Abstract
The Acinetobacter pcaIJFBDKCHG operon encodes the six enzymes that convert protocatechuate to citric acid cycle intermediates. Directly downstream from the operon are qui and pob genes encoding sets of enzymes that convert quinate and p-hydroxybenzoate, respectively, to protocatechuate. Prior to this investigation, the only known regulatory gene in the pca-qui-pob cluster was pobR, which encodes a transcriptional activator that responds to p-hydroxybenzoate and activates transcription of pobA. The pca and qui genes were known to be expressed in response to protocatechuate, but a protein that mediated this induction had not been identified. This study was initiated by characterization of a spontaneous mutation that mapped upstream from pcaI and prevented expression of the pca genes. Sequencing of wild-type DNA extending from the translational start of pcaI through and beyond the location of the mutation revealed a 282-bp intergenic region and a divergently transcribed open reading frame, designated pcaU. Downstream from pcaU are two open reading frames encoding proteins similar in amino acid sequence to those associated with the oxidation of acyl thioesters. Inactivation of pcaU reduced the induced expression of pca structural genes by about 90% and impeded but did not completely prevent growth of the mutant cells with protocatechuate. PcaU was expressed in Escherichia coli and shown to bind to a portion of the pcaI-pcaU intergenic region containing a sequence identical in 16 of 19 nucleotide residues to a segment of the pob operator. Further similarity of the two regulatory systems is indicated by 54% amino acid sequence identity in the aligned primary structures of PobR and PcaU. The pob and pca systems were shown to differ, however, in the relative orientations of transcriptional starts with respect to the site where the activator binds to DNA, the size of the intergenic region, and the tightness of transcriptional control. The spontaneous mutation blocking pca gene expression was located in the promoter for the pca operon. The 19-nucleotide residue operator sequences were shown to be parts of a consensus associated with transcriptional activation of genes associated with protocatechuate catabolism. Two different binding sites for Pseudomonas putida PcaR differ from the consensus in only a single nucleotide residue, and DNA directly downstream from Acinetobacter pcaU contains a 19-bp segment differing from the consensus in only two residues. PcaU was shown to bind to DNA containing this segment as well as to the DNA in the pcaU-pcaI intergenic region.
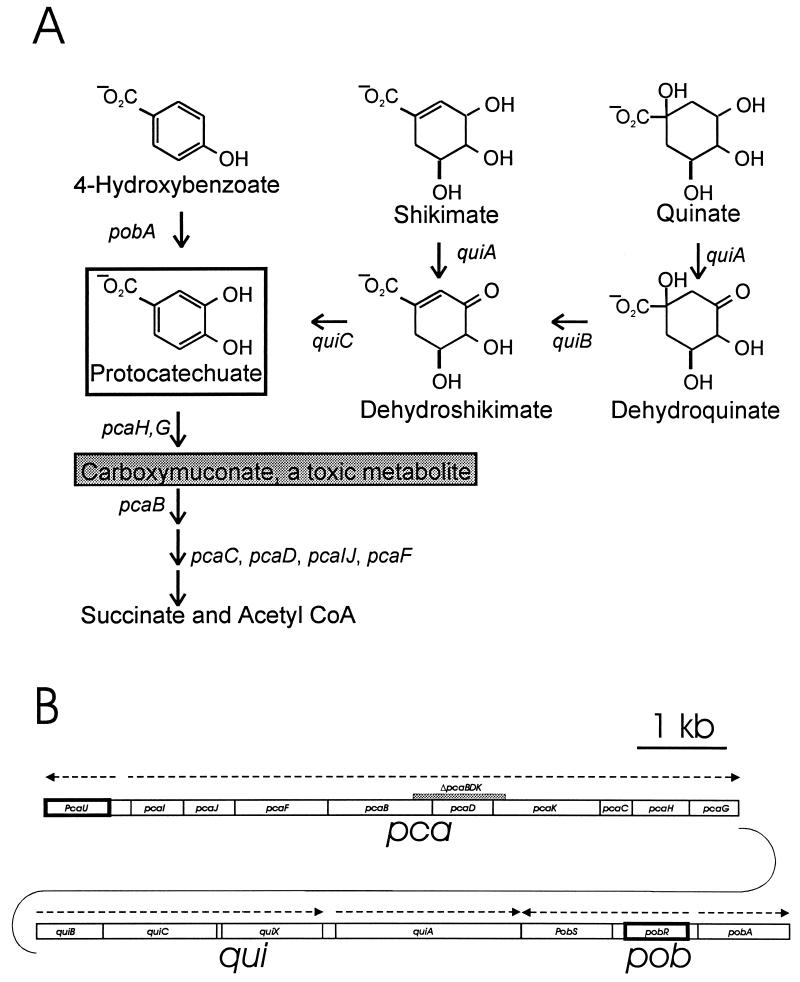
Protocatechuate supports the growth of diverse microorganisms (14–16, 19, 26, 27, 36, 39, 40, 42, 43, 48, 49, 57, 58, 60, 61, 64). The growth substrate may be utilized directly or may be formed as a metabolite in the dissimilation of compounds produced by plants in the natural environment (Fig. 1A). Representatives of the bacterial genus Acinetobacter are ubiquitous in terrestrial habitats (3), and metabolic pathways for utilization of the compounds depicted in Fig. 1A have been characterized. Furthermore, structural genes associated with the pathways have been shown to comprise the pca-qui-pob cluster (17, 34) (Fig. 1B) in Acinetobacter strain ADP1, an organism exceptionally amenable to genetic analysis by natural transformation (2, 10, 25, 31, 32).
FIG. 1.
Organization of genes associated with protocatechuate catabolism in Acinetobacter. (A) Various growth substrates are catabolized through pathways converging upon protocatechuate (open box). In Acinetobacter, protocatechuate elicits expression of all of the depicted genes except pobA, which is expressed in response to 4-hydroxybenzoate. Protocatechuate is converted to succinate and acetyl CoA by the consecutive actions of enzymes encoded by the pca genes. Null mutations in pcaB cause accumulation of the toxic metabolite carboxymuconate from protocatechuate; exposure of cells containing the ΔpcaBDK1 deletion to growth media supplemented with protocatechuate selects colonies that fail to express pcaH and -G. Most of these colonies are double mutants containing ΔpcaBDK1 and a mutation in pcaH or -G (21). In this paper, we describe another mutation, pcaP1, that blocks the promoter for the pca operon. Characterization of DNA flanking this mutation revealed pcaU. (B) Genes required for catabolism of the compounds shown in panel A are grouped within the 20 kb of contiguous DNA forming the pca-qui-pob cluster in the Acinetobacter chromosome. Directions of transcription are indicated by dashed arrows, and the position of ΔpcaBDK1 is shown by the grey rectangle. As described in this communication, expression of the pca genes in response to protocatechuate is governed by the divergently transcribed activator encoded by pcaU (dark border). The pobA gene (10) encodes 4-hydroxybenzoate hydroxylase and is regulated independently of the pca and qui genes by the divergently transcribed pobR (dark border) (9, 11).
Prior to this investigation, the only studied regulatory gene in the Acinetobacter pca-qui-pob cluster was pobR (9, 11, 20), which encodes a transcriptional activator promoting expression of pobA in response to 4-hydroxybenzoate (Fig. 1). Protocatechuate was known to elicit expression of the pca and qui structural genes (5, 6, 18) (Fig. 1), but a regulatory gene governing their transcription had not been identified. Analysis of pca structural-gene transcription in Pseudomonas putida (28, 46, 52) and Agrobacterium tumifaciens (47) revealed pcaR genes encoding transcriptional activators that respond to β-ketoadipate, an intermediate formed in protocatechuate catabolism. β-Ketoadipate does not induce pca-encoded enzymes in Acinetobacter and has even been used to select strains in which the enzymes are expressed constitutively (6, 50).
Among spontaneous Acinetobacter mutant strains defective in expression of pcaH and -G were a few containing mutations outside of the pca operon (21). We describe here the characterization of one such mutation as a nucleotide substitution in the promoter of the pca structural genes. Characterization of flanking DNA revealed pcaU, a divergently transcribed gene that activates transcription of the pca genes but that is not required for their expression at levels sufficient to support slow growth.
The Acinetobacter pcaU nucleotide sequence reveals that its product is a member of the PobR family, a subset of a sparsely represented group of transcriptional regulators in the GylR superfamily (55). Members of the PobR protein family regulate catabolic pathways for natural products from plants and include Acinetobacter PobR (9, 11), PcaR from P. putida (28, 46, 52), and A. tumifaciens (47). Since PcaU responds to protocatechuate and not to p-hydroxybenzoate or β-ketoadipate, it is apparent that members of the PobR family have been adapted to respond specifically to three different metabolite inducers in the 4-hydroxybenzoate catabolic pathway. Remarkably conserved during this divergence were the operators where the regulatory proteins appear to exert their different controls.
MATERIALS AND METHODS
Organisms, plasmids, and growth conditions.
Strains and plasmids used in this study are presented in Table 1. All organisms were grown at 37°C. Minimal medium (21) for Acinetobacter was supplemented with 10 mM succinate, 10 mM glucose, 5 mM p-hydroxybenzoate, or 5 mM protocatechuate. Escherichia coli strains were grown in Luria-Bertani medium (53). Growth media were supplemented with ampicillin (75 μg/ml), kanamycin (50 μg/ml), streptomycin (10 μg/ml), or spectinomycin (40 μg/ml) as required.
TABLE 1.
Acinetobacter strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Acinetobacter strains | ||
| ADP1 | Wild type (strain BD413) | 32 |
| ADP6 | pcaG6 | 21 |
| ADP92 | pcaU1::ΩSmrSpcr | This study |
| ADP331 | ΔpcaU2, a 552-bp deletion between NcoI and MluI restriction sites in pcaUa | This study |
| ADP500 | ΔpcaB′DK′1 ΔcatD101::Kmr | 21 |
| ADP5126 | ΔpcaB′DK′1 ΔcatD101::KmrpcaP1 | 21 |
| ADP6126 | ΔcatD101::KmrpcaP1 | This study |
| Plasmids | ||
| pKOK6 | Contains promoterless lacZ-Kmr cassette for constructing transcriptional fusions | 35 |
| pHP45Ω | Contains ΩSmrSpcr cassette terminating transcription and translation | 51 |
| pRK415 | Tcrlacp/o | 33 |
| pUC18 | Aprlacp/o | 62 |
| pUC19 | Aprlacp/o | 62 |
| pWH660+ | Shuttle vector for transfer of genes between E. coli and Acinetobacter | 30 |
| pWH661+ | Xho site deleted from pWH660+ | This study |
| pZR1 | 11,062-bp EcoRI fragment containing pcaIJFBDKCHGquiBCX′ and inserted in pUC18 | 13 |
| pZR9 | 5,403-bp Sau3A fragment containing pcaU′-pcaIJFBD′ and inserted in pUC19 | This study |
| pZR15 | 7-kbp HpaII fragment containing pcaU and inserted in pUC19 | This study |
| pZR17 | 2,792-bp EcoRI fragment containing pcaU and inserted in pUC19 | This study |
| pZR18 | 2,618-bp HindIII-HpaII fragment containing pcaU and inserted in pUC19 | This study |
| pZR22 | 2,618-bp insert from pZR18 integrated in HindIII-SstI cleavage sites of pWH661+ | This study |
| pZR23 | 2,416-bp HindIII-EcoRI fragment from pZR17 inserted into HindIII-EcoRI cleavage sites of pUC18 | This study |
| pZR26 | SalI fragment containing lacZ-Kmr cassette from plasmid KOK6 inserted in Xho site of pZR22 | This study |
| pZR28 | SalI fragment containing lacZ-Kmr cassette from plasmid KOK6 inserted in Xho site of pZR92 | This study |
| pZR33 | pZR18 modified by ΔpcaU2 deletion (552-bp deletion of NcoI-MluI fragment) | This study |
| pZR91 | ΩSmrSpcr cassette in XhoI site of pZR9 | This study |
| pZR92 | 5,403-bp Sau3A insert from pZR9 in PstI-SstI site of pRK415 | This study |
See Fig. 3.
Gene transfer by natural transformation or by conjugation.
Recipient Acinetobacter cultures were grown in shake cultures overnight with 10 mM succinate. Additional succinate was added to a final concentration of 5 mM, and after 30 min of incubation, 0.1 ml of cell suspension was spread on plates containing selective medium. Solutions containing linear DNA fragments (21) or replicas of colonies (2) were placed upon cell lawns. Transformant colonies appeared after incubation for 1 or 2 days. Large plasmids were introduced into Acinetobacter by conjugation from E. coli S17-1 followed by selection for resistance to the appropriate antibiotics (54).
DNA and RNA manipulations.
General DNA manipulations were performed by using published procedures (53). The alkaline lysis method (4) was used for preparation of plasmid DNA. Chromosomal Acinetobacter DNA was isolated as described previously (21). Cloned double-stranded DNA was sequenced with the Sequenase kit version 2.0 (United States Biochemical, Cleveland, Ohio) and either the supplied sequencing primers or custom-designed primers (23). Chromosomal DNA was sequenced directly as described elsewhere (21). Homology searches were conducted with the BLAST network service, and screening of nucleotide sequences for repetitions was conducted with the DNAStar software package (DNAStar Corporation, Madison, Wis.). Oligonucleotides were synthesized at Yale Medical School, New Haven, Conn.
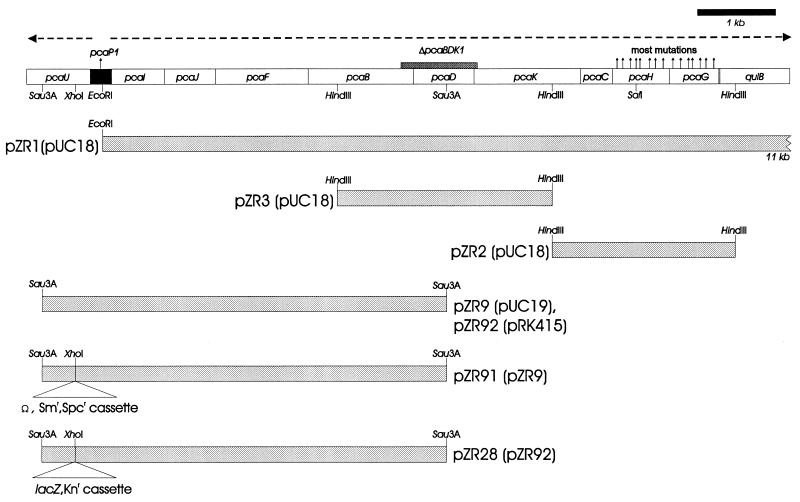
Southern (22) and Northern (23) blot analyses were performed as described previously. DNA probes were labelled with [α-32P]dATP by using a random prime labelling kit (Boehringer, Mannheim, Germany). Previously described procedures (23) were used for RNA isolation and analysis of transcription start points. The DNA probe for Northern blots of transcripts of the pca operon was a 1,370-bp SalI-HindIII restriction fragment containing pcaH and -G; the DNA probe for pcaU transcripts was a 705-bp Sau3A-EcoRI restriction fragment from pZR9 (Fig. 2).
FIG. 2.
Restriction fragments used to characterize mutations blocking expression of pcaH and -G. Open rectangles represent the pca genes drawn to a scale proportionate to their length; the black rectangle represents the pcaU-pcaI intergenic region. Dashed horizontal arrows indicate the directions of transcription. Vertical arrows above the genes indicate the locations of mutations blocking expression of pcaH and -G, the structural genes for protocatechuate oxygenase (Fig. 1). Large shaded rectangles indicate the inserts within each recombinant plasmid; the name of the corresponding parental plasmid is enclosed in parentheses. Strains containing both ΔpcaBDK1 and null pcaH or -G mutations can be restored to wild type by transformation with the insert from pZR1. Strains containing null pcaH or -G mutations can be restored to wild type by transformation with the insert from pZR2. Strain ADP5126(ΔpcaB′DK′1, ΔcatD101::Kmr pcaP1) is unusual in that it does not recover wild-type pca functions when it is transformed with pZR1 (21). Restoration of wild-type pcaD to this strain by transformation with pZR3 yielded a recombinant [strain ADP6126(ΔcatD101::Kmr pcaP1)] that grew extremely slowly with p-hydroxybenzoate; the organism’s inability to metabolize protocatechuate was further indicated by the accumulation of the compound in the growth medium. Enzymatic analysis showed that pca genes are not expressed constitutively in strain ADP6126. The pca function missing in ADP6126 was not supplied by pZR2 but, as described in this paper, was provided by pZR9. The Sau3A restriction fragment in pZR9 contains a portion of pcaU, the pcaU-pcaI intergenic region, and pca structural genes extending from pcaI to the Sau3A site within pcaD.
For determination of transcription starts by primer extension, the primer used for pcaU was 5′-TTGAGTTGTGAAGAATT, which complements the coding strand 88 to 72 bp downstream from the position determined to be the transcriptional start. The primer used for the pca operon was 5′-TCGCTGCACTTTTATCT, which complements the coding strand at a position 140 to 124 bp downstream from the position determined to be the transcriptional start.
Enzyme and protein determinations.
Previously described procedures were used for assay of protocatechuate 3,4-dioxygenase (13) and β-galactosidase (41). Proteins were separated by denaturing sodium dodecyl sulfate-gel electrophoresis as described by Laemmli (37) with a molecular mass standard for the low range (14.4 to 97.4 kDa) from Bio-Rad. Protein concentrations were determined by using established procedures (38).
Gel mobility shift assays.
Published procedures (1) were used to demonstrate the binding of PcaU to DNA fragments. For measurement of PcaU binding to DNA upstream of pcaU, the probe was prepared as a restriction fragment and labelled at the 3′ ends with the Klenow fragment of DNA polymerase; the labelled fragments were purified by ion-exchange chromatography. A labelled fragment containing 10,000 cpm was incubated for 15 min at 30°C in 12% (vol/vol) glycerol, 300 μg of bovine serum albumin per ml, 1 μg poly(dI-dC), crude extract, and competitor DNA in a total volume of 15 μl. Samples were separated in a 4% nondenaturing acrylamide gel in low-ionic-strength buffer (6.7 mM Tris-HCl, pH 7.9; 3.3 mM sodium acetate; 1 mM EDTA). After electrophoresis, the gel was dried and exposed to X-ray film.
For measurement of PcaU binding to DNA downstream of pcaU, the DNA probe was prepared by PCR, gel purified, labelled with digoxigenin-11-dUTP with the Klenow fragment, and repurified in an acrylamide gel. Conditions for the binding reaction were the same as those described above. For the shift assay (1), 10 ng of labelled DNA was employed in each incubation. After electrophoresis for 1 h in a 4% nondenaturing acrylamide gel in low-ionic-strength buffer, DNA was transferred by capillarity with 10× saline sodium citrate buffer to a nylon membrane over 5 h and fixed for 2 min with UV light. The membrane was soaked overnight in washing buffer, and chemiluminescent detection was achieved by following the instructions of the manufacturer (Genius System; Boehringer Mannheim).
Introduction of designed mutations into bacterial strains.
Acinetobacter strain ADP92 was formed by introduction of the ΩSmrSpcr cassette from plasmid pHP45Ω into the sole XhoI site of pcaU in plasmid pZR9. The ΩSmrSpcr cassette was removed from pHP45Ω by cleavage with EcoRI and PstI. The ends of both DNA preparations were filled in with the Klenow fragment, ligated, and transformed into E. coli DH5α. Clones with a plasmid containing the cassette within pcaU were selected on Luria-Bertani medium supplemented with streptomycin and spectinomycin. The resulting plasmid, designated pZR91 (Fig. 2), was cut with EcoRI, releasing a 2.7-kb DNA fragment. The DNA fragment was eluted from an agarose block after preparative agarose gel electrophoresis and used as a donor in transformation of the wild-type Acinetobacter strain ADP1. Selection for streptomycin and spectinomycin resistance produced strain ADP92.
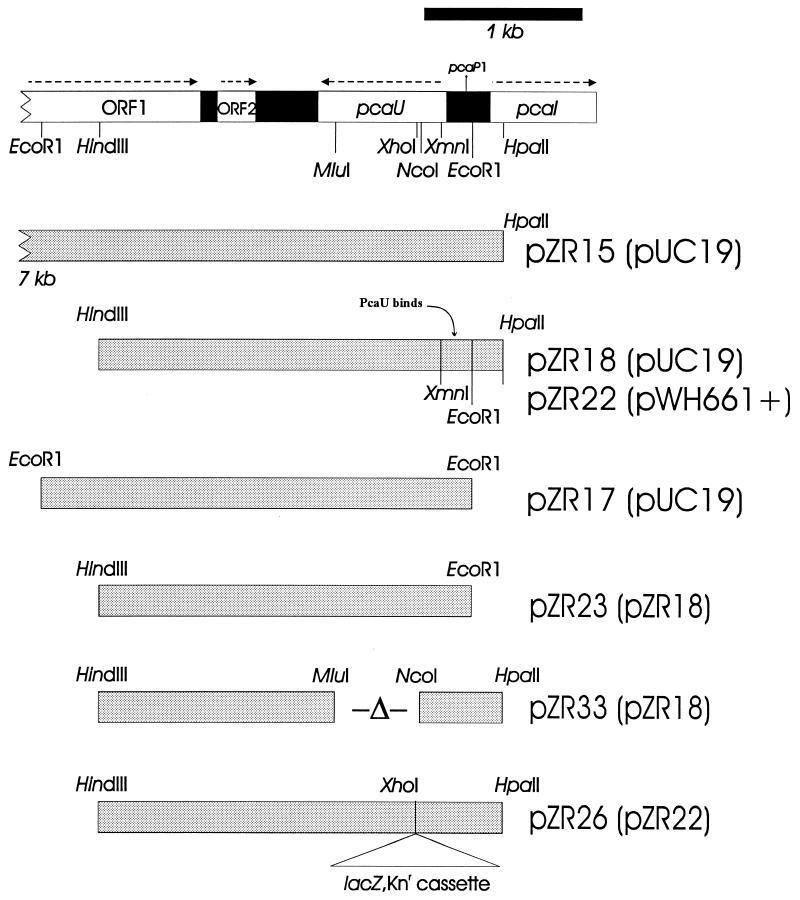
The 552-bp pcaU deletion in strain ADP331 was created by removal of DNA between the NcoI and MluI sites in pZR33 (Fig. 3). After digestion of the plasmid with these enzymes, the resulting fragments were filled in with the Klenow fragment, ligated, and transformed into E. coli DH5α. The presence of the desired deletion in pZR33 was confirmed by restriction analysis and DNA sequencing. After digestion of pZR33 with HindIII and SstI, DNA containing the deletion was introduced into wild-type Acinetobacter strain ADP1 by natural transformation. Recombinant colonies on succinate plates were identified by colony hybridization with the NcoI-MluI fragment as a probe to screen for clones in which it was lacking. The presence of the NcoI-MluI deletion in strain ADP331 was confirmed by PCR.
FIG. 3.
Recombinant plasmids containing all or part of pcaU. Symbols and markings are the same as described for Fig. 2.
Nucleotide sequence accession number.
The nucleotide sequence data presented in this paper has been deposited with GenBank under accession no. U04359.
RESULTS
The pcaP1 mutation is upstream of pcaI and blocks expression of pca structural genes.
Strains blocked in expression of pcaH and -G, structural genes for protocatechuate 3,4-dioxygenase, can be selected by demanding growth of organisms carrying the ΔpcaBDK1 mutation (Fig. 1) with succinate in the presence of protocatechuate. A previous study (21) showed that most of the selected strains contain, in addition to ΔpcaBDK1, mutations in pcaH and -G. As described in the legend to Fig. 2, strain ADP6126 contains an exceptional mutation, pcaP1, which lies outside the chromosomal region occupied by the pca structural genes.
In order to isolate wild-type DNA corresponding to pcaP1, a gene bank of partially digested Sau3A fragments of Acinetobacter chromosomal DNA inserted in the BamHI site of pUC19 was prepared in E. coli. An E. coli clone containing a plasmid with the desired insert was identified by replica plating E. coli colonies containing a library of the cloned fragments on a lawn of strain ADP6126 spread upon plates containing 4-hydroxybenzoate as the sole carbon source (2). An E. coli clone that was able to replace pcaP1 with wild-type Acinetobacter DNA yielded a 5,403-bp Sau3A insert, and the recombinant plasmid in this strain was named pZR9 (Fig. 2).
Sequencing of DNA replacing pcaP1 revealed both pcaU and a 282-bp pcaU-pcaI intergenic region.
The nucleotide sequence of the Sau3A insert in pZR9 overlapped the known sequence of the pZR1 EcoRI insert by 4,698 bp and contained 705 bp of previously uncharacterized DNA. Sequencing the latter DNA segment revealed a portion of a divergent open reading frame with two possible translational origins, either 282 or 294 bp from the start of pcaI (Fig. 2). In order to complete the nucleotide sequence of the newly discovered open reading frame, the 705-bp Sau3A-EcoRI fragment was used as a probe in Southern hybridization of Acinetobacter DNA restriction fragments to find an endonuclease that generated a fragment of appropriate size and location for further analysis. On the basis of this information, a 7-kb HpaII fragment was cloned into the AccI site of pUC19. The insert of the new plasmid, pZR15, extends through and beyond the newly characterized chromosomal region. Sequencing of pZR15 subclones (Fig. 3) revealed an open reading frame, designated pcaU, encoding a protein of either 274 or 278 residues (Fig. 4) that is a member of the PobR family of transcriptional activators (29). Among known members of this family, Acinetobacter PobR (9, 11), the transcriptional activator of pobA, is most similar to PcaU, to which it displays 54% amino acid sequence identity (Fig. 4). Among other known representatives of the family, the closest evolutionary affinity was exhibited by P. putida PcaR (52), which displays 31% amino acid sequence identity to Acinetobacter PcaU.
FIG. 4.
Similar deduced amino acid sequences of Acinetobacter PcaU and PobR. The aligned amino acid sequences are flanked by their encoding nucleotide sequences. Asterisks mark identical residues in the aligned amino acid sequences; nucleotide sequences extending both upstream and downstream from pcaU are shown. Two methionine codons, separated by nine nucleotide residues, are plausible start codons for pcaU translation; these codons are underlined. Because of uncertainty about the start codons, it is possible to conclude only that PcaU contains either 274 or 278 residues. The weight matrix comparison method of Dodd and Egan (12) assessed the likelihood of the shaded PcaU sequence being a helix-turn-helix DNA binding motif to be 100%, on the basis of a standard deviation score of 4.6; the corresponding value for the shaded PobR sequence is 5.2 (9). Restriction sites used in this investigation are labelled and marked by double overlining. Dashed arrows both upstream and downstream of pcaU indicate inverted repetitions that subsequent experiments demonstrated to be in sites where PcaU binds to DNA. Double overlining and underlining mark the EcoRI and XmnI sites bordering an upstream DNA fragment that was shown to bind PcaU. Double-dashed arrows indicate the positions of primers used to amplify a downstream DNA fragment that binds PcaU.
Two open reading frames apparently associated with β-oxidation of acyl CoA thioesters are located downstream from pcaU.
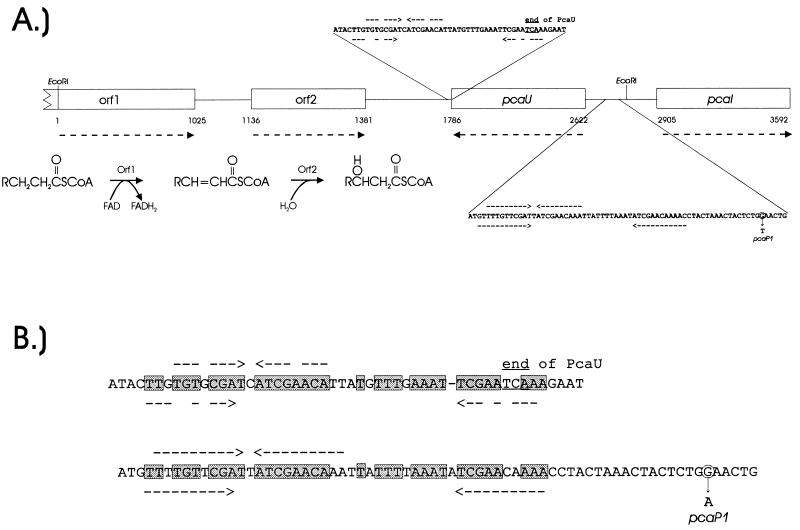
The newly obtained nucleotide sequence revealed a portion of an open reading frame potentially encoding a polypeptide of 340 residues (Fig. 5). This sequence corresponds to the carboxyl-terminal portion of an enzyme family of flavin adenine dinucleotide-containing acyl coenzyme A (CoA) dehydrogenases generally associated with the oxidation of the fatty acid thioesters. A neighboring open reading frame (Fig. 5) potentially encodes an 81-residue protein that is a member of a family of enoyl-CoA dehydratases also associated with metabolic pathways for fatty acyl CoA oxidation. Thus, both open reading frames appear to be associated with a physiological function demanding the β-oxidation of acyl CoA thioesters, metabolic steps that are not associated with protocatechuate catabolism.
FIG. 5.
Functions of nucleotide sequences downstream and upstream from pcaU. (A) Two open reading frames, designated orf1 and orf2, encode amino acid sequences closely resembling those of enzymes associated with β-oxidation of fatty acids. Dashed arrows below gene designations show directions of transcription. The other dashed arrows indicate positions of inverted repetitions that were depicted in Fig. 4 and shown in later experiments to be within sites where PcaU binds to DNA. To provide a frame of reference for subsequent observations, the position of the nucleotide substitution causing the pcaP1 mutation also is shown here, as is the EcoRI site between pcaU and pcaI. (B) Alignment of nucleotide sequences containing inverted repetitions downstream and upstream from pcaU. Identical residues in the aligned sequences are in shaded boxes.
Expression of PcaU from its cloned gene in E. coli.
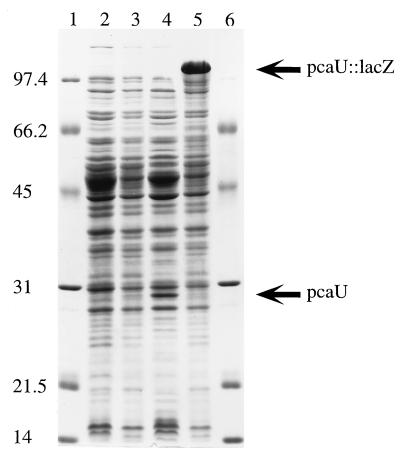
The open reading frame inferred to be pcaU encodes a polypeptide with a predicted molecular mass of either 31,610 or 31,092 Da. A cloned DNA fragment containing the gene was used to demonstrate synthesis of the corresponding protein in E. coli. The HindIII-HpaII restriction fragment containing pcaU was placed under the control of the lac promoter in the broad-host-range plasmid pWH661+, giving rise to pZR22; pZR18 contains the same insert in the opposite orientation (Fig. 3). As shown in Fig. 6, E. coli DH5α(pZR22) expresses substantial quantities of a 29,000-Da protein at a level that is below the level of detection in E. coli DH5α(pZR18). Insertion of lacZ into the XhoI site of pcaU disrupts the gene in pZR26, which in other respects is identical to pZR22 (Fig. 3). Figure 6 demonstrates that the E. coli strain containing the pcaU::lacZ insert replaces the protein corresponding to PcaU with a very large protein likely to correspond to LacZ.
FIG. 6.
Expression of pcaU in E. coli. Denaturing sodium dodecyl sulfate-gel (12.5% [wt/vol] acrylamide) electrophoresis of crude extracts (100 μg of protein per lane) of E. coli DH5α strains containing the following: lanes 1 and 6, molecular weight standards; lane 2, the unmodified vector pWH661+; lane 3, pZR18 containing pcaU oriented so that its transcription is not under the control of lacP; lane 4, pZR22 containing pcaU oriented so that its transcription is initiated by lacP; lane 5, pZR26 (pZR22 modified by insertion of a lacZ-Knr cassette into pcaU).
PcaU binds to DNA containing a consensus operator sequence located between the pcaU and pcaI promoters.
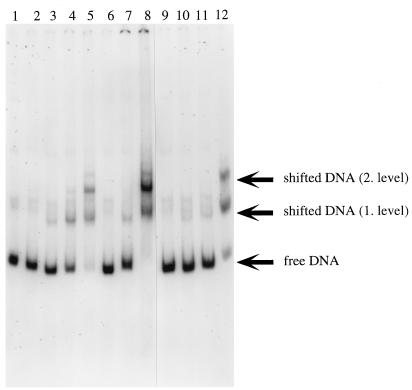
Extracts of E. coli DH5α(pZR22) containing PcaU were used to observe binding of the protein to DNA fragments containing a portion of the pcaU-pcaI intergenic region. The initial probe for gel shift assays was the 417-bp XmnI-HpaII fragment prepared from pZR18 (Fig. 3). This DNA was divided into a 201-bp HpaII-EcoRI fragment containing a portion of pcaI and a 216-bp XmnI-EcoRI fragment containing a portion of pcaU (Fig. 3). The latter fragment and the 417-bp fragment bound PcaU; the 201-bp HpaII-EcoRI fragment did not. Results obtained with the XmnI-EcoRI fragment are shown in Fig. 7. Two shifted bands were observed (Fig. 7). The addition of protocatechuate to the binding reaction did not significantly change the observed protein-DNA interaction.
FIG. 7.
Binding of PcaU to a DNA fragment containing a portion of the pcaU-pcaI intergenic region. The probe for this gel shift experiment was the 216-bp XmnI-EcoRI fragment containing a portion of pcaU (Fig. 4). Lane 1 depicts migration of the probe without added extract. Incubation of the probe with 15 μg of protein from E. coli DH5α(pWH661+) (lane 2) or E. coli DH5α(pZR26) (lane 6) did not shift the migration pattern, whereas incubation of the probe with the same amount of protein from E. coli DH5α(pZR22) gave rise to two bands (lanes 5 and 12). Lesser amounts of protein from E. coli DH5α(pZR22) produced little or no shift: the probe had been incubated with 5.2 μg of protein for the preparation run in lanes 4 and 11; 1.5 μg of protein was provided for lanes 3 and 10; 0.15 μg of protein was provided for lane 9. The tests whose results are shown in lanes 9, 10, 11, and 12 included 1.5 mM protocatechuate in the assay. Binding of the probe to 15 μg of protein contained in an extract from E. coli DH5α(pZR22) appears to have been competitively removed by 1.8 μg of pZR23 DNA (which contains the 216-bp XmnI-EcoRI segment [lane 7]) and not by 1.8 μg of pWH661+ DNA (which is the pZR22 vector lacking the insert [lane 8]).
Identification of consensus operator sequences.
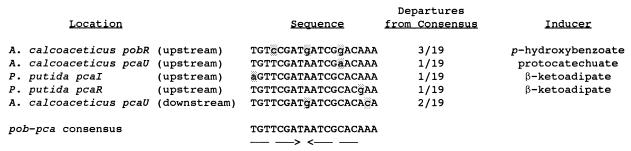
The 216-bp XmnI-EcoRI fragment that is bound by PcaU contains a 19-bp segment that displays 84% nucleotide sequence identity to the Acinetobacter pobR operator. Comparison of these sequences with operators governing expression of P. putida pca genes in response to PcaR revealed a consensus sequence which exhibits identity in at least 16 of the 19 residues in each comparison (Fig. 8). On the basis of this evidence, it is reasonable to propose that the newly sequenced 19-bp segment represents a portion of the pcaU operator. Thus, it is apparent that three chemically different inducers (p-hydroxybenzoate, protocatechuate, and β-ketoadipate, respectively) exercise precise transcriptional control through the interaction of homologous proteins (PobR, PcaU, and PcaR, respectively) with DNA segments containing closely similar nucleotide sequences (Fig. 8).
FIG. 8.
Consensus nucleotide sequences at DNA locations where transcription is regulated by metabolites in the β-ketoadipate pathway. The nucleotide sequence of the 216-bp XmnI-EcoRI fragment that binds PcaU contains a segment that displays identity to the pobR operator at 16 of 19 positions. The pobR operator is also closely similar to putative operators that are governed by PcaR and the inducer β-ketoadipate in P. putida. A scan for similar nucleotide sequences in the 20-kb Acinetobacter pca-qui-pob cluster revealed an additional close match directly downstream from pcaU. Arrows, inverted repetitions conserved in the consensus sequence.
Demonstration of a PcaU-binding site directly downstream from pcaU.
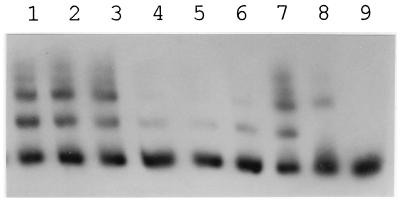
The availability of a consensus nucleotide sequence for DNA that may bind transcriptional regulators in the PobR family prompted a scan of the 20-kb Acinetobacter pca-qui-pob cluster for additional sequences displaying identity to the consensus sequence at more than 15 of 19 aligned residues. The survey revealed only one additional sequence, a DNA segment directly downstream from pcaU that differed from the consensus sequence in only 2 of 19 positions (Fig. 8). More-extensive comparison of the downstream sequence with the putative operator upstream from pcaU revealed that 33 of 43 aligned residues were identical after the introduction of a gap corresponding to one nucleotide in the downstream sequence (Fig. 5). This observation warranted a direct determination of the ability of PcaU to bind to DNA downstream from pcaU, and such evidence (Fig. 9) was obtained with a 300-bp PCR fragment containing the downstream sequence (Fig. 4). Thus, it appears that there are two binding sites for PcaU, one upstream and one downstream from pcaU.
FIG. 9.
Binding of PcaU to DNA directly downstream from pcaU. Probe (10 ng per reaction mix) was preincubated with 10 μg of protein from E. coli DH5α(pZR22) (lanes 1 through 7) or from E. coli DH5α(pWH661+) (lane 8) or without protein (lane 9). Additional components were as follows: 50, 30, and 10 ng of an unlabelled 297-bp PCR DNA fragment from the unrelated vanA gene from Acinetobacter (lanes 1 through 3, respectively); and 50, 30 and 10 ng of the unlabelled PCR fragment used to construct the probe (lanes 4 through 6, respectively). As with PcaU bound to the upstream site, several protein bands were observed, suggesting that bound PcaU exists in oligomeric forms.
PcaU is an activator of pca structural genes.
The physiological function of pcaU was explored by the preparation of strains carrying mutated forms of the gene. One of these, strain ADP92, contains the mutation pcaU1::ΩSmrSpcr (Fig. 2). This insertion would be expected to terminate both transcription and translation of pcaU at the XhoI site within its presumed helix-turn-helix DNA binding motif (Fig. 4). The other mutant strain, ADP331, was prepared from plasmid pZR33 (Fig. 3) and lacks the NcoI-MluI restriction fragment (Fig. 4) corresponding to two-thirds of pcaU. Both ADP92 and ADP331 expressed the full set of pca structural genes, as judged by their ability to grow, albeit more slowly than wild-type cells, on plates containing p-hydroxybenzoate as the carbon source.
The influence of the pcaU mutations on enzyme synthesis was examined by comparing the levels of protocatechuate oxygenase in uninduced (glucose-grown) cultures with the levels in cultures that had been induced by exposure of glucose-grown cells to 5 mM protocatechuate for 1 h prior to harvest. The mutations blocking pcaU reduced the induced level of protocatechuate oxygenase to between 10 and 20% of the fully induced level of 1.0 U/mg of protein found in wild-type cells. Uninduced mutant cultures formed the enzyme at levels corresponding to less than 5% of the specific activity found in induced cultures of wild-type cells. Thus, cells lacking a functional pcaU gene are impaired in pca gene expression but may exhibit a low level of inducible response to protocatechuate.
Study of the activity of PcaU in trans necessitated introduction of a recA mutation into the host strain (24). Results with this organism are not strictly comparable to those with the wild type, but it was evident that the plasmid-borne wild-type pcaU gene enhanced inducibility in an organism in which chromosomal pcaU is blocked. When expressed from the plasmid pZR22, pcaU allowed a 10-fold increase in inducible response, from 0.05 to 0.5 U of protocatechuate oxygenase/mg of protein, in cells containing the insertional mutations pcaU1::ΩSmrSpcr and recA100::Tn5.
Repression of pcaU transcription is relieved by protocatechuate.
In order to examine the influence of pcaU on its own expression, a promoterless lacZ:Kmr cassette was introduced into the XhoI site of pZR92, giving rise to the transcriptional fusion pcaU::lacZ in pZR28 (Fig. 2). Expression of the lacZ insert from the pcaU promoter was examined in cells containing recA100::Tn5. There was no functional pcaU in strains containing the chromosomal pcaU1::ΩSmrSpcr mutation, and during growth with succinate these organisms expressed lacZ from the plasmid at levels corresponding to 1,100 Miller units; the addition of protocatechuate to the growth medium increased these levels slightly to 1,400 Miller units. In the presence of wild-type chromosomal pcaU, expression of lacZ from the plasmid corresponded to levels between 300 and 350 Miller units during growth with succinate. When protocatechuate was added to the growth medium of these cells, lacZ expression increased to levels corresponding to 1,200 Miller units. Thus PcaU appears to cause a three- to fourfold repression of pcaU expression under these conditions, and this repression is relieved by protocatechuate.
Regulation by protocatechuate is exerted at the transcriptional level; the locations of the transcriptional starts of pcaI and pcaU.
Primer extension (Fig. 10A) revealed the beginning of the pcaI transcript, 119 bp upstream from the gene and 6 bp distal to the intergenic EcoRI site (Fig. 11). Primer extension (Fig. 10B) also showed that the pcaU transcript starts 31 bp upstream from pcaU (Fig. 11) and 132 bp from the transcriptional start for pcaI (Fig. 11). The starts of both transcripts are apparent in extracts of cells grown under inducing conditions and below detectable levels in uninduced cells (Fig. 10). This finding allows the conclusion that protocatechuate stimulates transcription of both pcaU and the pca operon. Further evidence that protocatechuate increases transcription of pcaU in wild-type cells emerged from Northern blots showing the formation of transcripts corresponding to both pcaU and the pca operon in response to growth with either p-hydroxybenzoate or protocatechuate (results not shown).
FIG. 10.
Transcriptional starts of the pca operon (A) and pcaU (B). Primer extension was used to determine the transcriptional starts. The cDNA from reverse transcription was loaded onto sequencing gels next to sequencing reactions with the same primers (lanes GATC); 10 μg of RNA was analyzed in each reaction. The RNA was isolated from cells after growth with succinate (lane 1) or p-hydroxybenzoate (lane 2) as the carbon source.
FIG. 11.
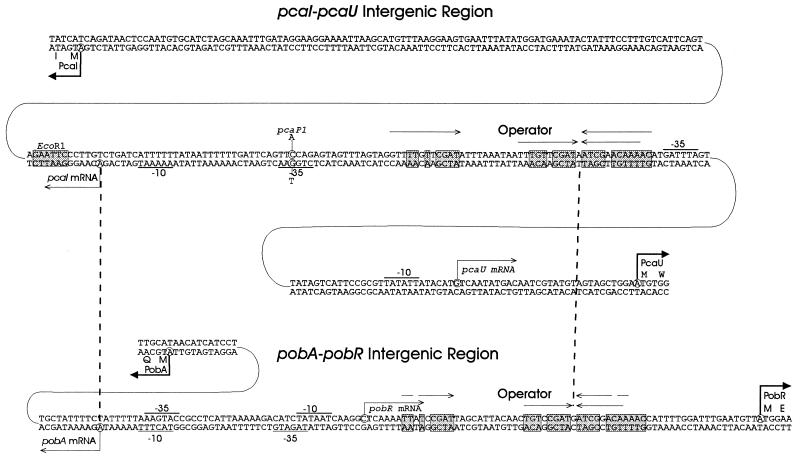
Conserved and divergent elements in the respective genetic regions governed by PcaU and PobR. Dashed vertical lines mark sites where the pcaI-pcaU and pobA-pobR regions have been aligned. Aligned positions are the transcriptional starts of structural genes (pcaI and pobA, respectively) and the highly conserved operators. Also shown in the pcaI-pcaU region are the translational start of PcaI, the EcoRI site that allows separation of pcaU from the pca structural genes, the A strings that are probable locations favoring DNA bending, the location of the pcaP1 promoter, and both the transcriptional and translational starts of pobR. Comparison of these sites with the aligned pobA-pobR sequence reveals substantial differences in the functional organization of DNA. Arrows, inverted repetitions; shaded boxes, conserved residues; underlining, putative promoter sequences; circles, nucleotides replaced by pcaP1.
The location of pcaP1 is consistent with its being a promoter mutation.
The nucleotide sequence of DNA containing pcaP1 showed a single nucleotide substitution consistent with a change at the −35-bp promoter region for the pcaI transcript (Fig. 11).
DISCUSSION
An overview of the genetic region containing pcaU.
The available evidence supports the conclusion that PcaU activates transcription of the pca operon by binding to an operator between pcaI and pcaU (Fig. 11). The first indication of where transcriptional regulation is exerted upstream of pcaI was given by the phenotype conferred by pcaP1, which mapped in the intergenic region. The mutation blocks expression of pca structural genes, and its location, 35 bp upstream from the position where pcaI transcription begins, suggests that it blocks promoter function (Fig. 11).
Translation of the open reading frame designated pcaU begins either 282 or 294 bp upstream from the translational start of pcaI, and the pcaI-pcaU intergenic region exhibits considerable complexity. Between the transcriptional starts of pcaI and pcaU is a presumptive pca operator which bears striking nucleotide sequence similarity to the operator governing pob expression (Fig. 11). A scan of the 20-kb pca-qui-pob region revealed that directly downstream from pcaU is an additional nucleotide sequence resembling the pcaU operator (Fig. 5). This raised the possibility that PcaU may bind to two different locations, one in the pcaI-pcaU intergenic region and the other downstream from pcaU. This inference was supported by direct measurement of PcaU binding to the two genetic regions (Fig. 8 and 9). Simultaneous binding to both regions by PcaU could allow the protein to regulate its own synthesis by looping (56) of DNA containing pcaU.
An intergenic region of 404 bp separates pcaU from open reading frames, here designated orf1 and orf2, that, on the basis of nucleotide sequence comparison, appear to be associated with the β-oxidation of CoA thioesters of carboxylic acids (Fig. 5). Whatever the natural substrates of these enzymes are, they are not metabolites formed in the catabolism of protocatechuate, quinate, or p-hydroxybenzoate. Thus, the chromosomal cluster of catabolic genes including pca-qui-pob extends to additional genes with as yet unknown function.
Transcriptional controls exerted by PcaU.
PcaU is a transcriptional activator of the pca operon. Inactivation of pcaU lowers induced expression of pcaH and -G by 80 to 90% and severely reduces the rate of growth of cells with p-hydroxybenzoate. When expressed from a plasmid in trans, pcaU can foster a 10-fold induction of pca structural genes in response to protocatechuate. Analysis of pcaU transcriptional fusions demonstrated that the wild-type gene product represses expression of its gene about threefold, and this repression is relieved by protocatechuate. The induction of transcripts for both pcaU and the pca operon in response to protocatechuate was demonstrated by primer extension and by Northern blot analysis.
The level of expression of the pca operon in the absence of PcaU is fairly high. Two possible factors may contribute to the background expression of the pca operon. First, there may be additional transcriptional activators that to some extent mimic the effect of PcaU in Acinetobacter. Second, the structure of the pca operon promoter may allow a significant level of constitutive transcription. In any case, the observed background of gene expression fits with the notion of regulatory noise, the hypothesis that transcriptional controls allowing a low level of background expression are candidates well-suited to being borrowed in the evolution of new regulatory mechanisms (8).
Comparison of PcaU and PobR.
PcaU contains either 274 or 278 amino acid residues, and most of these are in a sequence closely resembling the amino acid sequence of PobR. Introduction of a single gap into the PcaU sequence allows an alignment in which PcaU and PobR exhibit 54% identity over 255 amino acid residues (Fig. 4). The similarity extends through the presumed helix-turn-helix DNA-binding region (Fig. 4) where 13 of 22 amino acid residues are identical. The proteins bear no significant sequence similarity in their NH2-terminal regions which are highly charged.
Comparison of the pcaI-pcaU and pobA-pobR intergenic regions.
The common ancestry of PcaU and PobR can be inferred from the similarity of their amino acid sequences (Fig. 4), and this conclusion is strengthened by the resemblance of the operators to which the respective proteins probably bind (Fig. 11). Perhaps more remarkable are the variations achieved by the two regulatory systems during their evolutionary divergence. At the phenotypic level, control exercised by PcaU appears to be lax, whereas regulation exerted by PobR is quite tight: in the absence of either p-hydroxybenzoate or a functional pobR gene, pobA is not expressed at detectable levels. Knockout mutations in pobR completely block growth with p-hydroxybenzoate, whereas knockout mutations in pcaU do not prevent slow growth supported by the pca structural genes. Differences extend to control of regulatory gene expression: pcaU and pobR are repressed by their respective protein products, but repression exerted by PcaU is relieved by its effector, protocatechuate, whereas p-hydroxybenzoate, the PobR effector, does not relieve the repression exerted by this protein upon its gene (11).
The pobA-pobR intergenic region is much more tightly structured than the pcaU-pcaI intergenic region. Only 21 bp separate the transcriptional and translational starts of pobA (Fig. 11). In contrast, the transcriptional and translational starts of pcaI are separated by 118 bp of DNA with unknown function (Fig. 11). The pca operator lies between the promoters for pcaI and pcaU (Fig. 11). In contrast, extremely tight organization superimposes the pobA and pobR promoters upon each other and places the pob operator between the transcriptional and translational starts of pobR (Fig. 11).
DNA arrangements and rearrangements during evolution.
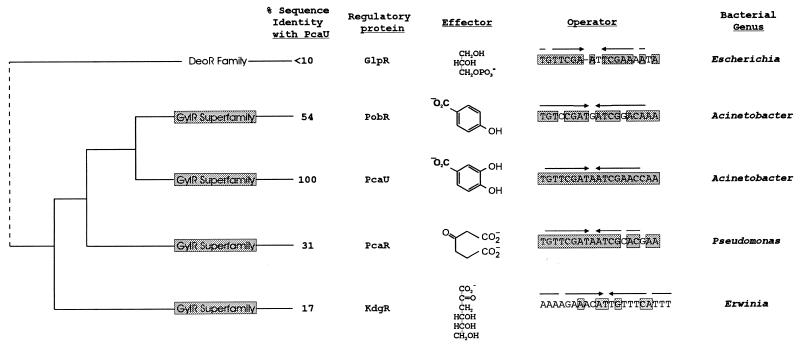
The overall pattern that emerges from a comparison of pcaU and pobR is that of conservation of similar transcriptional activators and consensus operators against a background of extensive evolutionary divergence in the Acinetobacter pca-qui-pob gene cluster. The common themes are maintained in a broader biological context. PcaU and PobR are members of a somewhat sparsely represented transcriptional-activator family that has been called upon during evolution of the β-ketoadipate pathway (9, 29, 47). The closest known relatives of Acinetobacter PcaU and PobR are P. putida PcaR (52) and Erwinia chrysanthemi KdgR (45). All four proteins respond to chemically different inducing metabolites (Fig. 12). Three of the proteins (PcaU, PobR, and PcaR) are associated with the same physiological function, growth with p-hydroxybenzoate through the β-ketoadipate pathway. Thus, it appears that a critical stage in the evolution of controls for the β-ketoadipate pathway was the selection of an ancestor of the PobR family as a transcriptional activator. Subsequent events resulted in modified transcriptional regulators that respond to different inducers.
FIG. 12.
Ancestry of regulatory proteins and the operators to which they bind. Acinetobacter PobR (9), Acinetobacter PcaU, Pseudomonas PcaR (52), and Erwinina KdgR (45) have a common ancestry and respond to chemically different effectors. E. coli GlpR (7) is a member of a different evolutionary family of regulatory proteins, but comparison of identical nucleotide residues (represented by shading) raises the possibility of a common ancestry for the glp operator and operators associated with aromatic catabolism in Acinetobacter and Pseudomonas. Arrows, inverted repetitions.
It is noteworthy that the three transcriptional regulators associated with the β-ketoadipate pathway interact with closely similar operators, and the consensus nucleotide sequences in these operators are not apparent in the operators with which KdgR interacts (44, 45) (Fig. 12). The operators associated with the β-ketoadipate pathway are closely similar to the E. coli glpR operator (63) which interacts with a transcriptional activator (7) from the separate DeoR (59) family of transcriptional regulators (Fig. 12). The close similarities of the operator sequences might be the product of convergent evolution. Alternatively, it is possible that DNA rearrangements may have produced novel combinations of operators and regulatory proteins during the evolution of transcriptional controls.
ACKNOWLEDGMENTS
This research was supported by grants from the Army Research Office and the National Science Foundation. U.G. was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft. A.S. was supported by a postdoctoral fellowship from the Spanish Ministerio de Educacion y Ciencia.
We are indebted to San Tran and Stephanie Haus for technical assistance, to David Young for assistance in the identification of a consensus sequence, and to Wayne Coco and David D’Argenio for improvements made to the manuscript.
Footnotes
Publication 17 from the Biological Transformation Center in the Yale Biospherics Institute.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1994. [Google Scholar]
- 2.Averhoff B, Gregg-Jolly L, Elsemore D, Ornston L N. Genetic analysis of supraoperonic clustering by use of natural transformation in Acinetobacter calcoaceticus. J Bacteriol. 1992;174:200–204. doi: 10.1128/jb.174.1.200-204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann P. Isolation of Acinetobacter from soil and water. J Bacteriol. 1968;96:39–42. doi: 10.1128/jb.96.1.39-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canovas J L, Stanier R Y. Regulation of the enzymes of the β-ketoadipate pathway in Moraxella calcoacetica. I. General aspects. Eur J Biochem. 1967;1:289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- 6.Canovas J L, Wheelis M L, Stanier R Y. Regulation of the enzymes of the β-ketoadipate pathway in Moraxella calcoacetica. II. The role of protocatechuate as inducer. Eur J Biochem. 1968;3:293–304. doi: 10.1111/j.1432-1033.1968.tb19529.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi Y L, Kawase S, Nishida T, Sakai H, Komano T, Kawamukai M, Utsumi R, Kohara Y, Akiyama K. Nucleotide sequence of the glpR gene encoding the repressor for the glycerol-3-phosphate regulon of Escherichia coli K12. Nucleic Acids Res. 1988;16:7732. doi: 10.1093/nar/16.15.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lorenzo V, Perez-Martin J. Regulatory noise in prokaryotic promoters: how bacteria learn to respond to novel environmental signals. Mol Microbiol. 1996;19:1177–1184. doi: 10.1111/j.1365-2958.1996.tb02463.x. [DOI] [PubMed] [Google Scholar]
- 9.DiMarco A A, Averhoff B, Ornston L N. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J Bacteriol. 1993;175:4499–4506. doi: 10.1128/jb.175.14.4499-4506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMarco A A, Averhoff B A, Kim E E, Ornston L N. Evolutionary divergence of pobA, the structural gene encoding p-hydroxybenzoate hydroxylase in an Acinetobacter calcoaceticus strain well-suited for genetic analysis. Gene. 1993;125:25–33. doi: 10.1016/0378-1119(93)90741-k. [DOI] [PubMed] [Google Scholar]
- 11.DiMarco A A, Ornston L N. Regulation of p-hydroxybenzoate hydroxylase synthesis by PobR bound to an operator in Acinetobacter calcoaceticus. J Bacteriol. 1994;176:4277–4284. doi: 10.1128/jb.176.14.4277-4284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doten R C, Ngai K L, Mitchell D J, Ornston L N. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J Bacteriol. 1987;169:3168–3174. doi: 10.1128/jb.169.7.3168-3174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doten R C, Ornston L N. Protocatechuate is not metabolized via catechol in Enterobacter aerogenes. J Bacteriol. 1987;169:5827–5830. doi: 10.1128/jb.169.12.5827-5830.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durham D R, Stirling L A, Ornston L N, Perry J J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980;19:149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- 16.Earhart C A, Radhakrishnan R, Orville A M, Lipscomb J D, Ohlendorf D H. Preliminary crystallographic study of protocatechuate 3,4-dioxygenase from Brevibacterium fuscum. J Mol Biol. 1994;236:374–376. doi: 10.1006/jmbi.1994.1143. [DOI] [PubMed] [Google Scholar]
- 17.Elsemore D A, Ornston L N. The pca-pob supraoperonic cluster of Acinetobacter calcoaceticus contains quiA, the structural gene for quinate-shikimate dehydrogenase. J Bacteriol. 1994;176:7659–7666. doi: 10.1128/jb.176.24.7659-7666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsemore D A, Ornston L N. Unusual ancestry of dehydratases associated with quinate catabolism in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:5971–5978. doi: 10.1128/jb.177.20.5971-5978.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fewson C A. Microbial metabolism of mandelate: a microcosm of diversity. FEMS Microbiol Rev. 1988;4:85–110. doi: 10.1111/j.1574-6968.1988.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 20.Gerischer U, D’Argenio D A, Ornston L N. IS1236, a newly discovered member of the IS3 family, exhibits varied patterns of insertion into the Acinetobacter calcoaceticus chromosome. Microbiology. 1996;142:1825–1831. doi: 10.1099/13500872-142-7-1825. [DOI] [PubMed] [Google Scholar]
- 21.Gerischer U, Ornston L N. Spontaneous mutations in pcaH and -G, structural genes for protocatechuate 3,4-dioxygenase in Acinetobacter calcoaceticus. J Bacteriol. 1995;177:1336–1347. doi: 10.1128/jb.177.5.1336-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerischer U, Dürre P. Cloning, sequencing, and molecular analysis of the acetoacetate decarboxylase gene from Clostridium acetobutylicum. J Bacteriol. 1990;172:6907–6918. doi: 10.1128/jb.172.12.6907-6918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerischer U, Dürre P. mRNA analysis of the adc gene region of Clostridium acetobutylicum during shift to solventogenesis. J Bacteriol. 1992;174:426–433. doi: 10.1128/jb.174.2.426-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregg-Jolly L A, Ornston L N. Properties of Acinetobacter calcoaceticus recA and its contribution to intracellular gene conversion. Mol Microbiol. 1994;12:985–992. doi: 10.1111/j.1365-2958.1994.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 25.Gregg-Jolly L A, Ornston L N. Recovery of DNA from the Acinetobacter calcoaceticus chromosome by gap repair. J Bacteriol. 1990;172:6169–6172. doi: 10.1128/jb.172.10.6169-6172.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grund E, Knorr C, Eichenlaub R. Catabolism of benzoate and monohydroxylated benzoates by Amycolatopsis and Streptomyces spp. Appl Environ Microbiol. 1990;56:1459–1464. doi: 10.1128/aem.56.5.1459-1464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer A, Stolz A, Knackmuss H. Purification and characterization of a novel type of protocatechuate 3,4-dioxygenase with the ability to oxidize 4-sulfocatechol. Arch Microbiol. 1996;166:92–100. doi: 10.1007/s002030050361. [DOI] [PubMed] [Google Scholar]
- 28.Harwood C S, Nichols N N, Kim M K, Ditty J L, Parales R E. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 30.Hunger M, Schmucker R, Kishan V, Hillen W. Analysis and nucleotide sequence of an origin of DNA replication in Acinetobacter calcoaceticus and its use for Escherichia coli shuttle plasmids. Gene. 1990;87:45–51. doi: 10.1016/0378-1119(90)90494-c. [DOI] [PubMed] [Google Scholar]
- 31.Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972;112:917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juni E, Janik A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 34.Kloos D U, DiMarco A A, Elsemore D A, Timmis K N, Ornston L N. Distance between alleles as a determinant of linkage in natural transformation of Acinetobacter calcoaceticus. J Bacteriol. 1995;177:6015–6017. doi: 10.1128/jb.177.20.6015-6017.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kokotek W, Lotz W. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene. 1989;84:467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- 36.Krooneman J, Wieringa E B, Moore E R, Gerritse J, Prins R A, Gottschal J C. Isolation of Alcaligenes sp. strain L6 at low oxygen concentrations and degradation of 3-chlorobenzoate via a pathway not involving (chloro)catechols. Appl Environ Microbiol. 1996;62:2427–2434. doi: 10.1128/aem.62.7.2427-2434.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;176:265–275. [PubMed] [Google Scholar]
- 39.Mashetty S B, Manohar S, Karegoudar T B. Degradation of 3-hydroxybenzoic acid by a Bacillus species. Indian J Biochem Biophys. 1996;33:145–148. [PubMed] [Google Scholar]
- 40.Middelhoven W J. Catabolism of benzene compounds by ascomycetous and basidiomycetous yeasts and yeastlike fungi. A literature review and an experimental approach. Antonie Leeuwenhoek. 1993;63:125–144. doi: 10.1007/BF00872388. [DOI] [PubMed] [Google Scholar]
- 41.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 42.Milstein O, Trojanowski J, Huttermann A, Gressel J. Catabolism of single ring aromatic acids by four Aspergillus species. Microbios. 1988;55:7–16. [PubMed] [Google Scholar]
- 43.Morawski B, Eaton R W, Rossiter J T, Guoping S, Griengl H, Ribbons D W. 2-Naphthoate catabolic pathway in Burkholderia strain JT 1500. J Bacteriol. 1997;179:115–121. doi: 10.1128/jb.179.1.115-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasser W, Reverchon S, Condemine G, Robert-Baudouy J. Specific interactions of Erwinia chrysanthemi KdgR repressor with different operators of genes involved in pectinolysis. J Mol Biol. 1994;236:427–440. doi: 10.1006/jmbi.1994.1155. [DOI] [PubMed] [Google Scholar]
- 45.Nasser W, Reverchon S, Robert-Baudouy J. Purification and functional characterization of the KdgR protein, a major repressor of pectinolysis genes of Erwinia chrysanthemi. Mol Microbiol. 1992;6:257–265. doi: 10.1111/j.1365-2958.1992.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 46.Parales R E, Harwood C S. Regulation of the pcaIJ genes for aromatic acid degradation in Pseudomonas putida. J Bacteriol. 1993;175:5829–5838. doi: 10.1128/jb.175.18.5829-5838.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parke D. Acquisition, reorganization, and merger of genes—novel management of the β-ketoadipate pathway in Agrobacterium tumefaciens. FEMS Microbiol Lett. 1997;146:3–12. [Google Scholar]
- 48.Parke D. Positive regulation of phenolic catabolism in Agrobacterium tumefaciens by the pcaQ gene in response to β-carboxy-cis,cis-muconate. J Bacteriol. 1993;175:3529–3535. doi: 10.1128/jb.175.11.3529-3535.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parke D, Ornston L N. Enzymes of the β-ketoadipate pathway are inducible in Rhizobium and Agrobacterium spp. and constitutive in Bradyrhizobium spp. J Bacteriol. 1986;165:288–292. doi: 10.1128/jb.165.1.288-292.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel R N, Mazumdar S, Ornston L N. β-Ketoadipate enol-lactone hydrolases I and II from Acinetobacter calcoaceticus. J Biol Chem. 1975;250:6567–6577. [PubMed] [Google Scholar]
- 51.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 52.Romero-Steiner S, Parales R E, Harwood C S, Houghton J E. Characterization of the pcaR regulatory gene from Pseudomonas putida, which is required for the complete degradation of p-hydroxybenzoate. J Bacteriol. 1994;176:5771–5779. doi: 10.1128/jb.176.18.5771-5779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 54.Simon R, O’Connell M, Labes M, Puhler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- 55.Smith C P, Chater K F. Structure and regulation of controlling sequences for the Streptomyces coelicolor glycerol operon. J Mol Biol. 1988;204:569–580. doi: 10.1016/0022-2836(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 56.Soisson S M, MacDougall-Shackleton B, Schleif R, Wolberger C. Structural basis for ligand-regulated oligomerization of AraC. Science. 1997;276:421–425. doi: 10.1126/science.276.5311.421. [DOI] [PubMed] [Google Scholar]
- 57.Suemori A, Nakajima K, Kurane R, Nakamura Y. o-, m- and p-hydroxybenzoate degradative pathways in Rhodococcus erythropolis. FEMS Microbiol Lett. 1995;125:31–35. doi: 10.1111/j.1574-6968.1995.tb07331.x. [DOI] [PubMed] [Google Scholar]
- 58.Turner J E, Allison N, Fewson C A. Metabolic characterisation of a novel vanillylmandelate-degrading bacterium. Arch Microbiol. 1996;166:252–259. doi: 10.1007/s002030050381. [DOI] [PubMed] [Google Scholar]
- 59.Valentin-Hansen P, Hojrup P, Short S. The primary structure of the DeoR repressor from Escherichia coli K-12. Nucleic Acids Res. 1985;13:5927–5936. doi: 10.1093/nar/13.16.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasudevan N, Mahadevan A. Degradation of indulin by Candida albicans. Biochem Int. 1992;26:317–325. [PubMed] [Google Scholar]
- 61.Wheeler K A, Lamb H K, Hawkins A R. Control of metabolic flux through the quinate pathway in Aspergillus nidulans. Biochem J. 1996;315:195–205. doi: 10.1042/bj3150195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 63.Zhao N, Oh W, Trybul D, Thrasher K S, Kingsbury T J, Larson T J. Characterization of the interaction of the glp repressor of Escherichia coli K-12 with single and tandem glp operator variants. J Bacteriol. 1994;176:2393–2397. doi: 10.1128/jb.176.8.2393-2397.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zylstra G J, Olsen R H, Ballou D P. Genetic organization and sequence of the Pseudomonas cepacia genes for the α and β subunits of protocatechuate 3,4-dioxygenase. J Bacteriol. 1989;171:5915–5921. doi: 10.1128/jb.171.11.5915-5921.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]