Abstract
Burgeoning evidence demonstrates that effects of environmental exposures can be transmitted to subsequent generations through the germline without DNA mutations1,2. This phenomenon remains controversial because underlying mechanisms have not been identified. Therefore, understanding how effects of environmental exposures are transmitted to unexposed generations without DNA mutations is a fundamental unanswered question in biology. Here, we used an established murine model of male-specific transgenerational obesity to show that exposure to the obesogen tributyltin (TBT) elicited heritable changes in chromatin interactions (CIs) in primordial germ cells (PGCs). New CIs were formed within the Ide gene encoding Insulin Degrading Enzyme in the directly exposed PGCs, then stably maintained in PGCs of the subsequent (unexposed) two generations. Concomitantly, Ide mRNA expression was decreased in livers of male descendants from the exposed dams. These males were hyperinsulinemic and hyperglycemic, phenocopying Ide-deficient mice that are predisposed to adult-onset, diet-induced obesity. Creation of new CIs in PGCs, suppression of hepatic Ide mRNA, increased fat mass, hyperinsulinemia and hyperglycemia were male-specific. Our results provide a plausible molecular mechanism underlying transmission of the transgenerational predisposition to obesity caused by gestational exposure to an environmental obesogen. They also provide an entry point for future studies aimed at understanding how environmental exposures alter chromatin structure to influence physiology across multiple generations in mammals.
Introduction
Several possible mechanisms have been proposed to explain transgenerational inheritance2. These included epigenetic alterations such as DNA methylation1, histone methylation3, histone retention4,5, and the transmission of small, non-coding RNAs6. Each has some merit for transmitting the results of direct exposure. However, none provides a mechanistic understanding for how phenotypes can be transmitted to unexposed descendants2. DNA methylation is erased genome-wide twice each generation in mammals7. This makes it unclear how the large number of differentially methylated regions we previously observed in white adipose tissue (WAT) of male F4 tributyltin (TBT)-group mice8 could have been transmitted to an unexposed generation. How histone methylation at specific loci can be transmitted across generations and why only some histones are retained during spermatogenesis are unknown. It remains uncertain how expression of non-coding RNAs, such as those that were reported in F1 sperm and seminal fluid9 could be transmitted to multiple future generations. Our preceding studies suggested that stable alterations of higher-order chromatin structures might provide a unifying theory to explain mammalian transgenerational inheritance8,10. Support for such a model requires the identification of persistently altered regions of higher-order chromatin structure. Therefore, we sought to identify regions of persistently altered chromatin interactions (CIs) in PGCs from mice exposed to TBT (F1) and unexposed (F2, F3) generations and associate these specific changes with the transgenerational obesity phenotypes observed.
Ancestral TBT exposure led to a transgenerational predisposition to increased WAT mass
In the new transgenerational experiment 4 (T4) detailed here, effects of ancestral TBT exposure throughout gestation were confirmed to be much stronger in male versus female F2 and F3 generation C56BL/6J mice. This confirms our previous transgenerational experiments denoted as T111, T28 and T312 and another study using OG2 C56BL/6J mice13. Salient findings included increased WAT depot size, more overall body fat and in some cases, increased body weight (Fig. 1). After the diet was changed from a standard chow diet (SD) to a higher fat diet (HFD) at 5 weeks of age, only at 11 weeks did male F2 animals ancestrally exposed to TBT accumulate significantly more WAT than controls (Fig. 1a); body weight did not differ between groups. In contrast, F3 male animals did not show increased body weight or fat mass when switched to the HFD at 5 weeks (Extended data Fig. 1). We hypothesized that HFD challenge had started too soon compared with previous experiments8,12. Therefore, we switched sibling DMSO- and TBT-group F3 animals that had been maintained on the SD to HFD at 17 weeks of age. TBT group F3 males rapidly accumulated body weight and fat mass compared with controls; these differences became statistically significant at 19 (body fat) or 20 weeks (body weight) (Fig. 1b). No effects of ancestral TBT exposure on fat accumulation were observed in females (Fig. 1c, d).
Figure 1. Mice ancestrally exposed to TBT exhibited increased fat content in F2 and F3 male descendants.
Body weight and relative body composition of a, F2 male descendants (n = 15), b, F3 male descendants (n = 16), c, F2 female descendants (n = 17), and d, F3 female descendants (n = 16) throughout the time course of the experiment. Gray area indicates the period of diet challenge. Statistical significance was determined using two-way ANOVA. Pair-wise Bonferroni post-hoc tests were used to compare different groups. Data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01.
Ancestral TBT exposure caused transgenerationally stable changes in chromatin interactions in the genome of male PGCs
To determine whether CIs were stably altered after ancestral TBT exposure, we performed Hi-C seq of PGCs isolated from E13.5 embryonic gonads of F1-F3 mice, pooled by litter and sex. Approximately 10,000–15,000 FACS-enriched PGCs were subjected to Hi-C sequencing, data generation and analysis (Extended data Fig. 2). Successful detection of a known set of TADs around the HoxD gene cluster14 demonstrated the validity of our Hi-C seq data (Extended data Fig. 2i, j)
PGCs in F1 embryos were directly exposed to TBT while the embryos were within the treated F0 dams. Those isolated from F2 or F3 embryos were not exposed. PGCs in F2 embryos became gametes producing F3 animals, which showed a transgenerational predisposition to diet-induced obesity8,11–13. The global profiles of chromatin contacts were well conserved in PGC genomes across sex, F0 exposure to TBT, or F1-F3 generations (Extended data Fig. 3a). The median distance between two chromatin contacts was approximately 1 megabase (Extended data Fig. 3b), which agrees with the previously reported size of TADs in the mouse genome (880 kb)15.
Applying a strict criterion of detection (Differential Chromatin Interaction Scores > 3.0), we identified 20 autosomal differential chromatin interactions (DCIs) conserved between F1 and F2 male PGCs and only one DCI in chromosome 19 that was conserved across all generations (F1-F3) of male PGCs (Extended data Fig. 3c).
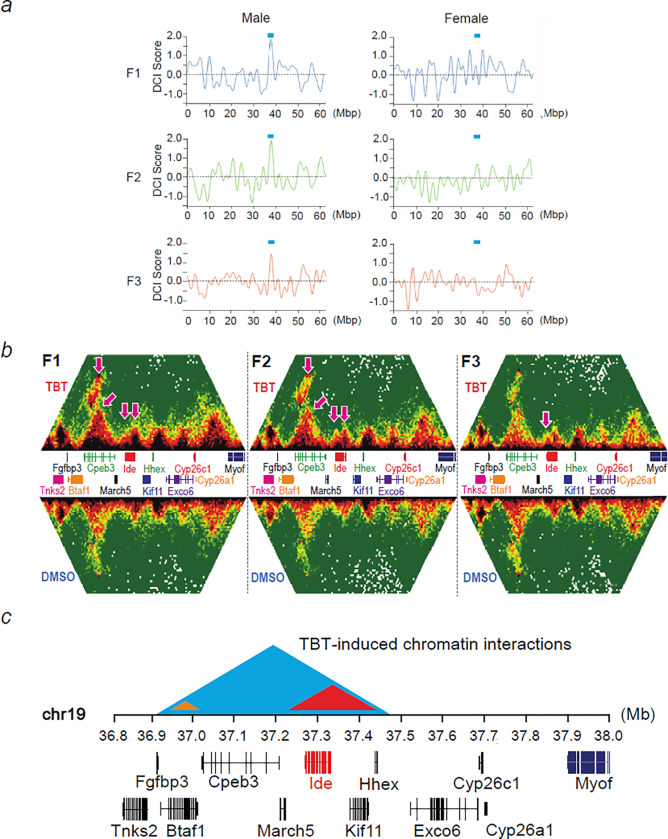
The DCI score plots shown in Fig. 2a demonstrate a region containing significant DCIs – which were gained in the TBT group compared to the DMSO group – well conserved in chromosome 19 of male PGCs across F1-F3 generations (chr19:36,920,000–37,420,000; blue horizontal bar) but not in female PGCs. Formation of new CIs in this region in the genome of the TBT-group PGCs was confirmed by direct visual inspection of normalized chromatin contact matrix data of male PGCs isolated from F1-F3 embryonic testes although these new contacts became weaker in F3 embryonic testes (Fig. 2b). In contrast, there were no changes in CIs in this region among female PGCs across generations after ancestral TBT exposure compared with vehicle controls (Extended data Fig. 4a). Visual inspection of DCIs for the whole chromosome 19 of male PGCs isolated from the F2 embryos identified only a single region displaying discernible DCIs (Extended data Fig. 4b, top panel), which was confirmed to be identical to the region described above (Extended data Fig. 4b, zoom-in panels). Detailed examination of this region revealed three DCIs – namely, two small DCIs around chr19:36,920,000–37,020,000 (DCI-1) and chr19:37220000–37420000 (DCI-2) and a large DCI (DCI-3) spanning over the two small DCIs (Fig. 2c and Extended data Fig. 4b). The nested structure involving these three DCIs convincingly supports TBT-induced formation and transgenerational persistence of these DCIs. To increase our confidence that the two small DCIs (DCI-1 and DCI-2) involved in the longer-range DCI (DCI-3) are not problematic genomic regions, we confirmed that DCI1–3 are not flagged by the ENCODE blacklist16, which are genomic regions unsuitable for deep sequencing-based analyses due to unusual structures. We also confirmed that DCI1–3 are not involved in any segmental duplication as detected by SDquest17, eliminating the possibility that DCI1 and DCI2 are deep sequencing artifacts stemming from significantly similar sequences locating nearby. Interestingly, DCI2 contained the Ide gene, which encodes insulin degrading enzyme (Fig. 2b,c). Hepatic Ide is responsible for the majority of insulin clearance18. These results demonstrated formation of transgenerational, germline-transmitted alterations in CIs after exposure of pregnant F0 female mice to the obesogen, TBT.
Figure 2. Transgenerationally transmitted differential chromatin interactions (DCI).
a, DCI scores of the whole chromatin 19 in mouse primordial germ cells isolated from F1-F3 embryos were determined by Bart3D and smoothened for plotting. Blue horizontal bars indicate the location of DCI at the Ide gene. b, Chromatin contact plots of primordial germ cells isolated from F1-F3 male embryos after F0 exposure to TBT (top) or DMSO (bottom). Arrows indicate DCIs gained by the F0 exposure to TBT. c, Locations of genes near the transgenerationally conserved DCIs caused by F0 exposure to TBT. The Ide gene is shown in red.
Ancestral TBT exposure induced CI formation in the Ide gene in male livers
To determine whether the DCIs identified in PGCs were also found in the Ide gene in somatic tissues, we sought to examine CTCF binding status in F3 livers because liver is the primary site of Ide expression and F2 PGCs give rise to F3 descendants. Five CTCF binding sites predicted by the JASPAR Transcription Factor Binding Site Database lie near the CI (A-E) together with two known CTCF binding sites (F, G) (Chr19:37,320,000 in mm10) (Fig. 3a). ChIP-qPCR analysis was performed on CTCF or RAD21 chromatin pulldown samples. We observed increased CTCF binding in four regions (B, C, F, G) within the Ide gene in male livers and infer that these may form a small chromatin loop (Fig. 3b). These findings were reproduced with different primer sets (Extended data Fig. 5). No significant enrichment was noted in TBT female samples (Fig. 3c). We did not detect enrichment of RAD21 binding on the Ide gene (Fig. 3d, e).
Figure 3. Mice ancestrally exposed to TBT showed increased CTCF binding at the Ide gene on chromosome 19.
a, Five potential CTCF binding sites (A to E) in IDE gene of chromosome 19 predicted by JASPAR on the UCSC Genome Browse and two known CTCF binding sites (F and G) were analyzed. Chromatin immunoprecipitation (ChIP) and quantitative real time RT-PCR (qPCR) assays using antibody against CTCF in F3 b, male or c, female descendants livers. ChIP and qPCR assays using antibody against RAD21 in F3 d, male or e, female descendants livers. Normal rabbit IgG was used as a non-specific antibody control. Unpaired t-tests were used for qPCR analysis. Data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01; ***p < 0.001.
Ancestral TBT exposure led to a transgenerational, male-specific hyperinsulinemia
Formation of transgenerationally persistent, novel CIs in male PGCs within the Ide gene (Fig. 2, Extended data Figs. 3, 4) prompted us to hypothesize that expression of Ide mRNA might be affected by ancestral TBT exposure. Strikingly, significant decreases in Ide mRNA expression were found in the livers of F2 and F3 adult males (Fig. 4a) but not in gonadal WAT (Fig. 4b), skeletal muscle (Fig. 4c) or in females other than F2 female livers (Extended data Fig. 6a-c). Notably, Ide mRNA expression was also decreased in F3 male livers from the previously published T3 experiment12 (Extended data Fig. 6d) indicating that this phenotype is reproducible across experiments.
Figure 4. Male mice ancestrally exposed to TBT showed decreased Ide gene expression in liver and suffer from hyperinsulinemia before and after six weeks of diet challenge.
The relative mRNA levels of Ide gene were assayed by quantitative PCR in a, liver, b, gonadal white adipose tissue (gWAT), and c, soleus muscle of F2 (n = 15) and F3 (n =16) male descendants with the expression normalized to GAPDH. Data are expressed as mean fold change ± SEM and assayed in duplicate. *p < 0.05 and **p < 0.01, compared with DMSO groups by unpaired t-test. d, Plasma glucose and e, insulin of male mice before diet challenge (F2 at 5 weeks age and F3 at 17 weeks age) were measured and f HOMA-IR indexes were calculated accordingly. g, Plasma glucose and h, insulin of male mice after six weeks of diet challenge (F2 at 11 weeks age and F3 at 22 weeks age) were measured and i, HOMA-IR indexes were calculated accordingly. Statistical significance was determined using two-way ANOVA. Pair-wise Bonferroni post-tests were used to compare different groups. Data are presented as mean ± s.e.m. *p < 0.05; **p < 0.01; ***p < 0.001.
No differences in fasting blood glucose levels were observed in either males (Fig. 4d) or females (Extended data Fig. 7a) prior to diet challenge, as we previously reported8. However, plasma insulin levels in TBT group males were significantly higher (Fig. 4e), in accord with the decreased hepatic Ide mRNA expression (Fig. 4a) whereas no significant differences were observed in females (Extended data Fig. 7b). We calculated homeostasis model assessment of insulin resistance (HOMA-IR) to estimate insulin resistance and found that TBT-group male descendants showed higher potential for insulin resistance than did DMSO descendants (Fig. 4f). Females showed no differences (Extended data Fig. 7c). After 6 weeks of HFD diet, TBT male descendants were hyperglycemic (Fig. 4g) and hyperinsulinemic (Fig. 4h), in addition to having increased fat content (Fig. 1a, b). Females showed no effects (Extended data Fig. 7 d, e). HOMA-IR index was strikingly increased in the male (Fig. 4i) but not female (Extended data Fig. 7f) TBT animals, suggesting a high likelihood of insulin resistance compared with vehicle controls.
To address whether insulin secretion or insulin breakdown was responsible for increased insulin levels in TBT-group males, we measured C-peptide (Extended data Fig. 8) and calculated C-peptide:insulin ratios (Extended data Fig. 9). Analysis of C-peptide levels revealed that there were no differences between DMSO and TBT group males or females before HFD challenge (Extended data Fig. 8a, c), or females after HFD challenge (Extended data Fig. 8d). C-peptide levels increased ~ 50% in DMSO and TBT-group males after HFD challenge, indicating that HFD led to increased insulin secretion (Extended data Fig. 8b). Measurement of the ratios of C-peptide:insulin indicated that increased insulin levels observed in TBT-group males before and after HFD were likely the result of impaired insulin clearance resulting from decreased IDE production (Extended data Fig. 9a, b). Females were unaffected (Extended data Fig. 9c, d). In accord with our previous publications8,12, plasma leptin levels were strongly increased in TBT-group males, but not females after HFD challenge (Extended data Fig. 10).
Discussion
Obesogens are chemicals that lead to increased WAT mass in exposed organisms2,19. TBT activated the nuclear receptors peroxisome proliferator activated receptor gamma (PPARγ) and its heterodimeric partner, retinoid ‘X’ receptor (RXR)20,21, leading to increased commitment of multipotent mesenchymal stromal stem cells to the adipose lineage and differentiation of pre-adipocytes into mature adipocytes22. TBT promoted fat accumulation in vivo in a variety of model systems, including mice2,19. Our previous experiments demonstrated increased WAT accumulation when dietary fat was elevated modestly (21.6% vs 13.1% calories from fat) in male F3 and F4 descendants of pregnant F0 mouse dams exposed to TBT throughout pregnancy11,12 or pregnancy and lactation8,10,13. TBT exposure of pregnant F0 dams resulted in a stable, male-specific predisposition to obesity in exposed (F1 were exposed in utero, F2 were exposed as germ cells in F1) and unexposed (F3, F4) descendants.
We previously identified blocks of iso-directional, differentially methylated DNA (isoDMBs) in WAT of F4-generation male mice after exposure of F0 dams to TBT throughout pregnancy and lactation8,10. Genomic DNA regions in WAT where isoDMBs were under-methylated compared to controls were enriched in metabolic genes such as leptin and these regions were less accessible in F3 and F4 sperm of the TBT group than in controls8. We proposed that ancestral TBT exposure caused changes in higher-order chromatin structure that were then inherited or reconstructed every generation, ultimately resulting in changes in chromatin accessibility and DNA methylation that altered expression of adipogenic and metabolic genes compared with controls8,10.
Transgenerational inheritance of altered chromatin structure offers an attractive unifying model that provides a common basis to integrate how disparate mechanisms such as DNA methylation, histone methylation, histone retention, and noncoding-RNA expression might be coordinately regulated across the generations. Higher-order chromatin structure is often reflected by the presence or absence of chromatin TADs and loops that modulate accessibility to DNA and histone-modifying enzymes, to histones and to the transcription machinery15. Support for such a model required the identification of persistently altered regions of higher-order chromatin structure. Here we used Hi-C analysis to identify CIs whose presence was stably altered in PGCs by direct or ancestral TBT exposure. Critically, the most high-scoring CI identified objectively was on chromosome 19 within the Ide gene. While we have only assessed the presence of CIs in PGCs from the current (T4) experiment, expression of Ide mRNA was also reduced in livers from a previous experiment (T3)12. Reduced Ide expression did not affect fasting glucose levels in the current T4 experiment, but significantly altered basal insulin levels, then led to strong increases in both glucose and insulin levels in HFD-challenged F2 and F3 males. This increase in insulin was not the result of increased insulin secretion because C-peptide levels did not change between vehicle and TBT groups. Since only the male animals in our transgenerational experiments responded to diet challenge and then only increased WAT mass after HFD diet was initiated, decreased hepatic Ide expression in males appears to be a strong component of the transgenerational susceptibility to obesity. Previous gene knockout studies confirmed a role for IDE in insulin clearance; Ide loss-of-function produced hyperinsulinemia and age-dependent glucose-intolerance18. It is also notable that leptin levels were increased in TBT-group males since it is known that hyperinsulinemia and insulin resistance impair leptin signaling, leading to leptin resistance23.
Together with our previous studies8,10, the new data presented here support a model in which transgenerational, non-genetic propagation of environmentally-induced phenotypes relied on alterations in chromatin structure. Altered chromatin structure necessarily change the accessibility of DNA and histones to modifying enzymes such as DNA and histone methyl transferases, the location and retention of histones and the expression of various genes, including those for non-coding RNAs. These mechanisms could interact and be preserved across generations and in various types of differentiated cells10.
Methods
Chemicals and Reagents -
TBT, dexamethasone, isobutylmethylxanthine, insulin were purchased from Sigma-Aldrich (St. Louis, MO). Rosiglitazone (ROSI) was purchased from Cayman Chemical (Ann Arbor, MI). Embryoid Body Dissociation Kit (#130–096-348) was purchased from Miltenyi Biotec (North Rhine-Westphalia, Germany). Zombie Red Dye (#77475) was purchased from BioLegend (San Diego, CA). PE Mouse anti-SSEA-1 (#560142) and Alexa-Fluor 647 Mouse anti-CD61 (#563523) were purchased from BD Biosciences (Franklin Lakes, NJ). Blood glucose meter kits (BG1000) were purchased from Clarity Diagnostics (Boca Raton, FL). Mouse Leptin ELISA Kit (#90030), Mouse C-peptide ELISA kit (#80954) and mouse insulin ELISA kit were purchased from Crystal Chem (Elk Grove Village, IL, USA). Arima-HiC Kit (A510008) was purchased from Arima Genomics (San Diego, CA). Ultra-pure formaldehyde (#18508) was purchased from Ted Pella Inc (Redding, CA). MinElute Reaction Cleanup Kit (#28206) was purchased from Qiagen (Hilden, Germany).
Animal maintenance and exposure-:
C57BL/6J mice were purchased from the Jackson Laboratory (Sacramento, CA) and housed in micro-isolator cages in a temperature-controlled room (21–22°C) with a 12 h light/dark cycle. Water and food were provided ad libitum unless otherwise indicated. Animals were treated humanely and with regard for alleviation of suffering. All procedures conducted in this study were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. At the moment of euthanasia, each mouse was assigned a code, known only to a lab member not involved in the dissection process. All tissue harvesting was performed with the dissector blinded to which groups the animals belonged. Group sizes were based on our prior experiments and a priori power analysis
For this new transgenerational experiment, denoted as T4; we purchased 50 male and 148 female C57BL/6J mice (5 weeks of age). Female mice (74 females per treatment group) were randomly assigned to the different F0 treatment groups and exposed via drinking water to 50 nM TBT or 0.1% DMSO vehicle (both diluted in 0.5% carboxymethyl cellulose in water to maximize solubility), for 7 days prior to mating as we have described8,12. One male was housed with two vehicle or 50 nM TBT exposed F0 females per cage to breed during the dark cycle (6PM to 6AM). Vaginal plug appearance was defined as embryonic day (E) 0.5. Treatment was removed during mating, then resumed for F0 females after copulation plugs detected (and males removed) then maintained until pups were born (Extended data Fig. 11). This TBT concentration was chosen based on our previous studies8,11,12,24 and is five-fold lower than the established no observed adverse effect level (NOAEL)25. While chemicals were administered to the dams throughout pregnancy, sires were never exposed to the treatment. No statistically significant differences were observed in the number of pups or the sex ratio per litter among the different groups (Extended data Fig. 12). It should be noted that F2 descendants were exposed to TBT as germ cells in the exposed F1 embryos. F3 descendants were not exposed to TBT.
From each generation, we randomly chose only 1 male and 1 female per litter for endpoint analysis and another 1 male and 1 or 2 females per litter for breeding to produce the next generation. There were insufficient animals in the F1 generation to both breed the F2 generation and analyze phenotypes in the diet challenge, so we only bred the F1 animals. To randomize the breeding process as much as possible, we did not breed siblings and never bred females from the same litter with the same male. Control animals were bred to each other and TBT-exposed animals were bred to each other.
Diet challenge and body composition analysis -
Animals from control and treatment groups were maintained on a standard diet (SD) (PicoLab 5053; 24.5% Kcal from protein, 13.1% Kcal from fat, and 62.3% Kcal from carbohydrates) from weaning onward. In diet challenge experiments, F2 (14 males and 14 females for each group) and F3 (15 males and 15 females for each group) were switched to higher fat diet (HFD) (PicoLab Rodent Chow, 5058) whereas control groups (F2: 15 males and 15 females; F3: 12 males and 12 females) were maintained on the SD (PicoLab Rodent Chow, 5053). Body weight and body composition were measured weekly for each animal using EchoMRI™ Whole Body Composition Analyzer, which provides lean, fat and water content information. Total water weight includes free water mainly from the bladder and water contained in lean tissue. F2 descendants started diet challenge at 4 weeks of age for 8 weeks when a significant fat content increase was confirmed and persisted. F3 descendants starting the diet challenge at week 5 had not become significantly fatter by week 17. Therefore, we switched the control F3 animals to the HFD at week 17 for 5 weeks. Fat content was significantly increased in this group by week 19. Mice were fasted for 12 hours prior to euthanasia and tissue collection.
Blood was collected via the saphenous vein at week 4 and week 12 (before and after diet challenge) for F2, and at weeks 4, 12, and 22 for F3. Blood was collected into heparinized tubes, then centrifuged for 15 minutes at 5,000 RPM at 4°C. Resulting plasma was transferred to a clean tube and preserved at −80°C. Animals were euthanized by isofluorane exposure followed by cardiac exsanguination after 4 hours fasting. Blood was drawn into a heparinized syringe and centrifuged for 15 minutes at 5,000 rpm at 4°C. Resulting plasma was transferred to a clean tube and preserved at −80°C. We measured plasma leptin levels to confirm the previously reported phenotypes8. Inguinal white adipose tissue (iWAT), gWAT, pancreas, spleen, liver, interscapular brown adipose tissue (iBAT), and soleus muscle were flash frozen in liquid N2 then stored at −80°C for subsequent analysis. Feces was freshly collected from animals prior to, and during the diet challenge at week 4 and week 12 for F2, week 4, 12, and 22 for F3, and stored at −80°C.
PGC isolation -
A randomly-selected subset of pregnant females was euthanized 13 days (E13.5) after vaginal plug detection. E13.5 embryos were isolated from euthanized pregnant dams, and E13.5 gonads containing primordial germ cells (PGCs) were isolated using Leica MZ9.5 Binocular Stereo Microscope. Gonads were identified and sexed by their characteristic morphology at E13.5 (Extended data Fig. 13a, b) and sex was verified by PCR26. Primer sequences are given in Extended data Table 1. Gonads from same-gender embryos in each litter were pooled prior to tissue dissociation. Gonads were enzymatically digested using Embryoid Body Dissociation Kit (Miltenyi Biotec). Next, total dissociated gonad cells were stained with Zombie Red Dye (BioLegend), PE Mouse anti-SSEA-1 (BD Pharmingen), and Alexa-Fluor 647 Mouse anti-CD61 (BD Pharmingen). Primordial germ cells were purified based on the expression of Zombie Red−/SSEA-1+/CD61+ using BD FACS Aria II Cell Sorter (BD Bioscience). Somatic gonad cells were purified based on the expression of Zombie Red−/SSEA-1−/CD61. The gating strategy and purity of the isolated cells is shown in Extended data Fig. 14.
Hi-C data generation -
Five litters of each group with ~ 20,000 PGCs (Zombi Red−/SSEA-1+/CD61+) were designated to proceed for Hi-C sample preparation using Arima Hi-C Kit following the manufacturer’s instructions for low-input Hi-C sequencing. This low-input protocol supported quantitative determination of topologically associating domains (TADs) from 10,000 human cells (Arima Genomics Application Note: “Unlock Low-Input 3D Genome Analysis with the Arima-HiC Kit”, Arima Genomics), which was confirmed by our current study (Extended data Fig. 2a-2d). Briefly, cells were fixed with formaldehyde (Ted Pella) to crosslink chromatin contacts. Genomic DNA was isolated from the fixed cells and digested using a restriction enzyme cocktail. The digested 5’-overhanging ends were filled with biotinylated nucleotides, and spatially proximal digested ends were ligated. Proximally ligated DNA fragments, which capture chromatin contacts were purified, fragmented by sonication, and enriched using streptavidin-conjugated beads. Illumina sequencing libraries were synthesized from the solid phase-captured DNA fragments using the Swift Biosciences® Accel-NGS® 2S Plus DNA Library Kit (Swift). Libraries were sequenced using an Illumina NovoSeq 6000 deep sequencer to obtain 150 + 150 nt paired-end FASTQ reads.
Identification of differential topologically associating domains (dTADs) and genes -
After adaptor sequences and low-quality reads (< 30) were removed using the Trim Galore! tool, FASTQ reads were subjected to Hi-C seq analysis using the Hi-C Pro tool27. The Hi-C Pro quality control plots showed that least 300 million valid interaction pairs were generated for each group of embryos (> 60 million valid pairs per individual embryo) with greater than 60% long (> 20 kb) cis interactions, indicating successful generation of sufficient amounts of high-quality Hi-C data (Extended data Fig. 2e-2h). Using these data we were able to reproduce a known TAD profile surrounding the HoxD gene cluster14, which was demonstrated using a contact map (Extended data Fig. 2i) and a directionality index plot (Extended data Fig. 2j). Locations of chromatin contact boundaries and the Differential Chromatin Interaction (DCI) scores between TBT versus DMSO vehicle groups were calculated using the BART-3D software tool28. Distributions of TADs and dTADs were visualized using the OmicCircos29 and the HiTC30 R/Bioconductor tools. For visualization, DCI scores in chromosome 19 were smoothened with 40 kbp bins and rolling averaged with 11 bins.
Chromatin immunoprecipitation-Quantitative Polymerase Chain Reaction -
Chromatin immunoprecipitation (ChIP) was performed using the method by Abcam and optimized with the established method we previously described31. Briefly, 50 mg of liver tissue that had been snap-frozen in liquid N2 were thawed on ice in cold PBS and dispersed into single cell suspensions using a 100 μm cell strainer (#22363549; Fisher Brand, PA). Cells were washed twice with PBS containing protease inhibitor cocktail (#ab201111; Abcam, Cambridge, UK) then resuspended and fixed at room temperature for 10 minutes with 1% paraformaldehyde (Fisher Chemical, PA) in DMEM, followed by an ice-cold phosphate-buffered saline wash, and then quenched for 5 minutes with 125 mM glycine at room temperature. Fixed cells were washed, collected by centrifugation, then resuspended in phosphate-buffered saline at 107 cells/mL. To isolate nuclei, cell pellets were lysed at 4°C for 10 minutes with a gentle detergent recipe consisting of 50 mM HEPES-KOH, pH 7.5, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% Nonidet P-40, 0.25% Triton X-100, Protease Inhibitor Cocktail (#ab201111; Abcam, Cambridge, UK). Nuclei were recovered by centrifugation at 8000 × g for 15 minutes, washed for 10 minutes at room temperature (10 mM Tris-HCl, pH 8.0, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, protease inhibitors (#ab201111; Abcam, Cambridge, UK), and lysed in 300 μL nuclear lysis buffer (10 mM Tris-HCl, pH 8.0, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.1% Na-deoxycholate, 0.5% N-lauroylsarcosine, protease inhibitors (#ab201111; Abcam, Cambridge, UK). Chromatin samples were prepared by sonicating in 0.5 mL thin-walled polymerase chain reaction tubes (BrandTech, CT) using a QSonica Q800R2 (QSonica, CT) with the following settings: 30 seconds on/30 seconds off, amplitude 40% repeated for 30 minutes. Triton X-100 (1%) was added to sonicated lysates prior to high-speed, cold centrifugation to remove debris. A total of 5 μg DNA was immunoprecipitated with preblocked protein A/G Dynabeads (Thermo Fisher Scientific, MA) complexed to 2.5 μg antibody (anti-CTCF, ab128873, anti-RAD21, ab217678, or Isotype IgG control, ab171870, Abcam, Cambridge, UK). Beads were washed three times with LiCl buffer (50 mM HEPES-KOH, pH 7.5, 500 mM LiCl, 1 mM EDTA, 1% Nonidet P-40, 0.7% Na-deoxycholate) and once with Tris-EDTA buffer plus 50mM NaCl. To release chromatin from beads, pelleted beads were resuspended in elution buffer (50mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% sodium dodecyl sulfate) and incubated at 65°C for 30 minutes. Cross-link reversal was performed overnight at 65°C. DNA samples were purified using Qiaquick PCR Cleanup kit (#28106, Qiagen, Germantown, MD) following RNase A (0.2 mg/mL, 2 hours, 37°C) and proteinase K (0.2 mg/mL, 2 hours, 55°C) treatment. Input DNA content was determined by spectrophotometry (Nanodrop, Thermo Fisher Scientific, MA). For analysis of candidate loci, real-time PCR was performed using SYBRTM Green PCR Master Mix (Thermo Fisher Scientific, MA) on a Roche LightCycler 480 II (Roche, Switzerland) according to the recommended protocol. Enrichment of the ChIP target were presented as fold difference between specific Ab-immunoprecipitated samples and the immunoprecipitated total input with an IgG control. Primer sequences of the examined loci are listed in Extended data Table 1. Multiple primer sets were tested for each site. For sites B and C, 2 of 2 primer sets showed significant enrichment. For binding site F, 1 of 3 primer sets showed enrichment and for site G, 2 of 3 showed enrichment.
Quantitative real time reverse transcriptase polymerase chain reaction -
Tissue that had been previously snap-frozen in liquid N2 was cut into ~ 20 mg pieces and lysed with Trizol following the manufacturer’s recommended protocol (Thermo Fisher Scientific, MA); total RNA was recovered after isopropanol precipitation (Fisher Chemical, PA). Complementary DNA was synthesized from 5 μg total RNA using SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific, MA) according to the manufacturer’s instructions. Gene expression was assessed with real-time quantitative polymerase chain reaction (qPCR) using SYBRTM Green PCR Master Mix (Thermo Fisher Scientific, MA) on a Roche LightCycler 480 II (Roche, Switzerland). Primer sequences of the examined genes were listed in Extended data Table 1. Cycle threshold values were quantified as the second derivative maximum using LightCycler software (Roche, Switzerland). The 2−ΔΔCt method32 was used to analyze RT-qPCR data and determine relative quantification corrected for primer efficiency. Ide expression was normalized to the housekeeping gene, GAPDH, and compared to DMSO descendants group. Error bars represent the SEM from 15 to 17 biological replicates, calculated using standard propagation of error.
Measurement of insulin and C-peptide -
C-peptide serum levels were measured by EIA (Crystal Chem #80954; Elk Grove Village, IL, USA) at two different time points (before and after diet challenge) in plasma from blood samples drawn after overnight (12 hours) fasting. Insulin levels were measured by EIA (Crystal Chem #90080; Elk Grove Village, IL, USA) in plasma from blood samples drawn after overnight (12 hours) fasting.
Acknowledgements -
Supported by grants from the National Institutes of Health, USA (R01ES023316 and R01ES031139) and the John Templeton Foundation to B.B. and T.S. We thank Drs. Sha Sun (University of California, Irvine, CA USA), Xiao-Min Ren (Kunming University of Science and Technology, China), Angel Nadal (Universidad Miguel Hernández de Elche. Spain), Ivan Quesada (Universidad Miguel Hernández de Elche. Spain), Hanjun Lee (Whitehead Institute, Cambridge, MA, USA), and Rob Lustig (University of California, San Francisco, CA USA) for critically reading the manuscript and Dr. Hanjun Lee for suggesting BART-3D. We wish to thank Drs. Raquel Chamorro-Garcia (University of California, Santa Cruz) and Grant MacGregor (University of California, Irvine) for advice and assistance during the early stages of this project.
Footnotes
Competing interests - B.B. is a named inventor on U.S. patents related to PPARγ. The remaining authors declare no competing financial interests.
Contributor Information
Bruce Blumberg, University of California.
Richard Cheng-An Chang, University of California.
Riann Egusquiza, DTxPharma.
Angélica Amato, University of Brasília.
Zhuorui Li, University of California, Irvine.
Erika Joloya, University of California, Irvine.
Hailey Wheeler, University of California, Irvine.
Angela Nguyen, University of California, Irvine.
Keiko Shioda, Massachusetts General Hospital.
Junko Odajima, Massachusetts General Hospital.
Michael Lawrence, University Massachusetts General Hospital.
Toshi Shioda, Massachusetts General Hospital.
Data availability statement -
Hi-C datasets will be available at Gene Expression Omnibus (GSE218701).
References
- 1.Skinner M. K. Environmental Epigenetics and a Unified Theory of the Molecular Aspects of Evolution: A Neo-Lamarckian Concept that Facilitates Neo-Darwinian Evolution. Genome Biol Evol 7, 1296–1302, doi: 10.1093/gbe/evv073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohajer N., Joloya E. M., Seo J., Shioda T. & Blumberg B. Epigenetic Transgenerational Inheritance of the Effects of Obesogen Exposure. Front Endocrinol (Lausanne) 12, 787580, doi: 10.3389/fendo.2021.787580 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camacho J. et al. The Memory of Environmental Chemical Exposure in C. elegans Is Dependent on the Jumonji Demethylases jmjd-2 and jmjd-3/utx-1. Cell Rep 23, 2392–2404, doi: 10.1016/j.celrep.2018.04.078 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben Maamar M., Sadler-Riggleman I., Beck D. & Skinner M. K. Epigenetic Transgenerational Inheritance of Altered Sperm Histone Retention Sites. Sci Rep 8, 5308, doi: 10.1038/s41598-018-23612-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner M. K. et al. Alterations in sperm DNA methylation, non-coding RNA and histone retention associate with DDT-induced epigenetic transgenerational inheritance of disease. Epigenetics Chromatin 11, 8, doi: 10.1186/s13072-018-0178-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carone B. R. et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096, doi: 10.1016/j.cell.2010.12.008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seisenberger S. et al. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48, 849–862, doi: 10.1016/j.molcel.2012.11.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamorro-Garcia R. et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun 8, 2012, doi: 10.1038/s41467-017-01944-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma U. et al. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev Cell 46, 481–494 e486, doi: 10.1016/j.devcel.2018.06.023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz-Castillo C., Chamorro-Garcia R., Shioda T. & Blumberg B. Transgenerational Self-Reconstruction of Disrupted Chromatin Organization After Exposure To An Environmental Stressor in Mice. Scientific reports 9, 13057, doi: 10.1038/s41598-019-49440-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamorro-Garcia R. et al. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect 121, 359–366, doi: 10.1289/ehp.1205701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamorro-Garcia R. et al. Transgenerational metabolomic fingerprints in mice ancestrally exposed to the obesogen TBT. Environ Int 157, 106822, doi: 10.1016/j.envint.2021.106822 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shioda K., Odajima J., Blumberg B. & Shioda T. Transgenerational Transcriptomic and DNA Methylome Profiling of Mouse Fetal Testicular Germline and Somatic Cells after Exposure of Pregnant Mothers to Tributyltin, a Potent Obesogen. Metabolites 12, doi: 10.3390/metabo12020095 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Carballo E. et al. The HoxD cluster is a dynamic and resilient TAD boundary controlling the segregation of antagonistic regulatory landscapes. Genes Dev 31, 2264–2281, doi: 10.1101/gad.307769.117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu M. & Ren B. The Three-Dimensional Organization of Mammalian Genomes. Annu Rev Cell Dev Biol 33, 265–289, doi: 10.1146/annurev-cellbio-100616-060531 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amemiya H. M., Kundaje A. & Boyle A. P. The ENCODE Blacklist: Identification of Problematic Regions of the Genome. Sci Rep 9, 9354, doi: 10.1038/s41598-019-45839-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pu L., Lin Y. & Pevzner P. A. Detection and analysis of ancient segmental duplications in mammalian genomes. Genome Res 28, 901–909, doi: 10.1101/gr.228718.117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Casimiro C. M. et al. Modulation of Insulin Sensitivity by Insulin-Degrading Enzyme. Biomedicines 9, doi: 10.3390/biomedicines9010086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heindel J. J. et al. Obesity II: Establishing causal links between chemical exposures and obesity. Biochem Pharmacol 199, 115015, doi: 10.1016/j.bcp.2022.115015 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanayama T., Kobayashi N., Mamiya S., Nakanishi T. & Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol 67, 766–774, doi: 10.1124/mol.104.008409 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Grun F. et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Molecular endocrinology (Baltimore, Md: 20, 2141–2155, doi: 10.1210/me.2005-0367 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Black B. E. & Cleveland D. W. Epigenetic centromere propagation and the nature of CENP-a nucleosomes. Cell 144, 471–479, doi: 10.1016/j.cell.2011.02.002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazarians-Armavil A., Menchella J. A. & Belsham D. D. Cellular insulin resistance disrupts leptin-mediated control of neuronal signaling and transcription. Mol Endocrinol 27, 990–1003, doi: 10.1210/me.2012-1338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamorro-García R. et al. Effects of Perinatal Exposure to Dibutyltin Chloride on Fat and Glucose Metabolism in Mice, and Molecular Mechanisms,. Environ Health Perspect 126, 057006, doi: 10.1289/EHP3030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos J. G., De Klerk A., Krajnc E. I., Van Loveren H. & Rozing J. Immunotoxicity of bis(tri-n-butyltin)oxide in the rat: effects on thymus-dependent immunity and on nonspecific resistance following long-term exposure in young versus aged rats. Toxicol Appl Pharmacol 105, 144–155 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Clapcote S. J. & Roder J. C. Simplex PCR assay for sex determination in mice. Biotechniques 38, 702, 704, 706, doi: 10.2144/05385BM05 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Servant N. et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol 16, 259, doi: 10.1186/s13059-015-0831-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., Zhang Y. & Zang C. BART3D: Inferring transcriptional regulators associated with differential chromatin interactions from Hi-C data. Bioinformatics, doi: 10.1093/bioinformatics/btab173 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y. et al. OmicCircos: A Simple-to-Use R Package for the Circular Visualization of Multidimensional Omics Data. Cancer Inform 13, 13–20, doi: 10.4137/CIN.S13495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Servant N. et al. HiTC: exploration of high-throughput ‘C’ experiments. Bioinformatics 28, 2843–2844, doi: 10.1093/bioinformatics/bts521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoucri B. M. et al. Retinoid X Receptor Activation Alters the Chromatin Landscape To Commit Mesenchymal Stem Cells to the Adipose Lineage. Endocrinology 158, 3109–3125, doi: 10.1210/en.2017-00348 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Hi-C datasets will be available at Gene Expression Omnibus (GSE218701).