Abstract
We previously reported that opposite arm adaptation to visuomotor rotations improved the initial direction of right arm movements in right-handers, whereas it only improved the final position accuracy of their left arm movements. We now investigate the pattern of interlimb transfer following adaptation to 30° visuomotor rotations in left-handers to determine whether the direction of transfer depends on handedness. Our results indicate unambiguous transfer across the arms. In terms of final position accuracy, the direction of transfer is opposite to that observed in right-handers, such that transfer only occurred from the left to the right arm movements. Directional accuracy also showed the opposite pattern of transfer to that of right-handers: initial movement direction, calculated at peak tangential acceleration, transferred only from right to left arms. When movement direction was measured later in the movement, at peak tangential velocity, asymmetrical transfer also occurred, such that greater transfer occurred from right to left arms. However, a small, but significant influence of opposite arm adaptation also occurred for the left arm, which might reflect differences in the use of the nondominant arm between left- and right-handers. Overall, our results indicate that left-handers show a mirror-imaged pattern of interlimb transfer in visuomotor adaptation to that previously reported for right-handers. This pattern of transfer is consistent with the hypothesis that asymmetry in interlimb transfer is dependent on differential specialization of the dominant and nondominant hemisphere/limb systems for trajectory and positional control, respectively.
Keywords: Visuomotor adaptation, Generalization, Motor learning, Motor control, Intermanual
Introduction
Initial practice of a novel motor task with one arm often leads to an improvement in subsequent performance with the other arm. This tendency to transfer motor learning across the arms has long been demonstrated for a variety of tasks, including finger tapping, prism adaptation, ball catching and adaptation to novel visuomotor or dynamic conditions. Interestingly, the majority of these studies have reported asymmetrical transfer (e.g., Laszlo et al. 1970; Hicks 1975; Taylor and Heilman 1980; Parlow and Kinsbourne 1989; Marzi et al. 1991; Halsband 1992; Dizio and Lackner 1995; Thut et al. 1996; Sainburg and Wang 2002; Criscimagna-Hemminger et al. 2003; Malfait and Ostry 2004; Wang and Sainburg 2004a, b), such that the transfer of information obtained during initial training is greater in one direction than in the other. This asymmetry in transfer has previously been attributed to one of two general explanations: (1) that one arm/hemisphere system is superior to the other in motor learning, or (2) that following learning, the access of the two arm controllers to memory resources is asymmetrical. The first hypothesis is characterized by the proficiency model (Laszlo et al. 1970; also see Parlow and Kinsbourne 1989), which postulates that the dominant hemisphere is superior to its counter-part in motor learning. As a result, more information is available for transfer to the nondominant arm, following dominant arm learning. The second hypothesis is characterized by the callosal access model proposed by Taylor and Heilman (1980), which states that the information obtained during adaptation with either arm is stored in the dominant brain hemisphere. This hypothesis predicts better transfer to the dominant arm due to more direct (intrahemispheric) access. However, neither model is adequate in explaining two major features of interlimb transfer that have been reported for visuomotor adaptations. First, different features of task performance, directional accuracy and final position accuracy, transfer in different directions (see Sainburg and Wang, 2002), yet each model predicts transfer in only one direction. Second, task performance of the two arms while adapting to visuomotor rotations improves to the same extent and at the same rate, which suggests symmetric adaptation, and thus contradicts the proficiency model (Sainburg 2002; Sainburg and Wang 2002; Wang and Sainburg 2006).
Through a series of studies in right-handers, we previously characterized the patterns of interlimb transfer that occur following adaptation to visuomotor rotations (Sainburg and Wang 2002; Wang and Sainburg 2003, Wang and Sainburg 2004a, b). When the nondominant arm adapts first, subsequent performance with the dominant arm shows a substantial improvement only in initial direction accuracy, as compared to naïve performance. On the other hand, when the dominant arm first adapts to the rotation, subsequent nondominant arm performance improves only in terms of final position accuracy, and not directional accuracy. Because the dominant arm of right-handers appears better adapted for control of trajectory direction and the nondominant arm for control of final limb position (Sainburg and Kalakanis 2000; Bagesteiro and Sainburg 2002; Sainburg 2002), we hypothesized that each arm controller utilizes the information obtained during opposite arm adaptation according to that controller’s proficiency for specifying particular features of movement. That is, the dominant arm controller is specialized for trajectory control, and thus might better utilize movement direction information, whereas the nondominant controller, which appears specialized for controlling final limb position, utilizes opposite arm adaptation to improve final position accuracy. This hypothesis is consistent with previous findings that these two features of movement control are mediated by independent neural mechanisms (Hirayama et al. 1993; Dizio and Lackner 1995; Gottlieb 1996; Lackner and Dizio 1994; Sainburg et al. 1999).
Our hypothesis that interlimb transfer depends on the proficiency of each arm controller for different aspects of movement leads to the prediction that the direction of transfer should also vary with handedness. We now directly test this prediction by investigating interlimb transfer of visuomotor rotations in left-handers. We predict that left-handers should show similar patterns of transfer to those previously revealed in right-handers. Specifically, initial direction accuracy should transfer from nondominant to dominant arm, while final position accuracy should transfer from dominant to nondominant arm. Alternatively, it is plausible that asymmetries in interlimb transfer are dependent on factors other than limb dominance. For example, Boulinguez and colleagues (2001) recently reported left arm advantages in reaction time for making judgments about the trajectories of a moving visual stimulus. Left arm advantages occurred in both left- and right-handers, leading the authors to conclude that these advantages resulted from right-hemisphere specializations that were independent of handedness.
Materials and methods
Subjects
Subjects were 12 neurologically intact left-handed adults (six females, six males), aged from 18 to 30 years old. Subjects were recruited from the university community, and were paid for their participation. Informed consent was solicited prior to participation. Left-handedness was assessed using the 10-item version of the Edinburgh inventory (Oldfield 1971).
Apparatus
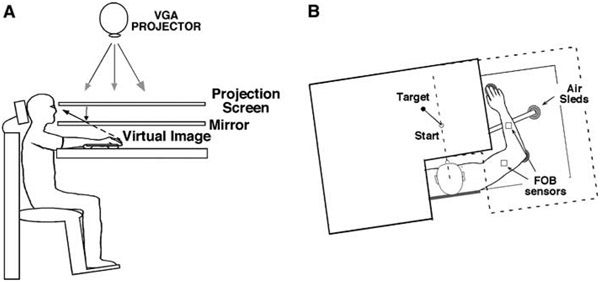
Subjects sat facing a table with either the right arm or the left arm supported over a horizontal surface, positioned just below shoulder height, by a friction-less air jet system (Fig. 1a). A start circle, target, and cursor representing the index finger position were projected on a horizontal back-projection screen positioned above the arm (Fig. 1b). A mirror, positioned parallel and below this screen, reflected the visual display, so as to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display ensured that this projection was veridical. Position and orientation of each limb segment was sampled at 103 Hz using the Flock of Birds® (Ascension-Technology, Burlington, VT) magnetic 6-DOF movement recording system. The position of the following three bony landmarks was digitized using a stylus rigidly attached to a FOB sensor: (1) index finger tip, (2) the lateral epicondyle of the humerus, and (3) the acromion, directly posterior to the acromioclavicular joint. This sensor was then attached to a rigid upper arm cuff. Another FOB sensor was attached to a rigid forearm support. Thus, the position of the body landmarks relative to these sensors remained constant throughout the experiment, and these positions were computed by our custom software as sensor data was received from the Flock of Birds®.
Fig. 1.
Experimental setup. A side view subjects were seated in a dentist-type chair with the arm supported by an air jet system that removed the effects of friction on arm movement. Targets and the cursor representing finger position were back-projected on a screen placed above the arm. a mirror placed below this screen reflected the image, such that the projection was perceived in the plane of the arm. b Top view the positions of the Flock of Birds sensors are shown
Experimental design
The experimental design of the current study is identical to that of our previous study (Sainburg and Wang 2002). The starting circle and targets were presented in midline, such that the two arms shared the task-space while adapting to visuomotor rotations. Prior to movement, one of eight targets (2 cm in diameter; 13 cm away from the starting position), presented in a pseudorandom sequence, was displayed on the horizontal tabletop. Subjects were asked to move the finger from the starting circle (1.5 cm in diameter) to the target using a single, rapid motion in response to an auditory ‘go’ signal. During the movement, visual feedback was provided by a screen cursor. At the end of each trial, knowledge of results was provided in the form of a hand-path, and by points awarded for spatial accuracy (2D distance between the target and the final finger position): 1 point for accuracy <4 cm, 3 points for accuracy <2 cm, and 10 points for accuracy <1 cm. No points were given for movements that took longer than 400 ms.
The experiment consisted of two sessions: baseline (no visual rotation) and exposure (visual rotation) sessions. During the baseline session, the cursor representing the location of index Wnger tip was always veridical, whereas during the exposure session, the visual display was perturbed in such a way, that the cursor position was rotated 30° counterclockwise (CCW) relative to the start circle. Subjects performed two blocks of trials in each session, one block with each arm. Half the subjects performed with the left arm first and then the right arm (group LR), while the other half performed with the right arm first and then the left arm (group RL). Each block comprised 192 trials, divided into 24 cycles, with each cycle containing all eight of the targets consecutively. Each block of trials was separated by a 10-min break. Table 1 shows the sequence of the experimental sessions and blocks for each subject group.
Table 1.
Experimental design
| Group | Baseline (no rotation) |
Exposure (30° CCW rotation) |
||
|---|---|---|---|---|
| NP | OAA | NP | OAA | |
| LR (n = 6) | L | R | L | R |
| RL (n = 6) | R | L | R | L |
NP naïve performance, OAA performance following opposite arm adaptation
Data analysis
Three measures of performance were calculated for three diVerent phases of movement: hand-path direction error at peak tangential arm acceleration (Amax) for the initial phase, hand-path direction error at peak tangential arm velocity (Vmax) for a later phase, and final position error for the final phase. Direction errors were calculated as the angular diVerence between the vectors defined by the target and by the hand-path position at movement start and at Vmax or Amax. Final position error was calculated as the 2D distance between the index finger at movement termination and the center of the target. In addition to these three measures, linearity errors were also measured to determine the straightness of hand-paths, which was calculated as the minor axis (the largest distance, perpendicular to the major axis, between any two points in the path) divided by the major axis (the largest distance between any two points in the path).
A repeated-measures ANOVA was conducted with group (LR, RL) as a between-subject factor, and hand and cycle as within-subject factors. Because the purpose of this study was to examine the eVect of initial training with one arm on subsequent performance with the other arm, the comparison between the arms was not of main interest in this study. Rather, we were more interested in post hoc pair-wise comparisons using Tukey tests, which were made between naïve performance and performance following opposite arm adaptation for the dominant arm blocks (left arm performances by LR and RL groups), as well as for the nondominant arm blocks (right arm performances by LR and RL groups). This effect of opposite arm adaptation was assessed for the first three epochs (mean of cycles 1 and 2, 3 and 4, 5 and 6) and for the last epoch (mean of cycles 23 and 24) only, in order to examine initial information transfer and the extent of Wnal adaptation, respectively. In order to examine differences in the time course of adaptation between naïve performance and that following opposite arm adaptation, additional post hoc comparisons were made between the measure from each individual epoch and that from the final epoch (i.e., final adaptation value) of the exposure session. The first epoch whose value was not significantly different from the final adaptation value was considered the first adapted epoch.
Results
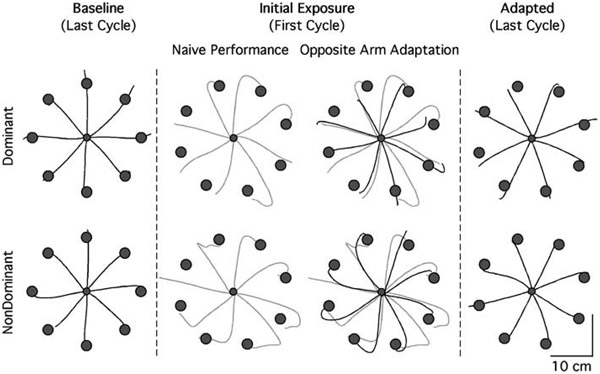
Figure 2 shows typical hand-paths of representative subjects during the final phase of the baseline session, and during the initial and final phases of the adaptation session. In column 3, naïve performance (gray lines) and performance following opposite arm adaptation (black lines) are illustrated for each arm separately, and differences in accuracy between these two sets of hand-paths represent the effect of opposite arm adaptation. On initial exposure to the visuomotor rotation during naïve performance, hand-paths are initially directed approximately 20–30° CCW to the target (column 2). During the performance following opposite arm adaptation, the initial direction of the hand-paths is substantially less deviated than that observed during naïve performance (column 3) for both arms, although the effect of opposite arm training appears to be greater for the left arm. In addition, the hand-paths of the right arm appear to be more curved than that of the left arm. Following adaptation to the visuomotor rotation, hand-paths are directed relatively straight to the target and become substantially more accurate (column 4).
Fig. 2.
Hand-paths of representative subjects. Dominant hand paths are shown along the top row, whereas nondominant hand paths are shown below. Each column shows hand-paths of eight consecutive trials, one for each target direction. Column 2 shows hand-paths of the first eight trials during naive performance; column 3 shows those during naïve performance (gray lines) and those during performance following opposite arm adaptation (black lines)
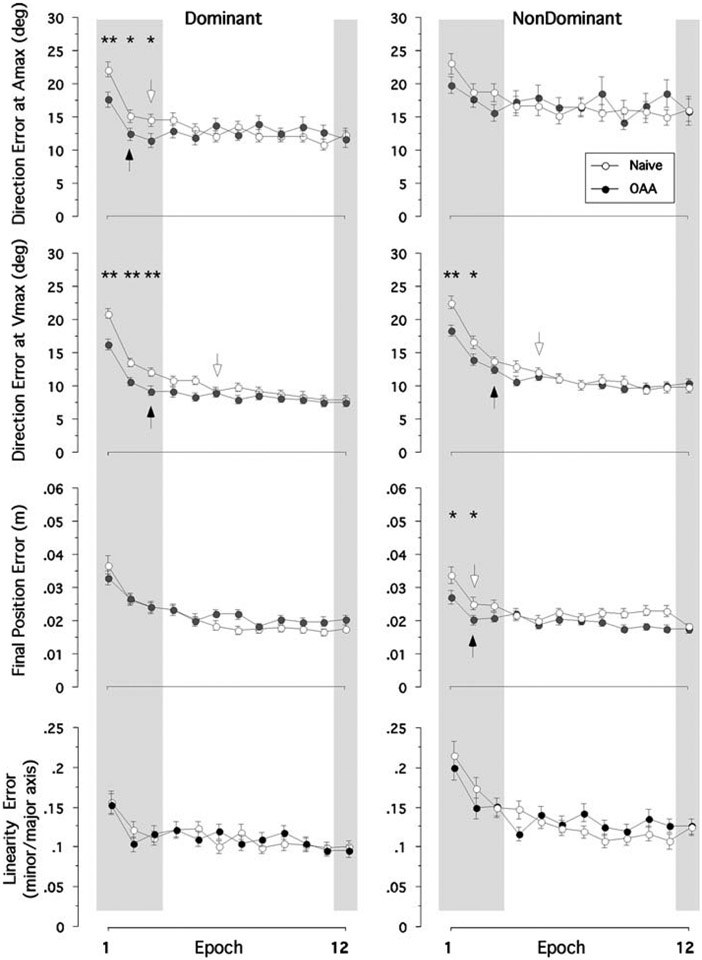
We calculated the four performance measures to quantify the beneficial effect of opposite arm training (Fig. 3). According to the repeated-measures ANOVA, there was a significant 3-way interaction effect among hand, group and cycle for all three measures (P<0.05). We, thus, performed post hoc pair-wise comparisons using Tukey tests between naïve performance and performance following opposite arm adaptation at the first three and the last epochs, for the two arms separately. Arrows in Fig. 3 represent the first adapted epoch for each arm performance during the exposure session. Direction errors at Amax, reflecting the initial phase of movement, were significantly lower in the performance following opposite arm adaptation than in naïve performance with the left arm (P<0.001), but not with the right arm (P>0.05), for the first three epochs. At a later phase of movement, the effect of opposite arm adaptation was observed for both arms, in that the direction errors at Vmax were significantly lower in the performance following opposite arm adaptation than in naïve performance for both arms (P<0.01) at the first epoch. However, the effect of opposite arm training appeared to be more beneficial for the left arm, because this effect remained to be strong at the second and third epochs (P=0.003 and 0.002, respectively) for the left arm, whereas it was significant for the right arm until the second epoch (P=0.02) only. With regard to the final position errors, the beneficial effect of opposite arm adaptation was only significant for the right arm at the first and second epochs (P<0.05). In addition, the effect of opposite arm adaptation was not significant for either arm in terms of linearity errors. At the last epoch (mean of cycles 23 and 24), there was no difference between the two performance conditions in any measures.
Fig. 3.
Mean performance measures of direction errors at Vmax and Amax, final position error and linearity error. Every data point shown on X axis represents the average of 16 consecutive trials (epoch) across all subjects (mean ± SE). Performance measures for naïve performance (open circles) and performance following opposite arm adaptation (filled circles) are shown separately. **Significant difference between naïve performance and performance following opposite arm adaptation (OAA) at P<0.01; *Significant difference at P<0.05. Arrows represent the first adapted epoch for each arm performance
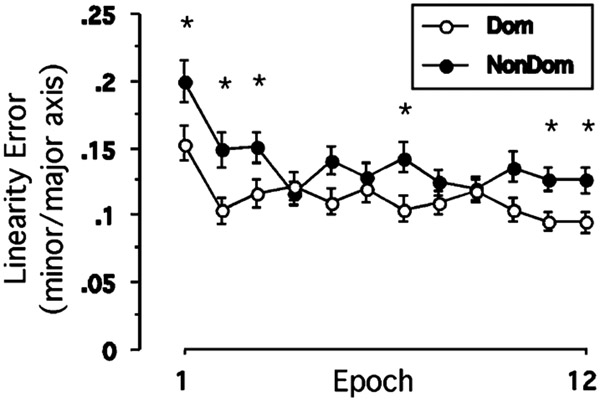
These data indicate that the two arms use different strategies to improve their performance following opposite arm adaptation. The left arm improves its performance by adjusting the initial direction (average 4.5° as compared to naïve performance) as early as 98 ms after the onset of movement (average time to Amax). On the other hand, the right arm does not substantially adjust its direction until 158 ms after the onset of movement (average time to Vmax), and yet shows substantial improvement (average 7 mm as compared to naïve performance) in final position accuracy. This indicates that the right arm continues to change its direction throughout the movement to improve its performance, probably by utilizing online feedback. We, thus, compared between the linearity errors of the two arms (performance following opposite arm adaptation) to see if the performance of one arm is more linear than that of the other arm. As illustrated in Fig. 4, the linearity errors were significantly higher for the right arm at most epochs throughout the session (P<0.05), thus indicating that the right arm hand-paths were substantially more curved than those of the left arm during performance following opposite arm adaptation.
Fig. 4.
Mean performance measure of linearity error. Every data point shown on X axis represents the average of 16 consecutive trials (epoch) across all subjects(mean ± SE). Performance measures for dominant arm performance (open circles) and for nondominant arm performance (filled circles) are shown separately. *Significant difference between naïve performance and performance following opposite arm adaptation (OAA) at P<0.05
Discussion
Patterns of interlimb transfer depend on handedness
In the present study, we hypothesized that the pattern of interlimb transfer following visuomotor rotation adaptation is dependent on the proficiency of each limb system for different features of control. We thus predicted that left-handers would show the same pattern of transfer, with respect to handedness, as that of right-handers: initial direction information should transfer from nondominant to dominant arm, and final position accuracy should transfer from dominant to nondominant arm (Sainburg and Wang 2002; Wang and Sainburg 2003). Alternatively, asymmetry in transfer might arise from hemispheric specializations for factors that are independent of handedness. Our results in left-handers unambiguously support the former hypothesis. In terms of directional accuracy, initial movement direction, calculated at peak tangential acceleration, transferred only from nondominant to dominant arm. Similarly, movement direction measured at a later phase of movement, reflected by direction errors at peak tangential velocity, showed asymmetrical transfer, with the largest effects transferring from nondominant to dominant. Final position accuracy, measured at the final phase of movement, was also similar to that of right-handers with respect to handedness, in that transfer only occurred from dominant to nondominant arm. These findings provide strong support to the hypothesis that asymmetry in interlimb transfer is dependent on interlimb differences in proficiencies for controlling different aspects of movement, which varies with handedness.
Differences in interlimb transfer between right- and left-handers
The patterns of interlimb transfer revealed in the current study were consistent with our previous findings in right-handers: Initial movement direction transferred only from nondominant to dominant arm, and final position only from dominant to nondominant arm. However, in contrast to our previous findings in right-handers, when movement direction was calculated later in the movement, at the time of peak tangential velocity, significant transfer also occurred from the dominant to nondominant arm. The fact that this effect was not apparent earlier in the movement suggests that such transfer did not directly affect the planning of movement direction. Instead, following opposite arm adaptation, the nondominant arm appeared to correct initial errors in direction, which is consistent with a nondominant arm advantage observed in right-handers for feedback-mediated corrections to movements (Sainburg and Wang 2002; Bagesteiro and Sainburg 2002; Sainburg and Schaefer 2004). Nevertheless, transfer of direction information to the nondominant arm occurred in left-handers in the current study, but not in right-handers in our previous studies. We speculate that this might result from differences in experience between left- and right-handers that arises from the fact that left-handers live in a culture that is right-hand biased. The majority of tools and a large part of the man-made environment are designed for right-handers (Hardyck and Petrinovich 1977). Left-handers living in this right-biased world are often forced to use their nondominant hand and arm to use the tools designed for right-handers, which may enable left-handers to use their nondominant arm more flexibly as compared with right-handers (Bryden 1982). It is, thus, plausible that the nondominant arm of left-handers has benefited from these environmental factors with regard to the development of coordination and sensorimotor transformations, such as studied here. It should, nevertheless, be emphasized that the patterns of transfer as measured by initial movement direction and final position showed no differences between left- and right-handers, and that the direction of asymmetry in transfer of direction, even as measured at peak velocity, was consistent with our previous studies in right-handers.
Independent control of trajectory and final limb posture during reaching
Our findings that different features of movement (i.e., initial direction and final position accuracy) transfer across the arms in different directions suggest that separate neural processes mediate these two features of movement (Sainburg and Wang 2002). In fact, it has been suggested that movement trajectory and final limb position may be represented and controlled independently in the CNS (Lackner and DiZio 1994; DiZio and Lackner 1995). This argument is consistent with a recent report by Kurtzer et al. (2005) who examined how individual neurons in the primary motor cortex of macaque monkeys represent mechanical loads during movement and posture tasks. They reported that half of the neurons that expressed load-related activity did so exclusively during either posture or movement task, and those neurons with activity during both tasks randomly switched their magnitude of response between the two tasks. Based on these findings, they suggested that two specialized control processes exist in the CNS: one for movement and the other for posture. These findings are also in agreement with the idea that initial movement direction and final limb posture are controlled by distinct neural modules, possibly through a two-phase model, in which the specification of initial movement direction is initiated through a forward dynamic controller and final position is achieved by specifying joint stiffiness about an equilibrium posture (Hirayama et al. 1993). This idea is consistent with a model operationalized by Gottlieb (1996). He proposed a model in which initial trajectory is controlled by open-loop mechanisms through a forward dynamic controller, while final position control is achieved by closed-loop mechanisms that specify a series of equilibrium positions. In this model, the weighting of each control strategy varied in time, such that the forward dynamic controller almost completely specified movement initiation, while the equilibrium controller almost completely accounted for position stabilization at the end of movement. Our current findings suggest that the two limb/hemisphere systems might be differentially specialized for each of these processes, which is in agreement with an idea that not only the left, but also the right hemisphere have specialized functions that are crucial for the realization of goal-directed motor behavior (Serrien et al. 2006).
We recently investigated whether, for the dominant arm of right-handers, visuomotor adaptation is best reflected by a remapping between the position of the visual targets and the final limb position or between the target vector and the movement vector (Wang and Sainburg 2005). Subjects first adapted to a 30° rotation during reaching movements made from a single starting location to four different target locations. After adaptation, generalization trials were introduced, during which movements were made under the same rotation condition but started from two new locations to either the previously practiced targets or new targets that reflected the previously experienced direction and distance. Our results showed that generalization was most complete for movements made toward the locations that reflected the previously trained direction and distance, but not toward the previously trained final positions. This indicated a remapping between the target and movement vectors. These results indicated that this visuomotor adaptation was best characterized by an adjustment in trajectory planning, and not the final limb control. However, our current results suggest that the nondominant arm may show the opposite pattern of results, such that for this arm visumotor adaptations may be better characterized by adjustments in final position control. Further research is necessary to test this hypothesis.
Interlimb differences in controlling feedforward control of trajectory and feedback mediated control of limb position
We have repeatedly demonstrated that the dominant arm of right-handers is more proficient in coordinating intersegmental dynamics for specifying trajectory, and the nondominant arm more proficient in controlling final limb positions (Bagesteiro and Sainburg 2002; Sainburg 2002; Sainburg and Kalakanis 2000). Based on these findings, we hypothesized that the dominant hemisphere/arm system is specialized for controlling trajectory direction and shape, whereas that of the nondominant system is specialized for control of limb position through impedance mechanisms (Bagesteiro and Sainburg 2002; Sainburg and Wang 2002). In fact, we have recently shown that even during single-joint elbow movements, the two arms employ qualitatively different mechanisms to achieve equivalent peak velocities and movement accuracies: the dominant arm movements vary peak acceleration in accord with intended movement distance, whereas the nondominant arm varies acceleration duration (Sainburg and Schaefer 2004). These two features of control have previously been associated with feedfoward and feedback-mediated control mechanisms, respectively (Brown and Cooke 1981, 1984, 1986; Ghez 1979; Ghez and Gordon 1987). Our current findings support the specializations of dominant and nondominant arms, as reflected by these previous studies and extend these findings to left-handers. In this study, only dominant arm movements benefited from opposite arm adaptation in terms of initial direction control, as measured at peak acceleration and likely reflected feedforward control mechanisms. Nondominant arm benefits of opposite arm adaptation only emerged later in the movement, when feedback mediated control mechanisms were likely employed.
The hypothesis that the dominant hemisphere/limb system is specialized for feedfoward control of trajectory and the nondominant system for feedback-mediated control of position is consistent with recent findings reported by Haaland and colleagues (2004), who examined control in the ipsilesional arm of stroke patients with unilateral brain damage. The ipsilesional limbs of such patients are the limbs that appear to be spared by the effects of the stroke, whereas the contralesional limbs tend to exhibit hemiparesis. The hemisphere ipsilateral to the affected limb is completely spared in these patients. Thus, if any deficits in the ipsilesional limb are observed, they must reflect the contributions of the damaged hemiphere to the control of this ipsilesional limb, which is a phenomenon that is well supported by recent imaging studies (Kutas and Donchin 1974; Kawashima et al. 1998; Matsunami and Hamada 1981; Macdonell et al. 1991; Kim et al. 1993; Dassonville et al. 1997). In a study of rapid reaching movements in right-handed stroke patients, Haaland et al. revealed that left (dominant) hemisphere damage produced deficits in control of movement speed, whereas right (nondominant) hemisphere damage produced deficits in final position accuracy. These findings were consistent with those of a previous study by Winstein and Pohl (1995), which showed that dominant hemisphere lesions result in a prolonged acceleration phase of rapid targeted movements, whereas nondominant lesions prolong the deceleration phase of motion. Collectively, these findings support a specialized role of the dominant hemisphere in initial trajectory control, and of the nondominant hemisphere in control of final limb position. Our current findings suggest that asymmetries in interlimb transfer are directly determined by these hemispheric specializations.
Acknowledgments
This research was supported by National Institutes of Health grants R01HD39311 and NRSA 1-F32-NS-46239-1.
References
- Bagesteiro LB, Sainburg RL (2002) Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88:2408–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulinguez P, Velay JL, Nougier V (2001) Manual asymmetries in reaching movement control. II: study of left-handers. Cortex 37:123–138 [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD (1981) Responses to force perturbations preceding voluntary human arm movements. Brain Res 220:350–355 [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD (1984) Initial agonist burst duration depends on movement amplitude. Exp Brain Res 55:523–527 [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD (1986) Initial agonist burst is modified by perturbations preceding movement. Brain Res 377:311–322 [DOI] [PubMed] [Google Scholar]
- Bryden MP (1982) Laterality: functional asymmetry in the intact brain. Academic, New York [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R (2003) Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89:168–176 [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J (1997) Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci USA 94:14015–14018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizio P, Lackner JR (1995) Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol 74:1787–1792 [DOI] [PubMed] [Google Scholar]
- Ghez C (1979) Contributions of central programs to rapid limb movement in the cat. In: AhaW VJ (ed) Integration in the nervous system. Igaku-Shoin, Tokyo, New York [Google Scholar]
- Ghez C, Gordon J (1987) Trajectory control in targeted force impulses. I. Role of opposing muscles. Exp Brain Res 67:225–240 [DOI] [PubMed] [Google Scholar]
- Gottlieb GL (1996) On the voluntary movement of compliant (inertial-viscoelastic) loads by parcellated control mechanisms. J Neurophysiol 76:3207–3229 [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR (2004) Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain 127:1145–1158 [DOI] [PubMed] [Google Scholar]
- Halsband U (1992) Left hemisphere preponderance in trajectorial learning. Neuroreport 3:397–400 [DOI] [PubMed] [Google Scholar]
- Hardyck C, Petrinovich LF (1977) Left-handedness. Psychol Bull 84:385–404 [PubMed] [Google Scholar]
- Hicks RE (1975) Intrahemispheric response competition between vocal and unimanual performances in normam adult human males. J Comp Physiol Psychol 89:50–60 [DOI] [PubMed] [Google Scholar]
- Hirayama M, Kawato M, Jordan MI (1993) The cascade neural network model and a speed-accuracy trade-off of arm movement. J Mot behav 25:162–174 [DOI] [PubMed] [Google Scholar]
- Kawashima R, Matsumura M, Sadato N, Naito E, Waki A, Nakamura S, Matsunami K, Fukuda H, Yonekura Y (1998) Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements—a PET study. Eur J Neurosci 10:2254–2260 [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP (1993) Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science 261:615–617 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH (2005) Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci 8:498–504 [DOI] [PubMed] [Google Scholar]
- Kutas M, Donchin E (1974) Studies of squeezing: handedness, responding hand, response force, and asymmetry of readiness potential. Science 186:545–548 [DOI] [PubMed] [Google Scholar]
- Lackner JR, Dizio P (1994) Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol 72:299–313 [DOI] [PubMed] [Google Scholar]
- Laszlo JI, Baguley RA, Bairstow PJ (1970) Bilateral transfer in tapping skill in the absence of peripheral information. J Mot Behav 2:261–271 [DOI] [PubMed] [Google Scholar]
- Macdonell RA, Shapiro BE, Chiappa KH, Helmers SL, Cros D, Day BJ, Shahani BT (1991) Hemispheric threshold differences for motor evoked potentials produced by magnetic coil stimulation. Neurology 41:1441–1444 [DOI] [PubMed] [Google Scholar]
- Malfait N, Ostry DJ (2004) Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24:8084–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi CA, Bisiacchi P, Nicoletti R (1991) Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia 29:1163–1177 [DOI] [PubMed] [Google Scholar]
- Matsunami K, Hamada I (1981) Characteristics of the ipsilateral movement-related neuron in the motor cortex of the monkey. Brain Res 204:29–42 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M (1989) Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn 11:98–113 [DOI] [PubMed] [Google Scholar]
- Sainburg RL (2002) Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142:241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D (2000) Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83:2661–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Schaefer SY (2004) Interlimb differences in control of movement extent. J Neurophysiol 92:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J (2002) Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghez C, Kalakanis D (1999) Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81:1040–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP (2006) Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci 7:160–6 [DOI] [PubMed] [Google Scholar]
- Taylor HG, Heilman KM (1980) Left-hemisphere motor dominance in righthanders. Cortex 16:587–603 [DOI] [PubMed] [Google Scholar]
- Thut G, Cook ND, Regard M, Leenders KL, Halsband U, Landis T (1996) Intermanual transfer of proximal and distal motor engrams in humans. Exp Brain Res 108:321–327 [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2003) Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2004a) Interlimb transfer of novel inertial dynamics is asymmetrical. J Neurophysiol 92:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2004b). Limitations in interlimb transfer of visuomotor rotations. Exp Brain Res 155:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2005) Adaptation to visuomotor rotations remaps movement vectors, not final positions. J Neurosci 25:4024–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2006) The symmetry of interlimb transfer depends on workspace locations. Exp Brain Res 170:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS (1995) Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res 105:163–174 [DOI] [PubMed] [Google Scholar]