Abstract
Two Bacillus stearothermophilus wild-type strains were investigated regarding a common recognition and binding mechanism between the S-layer protein and the underlying cell envelope layer. The S-layer protein from B. stearothermophilus PV72/p6 has a molecular weight of 130,000 and assembles into a hexagonally ordered lattice. The S-layer from B. stearothermophilus ATCC 12980 shows oblique lattice symmetry and is composed of subunits with a molecular weight of 122,000. Immunoblotting, peptide mapping, N-terminal sequencing of the whole S-layer protein from B. stearothermophilus ATCC 12980 and of proteolytic cleavage fragments, and comparison with the S-layer protein from B. stearothermophilus PV72/p6 revealed that the two S-layer proteins have identical N-terminal regions but no other extended structurally homologous domains. In contrast to the heterogeneity observed for the S-layer proteins, the secondary cell wall polymer isolated from peptidoglycan-containing sacculi of the different strains showed identical chemical compositions and comparable molecular weights. The S-layer proteins could bind and recrystallize into the appropriate lattice type on native peptidoglycan-containing sacculi from both organisms but not on those extracted with hydrofluoric acid, leading to peptidoglycan of the A1γ chemotype. Affinity studies showed that only proteolytic cleavage fragments possessing the complete N terminus of the mature S-layer proteins recognized native peptidoglycan-containing sacculi as binding sites or could associate with the isolated secondary cell wall polymer, while proteolytic cleavage fragments missing the N-terminal region remained unbound. From the results obtained in this study, it can be concluded that S-layer proteins from B. stearothermophilus wild-type strains possess an identical N-terminal region which is responsible for anchoring the S-layer subunits to a secondary cell wall polymer of identical chemical composition.
Crystalline bacterial cell surface layers (S-layers) represent the outermost cell envelope component of many eubacteria and archaebacteria. S-layer lattices can show oblique, square, or hexagonal symmetry, and they are composed of identical protein or glycoprotein subunits with the ability to assemble into two-dimensional crystalline arrays (4, 35).
For most Bacillus stearothermophilus wild-type strains, oblique and square S-layer lattices have been identified, and only a single strain, B. stearothermophilus PV72/p6, exhibited an S-layer lattice with hexagonal symmetry (25, 36). From this observation, it was concluded that in contrast to other Bacillus species such as B. sphaericus (6, 11), the lattice type has no taxonomical relevance. In addition to this morphological heterogeneity, S-layer proteins from B. stearothermophilus wild-type strains showed quite different molecular weights (25, 36). The different cleavage products obtained by peptide mapping further indicated the absence of extended structurally homologous domains (30). Since S-layer proteins from selected wild-type strains had identical N-terminal regions (7, 30) and chemical analysis revealed comparable compositions of their peptidoglycan-containing sacculi (28), the question arose as to whether there was a species-specific binding and recognition mechanism common to the S-layer proteins and the rigid cell wall layer.
In previous studies, oxygen-induced variant formation and oxygen-induced changes in S-layer protein synthesis have been reported for various B. stearothermophilus wild-type strains (28–30). During variant formation, the S-layer proteins from three different wild-type strains forming either oblique, square, or hexagonal lattices were replaced by a common type of S-layer protein with a molecular weight of 97,000 which assembled into an oblique lattice. Detailed investigation of the dynamics of variant formation for B. stearothermophilus PV72/p6 showed that change in S-layer protein synthesis is a synchronous process in most if not in all individual cells of the culture (28, 34). The S-layer protein produced by the B. stearothermophilus PV72/p6 wild-type strain and the S-layer protein from the oxygen-induced p2 variant (B. stearothermophilus PV72/p2) are encoded by different genes (16–18). Multiple recombination events involving chromosomal and plasmid DNA seem to be responsible for oxygen-induced S-layer variation (32).
Chemical analysis of peptidoglycan-containing sacculi from B. stearothermophilus PV72/p6 and the p2 variant strongly indicated that they consist of peptidoglycan of the A1γ-chemotype (31) and a secondary cell wall polymer of different chemical composition (28). In addition, the secondary cell wall polymer of the p2 variant has been characterized in more detail (27). It has an estimated molecular weight of 24,000, is mainly composed of GlcNAc and ManNAc, and was found to anchor the S-layer protein via its N-terminal region to the peptidoglycan-containing layer (27).
In this study, we investigated two B. stearothermophilus wild-type strains for the presence, chemical composition, and function of a secondary cell wall polymer. The S-layer protein from B. stearothermophilus PV72/p6 has a molecular weight of 130,000 and assembles into a hexagonally ordered S-layer lattice (36). The S-layer from B. stearothermophilus ATCC 12980 shows oblique lattice symmetry and is composed of subunits with a molecular weight of 122,000 (7).
MATERIALS AND METHODS
Organisms, growth conditions, and cell wall preparations.
For production of biomass, B. stearothermophilus PV72/p6 was grown in continuous culture on synthetic PVIII medium at 57°C at a dilution rate of 0.2 h−1 in a 7-liter Applikon (Schiedam, The Netherlands) bioreactor. The glucose concentration was 3.0 g/liter of PVIII medium (33, 34). B. stearothermophilus ATCC 12980 was grown on complex SVIII medium (7, 28) in a 5-liter Braun (Melsungen, Germany) bioreactor in batch culture until the end of exponential growth. The rate of aeration was adjusted to 4.0 liter of air/min, which corresponded to oxygen-limited growth. The pH value of the culture was kept constant at 7.2 by addition of 1 M NaOH or 2 M H2SO4. Cells were separated from spent medium by continuous centrifugation at 16,000 × g at 4°C and were stored at −20°C. Cell wall fragments were prepared as previously described (25, 27, 28).
Preparation of S-layer self-assembly products and peptidoglycan-containing sacculi.
Wet pellets of cell wall fragments (obtained by centrifugation at 40,000 × g for 20 min at 4°C) were suspended in a 10-fold volume of a guanidine hydrochloride (GHCl) solution (5 M GHCl in 50 mM Tris-HCl buffer [pH 7.2]) and stirred for 20 min at 4°C. After centrifugation at 40,000 × g for 20 min at 4°C, the supernatant containing the extracted S-layer protein was carefully removed, centrifuged twice at 40,000 × g, and dialyzed against distilled water at 4°C overnight. S-layer self-assembly products were sedimented by centrifugation at 20,000 × g for 20 min, washed at least three times with distilled water, frozen at −20°C, and lyophilized. For investigating S-layer self-assembly products by negative staining and transmission electron microscopy, GHCl extracts were dialyzed against 10 mM KCl and 10 mM CaCl2 at 20°C overnight. Electron microscopy was performed with a Philips CM100 transmission electron microscope.
For chemical analyses and extraction of the secondary cell wall polymer, peptidoglycan-containing sacculi were once washed with 5 M GHCl and twice with 50 mM Tris-HCl buffer (pH 7.2). To inactivate autolysins, peptidoglycan-containing sacculi were incubated in sodium dodecyl sulfate (SDS) solution (1% in distilled water) for 30 min at 100°C (27), and the pellet was subsequently extensively washed with distilled water. The purity of S-layer self-assembly products and peptidoglycan-containing sacculi was checked by SDS-polyacrylamide gel electrophoresis (PAGE), negative staining, and ultrathin sectioning as described elsewhere (25).
Extraction of the secondary cell wall polymer.
For cleaving phosphodiester linkages between the secondary cell wall polymer and the peptidoglycan backbone, native peptidoglycan-containing sacculi were extracted with 48% hydrofluoric acid (HF) for 7 to 96 h at 4°C (15). After centrifugation at 40,000 × g for 20 min at 4°C, the pellets were once washed with HF and three times with distilled water, frozen at −20°C, and lyophilized. The extracted peptidoglycan-containing sacculi were used for chemical analyses and recrystallization experiments.
The clear supernatant obtained after extraction of the peptidoglycan-containing sacculi with 48% HF for 96 h was carefully removed. The secondary cell wall polymer was precipitated by addition of the fivefold volume of chilled ethanol (−20°C) absolute (8). After incubation for 18 h at −20°C, the precipitated cell wall polymer was sedimented at 20,000 × g for 15 min at −10°C, washed twice with chilled ethanol, and finally dissolved in distilled water. The clear solution containing the secondary cell wall polymer was dialyzed against distilled water for 24 h at 4°C (Biomol membrane type 8; molecular weight cutoff, 12,000 to 16,000), frozen at −20°C, and lyophilized. For obtaining information on the molecular weight, 2 mg of the secondary cell wall polymer was dissolved per ml of 150 mM NaCl in 50 mM Tris-HCl buffer (pH 7.8), and 2 ml was applied to a Sephacryl S-200 HR column (Pharmacia, Uppsala, Sweden) which was calibrated as previously described (27). The homogeneity of the secondary cell wall polymer was further examined by reversed-phase high-pressure liquid chromatography (RP-HPLC) as described elsewhere (5).
Recrystallization experiments.
Native and HF-extracted peptidoglycan-containing sacculi were used for S-layer recrystallization experiments. For this purpose, 2.5 mg of lyophilized peptidoglycan-containing sacculi and 2.5 mg of S-layer self-assembly products were suspended in 5 ml of 5 M GHCl in 50 mM Tris-HCl buffer (pH 7.2), stirred for 20 min at 20°C, and dialyzed against distilled water overnight at 4°C. After recrystallization, the samples were investigated by negative staining and ultrathin sectioning (25). For studying the influence of Ca2+ ions on the binding and recrystallization process, dialysis was also performed against 10 mM CaCl2 and 10 mM EDTA.
Chemical analyses.
Native and HF-extracted peptidoglycan-containing sacculi and the secondary cell wall polymer from both organisms were subjected to amino acid, amino sugar, and neutral sugar analyses. For amino acid and amino sugar analyses, the samples were hydrolyzed with 6 N HCl for 6 h at 110°C. After modification with sodium borohydride and o-phthalaldehyde, amino acids and amino sugars were analyzed by HPLC (1). Neutral sugars were liberated by hydrolysis with 2.2 M trifluoroacetic acid (TFA) for 4 h at 110°C. After drying with nitrogen, the samples were dissolved in distilled water and applied to a DIONEX DX-300 gradient chromatography system (5).
Proteolytic degradation of the S-layer proteins with endoproteinase Glu-C (Staphylococcus aureus V8 protease) and affinity studies.
For proteolytic degradation of the S-layer proteins, 1-mg aliquots of S-layer self-assembly products were dissolved per ml of 2 M GHCl in 50 mM Tris-HCl buffer (pH 7.8) and incubated with endoproteinase Glu-C (Sigma P 6181; 40 μg/mg of S-layer protein) for 1 h at 37°C. After dialysis for 18 h at 4°C and centrifugation at 40,000 × g for 20 min, the clear supernatant was subjected to SDS-PAGE. For affinity studies, 1 mg of native peptidoglycan-containing sacculi was suspended per ml of clear supernatant and stirred for 1 h at 20°C. After centrifugation at 40,000 × g for 20 min, both the supernatant and the pellet were subjected to SDS-PAGE. Blotting to polyvinylidene fluoride membranes (Immobilon PSQ; Millipore) and N-terminal sequencing were performed as previously described (7). Peptide mapping of the S-layer proteins from both B. stearothermophilus wild-type strains was carried out in 0.1% SDS in 50 mM Tris-HCl buffer (pH 7.8). The incubation time with endoproteinase Glu-C (10 μg/mg of S-layer protein) was 1 h at 37°C. The reaction was stopped by heating the samples for 10 min at 100°C. After separation on SDS–6 or 10% gels and blotting to polyvinylidene membranes, selected protein bands from the S-layer protein of B. stearothermophilus ATCC 12980 were subjected to N-terminal sequencing.
Investigation of the affinity between proteolytic cleavage fragments of the S-layer protein from B. stearothermophilus PV72/p6 and the isolated secondary cell wall polymer.
Proteolytic degradation of the S-layer protein from B. stearothermophilus PV72/p6 in the presence of 2 M GHCl with endoproteinase Glu-C and affinity studies were carried out as described above. After sedimentation of native peptidoglycan-containing sacculi with bound proteolytic cleavage fragments at 40,000 × g for 20 min at 4°C, the pellet was extracted with 2 M GHCl and the suspension was centrifuged under conditions described above. The clear supernatant containing 2 mg of the GHCl-extracted proteolytic cleavage fragments was carefully removed, and 500 μg of isolated secondary cell wall polymer was added. After dialysis against 150 mM NaCl in 50 mM Tris-HCl buffer (pH 7.8) for 48 h at 4°C, this solution was applied to a Sephacryl S-200 HR column. For determination of protein, the absorption of the eluate was measured at 280 nm. Elution of the secondary cell wall polymer was monitored via the refraction index (RI) by using an RI detector.
Production of antiserum against the SbsA and the S-layer proteins from B. stearothermophilus ATCC 12980.
Production of polyclonal rabbit antisera and immunoblotting were performed as described previously (7).
RESULTS
Chemical analyses of peptidoglycan-containing sacculi from B. stearothermophilus PV72/p6 and ATCC 12980.
The results from amino acid and amino sugar analysis of peptidoglycan-containing sacculi from both organisms are summarized in Table 1. With the exception of glucosamine, the molar ratios of all peptidoglycan constituents (Glu, Ala, [Dap], diaminopimelic acid and muramic acid) corresponded to the directly cross-linked Alγ chemotype typical of B. stearothermophilus wild-type strains (31). In comparison to muramic acid, Glu, and Dap, the glucosamine content was at least 1.5 times higher than the theoretical value (28). As determined by sugar HPLC, the glucose content of native peptidoglycan-containing sacculi was in the range of 10%. The increased molar ratio between glucosamine and all other peptidoglycan constituents (Table 1) and the relatively high glucose content indicated the presence of a secondary cell wall polymer in the peptidoglycan-containing sacculi of both B. stearothermophilus wild-type strains (28).
TABLE 1.
Chemical analysis of native and HF-extracted (48%; 96 h) peptidoglycan-containing sacculi from B. stearothermophilus PV72/p6 and ATCC 12980
| Amino acid | Molar ratioa
|
||
|---|---|---|---|
| Theoretical value | Native peptidoglycan-containing sacculi | HF-extracted peptidoglycan-containing sacculi | |
| Glucosamine | 1 | 1.52/1.57 | 0.94/0.95 |
| Muramic acid | 1 | 0.99/0.91 | 0.89/0.99 |
| Ala | 1.5 | 1.51/1.43 | 1.46/1.44 |
| Dap | 1 | 1.01/1.12 | 0.96/0.98 |
All values were related to Glu = 1. The theoretical values represent the molar ratios typical of the peptidoglycan A1γ chemotype (31).
Extraction of the secondary cell wall polymer from native peptidoglycan-containing sacculi and recrystallization experiments.
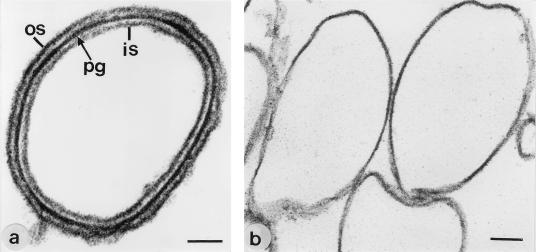
Native peptidoglycan-containing sacculi were treated with 48% HF for 7 to 96 h, and the remaining glucose was taken as a marker for the extent of extraction of the secondary cell wall polymer. As shown in Table 2, binding and recrystallization of the S-layer protein from both organisms was observed on native peptidoglycan-containing sacculi and on those extracted with 48% HF for 7 h from which 40 to 50% of the glucose had been removed. After recrystallization of the S-layer protein, complete outer and inner S-layers were observed in ultrathin-sectioned preparations (Fig. 1a). Peptidoglycan-containing sacculi extracted with 48% HF for 48 h still contained 5 to 10% of the amount of glucose detected in native samples. In ultrathin-sectioned preparations, binding and recrystallization of the S-layer protein were observed on a low number of peptidoglycan-containing sacculi only (Table 2). The S-layer protein did not bind and recrystallize on peptidoglycan-containing sacculi extracted with 48% HF for 72 or 96 h (Fig. 1b) from which glucose had completely been removed. Amino acid and amino sugar analysis further revealed that the molar ratio between glucosamine, Glu, Dap, and muramic acid had decreased to approximately 1 which corresponded to pure peptidoglycan of the A1γ chemotype (Table 1).
TABLE 2.
Extraction of the secondary cell wall polymer from native peptidoglycan-containing sacculi of B. stearothermophilus PV72/p6 and ATCC 12980 with 48% for 7 h to 96 ha
| Sacculi | % Glucose extracted | Recrystallization |
|---|---|---|
| Native | 0 | + |
| 7 h | 40–50 | + |
| 48 h | 90–95 | − (maximum 5% positive) |
| 72 h | 100 | − |
| 96 h | 100 | − |
Glucose as an essential component of the secondary cell wall polymer was used as marker for the extent of extraction. Binding and recrystallization of the S-layer protein on the differently extracted peptidoglycan-containing sacculi was checked by ultrathin sectioning.
FIG. 1.
Electron micrographs of ultrathin-sectioned preparations from (a) native and (b) HF-extracted (48%, 96 h, 4°C) peptidoglycan-containing sacculi from B. stearothermophilus PV72/p6. Recrystallization of the S-layer protein was observed only on native peptidoglycan-containing sacculi. os, outer S-layer; pg, peptiodoglycan-containing layer; is, inner S-layer. Bars, 100 nm.
Ultrathin sectioning, freeze-drying, and metal shadowing confirmed that the S-layer proteins from both B. stearothermophilus wild-type strains could bind and recrystallize into the characteristic lattice type (hexagonal for PV72/p6 and oblique for ATCC 12980) on peptidoglycan-containing sacculi from the other organism (not shown). Moreover, binding and recrystallization of the S-layer protein was independent on the presence of Ca2+ ions.
Chemical analyses of the HF-extracted secondary cell wall polymers.
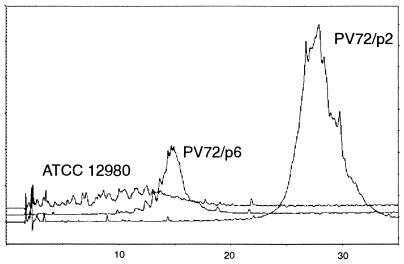
The secondary cell wall polymer from both organisms could completely be extracted from native peptidoglycan-containing sacculi with 48% HF for 96 h, indicating the presence of phosphodiester linkages between the polymer chains and the peptidoglycan backbone (3, 15). The HF-extracted secondary cell wall polymers were precipitated with chilled ethanol, dialyzed against distilled water, and purified by gel permeation chromatography using a Sephacryl S-200 HR column. Both eluted as a single peak with an estimated molecular weight of 50,000. After application of the secondary cell wall polymer from B. stearothermophilus PV72/p6 to RP-HPLC as described previously (5), a single homogeneous peak was obtained (Fig. 2). On the contrary, the elution profile of the secondary cell wall polymer from B. stearothermophilus ATCC 12980 showed that this material was rather polydisperse (Fig. 2), which can be explained by the properties of the biomass used for isolation of the secondary cell wall polymer. In contrast to B. stearothermophilus PV72/p6, which was grown in continuous culture at constant specific growth rate under steady-state conditions, biomass from B. stearothermophilus ATCC 12980 was harvested from batch culture under otherwise identical conditions. The influence of the growth conditions on the homogeneity of the secondary cell wall polymer was supported by using biomass from B. stearothermophilus PV72/p2 (27) which was also grown in continuous culture at constant specific growth rate. As described for the B. stearothermophilus PV72/p6 wild-type strain, a single homogeneous peak was obtained when the secondary cell wall polymer from the p2 variant was applied to RP-HPLC (Fig. 2).
FIG. 2.
Elution profile of the secondary cell wall polymers from B. stearothermophilus ATCC 12980, PV72/p6, and PV72/p2 after application to RP-HPLC as described previously (6). Only the secondary cell wall polymers from B. stearothermophilus PV72/p6 and PV72/p2 eluted as a single peak, showing that this material was homogeneous. In contrast to B. stearothermophilus ATCC 12980, which was grown in batch culture, B. stearothermophilus PV72/p6 and PV72/p2 were grown in continuous culture at constant specific growth rate. The absorption at 220 nm is plotted versus the elution time in minutes.
Amino sugar and neutral sugar analysis revealed that the secondary cell wall polymers from both B. stearothermophilus wild-type strains contained glucose and glucosamine. The maximum amount of gluosamine was liberated by hydrolysis with 6 N HCl for 6 h, while the highest amount for glucose was determined when hydrolysis was performed with 2.2 N TFA for 4 h. The maximum of each component was used for calculation of the molar ratio, which was 1 to 1, glucose to glucosamine. In addition to the peak corresponding to glucosamine, two further peaks were detected by amino acid and sugar HPLC in samples hydrolyzed with 6 N HCl. As estimated from the peak areas, the molar ratio between glucosamine and the two other (still unidentified) components was 1 to 0.2. Modification of HCl- and TFA-hydrolyzed samples with 2-aminoacridone and separation by PAGE as described previously (14) confirmed that the two secondary cell wall polymers have the same chemical composition. The secondary cell wall polymers represented about 20% of the peptidoglycan-containing sacculi dry weight.
Binding of proteolytic cleavage fragments to native peptidoglycan-containing sacculi.
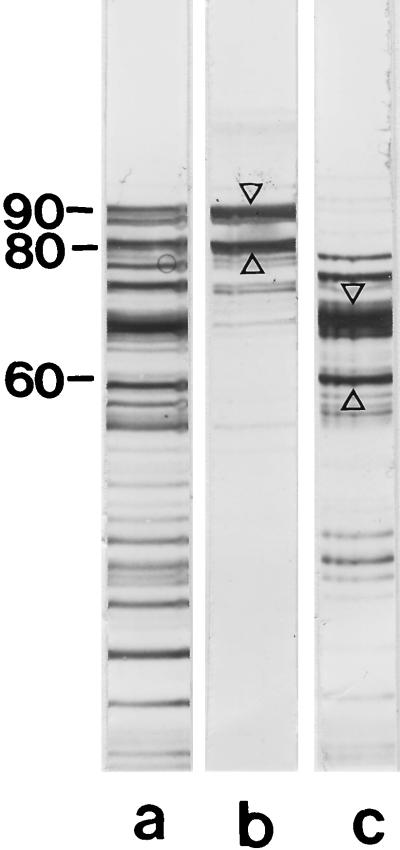
For proteolytic cleavage of the S-layer proteins from both B. stearothermophilus wild-type strains, we used endoproteinase Glu-C, which specifically attacks proteins after glutamic and aspartic acid residues. In case of B. stearothermophilus PV72/p6, S-layer self-assembly products were dissolved in 2 M GHCl. After proteolytic degradation of the S-layer protein, the solution was dialyzed against distilled water and insoluble material was removed by centrifugation. As shown by SDS-PAGE, the supernatant consisted of a series of proteolytic cleavage fragments (Fig. 3, lane a). After incubation with native peptidoglycan-containing sacculi, two proteolytic cleavage fragments showing apparent molecular weights of 90,000 and 80,000 on SDS-gels were detected in the pellet (lane b), indicating that they recognized native peptidoglycan-containing sacculi as binding site. All other proteolytic cleavage fragments remained in the clear supernatant (lane c). Both S-layer protein fragments (A and B [Table 3]) which had bound to native peptidoglycan-containing sacculi had an N-terminal region (ATDVATVVSQAKAQ) identical to that of the mature S-layer protein (18) and were therefore missing a 40,000- or 50,000-molecular-weight C-terminal fragment. N-terminal sequencing of two major proteolytic cleavage fragments (C and D [Table 3]) which did not recognize native peptidoglycan-containing sacculi as binding site and showed apparent molecular weights of 66,000 and 60,000 on SDS-gels (Fig. 3, lane c) led to the following result: AALTPK. This sequence could completely be detected on the S-layer protein from B. stearothermophilus PV72/p6, and the first amino acid corresponded to Ala in position 228 of the mature SbsA. In comparison to the whole S-layer protein, these two cleavage fragments were missing a 24,000-molecular-weight N-terminal fragment and either a 40,000- or 46,000-molecular-weight C-terminal fragment (Table 3). Thus, the affinity studies revealed that the N-terminal part of the S-layer protein from B. stearothermophilus PV72/p6 involving a maximum of 227 amino acids is responsible for anchoring the S-layer subunits to the peptidoglycan-containing layer.
FIG. 3.
(a) SDS-PAGE pattern of the S-layer protein from B. stearothermophilus PV72/p6 after proteolytic degradation with endoproteinase Glu-C in presence of 2 M GHCl, dialysis against distilled water, and removal of insoluble material by centrifugation. Only two high-molecular-weight cleavage fragments with apparent molecular weights of 90,000 and 80,000 recognized native peptidoglycan-containing sacculi as binding sites and were detected in the pellet (lane b), while the lower-molecular-weight cleavage fragments remained in the clear supernatant (lane c). Protein bands which were subjected to N-terminal sequencing are indicated by arrow. Molecular weights are given in thousands.
TABLE 3.
Cleavage fragments of the S-layer protein SbsA from B. stearothermophilus PV72/p6 produced with endoproteinase Glu-C
| Fragment | Mr according to SDS-PAGE | N-terminal sequence | Position of first amino acid on the mature SbsA | Mr of missing N/C-terminal fragmenta |
|---|---|---|---|---|
| A | 90,000 | ATDVAT | N terminus | ------/40,000 |
| B | 80,000 | ATDVAT | N terminus | ------/50,000 |
| C | 66,000 | AALTPK | 228 | 24,000/40,000 |
| D | 60,000 | AALTPK | 228 | 24,000/46,000 |
Based on the apparent molecular weight of the SbsA of 130,000 determined by SDS-PAGE.
In the case of B. stearothermophilus ATCC 12980, proteolysis with endoproteinase Glu-C was carried out after suspension of S-layer self-assembly products in 50 mM Tris-HCl buffer (pH 7.8) and incubation for 4 h at 37°C. Several cleavage fragments which had retained the ability to bind to native peptidoglycan-containing sacculi revealed the same N-terminal region as the mature S-layer protein (ATDVATVVSQAKAQ). On SDS-gels, the apparent molecular weights of these cleavage fragments were determined to be 101,000, 86,000, 76,000, 68,000, 55,000, and 47,000 (not shown).
Investigation of the affinity between proteolytic cleavage fragments from the S-layer protein of B. stearothermophilus PV72/p6 and the isolated secondary cell wall polymer.
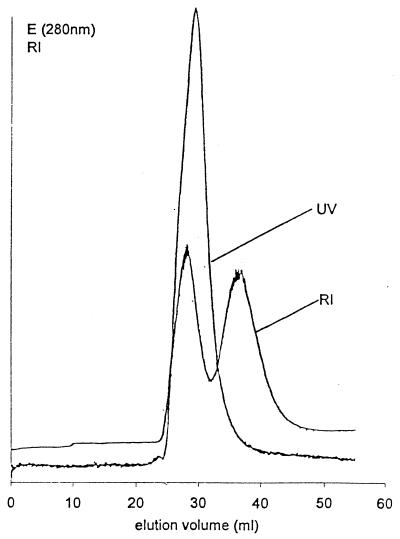
After addition of the isolated secondary cell wall polymer to the GHCl-extracted proteolytic cleavage fragments which had retained the ability to bind to native peptidoglycan-containing sacculi (Fig. 3, lane b), this mixture was dialyzed against buffer and applied to a Sephacryl S-200 HR column with a fractionation range of 5,000 to 250,000 for proteins. As shown in Fig. 4, the first peak strongly absorbed at 280 nm and gave a distinctly positive signal, with the RI detector indicating the common elution of the proteolytic cleavage fragments with the secondary cell wall polymer. On SDS-gels, both proteolytic cleavage fragments with apparent molecular weights of 90,000 and 80,000 were detected in comparable amounts in the fractions representing the first peak.
FIG. 4.
Elution profile of a mixture of proteolytic cleavage fragments from the S-layer protein from B. stearothermophilus PV72/p6 which had retained the affinity to bind to native peptidoglycan-containing sacculi (Fig. 3, lane b) and the isolated secondary cell wall polymer. Fractions representing the first peak contained comparable amounts of the proteolytic cleavage fragments with apparent molecular weights of 80,000 and 90,000 on SDS-gels. The second peak represented unbound secondary cell wall polymer.
In contrast to the first peak, the second peak eluted at a significantly lower molecular weight of about 50,000 and was recognized only by the RI detector (Fig. 4). Chemical analysis and comparison with the elution profile of the isolated secondary cell wall polymer confirmed that the second peak represented unbound cell wall polymer. As derived from the peak areas, approximately half of the amount of the added secondary cell wall polymer had bound to the two high-molecular-weight proteolytic cleavage fragments.
Self-assembly of the whole S-layer protein from B. stearothermophilus ATCC 12980 and of a proteolytic cleavage fragment.
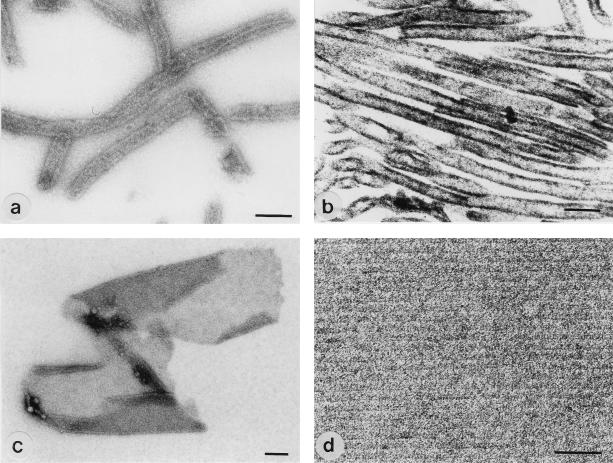
After disintegration of S-layer self-assembly products from B. stearothermophilus ATCC 12980 with 2 M GHCl, the clear solution was dialyzed against distilled water, 10 mM KCl, or 10 mM CaCl2 at 20°C. In negatively stained and ultrathin-sectioned preparations, monosheet cylinders with a diameter of about 100 nm could be observed. The oblique S-layer lattice was not visible or only poorly visible (Fig. 5a and b). When proteolysis of the S-layer protein from B. stearothermophilus ATCC 12980 was performed in presence of 2 M GHCl, a diffuse protein band with an apparent molecular weight ranging from 80,000 to 100,000 could be observed on SDS-gels (not shown). After dialysis against distilled water, mostly double-layer sheets with a maximum size of 3 μm and open-ended cylinders were obtained (Fig. 5c). In negatively stained preparations, sheets and cylinders consisting of the proteolytic cleavage fragments exhibited a highly ordered oblique lattice with lattice constants of a = 10.5 nm, b = 6.5 nm, and γ = 80° (Fig. 5d).
FIG. 5.
Negative-staining (a, c, and d) and ultrathin sectioning (b) of S-layer self-assembly products formed by the whole S-layer protein (a and b) and a proteolytic cleavage fragment of the S-layer protein (c and d) from B. stearothermophilus ATCC 12980. Bars: (a to c) 200 nm; (d) 50 nm.
Homology between the S-layer proteins from B. stearothermophilus PV72/p6 and ATCC 12980.
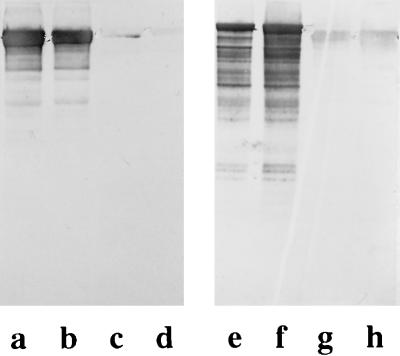
As shown by immunoblotting, polyclonal rabbit antiserum raised against the S-layer protein from either strain PV72/p6 or strain ATCC 12980 gave a strongly positive reaction with the respective type of S-layer protein but did not recognize the S-layer protein from the other wild-type strain (Fig. 6). Peptide mapping performed with endoproteinase Glu-C in the presence of 0.1% SDS led to a series of cleavage fragments with different molecular weights, but no protein bands common to the two S-layer proteins were obtained (Fig. 7, lanes a and b). After separation on SDS–6% gels, three cleavage fragments of the S-layer protein from B. stearothermophilus ATCC 12980 with apparent molecular weights of 25,000, 29,000, and 45,000 were subjected to N-terminal sequencing. The N-terminal region of the 29,000-molecular-weight fragment was ATDTNG, which could not be detected on the SbsA. The two other cleavage fragments had the same N-terminal region, TGQFP. Interestingly, a similar sequence (TGEFP) is located on the N-terminal region of the SbsA (18), starting with threonine in position 5q of the mature S-layer protein. In contrast to the S-layer protein from B. stearothermophilus PV72/p6, which did not cross-react with polyclonal rabbit antiserum raised against the S-layer protein from B. stearothermophilus ATCC 12980, four proteolytic cleavage fragments with apparent molecular weights from 27,000 to 30,000 gave a weakly positive reaction on immunoblots (Fig. 7, lane c). N-terminal sequencing of the three major protein bands (Fig. 7, lane a) revealed that they possess an N-terminal region identical to that of the mature S-layer protein. Thus, it could be demonstrated that except for the N terminus, the S-layer proteins from two B. stearothermophilus wild-type strains do not have extended structurally homologous domains.
FIG. 6.
Immunoblots demonstrating the specificity of polyclonal rabbit antiserum raised against the S-layer protein from B. stearothermophilus PV72/p6 and ATCC 12980. Lanes: a to d, anti-ATCC 12980 antiserum applied to whole cells from B. stearothermophilus ATCC 12980 (a and b) and B. stearothermophilus PV72/p6 (c and d); e to h, anti-PV72/p6-antiserum applied to whole cells from B. stearothermophilus PV72/p6 (e and f) and B. stearothermophilus ATCC 12980 (g and h). Dilutions of antisera: 1:5,000 in lanes a, c, f, and h and 1:10,000 in lanes b, d, e, and g.
FIG. 7.
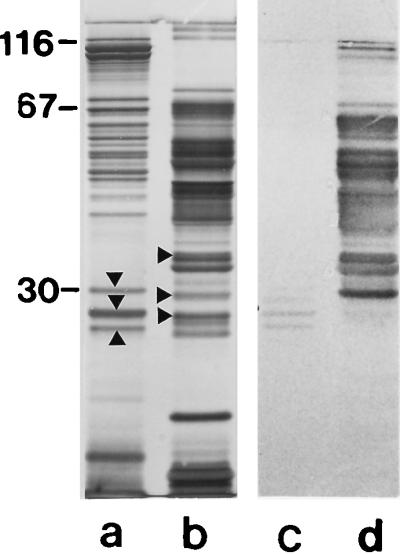
Lanes a and b, SDS-PAGE patterns of proteolytic cleavage fragments of the S-layer proteins from B. stearothermophilus PV72/p6 and ATCC 12980, respectively. Proteolytic cleavage fragments used for N-terminal sequencing are indicated by arrowheads. Lanes c and d, immunoblots obtained by applying the anti-ATCC 12980 antiserum to the proteolytically degraded S-layer proteins from B. stearothermophilus PV72/p6 and ATCC 12980, respectively. Four cleavage fragments of the S-layer protein from B. stearothermophilus PV72/p6 gave a weakly positive reaction with the anti-ATCC 12980 antiserum on immunoblots (lane c).
DISCUSSION
In this study, the S-layer protein and the secondary cell wall polymer from two B. stearothermophilus strains were investigated regarding a common recognition mechanism between both cell envelope components. With exception of the N terminus, no structurally homologous domains could be identified on the S-layer proteins, while a secondary cell wall polymer of identical chemical composition and molecular weight was detected in peptidoglycan-containing sacculi of both organisms. After complete extraction of the secondary cell wall polymer, the S-layer subunits had lost the ability to bind to the peptidoglycan sacculus which according to chemical analysis represented peptidoglycan of the A1γ chemotype (31). Moreover, only proteolytic cleavage fragments possessing the complete N-terminal region had retained the affinity to bind to native peptidoglycan-containing sacculi or could associate with the isolated secondary cell wall polymer. Recently, a similar binding mechanism was reported for the oxygen-induced variant strain B. stearothermophilus PV72/p2 (27). The incubation of proteolytic S-layer cleavage fragments with native peptidoglycan-containing sacculi confirmed that the S-layer protein is bound via its N-terminal region to a secondary cell wall polymer (27).
The S-layer protein from the B. stearothermophilus PV72/p6 wild-type strain and the S-layer protein from the oxygen-induced p2 variant are encoded by different genes (16, 17) and have different N-terminal regions, and only the latter possesses a typical S-layer homologous (SLH) domain at the N-terminus (16).
In general, SLH domains were identified at the N-termini of several S-layer proteins or at the very C-terminal ends of cell-associated exoproteins and exoenzymes. SLH domains were suggested to anchor these proteins permanently or transiently to the peptidoglycan (9, 20, 22–24, 26). Despite the absence of a typical SLH domain, the S-layer protein from B. stearothermophilus PV72/p6 recognized native peptidoglycan-containing sacculi from the p2 variant as binding sites but not vice versa (28). In addition to the change in S-layer gene expression occurring during oxygen-induced variant formation, synthesis of a secondary cell wall polymer of different chemical composition was induced (28). Since the switch in S-layer protein synthesis is irreversible and was observed in only one direction, the S-layer protein from the B. stearothermophilus PV72/p6 wild-type strain remained bound to the whole cells even during the phase of variant formation (28, 34), guaranteeing complete coverage of the bacterial cell surface with an S-layer lattice. According to these findings, the S-layer protein from B. stearothermophilus ATCC 12980 recognized peptidoglycan-containing sacculi of the p2 variant as binding sites but not vice versa (data not shown).
Chemical analyses revealed that the secondary cell wall polymers from both B. stearothermophilus wild-type strains contain glucose and glucosamine in a molar ratio of 1 to 1. Preliminary nuclear magnetic resonance studies confirmed that glucosamine is quantitatively N-acetylated. Since the secondary cell wall polymer could be extracted with HF, the polymer chains are most probably attached via phosphodiester bonds to the C-6 of muramic acid, which is the commonly observed linkage type between teichoic or teichuronic acids and the peptidoglycan backbone (3). As demonstrated by gel permeation chromatography and RP-HPLC, the secondary cell wall polymers from B. stearothermophilus PV72/p6 and the oxygen-induced p2 variant (27) eluted as a single homogeneous peak. In contrast to the usual assumption that secondary cell wall polymers are polydisperse, more detailed studies on their biosynthesis revealed that the polymer chains are fairly homogeneous in length (10) and do not vary under different growth conditions (10, 19). Moreover, the chain length of teichoic or teichuronic acids was frequently underestimated since hydrolysis and degradation had occurred under the acidic extraction conditions (3, 38, 39). As demonstrated in this study, the homogeneity of the secondary cell wall polymer from B. stearothermophilus strains was strongly dependent on growing the organisms in continuous culture at constant specific growth rate.
In contrast to the typical teichoic or teichuronic acids which contain large amounts of phosphate or uronic acids (3), the secondary cell wall polymers from B. stearothermophilus wild-type strains and the oxygen-induced p2 variant (27) possess considerable amounts of N-acetylated amino sugars. Interestingly, a secondary cell wall polymer composed of Gal, GlcNAc, and ManNAc in a molar ratio of 3 to 2 to 1 was detected in the cell envelope of B. anthracis (8), an S-layer-carrying organism, while the teichuronic acids of B. subtilis, B. megaterium, and B. licheniformis, which represent S-layer-deficient species (35), contained Rha, Glc, GlcNAc, ManNAc, GalNAc, and GlcA (21, 37, 38). Two strongly negatively charged cell wall polymers were extracted from the cell envelope of the alkalophilic Bacillus sp. (2). Moreover, it could be demonstrated that teichuronic acids are constitutive in B. megaterium and B. cereus and are synthesized even under conditions of excess phosphate (39). According to these findings, the chemical composition of peptidoglycan-containing sacculi from B. stearothermophilus PV72/p6 did not depend on the growth conditions in continuous culture and was the same under carbon, oxygen, nitrogen, and phosphate limitation (33). As shown in the present study, the compositions of the secondary cell wall polymers from two B. stearothermophilus wild-type strains were identical despite the use of synthetic or complex growth media.
Most of the biological functions ascribed to teichoic or teichuronic acids such as binding of bivalent cations or keeping the peptidoglycan sacculus in an expanded state by charge repulsion as well as binding of protons to create an acidic cell wall during bacterial growth are associated with their acidic nature (3). So far, specific interactions between a secondary cell wall polymer and a protein have been reported for the teichoic acids and the cell wall autolysin in B. subtilis (12, 13). To our knowledge, the secondary cell wall polymers from B. stearothermophilus are the first identified to function as specific binding sites for S-layer proteins (27).
This study clearly demonstrated that a common recognition mechanism exists between the N termini of the S-layer proteins and the secondary cell wall polymers from two B. stearothermophilus wild-type strains. The structural homology of the S-layer proteins is limited to the N terminus, which seems to be conserved within the species (7, 28, 29) and anchors the S-layer subunits to the underlying cell envelope layer. The location of the structurally nonrelated protein domains on the outer face of the S-layer lattice most probably leads to quite diverse cell surface properties even among closely related strains of the same species.
ACKNOWLEDGMENTS
This work was supported by the Austrian Science Foundation, project S72/02, and by the Ministry of Science and Transports.
We thank Harald F. Mayer for continuous cultivations and Thomas Dalik for amino acid analyses.
REFERENCES
- 1.Altmann F. Determination of amino sugars and amino acids in glycoconjugates using precolumn derivatization with o-phthalaldehyde. Anal Biochem. 1992;204:215–219. doi: 10.1016/0003-2697(92)90164-3. [DOI] [PubMed] [Google Scholar]
- 2.Aono R. Characterization of cell wall components of the alkalophilic Bacillus strain C-125: identification of a polymer composed of polyglutamate and poly-glucuronate. J Gen Microbiol. 1989;135:265–271. [Google Scholar]
- 3.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis, and turnover. In: Sonenshein A, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. N.Y., N.Y: Academic Press; 1993. pp. 381–410. [Google Scholar]
- 4.Beveridge T J. Bacterial S-layers. Curr Opin Struct Biol. 1994;4:204–212. [Google Scholar]
- 5.Bock K, Schuster-Kolbe J, Altman E, Stahl B, Christian R, Sleytr U B, Messner P. Primary structure of the O-glycosidically linked glycan chain of the crystalline surface layer glycoprotein of Thermoanaerobacter thermohydrosulfuricus L111-69. Galactosyl tyrosine as a novel linage unit. J Biol Chem. 1994;269:7137–7144. [PubMed] [Google Scholar]
- 6.Bowditch R D, Baumann P, Yousten A. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989;171:478–488. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egelseer E M, Schocher I, Sleytr U B, Sára M. Evidence that an N-terminal S-layer protein fragment triggers the release of a cell-associated high-molecular-weight amylase in Bacillus stearothermophilus ATCC 12980. J Bacteriol. 1996;178:5602–5609. doi: 10.1128/jb.178.19.5602-5609.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekwunife F S, Singh, J. J, Taylor K G, Doyle R J. Isolation and purification of a cell wall polysaccharide of Bacillus anthracis. FEMS Microbiol Lett. 1991;82:257–262. doi: 10.1016/0378-1097(91)90270-k. [DOI] [PubMed] [Google Scholar]
- 9.Etienne-Toumelin I, Sirard J-C, Duflot E, Mock M, Fouet A. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J Bacteriol. 1995;177:614–620. doi: 10.1128/jb.177.3.614-620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiedler F, Glaser L. The synthesis of poly(ribitolphosphate). II. On the mechanisms of poly(ribitolphosphate) polymerase. J Biol Chem. 1974;249:2690–2695. [PubMed] [Google Scholar]
- 11.Hastie A T, Brinton C C., Jr Specific interaction of the tetragonally arrayed protein layer of Bacillus sphaericus with its peptidoglycan sacculus. J Bacteriol. 1979;138:1010–1021. doi: 10.1128/jb.138.3.1010-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbold D R, Glaser L. Bacillus subtilis N-acetylmuramic acid l-alanine amidase. J Biol Chem. 1975;250:1676–1680. [PubMed] [Google Scholar]
- 13.Herbold D R, Glaser L. Interaction of N-acetylmuramic acid l-alanine amidase with cell wall polymers. J Biol Chem. 1975;250:7231–7238. [PubMed] [Google Scholar]
- 14.Jackson P. Polyacrylamide gel electrophoresis of reducing saccharides labeled with the fluorophore 2-aminoacridone: subpicomolar detection using an imaging system based on a cooled charge-coupled device. Anal Biochem. 1991;196:238–244. doi: 10.1016/0003-2697(91)90460-b. [DOI] [PubMed] [Google Scholar]
- 15.Jürgens U J, Weckesser J. Polysaccharide covalently linked to the peptidoglycan of the cyanobacterium Synechocystis sp. strain PCC6714. J Bacteriol. 1986;168:568–573. doi: 10.1128/jb.168.2.568-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuen B, Koch A, Asenbauer E, Sára M, Lubitz W. Molecular characterization of the second S-layer gene sbsB of Bacillus stearothermophilus PV72 expressed by oxidative stress. J Bacteriol. 1997;179:1664–1670. doi: 10.1128/jb.179.5.1664-1670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuen B, Sára M, Lubitz W. Heterologous expression and self-assembly of the S-layer protein SbsA of Bacillus stearothermophilus in Escherichia coli. Mol Microbiol. 1996;19:495–503. doi: 10.1046/j.1365-2958.1996.386918.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuen B, Sleytr U B, Lubitz W. Sequence analysis of the sbsA gene encoding the 130 kDa surface layer protein of Bacillus stearothermophilus PV72. Gene. 1994;145:115–120. doi: 10.1016/0378-1119(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 19.Lang W K, Archibald A R. Length of teichoic acid chains incorporated into walls of Bacillus subtilis grown under conditions of different phosphate supply. FEMS Microbiol Lett. 1982;13:93–97. [Google Scholar]
- 20.Lemaire M, Ohayon H, Gounon P, Fujino T, Beguin P. OlpB, a new outer layer protein of Clostridium thermocellum, and binding of its S-layer-like domains to components of the cell envelope. J Bacteriol. 1995;177:2451–2459. doi: 10.1128/jb.177.9.2451-2459.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lifely M R, Tarelli E, Baddiley J. The teichuronic acid from walls of Bacillus licheniformis ATCC 9945. Biochem J. 1980;191:305–318. doi: 10.1042/bj1910305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupas A, Engelhardt H, Peters J, Santarius U, Volker S, Baumeister W. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J Bacteriol. 1994;176:1224–1233. doi: 10.1128/jb.176.5.1224-1233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matuschek M, Burchhardt G, Sahm K, Bahl H. Pullulanase of Thermoanaerobacterium thermosulfurigenes EM1 (Clostridium thermosulfurogenes): molecular analysis of the gene, composite structure of the enzyme, and a common model for its attachment to the cell surface. J Bacteriol. 1994;176:3295–3302. doi: 10.1128/jb.176.11.3295-3302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matuschek M, Sahm K, Zibat A, Bahl H. Characterization of genes from Thermoanaerobacterium thermosulfurigenes EM1 that encode two glycosyl hydrolases with conserved S-layer like domains. Mol Gen Genet. 1996;252:493–496. doi: 10.1007/BF02173016. [DOI] [PubMed] [Google Scholar]
- 25.Messner P, Hollaus F, Sleytr U B. Paracrystalline cell wall surface layers of different Bacillus stearothermophilus strains. Int J Syst Bacteriol. 1984;34:202–210. [Google Scholar]
- 26.Olabarría G, Carrascosa J L, de Pedro M A, Berenguer J. A conserved motif in S-layer proteins is involved in peptidoglycan binding in Thermus thermophilus. J Bacteriol. 1996;178:4765–4722. doi: 10.1128/jb.178.16.4765-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ries W, Hotzy C, Schocher I, Sleytr U B, Sára M. Evidence that the N-terminal part of the S-layer protein of Bacillus stearothermophilus PV72/p2 recognizes a secondary cell wall polymer. J Bacteriol. 1997;179:3892–3898. doi: 10.1128/jb.179.12.3892-3898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sára M, Kuen B, Mayer H, Mandl F, Schuster K C, Sleytr U B. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and the S-layer-deficient variant T5 in continuous culture and studies of the cell wall composition. J Bacteriol. 1996;178:2108–2117. doi: 10.1128/jb.178.7.2108-2117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sára M, Pum D, Küpcü S, Messner P, Sleytr U B. Isolation of two physiologically induced variant strains of Bacillus stearothermophilus NRS 2004/3a and characterization of their S-layer lattices. J Bacteriol. 1994;176:848–860. doi: 10.1128/jb.176.3.848-860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sára M, Sleytr U B. Comparative studies on S-layer proteins from Bacillus stearothermophilus strains expressed during growth in continuous culture under oxygen-limited and non-oxygen-limited growth conditions. J Bacteriol. 1994;176:7182–7189. doi: 10.1128/jb.176.23.7182-7189.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleifer K H, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholz H, Kuen B, Lubitz W, Sára M. S-layer variation in Bacillus stearothermophilus PV72. FEMS Microbiol Rev. 1997;20:69–78. [Google Scholar]
- 33.Schuster K C, Mayer H F, Kieweg R, Hampel W A, Sára M. A synthetic medium for continuous culture of the S-layer carrying Bacillus stearothermophilus PV72 and studies on the influence of growth conditions on cell wall properties. Biotechnol Bioeng. 1995;48:66–77. doi: 10.1002/bit.260480110. [DOI] [PubMed] [Google Scholar]
- 34.Schuster K C, Pink T, Mayer H F, Sára M. Oxygen-triggered synchronisized variant formation of the S-layer carrying Bacillus stearothermophilus PV72 during continuous cultivation. J Biotechnol. 1997;54:15–28. [Google Scholar]
- 35.Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface proteins. Austin, Tex: Landes Company, Academic Press; 1996. [Google Scholar]
- 36.Sleytr U B, Sára M, Küpcü Z, Messner P. Structural and chemical characterization of S-layers of selected strains of Bacillus stearothermophilus and Desulfotomaculum nigrificans. Arch Microbiol. 1986;146:19–24. doi: 10.1007/BF00690152. [DOI] [PubMed] [Google Scholar]
- 37.Wright J, Heckels J E. The teichuronic acid of Bacillus subtilis W23 grown in a chemostat under phosphate limitation. Biochem J. 1975;147:186–189. doi: 10.1042/bj1470187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoneyama Y, Araki Y, Ito E. The primary structure of teichuronic acid in Bacillus subtilis AHU 1031. Eur J Biochem. 1984;141:83–89. doi: 10.1111/j.1432-1033.1984.tb08160.x. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama Y, Koike Y, Araki Y, Arakawa H, Yokohama K, Sasaki Y, Kawamura T, Ito E, Takao S. Distribution of mannosamine and mannosaminuronic acid among cell walls of Bacillus species. J Bacteriol. 1982;149:15–21. doi: 10.1128/jb.149.1.15-21.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]