Abstract
Background:
Lithium (Li) remains the treatment of choice for bipolar disorders (BP). Its mood-stabilizing effects help reduce the long-term burden of mania, depression and suicide risk in patients with BP. It also has been shown to have beneficial effects on disease-associated conditions, including sleep and cardiovascular disorders. However, the individual responses to Li treatment vary within and between diagnostic subtypes of BP (e.g. BP-I and BP-II) according to the clinical presentation. Moreover, long-term Li treatment has been linked to adverse side-effects that are a cause of concern and non-adherence, including the risk of developing chronic medical conditions such as thyroid and renal disease. In recent years, studies by the Consortium on Lithium Genetics (ConLiGen) have uncovered a number of genetic factors that contribute to the variability in Li treatment response in patients with BP. Here, we leveraged the ConLiGen cohort (N=2,064) to investigate the genetic basis of Li effects in BP. For this, we studied how Li response and linked genes associate with the psychiatric symptoms and polygenic load for medical comorbidities, placing particular emphasis on identifying differences between BP-I and BP-II.
Results:
We found that clinical response to Li treatment, measured with the Alda scale, was associated with a diminished burden of mania, depression, substance and alcohol abuse, psychosis and suicidal ideation in patients with BP-I and, in patients with BP-II, of depression only. Our genetic analyses showed that a stronger clinical response to Li was modestly related to lower polygenic load for diabetes and hypertension in BP-I but not BP-II. Moreover, our results suggested that a number of genes that have been previously linked to Li response variability in BP differentially relate to the psychiatric symptomatology, particularly to the numbers of manic and depressive episodes, and to the polygenic load for comorbid conditions, including diabetes, hypertension and hypothyroidism.
Conclusions:
Taken together, our findings suggest that the effects of Li on symptomatology and comorbidity in BP are partially modulated by common genetic factors, with differential effects between BP-I and BP-II.
Keywords: Bipolar disorder, lithium treatment, psychiatric symptoms, comorbidity, genetics
BACKGROUND
Lithium (Li) is the first-line maintenance treatment for bipolar disorders (BP). Multiple beneficial properties have been attributed to Li, including mood stabilization, cardio- and neuroprotection, circadian regulation, immunomodulation, and suicide prevention in patients with BP [Geoffroy PA et al., 2016; Volkmann C et al., 2020; Xu N et al., 2021; Queissner R et al., 2021; Miller BJ & McCall WV, 2022; Rybakowski JK, 2022; Chen PH et al., 2023; Szałach ŁP et al., 2023]. Li is not exempt from acute side-effects, the most frequent being gastrointestinal complaints, that may cause non-adherence. However, it is the long-term adverse effects, including thyroid and kidney problems [Volkmann C et al., 2020; Ferensztajn-Rochowiak E et al., 2021], that cause most concern.
Individual responses to Li vary according to the clinical presentation of the disease. Reportedly, only about 30% of patients with BP have a full response to Li treatment. Various clinical, psychosocial and demographic factors that affect Li response have been described [Nunes A et al., 2020; Ferensztajn-Rochowiak E et al., 2021]. Moreover, genetic studies have established Li response as a polygenic trait [Papiol S et al., 2022]. Previous work performed by the Consortium on Lithium Genetics (ConLiGen) has offered significant insights into the molecular mechanisms contributing to Li response [Amare AT et al., 2023], as well as the links with the polygenic scores of other psychiatric disorders [Amare AT et al., 2018; Schubert KO et al., 2021; Coombes BJ et al., 2021] and with suicidal behavior [Yoshida T et al., 2019] in BP. However, the relationships between Li response and disease features, particularly comorbidity, remain largely unexplored. Moreover, most studies have made no distinction between different diagnostic groups. Here, we used data from ConLiGen participants (N = 2,064) to explore how the genetic factors that contribute to Li response variability in patients with BP are associated with specific psychiatric symptoms and the polygenic load (i.e. genetic risk) for medical comorbid conditions, and whether these relationships differ between BP types I and II.
METHODS
Study population
The ConLiGen cohort has been described elsewhere [Hou L et al., 2016]. Briefly, between 2003 and 2013, ConLiGen recruited over 2,500 Li-treated individuals with bipolar spectrum disorders at various sites in Europe, the United States, Australia and East-Asia. The inclusion criteria consisted of a diagnosis of bipolar disorder type I (BP-I) or type II (BP-II), schizoaffective bipolar disorder or bipolar disorder not otherwise specified in accordance with the criteria established in the Diagnostic and Statistical Manual of Mental Disorders (DSM) versions III or IV, as well as Li treatment that lasted a minimum of six months with no additional mood stabilizers. Long-term responses to Li treatment were assessed using the Alda scale, where an A subscale rates the degree of response in the range 0–10 and a B subscale reflects the relationship between improvement and treatment. A total score, ranging from 0–10, is obtained by subtracting the B score from the A score [Manchia M et al., 2013]. Negative scores are set to 0. Here, we used a sample of 2,064 ConLiGen participants with complete covariate phenotypes: sex, age-at-onset (AAO), age at recruitment (i.e. sample collection), diagnosis and recruitment site (used to establish population).
The Ethics Committee at the University of Heidelberg provided central approval for ConLiGen. Written informed consent from all participants was obtained according to the study protocols of each of the participating sites and their institutions. All procedures were performed in accordance with the guidelines of the Declaration of Helsinki.
Genotype data
Genotyping, quality control (QC) and imputation of the ConLiGen cohort has been described elsewhere [Hou L et al., 2016]. Briefly, DNA genotyping by array was performed from peripheral blood samples in two batches of similar composition, originally referred to as “GWAS1” (N = 1,162) and “GWAS2” (N = 1,401). Standard procedures for QC and imputation using the 1000 Genomes Project reference panel were employed. Here, we used an updated ConLiGen dataset we previously described in detail [Herrera-Rivero M et al., 2023], in which we re-imputed the combined ConLiGen batches using the Haplotype Reference Consortium (HRC) panel. This procedure increased the number of markers and the quality of the dataset, increasing its suitability for polygenic score (PGS) analyses. Single nucleotide polymorphisms (SNPs) in 37 genes that were previously reported to contribute to Li response in ConLiGen following a gene-level genome-wide analysis [Amare et al., 2023] were extracted from the dataset using a window of ± 1 kb from the start and end positions of the gene (according to the Ensembl hg19 genome build). Our final dataset contained 9,374 SNPs corresponding to 34 Li response-linked genes and 2,064 individuals with BP, from which 1,669 had a diagnosis of BP-I and 370 of BP-II.
Phenotypes
Li response: We used the total Alda score as a measure of Li response. This was available for all 2,064 individuals included in our study.
Psychiatric symptoms: Here, the psychiatric symptoms corresponded to the numbers of episodes of depression and mania, the presence of psychosis, alcohol and substance abuse, and of suicidal ideation. These variables were available for a maximum of 853 individuals from the GWAS1 batch.
Genetic risk for medical comorbidities: Based on the literature, we identified various conditions that are comorbid in BP and searched the PGS Catalog [Lambert SA et al., 2021] for publicly available PGSs for these. Weight files for the calculation of PGSs for various traits, such as disorders of sleep and metabolism, were downloaded from the PGS Catalog and used for allelic scoring in the total ConLiGen sample with plink 1.9 [Chang CC et al., 2015]. Standardized sum scores were used for analysis. Because of incomplete compatibility between PGS SNPs and variants in the ConLiGen dataset, only PGSs with compatibility > 78% were used. These corresponded to the following traits: chronotype (PGS ID: PGS002209), sleep duration (PGS ID: PGS002196), insomnia (PGS ID: PGS002149), hypertension (PGS ID: PGS002047), hypothyroidism (PGS ID: PGS001816) and type 2 diabetes (PGS ID: PGS003118) [Privé F et al., 2022; Ma Y et al., 2022] (Suppl.Table.1). Traits excluded due to lower compatibility included cardiovascular disorders, obesity, migraine and asthma.
Statistical analyses
Associations between total Alda scores and psychiatric symptoms were tested using robust linear/logistic regression models with the “robustbase” R package (nmax=853). Models were adjusted for sex, AAO and age. Associations between total Alda scores and PGSs for comorbid conditions were tested using partial Spearman correlation with the “ppcor” R package (nmax=2,064). Models were adjusted for sex, AAO, age and population. SNP-phenotype associations were tested using linear/logistic regression models with plink 1.9. Models were adjusted for sex, AAO, age, population, total Alda score and the first eight dimensions coming from a principal components analysis of the genotypes. When testing associations using all individuals, all models were also adjusted for the differential BP diagnosis. All associations were also tested separately for BP-I and BP-II. For exploratory purposes, significance was set to nominal (i.e. unadjusted) p < 0.05 and p < 0.01 for total Alda score and SNP-phenotype associations, respectively.
RESULTS
To explore how Li response genes are associated with specific psychiatric symptoms and the poygenic load for medical comorbid conditions, and whether these relationships differ between BP types I and II, we used a sample of 2,064 individuals with BP from the ConLiGen cohort. From these, 1,197 (58%) were females, 1,669 (80.1%) had a diagnosis of BP-I and 370 (17.9%) were diagnosed with BP-II. The mean AAO in the sample was 25 ± 11 years, while the mean age at recruitment was 47 ± 14 years. The mean total Alda score was 4.22 ± 3.16 points, with 29.8% of the patients being categorized as good responders (total Alda score ≥ 7). Compared to BP-I, BP-II patients were slightly older at disease onset (28 ± 12 vs 24 ± 10 years) and recruitment (50 ± 14 vs 47 ± 14 years), and had higher rates of females (61.9% vs 57.2%) and good Li responders (34.1% vs 28.2%). However, the mean total Alda scores were very similar (4.6 ± 3.2 vs 4.2 ± 3.1 points).
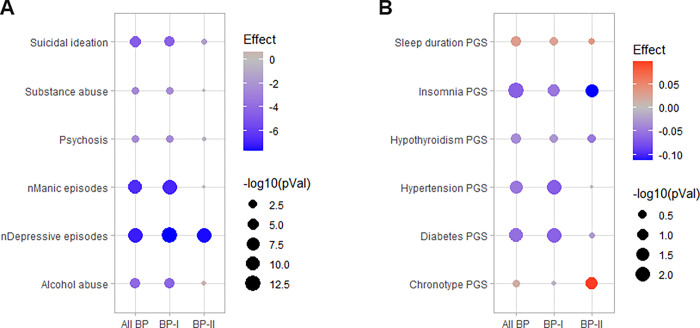
First, we explored the association between Li response and psychiatric symptoms/PGSs for comorbid conditions. Using a nominal significance threshold (p < 0.05), we found that the total Alda scores showed a negative relationship with all psychiatric symptom variables in all BP (nmax=835) and BP-I (nmax=665) individuals. However, in BP-II individuals (nmax=153), the total Alda scores showed a negative relationship only with the number of depressive episodes (Fig. 1A). These observations suggest that better responses to Li treatment diminish the burden of most psychiatric symptoms in patients with BP-I, but only that of depression in patients with BP-II. Noticeably, these results survived false discovery rate correction (FDR < 0.05). Furthermore, the total Alda scores also correlated negatively with the PGSs for diabetes and hypertension in all BP (N = 2,064) and BP-I (N = 1,669) individuals, and with the PGS for insomnia in all BP, BP-I and BP-II (N = 370) individuals (Fig. 1B). This suggested that better Li response correlates with lower genetic burden predisposing to insomnia in patients with BP in general, and to diabetes and hypertension in patients with BP-I diagnosis in particular. However, none of the nominal associations with PGSs survived FDR correction in our sample.
Figure 1.
Links between phenotypes and Li responses in ConLiGen. A) Association test results between total Alda scores and psychiatric symptoms. Shown are the nominal p-values (−log10) and z-values (effect) obtained from robust linear/logistic regression models. B) Correlation test results between total Alda scores and PGSs for comorbid conditions. Shown are the nominal p-values (−log10) and correlation coefficients (effect) obtained from partial correlation models using the Spearman method.
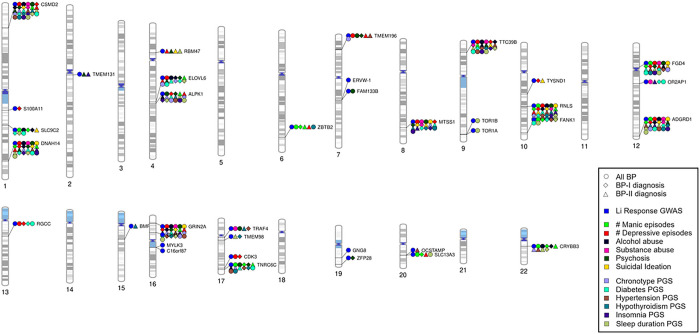
Second, we explored the association between genes previously linked to Li response and psychiatric symptoms/PGSs for comorbid conditions. Using a nominal significance threshold (p < 0.01) as indicative of suggestive association, we found that 32 of the 34 genes tested were suggested to associate with specific psychiatric symptoms and/or PGSs for comorbid conditions (Fig. 2, Suppl.Tables.2–7). The most significant hits were for the number of manic episodes, with SLC13A3 as top gene in BP-I and TNRC6C in BP-II, followed by the number of depressive episodes, with MTSS1 as top gene in BP-I and DNAH14 in BP-II (Table 1). These results suggest some candidate genes that might be involved in Li effects with respect to episodes of mania and depression in BP.
Figure 2.
Visual integration of nominal findings for Li response genes. Shapes depict the diagnostic group analyzed while colors refer to the phenotypes nominally associated with the gene in our analyses, except for the blue color, which localized even the genes not analyzed in this study that were reported by Amare et al., 2023 as contributors to Li response in ConLiGen.
Table 1.
Phenotype-based summary of findings for the association analyses between Li response genes and psychiatric symptoms/PGSs for comorbid conditions in ConLiGen.
| Phenotype | N | # Cases | # Controls | # SNPs p <0.01 | # Genes | Top gene | Top# SNPs p< 0.01 | Lowest p |
|---|---|---|---|---|---|---|---|---|
| All BP | ||||||||
| # Manic episodes | 724 | - | - | 38 | 9 | SLC13A3 | 11 | 2.48E-08 |
| # Depressive episodes | 789 | - | - | 225 | 12 | FGD4 | 75 | 5.15E-06 |
| Alcohol abuse | 835 | 140 | 695 | 114 | 9 | ELOVL6 | 5 | 1.11E-04 |
| Substance abuse | 832 | 135 | 697 | 143 | 9 | ADGRD1 | 45 | 4.17E-04 |
| Psychosis | 692 | 342 | 350 | 55 | 11 | GRIN2A | 12 | 7.83E-04 |
| Suicidal ideation | 660 | 321 | 339 | 10 | 6 | DNAH14 | 1 | 2.31E-03 |
| Insomnia PGS | 2064 | - | - | 57 | 8 | CSMD2 | 6 | 1.73E-04 |
| Sleep duration PGS | 2064 | - | - | 211 | 12 | DNAH14 | 133 | 1.12E-04 |
| Chronotype PGS | 2064 | - | - | 81 | 7 | GRIN2A | 47 | 4.06E-04 |
| Diabetes PGS | 2064 | - | - | 111 | 12 | CSMD2 | 33 | 6.28E-04 |
| Hypertension PGS | 2064 | - | - | 34 | 7 | TTC39B | 5 | 9.57E-05 |
| Hypothyroidism PGS | 2064 | - | - | 82 | 7 | MTSS1 | 42 | 4.73E-04 |
| BP-I diagnosis | ||||||||
| # Manic episodes | 641 | - | - | 48 | 10 | SLC13A3 | 11 | 2.15E-08 |
| # Depressive episodes | 632 | - | - | 193 | 13 | MTSS1 | 12 | 1.52E-06 |
| Alcohol abuse | 665 | 129 | 536 | 131 | 9 | CSMD2 | 52 | 1.34E-04 |
| Substance abuse | 662 | 121 | 541 | 121 | 5 | ADGRD1 | 52 | 4.13E-04 |
| Psychosis | 564 | 318 | 246 | 87 | 10 | CSMD2 | 21 | 7.17E-04 |
| Suicidal ideation | 530 | 264 | 266 | 41 | 6 | MTSS1 | 1 | 2.15E-04 |
| Insomnia PGS | 1669 | - | - | 48 | 6 | ALPK1 | 4 | 3.92E-04 |
| Sleep duration PGS | 1669 | - | - | 174 | 11 | RNLS | 3 | 4.37E-05 |
| Chronotype PGS | 1669 | - | - | 35 | 5 | RNLS | 2 | 1.76E-04 |
| Diabetes PGS | 1669 | - | - | 74 | 13 | TTC39B | 1 | 6.78E-04 |
| Hypertension PGS | 1669 | - | - | 29 | 7 | TTC39B | 1 | 6.81E-04 |
| Hypothyroidism PGS | 1669 | - | - | 38 | 8 | CSMD2 | 4 | 6.95E-04 |
| BP-II diagnosis | ||||||||
| # Manic episodes | 68 | - | - | 113 | 10 | TNRC6C | 3 | 3.76E-79 |
| # Depressive episodes | 141 | - | - | 128 | 11 | DNAH14 | 6 | 3.12E-08 |
| Alcohol abuse | 153 | 7 | 146 | 7 | 5 | TNRC6C | 2 | 1.80E-03 |
| Substance abuse | 153 | 8 | 145 | 0 | 0 | - | - | - |
| Psychosis | 115 | 12 | 103 | 353 | 7 | TMEM131 | 46 | 1.08E-03 |
| Suicidal ideation | 118 | 48 | 70 | 79 | 7 | TTC39B | 24 | 2.49E-03 |
| Insomnia PGS | 370 | - | - | 209 | 7 | GRIN2A | 38 | 2.65E-04 |
| Sleep duration PGS | 370 | - | - | 64 | 9 | DNAH14 | 16 | 2.95E-04 |
| Chronotype PGS | 370 | - | - | 32 | 7 | GRIN2A | 19 | 1.81E-03 |
| Diabetes PGS | 370 | - | - | 97 | 9 | MTSS1 | 6 | 2.01E-04 |
| Hypertension PGS | 370 | - | - | 130 | 10 | TMEM196 | 27 | 3.21E-04 |
| Hypothyroidism PGS | 370 | - | - | 70 | 7 | BMF | 12 | 1.92E-04 |
Taken together, 22 of the 34 genes tested were nominally associated with at least one psychiatric symptom and one PGS in at least one of the tests performed (i.e. all BP, BP-I and BP-II). Noticeably, some of the Li response genes were suggested to associate with all the phenotypes that we studied in at least one of the tests. We also observed that genes with the most overlaps, including RNLS, GRIN2A, CSMD2, DNAH14 and TTC39B (Table 2), represented the most significant hits obtained in BP-I or BP-II for various PGSs for comorbid conditions (Table 1). This suggests that Li effects on medical comorbid conditions might also involve shared genetic factors, although with small independent effects.
Table 2.
Gene-based summary of findings for the association analyses between Li response genes and psychiatric symptoms/PGSs for comorbid conditions in ConLiGen.
| Gene | Chr | Gene start (−1kb) | Gene end (+ 1kb) | # tested SNPs | Psychiatric phenotype count | PGS phenotype count | Max. # phenotypes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | BP-I | BP-II | All | BP-I | BP-II | ||||||
| CSMD2 | 1 | 33978609 | 34632443 | 1064 | 4 | 5 | 3 | 5 | 5 | 5 | 12 |
| S100A11 | 1 | 152003982 | 152021383 | 14 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| SLC9C2 | 1 | 173468603 | 173573233 | 179 | 2 | 2 | 1 | 1 | 2 | 0 | 5 |
| DNAH14 | 1 | 225082964 | 225587996 | 1417 | 5 | 5 | 3 | 3 | 3 | 5 | 11 |
| TMEM131 | 2 | 98371799 | 98613388 | 358 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| RBM47 | 4 | 40424272 | 40633892 | 164 | 0 | 0 | 3 | 0 | 0 | 1 | 4 |
| ELOVL6 | 4 | 110966002 | 111121355 | 261 | 2 | 2 | 3 | 2 | 3 | 1 | 7 |
| ALPK1 | 4 | 113205665 | 113364776 | 301 | 1 | 2 | 3 | 4 | 3 | 4 | 10 |
| ZBTB2 | 6 | 151684252 | 151713683 | 43 | 1 | 1 | 2 | 1 | 0 | 0 | 3 |
| TMEM196 | 7 | 19757933 | 19814221 | 108 | 2 | 1 | 1 | 1 | 0 | 1 | 4 |
| ERVW-1 | 7 | 92096694 | 92108300 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FAM133B | 7 | 92189107 | 92220708 | 50 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| MTSS1 | 8 | 125562031 | 125741730 | 499 | 4 | 4 | 1 | 2 | 3 | 4 | 8 |
| TTC39B | 9 | 15162620 | 15308358 | 408 | 3 | 3 | 4 | 4 | 5 | 2 | 11 |
| TOR1B | 9 | 132564432 | 132574560 | 20 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| TOR1A | 9 | 132574223 | 132587413 | 32 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| TYSND1 | 10 | 71896737 | 71907432 | 40 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| RNLS | 10 | 90032621 | 90345287 | 628 | 5 | 5 | 3 | 6 | 6 | 4 | 12 |
| FANK1 | 10 | 127584108 | 127699161 | 250 | 1 | 1 | 0 | 2 | 3 | 0 | 4 |
| FGD4 | 12 | 32551463 | 32799984 | 882 | 5 | 3 | 3 | 5 | 2 | 3 | 12 |
| OR2AP1 | 12 | 55967199 | 55970128 | 7 | 1 | 0 | 0 | 1 | 1 | 1 | 3 |
| ADGRD1 | 12 | 131437452 | 131627014 | 603 | 5 | 5 | 1 | 6 | 3 | 4 | 12 |
| RGCC | 13 | 42030695 | 42046018 | 35 | 1 | 1 | 0 | 1 | 1 | 0 | 2 |
| BMF | 15 | 40379091 | 40402093 | 16 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| GRIN2A | 16 | 9851376 | 10277611 | 1624 | 5 | 4 | 3 | 3 | 5 | 4 | 12 |
| CHP2 | 16 | 23764948 | 23771272 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MYLK3 | 16 | 46739891 | 46825319 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C16orf87 | 16 | 46829519 | 46866323 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TRAF4 | 17 | 27070002 | 27078974 | 8 | 2 | 0 | 0 | 0 | 1 | 1 | 4 |
| TMEM98 | 17 | 31253928 | 31273124 | 33 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| CDK3 | 17 | 73995987 | 74003080 | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| TNRC6C | 17 | 75999249 | 76105916 | 153 | 2 | 2 | 2 | 3 | 2 | 2 | 7 |
| GNG8 | 19 | 47136333 | 47138942 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZFP28 | 19 | 57049317 | 57069169 | 46 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| OCSTAMP | 20 | 45168585 | 45180213 | 10 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| SLC13A3 | 20 | 45185463 | 45305714 | 58 | 1 | 1 | 1 | 1 | 0 | 0 | 3 |
| CRYBB3 | 22 | 25594817 | 25604330 | 31 | 2 | 2 | 1 | 0 | 1 | 3 | 5 |
Overall, our results suggested that genes linked to Li response also contribute to modulate the clinical presentation of BP, and that these contributions vary between BP-I and BP-II diagnoses in many instances. We corroborated the latter observation by looking into the overlapping and non-overlapping genes between the BP-I and BP-II analyses (Table 3). Here, we observed that, for example, GRIN2A was suggested to relate to the number of depressive episodes, the presence of alcohol abuse, and the polygenic contribution to chronotype, diabetes and hypertension in both major types of BP. However, it was suggested to be linked to the presence of psychosis and suicidal ideation, and the polygenic contribution to sleep duration and hypothyroidism in BP-I only, while relating to the number of manic episodes and the genetic load for insomnia only in BP-II.
Table 3.
Li response genes nominally associated with psychiatric symptoms/PGSs for comorbid conditions in ConLiGen. Shown are the overlapping and non-overlapping genes between BP-I and BP-II diagnostic groups.
| Phenotype | BP-I only | BP-II only | Overlap |
|---|---|---|---|
| # Manic episodes | ADGRD1, FANK1, FGD4, SLC13A3, SLC9C2 | ALPK1, CSMD2, ELOVL6, GRIN2A, TTC39B | CRYBB3, DNAH14, RNLS, TNRC6C, ZBTB2 |
| # Depressive episodes | ADGRD1, CDK3, MTSS1, RGCC, S100A11, TTC39B, TYSND1 | ELOVL6, RBM47, SLC13A3, TMEM196, ZBTB2 | ALPK1, CSMD2, DNAH14, FGD4, GRIN2A, RNLS |
| Alcohol abuse | ADGRD1, CRYBB3, CSMD2, DNAH14, ELOVL6, RNLS, SLC9C2 | ALPK1, FGD4, TNRC6C | GRIN2A, TTC39B |
| Substance abuse | ADGRD1, CSMD2, MTSS1, RNLS, TTC39B | - | - |
| Psychosis | ALPK1, FGD4, GRIN2A, MTSS1, TMEM196, TNRC6C, ZFP28 | ADGRD1, RBM47, TMEM131, TTC39B | CSMD2, DNAH14, ELOVL6 |
| Suicidal ideation | ADGRD1, CSMD2, DNAH14, GRIN2A, MTSS1 | FGD4, MTSS1, RBM47, SLC9C2, TTC39B, TYSND1 | RNLS |
| Insomnia PGS | ALPK1, CSMD2, TTC39B | ADGRD1, GRIN2A, OR2AP1, TMEM131 | DNAH14, MTSS1, RNLS |
| Sleep duration PGS | ELOVL6, FANK1, GRIN2A, RNLS, SLC9C2 | FGD4, RBM47, TMEM98 | ADGRD1, ALPK1, CRYBB3, CSMD2, DNAH14, TTC39B |
| Chronotype PGS | ELOVL6, RNLS | CRYBB3, DNAH14, MTSS1, TNRC6C | ALPK1, CSMD2, GRIN2A |
| Diabetes PGS | ADGRD1, FANK1, OR2AP1, SLC9C2, TTC39B | ALPK1, TNRC6C | CSMD2, DNAH14, ELOVL6, FGD4, GRIN2A, MTSS1, RNLS |
| Hypertension PGS |
FANK1, TNRC6C, TRAF4 | ADGRD1, CRYBB3, CSMD2, DNAH14, OCSTAMP, TMEM196 | FGD4, GRIN2A, RNLS, TTC39B |
| Hypothyroidism PGS | GRIN2A, TMEM98, TNRC6C, TTC39B | ALPK1, BMF, TRAF4 | ADGRD1, CSMD2, MTSS1, RNLS |
DISCUSSION
We showed that positive responses to Li treatment in patients with BP are generally more beneficial to those patients diagnosed with BP-I than to those with a BP-II diagnosis, and that genes linked to Li response also contribute to the clinical presentation of the disorder in terms of psychiatric symptomatology and, potentially, the risk of medical comorbid conditions. This may partly explain why Li responses usually vary according to clinical features, and why clinical and psychosocial factors can only partially predict Li responses [Tondo L et al., 2001; Ferensztajn-Rochowiak E et al., 2021].
Often, the efficacy of Li treatment in BP is assessed without making distinction between BP types and/or is focused on manic-depressive episodes, with disregard of other disease-associated afflictions. However, it is plausible that the beneficial effects of Li treatment on psychiatric symptomatology are related to its effects on other health issues associated with BP, such as improving inflammation and sleep [Geoffroy PA et al., 2016; Szałach ŁP et al., 2023]. Moreover, some studies have shown that Li impacts differently the frequency and duration of mood episodes in BP-I and BP-II [Tondo L et al., 2001], which might relate to stronger effects on acute manic than depressive episodes [Fountoulakis KN et al., 2022]. The results of our genetic study are in agreement with such observations. Indeed, we found that Li response genes were more strongly associated with manic than depressive episodes in both BP-I and BP-II. In addition, Li response genes were modestly but differentially associated with other features relevant to the clinical presentation, including, for example, suicidal ideation, psychosis and polygenic load for insomnia and hypothyroidism, in both BP-I and BP-II. Nevertheless, the fact that the results of our genetic analyses did not exactly match those obtained for the total Alda score, where the positive effects of Li showed a clear bias towards BP-I, also suggest important gene-environment interactions.
Despite the exploratory character of our genetic study, we believe that it also offers some valuable insights into the molecular mechanisms underlying inter-individual variability in Li response. For example, renalase (RNLS) was one of the most highlighted genes in our study. In addition to its link to Li response in BP [Amare AT et al., 2023], serum renalase levels have been reported to be lower in patients with schizophrenia (SCZ) than in control individuals [Catak Z et al., 2019], and Li response was previously shown to inversely associate with the genetic risk for SCZ [Amare AT et al., 2018]. RNLS is thought to modulate blood pressure and cardiac function, and has been associated with metabolic and cardiovascular alterations as well as kidney disease [Vijayakumar A & Mahapatra NR, 2022], all of which are affected by Li. Similar are the cases of CSMD2 and GRIN2A, which are involved in the control of the complement cascade and N-methyl-D-aspartate (NMDA) receptor activity, respectively. Polymorphisms in both genes have also been associated with SCZ [Tang J et al., 2006; Håvik B et al., 2011] and their respective functions are reported targets of Li effects [Ghasemi M & Dehpour AR, 2011; Yu Z et al., 2015].
CONCLUSIONS
Taken together, our findings suggest that the effects of Li on symptomatology and comorbidity in BP are partially modulated by common genetic factors, with differential effects between BP-I and BP-II. These findings might pave the way towards the development of more personalized treatment strategies for patients with BP.
Funding
The study was supported by the joint project „Individualisation in Changing Environments“ (InChangE) of the universities of Münster and Bielefeld, Germany. The project received funding from the programme “Profilbildung 2020”, an initiative of the Ministry of Culture and Science of the State of Northrhine Westphalia. The sole responsibility for the content of this publication lies with the authors.
The primary sources of funding for ConLiGen were grants RI 908/7-1, FOR2107 and RI 908/11-1 from the Deutsche Forschungsgemeinschaft (Marcella Rietschel) and grant NO 246/10-1 (Markus M. Nöthen) and grant ZIA-MH00284311 from the Intramural Research Program of the National Institute of Mental Health (ClinicalTrials.gov identifier: NCT00001174). The genotyping was funded in part by the German Federal Ministry of Education and Research through the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders), under the auspices of the e:Med Programme (Thomas G. Schulze, Marcella Rietschel and Markus M. Nöthen). The Canadian part of the study was supported by grant #166098 from the Canadian Institutes of Health Research and by a grant from Genome Atlantic/Research Nova Scotia (Martin Alda). Collection and phenotyping of the Australian University of New South Wales sample was funded by Program Grant 1037196 from the Australian National Health and Medical Research Council (Philip B. Mitchell, Peter R. Schofield, Janice M. Fullerton), and acknowledges support from Lansdowne Foundation, Betty Lynch OAM (dec) and the Janette Mary O’Neill Fellowship. AT Amare is currently supported by National Health and Medical Research Council (NHMRC) Emerging Leadership (EL1) Investigator Grant (APP2008000). The collection of the Barcelona sample was supported by grants PI080247, PI1200906, PI12/00018, 2014SGR1636, 2014SGR398, and MSII14/00030 from the Centro de Investigación en Red de Salud Mental, Institut d’Investigacions Biomèdiques August Pi i Sunyer, the Centres de Recerca de Catalunya Programme/Generalitat de Catalunya, and the Miguel Servet II and Instituto de Salud Carlos III. The Swedish Research Council, the Stockholm County Council, Karolinska Institutet and the Söderström-Königska Foundation supported this research through grants awarded to Lena Backlund, Louise Frisen, Catharina Lavebratt and Martin Schalling. The collection of the Geneva sample was supported by grants Synapsy-The Synaptic Basis of Mental Diseases 51NF40-158776 and 32003B-125469 from the Swiss National Foundation. The work by the French group was supported by INSERM (Institut National de la Santé et de la Recherche Médicale), AP-HP (Assistance Publique des Hôpitaux de Paris), the Fondation FondaMental (RTRS Santé Mentale), and the labex Bio-PSY (Investissements d’Avenir program managed by the ANR under reference ANR-11-IDEX-0004-02). The collection of the Romanian sample was supported by a grant from UEFISCDI, Bucharest, Romania (grants PCCA-89/2012; PCE-203/2021) to Maria Grigoroiu-Serbanescu. The collection of the Czech sample was supported by the project Nr. LO1611 with a financial support from the MEYS under the NPU I program and by the Czech Science Foundation, grant Nr. 17-07070S. Biju Viswanath is funded by the Intermediate (Clinical and PublicHealth) Fellowship (IA/CPHI/20/1/505266) of the DBT/Wellcome Trust India Alliance.
Abbreviations
- AAO
age at disease onset
- BP
bipolar disorders
- ConLiGen
Consortium on Lithium Genetics
- Li
lithium
- PGS
polygenic score
- SNP
single nucleotide polymorphism
Footnotes
Competing interests
Eduard Vieta has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Abbvie, Almirall, Allergan, Angelini, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, GH Research, Glaxo-Smith-Kline, Janssen, Lundbeck, Orion, Otsuka, Pfizer, Roche, Rovi, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, the Brain and Behaviour Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the Stanley Medical Research Institute and Viatris. Michael Bauer has received grants from the Deutsche Forschungsgemeinschaft (DFG), and Bundesministeriums für Bildung und Forschung (BMBF), and served as consultant, advisor or CME speaker for the following entities: Allergan, Aristo, Janssen, Lilly, Lundbeck, neuraxpharm, Otsuka, Sandoz, Servier and Sunovion outside the submitted work. Sarah Kittel-Schneider has received grants and served as consultant, advisor or speaker for the following entities: Medice Arzneimittel Pütter GmbH and Takeda. Bernhard Baune has received grants and served as consultant, advisor or CME speaker for the following entities: AstraZeneca, Bristol-Myers Squibb, Janssen, Lundbeck, Otsuka, Servier, the National Health and Medical Research Council, the Fay Fuller Foundation, the James and Diana Ramsay Foundation. Tadafumi Kato received honoraria for lectures, manuscripts, and/or consultancy, from Kyowa Hakko Kirin Co, Ltd, Eli Lilly Japan K.K., Otsuka Pharmaceutical Co, Ltd, GlaxoSmithKline K.K., Taisho Toyama Pharmaceutical Co, Ltd, Dainippon Sumitomo Pharma Co, Ltd, Meiji Seika Pharma Co, Ltd, Pfizer Japan Inc., Mochida Pharmaceutical Co, Ltd, Shionogi & Co, Ltd, Janssen Pharmaceutical K.K., Janssen Asia Pacific, Yoshitomiyakuhin, Astellas Pharma Inc, Wako Pure Chemical Industries, Ltd, Wiley Publishing Japan, Nippon Boehringer Ingelheim Co Ltd, Kanae Foundation for the Promotion of Medical Science, MSD K.K., Kyowa Pharmaceutical Industry Co, Ltd and Takeda Pharmaceutical Co, Ltd. Tadafumi Kato also received a research grant from Takeda Pharmaceutical Co, Ltd. Peter Falkai has received grants and served as consultant, advisor or CME speaker for the following entities Abbott, GlaxoSmithKline, Janssen, Essex, Lundbeck, Otsuka, Gedeon Richter, Servier and Takeda as well as the German Ministry of Science and the German Ministry of Health. Eva Reininghaus has received grants and served as consultant, advisor or CME speaker for the following entities: Janssen and Institut Allergosan. Mikael Landén has received lecture honoraria from Lundbeck. Kazufumi Akiyama has received consulting honoraria from Taisho Toyama Pharmaceutical Co, Ltd. Scott Clark has received grants, or data and served as consultant, advisor or CME speaker for the following entities: Otsuka Austalia, Lundbeck Australia, Janssen-Cilag Australia, Servier Australia,Viatris. Bruno Etain received honoraria from Sanofi Aventis. The rest of authors have no conflicts of interest to disclose.
Ethics approval and consent to participate
The Ethics Committee at the University of Heidelberg provided central approval for ConLiGen. Written informed consent from all participants was obtained according to the study protocols of each of the participating sites and their institutions. All procedures were performed in accordance with the guidelines of the Declaration of Helsinki.
Contributor Information
Marisol Herrera-Rivero, University of Münster.
Mazda Adli, Charité - Universitätsmedizin Berlin.
Kazufumi Akiyama, Dokkyo Medical University School of Medicine.
Nirmala Akula, United States Department of Health and Human Services.
Azmeraw T. Amare, University of Adelaide
Raffaella Ardau, Hospital University Agency of Cagliari.
Bárbara Arias, University of Barcelona, CIBERSAM.
Jean-Michel Aubry, Geneva University Hospitals.
Lena Backlund, Karolinska Institutet.
Frank Bellivier, Université Paris Cité, Inserm UMR-S 1144.
Antonio Benabarre, Hospital Clinic, University of Barcelona, IDIBAPS.
Susanne Bengesser, Medical University of Graz.
Abesh Kumar Bhattacharjee, University of California San Diego.
Joanna M. Biernacka, Mayo Clinic
Armin Birner, Medical University of Graz.
Micah Cearns, University of Adelaide.
Pablo Cervantes, McGill University Health Centre.
Hsi-Chung Chen, National Taiwan University Hospital.
Caterina Chillotti, Hospital University Agency of Cagliari.
Sven Cichon, University Hospital of Basel.
Scott R. Clark, University of Adelaide
Francesc Colom, Hospital Del Mar.
Cristiana Cruceanu, McGill University.
Piotr M. Czerski, Poznan University of Medical Sciences
Nina Dalkner, Medical University of Graz.
Franziska Degenhardt, University of Bonn.
Maria Del Zompo, University of Cagliari.
J. Raymond DePaulo, Johns Hopkins University.
Bruno Etain, Université Paris Cité, Inserm UMR-S 1144.
Peter Falkai, Ludwig-Maximilian-University Munich.
Ewa Ferensztajn-Rochowiak, Poznan University of Medical Sciences.
Andreas J. Forstner, University of Bonn
Josef Frank, Central Institute of Mental Health, University of Heidelberg.
Louise Frisén, Karolinska Institutet.
Mark A. Frye, Mayo Clinic
Janice M. Fullerton, UNSW Sydney
Carla Gallo, Cayetano Heredia University.
Sébastien Gard, Hôpital Charles Perrens.
Julie S. Garnham, Dalhousie University
Fernando S. Goes, Johns Hopkins University
Maria Grigoroiu-Serbanescu, Alexandru Obregia Clinical Psychiatric Hospital.
Paul Grof, Mood Disorders Center of Ottawa.
Ryota Hashimoto, National Institute of Mental Health.
Roland Hasler, Geneva University Hospitals.
Joanna Hauser, Poznan University of Medical Sciences.
Urs Heilbronner, Ludwig-Maximilian-University Munich.
Stefan Herms, University of Bonn.
Per Hoffmann, University of Bonn.
Liping Hou, United States Department of Health and Human Services.
Yi-Hsiang Hsu, Harvard University.
Stéphane Jamain, Paris-Est Créteil University.
Esther Jiménez, Hospital Clinic, University of Barcelona, IDIBAPS.
Jean-Pierre Kahn, Centre Psychothérapique de Nancy - Université de Lorraine.
Layla Kassem, United States Department of Health and Human Services.
Tadafumi Kato, Juntendo University.
John Kelsoe, University of California San Diego.
Sarah Kittel-Schneider, University Hospital Würzburg.
Po-Hsiu Kuo, National Taiwan University Hospital.
Ichiro Kusumi, Hokkaido University Graduate School of Medicine.
Barbara König, Landesklinikum Neunkirchen.
Gonzalo Laje, United States Department of Health and Human Services.
Mikael Landén, University of Gothenburg.
Catharina Lavebratt, Karolinska Institutet.
Marion Leboyer, Paris-Est Créteil University.
Susan G. Leckband, VA San Diego Healthcare System
Mario Maj, University of Campania ‘Luigi Vanvitelli’.
Mirko Manchia, University of Cagliari.
Cynthia Marie-Claire, Université Paris Cité, Inserm UMR-S 1144.
Lina Martinsson, Karolinska Institutet.
Michael J. McCarthy, University of California San Diego
Susan L. McElroy, University of Cincinnati
Vincent Millischer, Karolinska Institutet.
Marina Mitjans, University of Barcelona.
Francis M. Mondimore, Johns Hopkins University
Palmiero Monteleone, University of Salerno.
Caroline M. Nievergelt, University of California San Diego
Tomas Novák, National Institute of Mental Health.
Markus M. Nöthen, University of Bonn
Claire O’Donovan, Dalhousie University.
Norio Ozaki, Nagoya University.
Sergi Papiol, Ludwig-Maximilian-University Munich.
Andrea Pfennig, University Hospital Carl Gustav Carus, Technische Universität Dresden.
Claudia Pisanu, University of Cagliari.
James B. Potash, Johns Hopkins University
Andreas Reif, University Hospital Frankfurt.
Eva Reininghaus, Medical University of Graz.
Hélène Richard-Lepouriel, Geneva University Hospitals.
Gloria Roberts, UNSW Sydney.
Guy A. Rouleau, McGill University
Janusz K. Rybakowski, Poznan University of Medical Sciences
Martin Schalling, Karolinska Institutet.
Peter R. Schofield, UNSW Sydney
Klaus Oliver Schubert, University of Adelaide.
Eva C. Schulte, Ludwig-Maximilian-University Munich
Barbara W. Schweizer, Johns Hopkins University
Giovanni Severino, University of Cagliari.
Tatyana Shekhtman, University of California San Diego.
Paul D. Shilling, University of California San Diego
Katzutaka Shimoda, Dokkyo Medical University School of Medicine.
Christian Simhandl, Sigmund Freud University Vienna.
Claire M. Slaney, Dalhousie University
Alessio Squassina, University of Cagliari.
Thomas Stamm, Charité - Universitätsmedizin Berlin.
Pavla Stopkova, National Institute of Mental Health.
Fabian Streit, Central Institute of Mental Health, University of Heidelberg.
Fasil Tekola-Ayele, National Institutes of Health.
Anbupalam Thalamuthu, UNSW Sydney.
Alfonso Tortorella, University of Perugia.
Gustavo Turecki, McGill University.
Julia Veeh, University Hospital Frankfurt.
Eduard Vieta, Hospital Clinic, University of Barcelona, IDIBAPS.
Biju Viswanath, National Institute of Mental Health and Neurosciences.
Stephanie H. Witt, Central Institute of Mental Health, University of Heidelberg
Peter P. Zandi, Johns Hopkins University
Martin Alda, Dalhousie University.
Michael Bauer, University Hospital Carl Gustav Carus, Technische Universität Dresden.
Francis J. McMahon, United States Department of Health and Human Services
Philip B. Mitchell, UNSW Sydney
Marcella Rietschel, Central Institute of Mental Health, University of Heidelberg.
Thomas G. Schulze, Ludwig-Maximilian-University Munich
Bernhard T. Baune, University of Münster
Availability of data and material
The data that support the findings of this study are available from ConLiGen, but restrictions apply to their availability.
References
- 1.Geoffroy PA, Samalin L, Llorca PM, Curis E, Bellivier F. Influence of lithium on sleep and chronotypes in remitted patients with bipolar disorder. J Affect Disord. 2016;204:32–9. 10.1016/j.jad.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Volkmann C, Bschor T, Köhler S. Lithium Treatment Over the Lifespan in Bipolar Disorders. Front Psychiatry. 2020;11:377. 10.3389/fpsyt.2020.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu N, Shinohara K, Saunders KEA, Geddes JR, Cipriani A. Effect of lithium on circadian rhythm in bipolar disorder: A systematic review and meta-analysis. Bipolar Disord. 2021;23(5):445–53. 10.1111/bdi.13070. [DOI] [PubMed] [Google Scholar]
- 4.Queissner R, Lenger M, Birner A, et al. The association between anti-inflammatory effects of long-term lithium treatment and illness course in Bipolar Disorder. J Affect Disord. 2021;281:228–34. 10.1016/j.jad.2020.11.063. [DOI] [PubMed] [Google Scholar]
- 5.Miller BJ, McCall WV. Insomnia and suicide as reported adverse effects of second-generation antipsychotics and mood stabilizers. J Clin Sleep Med. 2022;18(2):517–22. 10.5664/jcsm.9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybakowski JK. Antiviral, immunomodulatory, and neuroprotective effect of lithium. J Integr Neurosci. 2022;21(2):68. 10.31083/j.jin2102068. [DOI] [PubMed] [Google Scholar]
- 7.Chen PH, Hsiao CY, Chiang SJ, et al. Cardioprotective potential of lithium and role of fractalkine in euthymic patients with bipolar disorder. Aust N Z J Psychiatry. 2023;57(1):104–14. 10.1177/00048674211062532. [DOI] [PubMed] [Google Scholar]
- 8.Szałach ŁP, Lisowska KA, Cubała WJ, Barbuti M, Perugi G. The immunomodulatory effect of lithium as a mechanism of action in bipolar disorder. Front Neurosci. 2023;17:1213766. 10.3389/fnins.2023.1213766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferensztajn-Rochowiak E, Chłopocka-Woźniak M, Rybakowski JK. Ultra-long-term lithium therapy: all-important matters and a case of successful 50-year lithium treatment. Braz J Psychiatry. 2021;43(4):407–13. 10.1590/1516-4446-2020-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunes A, Ardau R, Berghöfer A, et al. Prediction of lithium response using clinical data. Acta Psychiatr Scand. 2020;141(2):131–41. 10.1111/acps.13122. [DOI] [PubMed] [Google Scholar]
- 11.Papiol S, Schulze TG, Heilbronner U. Lithium response in bipolar disorder: Genetics, genomics, and beyond. Neurosci Lett. 2022;785:136786. 10.1016/j.neulet.2022.136786. [DOI] [PubMed] [Google Scholar]
- 12.Amare AT, Thalamuthu A, Schubert KO, et al. Association of polygenic score and the involvement of cholinergic and glutamatergic pathways with lithium treatment response in patients with bipolar disorder. Mol Psychiatry. 2023. 10.1038/s41380-023-02149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Consortium on Lithium Genetics (ConLi + Gen), Amare AT, Schubert KO, et al. Association of Polygenic Score for Schizophrenia and HLA Antigen and Inflammation Genes With Response to Lithium in Bipolar Affective Disorder: A Genome-Wide Association Study. JAMA Psychiatry. 2018;75(1):65–74. 10.1001/jamapsychiatry.2017.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert KO, Thalamuthu A, Amare AT, et al. Combining schizophrenia and depression polygenic risk scores improves the genetic prediction of lithium response in bipolar disorder patients. Transl Psychiatry. 2021;11(1):606. 10.1038/s41398-021-01702-2. Published 2021 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coombes BJ, Millischer V, Batzler A, et al. Association of Attention-Deficit/Hyperactivity Disorder and Depression Polygenic Scores with Lithium Response: A Consortium for Lithium Genetics Study. Complex Psychiatry. 2021;7(3–4):80–9. 10.1159/000519707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida T, Papiol S, Plans L et al. The polygenic effect of the response to lithium on suicidal behavior. European Neuropsychopharmacology 29:S179. 2019. [Conference poster]. 10.1016/j.euroneuro.2019.08.124. [DOI] [Google Scholar]
- 17.Hou L, Heilbronner U, Degenhardt F, et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet. 2016;387(10023):1085–93. 10.1016/S0140-6736(16)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manchia M, Adli M, Akula N, et al. Assessment of Response to Lithium Maintenance Treatment in Bipolar Disorder: A Consortium on Lithium Genetics (ConLiGen) Report. PLoS ONE. 2013;8(6):e65636. 10.1371/journal.pone.0065636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera-Rivero M, Gutiérrez-Fragoso K, Thalamuthu A et al. Immunogenetics of lithium response and psychiatric phenotypes in patients with bipolar disorder. Preprint. Res Sq. 2023;rs.3.rs-3068352. Published 2023 Jun 26. 10.21203/rs.3.rs-3068352/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert SA, Gil L, Jupp S, et al. The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat Genet. 2021;53(4):420–5. 10.1038/s41588-021-00783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Privé F, Aschard H, Carmi S, et al. Portability of 245 polygenic scores when derived from the UK Biobank and applied to 9 ancestry groups from the same cohort. Am J Hum Genet. 2022;109(1):12–23. 10.1016/j.ajhg.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Patil S, Zhou X, Mukherjee B, Fritsche LG. ExPRSweb: An online repository with polygenic risk scores for common health-related exposures. Am J Hum Genet. 2022;109(10):1742–60. 10.1016/j.ajhg.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tondo L, Baldessarini RJ, Floris G. Long-term clinical effectiveness of lithium maintenance treatment in types I and II bipolar disorders. Br J Psychiatry. 2001;178(Suppl 41):184–S190. [PubMed] [Google Scholar]
- 25.Fountoulakis KN, Tohen M, Zarate CA Jr.. Lithium treatment of Bipolar disorder in adults: A systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100–15. 10.1016/j.euroneuro.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catak Z, Kocdemir E, Ugur K, et al. A Novel Biomarker Renalase and Its Relationship with its Substrates in Schizophrenia. J Med Biochem. 2019;38(3):299–305. 10.2478/jomb-2018-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijayakumar A, Mahapatra NR. Renalase: a novel regulator of cardiometabolic and renal diseases. Hypertens Res. 2022;45(10):1582–98. 10.1038/s41440-022-00986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J, Chen X, Xu X, et al. Significant linkage and association between a functional (GT)n polymorphism in promoter of the N-methyl-D-aspartate receptor subunit gene (GRIN2A) and schizophrenia. Neurosci Lett. 2006;409(1):80–2. 10.1016/j.neulet.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Håvik B, Le Hellard S, Rietschel M, et al. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol Psychiatry. 2011;70(1):35–42. 10.1016/j.biopsych.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Ghasemi M, Dehpour AR. The NMDA receptor/nitric oxide pathway: a target for the therapeutic and toxic effects of lithium. Trends Pharmacol Sci. 2011;32(7):420–34. 10.1016/j.tips.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Yu Z, Ono C, Aiba S, et al. Therapeutic concentration of lithium stimulates complement C3 production in dendritic cells and microglia via GSK-3 inhibition. Glia. 2015;63(2):257–70. 10.1002/glia.22749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from ConLiGen, but restrictions apply to their availability.