Abstract
Exposure to ambient air pollution, especially particulate matter with aerodynamic diameter <2.5 μm (PM2.5) and nitrogen dioxide (NO2), are environmental risk factors for Alzheimer’s disease and related dementia. The medial temporal lobe (MTL) is an important brain region subserving episodic memory that atrophies with age, during the Alzheimer’s disease continuum, and is vulnerable to the effects of cerebrovascular disease. Despite the importance of air pollution it is unclear whether exposure leads to atrophy of the MTL and by what pathways. Here we conducted a longitudinal study examining associations between ambient air pollution exposure and MTL atrophy and whether putative air pollution exposure effects resembled Alzheimer’s disease-related neurodegeneration or cerebrovascular disease-related neurodegeneration.
Participants included older women (n = 627; aged 71–87) who underwent two structural brain MRI scans (MRI-1: 2005–6; MRI-2: 2009–10) as part of the Women’s Health Initiative Memory Study of Magnetic Resonance Imaging. Regionalized universal kriging was used to estimate annual concentrations of PM2.5 and NO2 at residential locations aggregated to 3-year averages prior to MRI-1. The outcome was 5-year standardized change in MTL volumes. Mediators included voxel-based MRI measures of the spatial pattern of neurodegeneration of Alzheimer’s disease (Alzheimer’s disease pattern similarity scores [AD-PS]) and whole-brain white matter small-vessel ischemic disease (WM-SVID) volume as a proxy of global cerebrovascular damage. Structural equation models were constructed to examine whether the associations between exposures with MTL atrophy were mediated by the initial level or concurrent change in AD-PS score or WM-SVID while adjusting for sociodemographic, lifestyle, clinical characteristics, and intracranial volume.
Living in locations with higher PM2.5 (per interquartile range [IQR]=3.17μg/m3) or NO2 (per IQR=6.63ppb) was associated with greater MTL atrophy (βPM2.5 = −0.29, 95% confidence interval [CI]=[−0.41,−0.18]; βNO2 =−0.12, 95%CI=[−0.23,−0.02]). Greater PM2.5 was associated with larger increases in AD-PS (βPM2.5 = 0.23, 95%CI=[0.12,0.33]) over time, which partially mediated associations with MTL atrophy (indirect effect= −0.10; 95%CI=[−0.15, −0.05]), explaining approximately 32% of the total effect. NO2 was positively associated with AD-PS at MRI-1 (βNO2=0.13, 95%CI=[0.03,0.24]), which partially mediated the association with MTL atrophy (indirect effect= −0.01, 95% CI=[−0.03,−0.001]). Global WM-SVID at MRI-1 or concurrent change were not significant mediators between exposures and MTL atrophy.
Findings support the mediating role of Alzheimer’s disease-related neurodegeneration contributing to MTL atrophy associated with late-life exposures to air pollutants. Alzheimer’s disease-related neurodegeneration only partially explained associations between exposure and MTL atrophy suggesting the role of multiple neuropathological processes underlying air pollution neurotoxicity on brain aging.
Keywords: air pollution, medial temporal lobe, Alzheimer’s disease, cerebrovascular disease, epidemiology
Introduction
Cognitive impairment leading to Alzheimer’s disease and related dementias disproportionately impacts women,1 and is among the leading causes of disability and death worldwide.2 Clinical dementia stems from underlying neurologic disease which lead to neurodegeneration several years prior to cognitive and functional deficits.3 The medial temporal lobe (MTL), consisting of the hippocampus, parahippocampal gyrus, entorhinal cortex, and amygdala, is an important brain structure subserving episodic memory.4 After age 70, healthy older adults lose approximately 1.5–2% of their MTL volume annually on average.5 Individuals with Alzheimer’s disease, the most common dementia etiology, experience more than double the rate of MTL atrophy compared to healthy older adults.5 Although given less attention, atrophy of the MTL likely also occurs in vascular dementia and stroke which is the second most common cause of dementia.6–8 Various neurodegenerative disease processes differentially impact the various MTL subregions. For example, the entorhinal cortex (ERC) is the first MTL subregion impacted by Alzheimer’s disease.9 Volumetric changes to the ERC may also be pronounced, compared to other MTL structures in normal aging,10 are associated with longitudinal memory changes in cognitively normal individuals,11 and may also be an important biomarker associated with other dementia etiologies.12 Identifying environmental factors that contribute to MTL atrophy during the preclinical disease stage is important for dementia prevention.
Accumulating evidence has shown that late-life exposure to ambient air pollution is an environmental risk factor for dementia.13,14 Despite the importance of the MTL across the various dementia etiologies, there have been few studies examining associations between exposure to air pollution in later-life on atrophy of the MTL with most studies providing mixed findings.15–21 The pathways by which air pollution exposure may lead to the atrophy of the MTL in cognitively healthy individuals are also unclear. These pathways may include Alzheimer’s disease-related neurodegeneration, cerebrovascular disease, or other neuropathological factors. Studies with animals and humans report a link between air pollution exposure and Alzheimer’s disease neuropathology.22 Our previous epidemiological work also reports a link between air pollution and increased Alzheimer’s disease-related neurodegeneration over a five-year period.23,24 A larger body of studies has demonstrated the toxic effects of air pollution exposure on the vascular system including a link between air pollution and cerebrovascular disease.25,26 Air pollution exposure may also exert a neurotoxic effect on the MTL through other pathways as studies report links between air pollution exposure and other neuropathological factors implicated with MTL atrophy.27,28 Examining if putative associations between air pollution exposure and MTL atrophy resemble patterns of neurodegeneration consistent with Alzheimer’s disease or cerebrovascular disease will provide a better understanding of the neurotoxic effects of air pollution on the brain.
To address the knowledge gaps, the current study examined the longitudinal associations between ambient air pollution exposure and MTL atrophy in older women without dementia. We then examined whether putative air pollution exposure effects resembled AD-related neurodegeneration or cerebrovascular disease-related neurodegeneration.
Materials and methods
Participants and study design
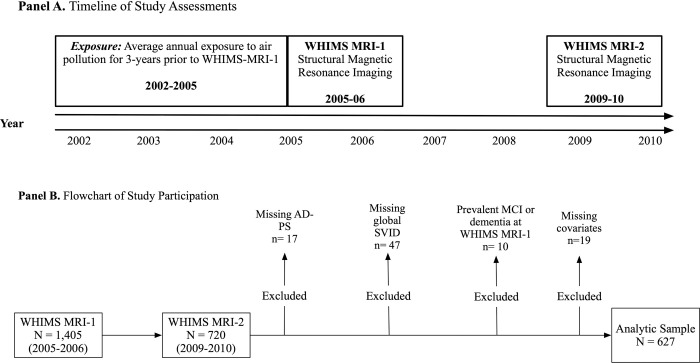
We analyzed longitudinal data from 627 community-dwelling older women who participated in the MRI substudy of the larger Women’s Health Initiative (WHI) Memory Study (WHIMS; n=7,479, age 65–79), which was an ancillary study of the WHI clinical trial of hormone therapy for postmenopausal women. Between April 2005 and January 2006, 1405 WHIMS participants, across 14 geographically diverse WHI centers, underwent a structural MRI scan (MRI-1). Between 2009–2010, 720 women completed a follow-up structural MRI scan (MRI-2). Since this study focused on mechanisms of exposure-related MTL atrophy at the preclinical stage of Alzheimer’s disease and related dementias, we excluded 11 women with probable mild cognitive impairment (MCI) or probable dementia at MRI-1 as determined through neuropsychological evaluation and clinical adjudication. Women with missing data on the mediators (n=64), and covariates (n=19) were also excluded, rendering a final analytic sample of 627 cognitively unimpaired older women. See Figure 1 for a timeline of study assessments (Panel A) and a flowchart of study participation (Panel B).
Figure 1.
A timeline of study assessments is presented in Panel A while a flowchart of study participation is presented in Panel B.
Air pollution exposure estimates
Participant residential address data were collected prospectively at each WHI assessment and geocoded.29 Regionalized universal kriging models30,31 were used to estimate the annual mean concentrations of ambient PM2.5 (in μg/m3) and NO2 (in ppb) at the participant’s residence for the 3 years prior to MRI-1. The models used US Environmental Protection Agency (EPA) monitoring data and over 300 geographic covariates in a partial least-squares regression to estimate air pollution exposure. The model includes geographic covariates (e.g., population density, distance to roads, and vegetation in the vicinity, etc.) to estimate exposures. These models are reliable and valid with high cross-validation with an R2 of 0.88 for PM2.5 and 0.85 for NO2. We calculated a 3-year weighted average of air pollution with the length of stay at each residential location within the 3-year time window as a weight to account for residential mobility.
Structural MRI acquisition and processing
Participants completed two structural MRI brain scans on 1.5T scanners at one of 14 WHIMS clinical centers using a standardized protocol developed by the WHIMS-MRI Quality Control Center at the University of Pennsylvania. The scan series for volumetric imaging used a 22cm field of view and a 256×256 acquisition matrix and the following image series: oblique axial spin density/T2-weighted spin echo images, oblique axial fast fluid-attenuated inversion recovery (FLAIR) T2-weighted spin echo image and oblique axial fast spoiled 3D T1-weighted gradient echo images. Details about the pulse sequence parameters were published previously.32,33
MTL regions were extracted using a multi-atlas region segmentation (MUSE) that transformed site-specific labeled atlases into a harmonized map.34,35 MUSE follows a voxel-based spatial adaptation strategy to transform multiple atlases with different warping algorithms and regularization parameters into an ensemble-based parcellation of anatomical reference labels to harmonize the T1-weighted volumetric images. This approach leads to robust segmentation accuracy and is superior to other multi-atlas segmentation and label fusion methods, especially for multi-site MRI investigations and longitudinal analyses.34,35 Total MTL volume was operationally defined as the summed bilateral gray matter volumes of the hippocampus, amygdala, parahippocampal gyrus (PHG), and entorhinal cortex (ERC). We estimated total MTL atrophy and subregional MTL atrophy by calculating the volume changes from MRI-1 to MRI-2 and standardized this change by the number of years between the two visits. Numerical changes were then further standardized on a z-score metric based on the means and standard deviations of the sample.
Assessment of Alzheimer’s disease-related neurodegeneration
We used Alzheimer’s disease pattern similarity (AD-PS) scores to estimate individual risk for Alzheimer’s disease-related neurodegeneration. AD-PS scores are metrics that reflect the degree of spatial similarity between a participant’s gray matter and the pattern of gray matter in individuals with Alzheimer’s disease. Scores were calculated using data-driven methods developed and validated using data from the Alzheimer’s disease neuroimaging initiative (ADNI).36 Briefly, a high-dimensional elastic net regularized logistic regression approach was used to derive a voxel-based model discriminating between cognitively unimpaired controls and those with clinically diagnosed dementia due to Alzheimer’s disease in ADNI based on gray matter probability maps. The class-conditional probabilities of membership to the Alzheimer’s disease group are called AD-PS scores. Discriminative maps derived from the model’s coefficients, which characterize the spatial pattern of gray matter differences, covered Alzheimer’s disease-vulnerable brain regions including the amygdala, hippocampus, PHG, thalamus, inferior temporal areas, and midbrain. Detailed technical information is provided elsewhere.37 The resulting AD-PS score can be interpreted as a measure of Alzheimer’s disease anatomical risk, with higher scores indicating higher Alzheimer’s disease risk. AD-PS scores were calculated at both MRI-1 and MRI-2. Our primary metrics of interest were AD-PS at MRI-1 and the change in AD-PS scores between the MRI visits.
Assessment of Small Vessel Ischemic Disease
To assess the potential mediating role of white matter small vessel ischemic disease (WM-SVID), white matter lesions were segmented using a deep learning based segmentation, DeepMRSeg.38 White matter was classified as normal or “abnormal”, and abnormal voxels were summed across regions to represent a global measure of WM-SVID volume.
Covariates of interest
At the WHI inception (1993–1998), a structured questionnaire was administered to gather information on the geographic region of residence (Northeast, South, Midwest, West), age, race/ethnicity (“White, non-Hispanic” and “Other ethnic backgrounds”), socioeconomic factors (education, family income, and employment status), and lifestyle factors (smoking status, alcohol use, and physical activity), and self-reported use of postmenopausal hormones. Body mass index (BMI) was determined from measured height and weight and depressive symptoms were recorded using the Center for Epidemiologic Studies Depression Scale-short form. Self-reported histories of cardiovascular disease (CVD; e.g., heart problems, problems with blood circulation, blood clots) and related risk factors (e.g., hypertension, treatment-dependent hypercholesterolemia, diabetes mellitus) were assessed using valid and reliable measures.39,40 As very few women endorsed a history of diabetes or hypercholesterolemia, we created a binary variable to indicate any versus no history of CVD to boost statistical power. In addition to the covariates collected at WHI inception, lifestyle factors, BMI, hypertension, and history of CVD were also updated at MRI-1. Socioeconomic status (SES) of residential neighborhoods was characterized using US Census tract-level data and estimated at both WHI inception and MRI-141 ; a higher neighborhood SES score indicated a more socioeconomically-favorable neighborhood.
Statistical analysis
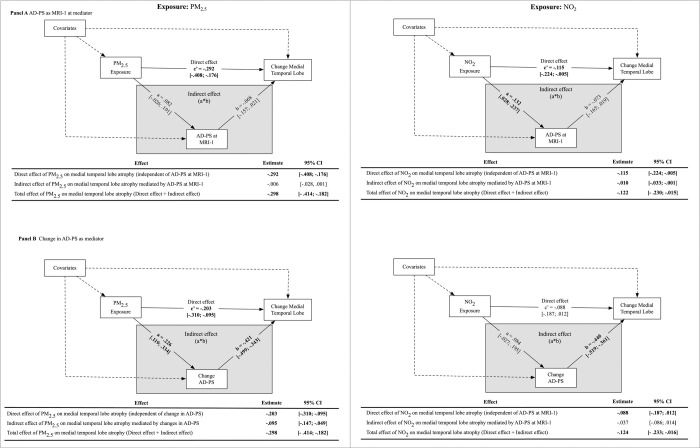
Multivariable linear regressions were used to estimate the associations between air pollution exposures and MTL volume changes in total MTL volume while adjusting for sociodemographic variables, lifestyle factors, clinical characteristics, and intracranial volume. We estimated the association between PM2.5 and NO2 on MTL volume change in separate models. Structural equation models (SEMs) were used to test four putative mediators of exposure-related MTL volume change in separate models: (1) AD-PS score at MRI-1, (2) 5-year change in AD-PS, (3) SVID volume at MRI-1, and (4) 5-year change in SVID volume. The SEM mediation used standard methods to estimate direct and indirect exposure effects on MTL volumes over time (Figure 2). The direct effect was defined as the effect of exposures on MTL change independent of the hypothesized mediator (Figure 2, path c). The indirect effect was defined as the effect of exposures on MTL change explained by the hypothesized mediator, which is a product of the exposure effect on the mediator (Figure 2, path a) and the effect of the mediator on MTL changes (Figure 2, path b). The total effect of exposures on MTL volume changes is the sum of the direct and indirect effects. All estimated parameters were adjusted for sociodemographic, lifestyle, clinical covariates, and ICV (Figure 2). The 95% confidence interval (CI) of the indirect effect was calculated using 5,000 bootstrapped samples. Mediation analyses were conducted using the MPLUS Automation package42 in R.
Figure 2.
Simplified illustration of the structural equation model (SEM) to estimate whether Alzheimer’s disease neurodegeneration or global small-vessel-ischemic disease mediate observed associations between air pollution and medical temporal lobe (MTL) atrophy.
Exploratory analyses assessed whether the magnitude of the observed mediations differed across MTL subregions. We conducted these analyses because prior studies suggest that air pollution exposure may differentially impact the various MTL subregions.21 Additionally, studies suggest heterogeneous patterns of atrophy across the various MTL subregions across various neurodegenerative disease processes.
Data availability
Access to all data elements used in this study may be made available following the established WHI policies.
Results
Descriptive characteristics of the sample
Participants were mostly non-Hispanic White women (n=592, 94%) with an average age of 77.42 ± 3.49 years. Table 1 compares the distribution of the 3-year average PM2.5 and NO2 exposures prior to MRI-1 by population characteristics. Compared to non-Hispanic White women, women who self-identified with other race/ethnic backgrounds tended to reside in locations with higher NO2 and PM2.5 exposures. Compared to women living in the South, women living in the Northeast and Midwest had higher PM2.5 exposures. Older women living in the South had lower NO2 exposures compared to those in other regions. Women who lived in the most socioeconomically-unfavorable neighborhoods at WHI inception or reported a history of postmenopausal hormone therapy had higher PM2.5 exposures compared to their counterparts, whereas women living in the most socioeconomically-favorable neighborhoods at WHI inception had higher NO2 exposures. Compared to their counterparts, higher NO2 exposures were also observed in current smokers, women who consumed one or more alcoholic drinks per day, or those with BMI<25 kg/m2.
Table 1.
Distribution of air pollution exposures by population characteristics in the Women’s Health Initiative Memory Study MRI cohort (N =627).
| PM2.5 (μg/m3) | NO2 (ppb) | ||||||
|---|---|---|---|---|---|---|---|
| Population Characteristics | N | mean ±SD | (25th, 50th, 75th) | p | mean ±SD | (25th, 50th, 75th) | p |
|
| |||||||
| Overall | 627 | 11.16 ±2.29 | (9.6, 11.2, 12.7) | 11.87 ±5.24 | (8.2, 11, 14.9) | ||
| Sociodemographic | |||||||
| Age at MRI-1 visit (years) | .41 | .02 | |||||
| 70–74 | 178 | 11.06 ±2.42 | (9.4, 10.6, 12.8) | 11.02 ±4.87 | (7.9, 10.5, 13.5) | ||
| 75–79 | 302 | 11.11 ±2.25 | (9.7, 11.2, 12.7) | 11.84 ± 5.06 | (8.2, 11.1, 15.0) | ||
| 80+ | 147 | 11.38 ± 2.20 | (9.8, 11.4, 12.8) | 12.96 ± 5.82 | (9.0, 11.4, 15.7) | ||
| Region of residence | <.01 | <.01 | |||||
| Northeast | 164 | 11.61 ± 2.39 | (9.4, 11.5, 13.9) | 13.25 ± 7.30 | (8.5, 10.9, 15.2) | ||
| South | 74 | 11.13 ± 1.63 | (9.6, 11.4, 12.4) | 7.87 ± 2.46 | (6.2, 8.0, 9.7) | ||
| Midwest | 231 | 11.48 ± 2.02 | (9.8, 11.3, 13.2) | 11.42 ± 3.56 | (8.5, 11.0, 14.3) | ||
| West | 158 | 10.24 ± 2.55 | (9.3, 11.0, 12.0) | 12.96 ± 4.64 | (9.4, 14.1, 16.5) | ||
| Race/Ethnicity | <.01 | <.01 | |||||
| White (not Hispanic) | 592 | 11.09 ± 2.31 | (9.5, 11, 12.7) | 11.57 ± 4.89 | (8.1, 10.9, 14.7) | ||
| Others | 35 | 12.35 ± 1.43 | (11.3, 12.5, 13.4) | 17.00 ± 7.77 | (11.1, 15.3, 18.8) | ||
| Formal education | .31 | .06 | |||||
| <= HS/GED | 190 | 11.27 ± 2.35 | (9.7, 11.1, 12.9) | 11.12 ± 4.33 | (8.2, 10.7, 14) | ||
| HS/GED but <4 yr college | 235 | 10.98 ± 2.23 | (9.5, 11.2, 12.6) | 12.07 ± 5.33 | (8.1, 11.2, 15.3) | ||
| >=Bacehlor’s degree | 202 | 11.27 ± 2.3 | (9.7, 11.2, 12.9) | 12.34 ± 5.82 | (8.5, 11.2, 15.3) | ||
| Employment status | .46 | .43 | |||||
| Currently working | 84 | 11.10 ± 2.43 | (9.6, 11.1, 12.8) | 12.53 ± 6.25 | (8.5, 11.0, 15.3) | ||
| Not working | 59 | 11.51 ± 2.24 | (10.2, 12, 13.1) | 12.03 ± 4.05 | (8.7, 11.9, 14.9) | ||
| Retired | 484 | 11.13 ± 2.27 | (9.6, 11, 12.7) | 11.74 ± 5.17 | (8.1, 10.9, 14.9) | ||
| Household income | .67 | .09 | |||||
| <35,000 | 307 | 11.17 ± 2.41 | (9.6, 11.4, 12.7) | 11.42 ± 4.96 | (7.8, 10.7, 14.3) | ||
| 35,000 to 74,999 | 240 | 11.10 ± 2.19 | (9.6, 11.1, 12.7) | 12.13 ± 5.42 | (8.4, 11.1, 15) | ||
| >=75,000 | 56 | 11.48 ± 2.07 | (10.2, 11.4, 13) | 13.22 ± 5.6 | (9.1, 12.2, 16) | ||
| don’t know | 24 | 10.90 ± 2.32 | (9.2, 10.2, 12.8) | 11.86 ± 5.43 | (8.9, 11.1, 14) | ||
| Neighborhood socioeconomic characteristics | .03 | <.01 | |||||
| <−3.63 | 151 | 11.61 ± 2.39 | (10.4, 12, 13.2) | 11.99 ± 6.19 | (7.8, 10.8, 14.8) | ||
| >= −3.63 and < −.26 | 162 | 11.19 ± 2.42 | (9.5, 11, 13.1) | 10.30 ± 4.04 | (7.6, 9.7, 12.0) | ||
| >=−.26 and <3.16 | 155 | 10.95 ± 2.31 | (9.4, 10.8, 12.4) | 11.81 ± 5.19 | (8.2, 11.1, 14.8) | ||
| >= 3.16 | 159 | 10.91 ± 1.98 | (9.6, 10.9, 12.1) | 13.41 ± 4.94 | (10, 13, 16.2) | ||
| Lifestyle | |||||||
| Smoking status | .85 | <.01 | |||||
| Never smoked | 367 | 11.2 ± 2.24 | (9.6, 11.3, 12.7) | 11.27 ± 4.27 | (8, 10.7, 14.4) | ||
| Past smoker | 235 | 11.09 ± 2.35 | (9.6, 11, 12.7) | 12.5 ± 6.05 | (8.4, 11.3, 15) | ||
| Current smoker | 25 | 11.24 ± 2.55 | (9.9, 11, 12.9) | 14.77 ± 7.84 | (10.2, 13.4, 17.1) | ||
| Alcohol use | .52 | .04 | |||||
| Non-drinker | 80 | 11.4 ± 2.05 | (9.8, 11.6, 12.8) | 10.41 ± 3.78 | (7.5, 9.6, 13.3) | ||
| Past drinker | 103 | 11.35 ± 2.47 | (9.8, 11.6, 13.2) | 11.87 ± 5.07 | (8.4, 10.8, 15.2) | ||
| <1 drink per day | 370 | 11.07 ± 2.31 | (9.5, 11, 12.7) | 12.02 ± 5.48 | (8.4, 11, 15.1) | ||
| >= 1 drink per day | 74 | 11.1 ± 2.21 | (10, 11, 12.3) | 12.68 ± 5.38 | (9.1, 12.3, 15.1) | ||
| Moderate to strenuous physical activity ≥ 20 minutes | .30 | .94 | |||||
| No activity | 344 | 11.23 ± 2.34 | (9.7, 11.4, 12.7) | 11.85 ± 5.04 | (8.3, 11.1, 15) | ||
| Some activity | 33 | 11.49 ± 2.21 | (9.5, 11.4, 13.3) | 12.4 ± 6.81 | (7.4, 11, 14.9) | ||
| 2–4 episodes/week | 137 | 11.21 ± 2.19 | (9.7, 11.1, 12.8) | 11.9 ± 4.97 | (8.5, 11.2, 14.7) | ||
| <4 episodes/week | 113 | 10.79 ± 2.25 | (9.3, 10.7, 12.3) | 11.73 ± 5.65 | (7.9, 10.5, 14.9) | ||
| Physical Health/Clinical factors | |||||||
| Body Mass Index | .81 | .04 | |||||
| <25 | 177 | 11.14 ± 2.17 | (9.7, 11.1, 12.7) | 12.59 ± 5.80 | (8.5, 11.8, 15.3) | ||
| 25–29 | 253 | 11.23 ± 2.32 | (9.5, 11.2, 12.8) | 11.87 ± 5.02 | (8.5, 10.9, 14.9) | ||
| >=30 | 197 | 11.09 ± 2.36 | (9.5, 11.2, 12.7) | 11.21 ± 4.90 | (7.5, 10.8, 13.8) | ||
| Cardiovascular disease risk indicator | .99 | .37 | |||||
| No | 339 | 11.16 ± 2.28 | (9.7, 11.2, 12.7) | 11.69 ± 5.03 | (8.2, 10.9, 14.7) | ||
| Yes | 288 | 11.16 ± 2.3 | (9.5, 11.1, 12.8) | 12.08 ± 5.47 | (8.3, 11.3, 15.1) | ||
| Any prior postmenopausal hormone use | .03 | .37 | |||||
| No | 319 | 11.36 ± 2.18 | (9.7, 11.3, 12.9) | 12.05 ± 5.17 | (8.5, 11, 15.3) | ||
| Yes | 308 | 10.96 ± 2.38 | (9.5, 11, 12.6) | 11.68 ± 5.31 | (8.1, 11, 14.3) | ||
| WHI hormone therapy assignment | .63 | .08 | |||||
| CEE-alone placebo | 114 | 11.06 ± 2.49 | (9.8, 11.2, 12.8) | 11.77 ± 5.53 | (7.8, 11.2, 14.8) | ||
| CEE-alone | 114 | 11.23 ± 2.58 | (9.7, 11.4, 13.1) | 11.76 ± 5.01 | (8.4, 11.1, 15) | ||
| CEE+MPA placebo | 196 | 11.31 ± 2.09 | (9.7, 11.3, 12.6) | 12.62 ± 5.48 | (8.9, 11.4, 15.5) | ||
| CEE+MPA | 203 | 11.03 ± 2.19 | (9.3, 10.7, 12.7) | 11.26 ± 4.89 | (8.1, 10.4, 14.1) | ||
Total effects of air pollution exposures on MTL atrophy
Each interquartile range (IQR) increase in PM2.5 (IQR = 3.17 μg/m3) was associated with 0.29 (95% CI= [−0.41, −0.18]) greater MTL atrophy, in standard deviation units. Increases in NO2 (per IQR 6.63 ppb) were also associated with greater atrophy in MTL volume, however, the magnitude of the effect was smaller (β=−0.12; 95% CI= [−0.23, −0.02]).
AD-PS mediation models
Results of the SEMs examining the mediating roles of Alzheimer’s disease-related neurodegeneration are presented in Figure 3. Higher NO2 exposures were associated with larger AD-PS scores at MRI-1 (Figure 3 Panel A; β=0.13; 95% CI= [0.03, 0.24]). Accordingly, exposure-associated increase in AD-PS scores at MRI-1 partially mediated the effect of NO2 on MTL atrophy (indirect effect β= −0.10; 95% CI= [−0.03, −0.01]), explaining approximately 7% of the total effect of NO2. The AD-PS scores at MRI-1 did not significantly mediate observed associations between PM2.5 and MTL atrophy (indirect effect β= −0.06; 95% CI= [−0.28, 0.01]).
Figure 3.
Results of mediation models examining whether Alzheimer’s disease related neurodegeneration (AD-PS) at Women’s Health Initiative Memory Study (WHIMS) MRI-1 (Panel A) or changes in AD-PS from MRI-1 to MRI-2 mediate associations between air pollution exposure on medial temporal lobe (MTL) atrophy (N = 627).
Figure 3B demonstrates mediation models with 5-year standardized change in AD-PS scores as the hypothesized mediator. Higher PM2.5 exposures were associated with larger increases in AD-PS scores over time (β=0.23; 95% CI= [0.12, 0.33]), and larger AD-PS score change from MRI-1 to MRI-2 was associated with greater MTL atrophy (β=−0.42; 95% CI=[−0.50, −0.34]). AD-PS change scores significantly mediated the effect of PM2.5 on MTL atrophy (indirect effect β=−0.10; 95% CI= [−0.15, −0.05]), explaining approximately 32% of the total effect. NO2 was not significantly associated with changes in AD-PS scores (β=0.08; 95% CI=−0.03, 0.20]). Changes in AD-PS score explained approximately 30% of the total effect of NO2 on MTL atrophy, although this estimated indirect effect was only marginally significant (β= −0.04; 95% CI= [−0.09, 0.01]).
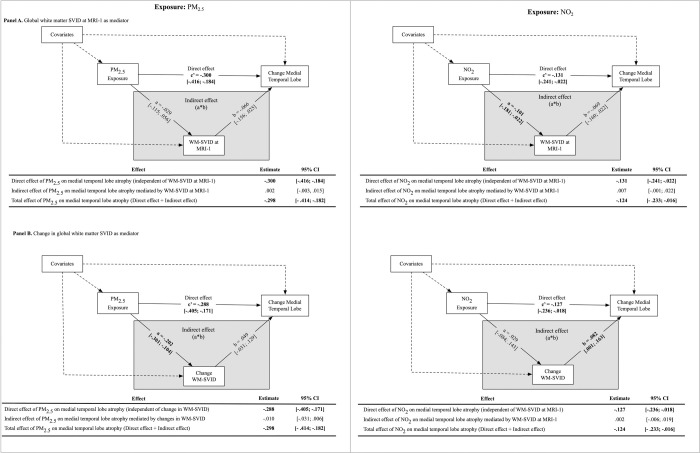
WM-SVID mediation models
Higher NO2 was associated with lower WM-SVID volume at MRI-1 (β=−0.10; 95% CI= [−0.18, −0.02]), and was not associated with WM-SVID volume changes over the 5-year period. By contrast, PM2.5 was not significantly associated with WM-SVID volume at MRI-1, but higher PM2.5 was associated with less increases WM-SVID volume over time (β=−0.20; 95% CI=[−0.30, −0.10]). Neither WM-SVID volume at MRI-1, nor change in WM-SVID volume over time, mediated the effect of either exposure on MTL atrophy (Figure 4).
Figure 4.
Results of mediation models examining whether global white matter small-vessel ischemic disease (WM-SVID) at Women’s Health Initiative Memory Study (WHIMS) MRI-1 (Panel A) or changes in global white matter small-vessel ischemic disease from MRI-1 to MRI-2 mediate associations between air pollution exposure on medial temporal lobe (MTL) atrophy (N = 627).
Secondary analysis of MTL subregions
We observed a heterogeneous effect of air pollution exposures across MTL subregions. Higher exposures of both PM2.5 (β=−0.44; 95% CI=[−0.56, −0.32]) and NO2 (β=−0.20; 95% CI=[−0.32, −0.09]) were associated with greater PHG atrophy over the 5-year period, whereas greater ERC atrophy was only associated with higher exposure to PM2.5 (β=−0.12; 95% CI=[−0.27, −0.01]), but not NO2 (β=−0.04; 95% CI=[−0.15, 0.06]). Neither exposure was significantly associated with volume changes in the amygdala or hippocampus.
There was a significant indirect effect of NO2 on ERC atrophy that was mediated by AD-PS scores at MRI-1, explaining approximately 47% of the total effect (Supplemental Fig. 1A). Change in AD-PS partially mediated the total effect of PM2.5 on ERC atrophy, explaining over 63% of the total effect (Supplemental Fig. 1B). Changes in AD-PS scores explained approximately 15% of the total effect of PM2.5 on PHG atrophy (Supplemental Figure 2). AD-PS change scores did not mediate the effect of NO2 on the ERC or PHG.
Discussion
The purpose of this study was to determine whether Alzheimer’s disease-related neurodegeneration or cerebrovascular damage explained the association between air pollution neurotoxicity and MTL atrophy in cognitively unimpaired older women. We found that higher NO2 and PM2.5 exposures were both associated with greater MTL atrophy over a 5-year period, which was partially explained by Alzheimer’s disease-related neurodegeneration but not WM-SVID. The effect of NO2 on MTL atrophy was only mediated by baseline AD-PS scores at MRI-1, whereas the effect of PM2.5 on MTL atrophy was only mediated by the change in AD-PS scores over time. Exploratory analyses showed that the mediation effect of AD-PS scores on exposure-related MTL atrophy was driven by cortical changes in the ERC and PHG, but not the amygdala or hippocampus. Collectively these results show that Alzheimer’s disease-related neurodegeneration, but not WM-SVID, contributes to MTL atrophy associated with late-life air pollution exposures in this sample of cognitively unimpaired older women.
Our results offer new insights into the explanatory mechanisms linking air pollution exposures to Alzheimer’s disease biomarkers and adverse cognitive outcomes that have been reported in prior work. The MTL is widely studied for its role in memory-related cognitive processes that can become impaired by Alzheimer’s disease neuropathological processes (e.g., neuroinflammation, lipid oxidation, abnormal beta-amyloid (Aβ) accumulation, tauopathy, neuronal damage, etc.). In rodents, air pollution exposures are associated with Alzheimer’s disease neuropathology in the MTL43–50, and contribute to impaired spatial learning and memory.44,48,51–54 Human studies also have linked exposures to Alzheimer’s disease biomarkers. Specifically, recent work from the Alzheimer’s & Families (ALFA) study revealed adverse associations. Higher NO2 and PM2.5 exposures were associated with higher brain fibrillar amyloid, as measured by higher centiloid values on positron emission tomography (PET) scans55 and with higher cerebrospinal fluid (CSF) levels of neurofilament light protein - a marker of neuronal injury. Ma et al.56 also linked PM2.5 exposures with CSF Aβ pathology in a large Chinese cohort of cognitively unimpaired adults.
Analyses of MTL subregions showed that the mediation effect of AD-PS change scores on PM2.5-related MTL atrophy was driven by cortical volume loss in the ERC and PHG, explaining over 60% and 15% of the total effects, respectively. This finding is consistent with numerous MRI studies that identify the ERC as one of the earliest brain regions to demonstrate structural damage and cell loss along the Alzheimer’s disease continuum.57–59 The ERC is also one of the earliest brain regions to exhibit pathological tau according to the original Braak & Braak model of Alzheimer’s disease neuropathology.60 Although the longitudinal effect of PM2.5 exposure on the ERC has not been studied in humans, mouse models of PM2.5 exposure have demonstrated increased levels of phosphorylated tau (p-tau) 61 and neuronal death62 in the ERC even at low levels of exposure.50,61,63 The PHG is also an initial site of preclinical brain atrophy and pathological tau deposition along the Alzheimer’s disease continuum64, yet our results showed that a large proportion (~85%) of PHG atrophy associated with PM2.5 exposure could not be explained by Alzheimer’s disease-like neurodegeneration. The neuropathological processes and cellular mechanisms underlying these regional differences are unclear, although there is preliminary evidence suggesting the PHG may be more sensitive to aging and mixed pathologies that also impact the MTL65, such as primary age-related tauopathy (PART)66 and transactive response DNA-binding protein 43 (TDP-43).67 However, literature on the neuropathological distinctions between Alzheimer’s disease, PART, and TDP-43 is still emerging, and more work is needed to understand the role of air pollution exposures on brain atrophy and cognition in the context of mixed pathology neurodegeneration.
An interesting finding of this study was the unique mediating effects of baseline AD-PS scores on NO2-related MTL atrophy versus the unique mediating effects of AD-PS change scores on PM2.5-related MTL atrophy. These results partially align with prior work from our group showing that the adverse effects of PM2.5 on cognitive outcomes in older women were partially explained by AD-PS change scores only (not baseline AD-PS scores), though NO2 exposure effects were not examined.23 The underlying mechanisms that contribute to pollutant-specific effects are unknown, but could be due to differences in epigenetic changes that are initiated by NO2 versus PM2.5 exposure. For example, a large study of middle-aged Dutch individuals revealed significant associations between NO2 exposure and genome-wide DNA methylation at several CpG sites on genes that influence lung function, but there were no genome-wide DNA methylation associations with PM2.5.68 In a separate epigenome-wide study of non-Hispanic White women in the U.S., higher NO2 exposure was associated with accelerated methylation-based “biological aging”, yet the effects of PM2.5 on biological aging depended on PM component profiles and the presence of other pollutants.69 Although the aforementioned studies did not focus on methylation patterns related to the brain, one possible interpretation of these studies and the results presented herein is that epigenetic changes activated by NO2 are involved in the initiation of Alzheimer’s disease pathological processes, whereas epigenetic and other neurobiological changes activated by PM2.5 are more closely linked to disease progression. In support of this hypothesis, the mediation effects of baseline AD-PS scores, rather than AD-PS change scores, on exposure-related MTL atrophy represent a stronger causal model of air pollution neurotoxicity since the predictor (exposures measured 3 years prior to MRI-1), mediator (baseline AD-PS at MRI-1) and outcome (MTL volume change over 5 years) variables were each measured at different time points.43 While differences in epigenetic changes offer an intriguing explanation for our pollutant-specific results, more work is needed to determine the role of pollutant-specific effects on DNA methylation in relation to brain integrity and Alzheimer’s disease-related neurodegeneration. Alternatively, NO2 and PM2.5 may simply represent surrogate markers for other pollutants with distinct neurotoxic properties.70–73
In contrast to the observed mediating effects of AD-PS scores on exposure-related MTL atrophy, WM-SVID was not significantly associated with and did not significantly mediate any of the observed associations between air pollution exposures and MTL atrophy in this study. Although the lack of association seems to contradict the extensive literature on the cardiovascular toxicity of airborne particles, several human studies have failed to find an association between exposures and MRI measures of WM-SVID.16,19,74,75 However, it is important to note that small vessel disease is an umbrella term for various pathologies of the brain microvascular system (e.g., lacunes, microbleeds, white matter hyperintensities (WMHs), enlarged perivascular spaces, etc.) that each requires unique neuroimaging techniques for detection.76 Prior studies (including the present study) of exposure-related SVD have focused on WMHs due to their high prevalence in older age and relative ease of measurement, but WMHs may not capture microvascular brain damage that results from air pollution neurotoxicity. The present study did not have access to alternative MRI markers of SVID that may mediate exposure-related MTL atrophy, but this is an important research direction for future work. The older age and generally good health of the sample may also indicate a survivor bias, such that individuals with significant WM-SVID would be deceased or cognitively impaired, and therefore would not meet the inclusion criteria for this study. We attempted to adjust for this potential bias in our analyses, but the lack of mediating effect remained.
This study has several major strengths. First, our longitudinal design allowed us to examine the mechanisms linking air pollution exposures to intraindividual changes in MTL volume, providing greater insight into the potential causal pathways of exposure-related MTL atrophy. Second, our richly phenotyped sample allowed us to adjust our mediation models for numerous potential confounding variables (e.g., socioeconomic, lifestyle, health factors, etc.) that have been associated with MTL atrophy in prior work. Third, the MUSE MRI protocol is another strength of our design as it uses an ensemble brain atlas for image segmentation that mitigates the impact of site-specific sources of noise. Finally, our serial mediation analyses allowed us to demonstrate the complete neuropathophysiological cascade linking exposures to increased, anatomical Alzheimer’s disease risk, greater MTL atrophy, and worse cognitive performance on tests of memory, which has not been done previously.
There are several limitations of this study that should also be acknowledged. First, results in this older all-female sample may not generalize to males or younger women. Participants were also in generally good health, which may limit generalizability to the broader population. Second, we cannot make inferences about the potential role of air pollution exposures on Alzheimer’s disease or SVID-related MTL atrophy during midlife as this study focused on neuroimaging associations with air pollution exposures during late life only. This may also explain the lack of a mediating effect of WM-SVID on MTL atrophy, as midlife vascular risk burden (rather than late life) is linked to greater late-life Alzheimer’s disease brain pathology77, hypoperfusion78, and brain volume.79 Third, our measure of anatomical Alzheimer’s disease risk did not account for individual differences in amyloid or tau positivity status as these biomarkers were not available for analysis. Fourth, we do not know whether structural changes in white matter contributed to exposure-related MTL atrophy as AD-PS scores were based on the spatial pattern of gray matter degeneration only. Finally, our measure of ambient air pollution exposures did not account for personal exposures at other locations (e.g., occupational), but it is unlikely this influenced the results as epidemiologic studies have shown that the associations between ambient exposure and health outcomes are not confounded by other sources of environmental pollutants.80–83
Conclusions
Findings from the present study of cognitively unimpaired older women provide insight into the neuropathological processes by which ambient air pollution exposures contribute to MTL atrophy and subsequent cognitive difficulties. Our data support the role of AD-like neurodegeneration, but not SVID, as a putative pathway leading to exposure-related MTL atrophy over a 5-year period. The mediating effect of AD-like neurodegeneration varied across MTL subregions and explained the largest portion of the exposure effect on ERC atrophy, and only ~30% of the total effect of air pollution exposure on total MTL atrophy, implying the importance of different neuropathologies. Future studies are needed to better understand the other neuropathological processes that explain associations between air pollution neurotoxicity and MTL atrophy in older adults.
Supplementary Material
Acknowledgements
We would like to acknowledge and thank Christos Davatzikos, Ph.D. and his research group at the University of Pennsylvania for providing the multi-atlas region segmentation (MUSE) processed estimates of the medial temporal lobe volume as well as the deep learning-based estimates of white matter small vessel ischemic disease.
Funding
The air pollution models were developed under a STAR research assistance agreement, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the US Environmental Protection Agency (EPA). This study is supported by R01AG033078 (PI: Dr. Chen), RF1AG054068 (PI: Dr. Chen), R01ES025888 (PI: Drs. Chen & Kaufman), P01AG055367, the Southern California Environmental Health Sciences Center (5P30ES007048), and the Alzheimer’s Disease Research Center at USC (NIA; P50AG005142 and P30AG066530). Dr. Salminen is support by grant 1R01ES033961-01, which is co-funded by NIEHS and NIA. Dr. Younan is also supported by a grant from the Alzheimer’s Association (AARF-19-591356). Dr. Espeland receives funding from the Wake Forest Alzheimer’s Disease Core Center (P30AG049638-01A1). Dr. Resnick is supported by the Intramural Research Program, National Institute on Aging, NIH. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. A list of contributors to WHI is available at https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Long-List.pdf
Abbreviations:
- Aβ
beta-amyloid
- ADNI
Alzheimer’s disease neuroimaging initiative
- AD-PS
AD pattern similarity
- ADRD
Alzheimer’s disease and related dementias
- ALFA
Alzheimer’s & Families
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- ERC
entorhinal cortex
- MCI
mild cognitive impairment
- MTL
medial temporal lobe
- MUSE
multi-atlas region segmentation
- NO2
nitrogen dioxide
- PART
primary age-related tauopathy
- PHG
parahippocampal gyrus
- PM2.5
particulate matter with aerodynamic diameter <2.5 μm
- p-tau
phosphorylated tau
- SEM
structural equation model
- SES
socioeconomic status
- TDP-43
DNA-binding protein 43
- WHI
Women’s Health Initiative
- WHIMS
Women’s Health Initiative Memory Study
- WM-SVID
white matter small vessel ischemic disease
Footnotes
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1.Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15(5):451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacigalupo I, Mayer F, Lacorte E, et al. A Systematic Review and Meta-Analysis on the Prevalence of Dementia in Europe: Estimates from the Highest-Quality Studies Adopting the DSM IV Diagnostic Criteria. J Alzheimers Dis. 2018;66(4):1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aisen PS, Cummings J, Jack CR, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimer’s Research & Therapy. 2017;9(1). doi: 10.1186/s13195-017-0283-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR Jr, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51(4):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Heijer T, Launer LJ, Prins ND, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64(2):263–267. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Yang Y, Wang T, Nie S, Yin H, Liu J. Correlation between White Matter Hyperintensities Related Gray Matter Volume and Cognition in Cerebral Small Vessel Disease. J Stroke Cerebrovasc Dis. 2020;29(12):105275. [DOI] [PubMed] [Google Scholar]
- 8.Kalaria RN, Ihara M. Medial temporal lobe atrophy is the norm in cerebrovascular dementias. Eur J Neurol. 2017;24(4):539–540. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Hao L, Zhang Y, Zuo C, Wang D. Entorhinal cortex volume, thickness, surface area and curvature trajectories over the adult lifespan. Psychiatry Res Neuroimaging. 2019;292:47–53. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong NM, An Y, Shin JJ, et al. Associations between cognitive and brain volume changes in cognitively normal older adults. Neuroimage. 2020;223:117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman JG, Stebbins GT, Bernard B, Stoub TR, Goetz CG, deToledo-Morrell L. Entorhinal cortex atrophy differentiates Parkinson’s disease patients with and without dementia. Mov Disord. 2012;27(6):727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air Pollution and Dementia: A Systematic Review. J Alzheimers Dis. 2019;70(s1):S145–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JC, Wang X, Wellenius GA, et al. Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Ann Neurol. 2015;78(3):466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilker EH, Preis SR, Beiser AS, et al. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46(5):1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crous-Bou Alemany. Air pollution and biomarkers of Alzheimer’s disease in cognitively unimpaired individuals. Alzheimers Dement. Published online 2020. https://acuresearchbank.acu.edu.au/item/8x8y7/air-pollution-and-biomarkers-of-alzheimer-s-disease-in-cognitively-unimpaired-individuals [DOI] [PubMed] [Google Scholar]
- 18.Casanova R, Wang X, Reyes J, et al. A Voxel-Based Morphometry Study Reveals Local Brain Structural Alterations Associated with Ambient Fine Particles in Older Women. Front Hum Neurosci. 2016;10:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Power MC, Lamichhane AP, Liao D, et al. The Association of Long-Term Exposure to Particulate Matter Air Pollution with Brain MRI Findings: The ARIC Study. Environ Health Perspect. 2018;126(2):027009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges DW, Erickson LD, Kunzelman J, Brown BL, Gale SD. Association between exposure to air pollution and hippocampal volume in adults in the UK Biobank. Neurotoxicology. 2019;74:108–120. [DOI] [PubMed] [Google Scholar]
- 21.Cho J, Noh Y, Kim SY, et al. Long-Term Ambient Air Pollution Exposures and Brain Imaging Markers in Korean Adults: The Environmental Pollution-Induced Neurological EFfects (EPINEF) Study. Environ Health Perspect. 2020;128(11):117006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilian J, Kitazawa M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease - Evidence from epidemiological and animal studies. Biomed J. 2018;41(3):141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younan D, Wang X, Casanova R, Barnard R. PM2. 5 Associated With Gray Matter Atrophy Reflecting Increased Alzheimer Risk in Older Women. Neurology. Published online 2021. https://n.neurology.org/content/96/8/e1190.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younan D, Petkus AJ, Widaman KF, Wang X. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain. Published online 2020. https://academic.oup.com/brain/article-abstract/143/1/289/5628036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8(2):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downward GS, van Nunen EJHM, Kerckhoffs J, et al. Long-Term Exposure to Ultrafine Particles and Incidence of Cardiovascular and Cerebrovascular Disease in a Prospective Study of a Dutch Cohort. Environ Health Perspect. 2018;126(12):127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata H, Barnhill LM, Bronstein JM. Air Pollution and the Risk of Parkinson’s Disease: A Review. Mov Disord. 2022;37(5):894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Block ML, Wu X, Pei Z, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18(13):1618–1620. [DOI] [PubMed] [Google Scholar]
- 29.Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160(10):1023–1029. [DOI] [PubMed] [Google Scholar]
- 30.Young MT, Bechle MJ, Sampson PD, et al. Satellite-Based NO2 and Model Validation in a National Prediction Model Based on Universal Kriging and Land-Use Regression. Environ Sci Technol. 2016;50(7):3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampson PD, Richards M, Szpiro AA, et al. A regionalized national universal kriging model using Partial Least Squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ . 2013;75:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resnick SM, Espeland MA, Jaramillo SA, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72(2):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coker LH, Hogan PE, Bryan NR, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erus G, Doshi J, An Y, Verganelakis D, Resnick SM, Davatzikos C. Longitudinally and inter-site consistent multi-atlas based parcellation of brain anatomy using harmonized atlases. Neuroimage. 2018;166:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doshi J, Erus G, Ou Y, et al. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casanova R, Hsu FC, Sink KM, et al. Alzheimer’s disease risk assessment using large-scale machine learning methods. PLoS One. 2013;8(11):e77949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casanova R, Barnard RT, Gaussoin SA, et al. Using high-dimensional machine learning methods to estimate an anatomical risk factor for Alzheimer’s disease across imaging databases. Neuroimage. 2018;183:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doshi J, Erus G, Habes M, Davatzikos C. DeepMRSeg: A convolutional deep neural network for anatomy and abnormality segmentation on MR images. arXiv [eessIV]. Published online July 3, 2019. http://arxiv.org/abs/1907.02110 [Google Scholar]
- 39.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004;160(12):1152–1158. [DOI] [PubMed] [Google Scholar]
- 40.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31(2):193–202. [DOI] [PubMed] [Google Scholar]
- 41.Roux AVD, Merkin SS, Arnett D, et al. Neighborhood of Residence and Incidence of Coronary Heart Disease. N Engl J Med. 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 42.Hallquist MN, Wiley JF. MplusAutomation: An R Package for Facilitating Large-Scale Latent Variable Analyses in Mplus. Struct Equ Modeling. 2018;25(4):621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole TB, Coburn J, Dao K, et al. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology. 2016;374:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan W, Yun Y, Ku T, Li G, Sang N. NO2 inhalation promotes Alzheimer’s disease-like progression: cyclooxygenase-2-derived prostaglandin E2 modulation and monoacylglycerol lipase inhibition-targeted medication. Sci Rep. 2016;6:22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cacciottolo M, Wang X, Driscoll I, et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7(1):e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hullmann M, Albrecht C, van Berlo D, et al. Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part Fibre Toxicol. 2017;14(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salvi A, Liu H, Salim S. Involvement of oxidative stress and mitochondrial mechanisms in air pollution-related neurobiological impairments. Neurobiology of Stress. 2020;12:100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehsanifar M, Jafari AJ, Montazeri Z, Kalantari RR, Gholami M, Ashtarinezhad A. Learning and memory disorders related to hippocampal inflammation following exposure to air pollution. J Environ Health Sci Eng. 2021;19(1):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahu B, Mackos AR, Floden AM, Wold LE, Combs CK. Particulate Matter Exposure Exacerbates Amyloid-β Plaque Deposition and Gliosis in APP/PS1 Mice. J Alzheimers Dis. 2021;80(2):761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patten KT, Valenzuela AE, Wallis C, et al. The Effects of Chronic Exposure to Ambient Traffic-Related Air Pollution on Alzheimer’s Disease Phenotypes in Wildtype and Genetically Predisposed Male and Female Rats. Environmental Health Perspectives. 2021;129(5). doi: 10.1289/ehp8905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Win-Shwe TT, Yamamoto S, Fujitani Y, Hirano S, Fujimaki H. Spatial learning and memory function-related gene expression in the hippocampus of mouse exposed to nanoparticle-rich diesel exhaust. Neurotoxicology. 2008;29(6):940–947. [DOI] [PubMed] [Google Scholar]
- 52.Ku T, Li B, Gao R, et al. NF-κB-regulated microRNA-574–5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part Fibre Toxicol. 2017;14(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, Li Q, Ma J, Zhao Y. PM2.5 impairs neurobehavior by oxidative stress and myelin sheaths injury of brain in the rat. Environ Pollut. 2018;242(Pt A):994–1001. [DOI] [PubMed] [Google Scholar]
- 54.Hajipour S, Farbood Y, Gharib-Naseri MK, et al. Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci. 2020;242:117210. [DOI] [PubMed] [Google Scholar]
- 55.Alemany S, Crous-Bou M, Vilor-Tejedor N, et al. Associations between air pollution and biomarkers of Alzheimer’s disease in cognitively unimpaired individuals. Environ Int. 2021;157:106864. [DOI] [PubMed] [Google Scholar]
- 56.Ma YH, Chen HS, Liu C, et al. Association of Long-term Exposure to Ambient Air Pollution With Cognitive Decline and Alzheimer’s Disease-Related Amyloidosis. Biol Psychiatry. Published online May 18, 2022. doi: 10.1016/j.biopsych.2022.05.017 [DOI] [PubMed] [Google Scholar]
- 57.Márquez F, Yassa MA. Neuroimaging Biomarkers for Alzheimer’s Disease. Mol Neurodegener. 2019;14(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maass A, Lockhart SN, Harrison TM, et al. Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J Neurosci. 2018;38(3):530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jack CR Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62(4):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16(3):271–278; discussion 278–84. [DOI] [PubMed] [Google Scholar]
- 61.Lee SH, Chen YH, Chien CC, et al. Three month inhalation exposure to low-level PM2.5 induced brain toxicity in an Alzheimer’s disease mouse model. PLoS One. 2021;16(8):e0254587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen TF, Lee SH, Zheng WR, et al. White matter pathology in alzheimer’s transgenic mice with chronic exposure to low-level ambient fine particulate matter. Part Fibre Toxicol. 2022;19(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen TF, Lee SH, Zheng WR, et al. White Matter Pathology in Alzheimer’s Transgenic Mice With Chronic Exposure to Low-Level Ambient Fine Particulate Matter. doi: 10.21203/rs.3.rs-907892/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell TW, Mufson EJ, Schneider JA, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol. 2002;51(2):182–189. [DOI] [PubMed] [Google Scholar]
- 65.Flores R, Wisse LEM, Das SR, et al. Contribution of mixed pathology to medial temporal lobe atrophy in Alzheimer’s disease. Alzheimer’s & Dementia. 2020;16(6):843–852. doi: 10.1002/alz.12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hickman RA, Flowers XE, Wisniewski T. Primary Age-Related Tauopathy (PART): Addressing the Spectrum of Neuronal Tauopathic Changes in the Aging Brain. Curr Neurol Neurosci Rep. 2020;20(9):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: Their relation to AD common pathology. Neuropathology. 2009;29(5):566–573. doi: 10.1111/j.1440-1789.2009.01017.x [DOI] [PubMed] [Google Scholar]
- 68.de F.C. Lichtenfels AJ, van der Plaat DA, de Jong K, et al. Long-term air pollution exposure, genome-wide DNA methylation and lung function in the LifeLines cohort study. Environ Health Perspect. 2018;126(02). doi: 10.1289/ehp2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White AJ, Kresovich JK, Keller JP, et al. Air pollution, particulate matter composition and methylation-based biologic age. Environ Int. 2019;132:105071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito K, Thurston GD, Silverman RA. Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol. 2007;17 Suppl 2(S2):S45–60. [DOI] [PubMed] [Google Scholar]
- 71.Sarnat JA, Schwartz J, Catalano PJ, Suh HH. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ Health Perspect. 2001;109(10):1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyzga RE, Rohr AC. Long-term particulate matter exposure: Attributing health effects to individual PM components. J Air Waste Manag Assoc. 2015;65(5):523–543. [DOI] [PubMed] [Google Scholar]
- 73.Evangelopoulos D, Katsouyanni K, Keogh RH, et al. PM2.5 and NO2 exposure errors using proxy measures, including derived personal exposure from outdoor sources: A systematic review and meta-analysis. Environ Int. 2020;137(105500):105500. [DOI] [PubMed] [Google Scholar]
- 74.Kulick ER, Wellenius GA, Kaufman JD, et al. Long-Term Exposure to Ambient Air Pollution and Subclinical Cerebrovascular Disease in NOMAS (the Northern Manhattan Study). Stroke. 2017;48(7):1966–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furlong MA, Alexander GE, Klimentidis YC, Raichlen DA. Association of Air Pollution and Physical Activity With Brain Volumes. Neurology. 2022;98(4):e416–e426. doi: 10.1212/wnl.0000000000013031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuadrado-Godia E, Dwivedi P, Sharma S, et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J Stroke Cerebrovasc Dis. 2018;20(3):302–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conner SC, Pase MP, Carneiro H, et al. Mid-life and late-life vascular risk factor burden and neuropathology in old age. Ann Clin Transl Neurol. 2019;6(12):2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suri S, Topiwala A, Chappell MA, et al. Association of Midlife Cardiovascular Risk Profiles With Cerebral Perfusion at Older Ages. JAMA Netw Open. 2019;2(6):e195776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pase MP, Davis-Plourde K, Himali JJ, et al. Vascular risk at younger ages most strongly associates with current and future brain volume. Neurology. 2018;91(16):e1479–e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwartz J. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect. 2001;109 Suppl 3(Suppl 3):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore, MD. J Air Waste Manag Assoc. 2000;50(7):1184–1198. [DOI] [PubMed] [Google Scholar]
- 82.Krewski D, Burnett RT, Goldberg MS, et al. Validation of the Harvard Six Cities Study of Particulate Air Pollution and Mortality. New England Journal of Medicine. 2004;350(2):198–199. doi: 10.1056/nejm200401083500225 [DOI] [PubMed] [Google Scholar]
- 83.Krewski D, Burnett RT, Goldberg M, et al. Reanalysis of the Harvard Six Cities Study, part II: sensitivity analysis. Inhal Toxicol. 2005;17(7–8):343–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to all data elements used in this study may be made available following the established WHI policies.