ABSTRACT
Introduction:
This study aims to review the current role of endoscopic combined intrarenal surgery (ECIRS) in the management of renal stones, with a focus on its efficacy and safety. The secondary outcome was to highlight the tips and tricks to improve the urologist’s experience with ECIRS.
Methods:
A scoping review of the literature, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines, was performed, using ECIRS and flexible ureteroscopy and percutaneous nephrolithotomy as the search terms. All original articles were screened and included.
Results:
Thirty-three studies were included in the analysis. ECIRS showed a good efficacy and safety profile, with an excellent stone-free rate and a low rate of complications, mostly Clavien–Dindo I/II. With ECIRS, a reduction in the need for multiple access tracts was noted and direct visualization of the targeted calyx during the puncture increased the ability to attain transpapillary punctures, thereby reducing the amount of bleeding.
Conclusion:
ECIRS, as the first-line minimal access intervention, is safe and efficacious, particularly for achieving a stone-free status in patients with large complex stones in a single stage. The ability to gain access under direct vision and the reduction in the number of tracts, in both the supine and the prone positions, makes this procedure an attractive surgical choice.
INTRODUCTION
The management of complex renal stones has always been a major challenge for the practicing urologist. Different approaches and surgical strategies have been proposed over the time to achieve a one-step stone-free rate (SFR), thereby avoiding the need for multiple procedures with a consequent increase in the cost and the likelihood of complications.[1] Endoscopic combined intrarenal surgery (ECIRS) was first introduced in 2008,[2] combining an antegrade and a retrograde approach, with the aim to perform the puncture of the collecting system under direct vision and “to pass the ball” during the percutaneous fragmentation, thus speeding up the lithotripsy and achieving higher SFRs.[3] Despite the promising features of ECIRS, a clear advantage over the standard percutaneous nephrolithotomy (PCNL), in the terms of single-stage SFR, has still not been demonstrated.[4]
Several studies and systematic reviews have compared PCNL with ECIRS, pointing out the benefits and the advantages of this new approach.[5] However, the procedure has its own limitations, such as, the need for two experienced surgeons, two camera stack systems, and the availability of two energy sources.[6] Despite these shortcomings, there are advantages of ECIRS over the standard or even the mini-invasive PCNL and the increasing interest in this procedure has led to several trials comparing its feasibility and efficacy to PCNL.[7] The most interesting characteristics of ECIRS is the possibility to avoid multiple renal accesses and to provide a framework for an individualized approach for each patient.[8,9] To this aim, ECIRS has the potential to surpass PCNL in the management of patients with complex and/or multiple renal stones.[10]
With this scoping review, we aim to draw a picture of the current role of ECIRS in the management of large and complex renal stones, with an effort to better understand the most important features and highlighting the most used and efficient strategies such as the choice of patients’ position, instruments, and the post-surgical management.
MATERIALS AND METHODS
Literature search and article selection
A scoping review of the literature was performed by two independent authors (CN, VJ) and the conflicts were resolved by a senior author (BKS). A search on PubMed, Cochrane database and Google Scholar for the eligible studies was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines.[11] The MeSH terms and keywords employed were as follows: “endoscopic combined intrarenal surgery” and “flexible ureteroscopy and percutaneous nephrolithotomy.” Articles were first screened by the title and full-text and a full-text analysis of the eligible articles was then performed for inclusion. Studies in language other than English were excluded during the screening process. Studies included in this review were then subjected to a narrative synthesis for analysis.
Study inclusion
Randomized controlled trials (RCTs), quasi-RCTs, non-randomized comparative studies, and single-arm case series were considered for inclusion. Systematic reviews, meta-analyses, commentaries, editorials only, expert opinions, case reports, book chapters, reviews, and congress abstracts were excluded. Additional exclusion criteria were the absence of abstract, absence of outcome data, and incomplete technical description of the surgical technique. Preclinical and animal studies were also not considered for inclusion.
Patients’ characteristics
Patients who underwent ECIRS for urolithiasis were included in the study population. There were no limitations regarding the age, sex, body mass index, American Society of Anaesthesiologists score, presence of congenital or acquired abnormalities of the urinary tract, or urinary tract diversion. Studies reporting on staghorn stones, complex stones, and concomitant renal and ureteral stones were included, while those on the application of ECIRS for the treatment of cancer were excluded.
Types of interventions
Only those studies with a clear description about the application of simultaneous combined retrograde ureteroscopy (URS) and percutaneous lithotripsy, namely ECIRS or combined intrarenal surgery, were included. No limitation regarding the patient position (prone, prone modified, supine, supine modified, etc.,), the choice of instruments (rigid-flexible URS, access sheet, type of percutaneous access), and the type of energy source were applied.
Objectives and outcome measure
The primary outcome of this review was to assess the efficacy and safety of ECIRS, and the objectives were the SFR and the complication rate (according to Clavien–Dindo grading). Additional outcomes were: the operative time, length of hospital stay, hemoglobin drop, and a qualitative analysis of the reported complications. The secondary objective was to identify the tips and tricks for improving the outcomes of ECIRS regarding the patient’s position during the surgery, and the strategy for percutaneous access.
Literature screening
The literature search identified 509 studies and four hundred and twenty-nine of these were duplicates and were excluded after screening the title and the abstract. Eighty full-texts were screened for inclusion. A total of 47 studies were further excluded and 33 were finally included. Figure 1 shows the flow diagram of the literature search.
Figure 1.
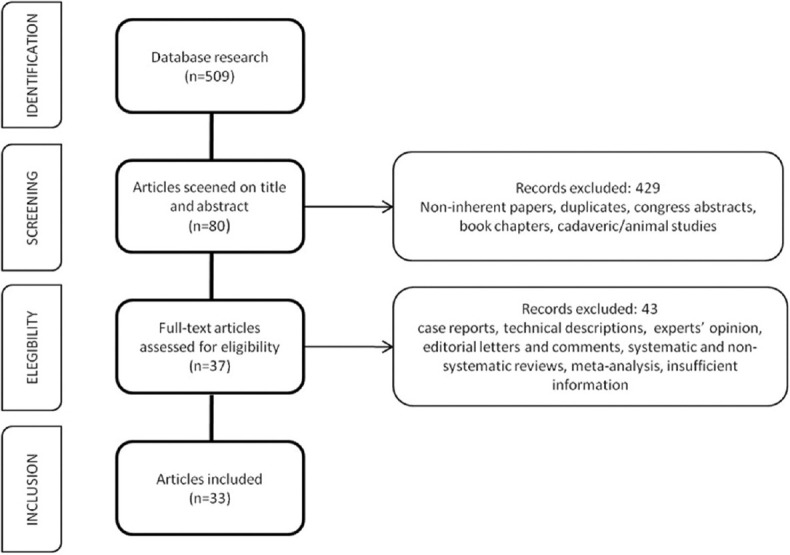
Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram
RESULTS
Among the 33 included studies, there were two prospective RCTs,[12,13] five prospective non-RCTs,[14-18] five prospective single-arm case series,[2,19-22] eleven retrospective comparative studies,[23-33] and ten retrospective single-arm case series.[6,34-42] The level of evidence for each study is summarized in Table 1. With only two prospective RCTs, the overall quality of evidence can be graded as “low” and the risk of bias as “serious.”
Table 1.
Characteristics and targets of the studies
| Author | Type of study | Level of evidence | Period of surgery | Number of patients | Target of study | Outcomes |
|---|---|---|---|---|---|---|
| Tabei, 2016 | RSACS | 4 | April 2010–October 2014 | 370 | Complication | Predictors of sepsis |
| Chen, 2022 | RCS | 3 | January 2018–September 2021 | 34 | Efficacy | ECIRS versus PCNL |
| Gao, 2019 | RCS | 3 | March 2013–December 2016 | 140 | Efficacy | PCNL versus URS versus ECIRS |
| Gokce, 2019 | PnRCT | 2 | September 2016–March 2018 | 137 | Efficacy | Antegrade versus retrograde nephroscopy |
| Goktug, 2023 | RCS | 3 | January 2007–January 2022 | 177 | Efficacy | PCNL versus ECIRS |
| Hamamoto, 2014 | PCS | 4 | February 2004–January 2013 | 161 | Efficacy | ECIRS versus mini-PCNL versus standard PCNL |
| Jung, 2018 | RSACS | 4 | August 2017–February 2018 | 30 | Efficacy | Tips and tricks of ECIRS |
| Jung, 2022 | RCS | 3 | August 2017–January 2019 | 100 | Efficacy | SWL versus ECIRS |
| Kallidonis, 2022 | PCS | 4 | January 2019–December 2021 | 33 | Efficacy | Nonpapillary puncture |
| Kuroda, 2015 | RCS | 3 | June 2010–September 2014 | 329 | Efficacy | success versus nonsuccess |
| Leng, 2017 | RCS | 3 | March 2014–January 2016 | 87 | Efficacy | ECIRS versus PCNL |
| Manikandan, 2016 | RSACS | 4 | June 2012–March 2016 | 43 | Efficacy | Simultaneous renal and ureteral calculi |
| Mishra, 2022 | PnRCT | 2 | January 2016–December 2019 | 60 | Efficacy | ECIRS versus PCNL/URS |
| Ping, 2016 | RSACS | 4 | January 2012–January 2016 | 26 | Efficacy | Post-PCNL residual stone |
| Schulster, 2019 | PCS | 4 | August 2017–January 2018 | 110 | Efficacy | Prediction of SFR |
| Scoffone, 2008 | PCS | 4 | April 2014–December 2007 | 127 | Efficacy | ECIRS in GMSV position |
| Tominaga, 2023 | RSACS | 4 | July 2019–December 2021 | 61 | Efficacy | Vacuum-assisted mini-ECIRS |
| Usui, 2020 | RCS | 3 | April 2009–May 2016 | 256 | Efficacy | Mini-ECIRS versus ECIRS |
| Wang, 2022 | RSACS | 4 | September 2017–January 2021 | 96 | Efficacy | Multiple stone |
| Wen, 2016 | PRCT | 1 | May 2012–Oct 2014 | 67 | Efficacy | Mini-PCNL versus ECIRS |
| Yamashita, 2017 | RSACS | 4 | February 2008–April 2015 | 75 | Efficacy | Predictors of SFR |
| Zaho, 2019 | PnRCT | 2 | January 2018–Oct 2019 | 140 | Efficacy | ECIRS versus mini-PCNL |
| Abouelgreed, 2022 | PRCT | 1 | Oct 2018–August 2021 | 66 | Position | Prone-split-leg versus GMSV |
| Hamamoto, 2014 | RSACS | 4 | December 2010–January 2013 | 60 | Position | Large renal calculi |
| Hamamoto, 2015 | RSACS | 4 | December 2010–August 2013 | 42 | Position | Staghorn calculi |
| Hamamoto, 2021 | RCS | 3 | January 2014–December 2018 | 218 | Position | prone-split-leg versus GMSV |
| Liu, 2022 | RCS | 3 | January 2018–June 2021 | 83 | Position | PCNL versus ECIRS |
| Otsuka, 2022 | RCS | 3 | January 2018–December 2022 | 226 | Position | LD versus GMSV |
| Hamamoto, 2017 | PCS | 4 | April 2014–July 2015 | 30 | Puncture system | Real-time virtual sonography versus US-guided puncture |
| Inoue, 2016 | RSACS | 4 | January 2013–September 2015 | 40 | Puncture system | Wideband doppler US for puncture |
| Sugino, 2018 | PnRCT | 2 | July 2013–April 2014 | 30 | Puncture system | US versus URS assisted |
| Taguchi, 2021 | RCS | 3 | January 2016–April 2020 | 313 | Puncture system | URS assisted versus US |
| Unno, 2021 | PnRCT | 2 | January 2016–April 2020 | 221 | Puncture system | Double lumen AS versus one-shot dilation |
PRCT=Prospective randomized controlled trial, PnRCT=Prospective nonrandomized controlled trial, PCS=Prospective case series, RCS=Retrospective comparative study, RSACS=Retrospective single arm case series, URS=Ureteroscopy, US=Ultrasound, ECIRS=Endoscopic combined intrarenal surgery, PCNL=Percutaneous nephrolithotomy, SWL=Shockwave lithotripsy, SFR=Stone-free rate, GWSV=Galdakao Modified Supine Valdivia, LD: Lateral decubitus, AS=Access sheath
Twenty-one studies reported the SFR as the primary objective, one studied reported on the safety profile as the primary outcome, six studies focused on the influence of patient’s position during the surgery, and five studies compared the different stratergies for renal puncture [Table 1]. Intraoperative variables (patient position, energy sources, instrumentation, strategy for renal puncture, and exit strategy) are summarized in Table 2. Table 3 shows the intra- and postoperative results.
Table 2.
Operative features
| Author | Position | Percutaneous access | Ureteroscope | UAS | URS energy | PCNL energy | Puncture guidance | Nephrostomy |
|---|---|---|---|---|---|---|---|---|
| Abouelgreed, 2022 | N/A | 18Fr Amplatz | 7.5F Flex X-2, Karl Storz | 10–12Fr | Hol: YAG | Pneumatic | Fluoroscopic | Yes, 18Fr |
| Chen, 2022 | GMSV | 17.5Fr or 22Fr | Flexible | N/A | Holmium laser | Pneumatic | US + fluoroscopic | Not always |
| Gao, 2015 | PSL | 18Fr | Flexible | N/A | Holmium laser | Ultrasonic | US + URS | Yes, 16Fr |
| Gokce, 2019 | N/A | 24Fr Amplatz | Flex X-2, Karl Storz | 9.5–11.5Fr | Laser | Laser or pneumatic | Fluoroscopic | No |
| Goktug, 2023 | GMSV | 30Fr Amplatz | 7.5F flexible | 9.5–11.5Fr | Holmium laser | Pneumatic or ultrasonic | URS + fluoroscopic | Yes, 14Fr |
| Hamamoto, 2014 | PSL | 18Fr Karl Storz | Flex X-2, Karl Storz | 12–14Fr | Hol: YAG | Pneumatic | US + fluoroscopic | Yes, 18Fr |
| Hamamoto, 2014 | PSL | 18Fr Karl Storz | Flex X-2, Karl Storz | 14Fr | Hol: YAG | Pneumatic | US + fluoroscopic | Yes, 18Fr |
| Hamamoto, 2015 | PSL | 18Fr Karl Storz | Flex X-2, Karl Storz | 12–14Fr | Hol: YAG | Pneumatic | US + fluoroscopic | Yes, 18Fr |
| Hamamoto, 2017 | PSL | 18Fr Karl Storz | URF-V2, Olympus | 12–14Fr | Hol: YAG | Pneumatic | US + URS/fluoroscopy | Not always |
| Hamamoto, 2021 | PSL or GMSV | 17.5–19.5Fr Karl Storz | URF-V2: Olympus; URF-P6: Olympus; Flex X-2: Karl Storz | Variable sizes | Hol: YAG | Pneumatic | US + fluoroscopic | No |
| Inoue, 2016 | GMSV | 18–19.5Fr Karl Storz | Flex X-2, Karl Storz | 9.5–11.5Fr | Holmium laser | Pneumatic | US | Yes, 14–16Fr |
| Jung, 2018 | GMSV | 18Fr cook medical; 24Fr boston scientific | Flexible | N/A | Holmium laser | Pneumatic | Preoperative nephrostomy | N/A |
| Jung, 2022 | GMSV | 18Fr cook medical; 15–16Fr Karl Storz; 24/30Fr boston scientific | Flexible | 11–13Fr | N/A | N/A | US + fluoroscopic | N/A |
| Kallidonis, 2022 | PSL | 22Fr or 30Fr | Flex-XC, Karl Storz | 12–14Fr | Hol: YAG | Pneumatic | Fluoroscopic | Yes, 20–24r or 16–18Fr |
| Kuroda, 2015 | GMSV | 24Fr or 30Fr | Flex X-2, Karl Storz | 11–13/13–15Fr | Hol: YAG | Pneumatic | URS/US + fluoroscopic | N/A |
| Leng, 2017 | Oblique supine | 16Fr | URF-P5, Olympus | N/A | Holmium laser | Holmium | US | Yes, 16Fr |
| Liu, 2022 | Modified PSL | 20Fr | 8–9.5F Wolf ureteroscope | 12–14Fr | Holmium laser | Pneumatic or Holmium laser | US + fluoroscopic | Yes, 18Fr |
| Manikandan, 2016 | GMSV | 16Fr Karl Storz | N/A | N/A | Pneumatic | Pneumatic | US + fluoroscopic | Not always, 12Fr |
| Mishra, 2022 | GMSV | 16Fr Karl Storz | 6–7.5F URS, Richard Wolf | N/A | N/A | Laser | US + fluoroscopic | No |
| Otsuka, 2022 | LD or GMSV | 26Fr | URF-P5, Olympus | N/A | N/A | Hol: YAG | US + URS | Not always |
| Ping, 2016 | GMSV | 24Fr | Olympus flexible ureteroscope | 14Fr | Holmium laser | Pneumatic or ultrasonic | US | Yes, Foley catheter |
| Schulster, 2019 | PSL | 18F3 or 30Fr Karl Storz | Flex-XC, Karl Storz | 11–13Fr | Hol: YAG | Pneumatic | URS + fluoroscopic | No |
| Scoffone, 2008 | GMSV | 24Fr or 30Fr | N/A | N/A | Laser | Pneumatic ultrasonic | US + fluoroscopy±URS | Yes |
| Sugino, 2018 | PSL | N/A | Flexible | 12–14Fr | N/A | N/A | US±URS | Not always |
| Tabei, 2016 | GMSV | 24Fr Karl Storz | Flex X-2, Karl Storz | 11–13/13–15Fr | Hol: YAG | Pneumatic | US + fluoroscopic | Not always, 14–22Fr |
| Taguchi, 2021 | PSL or GMSV | 16.5–17.5Fr or 21–22Fr Karl Storz | N/A | N/A | Hol: YAG | Pneumatic | US±URS | Not always |
| Tominaga, 2023 | VFF or PSL | 14–16Fr ClearPetra | URF-P7, Olympus | 9.5–11.5/10–12Fr | Hol: YAG | Hol: YAG | US + fluoroscopic | Yes, 14Fr |
| Unno, 2021 | PSL or GMSV | 16.5/19.5 Fr Karl Storz | N/A | N/A | Laser | Pneumatic | US + URS | Not always |
| Usui, 2020 | GMSV | 16.5Fr, 24Fr or 30Fr Karl Storz | Flexible | 11–13/13–15Fr | Hol: YAG | Pneumatic | US + fluoroscopic | ±14–20Fr |
| Wang, 2022 | Modified PSL | 22–24Fr | Flex X-2, Karl Storz | 12–14Fr | Hol: YAG | Pneumatic | US | No |
| Wen, 2016 | GMSV | 20Fr Amplatz | URF-V2, Olympus | 12–14Fr | Hol: YAG | Hol: YAG | US | Yes, 16Fr |
| Yamashita, 2017 | PSL | 16.5–19.5Fr | URF-P5/URF-V; Olympus | 12–14/14–16Fr | Hol: YAG | Pneumatic | US + fluoroscopic | Yes, 16Fr |
| Zaho, 2019 | GMSV | 16–18Fr | 7.5F flexible | 12–14Fr | Holmium laser | Holmium laser | US + fluoroscopic | Yes |
URS=Ureteroscopy, US=Ultrasound, GMSV=Galdakao Modified Supine Valdivia, PCNL=Percutaneous nephrolithotomy, UAS=Ureteral access sheath, PSL=Prone split legs, LD=Lateral decubitus, N/A=Not reported
Table 3.
Intra- and postoperative results
| Author | Mean stone size | SFR (%) | Definition of SF | Imaging for SFR | Mean operation time (min) | Mean hospital stay (days) | Hb drop (g/dL) | Number of tracts | CR (%) | Clavien >II |
|---|---|---|---|---|---|---|---|---|---|---|
| Abouelgreed, 2022 | 6.65–6.78 cm2 | 87.87–90.9 | <4 mm | KUB CT | 118.87 | 6.7 | 125 mL (loss) | 1 | 21.2 | None |
| Chen, 2022 | 21.4 cm3 | 70.6 | N/A | KUB X-ray + US | 140.12 | 4.3 | 1.66 | 1–5 | 70.6 | One IV |
| Gao, 2015 | 26 mm | 97.8 | <4 mm | KUB CT | 63.27 | 7.4 | 67.98 mL (loss) | 1 | 28.9 | None |
| Gokce, 2019 | 29.6 mm | 92.7 | Absence of residual fragments of any size | KUB CT | 80.6 | N/A | 0.8 | 1–2 | 5.8 | None |
| Goktug, 2023 | 9.00 cm2 | 64.4 | <4 mm | KUB X-ray + US | 70 | 2 | 1.6 | 1–2 | 15.5 | One IIIb, One IVb |
| Hamamoto, 2014 | 39.2 mm | 87 | <4 mm | KUB X-ray + US | 120.5 | 7.0 | 1.04 | 1 | 10 | None |
| Hamamoto, 2015 | 45.8 mm | 83.3 | <4 mm | KUB X-ray | 143.2 | 6.8 | 1.14 | 1 | 21.4 | None |
| Hamamoto, 2017 | 33.5–30.5 mm | 73.3–87.7 | <4 mm | KUB CT | 102.2–102.9 | 5 | 0.93–1.39 | 1–4 | 20-33 | None |
| Hamamoto, 2014 | 39.2 mm | 86.7 | <4 mm | KUB X-ray + US | 120.5 | 7.0 | 1.06 | 1 | 10 | None |
| Hamamoto, 2021 | 3.93–5.29 cm2 | 78.8–76.0 | <3 mm | KUB X-ray + US | 106.5–126.0 | N/A | 0.8–1.0 | 1–2 | 20.0–31.3 | One III |
| Inoue, 2016 | 45.5 mm | 97.5% | <4 mm | KUB X-ray + US | 158.4±51 | 12.8 | 0.54 | 1 | 7.3 | None |
| Jung, 2018 | 28.86 mm | 80 | N/A | N/A | 89.23±36.14 | 3.6 | N/A | 1 | N/A | N/A |
| Jung, 2022 | 28.7 mm | 70.0 | <3 mm | KUB X-ray | 82.23±35.68 | 2 | 1.21 | 1 | N/A | N/A |
| Kallidonis, 2022 | 35.0 mm | 90.9 | Absence of residual fragments of any size | KUB X-ray + US/KUB CT | 47 (36–65) | 3 | 1.2 | 1–3 | 9.1 | None |
| Kuroda, 2015 | 4.41–13.08 cm2 | 65.3 | <4 mm | KUB CT | 125.53–132.44 | 7.2–9.0 | N/A | 1–3 | 15.5 | Five V |
| Leng, 2017 | 5.171 cm | 90.9 | <4 mm | KUB CT | 87.500 | 9.7 | 1.56 | 1 | 6.8 | None |
| Liu, 2022 | 48.7 mm | 84.4 | <4 mm | KUB X-ray/CT | 105.0 | 10.0 | 2.4 | 1 | 15.5 | None |
| Manikandan, 2016 | 28.0 mm | 97.7 | <3 mm | KUB X-ray/CT | 132.1 | 6 | 1.25 | 1–3 | 32.5 | Two III |
| Mishra, 2022 | 16.8 mm | 98.3 | N/A | KUB X-ray | 42.1 | 27.7 h | 0.07 | 1 | 20.1 | None |
| Otsuka, 2022 | 20.5–21.6 mm2 | 91.6–97.2 | <2 mm | KUB X-ray | 72–81 | 5 | 1.2–1.3 | N/A | 28–31.9 | One III |
| Ping, 2016 | 39 mm | 96.2 | <4 mm | KUB X-ray | 78.2 | 4.7 | 0.6 | N/A | N/A | None |
| Schulster, 2019 | 33.3 mm | 76 | <2 mm | KUB CT | N/A | N/A | N/A | N/A | N/A | N/A |
| Scoffone, 2008 | 23.8 mm | 81.9 | Absence of residual fragments of any size | KUB CT | 70 | 5.1 | N/A | 1 | 38.6 | Eight III |
| Sugino, 2018 | 4.95–5.31 cm2 | 80–93.3 | <4 mm | KUB X-ray | 92.1–97.6 | 5.0–7.8 | 0.88–1.13 | N/A | N/A | N/A |
| Tabei, 2016 | 7.14–8.83 cm2 | N/A | N/A | N/A | 126.5–123.3 | N/A | N/A | 1 to multiple | 20.7 | Eight III |
| Taguchi, 2021 | 6.22 cm3 | 65.8–72.6 | <3 mm | KUB CT + X-ray | 110 | 5.0 | 1.3 | 1–2 | 35.8 | Eight III, Three IV |
| Tominaga, 2023 | 8.48 cm3 | 91.8 | <4 mm | KUB X-ray/CT | 117 | 11.0 | N/A | 1 or multiple | 65.6 | One IV |
| Unno, 2021 | 6.08–6.05 cm3 | N/A | Absence of residual fragments of any size | KUB CT | 105.00–121.00 | 4–5 | 0.4–0.5 | N/A | N/A | N/A |
| Usui, 2020 | 5.56–6.48 cm2 | 61.1–52.0 | <4 mm | KUB CT | 131.16–132.60 | 7.3–8.7 | 1.95–2.03 | 1 | 31.2–44.2 | One III, Four IV |
| Wang, 2022 | 32.5 mm | 78.1 | <2 mm | KUB CT | 82.2 | 6.5 | 0.9 | 1 | 16.7 | None |
| Wen, 2016 | 6.89 cm2 | 87.88 | <4 mm | KUB X-ray/CT | 105.33 | 9.7 | 77.21 mL (loss) | 1–2 | 48.5 | Two IV |
| Yamashita, 2017 | 4.17 cm2 | 69.3 | <4 mm | KUB CT | 124 | 9 | N/A | N/A | 32.0 | Three III |
| Zaho, 2019 | 6.40 cm2 | 88.06 | <4 mm | KUB X-ray/CT | 79.77 | 3 | 0.4 | 1 | 7.5 | None |
SFR=Stone free rate, CT=Computer tomography, KUB=Kidney-ureter-bladder, US=Ultrasound, Hb=Hemoglobin, CR=Complication rate, N/A=Not reported
Efficacy: Stone-free rate
Stone-free status was defined variably among the included studies, ranging from <4 mm (in eighteen studies) to smaller sized fragment to the complete absence of any residual fragment. A heterogeneity was also found in the choice of imaging for the detection of residual stones: computed tomography (CT) kidney–ureter–bladder (KUB), X-ray, ultrasound (US), or a combination of these modalities were variably used. The followup imaging to assess the postoperative SFR was also planned at a variable time post-operatively. The mean SFR was >80%, ranging from 52% to 98.3%. Smaller stone volume and lower number of involved calyces positively correlated with a higher SFR. In ECIRS, an optimal stone clearance rate could be achieved despite the use of smaller instrumentation such as mini-PCNL and with a single-access tract.
Safety: Complications
The reported rate of complications ranged from 5.8% to 70.6%, but most of the complications were classified as Clavien–Dindo I or II, with occasional reports of grades III or IV complications and none reported a grade V complication. According to the studies that compared ECIRS with PCNL, the former has a superior safety profile with a lower rate of complications. Trials with higher rates of perioperative complications denoted longer operative time and higher stone burden as the risk factors for higher complication rates. In particular, one study[39] focused on identifying the risk factors for sepsis and found that the number of involved calyces, stone surface, and previous history of febrile urinary tract infection (UTI) correlated with higher rates of sepsis. On analyzing the procedure-related factors that could influence the risk of complications, only one study that compared the patient’s positions, found that the prone split-leg (PSL) position was associated with an increased risk of postoperative febrile UTI, without an increase in the risk of postoperative sepsis.[32] In this study, the authors reported a higher risk of injury to the urinary tract in the PSL cohort, a finding not reported in any of the other studies. The reported amount of blood loss was usually acceptable, with a median drop in the hemoglobin level of <2.5 g/dL, and a reported range of 0.4–2.4 mg/dL.
Efficiency: Operative time, hospital stay, number of access tracts
The reported operative time among the included studies was very variable, with a range of 42–140 min, and was probably affected by the different definitions of time calculation (i.e., from anesthesia to checkout time versus from cystoscopy to catheterization) or by the biases due to re-positioning of the patient during some of the procedures. Still, the operative time for ECIRS appears to be shorter as compared to that of PCNL and is not clearly affected by the choice of instruments. The length of hospital stay also varied significantly among the various studies, from shorter than 2 days to longer than 10 days.
Twenty-one studies reported on the feasibility to achieve a complete SFR with a single-access in all the patients. Six studies reported a need of up to two additional access tracts in a few cases, but 78.8%–98.6% of the patients were still managed by a single access tract. In the remaining six studies, the number of percutaneous tracts was not specified. The reduced need of multiple access tracts was more evident in the trials comparing ECIRS with standard or mini-invasive PCNL.
Tips and tricks: Position, puncture system, exit strategy
Among the studies, the most frequently used patient positions were the PSL and the Galdakao Modified Supine Valdivia (GMSV) position [Figure 2], but ECIRS was also performed in the lateral decubitus position.[33] Of the six studies that compared the patient’s position as the main outcome, a gold standard position for ECIRS could not be clearly identified. In fact, both the PSL and the GMSV positions had comparable SFR and complication rates.[12,32,34,35,40,43] ECIRS performed in the GMSV position seems to have slightly longer operative time as compared to the PSL and lateral decubitus positions, which could be explained by the larger exposed surface for the renal puncture in the PSL position that allows for a rapid antegrade access. Some of the authors who chose PSL for ECIRS gained the ureteric access in the lithotomy position, and some did not mention whether they changed the patient’s position or not. Others, for example, Hamamoto and Goktung, clarified that an ureteric access was obtained in the prone position in all the cases.[25,32]
Figure 2.

Galdakao Modified Supine Valdivia on the left and prone-split-leg on the right. The positions of surgeons are represented by red triangles and the endoscopic accesses are shown by green squares
Renal puncture was usually performed under the fluoroscopic and US guidance.[10,12-14,17-19,21,23,27,28,31,32,35-41] Some studies reported on the utilization of a ureteroscopic-assisted calyx puncture, which could be very useful, particularly in the absence of hydronephrosis.[15,30] When dealing with a very large or a staghorn stone that completely obstructs the target calyx and restricts the immediate direct visualization of the puncture needle, some authors reported an initial retrograde fragmentation of the calculus. Hence, by removing a part of the stone one can “make space” and uncover the calyx, thus allowing for the scope to negotiate into an optimal position.[25] URS-guided renal puncture seems to result in lower blood loss and higher accuracy when compared with the standard US-guided puncture.[15] Another proposed system for achieving a percutaneous access is the real-time virtual sonography, which showed promising results in the trial by Hamamoto et al.[20] but was not investigated further, and its validity is not currently confirmed.
Exit strategies were also not standardized among the studies. It appears that the tendency to perform a tubeless procedure or at least reserving the placement of the nephrostomy tube to the complicated procedures, has increased with time and this has been embraced by the most recent studies. When placed, the safety nephrostomy tubes were usually medium sized, ranging from 12Fr to 16Fr in most of the studies. The majority of the authors agree on routinely leaving a ureteral pigtail stent after the surgery.
DISCUSSION
ECIRS, despite being introduced in 2008, did not gain a significant popularity untill the last decade, when it gained a world wide attention, as is witnessed by the increasing number of publications. Gaining more and more popularity, ECIRS has been added to the European Association of Urology Guidelines as a good alternative for the management of complex renal stones as compared to the standard PCNL.[44]
In our study, nine trials compared the efficacy of standard or mini-invasive PCNL with that of the ECIRS. Of them, only one study did not report a significant difference between the two procedures,[23] while the other eight, and particularly those which recruited a higher number of patients, reported a significant advantage of ECIRS, both in the terms of efficacy (i.e., SFR) and safety (complication rate and postoperative pain). At the same time, when treating complex renal stones such as the staghorn stones, the superiority of ECIRS over URS or extracorporeal shockwave lithotripsy is undeniable, as is also the case with PCNL.[27]
The mean stone size reported in each study is presented in Table 3. The authors have measured the stone burden variably, with some reporting the maximum diameter, some the surface, and some the volume of the stones. With only one exception (Mishra 2022, with 16.8 ± 4.33 mm), all the studies reported a mean stone size of >2 cm, despite the different definitions used. We believe that the stone burden can be considered comparable among the studies.
There is a large variation in the definition of stone-free status reported in the various studies. While 18 of 33 studies accepted residual fragments <4 mm as nonsignificant, sometimes adding the absence of infection or any other symptom as a mandatory feature for defining the SFR. Others defined stone-free status with a threshold size for residual fragments as <3 mm or <2 mm, and only 4 studies defined success as the complete absence of any residual fragment at the follow up imaging.[2,14,16,19] Moreover, the imaging technique utilized to define the SFR was also very variable among the studies, with plain KUB X-ray, noncontrast-enhanced CT, or US being applied variably to verify the postoperative status, alone or in combination. When specified, the timing of radiological examination varied from day 1 post-ECIRS to 2- or 3-months following the surgery. It is well known that the reported rate of complete clearance is affected by the postoperative management schedules and the definition of SFR.
With these preconditions, it is difficult to satisfactorily state the superiority of ECIRS over PCNL in the terms of efficacy. However, at the same time, it is undeniable that despite the different definitions of SFR used, ECIRS obtained superior results as compared to PCNL,[13,17,18,21,23,24,28,29] contrary to the recent meta-analysis that showed a superior SFR in the favor of PCNL.[4] One clear advantage of ECIRS is indeed the direct visualization of all the renal cavities and the ureter, which helps the surgeons in achieving a complete clearance of the upper urinary tract and reduces the chances of a missed fragment.[45] Gökce et al. performed a comparative study to evaluate whether the retrograde URS detects a higher number of residual fragments as compared to the anterograde view or not.[14] In their study, the authors were able to reach a SFR of 92.7% (defined as the absence of any residual stone at the noncontrast-enhanced CT 7–14 days after surgery) through the addition of a URS-driven check of all the renal cavities, before exiting. This final step of ECIRS is therefore recommended to ensure a complete clearance.
PCNL has been shown to have excellent results for the management of large/complex stones, with a general SFR of 57% for the staghorn stones, 66% for the complex stones, 75% with the application of standard technique with multiple accesses and even as high as 78% for the simpler stones.[46] Our review found that the reported SFR varied between 52% and 98.3%, with a mean value of 80%. Our results are in line with those reported in a recent systematic review by Cracco and Scoffone,[47] where they found a high SFR of 61%–97%, especially in cases with smaller stones and standard accesses, usually through a single percutaneous puncture. Kuroda et al. analyzed the possible predictors of SFR in a study of 329 patients and found a negative correlation between the SFR and the stone surface area (total stone surface >400 mm2) and the number of involved calyces (more than 1 calyx involved).[26]
In the terms of complication rate, the reported results were variable, again being influenced by the way these were described. Ranging from 5.8% to 70% among the studies included in our review, the complication rate was highly influenced by the inclusion of mild asymptomatic haematuria or postoperative pain as a Clavien I complication. What is evident is that the Clavien grade >II complications were uncommon, being reported in only 13 of the 33 studies and completely absent in the others. Hematuria and uncomplicated postoperative fever on the other hand were quite frequent and were independent of the exit strategy applied. Tabei et al., in a retrospective study, analyzed the complication rate in 370 cases of ECIRS and found that the number of involved calyces (>4), stone surface area >500 mm2 and a history of febrile UTI independently predicted the development of systemic inflammation response syndrome.[39]
ECIRS has a low rate of bleeding-related complications, possibly due to the endoscopic assistance during the percutaneous access or the size of the tract used. There are two major advantages of ECIRS in the terms of renal puncture: (1) the direct visualization of the renal collecting system allows for a more accurate puncture and reduces the need of multiple attempts;[15,30] (2) the addition of URS to the standard PCNL provides the PCNL surgeon with the ability to use a basket to move the stones from one calyx to another, thus reducing the need of multiple percutaneous tracts.[4,47] Both these features help in reducing the intraoperative and postoperative blood loss, which is mainly related to the renal puncture.[48] In our review, the blood loss in patients undergoing ECIRS. was minimal, with a median drop in the hemoglobin levels of <2.5 g/dL. A correlation between the blood loss and the size of the percutaneous access tract could not be found in our study, and the blood loss was comparable between the mini- and the standard-percutaneous accesses. At the same time, it is well known that multiple percutaneous tracts can increase the risk of bleeding.[49] Moreover, the patient’s position did not influence the number of the percutaneous tracts performed. As shown in Table 3, a single-tract ECIRS was performed by many authors for both the procedures, while other used multiple accesses.
It is well known that larger the size of the percutaneous tract higher are the bleeding-related complications. But maintaining a size difference of 4F between the access tract and the nephroscope, the “rule of 4F”, helps with the outflow and maintains a low intrarenal pressure, thus reducing the chances of postoperative fever and sepsis.[50] On the same lines, the retrograde access during the ECIRS could facilitate intrarenal decompression through the ureteral access sheath (UAS).[51] Although the use of UAS is not mandatory, the majority of the studies included in our review, 24 of the 33 studies, used UAS in all the patients. When placed, the size of the UAS ranged from small size 9.5–11.5F to the larger ones (i.e., 14-16F), according to the surgeon’s preference and the patient’s characteristics. We believe the UAS is of great value while performing ECIRS, as it can help in maintaining a low intrarenal pressure, provides the possibility to retrieve fragments safely, and also reduces risk of injury to the ureter. Thus, the authors suggest that the UAS might be useful, especially in cases where a miniaturized percutaneous access is used or when a prolonged active role of the retrograde approach is expected.[47]
As the secondary outcome of this study, we wanted to evaluate the most common position the patient is placed into for ECIRS. GMSV is the most common position reported in the included studies, followed by the PSL position and lastly by the lateral decubitus position. The GMSV position allows an easy insertion of the ureteroscope and avoids anesthetic complications (i.e., respiratory and pharmacokinetic problems typical of obese patients), and thus is the most commonly used position.[43] The PSL position has certain advantages such as it allows for easy renal access, easy image driven puncture and allows for multiple percutaneous accesses.[52] A downside of the PSL position is that the percutaneous tract is in an anti-gravity position, and therefore the intra-renal pressure is higher resulting in higher fluid absorption.[34] Six studies compared the feasibility, efficacy, and safety of the different positions for ECIRS and found excellent and comparable outcomes, irrespective of the patient’s position. The only difference between the two most common positions was the surgical time, which appeared to be longer in the GMSV position probably due to the time taken in performing the renal puncture.[32,33] The choice of patient’s position during the surgery should rely on the surgeons preference, if not driven by anesthetic needs or anatomical abnormalities. Besides, there is a difference in the tilt of renal axis according to the various positions chosen for surgery, and the lower pole of the kidney may be displaced medially and ventrally in the oblique position because of the gravity.[33] As reported by Hamamoto et al., the PSL might be the position of choice when targeting the lower renal calyces, while the GMSV position makes the access to the middle calyces easier.[32]
A lack of standardization in the surgical procedure of ECIRS among the included studies is the main limitation of our review. Also, the variability in reporting SFR and in when and how it was assessed, made it difficult to have a clear image of the efficacy of ECIRS. Moreover, the heterogeneity in reporting of the complications affected the total complication rate reported in each study. We believe, that with the current rising interest in the ECIRS, a consensus would soon be reached and the technique would be standardized. ECIRS is undoubtedly an efficient procedure with a good safety profile and excellent intra- and postoperative outcomes, in particular for the management of complex and/or multiple large stones. With the reduced costs of laser-related endourological procedures and an increase in the role of minimally invasive PCNLs, ECIRS is bound to play a larger role in the future.[53,54] With a myriad of options available, the endourological procedure offered to a patient needs to be individualised with the use of patient-reported outcome measures (PROMs) in the coming future.[55,56]
The main downside of ECIRS, when compared to the standard PCNL, is the need of two experienced surgeons involved in the same procedure. The increase in the cost of this double procedure could be offset by the advantages in terms of shorter operative times, hospital stay, and complication rates. It also would mean a superior stone clearance in a single stage, possibly avoiding ancillary or second-look procedures.
CONCLUSION
ECIRS is a feasible and safe procedure that allows for an excellent clearance of complex and large stones and should be considered as the first treatment in patients with large staghorn calculi or multiple stones trapped in different renal calyces. ECIRS has a high single-stage SFR and low rate of major complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Geraghty RM, Davis NF, Tzelves L, Lombardo R, Yuan C, Thomas K, et al. Best practice in interventional management of urolithiasis: An update from the European Association of Urology guidelines panel for urolithiasis 2022. Eur Urol Focus. 2023;9:199–208. doi: 10.1016/j.euf.2022.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Scoffone CM, Cracco CM, Cossu M, Grande S, Poggio M, Scarpa RM. Endoscopic combined intrarenal surgery in Galdakao-modified supine Valdivia position: A new standard for percutaneous nephrolithotomy? Eur Urol. 2008;54:1393–403. doi: 10.1016/j.eururo.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 3.Scoffone C, Hoznek A, Cracco C. Supine Percutaneous Nephrolithotomy and ECIRS: The New Way of Interpreting PNL. Paris, France: Springer Science & Business Media; 2014. pp. 1–312. [Google Scholar]
- 4.Gauhar V, Castellani D, Cracco CM, Scoffone CM, Lim EJ, Rubilotta E, et al. Is endoscopic combined intrarenal surgery ready for primetime in endourology?. Outcomes from a systematic review and meta-analysis. Cent European J Urol. 2022;75:171–81. doi: 10.5173/ceju.2022.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdullatif VA, Sur RL, Abdullatif ZA, Szabo SR, Abbott JE. The safety and efficacy of endoscopic combined intrarenal surgery (ECIRS) versus percutaneous nephrolithotomy (PCNL): A systematic review and meta-analysis. Adv Urol 2022. 2022:1716554. doi: 10.1155/2022/1716554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung HD, Kim JC, Ahn HK, Kwon JH, Han K, Han WK, et al. Real-time simultaneous endoscopic combined intrarenal surgery with intermediate-supine position: Washout mechanism and transport technique. Investig Clin Urol. 2018;59:348–54. doi: 10.4111/icu.2018.59.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scoffone CM, Cracco CM. Invited review: The tale of ECIRS (endoscopic combined intrarenal surgery) in the Galdakao-modified supine Valdivia position. Urolithiasis. 2018;46:115–23. doi: 10.1007/s00240-017-1015-9. [DOI] [PubMed] [Google Scholar]
- 8.Hiller SC, Ghani KR. Frontiers of stone management. Curr Opin Urol. 2020;30:17–23. doi: 10.1097/MOU.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 9.Lim EJ, Osther PJ, Valdivia Uría JG, Ibarluzea JG, Cracco CM, Scoffone CM, et al. Personalized stone approach: Can endoscopic combined intrarenal surgery pave the way to tailored management of urolithiasis? Minerva Urol Nephrol. 2021;73:428–30. doi: 10.23736/S2724-6051.21.04443-8. [DOI] [PubMed] [Google Scholar]
- 10.Liu YH, Jhou HJ, Chou MH, Wu ST, Cha TL, Yu DS, et al. Endoscopic combined intrarenal surgery versus percutaneous nephrolithotomy for complex renal stones: A systematic review and meta-analysis. J Pers Med. 2022;12:532. doi: 10.3390/jpm12040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abouelgreed TA, Abdelaal MA, Amin MM, Elatreisy A, Shalkamy O, Abdrabuh AM, et al. Endoscopic combined intrarenal surgery in the prone split-leg position versus Galdakao-modified supine Valdivia position for the management of partial staghorn calculi. BMC Urol. 2022;22:163. doi: 10.1186/s12894-022-01115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen J, Xu G, Du C, Wang B. Minimally invasive percutaneous nephrolithotomy versus endoscopic combined intrarenal surgery with flexible ureteroscope for partial staghorn calculi: A randomised controlled trial. Int J Surg. 2016;28:22–7. doi: 10.1016/j.ijsu.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Gökce Mİ, Gülpinar O, Ibiş A, Karaburun M, Kubilay E, Süer E. Retrograde versus antegrade fl exible nephroscopy for detection of residual fragments following PNL: A prospective study with computerized tomography control. Int Braz J Urol. 2019;45:581–7. doi: 10.1590/S1677-5538.IBJU.2018.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugino T, Hamamoto S, Unno R, Taguchi K, Okada A, Yasui T. Effectiveness of ureteroscope-assisted renal puncture for endoscopic combined intrarenal surgery. Int J Urol. 2019;26:424–5. doi: 10.1111/iju.13865. [DOI] [PubMed] [Google Scholar]
- 16.Unno R, Taguchi K, Hamamoto S, Hattori T, Kawase K, Okada T, et al. Anovel approach in creating nephrostomy using a double-lumen access sheath during endoscopic combined intrarenal surgery. Transl Androl Urol. 2021;10:4181–91. doi: 10.21037/tau-21-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F, Li J, Tang L, Li C. A comparative study of endoscopic combined intrarenal surgery (ECIRS) in the Galdakao-modified supine Valdivia (GMSV) position and minimally invasive percutaneous nephrolithotomy for complex nephrolithiasis: A retrospective single-center study. Urolithiasis. 2021;49:161–6. doi: 10.1007/s00240-020-01207-5. [DOI] [PubMed] [Google Scholar]
- 18.Mishra DK, Agrawal MS, Shah M, Naganathan K, Hameed Z, Gauhar V. Ambulatory minimally invasive endoscopic combined intrarenal surgery in management of large impacted proximal ureteral calculi: A feasibility study at a tertiary referral center. J Endourol. 2023;37:251–6. doi: 10.1089/end.2022.0234. [DOI] [PubMed] [Google Scholar]
- 19.Kallidonis P, Tsaturyan A, Faria-Costa G, Ballesta Martinez B, Peteinaris A, Adamou C, et al. Nonpapillary prone endoscopic combined intrarenal surgery: Effectiveness, safety and tips, and tricks. World J Urol. 2022;40:3067–74. doi: 10.1007/s00345-022-04178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamamoto S, Unno R, Taguchi K, Ando R, Hamakawa T, Naiki T, et al. Anew navigation system of renal puncture for endoscopic combined intrarenal surgery: Real-time virtual sonography-guided renal access. Urology. 2017;109:44–50. doi: 10.1016/j.urology.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Hamamoto S, Yasui T, Okada A, Taguchi K, Kawai N, Ando R, et al. Endoscopic combined intrarenal surgery for large calculi: Simultaneous use of flexible ureteroscopy and mini-percutaneous nephrolithotomy overcomes the disadvantageous of percutaneous nephrolithotomy monotherapy. J Endourol. 2014;28:28–33. doi: 10.1089/end.2013.0361. [DOI] [PubMed] [Google Scholar]
- 22.Schulster M, Small AC, Silva MV, Abbott JE, Davalos JG. Endoscopic combined intrarenal surgery can accurately predict high stone clearance rates on postoperative CT. Urology. 2019;133:46–9. doi: 10.1016/j.urology.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZH, Lee KH, Tseng WH, Su CC, Hsieh KL, Lim CY, et al. Comparison of mini endoscopic combined intrarenal surgery and multitract minimally invasive percutaneous nephrolithotomy specifically for kidney staghorn stones: A single-centre experience. BMC Urol. 2022;22:93. doi: 10.1186/s12894-022-01030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao H, Zhang H, Wang Y, Li K, Du W, Wang X, et al. Treatment of complex renal calculi by digital flexible ureterorenoscopy combined with single-tract super-mini percutaneous nephrolithotomy in prone position: A retrospective cohort study. Med Sci Monit. 2019;25:5878–85. doi: 10.12659/MSM.915034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goktug HN, Ozturk U, Cimen S, Kaymak S, Dogan AE, Imamoglu MA. Comparison of the conventional PNL with ecirs in the treatment of complete staghorn kidney stones. J Coll Physicians Surg Pak. 2023;33:346–51. doi: 10.29271/jcpsp.2023.03.346. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda S, Ito H, Sakamaki K, Tabei T, Kawahara T, Terao H, et al. Development and internal validation of a classification system for predicting success rates after endoscopic combined intrarenal surgery in the modified Valdivia position for large renal stones. Urology. 2015;86:697–702. doi: 10.1016/j.urology.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Jung HD, Moon YJ, Almujalhem AJ, Alqahtani AA, Alkhureeb MA, Lee JY. The first 100 cases of endoscopic combined intrarenal surgery in Korea: Matched cohort analyses versus shock-wave lithotripsy. Yonsei Med J. 2022;63:440–5. doi: 10.3349/ymj.2022.63.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leng S, Xie D, Zhong Y, Huang M. Combined single-tract of minimally percutaneous nephrolithotomy and flexible ureteroscopy for staghorn calculi in oblique supine lithotomy position. Surg Innov. 2018;25:22–7. doi: 10.1177/1553350617741023. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Zheng B, Wen J, Mao H, Jiang T, Chen Q, et al. One-stage efficacy of single tract minimally invasive ECIRS in the improved prone frog split-leg position for staghorn stones. BMC Urol. 2022;22:54. doi: 10.1186/s12894-022-01003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taguchi K, Yamashita S, Hamamoto S, Deguchi R, Kawase K, Okada T, et al. Ureteroscopy-assisted puncture for ultrasonography-guided renal access significantly improves overall treatment outcomes in endoscopic combined intrarenal surgery. Int J Urol. 2021;28:913–9. doi: 10.1111/iju.14603. [DOI] [PubMed] [Google Scholar]
- 31.Usui K, Komeya M, Taguri M, Kataoka K, Asai T, Ogawa T, et al. Minimally invasive versus standard endoscopic combined intrarenal surgery for renal stones: A retrospective pilot study analysis. Int Urol Nephrol. 2020;52:1219–25. doi: 10.1007/s11255-020-02433-x. [DOI] [PubMed] [Google Scholar]
- 32.Hamamoto S, Okada S, Inoue T, Taguchi K, Kawase K, Okada T, et al. Comparison of the safety and efficacy between the prone split-leg and Galdakao-modified supine Valdivia positions during endoscopic combined intrarenal surgery: A multi-institutional analysis. Int J Urol. 2021;28:1129–35. doi: 10.1111/iju.14655. [DOI] [PubMed] [Google Scholar]
- 33.Otsuka I, Terada N, Iwamoto H, Kobayashi T, Kamoto T. Comparison of safety and efficacy in endoscopic combined intrarenal surgery performed in the lateral decubitus and Galdakao-modified supine Valdivia positions. Urology. 2023;172:49–54. doi: 10.1016/j.urology.2022.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Hamamoto S, Yasui T, Okada A, Takeuchi M, Taguchi K, Shibamoto Y, et al. Developments in the technique of endoscopic combined intrarenal surgery in the prone split-leg position. Urology. 2014;84:565–70. doi: 10.1016/j.urology.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Hamamoto S, Yasui T, Okada A, Koiwa S, Taguchi K, Itoh Y, et al. Efficacy of endoscopic combined intrarenal surgery in the prone split-leg position for staghorn calculi. J Endourol. 2015;29:19–24. doi: 10.1089/end.2014.0372. [DOI] [PubMed] [Google Scholar]
- 36.Inoue T, Kinoshita H, Okada S, Hamamoto S, Taguchi M, Murota T, et al. Wideband Doppler ultrasound-guided mini-endoscopic combined intrarenal surgery as an effective and safe procedure for management of large renal stones: A preliminary report. Urology. 2016;95:60–6. doi: 10.1016/j.urology.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 37.Manikandan R, Mittal JK, Dorairajan LN, Mishra AK, Sreerag KS, Verma A. Endoscopic combined intrarenal surgery for simultaneous renal and ureteral stones: A retrospective study. J Endourol. 2016;30:1056–61. doi: 10.1089/end.2016.0329. [DOI] [PubMed] [Google Scholar]
- 38.Ping H, Zhang JH, Wang MS, Xing NZ. Endoscopic combined intrarenal surgery for the treatment of postpercutaneous nephrolithotomy residual stones. Chin Med J (Engl) 2016;129:2885–7. doi: 10.4103/0366-6999.194659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabei T, Ito H, Usui K, Kuroda S, Kawahara T, Terao H, et al. Risk factors of systemic inflammation response syndrome after endoscopic combined intrarenal surgery in the modified Valdivia position. Int J Urol. 2016;23:687–92. doi: 10.1111/iju.13124. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Sun H, Xie D, Liu Z, Yu D, Ding D. Application of a new position in endoscopic combined intrarenal surgery: Modified prone split-leg position. BMC Urol. 2022;22:38. doi: 10.1186/s12894-022-00994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita S, Kohjimoto Y, Iba A, Kikkawa K, Hara I. Stone size is a predictor for residual stone and multiple procedures of endoscopic combined intrarenal surgery. Scand J Urol. 2017;51:159–64. doi: 10.1080/21681805.2017.1284897. [DOI] [PubMed] [Google Scholar]
- 42.Tominaga K, Inoue T, Yamamichi F, Fujita M, Fujisawa M. Impact of vacuum-assisted mini-endoscopic combined intrarenal surgery for staghorn stones: Analysis of perioperative factors of postoperative fever and stone-free status. J Endourol. 2023;37:400–6. doi: 10.1089/end.2022.0579. [DOI] [PubMed] [Google Scholar]
- 43.Ibarluzea G, Scoffone C, Cracco C, Poggio M, Porpiglia F, Terrone C, et al. Supine Valdivia and modified lithotomy position for simultaneous anterograde and retrograde endourological access. BJU Int. 2007;100:233–6. doi: 10.1111/j.1464-410X.2007.06960.x. [DOI] [PubMed] [Google Scholar]
- 44.Skolarikos A, Neisius A, Petřík A, Somani B, Thomas K, Gambaro G. EAU Guidelines on Urolithiasis. EAU Guidelines. Presented at the EAU Annual Congress Amsterdam. 2022. [[Last accessed on 2023 Jun 12]]. Available from: https://uroweb.org/guidelines/urolithiasis/chapter/introduction .
- 45.Cracco CM, Knoll T, Liatsikos EN, Osther PJ, Smith AD, Scarpa RM, et al. Rigid-only versus combined rigid and flexible percutaneous nephrolithotomy: A systematic review. Minerva Urol Nefrol. 2017;69:330–41. doi: 10.23736/S0393-2249.17.02841-7. [DOI] [PubMed] [Google Scholar]
- 46.de la Rosette J, Assimos D, Desai M, Gutierrez J, Lingeman J, Scarpa R, et al. The clinical research office of the endourological society percutaneous nephrolithotomy global study: Indications, complications, and outcomes in 5803 patients. J Endourol. 2011;25:11–7. doi: 10.1089/end.2010.0424. [DOI] [PubMed] [Google Scholar]
- 47.Cracco CM, Scoffone CM. Endoscopic combined intrarenal surgery (ECIRS) –Tips and tricks to improve outcomes: A systematic review. Turk J Urol. 2020;46:S46–57. doi: 10.5152/tud.2020.20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramón de Fata F, Pérez D, Resel-Folkersma L, Galán JA, Serrano A, Servera A, et al. Analysis of the factors affecting blood loss in percutaneous nephrolithotomy: A registry of the Spanish Association of Urology in the supine position. Actas Urol Esp. 2013;37:527–32. doi: 10.1016/j.acuro.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Arora AM, Pawar PW, Tamhankar AS, Sawant AS, Mundhe ST, Patil SR. Predictors for severe hemorrhage requiring angioembolization post percutaneous nephrolithotomy: A single-center experience over 3 years. Urol Ann. 2019;11:180–6. doi: 10.4103/UA.UA_75_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilhelm K, Müller PF, Schulze-Ardey J, Späth J, Suarez-Ibarrola R, Miernik A, et al. Characterization of flow-caused intrarenal pressure conditions during percutaneous nephrolithotomy in vitro. J Endourol. 2019;33:235–41. doi: 10.1089/end.2018.0769. [DOI] [PubMed] [Google Scholar]
- 51.Traxer O, Wendt-Nordahl G, Sodha H, Rassweiler J, Meretyk S, Tefekli A, et al. Differences in renal stone treatment and outcomes for patients treated either with or without the support of a ureteral access sheath: The clinical research office of the endourological society ureteroscopy global study. World J Urol. 2015;33:2137–44. doi: 10.1007/s00345-015-1582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duty B, Waingankar N, Okhunov Z, Ben Levi E, Smith A, Okeke Z. Anatomical variation between the prone, supine, and supine oblique positions on computed tomography: Implications for percutaneous nephrolithotomy access. Urology. 2012;79:67–71. doi: 10.1016/j.urology.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Somani BK, Robertson A, Kata SG. Decreasing the cost of flexible ureterorenoscopic procedures. Urology. 2011;78:528–30. doi: 10.1016/j.urology.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 54.Jones P, Elmussareh M, Aboumarzouk OM, Mucksavage P, Somani BK. Role of minimally invasive (micro and ultra-mini) PCNL for adult urinary stone disease in the modern era: Evidence from a systematic review. Curr Urol Rep. 2018;19:27. doi: 10.1007/s11934-018-0764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juliebø-Jones P, Keller EX, Haugland JN, Æsøy MS, Beisland C, Somani BK, et al. Advances in ureteroscopy: New technologies and current innovations in the era of tailored endourological stone treatment (TEST) J Clin Urol. 2023;16:190–8. [Google Scholar]
- 56.Mehmi A, Jones P, Somani BK. Current status and role of patient-reported outcome measures (PROMs) in endourology. Urology. 2021;148:26–31. doi: 10.1016/j.urology.2020.09.022. [DOI] [PubMed] [Google Scholar]


