SUMMARY
Background
Listeriosis is a known cause of community acquired bacterial meningitis/meningoencephalitis. It occurs more frequently in neonates, older people and immunocompromised hosts. Rarely, brain abscesses can complicate the course of infection, which poses a difficulty in the management and elevates morbidity and mortality. Myasthenia gravis is an autoimmune disease that often requires immunosuppressive treatment, which increases the risk for invasive infections.
Case description
A 75-year-old myasthenic patient, treated with prednisone and pyridostigmine, was diagnosed with invasive listeriosis. He presented with muscle weakness, dyspnea, hemiparesis and altered mental status. Brain imaging revealed multiple abscesses and blood cultures were positive for Listeria monocytogenes. Despite combination antibiotic therapy, he died 6 weeks after admission.
Systematic review
Ninety-six cases of brain abscesses from 1968 to 2023 were reviewed; the majority of the patients were men, 54 years-old on average, and had at least one risk factor for invasive listeriosis. The mortality exceeded 22%. Blood cultures and CSF/abscess cultures were positive in only 79.2% and 54.6%, respectively. The most common therapeutic approach was a combination regimen that included amoxicillin or ampicillin. Only 8 patients underwent surgery, of which one died.
Conclusion
This case highlights the importance of L. monocytogenes as a cause of brain abscesses in populations at risk, including myasthenic patients. The challenge of diagnosing and treating this condition is aggravated by the paucity of literature and the high mortality rate.
Keywords: Listeriosis, Listeria monocytogenes, brain abscess, meningoencephalitis, myasthenia gravis
INTRODUCTION
Listeria monocytogenes is a facultative anaerobic Gram-positive rod that can cause foodborne invasive disease, mainly in immunocompromised patients, extremes of age and pregnant women [1, 2]. Invasive disease is classified in three forms: bacteremia, neurolisteriosis and maternal–neonatal infection. Reported mortality ranges from 30–45% among treated patients to 100% in untreated patients [2, 3]. Brain abscess is a rare manifestation of neurolisteriosis, with fewer than a hundred cases reported, and it is a risk factor for poor prognosis [4].
Myasthenia gravis (MG) is an autoimmune disease marked by muscle weakness, and treatment with immunosuppressive drugs increases the risk for infections [5]. Nonetheless, only four cases of listeriosis in patients with MG have been previously reported [6–9].
We present the case of a 75-year-old MG patient diagnosed with invasive listeriosis complicated with meningoencephalitis and brain abscesses. Also, a literature review was carried out in Cochrane Library, LILACS, SciELO, MEDLINE, PubMed, and PMC (PubMed Central) databases.
CASE PRESENTATION
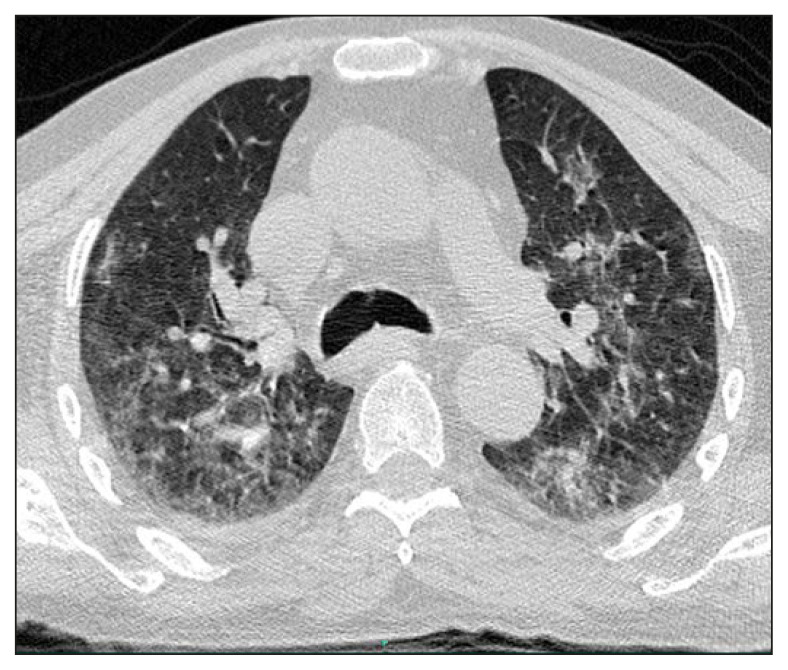
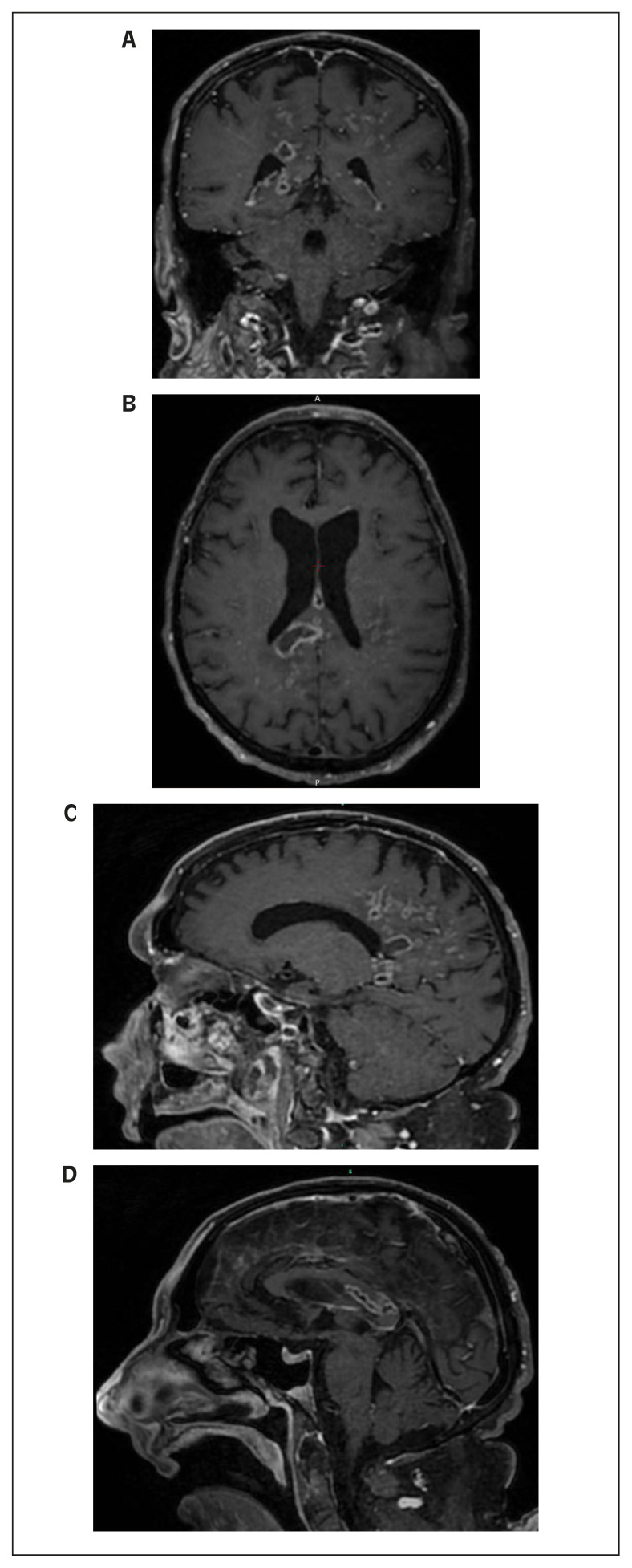
A 75-year-old male patient, with a previous history of MG, hypertension and regular ingestion of homemade artisanal cheese, was admitted to the hospital with a one-week history of worsening muscle weakness and dyspnea. He was taking 20 milligrams and 10 milligrams of prednisone on alternate days and pyridostigmine. On the second day of hospitalization, he developed altered mental status, right-sided hemiparesis and respiratory distress. A Computed Tomography (CT) scan of the brain was performed and the only abnormalities detected were global atrophy and microangiopathy. A chest CT showed bilateral ground-glass opacities with areas of consolidation and small bilateral pleural effusion (Figure 1). Blood cultures were drawn, piperacillin-tazobactam was initiated and orotracheal intubation was performed due to clinical deterioration. All four blood culture bottles (two aerobic and two anaerobic vials collected from two different sites) were positive in under an hour, and Listeria monocytogenes was identified by MALDI-TOF MS (Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry). Cerebrospinal fluid (CSF) analysis revealed lymphocytic meningitis (92 leukocytes/mm3, 87% lymphocytes), hypoglycorrhachia (19 mg/dL), hyperproteinorrhachia (176 mg/dL) and elevated lactate (4,52 mmol/L); direct examination and cultures were negative for bacteria, mycobacteria and fungi. Molecular testing for tuberculosis was also negative (RT-PCR, Xpert® MTB/RIF Ultra). Anti-infective therapy was changed to ampicillin plus trimethoprim-sulfamethoxazole (TMP-SMX). After ten days of combination antibiotic therapy, his mental status had not improved and Magnetic Resonance Imaging (MRI) of the brain revealed multiple ring-enhancing hypointense lesions in T1-weighted sequences in parietal lobes and corpus callosum (Figures 2A-D), compatible with brain abscesses. The neurosurgery team was consulted and surgical drainage was not indicated due to the deep, unfavorable location of the lesions in the brain. During treatment, he developed a maculopapular rash, with peripheral eosinophilia, aminotransferases and creatine kinase (CK) elevation and acute kidney injury. A skin biopsy was compatible with drug-induced exanthem. TMPSMX was discontinued due to drug-related toxicity concerns. Gentamicin was administered for five days and meropenem was chosen as the second drug of the regimen afterwards. Ampicillin was maintained as the primary drug at all times. He underwent multiple complications, including ventilator-associated pneumonia. Another brain MRI was performed after 5 weeks of medical therapy, with minimal improvement. Unfortunately, the patient died 6 weeks after admission.
Figure 1.
Chest CT showing bilateral ground-glass opacities with areas of consolidation and small bilateral pleural effusion.
Figure 2.
Brain MRI showing multiple ring-enhancing hypointense lesions in T1-weighted sequences in parietal lobes and corpus callosum.
DISCUSSION
As mentioned above, invasive listeriosis is a severe infection that predominantly affects older, immunocompromised patients, with very high mortality rates. Untreated, it is virtually always lethal [2, 3]. International guidelines recommend empiric coverage of L. monocytogenes with an aminopenicillin in the treatment of acute bacterial meningitis in populations at risk, including neonates, adults older than 50 years of age and immunocompromised patients [10–13]. In these groups, L. monocytogenes accounts for more than 20% of the cases of bacterial meningitis, while in the general population it is responsible for 4–8% of the cases [11–13]. Among immunocompromised patients, the ones with cellular immunity impairment, such as those receiving corticosteroid therapy, are particularly at risk, since immunity to L. monocytogenes is mediated by macrophages activated by T-cell lymphokines [13].
Clinically, patients with central nervous system (CNS) involvement usually have fever and altered mental status, but focal neurologic deficits, seizures and other neurologic signs can be present. CNS disease has been most frequently described as meningitis, encephalitis or, less commonly, rhombencephalitis, with brainstem and cerebellum involvement. Listerial brain abscesses are very rare, as they account for only 2% of neurolisteriosis cases in large observational studies [2, 14]. The optimal antibiotic regimen and duration for invasive listeriosis is unknown, as there are no high-quality large clinical trials available. The evidence guiding its management comes mainly from observational cohorts, in vitro studies and case reports. Drugs of choice include the aminopenicillins (amoxicillin and ampicillin) and penicillin G. TMP-SMX has been frequently used as an alternative or as adjuvant, and gentamicin traditionally as part of a combination regimen. Other alternatives described in literature and supported by the 2016 ESCMID guidelines are meropenem, linezolid and quinolones [11, 15]. The duration of therapy is usually ≥3 weeks, as shorter courses have been reported to have a higher relapse incidence [10, 11, 14]. In a nationwide prospective study (the MONALISA study), amoxicillin, gentamicin and TMPSMX were independently associated with three-month mortality reduction, as well as combination therapy of amoxicillin plus gentamicin for ≥ 3 days [2]. The literature on the management of brain abscesses is even more scarce, limited to case reports. Longer courses of antibiotic therapy (≥5–6 weeks of combination therapy) seem reasonable, based on available data. Adjuvant surgical drainage has been described with variable success [4, 14].
The diagnosis of invasive listeriosis can be made by blood cultures in bacteremic patients. Neurolisteriosis is diagnosed with a positive CSF culture, or positive blood cultures in patients with a compatible neurologic syndrome [2]. In patients with meningitis/meningoencephalitis, CSF culture is positive in approximately 84% of the cases, while blood cultures are positive in approximately 71% [2, 14]. Patients presenting with brain abscesses or rhombencephalitis can have a negative CSF culture in up to 59% of the cases [3, 4]. Accordingly, in an attempt to increase the accuracy of microbiological diagnosis, the use of culture-independent technologies has been increasing, such as multiplex PCR panel for meningitis/encephalitis, and real-time PCR assay for L. monocytogenes [16, 17]. In the case of our patient, microbiological diagnosis was made based on blood cultures, as tracheal aspirate cultures and CSF cultures were negative for L. monocytogenes. The pulmonary findings could be compatible with listerial pneumonia, which has been reported to cause ground-glass opacities, but pulmonary edema could also explain his chest CT abnormalities [18]. However, the CNS findings of meningoencephalitis and brain abscesses were unequivocal. Initially, he received piperacillin-tazobactam empirically, which has in vitro activity and has been reported as a treatment option for listeriosis [19]. The choice of the definitive antibiotic regimen in this case was ampicillin plus TMPSMX based on available literature suggesting the benefit of combination therapy, favorable pharmacokinetics in the treatment of CNS disease and concerns about the effect of the aminoglycoside on myasthenia gravis [2, 20, 21]. The same rationale was used to change the second drug to meropenem afterwards. This particular combination (ampicillin plus meropenem) has also been previously used successfully to treat listerial brain abscesses [4]. Unfortunately, like the myasthenic patient from Bossoni et al., 2015, and many others with invasive listeriosis, our patient had a poor clinical outcome, despite adequate medical treatment according to available literature [8].
In total, 96 cases of listerial brain abscesses from 1968 to 2023 have been reported (Table 1) [22–28]. Of these, 63.5% were male, and the average age was 54.3 years-old; the majority had risk factors for invasive listeriosis (79%). The mortality rate was 22.9%. Blood cultures were positive in only 57/72 (79.2%), and CSF or brain abscess cultures in 41/75 (54.6%). Combination therapy has been the most reported anti-infective strategy, with the aminopenicillins being the most prescribed drugs (in 68 cases). Other drugs commonly used were TMP-SMX, gentamicin, meropenem, linezolid and quinolones. Surgical therapy was performed in 8 patients, of which 1 died.
Table 1.
Summary of reported cases of Listeria monocytogenes brain abscesses (1968–2023).
| High-risk patients ¶ | Mortality rate | Positive blood cultures | Positive CSF§ or abscess cultures | Monotherapy | Combination therapy | Adjuvant surgical therapy |
|---|---|---|---|---|---|---|
| 76/96 (79%) | 22/96 (22.9%) | 57/72 (79.2%) | 41/75 (54.6%) | 28/89 (31.5%) | 61/89 (68.5%) | 8/91* (8.8%) |
At least one risk factor present, such as extremes of age and impaired immunity.
Cerebrospinal fluid.
One patient who underwent surgery died.
In conclusion, this case and literature review highlight the importance of L. monocytogenes as a highly lethal foodborne etiologic agent of brain abscesses, meningoencephalitis and disseminated disease in populations at risk, such as older people and immunocompromised patients, who should be advised to avoid eating raw food and unpasteurized dairy products [29]. The absence of high-quality clinical studies makes the management of listerial brain abscesses a tremendous challenge.
Footnotes
Consent for publication: Written consent for publication was obtained with the patient’s family prior to data collection.
Conflicts of interest: None.
Funding: No funding has been received for the present study.
REFERENCES
- 1. Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337(14):970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 2. Charlier C, Perrodeau É, Leclercq A, et al. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis. 2017;17(5):510–519. doi: 10.1016/S1473-3099(16)30521-7. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis. 1993;16(5):689–702. doi: 10.1093/clind/16.5.689. [DOI] [PubMed] [Google Scholar]
- 4. Zhang J, Huang S, Xu L, Tao M, Zhao Y, Liang Z. Brain abscess due to listeria monocytogenes: A case report and literature review. Medicine (Baltimore) 2021;100(31):e26839. doi: 10.1097/MD.0000000000026839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilhus NE, Romi F, Hong Y, Skeie GO. Myasthenia gravis and infectious disease. J Neurol. 2018;265(6):1251–1258. doi: 10.1007/s00415-018-8751-9. [DOI] [PubMed] [Google Scholar]
- 6. Kern RZ, Stewart JD. Listeria meningitis complicating treatment of myasthenia gravis with azathioprine and steroids. Neurology. 1986;36(7):1011–1012. doi: 10.1212/wnl.36.7.1011-b. [DOI] [PubMed] [Google Scholar]
- 7. Rodríguez D, Cid-Rodríguez MC, Núñez-Alvarez ML, González-Vázquez E, Lustres-Pérez M. Meningitis caused by Listeria monocytogenes in a patient with myasthenia gravis being treated with tacrolimus. Rev Neurol. 2005;40(6):383. [PubMed] [Google Scholar]
- 8. Bossoni AS, Freua F, Senaha SE, et al. Rhombencephalitis related to listeria infection in a myasthenic patient. J Neurol Scis. 2015;357:e432–e456. [Google Scholar]
- 9. Gertz K, Siebert E, Halle E, et al. Multiple supratentorial brain abscesses due to Listeria monocytogenes in a patient with myasthenia gravis. Clin Neurol Neurosurg. 2013;115(9):1923–1924. doi: 10.1016/j.clineuro.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 10. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 11. van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22(Suppl 3):S37–S62. doi: 10.1016/j.cmi.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 12. Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997 Oct 2;337(14):970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 13. Pagliano P, Ascione T, Boccia G, et al. Listeria monocytogenes meningitis in the elderly: epidemiological, clinical and therapeutic findings. Infez Med. 2016;24(2):105–111. [PubMed] [Google Scholar]
- 14. Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998;77(5):313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 15. Pagliano P, Arslan F, Ascione T. Epidemiology and treatment of the commonest form of listeriosis: meningitis and bacteraemia. Infez Med. 2017;25(3):210–216. [PubMed] [Google Scholar]
- 16. Le Monnier A, Abachin E, Beretti JL, et al. Diagnosis of Listeria monocytogenes meningoencephalitis by real-time PCR for the hly gene. J Clin Microbiol. 2011;49(11):3917–3923. doi: 10.1128/JCM.01072-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leber AL, Everhart K, Balada-Llasat JM, et al. Multicenter Evaluation of BioFire FilmArray Meningitis/ Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J Clin Microbiol. 2016;54(9):2251–2261. doi: 10.1128/JCM.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koufakis T, Chatzopoulou M, Margaritis A, Tsiakalou M, Gabranis I. Pneumonia by Listeria monocytogenes: A common infection by an uncommon pathogen. Case Rep Infect Dis. 2015;2015:627073. doi: 10.1155/2015/627073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thønnings S, Knudsen JD, Schønheyder HC, et al. Danish Collaborative Bacteraemia Network (DACOBAN). Antibiotic treatment and mortality in patients with Listeria monocytogenes meningitis or bacteraemia. Clin Microbiol Infect. 2016;22(8):725–730. doi: 10.1016/j.cmi.2016.06.006. doi: 10.1016/j.cmi.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 20. Nau R, Seele J, Djukic M, Eiffert H. Pharmacokinetics and pharmacodynamics of antibiotics in central nervous system infections. Curr Opin Infect Dis. 2018;31(1):57–68. doi: 10.1097/QCO.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 21. Sheikh S, Alvi U, Soliven B, Rezania K. Drugs That Induce or Cause Deterioration of Myasthenia Gravis: An Update. J Clin Med. 2021;10(7):1537. doi: 10.3390/jcm10071537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang C, Yi Z. Brain abscess caused by Listeria monocytogenes: a case report and literature review. Ann Palliat Med. 2022;11(10):3356–3360. doi: 10.21037/apm-22-383. doi: 10.21037/apm-22-383. [DOI] [PubMed] [Google Scholar]
- 23. Xu X, Shan Y, Cen Y, et al. Clinical characteristics and treatment of Listeria monocytogenes infections in the Central Nervous System. Infect Drug Resist. 2023;16:5899–5909. doi: 10.2147/IDR.S424012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steinbrecher M, Wolfert C, Maurer C, et al. Cerebral abscess due to Listeria monocytogenes infection in silent diabetes mellitus: Case presentation, treatment and patient outcome. IDCases. 2023;33:e01864. doi: 10.1016/j.idcr.2023.e01864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong V, et al. A Red Herring Case of Listeria Meningitis in an Immunocompetent Individual With COVID-19. Am J Respir Crit Care Med. 2023;207:A5278. [Google Scholar]
- 26. Zhang N, Sun W, Zhou L, Chen M, Dong X, Wei W. Multiple brain abscesses due to Listeria monocytogenes infection in a patient with systemic lupus erythematosus: A case report and literature review. Int J Rheum Dis. 2021;24(11):1427–1439. doi: 10.1111/1756-185X.14226. [DOI] [PubMed] [Google Scholar]
- 27. Cipriani D, Trippel M, Buttler KJ, Rohr E, Wagner D, Beck J, Schnell O. Cerebral Abscess Caused by Listeria monocytogenes: Case Report and Literature Review. J Neurol Surg A Cent Eur Neurosurg. 2022;83(2):194–205. doi: 10.1055/s-0041-1729174. [DOI] [PubMed] [Google Scholar]
- 28. Ogunleye OO, Karimi V, Gujadhur N. Listeria bacteremia presenting with cerebral abscess and endocarditis in an elderly patient with chronic immune thrombocytopenia. Cureus. 2021;13(7):e16601. doi: 10.7759/cureus.16601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC Centers for Control Disease Prevention, Division of Foodborne, Waterborne, and Environmental Diseases (DFWED) [Accessed 17 July 2023]. Last Reviewed: April 7, 2023. Available at: https://www.cdc.gov/listeria/prevention.html.