pg/mL, week 3 0.5 pg/mL, week 8 0.5 pg/mL and week 12 0.5 pg/mL | |||||||
| Estriol levels increased initially and normalised by week 12, and estradiol and estrone remained mostly undetectable throughout the study |
None of the studies included within this systematic review aimed to determine the rate of breast cancer recurrence within their study groups. Hirschberg et al and Melisko et al did aim to assess the risk of death or serious adverse events within their treatment groups as an endpoint and did not note any incidences of breast cancer recurrence following the use of vaginal estrogen products (Table 2).29,30
With regards to assessment of serum estrogen levels following treatment, Hirschberg et al noted a transient rise in median estriol (E3) levels at week-1 assessment point in their interventional group receiving E3 gel which normalised over the treatment period. At the 12-week assessment point and at 30 +/− 5 days following the last dose of E3 gel, median serum estrogen levels progressively returned to baseline levels. 29 The significance of these oscillations however was not measured. Melisko et al determined that within their intervention group receiving the Estring estradiol (E2) ring, no persistent increase in serum E2 levels was detected at 12 weeks when compared to baseline levels. This was defined as an E2 greater than 10 pg/mL and at least 10 pg/mL above baseline after treatment initiation on 2 consecutive tests at least 2 weeks apart. A persistent elevation in serum E2 levels was however noted in 12% of patients in the comparator group receiving intravaginal testosterone (IVT). Transient E2 elevation was seen in 11% of patients treated with a vaginal ring and in 12% of those treated with IVT (a transient elevation in E2 was defined as elevation without confirmation on consecutive blood draw with ongoing use of the assigned treatment). 30 Barton et al determined that serum E2 levels were significantly increased from baseline at 12 weeks in the intervention group receiving 6.5 mg/day DHEA (p < .5). However, no significant increase in concentrations of serum E2 was noted in the intervention group receiving 3.25 mg/day (p = .5). Additionally, no significant increase in E2 concentrations was noted from baseline in a sub-group analysis of all patients receiving AIs included within either intervention group (Table 2). 28
Discussion
To our knowledge, this is one of very few systematic reviews that has been conducted in order to determine the risks of breast cancer recurrence following the use of vaginal hormone or selective estrogen receptor modulating therapies amongst current published RCTs. Our study has determined that within current RCTs, no studies have aimed to specifically assess for episodes of breast cancer recurrence. However, no episodes of breast cancer recurrence have been noted by Hirschberg et al or Melisko et al who aimed to assess for serious adverse events or mortality following the use of vaginal estrogen products.29,30 Furthermore, no persistent increase in serum estrogen levels was detected following the use of the vaginal Estring or E3 gel, and no significant increases were seen following the use of 3.25 mg/d DHEA moisturiser gel in breast cancer survivors for the duration of the studies.28–30
Overall, data from non-randomised interventional studies suggest that vaginal estrogen for treatment of GSM may not be associated with an increased risk of recurrence or mortality from breast cancer in survivors. A recent cohort study conducted by Cold et al concluded that overall vaginal estrogen therapy was not associated with an increased risk of breast cancer recurrence or mortality. 15 However interestingly, Cold et al noted through a sub-group analysis that an increased risk of recurrence, but not mortality, was seen in patients receiving vaginal estrogen therapy with adjuvant AIs. 15 This study is the first to report this potential increased risk. Researchers of ongoing RCTs are continuing to evaluate serum estrogen levels and the risk of breast cancer recurrence in BC patients receiving adjuvant AIs and treated with vaginal estrogen therapies. These studies will be critical in helping to further elucidate the safety of these treatments. 31
Systemic absorption of vaginal estrogens would be of particular concern for women with contraindication to hormonal treatments, such as breast cancer survivors. This concern is particularly highlighted by Kendall et al who demonstrated a temporary elevation in serum estrogen level in breast cancer patients treated with AIs who received vaginal estrogen treatment. 32 However, it is important to note that vaginal estrogen was administered at 25 mcg dose (higher than the 10 mcg pessaries twice weekly commonly used now) in this study. Similar findings where serum estrogen levels were initially raised were additionally demonstrated within the study conducted by Hirschberg et al. However, it is notable that the rise seen in serum estrogen levels within these studies is transient with maximal concentrations being reached soon after application.29,32
In a recent meta-analysis conducted by Pavlovic et al, it was further determined that amongst the current literature treatment with vaginal estrogens is not associated with significant systemic absorption in postmenopausal women with a history of breast cancer. 33 This is thought to be due to the initial rapid absorption of estrogen through a thinner postmenopausal atrophic vaginal mucosa. This rate of absorption then begins to fall as the vaginal mucosa thickens and restores following continuous treatment. Furthermore, the initial concentration of serum estrogen following the application of vaginal estrogen has been found to be dose dependent as reported by a recently updated systematic review. 34
Serum estrogen levels may also be dependent on the formulation of vaginal hormone treatments. Research has determined that E3 is much less potent than E2 or estrone (E1), and in vivo does not exhibit conversion back to these more powerful natural estrogens.35,36 In addition, E3 is considered to be a much more short-acting estrogen. 37 Promestriene, a synthetic analogue of E2, has also demonstrated lower serum absorption rates and has been found to not alter to plasma gonadotrophin or E2 levels when applied topically. 38 Peripherally, DHEA appears to act through the local biosynthesis and action of its estrogenic and androgenic metabolites, E1, E2 and dihydrotestosterone. Studies have determined that these active androgens and estrogens exert their action within the same cells in which they were formed with little leakage of these hormones into the systemic circulation. 39 However, the clinical significance of any increase in serum estrogen following onset of vaginal hormone treatment no matter how transient is currently unknown, and further studies are required to determine what concentration rise in serum estrogen and for what duration would be significantly impactful on the long-term risk of breast cancer recurrence.
Although data are limited, no increase in incidence or risk of breast cancer recurrence has been noted in women with GSM treated with Ospemifene. In a meta-analysis of 6 RCTs comparing Ospemifene 60 mg/day versus placebo for side effects and safety, the data found that there was no significant increase in treatment-associated serious adverse effects including death. 20 Rather, data from animal studies have determined that Ospemifene may instead impart a chemoprotective effect as the drug was found to inhibit tumour growth and was not found to have pro-estrogenic effects on breast tissue. 40 A Post Authorisation Safety Study (PASS), a retrospective observational cohort analysis assessing healthy postmenopausal women, further determined that in practise Ospemifene treatment has demonstrated a low incidence of venous thromboembolism, and no increased risk of cerebrovascular events, vaginal bleeding, endometrial cancer and other gynaecological pathologies. 41 However, currently there are no RCTs available evaluating safety parameters such as serum estrogen levels or the risk of breast cancer recurrence within breast cancer survivors which would provide confirmation of previous observational findings.
The VIBRA pilot study determined that there were no severe adverse events recorded following the administration of 6.5 mg/d vaginal DHEA amongst breast cancer survivors. 42 Furthermore, no significant rise in serum estradiol levels was found at the 6 month assessment point. 42 However, these findings are to some extent different to the findings of Barton et al who found the higher 6.5 mg/d concentration of DHEA led to a significant increase in serum estradiol levels from baseline, and this was however not seen in those receiving the lower concentration 3.25 mg/d DHEA gel and not in those on AIs. 28 This was not determined to be due to a dose-dependent conversion of DHEA to systemic estradiol. 28 One possible explanation was stated to be due to normal variations in E2 levels between individuals; however, the reason why remains otherwise unknown. 21 This sub-group analysis is not possible within the VIBRA study as all the recruited patients’ for the study had been taking AIs as per the inclusion criteria. 42 Furthermore, it is important to note that the dose of AI prescribed to women treated for breast cancer may not completely inhibit the aromatase in the body and breast tissue and some conversion of androgens or precursors to estrogen cannot be ruled out and cannot be entirely assessed through the measurement of serum E2 levels alone. 43
Overall, we see a vital need for further study to determine changes in serum E2 and serum testosterone levels amongst patients’ prescribed both the 3.25 mg/d and 6.5 mg/d concentration of DHEA gel over a much longer follow-up period and to determine if any associated rise in these hormone levels has any significant impact on breast cancer recurrence rates.
Limitations
A major limitation is the lack of literature published within this area. We found only three small RCTs which could be included in this review, and none of the interventions studied are currently the first-line most recommended medications for management of GSM in the UK (such as 10 mcg estrogen pessaries or 0.1% or 0.01% E3 creams). Another significant limitation of this review is that the follow-up duration of the studies included was short and therefore may not accurately assess the long-term safety of vaginal hormone modulator preparations. This is echoed by a recent review conducted by Merlino et al who assessed the therapeutic choices for GSM in breast cancer survivors. They also suggested that the current data available demonstrate that vaginal treatments are effective for the therapy of GSM in breast cancer survivors and however also emphasised the need for larger clinical trials with longer follow-ups in this group of women to address safety concerns. 44
Cold et al demonstrate observational data that suggests a potential rise in breast cancer recurrence within a sub-group of patients receiving vaginal estrogen therapy with adjuvant AIs. Future RCTs will be vital in determining the safety of vaginal hormone treatments and in order to further qualify the risk within this sub-group of patients’. 15 In addition, Ospemifene, an approved selective estrogen receptor modulator for the management of GSM, was not assessed due to the lack of appropriate trials for this review.
Furthermore, the heterogenicity of interventions and formulations amongst the trials included within this assessment along with small sample sizes means that the trials may not be sufficiently powered to detect differences amongst these different treatments.
Clinical implications
Based on results from observational studies and other prospective and retrospective non-randomised studies, current clinical guidance is that vaginal estrogen preparations may be used for treatment of severe GSM in breast cancer survivors as second-line treatment option when lubricants or moisturisers have failed. It is recommended that such treatment be initiated in liaison with the oncology team and consideration should be given to switching from an AI to tamoxifen for endocrine therapy. Our review supports this approach based on the limited evidence obtained through the RCTs on this subject. Discussion of lack of evidence and long-term data and individualisation of benefits versus risks in relation to quality of life of women should be important components of clinical decision making.
Conclusions
Our review has determined that within the existing RCTs, no studies have aimed to specifically assess for breast cancer recurrence following the use of vaginal estrogens or hormone receptor modulators for treatment of GSM in breast cancer survivors. However, among current RCT studies observing for adverse effects of these preparations, none have reported an increased incidence of breast cancer recurrence. Additionally, none of the RCTs assessed within this review have reported a persistent increase in serum estrogen levels following the use of the vaginal estrogen products and no significant increase in serum estrogen levels following the use of low-concentration (3.25 mg/d) DHEA gel. Data reported amongst observational studies has identified a potential risk of recurrence amongst a sub-group of patients receiving vaginal estrogen therapy with adjuvant AIs, and therefore further RCTs using commonly used vaginal preparations with longer duration of follow-up and larger participant numbers are required to assess the risk of breast cancer recurrence in survivors receiving adjuvant treatments. Further trials assessing the risk of breast cancer recurrence in women taking approved selective estrogen receptor modulators for the management of GSM will also be vital in determining the safety of this therapy modality.
Practice points
The safety and the potential risk of breast cancer recurrence amongst breast cancer survivors using vaginal hormones and selective estrogen receptor modulators for the management of the genitourinary symptoms of menopause is currently debated.
Published data from randomised clinical trials shows that no studies have aimed to assess for breast cancer recurrence; however, among studies observing for serious adverse effects of vaginal estrogen, none have reported an increased incidence of recurrence. Furthermore, none of the studies have reported a significant increase in serum estrogen levels following the use of the vaginal estrogen and low-concentration DHEA gel.
Larger randomised trials studying approved vaginal hormone preparations and over a longer follow-up period will be vital to better assess the risk of breast cancer recurrence in survivors receiving these treatments.
Footnotes
Contributorship: IH and VST contributed to the conception and design of the article. IH performed the literature search and wrote the first draft of the review. IH and VST reviewed the drafts and approved the final version of the article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: VST.
Ethical approval
This study was deemed exempt by the University College London Research Ethics Committee under provision ‘Research involving information freely available in the public domain’ and therefore did not require ethical approval from the board prior to the conduction of the research.
ORCID iD
Ishrat Hussain https://orcid.org/0000-0002-5281-4028
References
- 1.Cancer Research UK . Breast cancer statistics, 2022. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero (Accessed 16 October 2022).
- 2.Broeckel JA, Thors CL, Jacobsen PB, et al. Sexual functioning in long-term breast cancer survivors treated with adjuvant chemotherapy. Breast Cancer Res Treat 2002; 75: 241–248. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause 2013; 20: 162–168. [DOI] [PubMed] [Google Scholar]
- 4.Falk SJ, Dizon DS. Sexual dysfunction in women with cancer. Fertil Steril 2013; 100: 916–921. [DOI] [PubMed] [Google Scholar]
- 5.Kim HK, Kang SY, Chung YJ, et al. The recent review of the genitourinary syndrome of menopause. J Menopausal Med 2015; 21: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickey M, Saunders CM, Stuckey BG. Management of menopausal symptoms in patients with breast cancer: an evidence-based approach. Lancet Oncol 2005; 6: 687–695. [DOI] [PubMed] [Google Scholar]
- 7.Sussman TA, Kruse ML, Thacker HL, et al. Managing genitourinary syndrome of menopause in breast cancer survivors receiving endocrine therapy. J Oncol Pract 2019; 15: 363–370. [DOI] [PubMed] [Google Scholar]
- 8.Lubián López DM. Management of genitourinary syndrome of menopause in breast cancer survivors: An update. World J Clin Oncol 2022; 13: 71–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cusack L, Brennan M, Baber R, et al. Menopausal symptoms in breast cancer survivors: management update. Br J Gen Pract 2013; 63: 51–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer--is it safe?), a randomised comparison: trial stopped. Lancet 2004; 363: 453–455. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg L, Iversen OE, Rudenstam CM, et al. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst 2008; 100: 475–482. [DOI] [PubMed] [Google Scholar]
- 12.NICE . Menopause: diagnosis and management. London, UK: National Institute for Health and Care Excellence,2015, www.NICE.org.uk (accessed 4 February 2023). [PubMed] [Google Scholar]
- 13.Marsden J, Marsh M, Rigg A. British Menopause Society consensus statement on the management of estrogen deficiency symptoms, arthralgia and menopause diagnosis in women treated for early breast cancer. Post Reprod Health 2019; 25: 21–32. [DOI] [PubMed] [Google Scholar]
- 14.Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause 2018; 25: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cold S, Cold F, Jensen M-B, et al. Systemic or vaginal hormone therapy after early breast cancer: a danish observational cohort study. J Natl Cancer Inst 2022; 114: 1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dew JE, Wren BG, Eden JA. A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric 2003; 6: 45–52. [PubMed] [Google Scholar]
- 17.Labrie F, Luu-The V, Labrie C, et al. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 2001; 22: 185–212. [DOI] [PubMed] [Google Scholar]
- 18.Labrie F, Archer DF, Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2018; 25: 1339–1353. [DOI] [PubMed] [Google Scholar]
- 19.Palacios S. Ospemifene for vulvar and vaginal atrophy: an overview. Drugs Context 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Donato V, Schiavi MC, Iacobelli V, et al. Ospemifene for the treatment of vulvar and vaginal atrophy: a meta-analysis of randomized trials. Part I: Evaluation of efficacy. Maturitas 2019; 121: 86–92. [DOI] [PubMed] [Google Scholar]
- 21.Di Donato V, Schiavi MC, Iacobelli V, et al. Ospemifene for the treatment of vulvar and vaginal atrophy: a meta-analysis of randomized trials. Part II: Evaluation of tolerability and safety. Maturitas 2019; 121: 93–100. [DOI] [PubMed] [Google Scholar]
- 22.Kingsberg SA, Larkin L, Krychman M, et al. WISDOM survey: attitudes and behaviors of physicians toward vulvar and vaginal atrophy (VVA) treatment in women including those with breast cancer history. Menopause 2019; 26: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hariton E, Locascio JJ. Randomised controlled trials—the gold standard for effectiveness research. BJOG An Int J Obstet Gynaecol. 2018; 125: 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panay N. British menopause society tool for clinicians: testosterone replacement in menopause. Post Reprod Health. 2022; 28:158–160. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Br Med J 2010; 8: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharifi N, Afshari F, Bahri N. The effects of yoga on quality of life among postmenopausal women: a systematic review study. Post Reprod Health 2021; 27: 215–221. [DOI] [PubMed] [Google Scholar]
- 28.Barton DL, Shuster LT, Dockter T, et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support Care Cancer 2018; 26: 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirschberg AL, Sánchez-Rovira P, Presa-Lorite J, et al. Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: a phase II, randomized, double-blind, placebo-controlled trial. Menopause 2020; 27: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial. JAMA Oncol 2017; 3: 313–319. [DOI] [PubMed] [Google Scholar]
- 31.Sulaica E, Han T, Wang W, et al. Vaginal estrogen products in hormone receptor-positive breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res Treat 2016; 157: 203–210. [DOI] [PubMed] [Google Scholar]
- 32.Kendall A, Dowsett M, Folkerd E, et al. Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 2006; 17: 584–587. [DOI] [PubMed] [Google Scholar]
- 33.Pavlović RT, Janković SM, Milovanović JR, et al. The safety of local hormonal treatment for vulvovaginal atrophy in women with estrogen receptor-positive breast cancer who are on adjuvant aromatase inhibitor therapy: meta-analysis. Clin Breast Cancer 2019; 19: e731–e740. [DOI] [PubMed] [Google Scholar]
- 34.Santen RJ, Mirkin S, Bernick B, et al. Systemic estradiol levels with low-dose vaginal estrogens. Menopause 2020; 27: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Vies J. The pharmacology of oestriol. Maturitas 1982; 4: 291–299. [DOI] [PubMed] [Google Scholar]
- 36.Head KA. Estriol: safety and efficacy. Alternative Med Rev 1998; 3: 101–113. [PubMed] [Google Scholar]
- 37.Al-Baghdadi O, Ewies AA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009; 12: 91–105. [DOI] [PubMed] [Google Scholar]
- 38.Del Pup L, Di Francia R, Cavaliere C, et al. A specific topic estrogen. Review of 40 years of vaginal atrophy treatment: is it safe even in cancer patients? Anti Cancer Drugs 2013; 24: 989–998. [DOI] [PubMed] [Google Scholar]
- 39.Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111: 178–194. [DOI] [PubMed] [Google Scholar]
- 40.Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97: 230–240. [DOI] [PubMed] [Google Scholar]
- 41.Bruyniks N, De Gregorio F, Gibbs T, et al. Safety of ospemifene during real-life use. J Gynecol Women’s Health 2018; 9: 555762. [Google Scholar]
- 42.Mension E, Alonso I, Cebrecos I, et al. Safety of prasterone in breast cancer survivors treated with aromatase inhibitors: the VIBRA pilot study. Climacteric. 2022. ;25 :476–482. [DOI] [PubMed] [Google Scholar]
- 43.Miller WR, Larionov AA. Understanding the mechanisms of aromatase inhibitor resistance. Breast Cancer Res . 2012. ;14 :1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merlino L, D'Ovidio G, Matys V, On Behalf Of Policlinico Umberto I Collaborators , et al. Therapeutic Choices for Genitourinary Syndrome of Menopause (GSM) in breast cancer survivors: a systematic review and update. Pharmaceuticals. 2023; 16: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]