Abstract
The bld mutants of Streptomyces coelicolor A3(2) are blocked at the earliest stage of sporulation, the formation of aerial hyphae, and are pleiotropically defective in antibiotic production. Using a phage library of wild-type S. coelicolor DNA, we isolated a recombinant phage which restored both sporulation and antibiotic production to strains carrying the single known bldD mutation. Nucleotide sequence analysis of a 1.3-kb complementing subclone identified an open reading frame, designated bldD, encoding a translation product of 167 amino acid residues. Nucleotide sequence analysis of the bldD-containing fragment amplified from the chromosome of a bldD mutant strain revealed a point mutation changing a tyrosine residue at amino acid position 62 to a cysteine. Although a comparison of the BldD sequence to known proteins in the databases failed to show any strong similarities, analysis of the BldD sequence for secondary structural elements did reveal a putative helix-turn-helix, DNA recognition element near the C terminus of the protein. A comparison of bldD transcript levels in the bldD+ and bldD mutant strains using both Northern blot analysis and S1 nuclease protection studies showed vast overexpression of bldD transcripts in the mutant, suggesting that BldD negatively regulates its own synthesis. High-resolution S1 nuclease mapping identified the transcription start point as a G residue 63 nucleotides upstream from the bldD start codon and 7 nucleotides downstream from −10 and −35 sequences resembling E. coli-like streptomycete promoters.
Streptomycetes are unusual among prokaryotes in that they undergo a complex cycle of morphological differentiation during their life cycle. Typically a spore germinates to form vegetative substrate hyphae that give rise to aerial hyphae, and the tips of the aerial hyphae then further differentiate to form chains of unigenomic spores. Coincident with the formation of aerial hyphae is the production of secondary metabolites, the most notable of which are antibiotics. Genetic studies of the extensively analyzed strain Streptomyces coelicolor A3(2) revealed several classes of bld (for bald) mutants that fail to form aerial hyphae. Many of these are also blocked in antibiotic production (sometimes called chemical or physiological differentiation). The existence of such mutants has suggested that the temporal coincidence seen between chemical and morphological differentiation results from shared global regulatory elements and further suggests that the bld genes may encode global regulators responsible for the switching on of those pathways.
Recently Willey et al. (37) showed that production of a small, spore-associated protein, SapB, is impaired in bld mutants and that aerial mycelium formation could be restored at the edges of bld mutant colonies closest to nearby SapB-producing colonies. On the basis of these experiments, SapB was proposed to be a morphogenetic protein that enables hyphae to extend into the air. Remarkably, production of SapB, and hence of aerial hyphae, could also be restored when some pairs of bld mutants were juxtaposed on the surface of agar plates, suggesting that differentiation is governed by a hierarchical cascade of intercellular signals (22, 38) and that the bld genes themselves directly or indirectly govern the production of the extracellular signals. One mutant (the single known bldD mutant) was capable of complementing all of the other bld mutants tested and therefore was placed at the top of the hierarchy. Together with the evidence suggesting that SapB is a nonribosomally synthesized protein, this finding led Willey et al. (38) to propose that bldD encodes a structural gene for a peptide synthetase involved in nonribosomal SapB production or, alternatively, a regulatory gene necessary for expression of such a peptide synthetase.
Many of the bld mutants exhibit a carbon source-dependent rescue of aerial mycelium formation (5, 19). In pursuit of the significance of this, Pope et al. (25) found that some of the characterized bld mutants (bldA, -B, -C, -D, -G, and -H) of S. coelicolor exhibit deregulated expression of the galP1 promoter for galactose utilization, with bldB showing a global defect in the regulation of carbon utilization. On the basis of these findings, they suggested that the bld mutants are not involved in morphogenesis per se, but are involved in assessing the nutritional environment of the cell, and that mutations in the bld loci are epistatic to morphogenesis.
To explain these diverse observations more fully, an understanding of the nature of bld gene products is necessary. The bldA gene is the most characterized bld gene. It encodes a leucyl-tRNA that recognizes the rare UUA codon in Streptomyces mRNA, and it has been proposed to function in translational regulation of differentiation and antibiotic production (16, 17). The bldK locus encodes genes specifying homologs of the subunits of the oligopeptide-permease family of ATP-binding cassette (ABC) membrane-spanning transporters (22). The bldB gene apparently encodes a small, highly negatively charged protein (GenBank accession no. U28930 [23b]) containing a putative DNA-binding sequence and which has been implicated in the regulation of catabolite control (25, 25a). Here we focus on bldD. Only one bldD mutant allele (bldD53) has been described (19). Phenotypically, the bldD53 mutant closely resembles bldA mutants: on minimal medium containing glucose as carbon source, the mutants of both classes produce none of the four antibiotics known to be produced by the wild-type strain, and they have a soft, fragmented colony surface lacking any aerial structures. Close examination of the colony surface has revealed a layer of malformed, prostrate hyphae that may be defective aerial hyphae with insufficient turgor to extend up into the air (7, 24). For both bldA and bldD mutants, the morphological defect, but not the loss of antibiotic production, can be overcome by replacing glucose in the medium with alternative, “permissive” carbon sources, such as mannitol.
We report here the cloning, nucleotide sequence analysis, and transcriptional studies on the wild-type and mutant bldD genes. bldD encodes a small, highly charged protein with an apparent ability to regulate negatively its own transcription. This apparent autoregulatory function of the gene product suggests that bldD may encode a transcription regulator protein.
MATERIALS AND METHODS
Strains and media.
S. coelicolor strains used in this study include M145 (prototrophic, SCP1− SCP2− [13]), J1501 (hisA1 uraA1 strA1 pgl SCP− SCP2− [6]), 1169 (hisA1 mthB2 pheA1 strA1 bldD53 NF SCP2* [19]), J774 (cysA15 pheA1 mthB2 bldD53 NF SCP2* [19]), and HU66 (hisA1 uraA1 strA1 pgl bldD53 NF SCP2* [38] [provided by J. Willey]). (It should be noted that with the exception of the bldD53 mutation, the chromosomes of strains J1501 and HU66 are isogenic.) Streptomyces lividans 66 (John Innes strain 1326) was the host for φC31 propagation and for the transfection of protoplasts. Media, culture conditions, and protoplast transformation and transfection were as described previously (13).
Escherichia coli host strains were MV1193 [Δ(lac-proAB) rpsL thi endA sbcB15 hsdR4 Δ(srl-recA)306::Tn10(Tetr) F′(traD36 proAB+ lacIq lacZΔM15) (43)], DH5αF′ (F′ supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 [Gibco-BRL]), and ET12567 (F− dam-13::Tn9 dcm-6 hsdM hsdR recF143 zjj-202::Tn10 galK2 galT22 ara14 lacY1 xyl-5 leuB6 thi-1 tonA31 rpsL136 hisG4 tsx78 mtl-1 glnV44 [18] [gift from D. MacNeil, Merck Sharp & Dohme Research Laboratories). Media and culture conditions were as in reference 29.
Plasmid and bacteriophage vectors.
Streptomyces vector KC304 (12) is a derivative of φC31 and contains the tsr (thiostrepton resistance) gene for vector selection; the vph (viomycin resistance) gene, flanked by BamHI sites, as a stuffer fragment for replacement by up to 6 kb of insert DNA; and the att-int region to allow efficient integration at single copy number into the chromosomal att site for φC31. KC304 and the Streptomyces high-copy-number plasmid vector pIJ486, containing tsr for vector selection (36), were manipulated as described previously (13). Prior to its use to transform S. coelicolor, the bifunctional E. coli-Streptomyces vector pSET152 (NRRL B-14792) (containing the multiple cloning site and replicon of pUC plasmids, the att-int region of φC31, and the apramycin resistance gene for vector selection in either E. coli or Streptomyces) (3) was replicated in the dam dcm host, E. coli ET12567, using standard procedures (29). Streptomyces plasmids were maintained by selection for resistance to thiostrepton (50 μg/ml) (a gift from S. Lucania, Squibb Institute for Medical Research, Princeton, N.J.) or Apralan (50 μg/ml) (Provel; Division of Eli Lilly Canada). The E. coli plasmids pIJ2925 (14), pUC118/119, and pBluescript II SK/KS (Stratagene) and the M13 derivatives mp18 and mp19 were manipulated as described in reference 29. The helper phage M13K07 was manipulated as previously described (35).
Library construction and screening.
A library of S. coelicolor M145 DNA fragments was constructed by isolating total genomic DNA (13), digesting it partially with Sau3AI, and ligating 2- to 7-kb fragments, purified on a sucrose density gradient, between the BamHI sites of the phage vector, KC304 (12). The ligation mixture was then used to transfect S. lividans 1326 protoplasts (13), and phage plaques were obtained. Pooled recombinant phages were soaked out of soft agar overlays (13) on nutrient agar plates showing near confluent lysis and stored at 4°C in Difco nutrient broth. Phage suspensions were titered and found to contain 108 PFU/ml.
Library screening involved spotting five 20-μl aliquots of the phage library suspension on a lawn of S. coelicolor 1169 mycelial fragments on the surface of each of two R2YE (13) agar plates. The lawns were allowed to grow for 2 weeks, and then the mycelia in and around the spotted areas were scraped from the surface of the plates, pooled, and resuspended in sterile distilled H2O. The mycelia were then homogenized to break the hyphae into small fragments, diluted, and plated to give about 100 single colonies/plate on minimal medium (13) containing glucose as a carbon source and thiostrepton (50 μg/ml) to select for phage-containing lysogens. Colonies showing aerial mycelium and the reddish pigmentation typical of the antibiotic-producing wild-type strain were sought as evidence of bldD complementation. Recombinant phages, containing the cloned bldD gene, were recovered from sporulating lysogens after replication onto Difco nutrient agar plates with soft nutrient agar overlays containing S. lividans 1326 spores (13) where free phages released from the lysogens resulted in plaque formation in the S. lividans lawn.
Subcloning and sequencing.
The ca. 3.5-kb bldD-complementing fragment from KC742 (see Results) was removed from the φC31 vector as a ca. 4.5-kb EcoRV fragment containing 1 kb of flanking φC31 vector DNA. The 4.5-kb blunt-ended fragment was ligated into SmaI-digested pIJ2925, and the ligation mixture was used to transform E. coli MV1193. The recombinant plasmid, designated pAU171, was then subcloned and the DNA sequence for the entire insert was determined by both manual and automated (Applied Biosystems model 373A; Department of Biological Sciences Sequencing Service, University of Alberta) sequence analysis. All primers were obtained from the Department of Biological Sciences Synthesis Service, University of Alberta.
Complementation of bldD mutant strains.
Digestion of pAU171 with SphI allowed the subcloning of a ca. 2.4-kb fragment that extended from the unique SphI site in the cloned bldD-containing DNA rightward to the SphI site located in the vector polylinker (Fig. 1). The fragment was ligated into the SphI site of pIJ2925, and the recombinant plasmid, designated pAU174, was isolated after transformation of E. coli MV1193 and selection for ampicillin resistance. A 1.3-kb SphI-XmnI fragment containing bldD was then further subcloned, after gel purification (41) and blunt ending (29) of the SphI site, into the EcoRV site of pSET152. The recombinant plasmid, designated pAU181, was isolated after transformation of E. coli DH5αF′ and selection for Apralan (apramycin) resistance (28). The plasmid, pAU181, was then passaged through E. coli ET12567 and used to transform protoplasts of three available S. coelicolor bldD53 strains, i.e., 1169 and its derivatives obtained by classical recombination, J774 and HU66. Apralan-resistant transformants were then selected and visually scored for their phenotype.
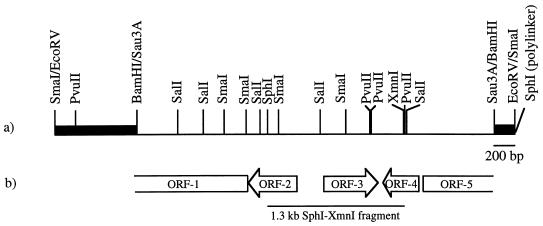
FIG. 1.
bldD-complementing DNA. (a) The restriction map of the 4.5-kb insert in pAU171. The thin line represents S. coelicolor M145 DNA, and the heavy black lines represent the flanking φC31 DNA. (b) Schematic diagram showing the location and orientation of three complete ORFs (ORF-2, ORF-3, and ORF-4) and two partial ORFs (ORF-1 and ORF-5) contained in the 3.5 kb bldD-complementing DNA fragment. The 1.3-kb SphI-XmnI subclone able to restore sporulation and antibiotic production to bldD mutants is shown below the ORF map.
The same 1.3-kb SphI-XmnI fragment was also transferred from pAU181, using flanking polylinker EcoRI and XbaI sites, into the same sites in the high-copy-number Streptomyces vector pIJ486 (36). The ligation mixture was used to transform S. coelicolor HU66, and thiostrepton-resistant transformants were selected. A transformant containing the recombinant plasmid was identified among thiostrepton-resistant transformants screened by colony hybridization. Colony hybridization was performed essentially as described by Davis and Chater (8) except that colonies were patched directly onto Whatman 541 filters laid on the surface of R2YE agar plates containing thiostrepton, and the filters were then hybridized and washed at 65°C, using procedure B described in reference 13. The probe was an [α-32P]dATP random-primer-labeled, 214-bp SmaI-PvuII fragment internal to the bldD gene.
Sequencing of the bldD mutant allele.
The mutant bldD gene was amplified from the chromosome of the bldD mutant strain S. coelicolor HU66, using the oligonucleotide primers BKL51 (5′GCGCGAATTCGGCGCGTTCGACGATCTCG; spanning nucleotides [nt] 152 to 170), located in the 5′ flanking region of bldD, and BKL47 (5′CTCGTTGCGCCGCGAGT; complementary to nt 1260 to 1278), located downstream of the bldD open reading frame (ORF) (Fig. 2). (Note that BKL51 contains a 10-nt nonhomologous extension [underlined] for use in S1 mapping experiments [see below].) PCR amplification was carried out with 1 μg of S. coelicolor HU66 genomic DNA as the template and 40 pmol of each primer in 100-μl reaction volumes; 2 U of Taq polymerase (Boehringer Mannheim) or 1.25 U of Expand polymerase was used in each reaction. Amplifications were performed separately with the Taq and Expand polymerases to ensure that any mutations revealed by sequence analysis of the products would not have arisen in vitro. The reaction mixtures were denatured at 95°C and then subjected to 30 cycles of 95°C for 30 s, 52°C for 30 s, and 68°C for 1 min. The major 1.1-kb amplification product from each of the two separate amplifications was purified from a 5% polyacrylamide gel by crushing and soaking (29) and sequenced directly (DNA Sequencing Service, Department of Biological Sciences, University of Alberta), using as primers the oligonucleotides BKL51 and BKL47 (described above), BKL37 (5′CGAGCTGGCGGACTTCT; nt 720 to 736), BKL41 (5′CGCCGTCATCTACGACC; nt 948 to 964), BKL50 (5′CCACGACGGCCTTCCAG; complementary to nt 654 to 670), MAE1 (5′GGAAGAGTCGGTGCGGA; nt 428 to 444), and MAE2 (5′GGTCGTAGATGACGGCG; complementary to nt 948 to 964) (Fig. 2).
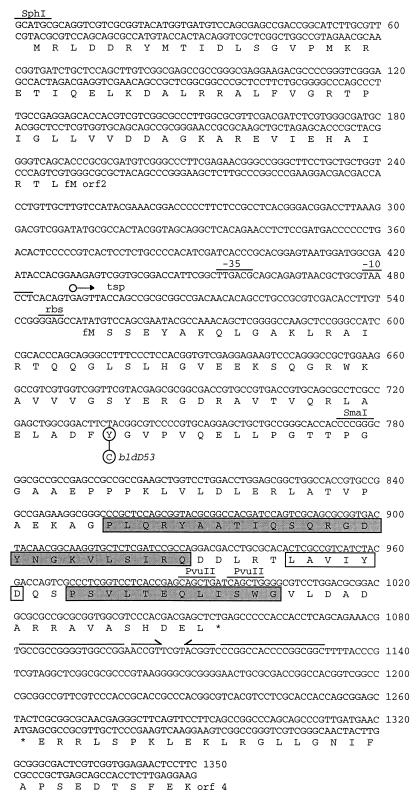
FIG. 2.
Nucleotide sequence of the 1.3-kb SphI-XmnI fragment containing bldD. The deduced amino acid sequences of bldD, and the two partial ORFs, orf2 and orf4, are also given in single-letter code. The tyrosine residue (Y) of BldD that is changed to a cysteine residue (C) by the A→G transition in the bldD53 mutant is circled. The hexameric −35 and −10 promoter sequences are shown, as is the ribosome-binding sequence (rbs) and the transcription start point (tsp; ○→) for bldD. An inverted repeat that may serve as a transcription termination signal is indicated by the solid black lines with arrowheads (a gap is shown over nucleotides that would not base pair). The putative HTH structural element is indicated by shaded boxes for the helical segments, and an open box indicates the turn. Selected restriction endonuclease sites corresponding to those shown in Fig. 1 are also indicated.
RNA isolation.
For RNA isolation, Streptomyces cultures were grown on cellophane discs on the surface of R2YE agar as previously described (17). RNA was extracted essentially as described elsewhere (13) except that mycelia were scraped directly from the cellophane discs into modified Kirby mix. The RNA was isolated at various time points as described in Results. The E. coli RNA used as a negative control was provided by Nicole Trepanier, Department of Biological Sciences, University of Alberta.
Northern blot analysis.
Northern blot analysis was performed as described by Williams and Mason (39). RNA (40 μg) was denatured with glyoxal and dimethyl sulfoxide and then size fractionated by electrophoresis at 4 V/cm on a 1.25% agarose gel, using a 10 mM Na2HPO4-NaH2PO4 (pH 7.0) recirculating buffer system. MW Markers III and V (625 ng; Boehringer), treated in the same way, served as DNA size markers. Capillary blot transfer to a Hybond-N (Amersham) membrane was done as previously described (29). For detection of bldD transcripts, the probe was an [α-32P]dATP random-primer-labeled, 214-bp SmaI-PvuII fragment internal to the bldD gene. Hybridization was performed overnight at 50°C in a solution containing 50% formamide (13) and 4 × 106 cpm of probe. After hybridization, the nylon filter was washed at the same temperature twice for 30 min each time in a solution containing 2× SSC (0.3 M NaCl, 0.03 M sodium citrate)–0.1% sodium dodecyl sulfate and then twice for 20 min each time in 0.2× SSC–0.1% sodium dodecyl sulfate. The signals were detected by using X-ray film. Molecular weight markers were stained on the surface of the nylon filter, using 0.2% methylene blue in 0.2 M sodium acetate (pH 4.7) (20). As a control for RNA loading levels, a probe for 16S rRNA was hybridized to the same blot. The 16S rRNA probe was an oligonucleotide, 5′CCGCCTTCGCCACCGGT, corresponding to a conserved region of Streptomyces 16S rRNA sequences. Hybridization and washing were performed at 55°C according to procedure B described in reference 13.
S1 nuclease mapping of bldD transcription start site.
The probe for S1 nuclease mapping of bldD was generated by PCR amplification of a 527-bp fragment by using pAU184, a pUC118 derivative containing the 1.3-kb bldD-containing DNA fragment from pAU181 inserted into the EcoRI and XbaI sites, as the template. The primers were a 17-mer synthetic oligonucleotide, BKL50, corresponding to a sequence internal to the bldD ORF, and a 27-mer synthetic oligonucleotide, BKL51, corresponding to a region 399 nt upstream of the bldD start codon and containing a 10-nt nonhomologous extension (see above). The amplified DNA was purified from a 5% polyacrylamide gel by crushing and soaking (29). The 5′ ends of the amplified DNA (about 2 pmol) were labeled with [γ-32P]ATP by using T4 polynucleotide kinase. The probe, labeled at both ends, was used without further treatment since the nonhomologous extension would be removed by S1 nuclease treatment and would not result in the appearance of labeled, protected fragments. A sequence ladder was generated by the dideoxy-chain termination method (30), using as the primer BKL50 and as the template helper phage-generated, single-stranded DNA arising from pAU183, which contains the same insert as pAU184 (see above) cloned in the opposite orientation. For each S1 nuclease protection reaction, 50 μg of RNA was hybridized to 50,000 Cerenkov cpm of the probe in formamide buffer as described previously (13) except that the hybridizations were carried out overnight and that glycogen (Boehringer) replaced the carrier tRNA. RNA extracted from E. coli was used as a control, and the samples were run under standard conditions on a 6% polyacrylamide sequencing gel.
Nucleotide sequence accession no. The nucleotide sequence data in this study have been deposited in GenBank under accession no. AF045549.
RESULTS
Cloning of the bldD gene.
Screening for bldD-containing recombinant phage in a library of S. coelicolor M145 DNA fragments (described above) was accomplished by looking for complementation of the antibiotic-negative and aerial mycelium-negative phenotypes of the bldD mutant strain, S. coelicolor 1169. The phage library was introduced into the bldD mutant, and thiostrepton-resistant lysogens were screened visually for those that had regained the ability to sporulate and to produce pigment attributable to the antibiotics actinorhodin and undecylprodigiosin. One such lysogen, presumed to carry a recombinant phage containing the bldD gene, was identified out of a total of 550 colonies screened. To show that the cloned DNA was responsible for the restoration of antibiotic production and sporulation, phages released from the lysogen (see Materials and Methods) were respotted on a S. coelicolor 1169 lawn and thiostrepton-resistant lysogens were again selected. The recombinant phage gave 100% transduction to a sporulation- and antibiotic-positive phenotype. It was not determined whether production of methylenomycin and calcium-dependent antibiotic, the other two antibiotics produced by S. coelicolor, was restored. One of the thiostrepton-resistant, bldD+ lysogens was chosen for DNA isolation, and the recombinant phage was designated KC742.
Analysis of sequence and ORFs.
The 3.5-kb partial Sau3AI fragment containing bldD was subcloned from KC742 as an EcoRV fragment that contained 1 kb of flanking φC31 vector DNA. The 4.5-kb EcoRV fragment was subcloned into pIJ2925, and the nucleotide sequence was determined (Fig. 1). FRAME (2) analysis (not shown) of the DNA sequence indicated the presence of two partial and three complete ORFs. Comparison of the sequences to the databases by using BLAST (1) analysis suggested that ORF-1 and ORF-2 determine part of the pyrimidine biosynthetic pathway (data not shown), and therefore they were presumed to be unrelated to bldD. Since ORF-3 was easily subcloned, using the unique SphI and XmnI sites, it was tested for its ability to complement the bldD53 mutation. The 1.3-kb SphI-XmnI fragment, when cloned (see Materials and Methods for details) into either the high-copy-number Streptomyces vector pIJ486 or the single-copy, att site-integrating vector pSET152 (to rule out the possibility of complementation by recombinational repair in the chromosome, using one of the partial ORFs contained on the fragment), was able to restore antibiotic production and sporulation to all available bldD mutant strains (the single bldD53 mutation exists in different genetic backgrounds [see Materials and Methods]). Southern blot analysis was used to confirm insertion of the bldD-containing plasmid pSET152 into the att site rather than into the chromosomal copy of bldD (data not shown). Figure 2 shows the nucleotide sequence of the 1.3-kb SphI-XmnI fragment containing ORF-3, which will be referred to hereafter as bldD. Comparison of the ORF-4 and ORF-5 sequences to entries in the databases failed to reveal any similarity to known proteins.
Confirmation that ORF-3 encodes bldD.
Although the complementation studies strongly suggested that ORF-3 encodes bldD, the ORF-3 sequence from the bldD strain, HU66, was determined to confirm that it contained a mutation. Direct sequencing of a PCR-amplified, S. coelicolor HU66-derived fragment of DNA corresponding to ORF-3 revealed an A→G point mutation changing a tyrosine residue at amino acid position 62 to a cysteine (Fig. 2). The same point mutation was found in DNA fragments amplified by using either the Taq or Expand polymerase.
The bldD gene encodes a 167-amino-acid protein with a deduced Mr of 18,167 and without extensive similarity to any known proteins in the databases, as determined by BLAST analysis. The deduced protein appears to be cytoplasmically localized, as indicated by the abundance of charged amino acids (22 basic and 21 acidic residues) and the absence of long stretches of hydrophobic amino acids. A proline/glycine-rich region near the center of the protein (amino acids 71 to 83) may suggest the existence of two separable domains. A MOTIF search (Wisconsin package version 8.1; Genetics Computer Group) indicated the presence of a Prosite K/HDEL motif, which serves as an endoplasmic reticulum targeting sequence in eukaryotic proteins (21), at the extreme C terminus; however, this motif has no known function in prokaryotic proteins. Although the MOTIF search failed to reveal the existence of other motifs, a manual comparison of the BldD sequence with the signature sequences of known eubacterial helix-turn-helix (HTH) proteins (Prosite defined; accessed through the Gopher search index) revealed the presence of a sequence (amino acid positions 129 to 154) matching 24 of 26 bases in the (rather degenerate) HTH signature sequence for the LysR family of transcriptional regulators (11, 34) (Fig. 3). Since the putative HTH did not yield a positive score when analyzed by the weight matrix scoring method for DNA-binding HTH sequences (9), attempts were made to authenticate its existence by using the inverse folding procedure of Bowie et al. (4) to identify structural or functional domains within BldD. To do this, the BldD sequence was systematically threaded onto the three-dimensional structure of all proteins whose fold has been determined by X-ray diffraction or nuclear magnetic resonance methods (Brookhaven Protein Data Bank). Such analysis resulted in a high score for a long HTH structural element in the C-terminal region of the protein, with the turn sequence and second helix overlapping the LysR signature sequence (Fig. 2). Putative DNA contact residues were seen at amino acid positions T144, E145, Q146, S149, and W150 in the second helical domain (23a).
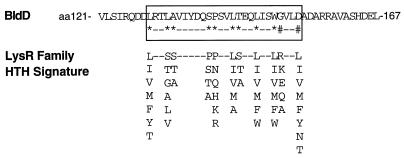
FIG. 3.
Alignment of putative HTH in BldD with the LysR family HTH signature sequence (Prosite; PS00044). Amino acid (aa) positions within BldD are indicated, and the HTH is boxed. ∗, amino acid matching the signature sequence; #, mismatch. All possible amino acids allowed at each position of the signature sequence are shown in a column, and positions where any amino acid is allowed are designated by dashes.
Analysis of bldD transcripts.
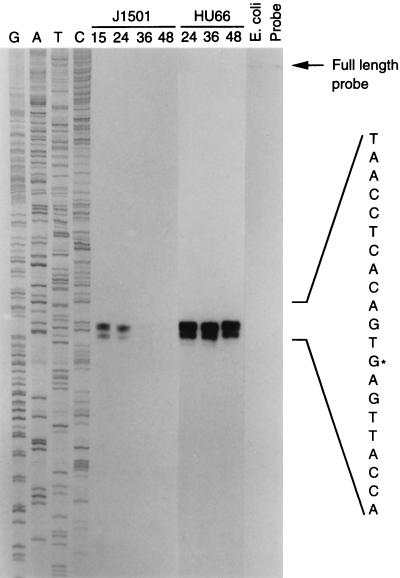
To determine the size and timing of expression of bldD transcripts, Northern blot analysis was performed with RNA isolated from S. coelicolor J1501 surface-grown cultures (Fig. 4). To address the effect of the bldD mutation on its own transcription, RNA samples isolated from surface-grown cultures of the bldD mutant, S. coelicolor HU66, were also included in the analysis. The probe was a 214-bp SmaI-PvuII, random-primer-labeled fragment internal to bldD. As seen in Fig. 4, a band of approximately 600 to 750 nt was detected in RNA samples from S. coelicolor J1501. The size of the band is consistent with bldD being expressed as a monocistronic transcript, and inspection of the nucleotide sequence just downstream of the bldD coding sequence revealed an inverted repeat that might serve as a transcription termination signal (ΔG = −57.6 kcal [Fig. 2]). To determine the location of the bldD promoter, high-resolution S1 nuclease mapping studies were performed on the same RNA samples as used for the Northern analysis. The RNA samples were hybridized with a 527-bp PCR-generated fragment labeled at the 5′ ends. A major protected fragment of 181 nt was detected (Fig. 5), suggesting a transcription start site corresponding to a G residue 63 nt upstream of the bldD translational start codon and just downstream of putative −10 and −35 sequences closely resembling the sequences of streptomycete E. coli-like promoters (32) and separated by the optimal 18-nt spacer (Fig. 2). (A shorter protected fragment of 176 nt was also detected; however, it was shown, by its absence in primer extension analysis and in S1 analysis using enzyme from a different supplier [data not shown], to be an S1 nuclease artifact].) Transcripts initiating at this position and terminating at the string of U residues following the inverted repeat downstream of the bldD translational stop codon would result in a transcript 632 nt in length, consistent with the lengths of the transcripts observed by Northern blot analysis.
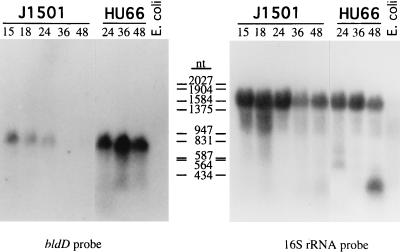
FIG. 4.
Northern blot analysis of bldD transcripts in RNA samples (40 μg) isolated from surface-grown S. coelicolor J1501 and HU66. RNA was isolated at various times (hours postinoculation) as indicated. The probe was a random-primer-labeled, 214-bp SmaI-PvuII fragment internal to bldD (Fig. 2). E. coli RNA was used as a negative control. Size markers were MW Markers III and V (Boehringer). As a control for RNA loading levels, the same blot was also probed with a 16S rRNA-specific oligonucleotide probe (shown on the right). The blot hybridized with the bldD-specific probe was exposed to X-ray film with an intensifying screen for 2 weeks, and the blot hybridized with the 16S rRNA-specific probe was exposed to X-ray film without an intensifying screen for 4 h.
FIG. 5.
High-resolution S1 nuclease mapping of the bldD transcriptional start site from RNA samples (50 μg) isolated at various times (shown above the lanes as hours postinoculation) from S. coelicolor J1501 and HU66 grown on the surface of agar plates. The gel was exposed to X-ray film without an intensifying screen for 72 h. GATC, bldD sequence ladder generated with the oligonucleotide (BKL50) used for probe preparation; ∗, the most probable transcription start site with the sequence given corresponding to the template strand; E. coli, control lane using RNA isolated from E. coli; Probe, control lane containing only the probe.
Both Northern blot analysis (Fig. 4) and S1 nuclease protection studies (Fig. 5) showed that bldD transcripts were also most abundant in early growth, the level of expression being highest in the earliest time point sample tested (15 h) and decreasing significantly after 24 h of growth. It should be noted that the 15-h cultures used in these experiments were probably already in transition phase between vegetative growth and differentiation because the surface-grown cultures differentiated very rapidly, with aerial hyphae being scant but present on the 18-h plates and with pigmented antibiotic visible by 24 h. In contrast, bldD transcripts were expressed at much higher levels and were approximately equally abundant in all RNA time point samples isolated from the bldD mutant strain S. coelicolor HU66. This apparent overexpression of bldD transcripts in the mutant lacking functional BldD protein suggests that the functional product plays a role in negative regulation of its own expression. The use of a 16S rRNA probe to compare RNA loading levels and RNA integrity showed that all sample lanes (except that for the E. coli, control RNA) had intact RNA and that the loading levels did not differ substantially among the lanes (Fig. 4).
DISCUSSION
As with many of the bld genes, mutation of bldD results in a global block in both antibiotic production and morphological differentiation, suggesting that the bldD gene product may exert a common regulatory influence over both processes. Perhaps the most compelling evidence in support of a regulatory role for BldD is the finding that bldD transcripts are overexpressed in the bldD mutant, suggesting that bldD is subject to negative autoregulation. Since negative autoregulation is an almost universal feature of positive transcription activators (27), and since bldD is required for the onset of antibiotic production and morphological differentiation, it seems likely that bldD exerts its global effects positively at the level of transcription of those pathways.
Although a comparison of the BldD amino acid sequence to those in the databases did not reveal any significant end-to-end similarity to known proteins, both a manual comparison with known prokaryotic HTH signature sequences and the use of the inverse folding procedure of Bowie et al. (4) to identify structural or functional domains within BldD did reveal a putative HTH DNA-binding motif near the C terminus of BldD. Since false positives are rarely seen when the inverse folding methodology is used (10, 23a), this finding, although it does not prove that the sequence adopts the structure, certainly warrants further experimental investigation of DNA-binding ability. Such studies will initially focus on the bldD promoter itself, since bldD appears to be autoregulatory. The secondary structure analysis also showed that the Pro/Gly-rich region near the center of the BldD protein (amino acid residues 71 to 83) exists as a disordered loop, suggesting that this region represents a junction between two distinct domains. Many bacterial transcription regulatory factors contain receiver and transmitter domains joined by flexible connectors (reviewed in reference 23).
It is interesting that the bldD53 mutation affects a tyrosine residue outside the putative HTH region and N terminal to the proposed hinge region, at position 62. The loss of this single tyrosine not only abolishes BldD activity but also results in vast overexpression of the bldD transcript, implying that it plays a critical role in either the function or the stability of the protein. A wide variety of regulatory proteins have been found to contain tyrosine residues that are essential for full activity. In E. coli, tyrosine residues have been shown to play a critical role in activation of chemotactic and other regulatory proteins (15, 42). In eukaryotes, tyrosine residues, often in a phosphorylated form, have been found to be critical for protein dimerization and other protein-protein interactions that contribute to signaling cascades (26, 33, 40). Further analysis of BldD stability, as well as the interaction of the wild-type and mutant forms of BldD with the bldD promoter, should shed some light on how the bldD53 mutation manifests its effects.
At the outset of this investigation, it was hoped that knowledge of the nature of BldD would help to establish its specific role in the onset of antibiotic production and differentiation. While it is possible that one aspect of its role may be, as proposed by Willey et al. (38), the direct activation of transcription of a peptide synthetase responsible for SapB production, it is clear that SapB expression is not the only target of BldD action. Since bldD mutants are pleiotropically defective in antibiotic production, and since they show defects in catabolite repression (25), the nature of the molecular interactions is undoubtedly more complex. At present little is known about the interconnections among the wide variety of pleiotropic genes that have been identified in S. coelicolor, including the bld genes that fall outside of the extracellular complementation scheme proposed by Willey et al. (38). Of special note for the purposes of this study are the bldD and bldB genes. bldD and bldB mutants both are pleiotropically defective in antibiotic production and morphological differentiation, yet their phenotypes are distinct: they show differences in carbon source dependency of aerial mycelium formation, in carbon catabolite deregulation (25), and in ADP-ribosylated protein profiles (31), and the bldB mutants do not fit into the extracellular complementation cascade. Despite this, the BldB and BldD proteins have remarkably similar features (see the accompanying report [25a]). Although they are clearly not homologs, and BldB is much smaller than BldD (only 10.8 kDa, compared to 18.2 kDa for BldD), both proteins have putative HTH sequences near their C termini, and both have tyrosine residues that appear to play a role in their function. While the differences seen between the bldB and bldD mutant phenotypes could suggest that these proteins are part of independent pathways for the activation of antibiotic production and differentiation, this remains to be determined. A model for the roles that they play cannot be formulated until we have a more thorough knowledge of the targets of BldD and BldB action and, on a broader scale, of the interplay between them and other known pleiotropic regulators.
ACKNOWLEDGMENTS
This work was supported by the Alberta Heritage Foundation for Medical Research and the Natural Sciences and Engineering Research Council of Canada and by the former Agricultural and Food Research Council of Great Britain and the John Innes Foundation.
We are very grateful to Gregory Petsko, Brandeis University, Waltham, Mass., for analyzing the BldD sequence for secondary structural elements and Jan Westpheling for critical reading of the manuscript. We thank Leigh Ann Giebelhaus for technical assistance, and we thank George Owttrim and Bill Klimke for assistance with the GCG programs.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bibb M J, Findlay P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use in the simple and reliable identification of protein coding sequences. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 3.Bierman M, Logan R, O’Brien K, Seno E T, Rao N, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 4.Bowie J U, Lüthy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991;253:164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- 5.Champness W C. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol. 1988;170:1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chater K F, Bruton C J, King A A, Suárez J E. The expression of Streptomyces and Escherichia coli drug resistance determinants cloned into the Streptomyces phage φC31. Gene. 1982;19:21–32. doi: 10.1016/0378-1119(82)90185-8. [DOI] [PubMed] [Google Scholar]
- 7.Chater K F, Merrick M J. Streptomycetes. In: Parish J H, editor. Developmental biology of prokaryotes. Vol. 1. Oxford, England: Blackwell; 1979. pp. 93–114. [Google Scholar]
- 8.Davis N K, Chater K F. The Streptomyces coelicolor whiB gene encodes a small transcription factor-like protein dispensable for growth but essential for sporulation. Mol Gen Genet. 1992;232:351–358. doi: 10.1007/BF00266237. [DOI] [PubMed] [Google Scholar]
- 9.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer D, Eisenberg D. Protein fold recognition using sequence-derived predictions. Protein Sci. 1996;5:947–955. doi: 10.1002/pro.5560050516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henikoff S, Haughn G W, Calvo J M, Wallace J C. A large family of bacterial activator proteins. Proc Natl Acad Sci USA. 1988;85:6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopwood D A, Bibb M J, Chater K F, Kieser T. Plasmid and phage vectors for gene cloning and analysis in Streptomyces. Methods Enzymol. 1987;153:116–166. doi: 10.1016/0076-6879(87)53052-x. [DOI] [PubMed] [Google Scholar]
- 13.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, England: John Innes Foundation; 1985. [Google Scholar]
- 14.Janssen G R, Bibb M J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993;124:133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- 15.Kanamaru K, Mizuno T. Signal transduction and osmoregulation in Escherichia coli: a novel mutant of the positive regulator, OmpR, that functions in a phosphorylation-independent manner. J Biochem. 1992;111:425–430. doi: 10.1093/oxfordjournals.jbchem.a123773. [DOI] [PubMed] [Google Scholar]
- 16.Lawlor E J, Baylis H A, Chater K F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2) Genes Dev. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 17.Leskiw B K, Mah R, Lawlor E J, Chater K F. Accumulation of bldA-specified tRNA is temporally regulated in Streptomyces coelicolor A3(2) J Bacteriol. 1993;175:1995–2005. doi: 10.1128/jb.175.7.1995-2005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacNeil D J, Gewain K M, Ruby C L, Dezeny G, Gibbons P H, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- 19.Merrick M J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976;96:299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- 20.Miller K. Gel electrophoresis of RNA. Focus. 1987;9:14–15. [Google Scholar]
- 21.Munro S, Pelham H R B. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 22.Nodwell J R, McGovern K, Losick R. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol Microbiol. 1996;22:881–893. doi: 10.1046/j.1365-2958.1996.01540.x. [DOI] [PubMed] [Google Scholar]
- 23.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 23a.Petsko, G. Personal communication.
- 23b.Piret, J., and M. Harasym. Unpublished data.
- 24.Plaskitt K A, Chater K F. Influences of developmental genes on localized glycogen deposition in colonies of a mycelial prokaryote, Streptomyces coelicolor A3(2): a possible interface between metabolism and morphogenesis. Philos Trans R Soc Lond B. 1995;347:105–121. [Google Scholar]
- 25.Pope M K, Green B D, Westpheling J. The bld mutants of Streptomyces coelicolor are defective in the regulation of carbon utilization, morphogenesis and cell-cell signalling. Mol Microbiol. 1996;19:747–756. doi: 10.1046/j.1365-2958.1996.414933.x. [DOI] [PubMed] [Google Scholar]
- 25a.Pope M K, Green B, Westpheling J. The bldB gene encodes a small protein required for morphogenesis, antibiotic production, and catabolite control in Streptomyces coelicolor. J Bacteriol. 1998;180:1556–1562. doi: 10.1128/jb.180.6.1556-1562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachez C, Sautiére P, Formstecher P, Lefebvre P. Identification of amino acids critical for the DNA binding and dimerization properties of the human retinoic acid receptor α. J Biol Chem. 1996;271:17996–18006. doi: 10.1074/jbc.271.30.17996. [DOI] [PubMed] [Google Scholar]
- 27.Raibaud O, Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- 28.Rao R N, Allen N E, Hobbs J N, Alborn W E, Kirst H A, Paschal J W. Genetic and enzymatic basis of hygromycin B resistance in Escherichia coli. Antimicrob Agents Chemother. 1983;24:689–695. doi: 10.1128/aac.24.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shima J, Penyige A, Ochi K. Changes in patterns of ADP-ribosylated proteins during differentiation of Streptomyces coelicolor A3(2) and its developmental mutants. J Bacteriol. 1996;178:3785–3790. doi: 10.1128/jb.178.13.3785-3790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strohl W R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992;20:961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Geer P, Wiley S, Gish G D, Lai V K-M, Stephens R, White M F, Kaplan D, Pawson T. Identification of residues that control specific binding of the Shc phosphotyrosine-binding domain to phosphotyrosine sites. Proc Natl Acad Sci USA. 1996;93:963–968. doi: 10.1073/pnas.93.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viale A M, Kobayashi H, Akazawa T, Henikoff S. rcbR, a gene coding for a member of the LysR family of transcriptional regulators, is located upstream of the expressed set of ribulose 1,5-bisphosphate carboxylase/oxygenase genes in the photosynthetic bacterium Chromatium vinosum. J Bacteriol. 1991;173:5224–5229. doi: 10.1128/jb.173.16.5224-5229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 36.Ward J M, Janssen G R, Kieser T, Bibb M J, Buttner M J, Bibb M J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986;203:468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- 37.Willey J, Santamaría R, Guijarro J, Geistlich M, Losick R. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation. Cell. 1991;65:641–650. doi: 10.1016/0092-8674(91)90096-h. [DOI] [PubMed] [Google Scholar]
- 38.Willey J, Schwedock J, Losick R. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 1993;7:895–903. doi: 10.1101/gad.7.5.895. [DOI] [PubMed] [Google Scholar]
- 39.Williams J G, Mason P J. Hybridization in the analysis of RNA. In: Hames B D, Higgens S J, editors. Nucleic acid hybridization. A practical approach. Oxford, England: IRL Press; 1985. pp. 139–178. [Google Scholar]
- 40.Wu R-Y, Durick K, Songyang Z, Cantley L C, Taylor S S, Gill G N. Specificity of LIM domain interactions with receptor tyrosine kinases. J Biol Chem. 1996;271:15934–15941. doi: 10.1074/jbc.271.27.15934. [DOI] [PubMed] [Google Scholar]
- 41.Zhen L, Swank R T. A simple and high yield method for recovering DNA from agarose gels. BioTechniques. 1993;14:894–898. [PubMed] [Google Scholar]
- 42.Zhu X, Amsler C D, Volz K, Matsumura P. Tyrosine 106 of CheY plays an important role in chemotaxis signal transduction in Escherichia coli. J Bacteriol. 1996;178:4208–4215. doi: 10.1128/jb.178.14.4208-4215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoller M J, Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol. 1987;154:329–349. doi: 10.1016/0076-6879(87)54083-6. [DOI] [PubMed] [Google Scholar]