SUMMARY
Dracunculiasis (Guinea Worm Disease) is a chronic disease that is primarily found in the arid and poor areas of our planet where water supply systems consist of open wells. This parasitic disease is transmitted to humans not only through the consumption of water contaminated with crustaceans harbouring larvae of Dracunculus medinensis, but also through the ingestion of paratenic (frogs) or transport hosts (fish). The natural progression of the disease is caused by adult worms invading connective tissues, leading to blistering and ulceration of the extremities, approximately one year after infection. In 1986, the Guinea Worm Eradication Program (GWEP) was launched and since then, the incidence of the disease has been reduced by over 99%. Indeed, the most recent global report from 2022 shows only 13 cases of human dracunculiasis worldwide, the lowest annual incidence ever reported. The new found knowledge of potential animal reservoirs and the recent discovery of possible edible paratenic hosts could pose challenges to the future eradication of this debilitating disease. Therefore, attempts to eradicate this parasitosis should not be postponed. Intensive research is needed in this neglected area of medicine, now that the goal is within reach.
Keywords: Guinea worm disease, dracunculiasis, tropical medicine, hygiene, parasitoses
INTRODUCTION
As recently observed during the COVID-19 pandemic, scientists study diseases with the ideal goal of finding a cure or a vaccine and to achieve the complete eradication of the pathogen. The World Health Organization (WHO) declared the global eradication of smallpox a success in 1980 after the last known case in 1977. A second infectious pathogen was targeted for eradication: Dracunculus medinensis (or guinea worm), a nematode that causes dracunculiasis in humans. Guinea worm disease (GWD) is a chronic neglected disease that has almost been eradicated for decades without definitive results [1]. Although infection control programmes have been highly effective in reducing the number of human cases, some countries are experiencing increasing numbers of D. medinensis infections in animals. There is preliminary evidence that alternative routes of infection (e.g. consumption of fish) may facilitate the transmission of Dracunculus spp. to definitive hosts [2, 3].
The aim of the present work is to briefly review the state of the art on dracunculiasis knowledge and clarify the factors contributing to dracunculiasis persistence in endemic areas.
HISTORICAL NOTES
Despite some unlikely hypotheses that dracunculiasis was described in the Old Testament, it is very probable that the parasitosis was endemic in Egypt and especially in the Blue Nile area [4]. Apart from the, albeit uncertain, testimony of the Ebers papyrus (c. 1550 BC), in the area of Deir el-Medina and Amarna there were widespread water supply systems consisting of open wells, present since the Paleolithic [5, 6]. These wells provided an ideal pabulum for the development of both cyclopids and anthropogenic water contamination [4, 5].
In the ancient West, dracunculiasis was completely unknown, thanks largely to the use of closed wells and aqueducts for irrigation, already present since at least the Minoan period. The few medical descriptions of this disease in the classical era are based on indirect evidence: only Rufus of Ephesus, a physician who lived at the time of the Emperor Trajan, reported observing the disease in Egypt and noting its spread via water. The other medical authors, including Galen, limit themselves to describing the disease with reference to Rufus. Even the Arabic medical authors show that they did not know the disease directly, so the medieval Western medical world treated the subject in an abstract way. So much so that, in the Renaissance, the disease was assimilated and identified with subcutaneous parasitoses in cattle and horses [7].
Instead, for obvious reasons, the disease was researched in colonial times, even though it always remained amongst the rare “exotic” diseases and thus of marginal interest to Western medicine. We would like to remind that the “traditional” therapy reported not only from antiquity but also by the doctors of the colonial period almost always consisted of tying a small weight to the parasite (after its spontaneous external presentation or after a skin incision) and not by progressive curling in a stick. This, and the fact that the disease was unknown in the West, seems to rule out once and for all that the snake of Asclepius’ rod, the symbol of the god and indeed of Asclepius’ doctors, could be a Dracunculus, as it is reported sometimes [7].
THE GUINEA WORM CLASSIFICATION AND ITS LIFE CYCLE
Dracunculus is a genus of nematode parasites in the family Dracunculidae, belonging to the order Callamanida. Among the Dracunculus species, the most relevant is D. medinensis, also known as Guinea worm. Dracunculidae are obligate parasites that usually settle in tissues and serous cavities of mammals, fish, reptiles, amphibians or birds. Adult female worms can grow up to 100 cm in length.
People usually become infected by drinking unfiltered water from ponds or other stagnant water sources contaminated with copepods containing Guinea worm larvae. These small crustaceans represent the intermediate host where immature larvae develop to the infective third stage larvae (L3) [8]. Recently, a food-borne route of transmission has been hypothesized after the demonstration of the existence of paratenic hosts, specifically frogs, transport hosts, such as fish, and animal reservoirs, mainly dogs but also cats and baboons [9, 10]. Guinea worm larvae can be released into the human or animal digestive tract, after ingestion of raw or undercooked aquatic animals, namely the paratenic or transport hosts, that might bear infected copepods. Therefore, instead of being exclusively a water-borne anthroponosis, dracunculiasis would also be a food-borne zoonosis [11].
L3 larvae penetrate the stomach and intestinal wall of the host and migrate to the abdominal and retroperitoneal connective tissues, where they develop into the adult stage and mate. About a year later, the female is ready to release her larvae. It then migrates under the skin, usually in the distal extremities and, when fully gravid, induces a painful swelling and ulcerative lesion. Indeed, when they are about to emerge to the surface, some larvae are released into the subdermis through a tear at the anterior end. The host’s reaction leads to the formation of a burning, painful blister, which bursts open after a few days and forms a flat ulcer. Subsequently, there is a pronounced inflammatory reaction against the cuticle of the entire worm, which prevents its removal. The bacteriologically sterile blister fluid contains larvae surrounded mainly by polymorphonuclear neutrophils with macrophages, lymphocytes and eosinophils [12].
The release of larvae into the environment can occur several times during different events. The copepod intermediate host may swallow these larvae, which then develop into the infective form, and the cycle begins again [13].
CLINICAL MANIFESTATIONS
Clinical manifestations of D. medinensis infection appear approximately one year after infection when fecund adult female worms appear near the surface of the skin (Figure 1). The initial presentation is a painful papule that enlarges over hours to days to form a blister that allows a portion of the worm to emerge from the skin. The blister may be accompanied by local erythema, urticaria, fever, nausea and pruritus [14]. The lesion then resolves quickly. Female worms sometimes burst in the tissues, resulting in a very large pus-filled abscess and severe cellulitis [15].
Figure 1.
Adult female worm emerging from the skin of lower extremities: From The Carter Center, with permission.
Complications can include secondary bacterial infections leading to sepsis, local abscesses and pyogenic arthritis. This is a possible expected articular complication of dracunculiasis. However, a previous examination of knee joint invasion by the adult female, showed live guinea-worm larvae in one case and dead ones in the other three cases. A less frequent intra-articular reaction would be expected in the absence of microfilariae. This second scenario could be due to the toxin released by the adult worms causing the arthritic lesion regardless of its actual location [16].
PATHOGENICITY AND HOST-PATHOGEN INTERACTION
Systemic manifestations of dracunculiasis are mainly limited to slight fever, urticarial rash with intense pruritus, nausea, vomiting, diarrhea and dizziness. However, there have been reports of organ involvement cases such as pericarditis or central nervous system infections as well as long term sequelae [17–19]. Indian records reported a dracunculiasis case fatality rate of 0.1%, which is largely overestimated and mostly secondary to bacterial overinfections [20, 21]. From a host-pathogen perspective, many aspects of Dracunculus “residency” in human hosts are still unknown. It is certain, however, that the immune response to Dracunculus spp. exposure does not prevent further infections. D. medinensis contains morphine and its active metabolite, morphine 6-glucuronide. Probably alkaloids acts as immunosuppressive and antinociceptive signal molecules [22]. White blood cells have been found to adhere to the Dracunculus cuticle especially in lesions of long duration [23]. Dracunculus usually elicits an antibody response, while, at the same time depressing IFN-γ production [24]. Antibody production is mainly induced by emerging adult worms rather than ingested infective larvae. During active infection, most human individuals respond with high levels of specific IgG1 and IgG4 and with relatively low levels of specific and total IgE [25]. It has been reported that individuals with a patent infection had lover serum IgE compared with prepatent, postpatent and uninfected individuals and it has been hypothesized that IgG4 (and possibly IgG1) may block protective immune response by occupying Fc receptors on effectors cells in the place of protective specific IgE [25, 26]. Cross reactions with Onchocerca antibodies may occur [27].
Serology is of little use in the disease diagnosis, which remains mainly clinical [28]. Conventional PCR and nematode DNA amplification have been used to some extent, but the turnaround time from the sample reception to species confirmation can be up to two weeks. Recently, a fast quantitative polymerase chain reaction (GW qPCR) test has shown high sensitivity and specificity in diagnosing suspected adult female Guinea worm infections in humans and in domestic or wild animals [29]. This approach could allow faster confirmation of Guinea worm infections, leading to more rapid interventions while improving our understanding of infection patterns.
ERADICATION PROGRAMS AND INFECTION CONTROL MEASURES
The Guinea Worm Eradication Program (GWEP) began in the late 1980s supported by the Carter Center and endorsed by the World Health Assembly [30]. At the beginning, the deadline was set to 1991, but was subsequently postponed to 2009, 2015, 2020 and 2030. Given the absence of a vaccine and effective treatment, the eradication strategy has solely been based on public health measures [31].
The eradication program effectively reduced 99% of cases, and is focused on:
ensuring access to safe drinking water,
active disease surveillance and case containment (with monetary incentives for reporting cases),
vector control (es. adding chemicals to water, temephos when applied to unsafe drinking-water sources at monthly intervals is effective in killing copepods),
health education and
certification of eradication [32].
Countries that have not recorded dracunculiasis cases for at least three years can apply to WHO for certification of eradication (with strict ICTs criterias). A crucial stage of the GWEP is placed on the certification of the absence of infection in animals. As a matter of fact, a current problem in some countries (such as Chad and Central Africa Republic) are infected domestic dogs, an active reservoir of the diseases [33]. However, today there are 197 dracunculus-free countries, with only 4 countries remaining endemic [34].
THE OBSTACLE OF ANIMAL RESERVOIRS AND EPIDEMIOLOGY OF ANIMAL CASES
The incidence of Dracunculiasis has been reduced by over 99% thanks to GWEP. One of the most relevant obstacles to GWD eradication is the emergency of a new reservoir of the disease in domestic dogs [35].
Sporadic Guinea worm infections were first described in dogs in Pakistan in 2005 [36]. From 2012, Dracunculiasis outbreaks in dogs have been reported in Africa. Chad has reported the majority of cases in dogs until now (833 in 2021, 1508 in 2020; 1935 in 2019). Cases in cats have also been described. Other countries where local outbreaks occurred in mammals include Ethiopia and Mali, where cats and baboons were also infected, and Angola, Cameroon and South Sudan [31, 37]. Most of these countries are implementing programs to control the infection in animals. According to the last WHO report on neglected tropical diseases, in 2021, a 46% reduction in animal infections was compared to 2020, as well as an additional 21% reduction in 2022 compared with 2021 [35].
Regarding these animal reservoirs, a new route of transmission has been described which likely explains recent epidemiological changes in GWD. As previously reported, this food-borne route is sustained by the ingestion of paratenic (frogs) or transport (fish) hosts [11]. This is reported also for other Dracunculus species, such as Dracunculus insignis [8]. The increase in dog infections is also linked to the fishing season. In Chad, GWD rates in dogs are higher at the end of the hot dry season and at the beginning of the wet season, when fishing is more intense [38].
No evidence of direct transmission from other mammals to humans has emerged [39].
EPIDEMIOLOGY OF HUMAN CASES
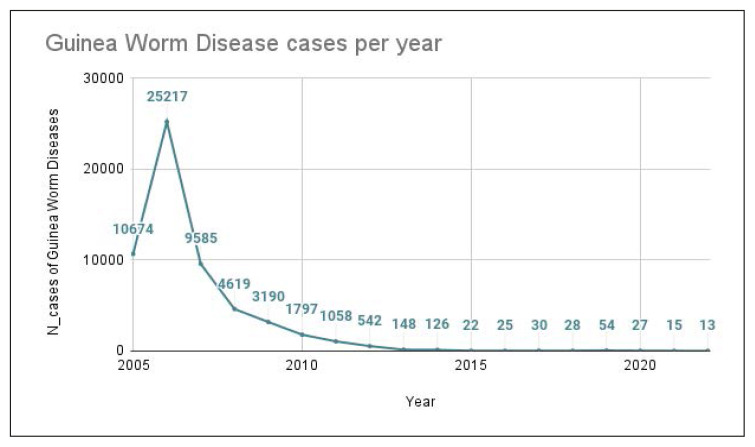
In 1986, when the Carter Centre began implementing the global Guinea Worm Eradication Program, approximately 3.5 million human cases occurred annually. The Carter Centre and the WHO have been collecting yearly cases of human GWD since 2005 to 2022; as shown in Figure 2 and Table 1.
Figure 2.
GWD yearly cases since 2005.
Table 1.
GWD yearly cases since 2005 as reported by the Carter Centre. Imported and exported cases are included in the count. Gray colour palette shows the progressive reduction of total cases.
| Year | Guinea Worm Disease cases |
|---|---|
| 2022 | 13 |
| 2021 | 15 |
| 2020 | 27 |
| 2019 | 54 |
| 2018 | 28 |
| 2017 | 30 |
| 2016 | 25 |
| 2015 | 22 |
| 2014 | 126 |
| 2013 | 148 |
| 2012 | 542 |
| 2011 | 1058 |
| 2010 | 1797 |
| 2009 | 3190 |
| 2008 | 4619 |
| 2007 | 9585 |
| 2006 | 25217 |
| 2005 | 10674 |
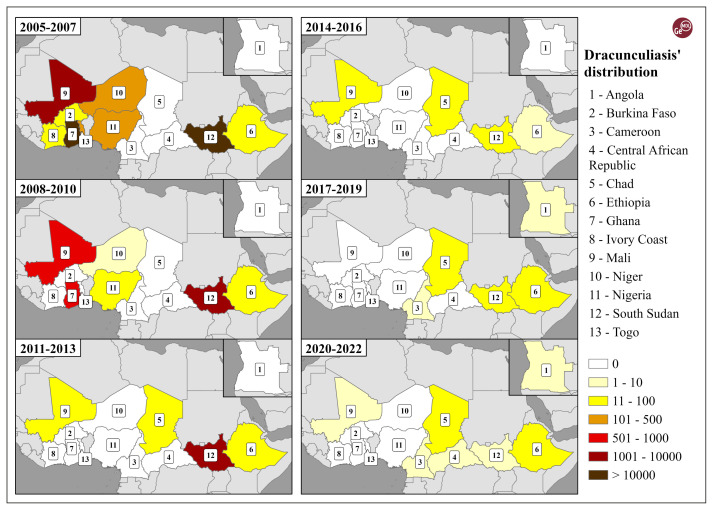
Owing to notable differences in the temporal and spatial distribution of dracunculiasis, we grouped data into triennial periods, starting from 2005 until 2022, to create more comparable GIS elaborations. Country data were combined from Carter Centre database, WHO and Centers for Disease Control and Prevention fact-sheets.
For representation purposes, we have used a yellow-brown colour scale, with lighter tones representing lower values, while darker tones are used for higher ones. In total, six classes were created, with the addition of a white class with values equal to 0 due to the diachronically recorded differences. Through ArcGIS Pro 3.0.3, we elaborated six maps (2005–2007, 2008–2010, 2011–2013, 2014–2016, 2017–2019, 2020–2022), grouped in a synthetic layout, showing the significant decline in dracunculiasis over time, particularly shown in Figure 3:
Figure 3.
Temporal variation and spatial distribution of dracunculiasis from 2005 until 2022 on the basis of triennial periods. Authors’ elaboration on The Carter Center data and World Bank boundaries.
– in the 2005–2007 period, 45,447 cases were recorded, with the highest numbers in Sudan/ South Sudan with 31,966 and Ghana with 11,475, in the sixth class (>10,000 cases), followed by Mali with 1,301, in the fifth class;
– in the 2008–2010 period 9,587 cases were recorded, with the highest numbers, once again, in Sudan/South Sudan with 8,049, now in the fifth class, followed by Ghana with 751 cases and Mali with 660, in the fourth class;
– in the 2011–2013 period 1,748 cases were recorded, with the highest values still in Sudan/South Sudan with 1,665, in the fifth class, followed by a small group of countries with a few dozen cases, such as, Chad, Mali and Ethiopia respectively with 34, 30 and 19 cases, while no other countries showed cases of dracunculiasis;
– in the 2014–2016 period, “only” 173 cases were recorded (if compared with the previous period), with the highest values once again in Sudan/ South Sudan but which had considerably decreased to 81 cases (even in the second class), followed by the same previous small group of countries, that is to say Mali with 45, Chad with 38 and Ethiopia with 9, while no other countries showed cases of dracunculiasis and with a notable reduction of the differences among the countries involved;
– in the 2017–2019 period, 112 cases were recorded, with (a further decrease and) some relevant changes, since the highest values were for the first time recorded in Chad with 80, followed with similar numbers by Ethiopia (with 15) and Sudan/South Sudan (with 14), and two other new countries showed isolated cases of dracunculiasis, that is to say Angola (2) and Cameroon (1);
– in the 2020–2022 period, “only” 55 cases were recorded (about half of the previous period). Chad remained the highest with 26 cases followed by Ethiopia (with 13) and Sudan/South Sudan (with 10), with some countries reporting isolated cases.
The most recent report from 2022 included only 13 cases of dracunculiasis worldwide; the lowest annual incidence ever reported (13% decrease from 2021). Of the 21 countries in Africa and Asia that were affected by dracunculiasis in the 1980s, the disease is now endemic only in Africa (Figure 3). As a matter of fact, in 2023 six human cases of Guinea worm disease were reported in Chad, five in South Sudan, one in Ethiopia and one in the Central African Republic [34].
In 2022 the document Abu Dhabi Declaration on the Eradication of Guinea Worm Disease strengthened the roadmap to GWD elimination in close consultation with endemic country leaders, national program leads, The Carter Center, and the World Health Organization [40]. After smallpox, Guinea Worm Disease could become the second human disease in history to be eradicated, and the first one to be eradicated without the use of a vaccine or drugs.
GUINEA WORM THERAPY
Modern treatment of GWD still relies on the ancient technique of a gentle, gradual extraction of the female parasite emerging from the skin [7]. This is a lengthy process that can take hours or days. One countermeasure is to squeeze the bump to free the adult worm from the carrying larvae making the parasite thinner and easier to remove [28].
There are no oral anthelmintic or effective drugs against dracunculiasis in humans. As a matter of fact, two previous unpublished field studies provided no evidence of effective transmission interruption using ivermectin and topical moxidectin [41]. Interestingly, a recent clinical trial in laboratory-bred ferrets tested the efficacy of flubendazole (FBZ), an anthelmintic drug, on GWD in a model animal system. The authors concluded that FBZ could be a promising anthelmintic treatment for GWD. Indeed, the high degree of blockage of the uterus of the female larva caused by FBZ could lead to the death of the adult worm [41].
Supportive therapies can be used to reduce oedema, pain, and the risk of wound superinfection [3]. While GWD-specific vaccines are not available, tetanus vaccination is recommended for patients with GWD [42]. Nevertheless, research in this field is still ongoing as shown by recent attempts to design a multi-epitope vaccine against D.medinensis using immune-informatics [43].
CONCLUSIONS
A parasitic disease historically confined to the subtropical areas of Africa and the Arabian Peninsula, dracunculiasis has recently been the focus of an eradication program promoted by the WHO. The campaign has demonstrated that environmental actions could achieve the eradication of an infectious disease with a limited geographical distribution. This contrasts with better-known programmes to eradicate globally spread pathogens such as smallpox through vaccination [44].
After decades of study and efforts to eradicate this interesting water-related human parasitic disease, there is growing concern about our previous certainties. Interestingly, in 2020, a young Vietnamese man who had never travelled to endemic areas of Africa contracted dracunculiasis. The nematode was extracted from the lower limbs and 18s DNA sequence analysis clustered it with the animal-infective Dracunculus insignis and Dracunculus lutrae [45]. We believe that these new findings on possible animal reservoirs and the recent discovery of potential edible paratenic hosts could mine the future eradication of this debilitating disease. To expedite the unaccomplished goal of GWD eradication and avoid future relapses more research is needed in this neglected field of medicine.
Footnotes
Conflict of interest: The authors declare no conflict of interest
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors
REFERENCES
- 1. Hsieh MH, Mentink-Kane MM. Smallpox and Dracunculiasis: the scientific value of infectious diseases that have been eradicated or targeted for eradication. Is Schistosomiasis Next? PLoS Pathog. 2016;12(1):e1005298. doi: 10.1371/journal.ppat.1005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Box EK, Cleveland CA, Garrett KB, et al. Copepod consumption by amphibians and fish with implications for transmission of species. Int J Parasitol Parasites Wildl. 2021;15:231–237. doi: 10.1016/j.ijppaw.2021.06.001. doi: 10.1016/j.ijppaw.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Lancet. Guinea worm disease eradication: a moving target. Lancet. 2019;393(10178):1261. doi: 10.1016/S0140-6736(19)30738-X. doi: 10.1016/S0140-6736(19)30738-X. [DOI] [PubMed] [Google Scholar]
- 4. Adamson PB. Dracontiasis in antiquity. Med Hist. 1988;32:204–209. doi: 10.1017/s0025727300048006. doi: 10.1017/s0025727300048006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller RL. Dqr, spinning and treatment of guinea worm in P. Ebers 875. J Egypt Archaeol. 1989;75(1):249–254. [Google Scholar]
- 6. Bouchet-Bert L. The Question of Dqr and Sterile Blades in P. Ebers 875. J Egypt Archaeol. 1998;84(1):224–228. [Google Scholar]
- 7. Simonetti O, Zerbato V, Di Bella S, Luzzati R, Cavalli F. Dracunculiasis over the centuries: the history of a parasite unfamiliar to the West. Infez Med. 2023;31:257–264. doi: 10.53854/liim-3102-15. doi: 10.53854/liim-3102-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cleveland CA, Garrett KB, Cozad RA, Williams BM, Murray MH, Yabsley MJ. The wild world of Guinea Worms: A review of the genus Dracunculus in wildlife. Int J Parasitol Parasites Wildl. 2018;7(3):289–300. doi: 10.1016/j.ijppaw.2018.07.002. doi: 10.1016/j.ijppaw.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eberhard ML, Cleveland CA, Zirimwabagabo H, Yabsley MJ, Ouakou PT, Ruiz-Tiben E. Guinea Worm (Dracunculus medinensis) Infection in a Wild-Caught Frog, Chad. Emerg Infect Dis. 2016;22:1961–1962. doi: 10.3201/eid2211.161332. doi: 10.3201/eid2211.161332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thiele EA, Eberhard ML, Cotton JA, et al. Population genetic analysis of Chadian Guinea worms reveals that human and non-human hosts share common parasite populations. PLoS Negl Trop Dis. 2018;12:e0006747. doi: 10.1371/journal.pntd.0006747. doi: 10.1371/journal.pntd.0006747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galán-Puchades MT. Dracunculiasis: water-borne anthroponosis vs. food-borne zoonosis. J Helminthol. 2020;94:e76. doi: 10.1017/S0022149X19000713. doi: 10.1017/S0022149X19000713. [DOI] [PubMed] [Google Scholar]
- 12.Muller R. The pathology of experimental Dracunculus infection and its relevance to chemotherapy. In: Soulsby EJL, editor. Pathophysiology of parasitic infection. Academic Press; New York NY: 1976. pp. 133–148. [DOI] [Google Scholar]
- 13.CDC-Centers for Disease Control Prevention. CDC - Guinea Worm Disease - Biology. [accessed on Jun. 9, 2023]. https://www.cdc.gov/parasites/guineaworm/biology.html .
- 14. Bennett MD, MACPJ, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases: 2-Volume Set. Elsevier Health Sciences. 2019:3444–3445. [Google Scholar]
- 15. Smith GS, Blum D, Huttly SR, Okeke N, Kirkwood BR, Feachem RG. Disability from dracunculiasis: effect on mobility. Ann Trop Med Parasitol. 1989;83:151–158. doi: 10.1080/00034983.1989.11812323. doi: 10.1080/00034983.1989.11812323. [DOI] [PubMed] [Google Scholar]
- 16. Kothari ML, Pardnani DS, Mehta L, Anand MP. Guinea-worm arthritis of knee joint. Br Med J. 1968;3(5615):435–436. doi: 10.1136/bmj.3.5615.435-c. doi: 10.1136/bmj.3.5615.435-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinare SG, Parulkar GB, Sen PK. Constrictive pericarditis resulting from dracunculosis. Br Med J. 1962;1(5281):845. doi: 10.1136/bmj.1.5281.845. doi: 10.1136/bmj.1.5281.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pittella JEH. Pathology of CNS parasitic infections. Handb Clin Neurol. 2013;114:65–88. doi: 10.1016/B978-0-444-53490-3.00005-4. doi: 10.1016/B978-0-444-53490-3.00005-4. [DOI] [PubMed] [Google Scholar]
- 19. Hours M, Cairncross S. Long-term disability due to guinea worm disease. Trans R Soc Trop Med Hyg. 1994;88:559–560. doi: 10.1016/0035-9203(94)90163-5. doi: 10.1016/0035-9203(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 20. Adeyeba OA. Secondary infections in dracunculiasis: bacteria and morbidity. Int J Zoonoses. 1985;12(2):147–149. [PubMed] [Google Scholar]
- 21. Cairncross S, Muller R, Zagaria N. Dracunculiasis (Guinea worm disease) and the eradication initiative. Clin Microbiol Rev. 2002;15:223–246. doi: 10.1128/CMR.15.2.223-246.2002. doi: 10.1128/CMR.15.2.223-246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu W, Baggerman G, Secor WE, et al. Dracunculus medinensis and Schistosoma mansoni contain opiate alkaloids. Ann Trop Med Parasitol. 2002;96:309–316. doi: 10.1179/000349802125000808. doi: 10.1179/000349802125000808. [DOI] [PubMed] [Google Scholar]
- 23. Reddy CR, Parvathi G, Sivaramappa M. Adhesion of white blood cells to guinea-worm larvae. Am J Trop Med Hyg. 1969;18:379–381. doi: 10.4269/ajtmh.1969.18.379. doi: 10.4269/ajtmh.1969.18.379. [DOI] [PubMed] [Google Scholar]
- 24. Knopp S, Amegbo IK, Hamm DM, Schulz-Key H, Banla M, Soboslay PT. Antibody and cytokine responses in Dracunculus medinensis patients at distinct states of infection. Trans R Soc Trop Med Hyg. 2008;102:277–283. doi: 10.1016/j.trstmh.2007.12.003. doi: 10.1016/j.trstmh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 25. Bloch P, Simonsen PE. Immunoepidemiology of Dracunculus medinensis infections II. Variation in antibody responses in relation to transmission season and patency. Am J Trop Med Hyg. 1998;59:985–990. doi: 10.4269/ajtmh.1998.59.985. doi: 10.4269/ajtmh.1998.59.985. [DOI] [PubMed] [Google Scholar]
- 26. Bloch P, Simonsen PE. Immunoepidemiology of Dracunculus medinensis infections I. Antibody responses in relation to infection status. Am J Trop Med Hyg. 1998;59:978–984. doi: 10.4269/ajtmh.1998.59.978. doi: 10.4269/ajtmh.1998.59.978. [DOI] [PubMed] [Google Scholar]
- 27. Garate T, Kliks MM, Cabrera Z, Parkhouse RM. Specific and cross-reacting antibodies in human respo nses to Onchocerca volvulus and Dracunculus medinensis infections. Am J Trop Med Hyg. 1990;42:140–147. doi: 10.4269/ajtmh.1990.42.140. doi: 10.4269/ajtmh.1990.42.140. [DOI] [PubMed] [Google Scholar]
- 28. Pellegrino C, Patti G, Camporeale M, et al. Guinea Worm Disease: a neglected diseases on the verge of eradication. Trop Med Infect Dis. 2022;7:366. doi: 10.3390/tropicalmed7110366. doi: 10.3390/tropicalmed7110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coker SM, Box EK, Stilwell N, et al. Development and validation of a quantitative PCR for the detection of Guinea worm (Dracunculus medinensis) PLoS Negl Trop Dis. 2022;16(10):e0010830. doi: 10.1371/journal.pntd.0010830. doi: 10.1371/journal.pntd.0010830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Awadi AR, Al-Kuhlani A, Breman JG, et al. Guinea worm (Dracunculiasis) eradication: update on progress and endgame challenges. Trans R Soc Trop Med Hyg. 2014;108:249–251. doi: 10.1093/trstmh/tru039. doi: 10.1093/trstmh/tru039. [DOI] [PubMed] [Google Scholar]
- 31. Molyneux DH, Eberhard ML, Cleaveland S, et al. Certifying Guinea worm eradication: current challenges. Lancet. 2020;396:1857–1860. doi: 10.1016/S0140-6736(20)32553-8. doi: 10.1016/S0140-6736(20)32553-8. [DOI] [PubMed] [Google Scholar]
- 32. Biswas G, Sankara DP, Agua-Agum J, Maiga A. Dracunculiasis (guinea worm disease): eradication without a drug or a vaccine. Philos Trans R Soc Lond B Biol Sci. 2013;368 doi: 10.1098/rstb.2012.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elom MO. Dracunculus medinensis (Guinea worm disease) elimination and eradication and the challenges of emerging non-human animal hosts: A review of the literature. Asian J Biol Life Sci. 2020:39–48. doi: 10.9734/ajob/2020/v10i430115. [DOI] [Google Scholar]
- 34.Guinea Worm Disease Reaches All-Time Low: Only 13* Human Cases Reported in 2022. [accessed on Jun. 22, 2023]. https://www.carter-center.org/news/pr/2023/2022-guinea-worm-world-wide-cases-announcement.html .
- 35.WHO - Global report on neglected tropical diseases. 2023. [accessed on Jun. 22, 2023]. https://www.who.int/teams/control-of-neglected-tropical-diseases/global-report-on-neglected-tropical-diseases-2023 .
- 36. Muhammad G, Khan MZ, Athar M, Saqib M. Dracunculus infection in a dog during the “post-eradication” period: the need for a longer period of surveillance. Ann Trop Med Parasitol. 2005;99:105–107. doi: 10.1179/136485905X19847. doi: 10.1179/136485905X19847. [DOI] [PubMed] [Google Scholar]
- 37. Hopkins DR. Progress Toward Global Eradication of Dracunculiasis - Worldwide, January 2021–June 2022. MMWR Morb Mortal Wkly Rep. 2022:71. doi: 10.15585/mmwr.mm7147a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodwin CED, Léchenne M, Wilson-Aggarwal JK, et al. Seasonal fishery facilitates a novel transmission pathway in an emerging animal reservoir of Guinea worm. Curr Biol. 2022;32:775–782. doi: 10.1016/j.cub.2021.11.050. e4. doi: 10.1016/j.cub.2021.11.050. [DOI] [PubMed] [Google Scholar]
- 39. The Lancet Infectious Diseases. Dracunculiasis - a case study for infection eradication. Lancet Infect Dis. 2019;19(11):1149. doi: 10.1016/S1473-3099(19)30488-8. doi: 10.1016/S1473-3099(19)30488-8. [DOI] [PubMed] [Google Scholar]
- 40.Carter Center - Guinea worm summit factsheet. [accessed on Jun. 22, 2023]. https://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/guinea-worm-summit-factsheet-eng.pdf .
- 41. Cleveland CA, Garrett KB, Box EK, et al. Investigating Flubendazole as an Anthelmintic Treatment for Guinea Worm (Dracunculus medinensis): Clinical Trials in Laboratory-Reared Ferrets and Domestic Dogs in Chad. Am J Trop Med Hyg. 2022;106:1456–1465. doi: 10.4269/ajtmh.21-1222. doi: 10.4269/ajtmh.21-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hopkins DR, Ruiz-Tiben E, Weiss AJ, Roy SL, Zingeser J, Guagliardo SAJ. Progress toward global eradication of Dracunculiasis - January 2017–June 2018. MMWR Morb Mortal Wkly Rep. 2018;67:1265–1270. doi: 10.15585/mmwr.mm6745a3. doi: 10.15585/ mmwr.mm6745a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarfraz MT, Rana MM. Designing a Multi-Epitope Vaccine against Dracunculus medinensis by employing immuno-informatics and in silico approaches. Preprints. 2021 doi: 10.20944/preprints202105.0400.v1. [DOI] [Google Scholar]
- 44. Russell CD. Eradicating infectious disease: can we and should we? Front Immunol. 2011;2:53. doi: 10.3389/fimmu.2011.00053. doi: 10.3389/fimmu.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thach PN, van Doorn HR, Bishop HS, et al. Human infection with an unknown species of Dracunculus in Vietnam. Int J Infect Dis. 2021;105:739–742. doi: 10.1016/j.ijid.2021.02.018. doi: 10.1016/j.ijid.2021.02.018. [DOI] [PubMed] [Google Scholar]