Abstract
Mutants blocked at the earliest stage of morphological development in Streptomyces species are called bld mutants. These mutants are pleiotropically defective in the initiation of development, the ability to produce antibiotics, the ability to regulate carbon utilization, and the ability to send and/or respond to extracellular signals. Here we report the identification and partial characterization of a 99-amino-acid open reading frame (ORF99) that is capable of restoring morphogenesis, antibiotic production, and catabolite control to all of the bldB mutants. Of the existing bld mutants, bldB is of special interest because the phenotype of this mutant is the most pleiotropic. DNA sequence analysis of ORF99 from each of the existing bldB mutants identified base changes either within the coding region of the predicted protein or in the regulatory region of the gene. Primer extension analysis identified an apparent transcription start site. A promoter fusion to the xylE reporter gene showed that expression of bldB is apparently temporally regulated and that the bldB gene product is involved in the regulation of its own expression.
Streptomycetes grow vegetatively as a branching mycelial mass. Presumably, in response to nutrient depletion, these multicellular bacteria initiate a complex morphogenetic program that involves both structural and biochemical adaptation. The first visible evidence of the initiation of development is the erection of aerial hyphae. As development proceeds, these hyphae coil and septate into uninucleoid compartments that give rise to spores, and the substrate mycelium begins to lyse. As these morphological changes occur, the organism produces a large number of secondary metabolites, many of which have antibiotic activity (8). Mutants blocked at the earliest stages of morphogenesis, those that fail to make aerial hyphae, are called bld mutants (9, 27). These mutants are also defective in antibiotic production (7, 8), catabolite repression (30), and cell-cell signaling (38, 39). Most of what we know about these genes and their roles in development comes from the study of bld mutants of Streptomyces coelicolor. At least 10 bld loci, bldA, bldB, bldC, bldD, bldF, bldG, bldH, bldI, bldK, and bld261, have been found in S. coelicolor (7, 9, 27, 28, 38, 39), and the highly pleiotropic phenotype of these mutations suggests, a priori, that they identify genes involved at an early stage in the initiation of development.
Only two bld genes, bldA and bldK, have been characterized at the molecular level. The bldA alleles reside in a gene for a leucyl-tRNA that recognizes the UUA codon (21). UUA is a rare codon in Streptomyces, and it has been suggested that this tRNA is involved in the translation of regulatory genes involved in antibiotic production and morphogenesis (12, 23, 24). While the level of bldA expression apparently increases at the initiation of development, the gene product is clearly present and active during vegetative growth (36). The role of this tRNA in catabolite control or in generating or responding to morphogenic signals remains unclear. The bldD gene has recently been cloned and characterized and is described in the accompanying report (10).
An important property of bld mutants is that they exhibit extracellular complementation (38, 39). This complementation apparently results from the diffusion of substances from one strain to another when patches of cells are grown in close proximity to each other on agar plates. Willey et al. (39) have suggested that the pattern of complementation observed among different mutant classes defines an intercellular signaling pathway involving as many as four extracellular factors. They postulated that the products of the bld genes are either directly or indirectly involved in the generation or uptake of extracellular signaling molecules such as SapB (39). Nodwell et al. (28) have recently shown that the bldK locus consists of five adjacent open reading frames (ORFs) that specify homologs of the subunits of the oligopeptide-permease family of ATP-binding cassette (ABC) membrane-spanning transporters. It has been inferred that bldK is an oligopeptide transporter and is perhaps responsible for the import of an extracellular signal required for the initiation of morphogenesis.
One of the most intriguing but poorly understood aspects of the bld phenotype is that growth on poor carbon sources is sufficient to restore partially the morphological and antibiotic defects of most of these mutants (7). An important exception is bldB (7, 27, 30). When grown on minimal medium agar plates containing glucose as the carbon source, bld mutants fail to erect aerial hyphae and are also defective in antibiotic production. When the mutants are grown on minimal medium containing mannitol, however, aerial hyphae and spore production are partially restored. While growth on mannitol partially rescues the morphogenic defect of bldA mutants, the cells remain deficient in antibiotic production (7, 27). In contrast, growth on mannitol rescues both sporulation and antibiotic production in bldH mutants (7). The most severely affected of the bld mutants, bldB, remains both morphologically and physiologically defective, failing to sporulate or produce antibiotics, regardless of carbon source (7, 27, 30).
In recent work, we have shown that bldA, bldB, bldC, bldD, bldG, and bldH mutants are defective in the regulation of the galP1 promoter, a glucose-sensitive and galactose-dependent promoter that directs expression of the galactose utilization operon, and that the bldB mutant is globally deregulated for catabolite control (30). Screens for mutants defective in catabolite control identified mutants that were at once resistant to glucose repression, defective in the regulation of antibiotic production (they overproduce antibiotics precociously), and bld (30). These observations strongly suggest that there is a direct connection between the regulation of carbon utilization and the initiation of morphogenesis in streptomycetes.
Of the existing bld mutants, bldB mutants are of special interest because their phenotype is the most pleiotropic. bldB mutants are completely defective in antibiotic production (7) and apparently globally defective in the regulation of carbon utilization (30), fail to initiate morphological development (27), and are the only bld mutants whose phenotype is not rescued by growth on poor carbon sources (7, 27, 30). Interestingly, bldB mutants also do not fit into the hierarchical signaling cascade proposed by Willey et al. (39). Harasym et al. (16) identified a 4-kb fragment from S. coelicolor (GenBank accession no. U28930) and showed that it complemented some of the bldB mutant alleles. They concluded from complementation analysis that the bldB locus contained at least two genes involved in morphological development. Here we report the cloning and sequencing of a portion of that fragment that complements all of the existing bldB mutant alleles. DNA sequence analysis of the complementing fragment identified a single small ORF capable of encoding a 99-amino-acid protein, and we suggest that this ORF is the bldB gene. DNA sequence analysis of this ORF from each of the existing bldB mutants identified base changes either within the coding region of the putative protein or in the regulatory region of the gene. Two of the mutations, bldB28 and bldB17, lie in the −10 region of the bldB promoter. bldB249 contains a base change in the putative ribosome binding site. The bldB112 mutation introduces a stop codon and would result in a truncated protein of 71 amino acids. The bldB186 mutation creates a frameshift and would result in a slightly larger protein of 134 amino acids. Two mutations, bldB15 and bldB43, identify the same tyrosine residue at position 21 to be important for bldB function. Primer extension analysis of the bldB transcript identified an apparent transcription start site. Analysis of bldB expression by using a bldB promoter fusion to the xylE reporter gene revealed that expression of bldB in wild-type cells is low during vegetative growth and increases as the cells enter stationary phase. bldB expression is apparently deregulated in a bldB mutant, suggesting that the bldB gene product is involved in the regulation of its own transcription.
MATERIALS AND METHODS
Bacterial strains and phages.
S. coelicolor A(3)2 and S. lividans 1326 (17) were used as hosts for plasmids and phages. The bldB strains used are described in Table 1. Subcloning of phage fragments or PCR-generated products was carried out according to standard E. coli techniques (25). KC628 (16) is a derivative of the S. coelicolor phage φC31. Escherichia coli DH5αMCR (Bethesda Research Laboratories) was used to prepare DNA for transformation into Streptomyces.
TABLE 1.
Strains of S. coelicolor containing bldB mutant and wild-type alleles
| Strain | Genotype or phenotype | Reference |
|---|---|---|
| J701 | bldB15 mthB2 agaA7 NF SCP2* | 27 |
| J703 | bldB28 mthB2 agaA7 NF SCP2* | 27 |
| J704 | bldB17 mthB2 agaA7 NF SCP2* | 27 |
| J669 | bldB43 mthB2 cysD18 agaA7 NF SCP2* | 27 |
| C112 | bldB112 hisA1 uraA1 strA1 SCP2− SCP1− Pgl− | 7 |
| C186 | bldB186 hisA1 uraA1 strA1 SCP2− SCP1− Pgl− | 7 |
| C249 | bldB249 hisA1 uraA1 strA1 SCP2− SCP1− Pgl− | 7 |
| KC628 | φC31 derivative; bldB+C+ ΔattP Vphr | 16 |
Plasmid constructions.
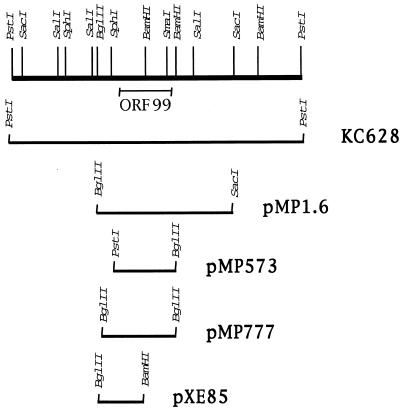
Phage KC628, containing a 4-kb PstI fragment from the S. coelicolor chromosome (Fig. 1), was provided by Jacqueline Piret (16). Plasmid pMP1.6 was generated by cloning a 1.6-kb BglII-SacI fragment from KC628 into the high-copy-number Streptomyces plasmid pIJ702 (17). This plasmid contains approximately 380 nucleotides upstream of the ORF99 translational start site, all of ORF99, and a portion of an adjacent ORF which is oriented on the opposite strand in the opposite direction. PCR primers were designed to amplify either a 573-bp fragment (upstream primer 5′-CCACCACTGCAGCCCGCGATTACTCTAGTCAC-3′; downstream primer 5′-TTGAGATCTCGTCGTGCTCATCTCGGGTAC-3′) that contains ORF99 as well as approximately 176 bp upstream or a 777-bp fragment (upstream primer 5′-GGCGGCGGAGATCTGTCAGGTCCTG-3′; downstream primer 5′-TTGAGATCTCGTCGTGCTCATCTCGGGTAC-3′) that included an additional 204 bases upstream. The 573-bp PCR fragment was cloned into the PstI/BglII sites of pIJ702, generating pMP573. Ligation mixtures were transformed into S. lividans 1326 and screened for disruption of the melanin gene contained on the vector. Plasmids were isolated from this strain and analyzed by restriction digestion before transformation into either A3(2) or the bldB mutant strains. The 777-bp PCR fragment contained engineered BglII sites on either end and was cloned into the BamHI site of pXE4, which contains an SCP2 replicon, generating pMP777. A BglII-BamHI fragment containing the 5′ end of ORF99 and upstream region was cloned into pXE4 (19), generating a promoter fusion to the xylE reporter gene, designated pXE85.
FIG. 1.
Restriction map and subclones of the 4-kb PstI fragment containing bldB. The PstI fragment originally identified by Harasym et al. (16) was used to generate subclones in the plasmids indicated at the right. Fragments subcloned into various plasmids are indicated as bars beneath the restriction map. KC628 contains the bldB-containing fragment on phage φC31. pMP1.6 and pMP573 are high-copy-number plasmids; pMP777 and pXE85 are low-copy-number plasmids.
Media and growth conditions.
Minimal medium plates supplemented with the appropriate amino acids were made as described previously (4). Thiostrepton (50 μg/ml) was included for selection of plasmids. Prior to plating of cells, the surface of the agar was covered with a sterile cellophane disc (19) to allow collection of cells without agar contamination for RNA isolation. Plates were incubated at 30°C. Genomic DNA was isolated from cells grown in liquid YEME medium as described previously (17) and used as the template for PCR amplifications. Following transformation with plasmid DNA, protoplasts were regenerated on R6 agar medium for 12 to 18 h at 30°C before antibiotic selection (18).
PCR amplification and DNA sequencing.
A 1.6-kb BglII-SacI fragment from KC628 was subcloned into pUC19, and the sequence was determined by using the pUC forward and reverse primers or primers designed internal to the sequence. PCR primers (described above) were then designed to amplify either a 573-bp product which contained the entire bldB ORF as well as 176 bp upstream of the translational start site or a slightly larger 777-bp product that contained 380 bp upstream of the ATG. These primers were engineered with the BglII or PstI restriction site. PCR products were purified by using Wizard PCR Prep (Promega) and then sequenced. DNA sequencing reactions were performed by using an fmol sequencing kit from Promega, with the addition of dimethyl sulfoxide to 10% (40). Terminal transferase was added at the end of the cycling profile (11).
RNA isolation and primer extension analysis.
RNA was isolated from minimal medium (4) plate-grown cultures by using standard RNA isolation techniques (17). Primer extension reactions were performed as previously described (26), using two different oligonucleotides complementary to the DNA sequence near the translation start site (5′-GTCCTCGTCCGGCACCTGGGC-3′ and 5′-GCGGGCTTTGACGTCCTC-3′) and a third oligonucleotide located approximately 120 bases downstream of the ATG (5′-GCTCCTCGTGTTCCTCCGTC-3′). The primer extension product shown in Fig. 3 was generated by using the primer 5′-GTCCTCGTCCGGCACCTGGGC-3′. The corresponding sequencing ladder was generated as described above, using the same end-labeled oligonucleotide. Twenty micrograms of total RNA was added for each primer extension reaction, and the entire reaction mixture was loaded onto gels.
FIG. 3.
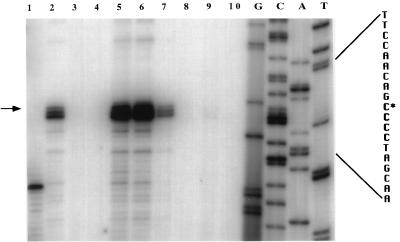
Primer extension analysis of bldB transcript. An oligonucleotide complementary to sequence immediately downstream of the putative translation start site (shown in Fig. 2) was used for extension reactions. Lane 1 is a tRNA control. RNA was isolated from J701 containing pMP1.6 after 2 days of growth (lane 2); J701 after 2 days of growth (lane 3); J704 after 2 days of growth (lane 4); J704 containing pMP573 after growth for 6 days (lane 5), 2 days (lane 6), or 1 day (lane 7); and A3(2) after growth for 3 days (lane 8), 2 days (lane 9), or 1 day (lane 10).
Catechol dioxygenase assays.
Detection of catechol 2,3-dioxygenase activity was performed as described previously (19). Strains containing pXE85, the bldB-xylE promoter fusion plasmid, were grown on solid medium, sprayed with a 0.1 M catechol solution, and scored for visual color change (clear to yellow) within 10 min of exposure. Quantitative assays (19) were performed with cells grown on agar plates containing minimal medium with mannitol as the carbon source or minimal medium with glucose as the carbon source.
RESULTS
Identification of the bldB gene.
In 1990, Harasym et al. (16) reported that a 4-kb PstI fragment of S. coelicolor DNA (shown in Fig. 1) contained in phage KC628 rescued the morphological defect of some of the known bldB mutants. They also showed that deletion of an internal BamHI fragment within the PstI fragment resulted in loss of rescue of the bldB phenotype. We cloned the portion of the PstI fragment, an internal BglII-SacI fragment, that included the region identified by this deletion on the high-copy-number plasmid pMP1.6 (Fig. 1) and determined the DNA sequence. Sequence analysis of this 1.6-kb fragment revealed the existence of one small ORF capable of encoding a protein of 99 amino acids, which we refer to as ORF99, and part of another ORF. A 573-bp fragment containing only ORF99 and approximately 176 bp upstream of the translational start site was amplified by using PCR from wild-type S. coelicolor genomic DNA. This PCR product was cloned into the high-copy-number Streptomyces plasmid pIJ702, generating plasmid pMP573 (Fig. 1). pMP573 was then transformed into protoplasts of the bldB mutant strains listed in Table 1.
Nine bldB mutant alleles, bldB15, bldB28, bldB17, bldB43, bldB57, bldB59 (27), bldB112, bldB186, and bldB249 (7), have been described. bldB57 and bldB59 did not survive storage and were not recovered from the John Innes strain collection (8a). In our experiments, KC628 completely complemented all of the existing bldB mutants except J703 (bldB28) and C249 (bldB249). Complementation of either J703 or C249 with KC628 resulted in rescue only on mannitol. Sporulation on mannitol is characteristic of a bldA mutant. The fact that complementation of J703 or C249 resulted in a bldA phenotype suggested that these strains might contain two mutations. Leskiw and Mah (22) showed that while there is no mutation in the bldA gene of J703, the level of bldA transcript in this strain is greatly reduced. It is likely, therefore, that J703 contains two mutations, one in the bldB gene (Fig. 2) and a second mutation that affects bldA expression. In recent experiments, C249 has also been shown to contain two mutations, one in bldB (Fig. 3) and another in bldA (22).
FIG. 2.
Physical map of the bldB mutations. The sequence of ORF99 is shown, with the predicted peptide sequence indicated beneath the coding region. Base changes identified in various bldB mutant strains are shown in bold above the sequence, with the bldB allele indicated. The putative helix-turn-helix motif is underlined and labeled. The transcription start site is indicated as +1.
ORF99 alone, contained on plasmid pMP573, rescued the morphological defect (scored as the ability to sporulate) of bldB mutants J701 (bldB15), J704 (bldB17), and J669 (bldB43) on all carbon sources tested, i.e., glucose, mannitol, galactose, and glycerol. pMP573 did not, however, rescue C112 (bldB112) or C186 (bldB186), which exist in a J1508 genetic background that does not contain the SCP1 plasmid. J701, J704, J669, and J703 contain SCP1 integrated in the chromosome. Schauer et al. (31) showed that plasmids containing a pIJ702 origin of replication, such as pMP573, are incompatible with SCP1, and while these plasmids may be maintained with selection in a strain that includes SCP1, the copy number is considerably lower than for strains that lack SCP1. Since the bldB mutant strains C112 and C186 lack SCP1, it is possible that the copy number of pMP573 was very high in these strains and the apparent failure of pMP573 to complement these mutants was actually due to plasmid instability or cell death. In an attempt to address this possibility, ORF99 was cloned into an SCP2, single-copy-number replicon to generate plasmid pMP777. pMP777 did rescue both C112 and C186. We suggest that the complementation observed in these strains results from the ORF99 gene product.
Physical mapping of the bldB mutations.
To examine the sequence of ORF99 in the existing bldB mutants, we generated a 573-bp PCR fragment from each of the bldB strains as well as the wild-type strain and determined the DNA sequence. The sequence of ORF99 in individual bldB mutants is shown in Fig. 2. Three of the mutations, bldB28, bldB17, and bldB249, map just upstream of the predicted ORF. bldB249 contains a base change in the putative ribosome binding site. The bldB112 mutation introduces a stop codon and would result in a truncated protein of 71 amino acids. The bldB186 mutation creates a frameshift and would result in a slightly larger protein of 134 amino acids. Two mutations, bldB15 and bldB43, identify the same tyrosine residue at position 21 to be important for bldB function. We conclude from these data and the genetic complementation analysis that ORF99 encodes the bldB gene.
Primer extension analysis of bldB RNA.
To identify the site of transcription initiation for bldB, primer extension experiments were performed. As shown in Fig. 3, bldB-specific reverse transcripts were easily detected in strains containing the wild-type bldB gene on either of the high-copy-number plasmids pMP1.6 and pMP573. Three different oligonucleotide primers were used in these experiments, and in all cases, one of four G residues located 37 bp upstream of the apparent ATG translation start codon was identified as the transcription start site (Fig. 2). The same start site was identified in assays using RNA isolated from wild-type S. coelicolor with no plasmid. While this chromosomal transcript of bldB was readily detected in these experiments and is the same size as the transcript generated from the plasmid copy, the amount of transcript detected was considerably less than in cells containing the bldB gene on plasmids. While these primer extension reactions were not rigorously quantitative, we used the same amount of RNA in each reaction and observed significantly stronger signals from cultures grown for 2 days than from those grown for 1 day. The results of this primer extension analysis place two of the bldB mutations, bldB28 and bldB17, in the −10 region of the bldB promoter.
Detection of bldB promoter activity by using a transcriptional fusion to the xylE reporter gene.
A DNA fragment containing sequences from 380 nucleotides upstream to 125 nucleotides downstream of the apparent transcription start site of bldB was subcloned upstream of a promoterless copy of the xylE reporter gene in the low-copy-number plasmid pXE4 (19), resulting in plasmid pXE85 (Fig. 1). The xylE gene product, catechol 2,3-dioxygenase, oxidizes catechol and results in a color change (clear to yellow) in colonies expressing the xylE gene. Visual inspection of colonies, grown on either glucose or mannitol as the carbon source, that contained the bldB-xylE fusion showed intense yellow color after exposure to catechol, while colonies containing only the xylE reporter gene with no promoter remained white (data not shown).
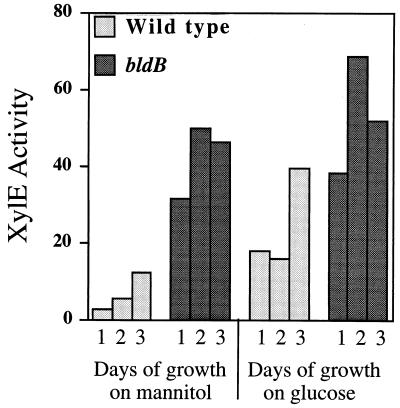
Expression from the bldB promoter was examined by using the bldB-xylE fusion contained in pXE85. The quantitative catechol dioxygenase assays in Fig. 4 show that expression of the bldB-xylE fusion in wild-type cells is low during vegetative growth and increases as cells enter stationary phase between 2 and 3 days of growth. This result is in good agreement with the primer extension analysis of bldB RNA shown in Fig. 3. Expression of the bldB-xylE fusion in a bldB mutant, however, is increased and apparently deregulated. This is true regardless of whether cells are grown on glucose or mannitol as the carbon source (Fig. 4). We conclude from these data that the apparent temporal expression of bldB is dependent on bldB itself and that bldB is likely involved in its own regulation.
FIG. 4.
Expression from the bldB-xylE fusion in wild-type and bldB mutant strains. The histogram shows results of catechol dioxygenase assays (specific activity) from cells grown for 1, 2, or 3 days on either glucose or mannitol as the carbon source. Expression of the bldB-xylE fusion was examined in an S. coelicolor wild-type strain A3(2) and J701, which contains the bldB15 allele. Specific activity is expressed as the rate of change in optical density at 375 nm per minute per milligram of protein. Duplicate assays gave nearly identical results and so error bars are not visible.
Structural analysis of the bldB gene product.
Neither the DNA sequence of the bldB gene nor the 99-amino-acid protein sequence deduced from the DNA sequence resembles any sequence in the GenBank database. When sequence comparisons fail to identify homologs, it is sometimes possible to deduce the likely fold that the putative protein sequence can adopt by threading that sequence onto known protein folds and scoring the resulting model for correctness (3). (Several hundred unique protein fold patterns have been identified by X-ray crystallography from more than a thousand different proteins.) A measure of physical stereochemical compatibility is obtained by assessing the fit of each residue of the protein of interest to the atomic coordinates of the corresponding residue of the protein fold (e.g., determining whether the fold places the hydrophobic groups in the interior and charged groups on the surface, whether it places the helix-forming residues in helices, etc.). Experience with this method suggests that false positives are not common (13), but it is easy to miss a correct fold. High scores are worth using as the basis for further experiments.
The bldB sequence was threaded (29a) onto all of the protein folds in the Brookhaven Protein Data Bank, using the algorithm of Bowie et al. (3). The three highest-scoring folds were all DNA binding proteins containing a helix-turn-helix DNA binding motif: the E. coli catabolite gene activator protein, 434 Cro protein, and λ repressor. In every case, the C-terminal region of bldB threaded well onto the helix-turn-helix region of each of these DNA binding proteins. These scores are a prediction and do not in any way prove that bldB is a DNA binding protein. This analysis does, however, suggest that experiments to test the possibility that bldB is a DNA binding protein are worthwhile. Several amino acids that could be important for protein-DNA interactions are also suggested by this analysis. These include glutamic acid residues at positions 62 and 69, a threonine at position 63, and an arginine at position 66.
DISCUSSION
We report the identification and partial characterization of the bldB gene of S. coelicolor. Genetic complementation analysis using subcloned portions of a 4-kb fragment of the S. coelicolor chromosome (16) identified a small ORF, ORF99, that restored sporulation to all of the known bldB mutants. Cloning and sequencing ORF99 from wild-type S. coelicolor and each of the bldB mutants identified base changes associated with this ORF in all of the existing bldB mutants. Three bldB alleles contained base changes in the 5′ untranslated region of the gene, two contained changes in the −10 region of the promoter, and one contained changes in the putative ribosome binding site of ORF99. One allele contained a frameshift in ORF99, one contained a stop codon within ORF99, and two independently isolated bldB mutants contained base changes that would change the same tyrosine residue in the amino-terminal portion of ORF99 to either a leucine or a cystine. We conclude from these data that ORF99 is the bldB gene.
Primer extension analysis identified an apparent transcription start site for bldB, placing base changes in two of the bldB mutant alleles in the −10 region of the promoter. The examination of bldB-specific reverse transcripts indicated that the level of bldB RNA is low during vegetative growth and increases at the initiation of development. Similarly, the level of expression of a bldB-xylE fusion was low level during vegetative growth and increased at the initiation of development. Taken together, these observations suggest that expression of bldB is temporally regulated.
Expression of the bldB-xylE fusion was increased and apparently deregulated in strains containing a bldB mutation, suggesting that the bldB gene product is required for regulation of bldB transcription. Interestingly, the level of bldB expression is higher in wild-type cells grown on glucose than in cells grown on mannitol. While there are no data to suggest that the bldB protein interacts directly with its own promoter, the bldB promoter is clearly a target of bldB activity. Structural analysis using protein threading of the predicted bldB peptide suggests the presence of a helix-turn-helix motif (5) in the carboxyl terminus with similarity to those of known DNA binding proteins. The BldB protein may function mechanistically in a manner similar to that of the AbrB protein of Bacillus subtilis. The abrB protein plays a key role in the expression of genes during the transition between vegetative and stationary-phase growth of B. subtilis. The AbrB protein consists of only 96 amino acids and has been shown to bind its own promoter (35) and repress its own synthesis during vegetative growth (34). Unlike bldB mutations, however, abrB mutations alone do not result in loss of sporulation; thus, while the mechanisms of binding and autoregulation may be the same, the biological functions of these proteins are clearly different.
One of the most interesting discoveries in this analysis of bldB is that two independently isolated bldB alleles identify the same residue, a tyrosine at position 21, to be important for bldB function. Tyrosine residues serve a number of roles in protein structure and function. Tyrosine residues are often important mediators of protein-protein interactions. Tyrosine is different from other hydrophobic, aromatic amino acids like phenylalanine and tryptophan in that its phenolic hydroxyl group is a strong hydrogen bond donor and acceptor. Consequently, tyrosines are often found on the surface of globular proteins or are partially buried with the hydroxyl group available for intermolecular hydrogen bonding (32). The tyrosine at position 21 may facilitate multimerization of BldB proteins for the kind of cooperative binding of DNA seen for AbrB which binds DNA as a hexamer (34). Alternatively, this tyrosine may facilitate protein-protein interaction between BldB and other proteins in the regulation of gene expression.
Tyrosine residues are also common sites for regulatory covalent modification, and these modifications, because they introduce charged groups, can act as switches either to create or abolish protein-protein contacts or to change the intramolecular conformation of proteins and thereby alter their activities. One intriguing possibility is that BldB is phosphorylated at tyrosine 21, and this phosphorylation event is required for the activity of bldB. While tyrosine phosphorylation in prokaryotes is not common, tyrosine residues are often the sites of phosphorylation in eukaryotic proteins. Waters et al. (37) demonstrated that eukaryotic protein kinase inhibitors blocked morphogenesis in Streptomyces and that the profile of tyrosine-phosphorylated proteins changed as Streptomyces initiated morphogenesis. Eukaryotic-type tyrosine kinases have also been implicated in signal transduction pathways affecting cell differentiation, pathogenicity, and secondary metabolism in a number of other bacteria, including Myxococcus xanthus (14, 15) and Salmonella typhimurium (29). The SpoOA protein, which plays an important role in the initiation of sporulation in B. subtilis, serves as an example of how phosphorylation activates regulatory proteins. Aspartate-phosphorylated SpoOA protein is a transcriptional activator required for the transcription of genes involved in the initiation of sporulation in B. subtilis (6). Unphosphorylated SpoOA is inactive as a transcriptional activator, and the regulation of the phosphorylation of SpoOA a key component of development.
The bldB gene is also clearly involved in catabolite repression in Streptomyces. Pope et al. (30) showed that bldB mutants are relieved of glucose repression of the galactose, glycerol, and agar utilization operons and that complementation of a bldB mutant with the wild-type bldB allele restores at once sporulation, antibiotic production, and glucose repression to these mutants. Kwakman and Postma (20) and Angell et al. (1, 2) in their studies of catabolite repression in Streptomyces showed that the glucose kinase protein, GlkA, of S. coelicolor is required for glucose repression of some genes even if the kinase activity is supplied by another gene. The observation that overexpression of glucose kinase resulted in a phenotype indistinguishable from that of a glkA mutant suggests that this overexpression might in fact titrate a protein that interacts with glucose kinase, resulting in loss of glucose repression (20). Since bldB mutants have the same phenotype as glkA mutants with respect to relief of glucose repression, it is possible that the bldB gene product interacts directly with glucose kinase to facilitate catabolite repression. In this model, the BldB-GlkA complex might interact directly with DNA regulatory regions to alter transcription levels of catabolite-controlled promoters or genes required for catabolite control. As no obvious DNA binding motif has been identified in glucose kinase, bldB may provide a DNA binding domain for this theoretical complex.
In the accompanying report, Elliot et al. (10) report the cloning and characterization of the bldD gene of S. coelicolor. Like bldB, the bldD gene may encode a small protein with DNA binding activity. Also like bldB, a tyrosine residue within the bldD coding sequencing is important for activity. While bldB and bldD may be similar in some ways, there are important differences. The phenotype of bldD mutants is clearly distinct from that of bldB mutants. Unlike bldB mutants, bldD mutants are not totally defective in antibiotic production, and their morphological defects are rescued by growth on poor carbon sources. There are other differences. S. coelicolor mutants that are resistant to 3-aminobenzamide or 3-methoxybenzamide are defective in ADP-ribosylation (33). These mutants are also defective in morphogenesis. In their study of such mutants, Shima et al. (33) showed that the patterns of ADP-ribosylated proteins in various bld mutants are different. In particular, the patterns detected in bldB and bldD mutants are totally different.
So how does one explain the function of a protein that is required for such diverse activities as the initiation of morphogenesis, the regulation of catabolite control, the global production of antibiotics, and the production of signals required for cell-cell communication? The highly pleiotropic nature of the bldB mutations suggests, a priori, that the bldB protein is required for an early event in the initiation of development in Streptomyces. We might argue that because bldB is the most pleiotropic of all known bld mutations, it identifies the earliest known gene in the initiation of development in Streptomyces. One of the keys to this puzzle comes from the observations of Willey et al. (38, 39). The ability of certain bld mutants to erect aerial hyphae when grown in close proximity to other bld mutants or wild-type S. coelicolor suggests that these mutants are not defective in the ability to make the structures associated with sporulation but are defective in the ability to send or receive extracellular signals required for the initiation of development. If one assumes that mutations such as bldB are not, in fact, involved in morphogenesis per se but are involved in assessing the nutritional environment of the cell, then it may not be so surprising that loss of this ability could affect the regulation of carbon utilization as well as other aspects of morphological development.
How does this fit with what is known about other bld mutants? The simplest explanation is that the bld mutants so far characterized identify at least two and very likely more than two independent pathways that lead to the initiation of morphogenesis in Streptomyces. So few bld genes have been identified that it is difficult to know at this point how or, in fact, whether their gene products interact. As Willey et al. (39) point out, the cascade of signals that they observe for extracellular complementation is true for only one set of nutritional conditions, i.e., rich medium with glucose as the carbon source. Clearly, more information is needed about the molecular nature of the genes identified by the bld mutants as well as saturation mutagenesis of the bld loci. Even at this early stage in the analysis of the bld mutants, it is clear that the genes they identify play key roles in the complex process of cellular differentiation in Streptomyces.
ACKNOWLEDGMENTS
We thank Keith Chater, Rich Losick, Karen McGovern, and Wendy Champness for supplying various bldB strains, for sharing unpublished results, and for helpful advice throughout the course of this work. We thank Jacqueline Piret for supplying KC628. We also thank Sidney Kushner, Eileen O’Hara, and Caroline Ingle for help with RNA isolation and characterization.
This work was supported by a grant from the National Science Foundation (DMB-9196067) to J.W.
REFERENCES
- 1.Angell S, Lewis C G, Buttner M J, Bibb M J. Glucose repression in Streptomyces coelicolor A3(2): a likely regulatory role for glucose kinase. Mol Gen Genet. 1994;244:135–143. doi: 10.1007/BF00283514. [DOI] [PubMed] [Google Scholar]
- 2.Angell S, Schwartz E, Bibb M J. The glucose kinase gene of Streptomyces coelicolor A3(2): its nucleotide sequence, transcriptional analysis, and role in glucose repression. Mol Microbiol. 1992;6:2833–2844. doi: 10.1111/j.1365-2958.1992.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 3.Bowie J, Luthy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991;253:164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- 4.Brawner M E, Auerbach J I, Fornwald J A, Rosenberg M, Taylor D P. Characterization of Streptomyces promoter sequences using the Escherichia coli galactokinase gene. Gene. 1985;40:191–201. doi: 10.1016/0378-1119(85)90042-3. [DOI] [PubMed] [Google Scholar]
- 5.Brennan R, Matthews B. The helix-turn-helix DNA binding motif. J Biol Chem. 1991;264:1903–1906. [PubMed] [Google Scholar]
- 6.Burbulys D, Trach K, Hoch J. The initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 7.Champness W. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol. 1988;170:1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champness W C, Chater K F. Regulation and integration of antibiotic production and morphological differentiation in Streptomyces spp. In: Piggot P J, Moran C P, Youngman P, editors. Regulation of bacterial development. Washington, D.C: American Society for Microbiology; 1994. pp. 61–93. [Google Scholar]
- 8a.Chater, K. Personal communication.
- 9.Chater K, Merrick M J. Approaches to the study of differentiation in Streptomyces coelicolor. London, England: Academic Press; 1976. [Google Scholar]
- 10.Elliot M, Damji F, Passantino R, Chater K, Leskiw B. The bldD gene of Streptomyces coelicolor A3(2): a regulatory protein involved in morphogenesis and antibiotic production. J Bacteriol. 1998;180:1549–1555. doi: 10.1128/jb.180.6.1549-1555.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fawcett T W, Bartlett S G. An effective method for eliminating “artifact banding” when sequencing double-stranded DNA templates. BioTechniques. 1990;9:46–48. [PubMed] [Google Scholar]
- 12.Fernandez-Moreno M A, Caballero J L, Hopwood D A, Malpartida F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell. 1991;66:769–780. doi: 10.1016/0092-8674(91)90120-n. [DOI] [PubMed] [Google Scholar]
- 13.Fischer D, Rice D, Bowie J, Eisenberg D. Assigning amino acid sequences to 3-dimensional protein folds. FASEB J. 1996;10:126–136. doi: 10.1096/fasebj.10.1.8566533. [DOI] [PubMed] [Google Scholar]
- 14.Frasch S, Dworkin M. Tyrosine phosphorylation in Myxococcus xanthus, a multicellular prokaryote. J Bacteriol. 1996;178:4084–4088. doi: 10.1128/jb.178.14.4084-4088.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanion W, Inouye M, Inouye S. Pkn9, a Ser/Thr protein kinase involved in the development of Myxococcus xanthus. Mol Microbiol. 1997;23:459–471. doi: 10.1046/j.1365-2958.1997.d01-1871.x. [DOI] [PubMed] [Google Scholar]
- 16.Harasym M, Zhang L, Chater K, Piret J. The Streptomyces coelicolor A3(2) bldB region contains at least two genes involved in morphological development. J Gen Microbiol. 1990;136:1543–1550. doi: 10.1099/00221287-136-8-1543. [DOI] [PubMed] [Google Scholar]
- 17.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, England: The John Innes Foundation; 1985. [Google Scholar]
- 18.Illing G T, Normansell I D, Peberdy J F. Genetic mapping in Streptomyces clavuligerus by protoplast fusion. J Gen Microbiol. 1989;135:2299–2305. doi: 10.1099/00221287-135-8-2299. [DOI] [PubMed] [Google Scholar]
- 19.Ingram C, Brawner M, Youngman P, Westpheling J. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J Bacteriol. 1989;171:6617–6624. doi: 10.1128/jb.171.12.6617-6624.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwakman J H, Postma P W. Glucose kinase has a regulatory role in carbon catabolite repression in Streptomyces coelicolor. J Bacteriol. 1994;176:2694–2698. doi: 10.1128/jb.176.9.2694-2698.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor E J, Baylis H A, Chater K F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2) Genes Dev. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 22.Leskiw B, Mah R. The bldA-encoded tRNA is poorly expressed in the bldI mutant of Streptomyces coelicolor. Microbiology. 1995;141:1921–1926. doi: 10.1099/13500872-141-8-1921. [DOI] [PubMed] [Google Scholar]
- 23.Leskiw B K, Lawlor E J, Fernandez-Abalos J M, Chater K F. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative Streptomyces mutants. Proc Natl Acad Sci USA. 1991;88:2461–2465. doi: 10.1073/pnas.88.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leskiw B K, Bibb M J, Chater K F. The use of a rare codon specifically during development? Mol Microbiol. 1991;5:2861–2867. doi: 10.1111/j.1365-2958.1991.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 25.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 26.Mattern S G, Brawner M E, Westpheling J. Identification of a complex operator for galP1, the glucose-sensitive, galactose-dependent promoter of the Streptomyces galactose operon. J Bacteriol. 1993;175:1213–1220. doi: 10.1128/jb.175.5.1213-1220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrick M J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976;96:299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- 28.Nodwell J R, McGovern K, Losick R. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol Microbiol. 1996;22:881–893. doi: 10.1046/j.1365-2958.1996.01540.x. [DOI] [PubMed] [Google Scholar]
- 29.Ostrovsky P, Maloy S. Protein phosphorylation on serine, threonine and tyrosine residues modulates membrane-protein interactions and transcriptional regulation in Salmonella typhimurium. Genes Dev. 1995;9:2034–2041. doi: 10.1101/gad.9.16.2034. [DOI] [PubMed] [Google Scholar]
- 29a.Petsko, G. Personal communication.
- 30.Pope M K, Green B D, Westpheling J. The bld mutants of Streptomyces coelicolor are defective in the regulation of carbon utilization, morphogenesis and cell-cell signalling. Mol Microbiol. 1996;19:747–756. doi: 10.1046/j.1365-2958.1996.414933.x. [DOI] [PubMed] [Google Scholar]
- 31.Schauer A T, Nelson A D, Daniel J B. Tn4563 transposition in Streptomyces coelicolor and its application to isolation of new morphological mutants. J Bacteriol. 1991;173:5060–5067. doi: 10.1128/jb.173.16.5060-5067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrauber H, Eisenhaber F, Argos P. Rotamers: to be or not to be? An analysis of amino acid side-chain conformations in globular proteins. J Mol Biol. 1993;230:592–612. doi: 10.1006/jmbi.1993.1172. [DOI] [PubMed] [Google Scholar]
- 33.Shima J, Penyige A, Ochi K. Changes in patterns of ADP-ribosylated proteins during differentiation of Streptomyces coelicolor A3(2) and its developmental mutants. J Bacteriol. 1996;178:3785–3790. doi: 10.1128/jb.178.13.3785-3790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauch M, Perego M, Burbulys D, Hoch J. The transition state transcription regulator AbrB of Bacillus subtilis is autoregulated during vegetative growth. Mol Microbiol. 1989;3:1203–1210. doi: 10.1111/j.1365-2958.1989.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 35.Strauch M, Spiegelman G, Perego M, Johnson W, Burbulys D, Hoch J. The transition state transcription regulator AbrB of Bacillus subtilis is a DNA binding protein. EMBO J. 1989;8:1615–1621. doi: 10.1002/j.1460-2075.1989.tb03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wezel G P, White J, Young P, Postma P W, Bibb M J. Substrate induction and glucose repression of maltose utilization by Streptomyces coelicolor A3(2) is controlled by malR, a member of the lacI-galR family of regulatory genes. Mol Microbiol. 1997;23:537–549. doi: 10.1046/j.1365-2958.1997.d01-1878.x. [DOI] [PubMed] [Google Scholar]
- 37.Waters B, Vujaklija D, Gold M, Davies J. Protein tyrosine phosphorylation in streptomycetes. FEMS Microbiol Lett. 1994;120:187–190. doi: 10.1111/j.1574-6968.1994.tb07028.x. [DOI] [PubMed] [Google Scholar]
- 38.Willey J, Santamaria R, Guijarro J, Geislich M, Losick R. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by Streptomyces coelicolor. Cell. 1991;65:641–650. doi: 10.1016/0092-8674(91)90096-h. [DOI] [PubMed] [Google Scholar]
- 39.Willey J, Schwedock J, Losick R. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 1993;75:895–903. doi: 10.1101/gad.7.5.895. [DOI] [PubMed] [Google Scholar]
- 40.Winship P A. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989;17:1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]