Abstract
Purpose:
To evaluate disease progression using static perimetry (SP) in patients with USH2A-related retinal degeneration, including Usher syndrome type 2 (USH2) and nonsyndromic autosomal recessive retinitis pigmentosa (ARRP).
Design:
Prospective, observational cohort study.
Methods
Setting:
16 clinical sites in Europe and North America.
Study Population:
Study participants with biallelic disease-causing sequence variants in USH2A with baseline best-corrected visual acuity (BCVA) letter score ≥54 (N=102).
Observation Procedures:
SP, BCVA, full-field stimulus thresholds (FST), spectral domain optical coherence tomography macular scans, and fundus-guided mesopic microperimetry (MP) also were performed at baseline and annually.
Main Outcome Measures:
Total hill of vision (VTOT), hill of vision in the central 30° (V30), VTOT-V30 (VPERIPH), and mean sensitivity.
Results:
The average decline (95% CI) was 2.05 (1.40, 2.70) decibel-steradian (dB-sr)/year for VTOT, 0.48 (0.32, 0.65) dB-sr/year for V30, 1.53 (0.97, 2.08) dB-sr/year for VPERIPH and 0.55 (0.40, 0.71) dB/year for mean sensitivity. Average percentage decline was 8.3 (5.5, 11.1) %/year for VTOT, 5.2 (3.0, 7.4) %/year for V30, 16.0 (9.5, 22.0) %/year for VPERIPH, and 5.1 (3.5, 6.7) %/year for mean sensitivity. Changes from baseline to Year 2 in all SP measures were highly correlated [rs ranging from 0.52 (V30 vs VPERIPH) to 0.98 (VTOT vs Vperiph)].
Conclusions:
Quantitative measures of SP declined significantly over 2 years in USH2A-related retinal degeneration. The annual percent rate of change was greatest for VTOT and VPERIPH, while V30 and mean sensitivity changed least, reflecting earlier and more severe peripheral degeneration compared to central loss.
Introduction
Disease-causing variants in the USH2A gene are among the most common causes of photoreceptor degeneration, either with congenital hearing loss (Usher syndrome type 2, USH2) or as nonsyndromic autosomal recessive retinitis pigmentosa (ARRP). Because the USH2A gene exceeds the carrying capacity of adeno-associated viral (AAV) vectors that have received regulatory approval for RPE65-related retinal degeneration,1–3 treatments for USH2A-related USH2 and ARRP have been more challenging than other autosomal recessive retinal degenerations to deliver. New therapeutic approaches including antisense oligonucleotide (ASO)4,5 and gene editing using clustered regular inter-spaced repeat (CRISPR/CAS)6–8 offer promising approaches, and clinical trials of ASO treatments for patients with mutations in the most commonly affected region of USH2A, exon 13,5 are enrolling patients (NCT05176717 and NCT05158296).
To inform the design and interpretation of clinical trials for USH2A-related retinal degeneration, natural history studies of disease progression are essential. The multicenter, international, longitudinal, prospective Rate of Progression of USH2A-related Retinal Degeneration (RUSH2A) Study will monitor disease progression, comparing quantitative static perimetry (SP) measures over 4 years. The study design and baseline characteristics of the RUSH2A study have been reported.9–11 Here we present change in SP and other measures of retinal function and structure after 2 years, midway through the study period. Although the rate of progression at the end of the study after 4 years will be evaluated later, we present the results at 2 years in the present manuscript as they may inform interpretation of treatment trial results and shape study design of future clinical trials for patients with USH2A-related USH2 and ARRP.
Methods
Study Design
The RUSH2A study design has been described previously (NCT03146078).9 Briefly, 127 participants were enrolled between August 2017 and December 2018 at 16 clinical sites in North America and Europe. The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review boards (IRBs) or ethics boards associated with each participating site.
Eligible participants at least 8 years of age had a clinical diagnosis of rod-cone degeneration. All participants had at least 2 disease-causing USH2A sequence variants, and ARRP participant variants were further documented as homozygous or heterozygous in trans. A committee reviewed all genetic reports to confirm the variants as pathogenic or likely pathogenic. The majority of testing was performed in the “study” eye, defined as the eye with better baseline best-corrected visual acuity (BCVA). The primary cohort included 105 participants with a baseline ETDRS letter score of 54 or greater (20/80 or better) in the study eye, central visual field at least 10 degrees diameter to a III4e target based on kinetic perimetry, and stable fixation. A secondary cohort of 22 participants with worse visual function was enrolled to complete a baseline visit only. The primary cohort was scheduled to be tested annually for 4 years after the baseline visit. The longitudinal data through 2 years of follow-up for the primary cohort were included in this report. Because of the COVID-19 pandemic, a protocol addendum allowed the annual visits to occur outside of the +/− 4-week window, up to 6 months after the target annual visit dates (i.e., 52, 104, 156 and 208 weeks from baseline visit date) or remotely (patient-reported outcome data only) if this was not possible.
Outcome Measures
The details of the static perimetry (SP) testing method have been described elsewhere. 9 Briefly, SP was performed using the Octopus 900 (Haag-Streit, Mason, OH) with the German Adaptive Thresholding Estimation (GATE) strategy and a custom centrally-condensed 186-point grid (historically called 185-point grid) to a size V stimulus.12 Four measures were included in the analyses herein: full-field hill of vision (VTOT), 30-degree hill of vision (V30), peripheral hill of vision (VPERIPH) defined as VTOT minus V30, and mean sensitivity.13,14 The custom grid was developed and VTOT, V30 and mean sensitivity were graded by the Casey Reading Center (Casey Eye Institute, Oregon Health Sciences University, Portland, Oregon, USA). The reliability factor (RF) for each testing session was defined as the sum of false-positive and false-negative answers divided by the total number of trial questions. SP was tested three times at the baseline visit, then once at each annual follow-up visit. The average of each SP measure from three repeated SP sessions at the baseline visit was used as the baseline value for analysis in this report.
Other aspects of visual function or retinal structure also were measured. After protocol refraction, best corrected visual acuity (BCVA) testing was performed using either the EVA tester or Early Treatment of Diabetic Retinopathy Study (ETDRS) charts with results recorded as the letter score.15,16 Full-field stimulus thresholds (FST) were determined using white, blue, and red stimuli (Espion E3 system, Diagnosys LCC, Lowell, MA).10,17 Fundus-guided mesopic (standard) microperimetry was performed using a Macular Integrity Assessment (MAIA-2) unit (iCare, Raleigh, NC) and summarized by mean sensitivity.18 The ellipsoid zone (EZ) area and central subfield thickness (CST) were derived from optical coherent tomography (OCT) volume scans (Heidelberg Spectralis HRA+OCT, Heidelberg Engineering GmbH, Heidelberg, Germany).18,19
Statistical Methods
Data from the primary cohort (N=105) were included in this report. In order to assess changes, 3 study eyes with only baseline data were excluded from analyses. To mitigate floor effects, a subset of the primary cohort, defined as the preserved visual field (VF) cohort, was analyzed including only study eyes with baseline VTOT > 5 dB-sr (N=88).
The distributions of SP measures at each visit were summarized using means, standard deviations (SDs), medians, interquartile ranges (IQRs) and ranges. Mixed effects models with a random intercept were used to estimate the annual rates of change and percentage rates of change using log transformed data with 95% confidence intervals (95% CI). Time was calculated as the number of days from baseline divided by 365.25. Models were applied to the entire cohort and to the preserved VF cohort as defined above. A model excluding unreliable test results (false positives ≥ 15%, N=8) and a model down-weighting outlier rates of change were also applied to the preserved VF cohort. For the outlier down-weighted model, the rate of decline for each participant was calculated from a simple linear regression model, then a robust regression model using M estimation with a Huber weighting function20,21 was used to calculate the weight to be applied in the mixed effects model for each eye in the preserved VF cohort. Associations among change in SP measures and with change in other measures (BCVA, FST, OCT and MP) from baseline to 2 years were assessed with Spearman correlation coefficients. Subgroup analyses (e.g. by clinical diagnosis) will be performed on the 4-year data when there are more data points.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and reported P-values are two-sided.
Results
Study Population
The number of participants completing baseline, 1-year and 2-year visits is shown in Figure 1. Among the 105 participants recruited into the primary cohort in the RUSH2A study, 1 participant died before the 1-year visit. For the rest of the 104 participants, 102 completed the 1-year in-office visit and 88 completed the 2-year in-office visit for assessment on visual functional and structural measures.
Figure 1.
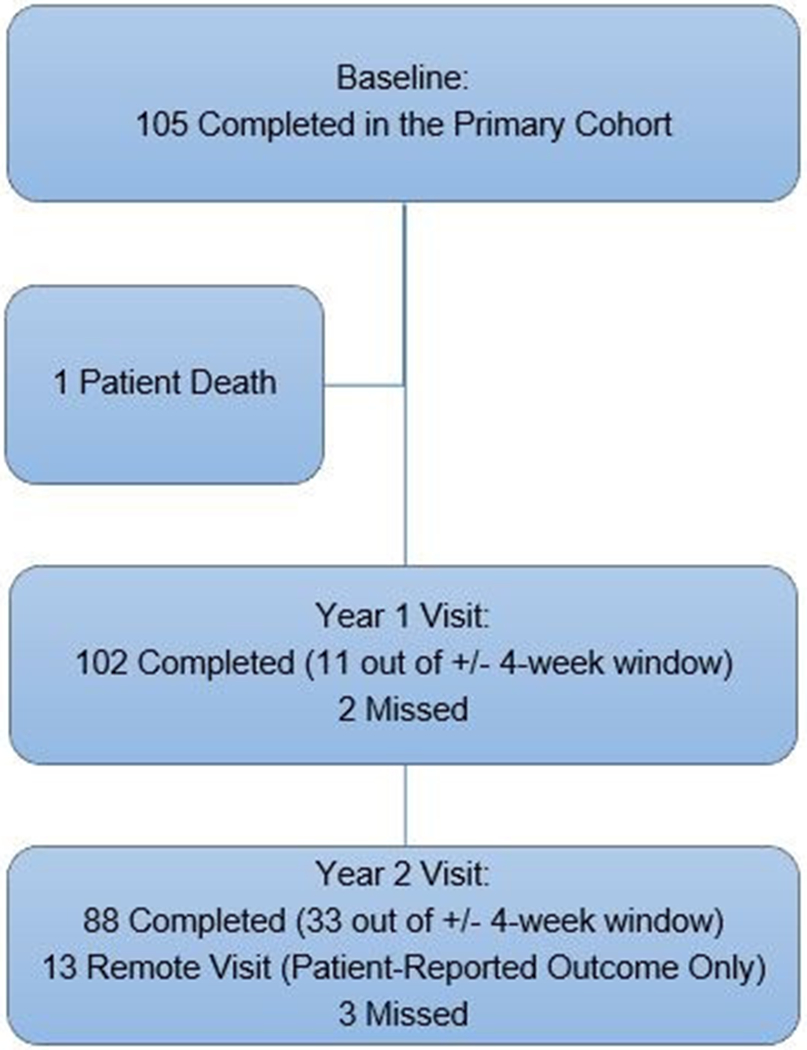
Flowchart
As shown in Table 1, among the 102 participants included in the entire analysis cohort, the clinical diagnosis was USH2 for 64 (63%) participants and ARRP for 38 (37%) participants. The mean age was 37 years (SD, 13), 58 (57%) were female, 91 (89%) were white, and 64 (63%) were enrolled in the US or Canadian sites. Median duration of disease at enrollment was 13 years (IQR, 7 to 20).
Table 1.
Participant characteristics at baseline for the analysis cohort (N=102)
| N (%) | |
|---|---|
| Clinical Diagnosis | |
| USH2 | 64 (63%) |
| ARRP | 38 (37%) |
| Age at Enrollment (years) | |
| <35 | 43 (42%) |
| 35-<45 | 35 (34%) |
| ≥45 | 24 (24%) |
| Mean ± SD | 37 ± 13 |
| Gender | |
| Female | 58 (57%) |
| Male | 44 (43%) |
| Race/Ethnicity | |
| White | 91 (89%) |
| Hispanic or Latino | 8 (8%) |
| Asian | 3 (3%) |
| Duration of Disease at Enrollment (years) a | |
| <10 | 34 (34%) |
| 10-<20 | 42 (42%) |
| ≥20 | 25 (25%) |
| Median (IQR) | 13 (7, 20) |
| Daily smoker ever | |
| Yes | 27 (26%) |
| No | 75 (74%) |
| Area | |
| USA and Canada | 64 (63%) |
| Europe | 38 (37%) |
One participant was missing age of onset (a participant-reported field based on their awareness of visual symptoms) and duration of disease (computed based on age of onset and date of enrollment)
Distribution of SP Measures
The distribution of 4 SP measures at each visit for the entire analysis cohort is shown in Table 2. Figure 2 provides a plot of the VTOT values for each eye showing an overall downward trend over time but with some eyes having increases (improvement) between adjacent measurements. The average VTOT was 33.1 (SD, 23.5) dB-sr at study baseline, 30.9 (SD, 23.4) dB-sr at the Year 1 visit, and 29.0 (SD, 22.6) dB-sr at the Year 2 visit. The average V30 was 10.2 (SD, 5.6) dB-sr at study baseline, 9.8 (SD, 5.5) dB-sr at Year 1 and 9.2 (SD, 5.4) dB-sr at Year 2. The average VPERIPH was 22.8 (SD, 18.8) dB-sr at study baseline, 21.0 (SD, 18.8) dB-sr at Year 1 and 19.7 (SD, 18.1) at Year 2. The average mean SP sensitivity was 11.7 (SD, 5.8) dB at baseline, 11.1 (SD, 5.7) dB at Year 1 and 10.6 (SD, 5.5) dB at Year 2.
Table 2.
Distribution of static perimetry measures at baseline and years 1 and 2
| Outcomes | Baseline | Year 1 | Year 2 |
|---|---|---|---|
| VTOT (dB-sr) | |||
| N | 98 a | 96 | 85 |
| Mean ± SD | 33.1 ± 23.5 | 30.9 ± 23.4 | 29.0 ± 22.6 |
| Median (IQR) | 29.8 (13.0, 51.2) | 24.5 (11.4, 50.1) | 22.8 (9.1, 44.4) |
| Range | 1.5 to 90.5 | 1.5 to 84.5 | 1.5 to 87.5 |
| V30 (dB-sr) | |||
| N | 102 | 100 | 87 |
| Mean ± SD | 10.2 ± 5.6 | 9.8 ± 5.5 | 9.2 ± 5.4 |
| Median (IQR) | 9.8 (5.4, 13.3) | 9.6 (5.3, 13.7) | 7.9 (4.8, 13.5) |
| Range | 1.5 to 22.7 | 1.5 to 22.3 | 1.5 to 21.8 |
| VPERIPH (dB-sr) | |||
| N | 98 a | 96 | 85 |
| Mean ± SD | 22.8 ± 18.8 | 21.0 ± 18.8 | 19.7 ± 18.1 |
| Median (IQR) | 19.3 (6.3, 37.7) | 14.6 (4.4, 38.8) | 14.1 (3.6, 32.1) |
| Range | 0.0 to 70.8 | 0.0 to 65.5 | 0.0 to 66.1 |
| Mean Sensitivity (dB) | |||
| N | 98 a | 96 | 85 |
| Mean ± SD | 11.7 ± 5.8 | 11.1 ± 5.7 | 10.6 ± 5.5 |
| Median (IQR) | 11.2 (7.1, 15.9) | 10.5 (6.9, 14.7) | 9.5 (6.4, 14.2) |
| Range | 2.4 to 24.6 | 2.1 to 24.3 | 2.2 to 24.6 |
Four participants had missing data due to wrong grid was used
Figure 2.
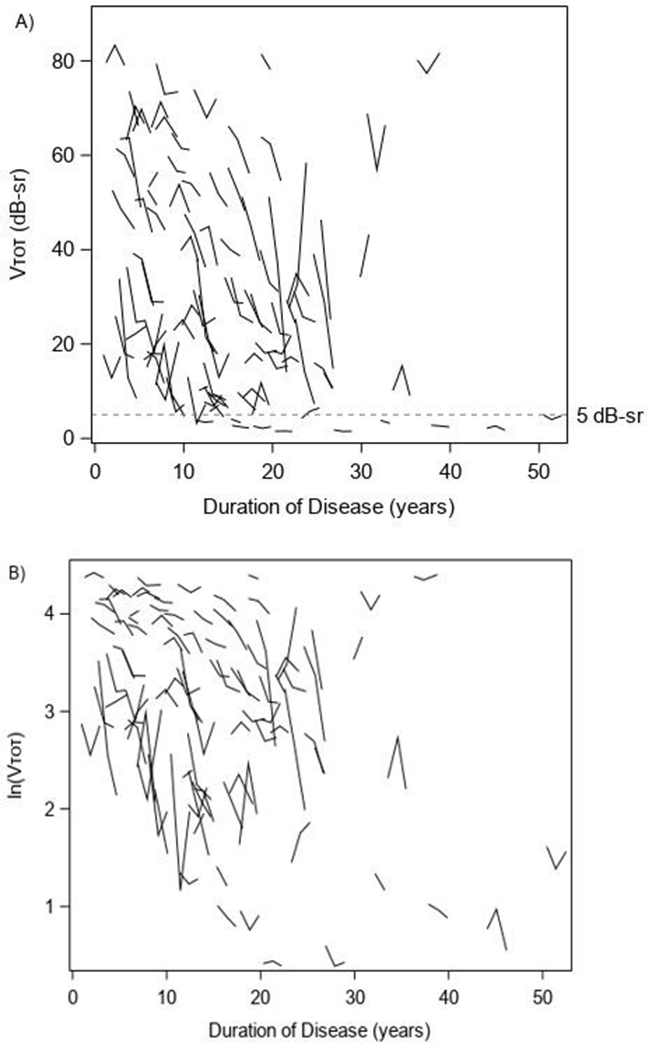
Vtot measurements x duration
Estimated Annual Change from Models
The average decline (95% CI) in VTOT was 2.05 (1.40, 2.70) dB-sr/year (Table 3) or 8.3 (5.5, 11.1) %/year (Table 4) in the entire cohort, but greater at 2.25 (1.54, 2.96) dB-sr/year or 8.8 (5.7, 11.7) %/year in the preserved VF cohort (with VTOT > 5 dB-sr at study baseline). The decline rate of V30 was 0.48 (0.32, 0.65) dB-sr/year or 5.2 (3.0, 7.4) %/year in the entire cohort versus 0.56 (0.40, 0.73) dB-sr/year or 5.9 (4.0, 7.9) %/year in the preserved VF cohort. The decline of VPERIPH was 1.53 (0.97, 2.08) dB-sr/year or 16.0 (9.5, 22.0) %/year in the entire cohort, and 1.68 (1.07, 2.29) dB-sr/year or 13.6 (7.7, 19.2) %/year in the preserved VF cohort. The decline rate for mean sensitivity was 0.55 (0.40, 0.71) dB/year or 5.1 (3.5, 6.7) %/year in the entire cohort and 0.60 (0.43, 0.77) dB/year or 5.4 (3.7, 7.1) %/year in the preserved VF cohort. Excluding unreliable observations or down-weighting outliers reduced the estimated percentage of decline compared to using all eyes in the preserved VF cohort. The outlier down-weighted models using the preserved VF cohort had similar point estimates compared with the entire cohort, but narrower confidence intervals.
Table 3.
Estimated average annual change in static perimetry measures
| Outcomes | Entire cohort (N=102) a | ------------Preserved visual field cohort b ----------- | ||
|---|---|---|---|---|
| All (N=88) | False positives <15% (N=80) c | Outliers down-weighted (N=88) d | ||
| VTOT (dB-sr/year) | ||||
| Annual Change e | −2.05 | −2.25 | −2.27 | −2.03 |
| 95% CI | (−2.70, −1.40) | (−2.96, −1.54) | (−2.92, −1.62) | (−2.57, −1.49) |
| V30 (dB-sr/year) | ||||
| Annual Change e | −0.48 | −0.56 | −0.52 | −0.52 |
| 95% CI | (−0.65, −0.32) | (−0.73, −0.40) | (−0.67, −0.37) | (−0.65, −0.38) |
| VPERIPH (dB-sr/year) | ||||
| Annual Change e | −1.53 | −1.68 | −1.75 | −1.56 |
| 95% CI | (−2.08, −0.97) | (−2.29, −1.07) | (−2.31, −1.18) | (−2.02, −1.10) |
| Mean Sensitivity (dB/year) | ||||
| Annual Change e | −0.55 | −0.60 | −0.58 | −0.54 |
| 95% CI | (−0.71, −0.40) | (−0.77, −0.43) | (−0.73, −0.43) | (−0.67, −0.41) |
Number of missing data: VTOT (4), VPERIPH (4), mean sensitivity (4)
Include participants with baseline VTOT >5 dB-sr
Tests with false positive rate <15%
Outliers were down-weighted using weighted mixed-effects model, weights were computed from robust regression modelling of estimate rate of decline from each participant
All P-values for testing the average annual change estimates against zero were <0.001
Table 4.
Estimated average annual percentage change in static perimetry measures based on log-transformed data
| Outcomes | Entire cohort (N=102) a | ------------ Preserved visual field cohort b ---------- | ||
|---|---|---|---|---|
| All (N=88) | False positives <15% (N=80) c | Outliers down-weighted (N=88) d | ||
| VTOT (%/year) | ||||
| Annual Change e | −8.3 | −8.8 | −8.3 | −7.3 |
| 95% CI | (−11.1, −5.5) | (−11.7, −5.7) | (−11.3, −5.2) | (−9.7, −4.8) |
| V30 (%/year) | ||||
| Annual Change e | −5.2 | −5.9 | −5.5 | −5.0 |
| 95% CI | (−7.4, −3.0) | (−7.9, −4.0) | (−7.4, −3.5) | (−6.8, −3.3) |
| VPERIPH (%/year) | ||||
| Annual Change e | −16.0 | −13.6 | −13.2 | −9.7 |
| 95% CI | (−22.0, −9.5) | (−19.2, −7.7) | (−19.1, −6.9) | (−13.7, −5.5) |
| Mean Sensitivity (%/year) | ||||
| Annual Change e | −5.1 | −5.4 | −5.1 | −4.9 |
| 95% CI | (−6.7, −3.5) | (−7.1, −3.7) | (−6.8, −3.4) | (−6.3, −3.5) |
Number of missing data: VTOT (4), VPERIPH (4), mean sensitivity (4)
Include participants with baseline VTOT >5 dB-sr
Tests with false positive rate <15%
Outliers were down-weighted using weighted mixed-effects model, weights were computed from robust regression modelling of estimate rate of decline from each participant
All P-values for testing the average annual change estimates against zero were <0.001
Correlation among Change in Visual Measures
The changes in all 4 SP measures from baseline to Year 2 were highly correlated with each other, with the Spearman correlation coefficient (r) ranging from 0.52 (V30 vs VPERIPH) to 0.98 (VTOT vs VPERIPH) (Table 5). None of the 4 SP measures were significantly correlated with BCVA, FST or microperimetry measures (with the exception of a mild correlation of V30 with microperimetry). There were mild correlations between the 4 SP measures with EZ area and CST without CME (r range from 0.24 to 0.40).
Table 5.
Spearman correlation coefficients (95% C.I.) for change in static perimetry measures with change in functional and structural measures from baseline to year 2
| VTOT | V30 | VPERIPH | Mean sensitivity on static perimetry | |
|---|---|---|---|---|
| VTOT, dB-sr | 1.00 | |||
| V30, dB-sr |
0.66
(0.52, 0.77) |
1.00 | ||
| VPERIPH, dB-sr |
0.98
(0.96, 0.98) |
0.52
(0.35, 0.66) |
1.00 | |
| Mean sensitivity on static perimetry, dB |
0.86
(0.79, 0.91) |
0.91
(0.87, 0.94) |
0.76
(0.66, 0.84) |
1.00 |
| BCVA, letters | 0.12 (−0.09, 0.33) |
0.06 (−0.15, 0.27) |
0.12 (−0.09, 0.33) |
0.07 (−0.15, 0.28) |
| FST white sensitivity, -dB | 0.11 (−0.14, 0.35) |
−0.10 (−0.15, 0.33) |
0.05 (−0.20, 0.29) |
0.10 (−0.15, 0.34) |
| FST blue sensitivity, -dB | −0.02 (−0.27, 0.23) |
0.07 (−0.17, 0.31) |
−0.07 (−0.31, 0.18) |
0.04 (−0.21, 0.28) |
| FST red sensitivity, -dB | 0.14 (−0.11, 0.38) |
0.07 (−0.18, 0.31) |
0.11 (−0.14, 0.35) |
0.09 (−0.16, 0.33) |
| Ellipsoid zone area, mm2 |
0.28
(0.07, 0.47) |
0.40
(0.21, 0.57) |
0.24
(0.02, 0.43) |
0.38
(0.18, 0.55) |
| Central subfield thickness a , microns |
0.38
(0.09, 0.62) |
0.24 (−0.06, 0.50) |
0.40
(0.10, 0.62) |
0.34
(0.04, 0.59) |
| Mean sensitivity on microperimetry, dB | 0.12 (−0.12, 0.35) |
0.25
(0.02, 0.46) |
0.08 (−0.16, 0.32) |
0.19 (−0.05, 0.41) |
Correlation coefficients and 95% C.I.s which are significantly different from 0 are bolded
Scans having cystoid macular edema excluded
Discussion
The RUSH2A study will provide longitudinal measures of disease progression over 4 years, including quantitative measures of visual field sensitivity from SP analyses and of retinal structure from SD-OCT measures. Analysis of SP measures after 2 years in eyes with USH2A-related retinal degeneration offers an early look at disease progression in this study population. Although the estimates of annual rates of progression based on 4 years of follow-up should be more precise, estimation of rates of disease progression through 2 years can inform study design for therapeutic trials. The current work reports significant rates of decline in all 4 SP metrics analyzed, including VTOT, VPERIPH, V30 and mean sensitivity after 2 years. The SP measures were significantly correlated with other SP measures, and there was a mild correlation with fundus-guided MP mean sensitivity, and with structural measures of EZ area and CST thickness from OCT scans.
Although all SP measures changed significantly after 2 years, the annual rate of absolute change was greatest for VTOT, followed by VPERIPH, while V30 and mean sensitivity changed least. If we consider rates of change as a percentage, accounting for the visual field remaining, the greatest change occurred in VPERIPH, followed by VTOT. The absolute change and percentage changes reported here are based on different model assumptions. The annual rates of absolute change reported in Table 3 assumed that the decline for each measure followed a linear pattern. An average decline of mean sensitivity 0.55 dB/year, transformed to linear scale (1/Lambert) using the standard formula,22 is equivalent to 11.9% change on a linear scale, and this percentage does not depend on the starting value of mean sensitivity (in dB) at baseline. The annual rates of percentage change reported in Table 4 assumed that the decline of each measure, after an additional log-transformation (e.g. log(dB) for mean sensitivity), followed a linear pattern, meaning that each year the same percentage of the value at the start of the year is lost.
As rod density is greatest in an elliptical ring at the eccentricity of the optic disc,23 visual field loss in retinitis pigmentosa begins with a midperipheral annular scotoma at this eccentricity, progressing to involve the peripheral visual field earliest with preservation of the central 30 degrees until later stages of disease.24,25 Therefore, the finding of greatest rates of change in VPERIPH and VTOT, and less change in the central measures of visual field, V30 and mean sensitivity, is consistent with prior reports of visual field loss in patients with rod-cone degeneration. 24,25
SP measures that reflect peripheral visual field, including VTOT and VPERIPH were significantly correlated. This was expected, since VTOT and VPERIPH are measured from the same test results and VPERIPH shares many points with VTOT. The measure of central visual field (V30) was weakly correlated with central vision as measured by fundus-guided microperimetry mean sensitivity. All visual field measures were also weakly correlated with measures of macular structure including EZ area and CST (except for between V30 and CST which were not significantly correlated). Structural measures that correspond to visual function could provide an objective correlate of macular disease progression that may be less variable than functional measures based on SP or fundus-guided microperimetry. Reports of the correlation between fundus-guided microperimetry and OCT at baseline (cite Lad paper, submitted) and after 2 years (Vincent paper, in preparation) will be reported separately.
Although the primary cohort criteria required participants to have at least 10 degrees central visual field at baseline based on kinetic perimetry with a III4e target, there were 10 participants who had static perimetry VTOT < 5 dB-sr at baseline. To mitigate concerns that additional visual field loss may not be measurable due to a floor effect, as was visually observed in Figure 2, we analyzed a preserved VF cohort (N=88) of participants with VTOT > 5 dB-sr. Greater annual absolute and percentage rates of change were observed in this cohort as compared with the entire cohort. Future studies should consider requiring VTOT at least 5 dB-sr for all participants who will be evaluated longitudinally with SP using VTOT as the primary outcome measure.
Although visual function test results provide clinically meaningful measures of disease progression, they can be challenging to measure reliably. In the present study, test results with at least 15% false positive results (N=8) were excluded, following best practices established by researchers who model visual field progression in glaucoma.26 This approach did not result in exclusion of participant data at baseline unless all 3 visual fields showed > 15% false positive results. Excluding unreliable observations reduced the estimated percentage of decline compared to using all eyes in the preserved VF cohort and narrowed the confidence intervals.
In addition, there was substantial inter-individual variability in progressive VF loss over 2 years (Figure 2). Larger sample sizes for clinical trials of treatment interventions are needed when inter-individual variability is larger. In addition, results from individual eyes that differ markedly from the results of the majority in a group (outliers) can lead to biased estimates of rates of change. We provided analyses in which the rates of change for individual eyes that were outliers in either direction (improvement or severe worsening in each SP measure) were down-weighted. We employed a frequently-used weighting approach (M-estimation with Huber weighting function) that is resistant to outliers having undue influence on the average annual change estimate,20,21 and the scatter plots for weights from robust regression models vs individual slope estimates for VTOT are shown in e-Figure 1.
The current results are limited by missed visits during the COVID-19 pandemic for 13 participants. We extended the window around annual visits, but some sites did not permit clinical studies that were non-interventional or required for the health of the patient during the pandemic. The widespread availability of effective vaccines and improved social distancing protocols have enabled observational, natural history studies to resume at all sites and we anticipate more complete data at 3- and 4-year longitudinal time points. Higher completion rates along with longitudinal data including 5 annual time points over 4 years will provide an opportunity to refine the models of SP measures developed for the 2-year data and result in more precise estimates of annual rates of change, correlation with other measures, and the ability to evaluate factors related to progression of disease as measured on SP.
Supplementary Material
c. Other Writing Committee Acknowledgements:
This work was made possible, in part, by NEI P30 EY002162 - Core Grant for Vision Research, and by an unrestricted grant from Research to Prevent Blindness, New York, NY.
Katarina Stingl and Carel Hoyng are members of the ERN-EYE (www.ern-eye.eu).
a. Funding/Support:
Funded by Foundation Fighting Blindness
b. Conflict of Interest and other Financial Disclosures:
All authors have completed and submitted the ICMJE disclosures form, and they are summarized below.
J. Duncan: receives grant support from Acucela, AGTC, Allergan/Abbvie, Biogen/NightstaRx, ProQR, PYC Therapeutics, Consulting: ConeSight, DTx Therapeutics, Editas, Eloxx, Eyevensys, Gyroscope, Helios, Nacuity, ProQR, PYC Therapeutics, Replay Therapeutics, Spark, SparingVision, Vedere Bio; Spouse: RxSight
D. Birch: Grant support: NEI EY09076, AGTC, 4D Molecular Therapeutics, ProQR, Allergan/Abbvie, Biogen/NightstaRx, Consulting: AGTC, DTx Therapeutics, Editas, Nacuity, ProQR, PYC Therapeutics, Novartis, 4D Molecular Therapeutics
A. Fahim: Grant support: NEI 1K08EY032991-01; Stock: Ionis Pharmaceuticals; Consulting: Janssen Pharmaceuticals.
M.Maguire: None.
J. Cheetham: Stock: Allergan/Abbvie; Consulting: DTx Therapeutics; Foundation Fighting Blindness.
M. Pennesi: 4D Molecular Therapeutics, Abbvie, Adverum, AGTC, Alnylam, Ascidian, Astellas, Bayer, Biogen, Bluerock Opsis, Chlogene, Editas, Eyevensys, Foundation Fighting Blindness, Gensight, Intergalactic Therapeutics, IvericBio, Janssen, Mogrify, Novartis, Ora, ProQR, Prime Editing, PYC Therapeutics, Rejuvitas, Reneuron, RegenexBio, Roche, Sanofi, Saliogen, SparingVision, Viewpoint Therpeutics, Equity: Atsena, DTx Therapeutics, Endogena, Nacuity Pharmaceuticals, Ocugen, DSMB: Gensight, Akous.
C.Y Weng: Consultant: Allergan/AbbVie, Alimera Sciences, Alcon, DORC, Regeneron, REGENXBIO, Genentech, Novartis
K. Stingl: Grant support: Orphan Disease Center/University of Pennsylvania, ProQR, FFB, Johnson& Johnson, Consulting: ProQR, ViGeneron, Novartis, SANTEN, Nayan Therapeutics with consultancy fees paid to University of Tuebingen to support research.
Footnotes
Meeting Presentations: ARVO 2022 and Retina Society 2022
References
- 1.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maguire AM, Russell S, Chung DC, et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology. 2021;128(10):1460–1468. [DOI] [PubMed] [Google Scholar]
- 3.Maguire AM, Russell S, Wellman JA, et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials. Ophthalmology. 2019;126(9):1273–1285. [DOI] [PubMed] [Google Scholar]
- 4.Slijkerman RW, Vache C, Dona M, et al. Antisense Oligonucleotide-based Splice Correction for USH2A-associated Retinal Degeneration Caused by a Frequent Deep-intronic Mutation. Mol Ther Nucleic Acids. 2016;5(10):e381. [DOI] [PubMed] [Google Scholar]
- 5.Dulla K, Slijkerman R, van Diepen HC, et al. Antisense oligonucleotide-based treatment of retinitis pigmentosa caused by USH2A exon 13 mutations. Mol Ther. 2021;29(8):2441–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanjurjo-Soriano C, Erkilic N, Baux D, et al. Genome Editing in Patient iPSCs Corrects the Most Prevalent USH2A Mutations and Reveals Intriguing Mutant mRNA Expression Profiles. Mol Ther Methods Clin Dev. 2020;17:156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry LE, McClements ME, MacLaren RE. Analysis of Pathogenic Variants Correctable With CRISPR Base Editing Among Patients With Recessive Inherited Retinal Degeneration. JAMA Ophthalmol. 2021;139(3):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Lillywhite J, Zhu W, et al. Generation and Genetic Correction of USH2A c.2299delG Mutation in Patient-Derived Induced Pluripotent Stem Cells. Genes (Basel). 2021;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan JL, Liang W, Maguire MG, et al. Baseline Visual Field Findings in the RUSH2A Study: Associated Factors and Correlation With Other Measures of Disease Severity. Am J Ophthalmol. 2020;219:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birch DG, Cheng P, Duncan JL, et al. The RUSH2A Study: Best-Corrected Visual Acuity, Full-Field Electroretinography Amplitudes, and Full-Field Stimulus Thresholds at Baseline. Transl Vis Sci Technol. 2020;9(11):9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannaccone A, Brewer CC, Cheng P, et al. Auditory and olfactory findings in patients with USH2A-related retinal degeneration-Findings at baseline from the rate of progression in USH2A-related retinal degeneration natural history study (RUSH2A). Am J Med Genet A. 2021;185(12):3717–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiefer U, Pascual JP, Edmunds B, et al. Comparison of the new perimetric GATE strategy with conventional full-threshold and SITA standard strategies. Invest Ophthalmol Vis Sci. 2009;50(1):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weleber RG, Smith TB, Peters D, et al. VFMA: Topographic Analysis of Sensitivity Data From Full-Field Static Perimetry. Transl Vis Sci Technol. 2015;4(2):14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith TB, Smith N, Weleber RG. Comparison of nonparametric methods for static visual field interpolation. Med Biol Eng Comput. 2017;55(1):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: Adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. [DOI] [PubMed] [Google Scholar]
- 16.Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 17.Roman AJ, Cideciyan AV, Aleman TS, Jacobson SG. Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiol Meas. 2007;28(8):N51–56. [DOI] [PubMed] [Google Scholar]
- 18.Lad EM, Duncan JL, Liang W, et al. Baseline Microperimetry and OCT in the RUSH2A Study: Structure–Function Association and Correlation With Disease Severity. Am J Ophthalmol. 2022;244:98–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hariri AH, Zhang HY, Ho A, et al. Quantification of Ellipsoid Zone Changes in Retinitis Pigmentosa Using en Face Spectral Domain–Optical Coherence Tomography. JAMA Ophthalmol. 2016;134(6):628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber PJ. Robust Regression: Asymptotics, Conjectures and Monte Carlo. Ann Stat. 1973;1(5):799–821, 723. [Google Scholar]
- 21.Chen C Robust Regression and Outlier Detection with the ROBUSTREG Procedure. Paper presented at: SAS Conference Proceedings: SAS Users Group International; April 14-17, 2002; Orlando, Florida. [Google Scholar]
- 22.Gardiner SK, Demirel S, Johnson CA, Swanson WH. Assessment of linear-scale indices for perimetry in terms of progression in early glaucoma. Vision Res. 2011;51(16):1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. [DOI] [PubMed] [Google Scholar]
- 24.Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease Progression in Patients with Dominant Retinitis Pigmentosa and Rhodopsin Mutations. Invest Ophthalmol Vis Sci. 2002;43(9):3027–3036. [PubMed] [Google Scholar]
- 25.Birch DG, Bernstein PS, Iannacone A, et al. Effect of Oral Valproic Acid vs Placebo for Vision Loss in Patients With Autosomal Dominant Retinitis Pigmentosa: A Randomized Phase 2 Multicenter Placebo-Controlled Clinical Trial. JAMA Ophthalmol. 2018;136(8):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montesano G, Garway-Heath DF, Ometto G, Crabb DP. Hierarchical Censored Bayesian Analysis of Visual Field Progression. Transl Vis Sci Technol. 2021;10(12):4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


