Abstract
Previous research has demonstrated hemisphere-specific motor deficits in ipsilesional and contralesional unimanual movements in patients with hemiparetic stroke due to MCA infarct. Due to the importance of bilateral motor actions on activities of daily living, we now examine how bilateral coordination may be differentially affected by right or left hemisphere stroke. To avoid the caveat of simply adding unimanual deficits in assessing bimanual coordination, we designed a unique task that requires spatiotemporal coordination features that do not exist in unimanual movements. Participants with unilateral left (LHD) or right hemisphere damage (RHD) and age-matched controls moved a virtual rectangle (bar) from a midline start position to a midline target. Movement along the long axis of the bar was redundant to the task, such that the bar remained in the center of and parallel to an imaginary line connecting each hand. Thus, to maintain midline position of the bar, movements of one hand closer to or further away from the bar midline required simultaneous, but oppositely directed displacements with the other hand. Our findings indicate that left (LHD), but not right (RHD) hemisphere-damaged patients showed poor interlimb coordination, reflected by significantly lower correlations between displacements of each hand along the bar axis. These left hemisphere-specific deficits were only apparent prior to peak velocity, likely reflecting predictive control of interlimb coordination. In contrast, the RHD group bilateral coordination was not significantly different than that of the control group. We conclude that predictive mechanisms that govern bilateral coordination are dependent on left hemisphere mechanisms. These findings indicate that assessment and training in cooperative bimanual tasks should be considered as part of an intervention framework for post-stroke physical rehabilitation.
Keywords: Stroke, Bimanual, Brain Lateralization
Introduction
Most daily self-care, leisure, and work activities require bilateral coordination of both hands, such as when stabilizing a jar with one hand while opening the lid with the other. Because of this, recovery of function following unilateral deficits, such as hemiparetic stroke, requires changes in intralimb control, but also requires improvements in the ability to coordinate the use of both hands together (McCombe Waller and Whitall 2008; Sainburg et al. 2013). In fact, Whitall and colleagues have argued that physical rehabilitation should address bilateral coordination because unimanual training alone will not specifically address the unique neural control mechanisms associated with bilateral control, a view supported by research findings (McCombe Waller and Whitall 2008; Sainburg et al. 2013). It is well established that bilateral coordination recruits neural circuits that are not recruited during unimanual movements alone (Brinkman 1984; Debaere et al. 2001; Donchin et al. 1998; Jäncke et al. 2000; Sadato et al. 1997). The supplementary motor area has been one of the areas pointed out as having a specialized role in bimanual movements (Sadato et al. 1997; Jäncke et al. 1998, 2000; Debaere et al. 2001). Neurons in the primary motor cortex have also showed bimanual-specific activations (Donchin et al. 1998).
Previous research has demonstrated asymmetry in performance of bilateral movements (Swinnen et al. 1996; Johansson et al. 2006; Dounskaia et al. 2010; Kagerer 2016), but it is not clear whether this asymmetry results from lateralization of unimanual control mechanisms, or whether bilateral coordination itself is lateralized. Woytowicz et al. (2018, 2020) have shown in healthy young and older adults that the performance of cooperative bilateral tasks is performed asymmetrically, such that the dominant arm is advantaged for trajectory control, and the non-dominant arm for stabilizing against mechanical interactions arising between the hands during manipulation. This distribution of control features is consistent with the complimentary dominance hypothesis hypothesized by Sainburg and Colleagues, in which the dominant hemisphere is specialized for predictive control of trajectories, and the non-dominant hemisphere for control of limb impedance (Sainburg 2002; Yadav and Sainburg 2014). Previous studies in stroke patients have supported the role of each hemisphere in these two aspects of control, demonstrating hemisphere-specific movement deficits in both the ipsilesional (Schaefer et al. 2009a, 2012) and contralesional arms (Mani et al. 2013) of stroke patients following unilateral middle cerebral artery infarct. It is plausible that previously reported asymmetries in bilateral coordination may simply result from these hemispheric specializations for unimanual movements of each limb. However, previous fMRI studies of bimanual tasks have suggested lateralization in bimanual coordination, itself. For example, Zhuang et al. (2005) reported asymmetries in left and right primary motor cortex during movements requiring bilateral coordination, and Jäncke et al. (2000) reported that the left supplementary motor area showed greater activation than its right hemisphere counterpart in bimanual tasks.
Given previous evidence of hemisphere-specific deficits in unimanual movements and of fMRI-recorded brain activations during bimanual movements, we now hypothesize hemispheric asymmetry in controlling the distinct coordination requirements of bilateral movements of the upper limbs. To test this hypothesis, we designed a task that requires spatiotemporal coordination features between the hands that do not exist in unimanual movements. Participants with unilateral left (LHD) or right hemisphere damage (RHD) and age-matched controls moved a virtual bar (rectangle) from a start position to a target, and were required to maintain the bar in midline. Movement along the long axis of the bar was redundant to the task, such that the bar remained in the center of and parallel to an imaginary line connecting each hand. Thus, to maintain the horizontal position of the bar, movements of one hand closer to or further away from the bar midline required simultaneous, but oppositely directed displacements with the other hand. We predict that spatiotemporal coordination between the hands early in movement, determined by predictive mechanisms should be disrupted by left but not right hemisphere damage. In contrast, we predict that lesions to right hemisphere should disrupt coordination during the stabilization phase of reaching movements. It should be stressed that we are not simply predicting that the deficits previously shown for unilateral movements should be expressed during bimanual movements, but rather that hemisphere-specific deficits in bimanual coordination will occur.
Materials and methods
Participants
A total of 24 subjects participated in this experiment (8 controls, 8 left hemisphere damage, 8 right hemisphere damage), after providing informed consent, which was approved by the institutional review board of Penn State University. All groups were matched for age (Control: 65.38 ± 10.16, LHD: 63.25 ± 13.47, and RHD 63.22 ± 9.82), and all stroke participants were classified as having mild impairment by the Fugl–Meyer scale (> 45). Fugl–Meyer scores for the RHD group were 58.7 ± 5.9, and 62.0 ± 5.9 for LHD. All control participants self-reported being right-handed, and stroke participants reported being right-handed prior to stroke. We restricted our inclusion to participants with primarily cortical stroke with mild to moderate deficits, which allowed us to compare intrahemispheric lesion location and size between groups, to ensure that differences in findings were due to hemisphere that was damaged, and not gross differences in intrahemispheric lesion location, including differences in subcortical involvement. This is also consistent with the previously published studies, allowing for direct comparison of the hemisphere-specific unimanual deficits found in those studies (Schaefer et al. 2012; Mani et al. 2014; Stewart et al. 2019; Buxbaum et al. 2020).
Out of the 16 stroke patients, we were able to obtain MRI brain images for 13 participants (6 LHD, 7 RHD), which are shown in Fig. 1. Three patients were unable to participate in structural MRI procedures due to medical contraindications. The origins of the brain images were reoriented to the anterior commissure using Statistical Parametric Mapping (SPM12) software (Friston 1995). Brain lesions were then manually traced by a trained technician on T2-weighted brain images using MRIcron software (Rorden and Brett 2000) and reviewed with a neurologist. The T2 scan was co-registered to the space of the T1 scan, then brain images and corresponding lesion maps were transformed onto a brain template based on older adults using the MR-segment-normalize algorithm of the Clinical Toolbox in SPM12 (Rorden et al. 2012). The volumes of the resulting normalized lesion maps were then analyzed with non-parametric mapping software (MRIcron) and compared between groups using a non-parametric Wilcoxon signed-rank test. There was no significant difference between groups in lesion volume (p = .1336), although there was some variation in lesion location, as shown in Fig. 1.
Fig. 1.
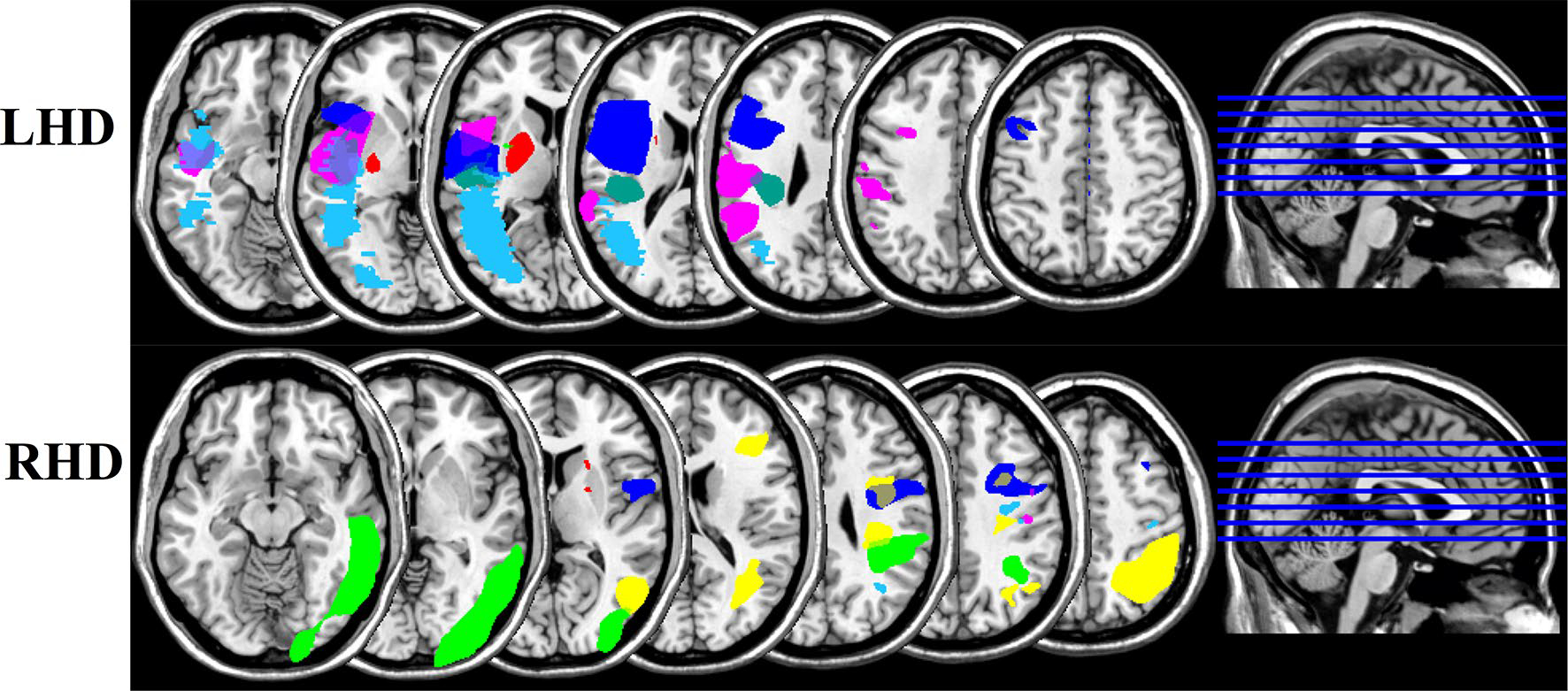
Overlap images showing lesion locations for the left hemisphere damaged group (LHD) and right hemisphere damaged group (RHD)
Experimental setup
Participants were seated at a 2D virtual–reality workspace in which stimuli from a TV screen were reflected by a mirror, with the participants’ arms under the mirror. Figure 2 shows this experimental set-up. Participants’ arm movements were tracked using 6 DOF magnetic sensors (Ascension TrackStar) placed on the hand and upper arm. All joints distal to the forearm were splinted. We digitized the location of the tip of the index finger, as well as multiple locations on the hand, and upper arm, and used custom software to estimate the locations of the wrist, elbow, and shoulder joints, relative to these digitized landmarks. Vision of the participants’ arms was occluded, while position of the index finger was provided as a cursor on the screen. Participants’ arms were supported on air sleds that reduced the effects of friction, and eliminated gravitational torques at the joints. The experimental session consisted of 200 total bimanual movements. The task was similar to the cooperative transport task employed by Sainburg et al. (2013) in which the left and right hands control a shared virtual object located halfway between each hand. Instead of a single cursor, this task represented the shared virtual object as a rectangular bar on the screen, with each hand controlling one end. Participants were first required to “grab” the virtual bar (20 cm across) by moving cursors representing the position of each hand to each end of the bar. Next, participants moved the bar into the start position, with each cursor placed into small start circles. Once in the start position, after 100 ms, participants were given an auditory start signal and the cursors disappeared, giving participants visual feedback of only the bar. The task required participants to move the bar with both hands quickly to two targets that were 25 cm away from the start position. Accuracy required the participants to move the bar not only to the correct distance, but also to stop with the bar horizontally oriented so that each end of the bar was in its respective target. As shown in Fig. 2, movement along the long axis of the bar was redundant, allowing the hands to move outside of the bar once the trial began, and requiring covariation of each hand to stabilize the location of the bar along the x-axis.
Fig. 2.
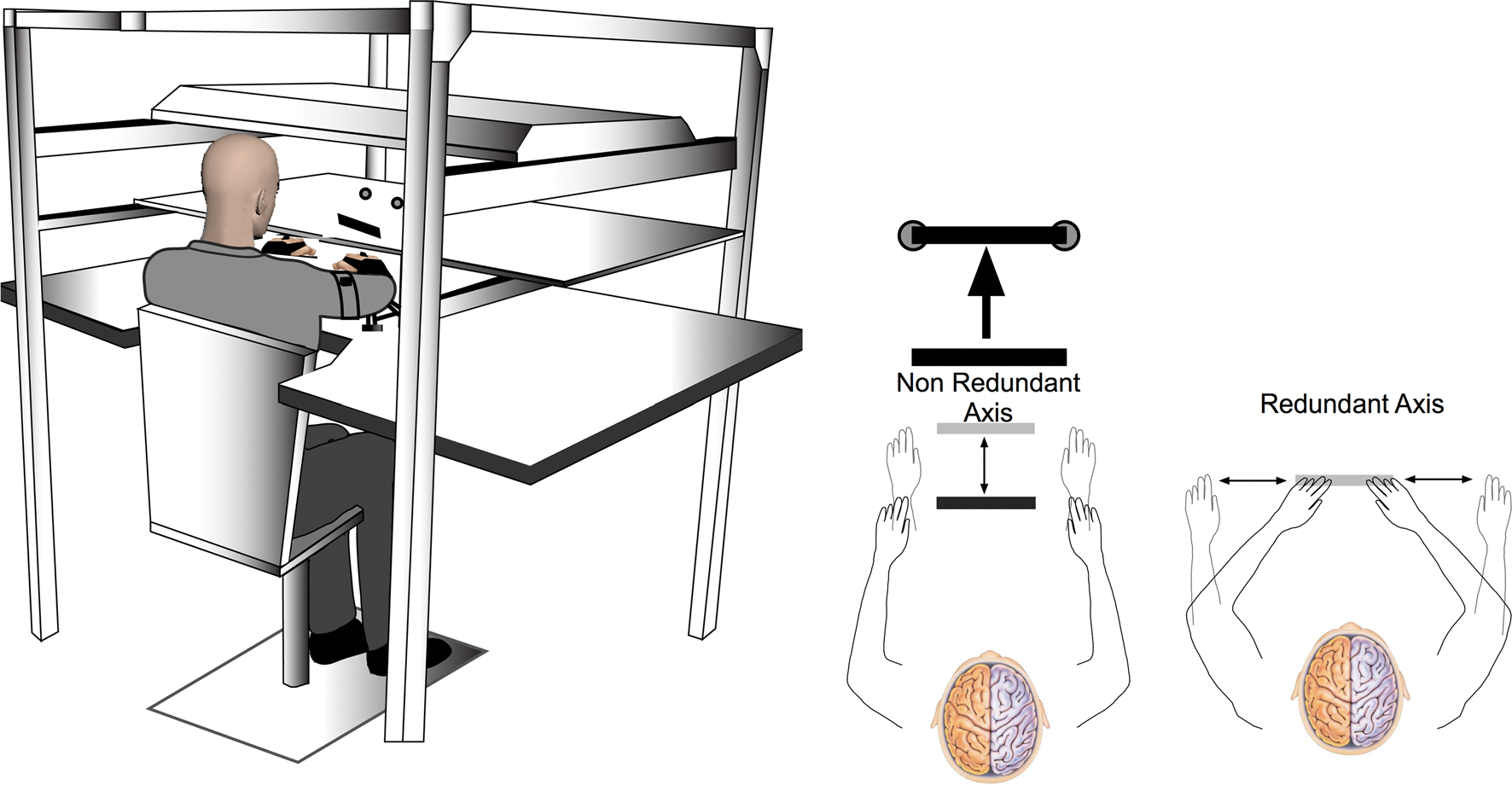
Experimental Set-up- A TV screen positioned above a mirror, creating a 2-D virtual reality workspace is shown on the left. Pictures on the right show how the participants were required to move the virtual bar
Kinematic analysis
We calculated arm segment positions and angles from digitized locations relative to the trackstar 6-DOF sensors. Data were collected from each sensor at 116 Hz. We digitized multiple positions on the hand, wrist and upper arm. Using custom software, we calculated 10 degrees of freedom per arm; however, because this task was restricted to the horizontal plane by air sled support, and all joints distal to the forearm were splinted, we analyzed only planar motion of the hand, as well as horizontal flexion/extension of the shoulder and elbow joint flexion/extension. All kinematic data were low-pass filtered at 8 Hz (3rd-order, dual-pass Butterworth) and differentiated to yield velocity and acceleration. Movement start was determined by identifying the time of peak velocity and searching backward in time for the first minimum below 8% of peak tangential velocity, or for zero velocity, whichever was identified first. Movement end was similarly determined by searching forward in time from peak velocity to find the first minimum below 8% of peak tangential velocity, thereby excluding any small, corrective submovements. Data from all subjects were de-identified and analysis was done by researchers who did not participate in data collection.
Statistical analysis
Most dependent measures were analyzed for differences between each group (LHD, RHD, Control) using a one-way ANOVA. Post hoc analysis (Tukey HSD) was used when warranted to compare the means of every treatment to the means of every other treatment; that is, it applies simultaneously to the set of all pairwise comparisons. Our use of the Tukey HSD controls for the family-wise Type 1 error rate and allows for pairwise comparisons of multiple groups (Barnette and McLean 2005). To test interlimb coordination, we calculated linear correlations of left-hand vs right-hand movement along the redundant x-axis of the bar within each trial. These correlations were separated into three phases to reflect different aspects of control: Phase 1, from movement start to peak velocity; Phase 2, from peak velocity to end of movement; and Phase 3, from the movement end to the end of the trial. We then performed pairwise comparisons of group mean slopes and correlation coefficients in each phase using the Steel–Dwass test, which is a non-parametric test that effectively controls for Type 1 errors associated with multiple comparisons of data that is not normally distributed (Dolgun and Demirhan 2017). We also checked that the number of data points in each trial was similar between groups, since systematic differences in the amount of data between groups could produce artifactual group differences in regression analysis, and confirmed that the number of data points between groups was not significantly different (F(2,21) = .6656, p = .5245).The alpha level for all statistics was set at 0.05, and only p values less than or equal to 0.1 were reported.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Stabilizing performance across trials
Participants were asked to quickly move the virtual bar with both hands along the anteroposterior axis and stop the bar with the sides of the bar inside the two targets. A successful trial required the participants to reach the targets with the bar horizontally oriented. Participants were also allowed to move along the axis of the bar, although this movement must be correlated between the left and right hands to limit bar deviation in the x-direction. As shown in Fig. 3, there were interesting differences in how the three groups moved along the redundant axis. Figure 3a shows a visual representation of how far the hands deviated from each end of the bar at the end of movement for each group on average, as well as how much the bar deviated from the center. Figure 3b shows the mean hand deviation along the axis of the bar at movement end. Our one-way ANOVA revealed no significant difference between the three groups (F(2,21) = 3.1022, p = .066); however, there was a trend for the LHD group to move less along this redundant axis. Crucial to the success of the task was the ability to coordinate the redundant movement between the hands to limit bar deviation. Figure 4 shows the correlation of left- and right-hand deviation along the bar axis with bar deviation across subjects for each of the three groups. For controls and RHD, there was very little correlation between movement of the hand along the bar axis and deviation of the bar, showing that they could move their hands along the bar without having much effect on the x-position of the bar and task error. The LHD group, however, showed a strong correlation of left- and right-hand movement with bar deviation, such that subjects who moved along the redundant axis of the bar more showed more bar deviation. This indicates that control and RHD participants coordinated their hands along the bar axis, to reduce task error; while, failure to do so led to task errors that were dependent on deviations of the hands along the bar axis in LHD patients.
Fig. 3.
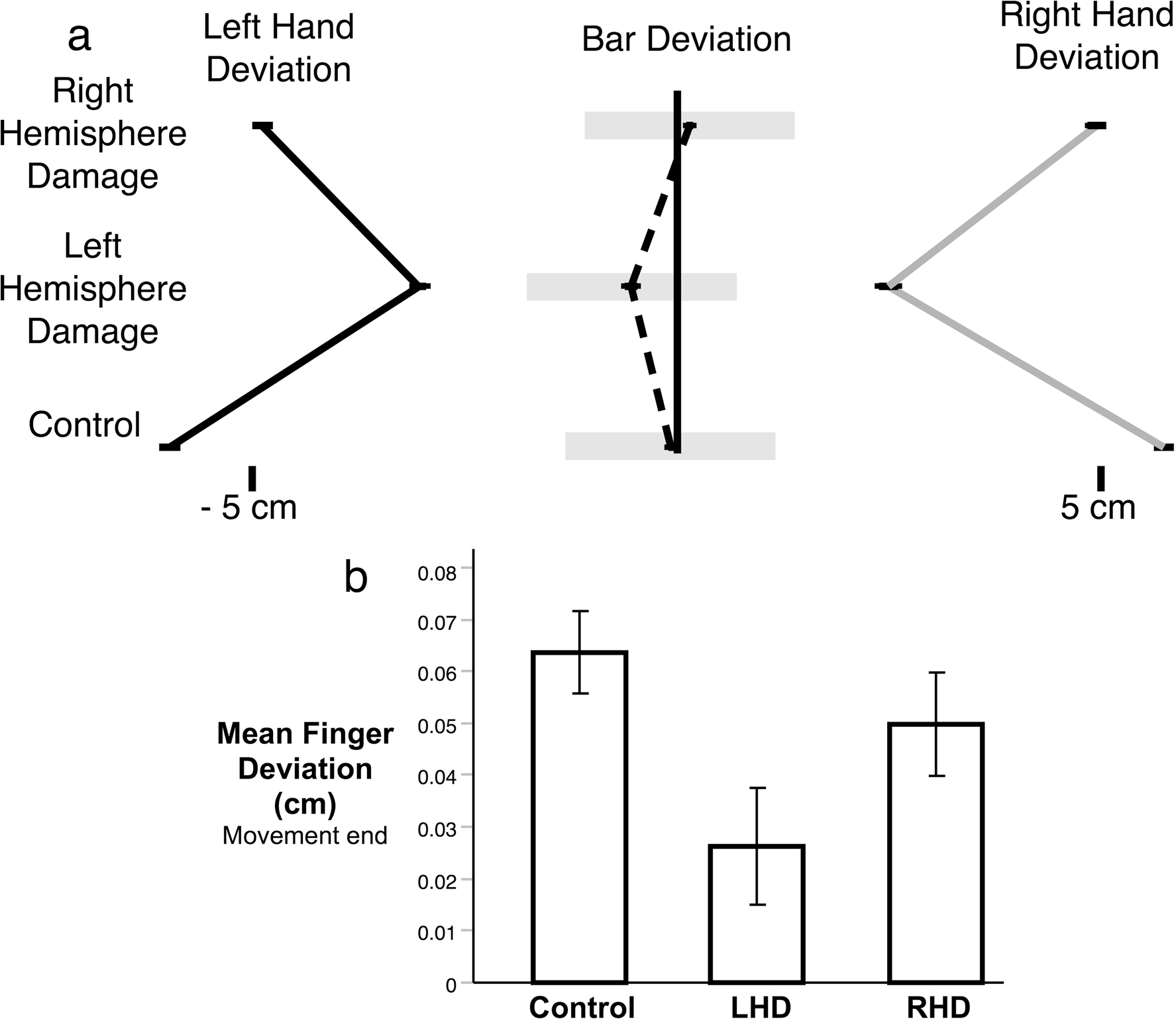
a) Scaled representation of the mean deviation of the left hand, right hand, and bar center along the x-axis of the bar for each group. b) Bar graph shows the mean deviation anlong the x-axis of the bar for each group at the end of movement
Fig. 4.
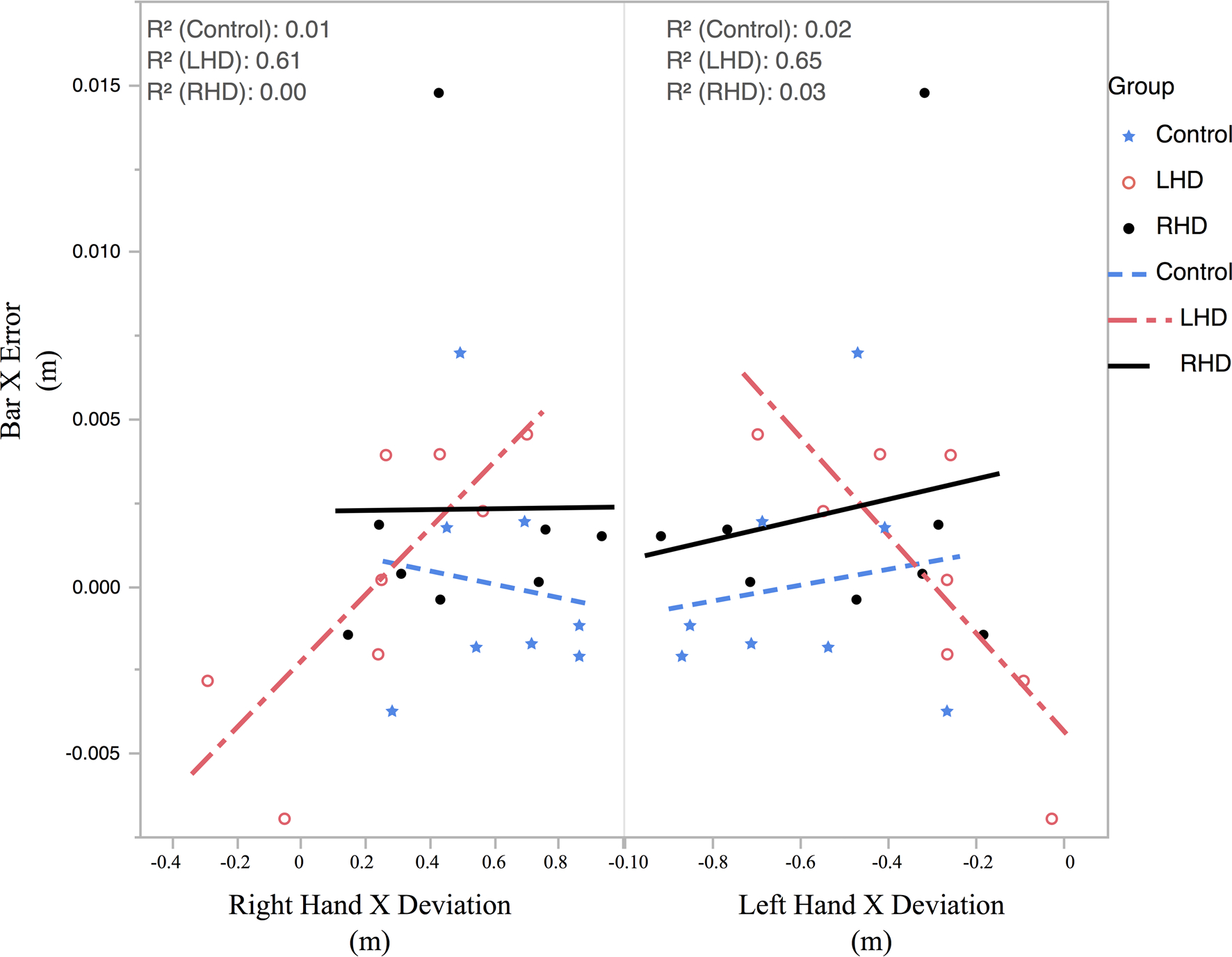
Correlations of left and right hand deviation along the bar axis with deviation of the bar center with Control in blue, LHD in red, and RHD in black. Each point represents mean of one participant
Figure 5 shows the correlation between the redundant axis (bar axis) movement between the right and left hands, for all trials of all subjects, separated by group. The graphs show the overall deviation at the end of movement for the right hand vs the left hand with each point representing a single trial and a gray circle showing the 95% confidence interval. The R-squared for the linear correlations between hands for each group is also shown. Although the Control group showed the most deviation, the deviation was also the most highly correlated between the hands with an R2 = .916. The RHD group movements were slightly less correlated than those of the Control group (R2 = .846), while the LHD group showed the lowest correlation (R2 = .569). Taken together, Figs. 4 and 5 show that the LHD subjects limit their deviation in the redundant axis, resulting in greater task errors.
Fig. 5.
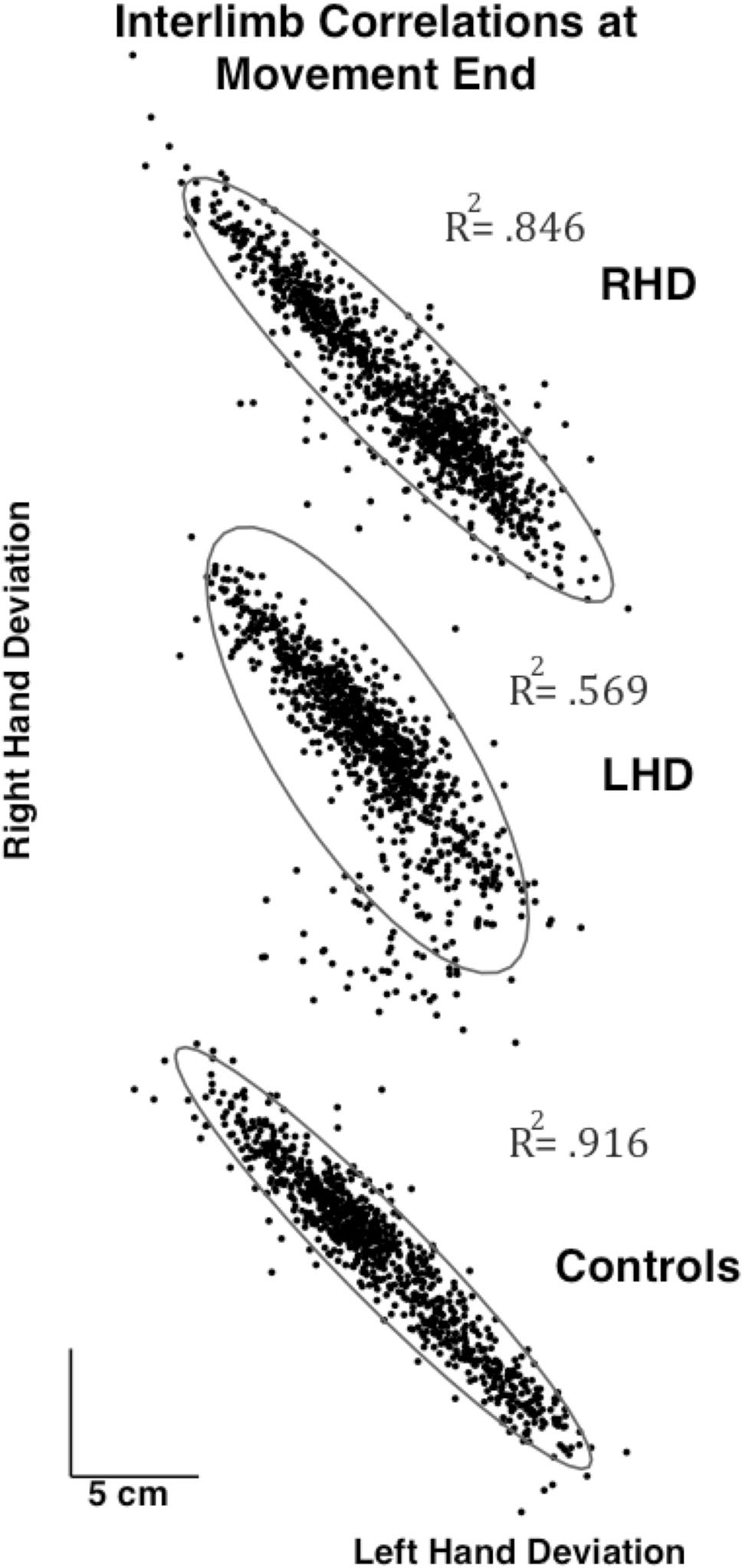
Interlimb correlations of movement along the bar x-axis for each group across trials
Stabilizing performance within trials
We now examine how the redundant axis movement was correlated between the hands within each trial. Figure 6 shows example movements and velocity profiles from subjects in each of the three groups (control, RHD, LHD). The lines representing stick figures of the arm are drawn between every 2 data points (17.2 ms). As shown in the examples, LHD participants moved slightly slower than controls; while, RHD participants moved at a similar speed to controls. In addition, the left-hand deviation in the x-axis vs the right-hand deviation in the x-axis is plotted in gray to the right of each example movement. The ability to correlate movements along the x-axis of the left and right hands is important for accuracy of the task and limiting deviation of the bar. As shown by Sainburg et al. (2013) in young healthy participants, success in this cooperative task required negative covariation between the hands to limit deviation of the bar along the x-axis. To further quantify the relationship between the arms, we also performed linear correlations between left and right redundant movement and broke that analysis into three phases as explained in the methods. Phase 1 of the movements (shown in black) is from movement start to peak velocity, reflecting the early predictive components of the movement, Phase 2 (shown in blue) is from peak velocity to movement end, and Phase 3 (shown in red) reflects the late corrections that occur after the initial cessation of movement. For each example movement, we included the fit line of the correlation for each phase along with the correlation coefficient (Pearson’s R). For the control, the left and right x-displacements in Phase 1 and Phase 2 are tightly negatively correlated, with a smaller positive correlation in the correction phase. The relationship between the hands looks similar for the RHD participant, although somewhat less tightly correlated than controls as shown by the lower R-values. The LHD participant shows very little correlation between the hands, particularly in the early phase of movement.
Fig. 6.
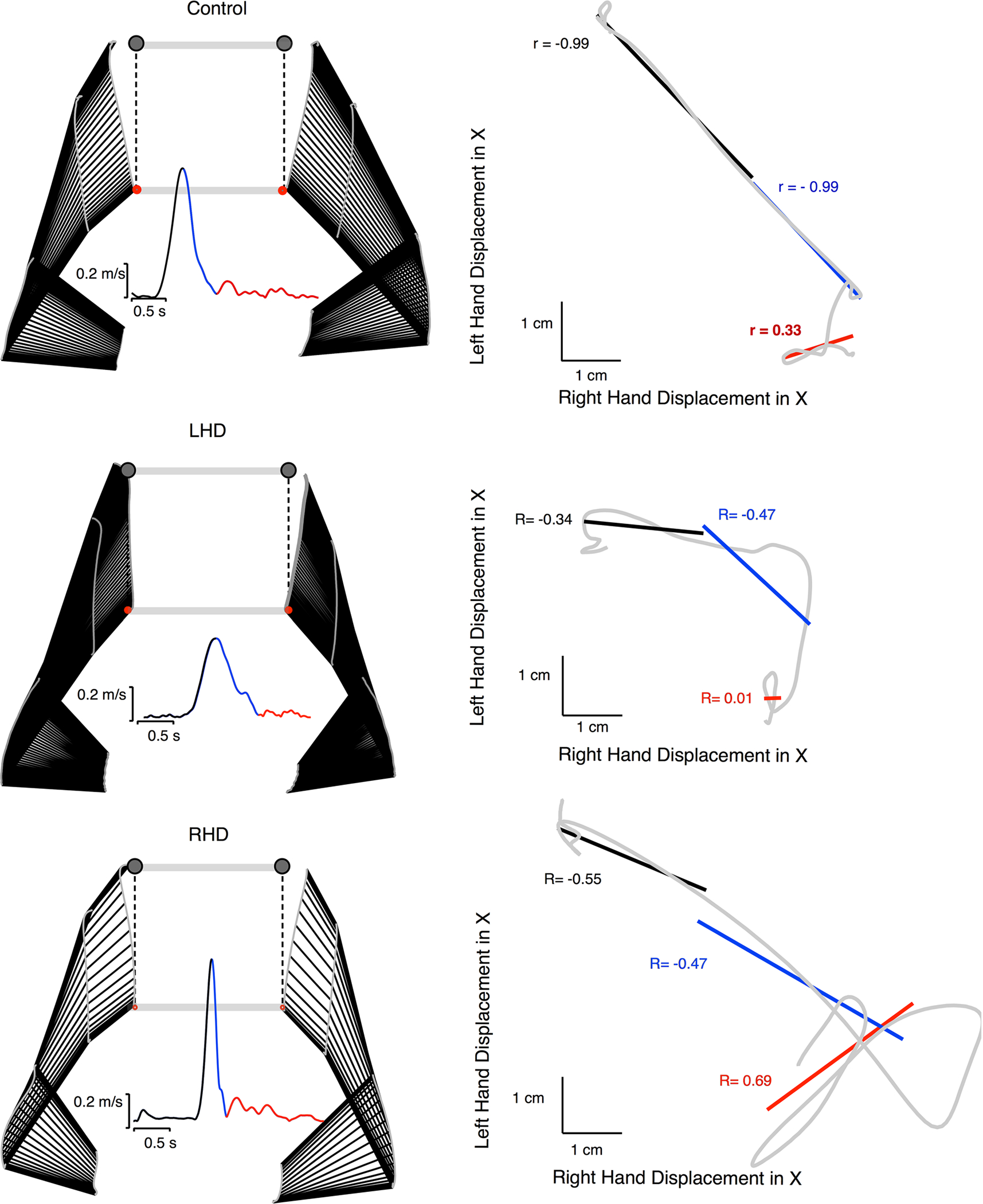
Correlations of redundant axis movements within example trials are shown for each group. Example movements and velocity profiles are shown (left) with stick figures of arm locations drawn every two data points (17.2 milliseconds). Left hand deviation in the x-axis vs the right hand deviation in the x axis are plotted in gray (right), with correlations broken into three phases represented in black (Phase 1), blue (Phase 2), and red (Phase 3)
Figure 7 shows the mean correlation coefficient and slope of the fit line within trials for each group in the three phases. As shown in the individual trials in Fig. 6, the stroke groups show less correlation between the hands than controls, with the LHD group showing the least correlation. The lack of interlimb coordination was particularly striking for the LHD group in the early phase of movement. Comparisons between groups using Steel–Dwass Tests revealed that LHD participants had a significantly smaller negative correlation coefficient (p = .0476) than controls in the first phase of movement, which is thought to reflect predictive aspects of control. The RHD group showed no significant difference in correlation coefficient from controls in any phase of movement. Both stroke groups show slightly lower correlations than controls in the second phase of movement, but these differences were not statistically significant. In the third corrective phase, all groups showed similar positive correlations. The trends are similar when looking at the slope of the correlations; however, the difference between LHD and Controls in the first phase did not reach significance (p = .0619).
Fig. 7.
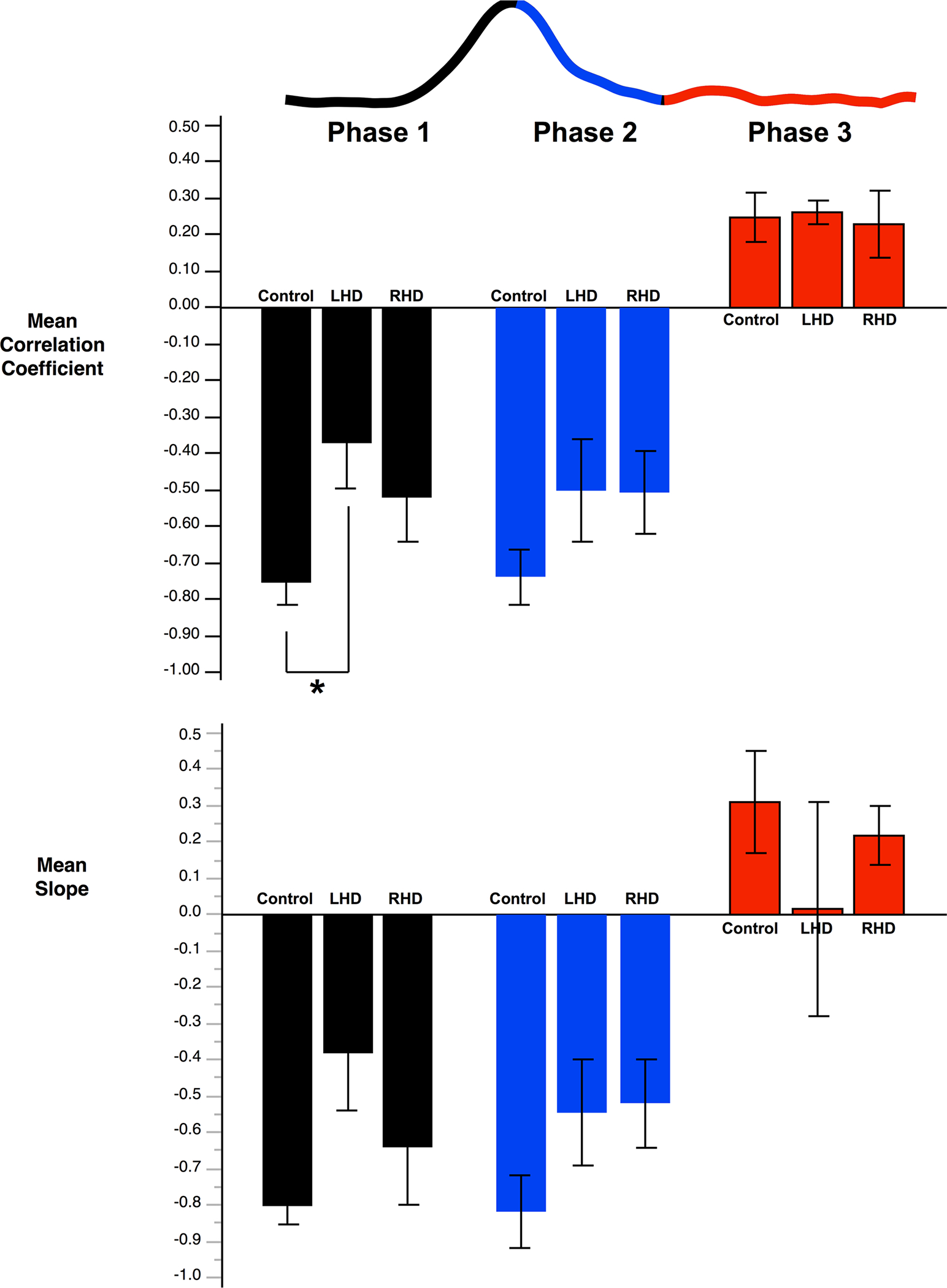
Mean correlation coefficient and slope of the fit line within trials for left vs right hand x-axis deviation in the three phases
Non‑redundant axis performance
As described earlier, success in this task required subjects to move the bar the proper distance and to limit tilt of the bar to keep both hands in their targets. Figure 8 shows those two measures that describe performance in the non-redundant axis of the task. Bar tilt at movement end was measured as the angle of the bar with respect to the horizontal. Bar center distance error was the absolute distance the center of the bar was from the point halfway between the left and right target circles. All three groups were able to perform the task fairly successfully in terms of the final position errors. For bar tilt and bar center distance error, there were no significant differences between groups, although there was a large amount of variance in the stroke groups.
Fig. 8.
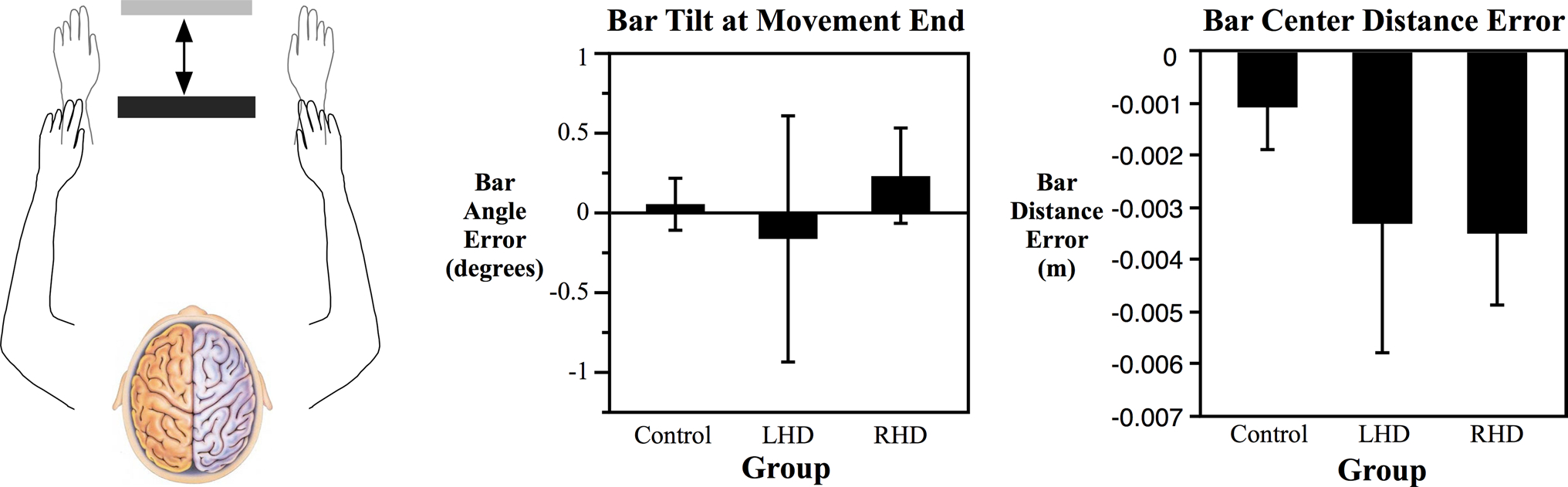
Mean bar angle error and bar distance error at the end of movement for each group
Discussion
This study examined performance in a cooperative bimanual task in RHD and LHD stroke patients compared to controls. We found significant differences in how each group moved along the axis of the bar. Specifically, the LHD group moved less along the redundant axis of the bar, and this movement was highly correlated with deviation of the bar and task error for the LHD group but not for Controls and RHD. The LHD group showed deficits in coordinating the two arms together, and this was most striking in the early predictive phase of movement, consistent with the left hemisphere’s specialization for predictive control of movement.
Although deficits in bimanual coordination resulting from stroke have been previously elucidated (Kang and Cauraugh 2014; Kantak et al. 2016), whether bilateral coordination deficits vary with the hemisphere of damaged has not previously been studied. One of the major differences between the LHD and RHD groups in this task was the degree to which they moved along the redundant axis of the bar. The LHD group restricted movement along this redundant axis more than controls; whereas, the RHD group moved similar to controls. This movement was also not as well correlated between the right and left hands across trials for the LHD group. This suggests that the LHD participants had difficulty implementing the use of redundant degrees of freedom in a way that did not affect task performance. In a previous study examining unimanual reaching movements with both arms, Freitas and Scholz (2009) found using UCM analysis that variance associated with task error (VORT) was greater for movements of the non-dominant left arm than the dominant right arm. They concluded that the right hemisphere may have more difficulty implementing the coordination needed to selectively increase motor abundance without also producing greater variability of left hand’s movement path. A recent study from our lab found a similar restriction of out-of-plane movement in the left non-dominant arm of healthy participants in a 3D reaching task (Schaffer and Sainburg 2017). In this study, the non-dominant arm moved less in redundant degrees of freedom, and any movement in this degree of freedom was highly correlated with task error in the non-dominant but not the dominant arm. Thus, coordination within redundant degrees of freedom that do not affect task error may require left hemisphere mechanisms in right-handers. Our current findings are consistent with this hypothesis, but more research is necessary to examine this idea and to examine the intrahemispheric functional neuroanatomy of this control.
The LHD group also had particular deficits in coordinating the two hands together in the early phase of movement. This early phase of movement, from start to peak velocity, is thought to rely largely on open-loop aspects of control (Scheidt and Ghez 2007). This finding is consistent with the previous studies on unimanual movements showing that the dominant hemisphere seems to be specialized for predictive control of intersegmental interactions (Sainburg 2002; Schaefer et al. 2009a). In the previous unimanual studies, LHD movements showed significant errors in initial trajectory, and in directional adaptation during visuomotor adaptation tasks (Schaefer et al. 2009b). While we cannot completely control for the contribution of unimanual trajectory planning deficits to bimanual coordination deficits in LHD, our analysis of coordination between the hands along the redundant axis focuses on an aspect of bimanual coordination that does not exist in unimanual movements, and that can be independent of unilateral planning deficits. This is because any deviations along the redundant axis of one arm can be compensated by opposite motions of the other arm. Thus, the redundant axis of motion along the bar reduced the importance of the accuracy of each hand’s trajectory, and emphasized the role of interlimb coordination in ensuring task accuracy. Our findings that these bimanual-specific predictive processes were disrupted by LHD but not RHD suggests that the deficits seen in this study are not simply individual unimanual deficits manifesting themselves in a bimanual task, but reflect the left hemisphere’s specialized contribution to bimanual coordination. Whether this type of planning taps into the same left hemisphere planning mechanisms used for unimanual movements, as reported in earlier studies (Winstein and Pohl 1995; Schaefer et al. 2009a, b), cannot be determined from the current findings, but direct comparison of unilateral control to bimanual control in LHD and RHD in future studies may help to address this question.
We did not find robust differences between groups in coordination near the end of movement or in the final position of the center of the bar. Given the right hemisphere’s specialization for positional control, we expected to see some deficits for the RHD group in these later components that rely upon impedance control, but that was not the case. These findings emphasize the bimanual coordination nature of this task, and the fact that hemisphere-specific deficits in unilateral movements do not directly translate to bimanual coordination deficits in right and left hemisphere-damaged stroke patients. In addition, the fact that the deficits observed in this task were associated with left hemisphere damage may reflect the nature of coordination necessary for the task. Although previous studies have reported both right (Duque et al. 2010) and left hemisphere (Jäncke et al. 2000) “dominance” for different bimanual tasks, we suggest that each hemisphere contributes its specialization to bimanual control. It is possible that had we tested the participants on a bimanual postural stabilization task or a task that emphasized different control features, such as compensation for unpredictable environmental conditions (Yadav and Sainburg, 2014) that more bimanual coordination deficits might have been seen in the RHD group; however, further studies are required to answer that question. In addition, it should be emphasized that many tasks, such as drawing or tracing (Duque et al. 2010), emphasize visuospatial mechanisms that appear to be most dependent on right hemisphere mechanisms (Kalbfleisch and Gillmarten 2013). Because all stroke patients in this study were right-handed, the LHD patients’ contralesional, most impaired arm, was the dominant arm; whereas, RHD patients were most impaired in their non-dominant arm. It has previously been shown that individuals with the dominant arm most affected following stroke often demonstrate less impairment than those with the non-dominant arm most affected (Harris and Eng 2006). However, other research suggests functional outcomes following stroke are equivalent for RHD and LHD patients (Fink et al. 2008). It is plausible that the effect of LHD on bimanual deficits might, at least in part, help to explain this apparent contradiction in the literature. While LHD patients may show lower unimanual contralesional impairments, deficits in bimanual coordination might prevent transfer to functional independence and activities of daily living, which depend heavily on bilateral performance. However, it should also be emphasized that we recently provided strong evidence that ipsilesional arm deficits tend to be greater in LHD than RHD patients, when patient groups were matched for contralesional impairment level (Maenza et al. 2020). We conclude that LHD-induced deficits in the ipsilesional arm can combine with ipsilesional unimanual coordination deficits to contribute to functional performance deficits in this group of patients.
Clinical implications
Many current strategies for stroke rehabilitation focus on the contralesional paretic limb. However, there has been an increasing focus of rehabilitation research and clinical rehabilitation on bimanual movements for stroke rehabilitation because of the functional importance of tasks that require both hands and the distinct mechanisms involved in bimanual movements (McCombe Waller and Whitall 2008). Our current findings indicate that hemisphere of damage is an important consideration for assessing and treating bimanual movements in stroke rehabilitation. While our restriction to participants with cortical stroke limits the generalizability of our results to the general stroke population with subcortical lesions and more severe paresis, our results provide evidence for specific bimanual coordination deficits resulting from left hemisphere damage. Bimanual coordination in most tasks involves not simply moving both arms at the same time, but coordinating them synergistically to compensate for one another and achieve the end goal. As most stroke rehabilitation strategies aim to improve one or both arms individually, it is important to consider that there may also be deficits in how the arms work together. We believe that these findings provide evidence that assessment and training in cooperative bimanual tasks should be considered as part of a functional framework for post-stroke rehabilitation.
Funding
This work was supported by the National Institutes of Health Grant #R01HD059783 to RLS.
Footnotes
Communicated by Winston D. Byblow.
References
- Barnette JJ, McLean JE (2005) Type I error of four pairwise mean comparison procedures conducted as protected and unprotected tests. J Mod Appl Stat Methods 4(2), Article 10. 10.22237/jmasm/1130803740 [DOI] [Google Scholar]
- Brinkman C (1984) Supplementary motor area of the monkey’s cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4:918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Varghese R, Stoll H, Winstein CJ (2020) Predictors of Arm Nonuse in Chronic Stroke: A Preliminary Investigation. Neurorehabilitation Neural Repair 34(6):512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Béatse E, Sunaert S, van Hecke P, Duysens J (2001) Brain areas involved in interlimb coordination: a distributed network. NeuroImage 14(5):947–958. 10.1006/nimg.2001.0892 [DOI] [PubMed] [Google Scholar]
- Dolgun A, Demirhan H (2017) Performance of nonparametric multiple comparison tests under heteroscedasticity, dependency, and skewed error distribution. Commun Stat-Simul C 46(7):5166–5183 [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E (1998) Primary motor cortex is involved in bimanual coordination. Nature 395(6699):274–278. 10.1038/26220 [DOI] [PubMed] [Google Scholar]
- Dounskaia N, Nogueira KG, Swinnen SP, Drummond E (2010) Limitations on coupling of bimanual movements caused by arm dominance: when the muscle homology principle fails. J Neurophysiol 102:2027–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Davare M, Delaunay L, Jacob B, Saur R, Hummel F, Olivier E (2010) Monitoring coordination during bimanual movements: where is the mastermind? J Cognit Neurosci 22(3):526–542. 10.1162/jocn.2009.21213 [DOI] [PubMed] [Google Scholar]
- Fink JN, Frampton CM, Lyden P, Lees KR (2008) Does hemispheric lateralization influence functional and cardiovascular outcomes after stroke? Stroke 39:3335–3340 [DOI] [PubMed] [Google Scholar]
- Freitas SMSF, Scholz JP (2009) Does hand dominance affect the use of motor abundance when reaching to uncertain targets? Hum Mov Sci 28(2):169–190. 10.1016/j.humov.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (1995) Statistical parametric mapping: ontology and current issues. J Cereb Blood Flow Metab 15:361–370 [DOI] [PubMed] [Google Scholar]
- Harris JE, Eng JJ (2006) Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehab Neural Repair 20(3):380–389. 10.1177/1545968305284528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Peters M, Schlaug G, Posse S, Steinmetz H, Müller-Gärtner H-W (1998) Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Cogn Brain Research 6(4):279–284 [DOI] [PubMed] [Google Scholar]
- Jäncke L, Peters M, Himmelbach M, Nösselt T, Shah J, Steinmetz H (2000) fMRI study of bimanual coordination. Neuropsychologia 38(2):164–174. 10.1016/S0028-3932(99)00062-7 [DOI] [PubMed] [Google Scholar]
- Johansson RS, Theorin A, Westling G, Andersson M, Ohki Y, Nyberg L (2006) How a lateralized brain supports symmetrical bimanual tasks. PLoS Biol 4(6):e158. 10.1371/journal.pbio.0040158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA (2016) Nondominant-to-dominant hand interference in bimanual movements is facilitated by gradual visuomotor perturbation. Neuroscience 318:94–103. 10.1016/j.neuroscience.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Kalbfleisch ML, Gillmarten C (2013) Left brain vs. right brain: findings on visual spatial capacities and the functional neurology of giftedness. Roeper Rev 35(4):265–275. 10.1080/02783193.2013.829549 [DOI] [Google Scholar]
- Kang N, Cauraugh JH (2014) Bimanual force variability and chronic stroke: asymmetrical hand control. Plos One 9(7):e101817. 10.1371/journal.pone.0101817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak SS, Zahedi N, McGrath RL (2016) Task-dependent bimanual coordination after stroke: relationship with sensorimotor impairments. Arch Phys Med Rehab 97(5):798–806. 10.1016/j.apmr.2016.01.020 [DOI] [PubMed] [Google Scholar]
- Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL (2013) Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain 136(4):1288–1303. 10.1093/brain/aws283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Przybyla A, Good DC, Haaland KY, Sainburg RL (2014) Contralesional Arm Preference Depends on Hemisphere of Damage and Target Location in Unilateral Stroke Patients. Neurorehabilitation Neural Repair 28(6):584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenza C, Good DC, Winstein CJ, Wagstaff DA, Sainburg RL (2020) Functional Deficits in the Less-Impaired Arm of Stroke Survivors Depend on Hemisphere of Damage and Extent of Paretic Arm Impairment. Neurorehabilitation Neural Repair 34(1):39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J (2008) Bilateral arm training: why and who benefits? NeuroRehabilitation 23(1):29–41 [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000) Stereotaxic display of brain lesions. Behav Neurol 12:191–200 [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO (2012) Age-specific CT and MRI templates for spatial normalization. Neuroimage 61:957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y (1997) Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci 17(24):9667–9674. 10.1016/S0168-0102(97)90507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL (2002) Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142(2):241–258. 10.1007/s00221-001-0913-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg R, Good D, Przybyla A (2013) Bilateral synergy: a framework for post-stroke rehabilitation. J Neurol Transl Neurosci 1(3):1025. https://www.ncbi.nlm.nih.gov/pubmed/24729985 [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL (2009a) Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia 47(13):2953–2966. 10.1016/j.neuropsychologia.2009.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL (2009b) Dissociation of initial trajectory and final position errors during visuomotor adaptation following unilateral stroke. Brain Res 1298:78–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Mutha PK, Haaland KY, Sainburg RL (2012) Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb Cortex 22(6):1407–1419. 10.1093/cercor/bhr237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer JE, Sainburg RL (2017) Interlimb differences in coordination of unsupported reaching movements. Neurosci 350:54–64. 10.1016/j.neuroscience.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Ghez C (2007) Separate adaptive mechanisms for controlling trajectory and final position in reaching. J Neurophysiol 98(6):3600–3613. 10.1152/jn.00121.2007 [DOI] [PubMed] [Google Scholar]
- Stewart JC, Lewthwaite R, Rocktashel J, Winstein CJ (2019) Self-efficacy and Reach Performance in Individuals With Mild Motor Impairment Due to Stroke. Neurorehabilitation Neural Repair 33(4):319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SP, Jardin K, Meulenbroek R (1996) Between-limb asynchronies during bimanual coordination: effects of manual dominance and attentional cueing. Neuropsychologia 34(12):1203–1213. 10.1016/0028-3932(96)00047-4 [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS (1995) Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res 105(1):163–174. 10.1007/bf00242191 [DOI] [PubMed] [Google Scholar]
- Woytowicz EJ, Westlake KP, Whitall J, Sainburg RL (2018) Handedness results from complementary hemispheric dominance, not global hemispheric dominance: evidence from mechanically coupled bilateral movements. J Neurophysiol 120(2):729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woytowicz EJ, Sainburg RL, Westlake KP, Whitall J (2020) Competition for limited neural resources in older adults leads to greater asymmetry of bilateral movements than in young adults. J Neurophysiol. 10.1152/jn.00405.2019 (Advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL (2014) Limb dominance results from asymmetries in predictive and impedance control mechanisms. PLoS One 9(4):e93892. 10.1371/journal.pone.0093892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Laconte S, Peltier S, Zhang K, Hu X (2005) Connectivity exploration with structural equation modeling: an fMRI study of bimanual motor coordination. NeuroImage 25:462–470. 10.1016/j.neuroimage.2004.11.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


