Abstract
Introduction
Tranexamic acid (TXA) use has become common in orthopedic surgeries. Despite the growing number of publications related to its use, no recent systematic reviews have been published examining TXA use in foot and ankle surgery. The purpose of this review article is to provide a summary of the current available literature regarding TXA use in foot and ankle surgery and to further the understanding of its safety and efficacy.
Methods
This systematic review utilized PubMed, Ovid, CINAHL, Clinical Key, Medline, and Embase, and the search was conducted through December 22, 2022. Key words used in the search included: “tranexamic acid,” “TXA,” “foot,” “ankle,” “calcaneal,” and “surgery.” The outcomes within the studies analyzed included measures of perioperative blood loss (intra-operative blood loss, 24-hour post-operative blood loss, blood loss from hour 24 to hour 48, post-operative hemoglobin (Hgb), and post-operative hematocrit [Hct]), as well as wound complications and vascular events. Meta-regression was included to assess the impact of age on between-study variation.
Results
Ten studies met preliminary inclusion criteria. Upon further inspection, eight met full inclusion criteria for the meta-analysis. Despite a growing amount of literature on the topic, there is still a paucity of literature published on TXA use in foot and ankle surgery. Current literature suggests that foot and ankle surgery patients treated with TXA may have reduced 24-hour post-operative blood loss (MD=−183.41 mL, 95% CI=−247.49 to −119.34 mL, p<0.001), increased post-operative hemoglobin (MD=0.71 g/dL, 95% CI=0.11 to 1.31 g/dL, p=0.020) and hematocrit (MD=2.66%, 95% CI=0.07 to 5.24%, p=0.040) when compared to similar patients not receiving TXA. The use of TXA in foot and ankle surgery did not lead to increased thromboembolic complications. Meta-regression indicated no clinically relevant association of age to between-study variation.
Conclusions
TXA was found to be a safe treatment that did affect wound healing or infection rates while decreasing perioperative blood loss. Further research should be performed to evaluate the long-term effects of TXA administration on patient outcomes after foot and ankle surgery.
Keywords: Tranexamic acid, foot injury, ankle injury, surgical blood loss, wound healing
INTRODUCTION
Tranexamic acid (TXA) is an anti-fibrinolytic drug that has been utilized in both surgical and non-surgical settings for decades.1 Non-surgically, TXA is used to prevent excessive bleeding during menstruation and epistaxis.2 Originally, TXA was utilized postpartum and in gastrointestinal (GI) surgeries and has since seen expanded indications, becoming a mainstay in many orthopedic procedures around the country, especially in total joint arthroplasty and trauma. The clinical rationale behind TXA use in surgery is the reduction of intra-operative and post-operative bleeding, subsequent reduction in wound complications, and improvement in patient outcomes.1
To our knowledge, two systematic reviews have been conducted on the use of TXA in foot and ankle surgery, with the most recent study’s data collection period ending in January 2022. A main conclusion of both reviews was the need for expanded literature to arrive at a definitive conclusion on the safety and efficacy of TXA use in foot and ankle surgery.3–4 Since the publication of the most recent review article, additional studies have been published, and a larger body of evidence is now available. The aim of this review article is to provide a summary of the current available literature regarding TXA use in foot and ankle surgery and to further the understanding of its safety and efficacy.
METHODS
Search Strategy
A reviewer (N.D.) performed a systematic review according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines as outlined on the PRISMA checklist. Databases utilized included PubMed, Ovid, CINAHL, Clinical Key, Medline, and Embase, and the search was conducted through May 21, 2022. Key words used in the search included: “tranexamic acid,” “TXA,” “foot,” “ankle,” “calcaneal,” and “surgery.” The basic search performed on Ovid utilized its related terms function. The search was repeated on December 22, 2022, to gather data that had been published since the original search. A flowchart of the systematic review process is presented in Figure 1.
Figure 1.
Literature search as per 2020 PRISMA guidelines.
Inclusion and Exclusion Criteria
The following inclusion criteria were utilized in identifying eligible studies for the systematic review: randomized controlled trials (RCT), cohort studies, case-control studies, and case series examining TXA use in foot and ankle surgery; studies listing the number and type of surgeries performed; studies specifying the dosage and timing of TXA administration; studies published during or after 2000; studies performed on humans; studies published in English; peer-reviewed; and full text available. The only additional inclusion criterion for the meta-analysis was the use of a comparison group within the study. Exclusion criteria for the systematic review included: studies published before 2000; studies not performed on humans; studies not published in English; studies not peer-reviewed; and reviews, individual case studies, technique papers, or opinion pieces. The only additional exclusion criterion for the meta-analysis was a lack of a comparison group as statistical analysis could not be run. Using these criteria, the titles of all papers identified during the literature review were screened by one reviewer (N.D.). Any paper not meeting the inclusion criteria for study design were excluded. The abstracts of the remaining papers were screened and full texts of any works that had not met exclusion criteria were reviewed. Papers included by the first reviewer were screened and confirmed by a second reviewer (J.E.). The outcomes within the studies analyzed included measures of perioperative blood loss (intra-operative blood loss, 24-hour post-operative blood loss, blood loss from hour 24 to hour 48, post-operative hemoglobin (Hgb), and post-operative hematocrit (Hct)), as well as wound complications and vascular events. Wound complications and vascular events were defined as infection, hematoma formation, necrosis, dehiscence, deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infarction (MI), acute coronary syndrome (ACS), cardiovascular event (CVA), limb ischemia (LI), gastrointestinal hemorrhage (GIH), nerve damage, and any other conditions specified as wound complications or vascular events within individual studies.
Data Preparation
Data extraction was performed by two authors (J.E. and N.D.). The Cochrane Collaboration’s Risk of Bias 2 (RoB2) tool was used to assess the risk of bias in the studies included. The RoB2 tool uses five domains to critique how a RCT was conducted and produces an overall rating of either low risk of bias, some concerns of bias, or high risk of bias.5 The quality of the studies included was scored using the Coleman methodology score (CMS).6 The CMS grades studies with a maximum score of 100. Studies receiving a score greater than 85 are excellent, 70–84 are good, 55–69 are fair, and less than 55 are poor.7 Due to the short-term nature of the studies included in this review, the CMS was modified (MCMS) by excluding the second and seventh sections of the CMS. This created a maximum score of 85, so MCMS values were divided by 85 and converted to a score out of 100 so that the qualitative designations of the CMS could be used.
Statistical Analysis
Primary treatment outcomes having data from three or more studies available were summarized in forest plots using RevMan 5.4.1 software (The Cochrane Collaboration, Copenhagen, Denmark). Random-Effects models were used to calculate mean differences with 95% confidence intervals for each outcome measure. Heterogeneity of studies was assessed using Chi-squared (χ2) and I2 statistics. For studies reporting outcomes as median, range, and sample size, means and standard deviations were estimated using methods described by Hozo et al.8 Meta-Regression was conducted in R (R Foundation for Statistical Computing, Vienna, AT) to test for the covariate effect of age between studies and was done using standardized within-study group averages for each outcome analyzed. Statistical significance was set at α=0.05 for all comparisons.
RESULTS
Literature Search
The initial literature search produced 1,125 papers, 38 of which were duplicates and removed. The titles of the remaining 1,087 papers were screened and 526 studies were excluded. The abstracts of the remaining 561 studies were screened and 545 excluded. The full texts of the remaining 16 studies were assessed using the inclusion and exclusion criteria established above. Seven studies met preliminary inclusion criteria.9–15 Upon further inspection, five met full inclusion criteria for the meta-analysis, and two articles were excluded from the meta-analysis due to lack of a comparison group.9–10,13–15 The second literature search produced an additional three studies that met full inclusion criteria for the meta-analysis, two of which were newly published, and one that had been incorrectly excluded during the initial search.16–18 This process is illustrated in Figure 1.
Study Characteristics
Eight studies with a combined 362 patients treated with TXA and 376 controls met inclusion and exclusion criteria for the meta-analysis.9–10,13–18 An additional two studies met inclusion and exclusion criteria for the systematic review and had a combined 266 patients who received TXA.11–12 One study contained two experimental groups receiving different doses of TXA.15 A summary of the studies can be seen in Table 1.
Table 1.
Study characteristics.
| Study | Type of Study (Level of Evidence) | n | Age (mean in years) | Procedure | TXA Route | TXA Dose | Included in Meta-analysis? | ||
|---|---|---|---|---|---|---|---|---|---|
| TXA | Non-TXA | TXA | Non-TXA | ||||||
| Ali et al., 202216 | Retro. Cohort (3) | 33 | 36 | 67.2? | 67.2? | Total Ankle Arthroplasty | IV | 1–2g | Yes |
| B. H. et al., 20219 | RCT (1) | 49 | 51 | 51.2 | 52.5 | * | IV | 10mg/kg body weight | Yes |
| Huang et al., 202210 | RCT (1) | 20 | 20 | 43.9 | 40.2 | Calcaneal Fx Fixation | Irrigation | 100 ml 0.5g/L | Yes |
| Moore et al., 202217 | Retro. Cohort (3) | 101 | 116 | 59.4 | 57.1 | ** | IV | 20mg/kg body weight | Yes |
| Nodzo et al., 201818 | Retro. Cohort (3) | 25 | 25 | 65.8 | 66.9 | Total Ankle Arthroplasty | IV | 1g | Yes |
| Steinmetz et al., 202013 | Retro. Cohort (3) | 55 | 64 | 62.7 | 63.5 | Total Ankle Arthroplasty | IV | 2g | Yes |
| Xie et al., 201514 | RCT (1) | 45 | 45 | 43.4 | 42.6 | Calcaneal Fx Fixation | IV | 15mg/kg body weight | Yes |
| Zhong et al., 202115 Group A | RCT (1) | 17 | 19 | 43.1 | 40.4 | Calcaneal Fx Fixation | IV | 200mg | Yes |
| Zhong et al., 202115 Group B | RCT (1) | 17 | 19 | 40.4 | 40.4 | Calcaneal Fx Fixation | IV | 400mg | Yes |
| Johns et al., 202011 | Retro. Cohort (3) | 241 | None | Not specified | None | *** | IV | 1g | No |
| Sadoun et al., 202112 | Retro. Cohort (3) | 25 | None | 61 | None | Total Ankle Arthroplasty | IV | 1g | No |
Bunionectomy, Ankle arthrodesis, Sesamoidectomy, Ankle fracture fixation, Tibia/fibula osteotomy, Hallux valgus fixation, Ankle replacement, Mid-foot fusion, Hindfoot osteotomy, Flatfoot reconstruction
Arthroplasty, Subtalar fusion, Ankle fusion, Double arthrodesis, Tibiotalocalcaneal fusion, Triple arthrodesis, Pantalar fusion
Trauma ankle hindfoot and ankle, Trauma midfoot/forefoot, Arthrodesis, Tendon repair/transfer, Elective reconstruction midfoot/hindfoot, Elective reconstruction forefoot, Infection/tumor, Amputation, Arthroscopy, Nerve Surgery, Arthroplasty, Hardware removal
Modified Coleman Methodology Score
The MCMS scores of the included studies are displayed in Table 2. Two studies had excellent scores, two good, one fair, and five poor. The overall grade of the studies included within the meta-analysis was fair, and the overall grade of the studies included within the systematic review was fair.
Table 2.
Modified Coleman Methodology Scores (MCMS).
| Study | CMS | MCMS |
|---|---|---|
| Ali et al., 202216 | 44 | 52 |
| B. H. et al., 20219 | 66 | 78 |
| Huang et al., 202210 | 74 | 87 |
| Moore et al., 202217 | 53 | 48 |
| Nodzo et al., 201818 | 48 | 56 |
| Steinmetz et al., 202013 | 40 | 47 |
| Xie et al., 201514 | 75 | 88 |
| Zhong et al., 202115 | 67 | 79 |
| Johns et al., 202011 | 36 | 42 |
| Sadoun et al., 202112 | 40 | 47 |
| Average of 10 studies in systematic review | 54.3 | 62.4 |
| Average of 8 studies in meta-analysis | 58.4 | 66.9 |
Risk of Bias 2 Tool
The results of the RoB2 assessment are displayed in Figure 2.
Figure 2.
RoB2 tool results. Green indicates low risk of bias, yellow indicates some risk of bias, and red indicated high risk of bias.
Calcaneal Fracture Fixation
Three RCTs examining TXA use in calcaneal fracture fixation were included in the review.10,14–15 The studies included a total of 183 subjects, 99 of whom were treated with TXA. Huang et al.10 published a study examining topical application of TXA during calcaneal fracture fixations using the extended lateral approach. Statistically significant findings included a decrease in 24-hour postoperative drain volume in the TXA group (63.3 vs. 181.0 mL, p=0.001), 48-hour post-operative drain volume in the TXA group (73.8 vs. 210.3 mL, p<0.001), and hemoglobin reduction (4.6 vs. 12.6 g/L, p<0.001). Hospital length of stay and hematocrit change were both reduced in the TXA group, though the difference was not statistically significant. No wound complications were reported in either group. The study concluded that topical application of TXA during calcaneal fracture fixation was safe and effective at reducing post-operative blood loss and did not lead to wound complications.10
In 2015, Xie et al.14 published a study of 90 patients that investigated TXA use in calcaneal fracture fixation. Twenty-four-hour post-operative blood loss was found to be significantly decreased in the TXA group (110 vs. 320 mL, p < 0.001). Additionally, post-operative hemoglobin (12.82 vs. 11.57 g/dL, p < 0.001) and post-operative hematocrit (36.1% vs. 33.9%, p=0.008) were found to be significantly higher in the TXA group. The TXA group had significantly fewer wound complications (3 vs. 10, p=0.036), but similar rates of vascular events (4 vs. 2, p=0.673) and adverse side effects (3 vs. 1, p=0.306). Adverse effects reported in the TXA group included one case of deep vein thrombosis, one case of acute coronary syndrome, and two cases of GIH. Adverse effects reported in the non-TXA group included one case of deep vein thrombosis and one case of GIH. The other outcomes measured failed to produce statistically significant results. Xie et al.14 concluded that a single dose of preoperative TXA could reduce post-operative blood loss and wound complications, without an increase in side effects following calcaneal fracture fixation.
Zhong et al.15 published a RCT of 53 patients that investigated the use of TXA during surgical fixation of Sanders III–IV calcaneal fractures. All surgeries were open reduction internal fixation (ORIF) via extended lateral approach. The RCT consisted of three groups. Group A consisted of 17 subjects, group B consisted of 17 subjects, and group C consisted of 19 subjects. Group A received 20 mL of 10 mg/mL TXA solution, group B received 20 mL of 20 mg/mL TXA solution, and group C received 20 mL of sterile saline. A statistically significant decrease (p<0.01) in 24-hour post-operative blood loss was found in both group A (110 mL) and group B (130 mL) compared to group C (360 mL). Post-operative hemoglobin and hematocrit levels were also found to be significantly higher (p=0.008 and p<0.001, respectively) in group A (12.3 g/dl, 38.1%) and group B (12.2 g/dl, 37.8%) in comparison to group C (10.8 g/dl, 32.2%). The remaining outcome measures failed to reach statistical significance. Group A and B each had three cases of wound complications, and group C had four. This study concluded that TXA use could effectively reduce post-operative blood loss, but did not significantly reduce wound complications when injected via drain following ORIF of Sanders III–IV calcaneal fractures.15
Total Ankle Arthroplasty
Four studies with 263 subjects have been published examining TXA use in total ankle arthroplasty.12–13,16,18 All four were retrospective reviews. Nodzo et al.18 published a study of 50 patients that examined TXA use in total ankle arthroplasty. The study was a retrospective review and included 25 subjects who received TXA and 25 who did not receive TXA. Patients who received TXA were given 1 g IV 20 minutes prior to tourniquet deflation. Post-operative blood loss was estimated from total drain output. Calculated blood loss was significantly reduced in the TXA group compared to the non-TXA group (649.9 mL vs. 906.8 mL, p=0.010). Two wound complications were reported in the TXA group, and five were reported in the non-TXA group, though this difference was not significant. The study concluded that TXA could be used to decrease post-operative hemarthrosis and reduce the risk of post-operative wound complications in total ankle arthroplasty.18
Steinmetz et al.13 published the second study in 2020. The study was a retrospective chart review of 119 patients who underwent total ankle arthroplasty, of which 55 received 1 g TXA IV prior to tourniquet inflation and 1 g TXA IV following tourniquet deflation. No outcome measure reached statistical significance. There were eight intra-operative and nine post-operative complications in the TXA group, and six intra-operative and five post-operative complications in the non-TXA group. Post-operative complications in the TXA group included five early wound complications (prior to three months), two late deep infections (after three months), and two patients with dorsal foot numbness. Post-operative complications in the non-TXA group included one early wound complication (prior to three months), one pulmonary embolism, two patients with dorsal foot numbness, and one patient with component malposition. The study concluded that TXA use may not be effective in reducing intra-operative blood loss, perioperative blood loss, transfusion rate, or wound complications in total ankle arthroplasties.13
The third study was a 2021 retrospective review by Sadoun et al.12 The primary goal of the study was to establish the efficacy of an outpatient total ankle replacement protocol. The chart review included 25 patients who had undergone an outpatient total ankle replacement, all of whom were treated with 1g TXA IV preoperatively and 1 g TXA IV post-operatively. The primary outcome measures of the study were not pertinent to the scope of this review. However, secondary outcomes of post-operative drain volume and wound complications were measured and reported. An average of 94.6 mL of drainage was measured per patient prior to discharge, and only one wound complication was recorded in the study. No conclusions pertinent to TXA use were made.12
Ali et al.16 published the last study in 2022. The study was a retrospective chart review of 69 patients who underwent total ankle arthroplasty, of which 33 received 1–2 g TXA IV prior to tourniquet inflation. The only outcome that demonstrated significant results was wound complications, of which the TXA group showed a reduction. Four wound complications were reported in the TXA group, whereas eight were reported in the non-TXA group (p=0.002). The study concluded that TXA use is safe, though a larger sample size is needed to conclude that it reduces wound complications.16
Multi-Surgery Studies
Three studies with 553 patients, and a total of 558 surgeries, investigated TXA use in patients who underwent a variety of foot and/or ankle surgeries.9,11,17 B. H. et al.9 published an RCT of 49 subjects that received TXA and 51 subjects that did not receive TXA during 1 of 10 different surgeries. The TXA group received 10 mg TXA/kg body weight IV prior to surgical incision and the control group received 10 mL saline/kg body weight IV prior to surgical incision. The only outcome found to be statistically significant was a reduction of intra-operative fentanyl consumption in the TXA group (0 vs. 50 mcg, p=0.03), although the study questions the clinical significance of this finding. No other reported measure reached statistical significance. The TXA group had a total of eight wound complications and two other adverse effects. The control group had eight wound complications as well and reported three instances of adverse effects. The adverse effects in the TXA group included one case of a headache and one case of nausea/vomiting. The adverse effects in the non-TXA group included two cases of headaches and one case of nausea/vomiting. Neither group reported any vascular events. B. H. et al.9 concluded that TXA use did not positively influence the measured outcomes, though there were no serious adverse effects.
Johns et al.11 published a retrospective review of 241 patients who received 1g TXA IV 10 minutes prior to surgical incision of 1 of 12 different types of foot or ankle surgeries. One case of superficial cellulitis, one deep operative site infection, four cases of delayed wound healing, one deep vein thrombosis, and two pulmonary emboli were reported. The study concluded that a preoperative bolus of TXA administered IV in foot and ankle surgeries was associated with a low risk of wound complications, infections, hematomas, thromboembolic events, and overall complication rates with minimal side effects.11
Moore et al.17 published a retrospective review of 212 subjects who had one of seven different types of foot or ankle surgeries. Five subjects underwent two surgeries each. TXA was administered in 101 of the 217 surgeries within the study. Subjects in the TXA group received 20 mg/kg body weight TXA IV prior to tourniquet deflation. The main outcome examined within this study was wound complications, though Moore et al.17 performed statistical analysis on individual categories of complications, which was not examined within this review. Within the TXA group, there were less infections requiring antibiotics, cases of delayed wound healing, reoperations, and wound complications in general. The study concluded that TXA has a favorable side effect profile and that its use should be considered in hindfoot/ankle fusion surgeries and total ankle arthroplasties.17
Intra-operative Blood Loss
Three included studies examined intra-operative blood loss, though none found statistically significant differences between TXA and non-TXA groups.12,16–17 Combined analysis of the three studies (Figure 3) produced no statistically significant difference in intra-operative blood loss between TXA and non-TXA groups (MD=2.31 mL, 95% CI=−5.89 to 10.50 mL, p=0.580). Heterogeneity was high (I2=84%, χ2 p=0.002), which suggests a large amount of difference between studies. Meta-Regression indicated age did not have a statistically significant influence on the effect sizes (I2=91.82%, R2=0.00%, p=0.566). The residual heterogeneity is likely attributed to the differing methods of measuring intra-operative blood loss and the inclusion of different surgeries.
Figure 3.
Pooled analysis for effect of TXA on intra-operative blood loss (mL). Estimated means and standard deviations (SD) for Steinmetz et al.13 calculated according to Hozo et al.8 Abbreviations: nTXA - not TXA; CI - confidence interval.
Post-operative Blood Loss
Four included studies with a total of five groups receiving TXA examined 24-hour post-operative blood loss.10,14–15,18 Three included studies with a total of four groups receiving TXA examined blood loss from 24 to 48 hours.10,14–15 All five groups receiving TXA had statistically significantly lower blood loss values at 24 hours. Pooled analysis (Figure 4) produced a statistically significant decrease in 24-hour post-operative blood loss (MD=−183.41 mL, 95% CI=−247.49 to −119.34 mL, p<0.001) with low heterogeneity (I2=28%, χ2 p=0.240) suggesting agreement between studies. Meta-Regression indicated age did not have a statistically significant influence on the effect sizes (I2=41.53%, R2=0.00%, p=0.568).
Figure 4.
Pooled analysis for effect of TXA on 24-hour post-operative blood loss (mL). Abbreviations: nTXA, no TXA; SD; standard deviation; CI, confidence interval.
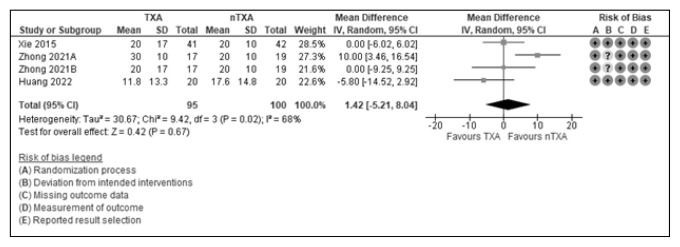
Zhong et al.15 found a statistically significant increase in blood loss from 24 to 48 hours in their A group (TXA) versus non-TXA. No other groups had statistically significant differences between TXA and non-TXA groups. Combined analysis (Figure 5) produced no statistically significant difference in post-operative blood loss from 24 to 48 hours between TXA and non-TXA groups (MD=1.42 mL, 95% CI=−5.21 to 8.04 mL, p=0.670). Moderate heterogeneity (I2=68%, χ2 p=0.020) suggested some differences between studies. Meta-Regression indicated age had a statistically significance influence on the effect sizes (I2=0.00%, R2=100.00%, p=0.002), however, the data indicated high collinearity and clinical relevance of this finding was unclear given the lack of association in intra-operative and 24-hour values. All blood loss values were below a relative low value of 42 mL, which may have contributed to the difference between studies. Blood loss values that low may not be clinically significant, even if statistically significant.
Figure 5.
Pooled analysis for effect of TXA on 24–48-hour post-operative blood loss (mL). Abbreviations: nTXA, no TXA; SD, standard deviation; CI, confidence interval.
Post-operative Hemoglobin
Five included studies with a total of six groups receiving TXA examined post-operative hemoglobin.10,13–16 Xie et al.14, and both Zhong et al.15 groups demonstrated statistically significant higher values of post-operative hemoglobin in patients who received TXA versus patients who did not receive TXA. Huang et al.10, Steinmetz et al.13, and Ali et al.16 did not find statistically significant differences in post-operative hemoglobin values. Pooled analysis (Figure 6) produced a statistically significant difference in post-operative hemoglobin in TXA groups versus non-TXA groups (MD=0.71 g/dL, 95% CI=0.11 to 1.31 g/dL, p=0.020). Heterogeneity was high (I2=73%, χ2 p=0.002), which suggested a moderate amount of difference between studies. Meta-Regression indicated age did not have a statistically significant influence on the effect sizes (I2=77.37%, R2=0.00%, p=0.994).
Figure 6.
Pooled analysis for effect of TXA on post-operative hemoglobin (g/ dL). Estimated means and standard deviations (SD) for Steinmetz et al.13 calculated according to Hozo et al.8 Abbreviations: nTXA, no TXA; CI, confidence interval.
Post-operative Hematocrit
Four included studies with a total of five groups receiving TXA examined post-operative hematocrit.10,13–15 Xie et al.14, and both Zhong et al.15 groups demonstrated statistically significant higher values of post-operative hematocrit in patients who received TXA versus patients who did not receive TXA. Both Huang et al.10 and Steinmetz et al.13 did not find statistically significant differences in post-operative hematocrit values. Pooled analysis (Figure 7) produced a statistically significant difference in post-operative hematocrit in TXA groups versus non-TXA groups (MD=2.66%, 95% CI=0.07 to 5.24%, p=0.040). Heterogeneity was high (I2=86%, χ2 p<0.001), which suggested a large amount of difference between studies. Meta-Regression indicated age did not have a statistically significant influence on the effect sizes (I2=81.23%, R2=24.46%, p=0.276).
Figure 7.
Pooled analysis for effect of TXA on post-operative hematocrit (%). Estimated means and standard deviations (SD) for Steinmetz et al.13 calculated according to Hozo et al.8 Abbreviations: nTXA, no TXA; CI, confidence interval.
Wound Complications and Vascular Events
Eight included studies with a total of nine groups receiving TXA measured wound complications and vascular events.9–10,13–18 None found statistically significant differences between TXA and non-TXA groups. Combined analysis of the eight studies (Figure 8) produced no statistically significant difference in wound complications and vascular events (MD=0.71, 95% CI=0.43 to 1.16, p=0.170). Heterogeneity was low (I2=37%, χ2 p=0.130), which suggested agreement between studies. Meta-Regression indicated age did not have a significant influence on the effect sizes (I2=46.96%, R2=0.00%, p=0.462).
Figure 8.
Pooled analysis for effect of TXA on wound complications and vascular events. Estimated means and standard deviations (SD) for Steinmetz et al.13 calculated according to Hozo et al.8 Abbreviations: nTXA, no TXA; CI, confidence interval.
DISCUSSION
Our findings demonstrate that TXA use in foot and ankle surgery does not appear to increase the incidence of wound complications and vascular events when compared to patients not treated with TXA. In addition, patients treated with TXA had lower post-operative 24-hour blood loss, and higher post-operative hemoglobin and hematocrit values when compared to patients not treated with TXA. Age was not found to have a significant impact on effect sizes for between-study heterogeneity, which suggests that treatment factors, such as sample collection methods and surgery type, are more likely associated with variations. An influence of age on 24 to 48-hour blood loss was indicated in our findings; however, this finding is difficult to interpret given the abnormal nature of the blood loss amount reported, as well as the lack of influence of age on blood loss at other time points.
Our findings do not support the postulation that decreased perioperative blood loss reduces the risk of wound complications in foot and ankle surgery. Correlation between perioperative blood loss and infection rate have not been studied in foot and ankle surgery. It is the authors’ suggestion that future research be conducted into the relationship between perioperative blood loss and infection rates in foot and ankle surgery, as the current sample size in the literature is not large enough to determine a difference or to state that no difference occurs.
All trials examining hemoglobin and hematocrit values demonstrated reduced post-operative decrease in hemoglobin and hematocrit.10,13–16 Without the complete data set from each individual study, analysis to determine statistical significance of these variables could not be completed. As such, post-operative hemoglobin and post-operative hematocrit were used as proxy measurements for perioperative blood loss instead.
Current literature suggests that foot and ankle surgery patients treated with TXA may have reduced 24-hour post-operative blood loss, increased post-operative hemoglobin and hematocrit when compared to similar patients not receiving TXA. There is no current data supporting the supposition that TXA use improves wound healing or infection rates, though the use of TXA in foot and ankle surgery did not lead to increased thromboembolic complications. Use of TXA in foot and ankle surgery should be determined on a patient-by-patient basis. Further research should be performed to evaluate the long-term effects of TXA administration on patient outcomes after foot and ankle surgery.
Footnotes
Conflicts of Interest: Dr. Damon Mar has served as a consultant for Agada Medical and Texas Back Institute. Dr. Bryan Vopat has served as a consultant for Artelon and holds a role within the American Orthopaedic Foot and Ankle Society.
Previous Presentations: American Orthopaedic Society for Sports Medicine 2023 Annual Meeting, July 15, 2023, Washington D.C., Kansas Chapter 2022 Annual Scientific Meeting & Hospitalist Day, October 27, 2022, Kansas City.
REFERENCES
- 1.Hunt BJ. The current place of tranexamic acid in the management of bleeding. Anaesthesia. 2015;70(Suppl 1):50–53e18. doi: 10.1111/anae.12910. [DOI] [PubMed] [Google Scholar]
- 2.Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev. 2013;(7):CD010562. doi: 10.1002/14651858.CD010562.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns WL, Walley KC, Jackson B, 3rd, Gonzalez TA. Tranexamic acid in foot and ankle surgery: A topical review and value analysis. Foot Ankle Spec. 2022;15(4):377–383. doi: 10.1177/1938640020983639. [DOI] [PubMed] [Google Scholar]
- 4.Salameh M, Attia AK, El Khatib S, Hantouly A, Hsu R, Blankenhorn B. Tranexamic acid utilization in foot and ankle surgery: A meta-analysis. Foot Ankle Int. 2022;43(10):1370–1378. doi: 10.1177/10711007221114139. [DOI] [PubMed] [Google Scholar]
- 5.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 6.Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: Clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2–11. doi: 10.1034/j.1600-0838.2000.010001002.x. [DOI] [PubMed] [Google Scholar]
- 7.Belk JW, McCarty EC, Houck DA, Dragoo JL, Savoie FH, Thon SG. Tranexamic acid use in knee and shoulder arthroscopy leads to improved outcomes and fewer hemarthrosis-related complications: A systematic review of level I and II studies. Arthroscopy. 2021;37(4):1323–1333. doi: 10.1016/j.arthro.2020.11.051. [DOI] [PubMed] [Google Scholar]
- 8.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.BHPP, Diskina D, Lin HM, Vulcano E, Lai YH. Use of tranexamic acid does not influence perioperative outcomes in ambulatory foot and ankle surgery-a prospective triple blinded randomized controlled trial. Int Orthop. 2021;45(9):2277–2284. doi: 10.1007/s00264-021-05131-0. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Guo H, Huang W, Tan X, Huang H, Zeng C. Topical application of tranexamic acid can reduce postoperative blood loss in calcaneal fractures: A randomized controlled trial. J Foot Ankle Surg. 2022;61(5):1056–1059. doi: 10.1053/j.jfas.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Johns WL, Walley KC, Seedat R, Jackson B, 3rd, Boukhemis K, Gonzalez T. Tranexamic acid use in foot and ankle surgery. Foot Ankle Orthop. 2020;5(4):2473011420975419. doi: 10.1177/2473011420975419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadoun M, Hardy A, Cladière V, Guichard L, Bauer T, Stiglitz Y. Outpatient total ankle replacement. Int Orthop. 2021;45(9):2429–2433. doi: 10.1007/s00264-021-05140-z. [DOI] [PubMed] [Google Scholar]
- 13.Steinmetz RG, Luick L, Tkach S, et al. Effect of tranexamic acid on wound complications and blood loss in total ankle arthroplasty. Foot Ankle Int. 2020;41(9):1117–1121. doi: 10.1177/1071100720934889. [DOI] [PubMed] [Google Scholar]
- 14.Xie B, Tian J, Zhou DP. Administration of tranexamic acid reduces post-operative blood loss in calcaneal fractures: A randomized controlled trial. J Foot Ankle Surg. 2015;54(6):1106–1110. doi: 10.1053/j.jfas.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhong L, Liu Y, Wang Y, Wang H. Effects of local administration of tranexamic acid on reducing postoperative blood loss in surgeries for closed, Sanders III–IV calcaneal fractures: A randomized controlled study. Indian J Orthop. 2021;55(Suppl 2):418–425. doi: 10.1007/s43465-021-00417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali M, Hassan A, Shah S, Rashid A, Naguib A. The effect of tranexamic acid on the outcome of total ankle replacement. Cureus. 2022;14(7):e26706. doi: 10.7759/cureus.26706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore AD, Smith BR, O'Leary RJ, Hoch CP, Gross CE, Scott DJ. Tranexamic acid associated with less wound complications in ankle and hindfoot surgery: Level III, retrospective cohort study. J Am Acad Orthop Surg. 2022;30(16):789–797. doi: 10.5435/JAAOS-D-21-01064. [DOI] [PubMed] [Google Scholar]
- 18.Nodzo SR, Pavlesen S, Ritter C, Boyle KK. Tranexamic acid reduces perioperative blood loss and hemarthrosis in total ankle arthroplasty. Am J Orthop. 2018;47(8) doi: 10.12788/ajo.2018.0063. [DOI] [PubMed] [Google Scholar]