Abstract
The structural genes encoding the two essential components A and B of hexaprenyl diphosphate synthase, which produce the precursor of the prenyl side chain of menaquinone-6, were cloned from Micrococcus luteus B-P 26.
Hexaprenyl diphosphate synthase (EC 2.5.1.33) (HexPS) catalyzes condensation of three molecules of isopentenyl diphosphate with farnesyl diphosphate (FPP) to afford (all-E)-hexaprenyl diphosphate (HexPP; C30), the precursor of the prenyl side chain of menaquinone-6. HexPS of Micrococcus luteus B-P 26 (2) and the heptaprenyl diphosphate (C35) synthase (EC 2.5.1.30) (HepPS) of Bacillus subtilis (16) are unique because they each consist of two dissociable components; the former are designated A and B (2), and the latter are designated I and II (4). The two components have no prenyltransferase activity unless they are combined (4). These two-component systems distinguish the medium chain (all-E)-prenyl diphosphate synthases from the other prenyltransferases which have homodimeric structures and catalyze the synthesis of shorter- or longer-chain prenyl diphosphates such as farnesyl, octaprenyl, and undecaprenyl diphosphates (13, 14).
The genes for the HepPS of Bacillus stearothermophilus consist of two cistrons encoding different components with molecular masses of 25 kDa (component I′) and 36 kDa (component II′), respectively (6). Zhang et al. (19) have recently identified two of the proteins (GerC1 and GerC3) encoded by the gerC locus of B. subtilis (12) as the dissociable heteromeric components I and II of the HepPS of the bacterium (4).
In order to study the significance and mechanism of the dissociable two-component systems of medium-chain prenyl diphosphate synthases, we cloned the HexPS genes of M. luteus B-P 26 and compared the deduced amino acid sequences to the corresponding subunits of heptaprenyl diphosphate synthases of the Bacillus species.
To amplify DNA fragments that might have typical motifs for prenyltransferases, we synthesized seven degenerate oligonucleotide primers designed on the basis of conserved amino acid regions of prenyltransferases (8, 9). An amplified product of approximately 500 bp (designated B500) was obtained by PCR with the pair P1 and N3 as primers [P1, 5′-GG(A,T,C)GG(A,T,C)AA(A,G)CGTA(A,T)TCGTCCTTTA-3′; N3, 5′-A TCTAAAATATCATC(C,T)TG(A,T)AT(C,T)TG(A,G)AA- 3′] with the genomic DNA template of M. luteus B-P 26. The amino acid sequence deduced from the nucleotide sequence of B500 contained the typical prenyltransferase motif DDXXD (1, 8) and showed 61% identity with the corresponding region of B. stearothermophilus FPP synthase (FPS) (8). This 500-bp PCR fragment was used for screening the prenyltransferase gene(s) from an M. luteus B-P 26 genomic library prepared in Escherichia coli JM109 harboring plasmids of pUC119 with inserts of 4- to 8-kb DNA fragments. Among 6,000 individual colonies, a single positive clone was found. This clone was further purified, and the plasmid, designated pFP00, was shown to carry a 10-kb DNA insert from M. luteus B-P 26. Product analysis of a prenyltransferase with increased activity in the cell-free homogenate of the clone indicated that pFP00 contained the FPS gene (fps) of M. luteus B-P 26 (Fig. 1A).
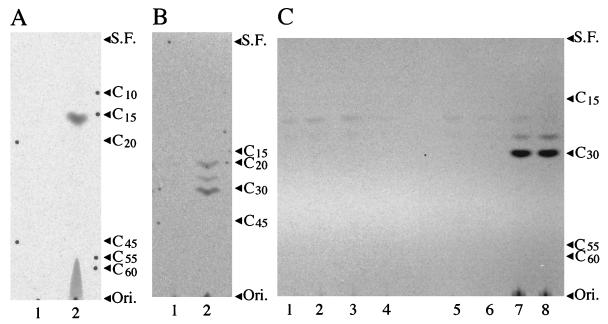
FIG. 1.
Autoradiograms of TLC of the prenyl alcohols obtained by enzymatic hydrolysis (3) of the products formed by the incubations of [1-14C]isopentenyl diphosphate and dimethylallyl diphosphate or FPP with the cell-free homogenates of E. coli transformants. (A) Incubations with dimethylallyl diphosphate. Products were derived from E. coli JM 109/pUC119 (control) (lane 1) and E. coli JM 109/pFP00 (lane 2). (B) Incubations with FPP. Products were derived from E. coli JM 109/pUC119 (control) (lane 1) and E. coli JM 109/pHX00 (lane 2). (C) Incubations with FPP. Products were derived from E. coli JM 109/pUC119 (control) (lane 1), E. coli JM 109/pREG1 (lane 2), E. coli JM 109/pREG2 (lane 3), E. coli JM 109/pREG3S (lane 4), the mixture of E. coli JM 109/pREG1 and E. coli JM 109/pREG2 (lane 5), the mixture of E. coli JM 109/pREG2 and E. coli JM 109/pREG3S (lane 6), the mixture of E. coli JM 109/pREG1 and E. coli JM 109/pREG3S (lane 7), and E. coli JM 109/pHX06 (lane 8). Each extract was analyzed by reversed phase TLC (type LKC-18; Whatman) with solvent systems of acetone-water (9:1 [B] or 19:1 [A and C]). Arrowheads indicate the positions of authentic prenyl alcohols as follows: C10, geraniol; C15, (all-E)-farnesol; C20, (all-E)-geranylgeraniol; C30, (all-E)-hexaprenol; C45, (all-E)-nonaprenol (solanesol); C55, beturaprenol-55, C60, beturaprenol-60; Ori., origin; S.F., solvent front.
Southern blot analysis with the radiolabeled B500 fragment, which was revealed to be a partial fragment of fps, gave a strong band at 7.5 kb and a faint band at 4.2 kb in the lane with EcoRI-digested genomic DNA (data not shown). We assigned the former, strong band to the FPS gene and the latter to the other prenyltransferase gene (2, 10) having some similarity. A subgenomic library of M. luteus B-P 26 was prepared from 4 to 6 kb of EcoRI-digested DNA, which contains the fragment weakly hybridizable with B500 and excludes the fps gene. Colony hybridization with B500 yielded 3 positive colonies carrying the same 5.7-kb DNA fragment among 1,200 colonies. The PCR primers were also used as probes to find coinciding positive signals. Cell-free homogenates of the clones showed evidently higher prenyl diphosphate synthase activities than endogenous prenyl diphosphate synthase activities of the host E. coli cells, and analysis of the reaction products by thin-layer chromatography (TLC) indicated that the clone produced HexPP along with some shorter-chain intermediate prenyl diphosphates (Fig. 1B). Then the clone, designated pHX00, was subjected to a deletion experiment and sequence determination to obtain a clone carrying pHX06 with a 2.4-kb DNA insert (Fig. 2) expressing HexPS activity.
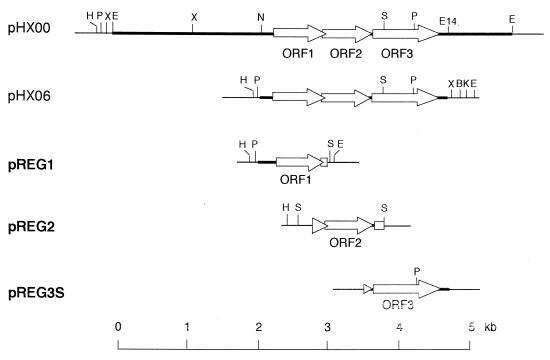
FIG. 2.
Schematic diagram of plasmids prepared. Only the inserted DNA regions in each plasmid are illustrated. Thick lines in each plasmid indicate the chromosomal DNA from M. luteus B-P 26, and thin lines indicate parts of the vector, pUC119. Open arrows show the three open reading frames found in the DNA region responsible for expression of HexPS. Abbreviations: H, HindIII; P, PstI; Hi, HincII; X, XbaI; B, BamHI; K, KpnI; S, SacI; E, EcoRI; E14, EcoT14I; N, NruI.
Analysis of the pHX06 nucleotide sequence showed three consecutive open reading frames, tentatively designated ORF1, ORF2, and ORF3. In order to determine the structural genes corresponding to HexPS, which had been shown to consist of two dissociable components (2), we prepared three plasmids, pREG1, pREG2, and pREG3S, having one of the three clones (Fig. 2), and examined the enzymatic activity of their protein products expressed in E. coli cells. Although none of the cell-free homogenates of the three clones showed any prenyltransferase activity alone (Fig. 1C), a significant level of prenyltransferase activity was observed when the homogenates of the pREG1 and pREG3S transformants were mixed together. As shown in Fig. 1C, the mixture of the homogenates of the pREG1 and pREG3S transformants gave a major spot of C30-polyprenol along with some amounts of shorter-chain prenols derived from the corresponding intermediate prenyl diphosphates. These results indicate that ORF1 and ORF3 encode the two essential components of the HexPP synthase. We named the genes hexs-a and hexs-b, respectively.
Comparison of the deduced amino acid sequences of the two components encoded by hexs-a and hexs-b with those for the other medium-chain prenyl diphosphate synthases indicated that the Hexs-b protein (component B) shows 38, 41, and 31% identity to component II′ (Heps-2) of HepPS of B. stearothermophilus (10), component II (GerC) of HepPS of B. subtilis (19), and HexPS of S. cerevisiae (1), respectively. On the other hand, Hexs-a (component A) has only 14 of 143 amino acid residues identical to components I and I′ of the HepPSs from the two Bacillus species (10% identity, as indicated by asterisks in Fig. 3), while there is 31% identity (69 of 220 residues, as indicated by shaded boxes in Fig. 3) between the latter two components. Moreover, component A of HexPS (Hexs-a) is shorter by about 100-amino-acid residues at the C-terminal side than the corresponding component I or I′ of HepPS. The three conserved regions in the structure of component A, as indicated by asterisks in Fig. 3, may participate in the dynamic interaction, as observed biochemically, between the two dissociable components forming a catalytically active complex in the presence of substrates and Mg2+ (17, 18).
FIG. 3.
Comparison of the deduced amino acid sequences of component A of HexPS from M. luteus B-P 26 (M. lut Hexs-a) with the HepPS equivalents from B. stearothermophilus and B. subtilis. B. sub Heps-1, component I of B. subtilis HepPS; B. st Heps-1, component I′ of B. stearothermophilus HepPS.
The protein encoded by hexs-b contains seven conserved regions, including the two aspartate-rich motifs DDXXD, which have been proved in several site-directed mutagenesis studies of FPSs to be essential for their catalytic function (5, 7, 11, 15). Thus, this component is reasonably considered to carry substantial sites for substrate binding and catalysis, while the other component seems to play an auxiliary but essential role for expression of catalytic function as medium-chain prenyl diphosphate synthase.
The primary structure of the protein encoded by ORF2 in this work shows a relatively high identity (60%) to that of the protein encoded by the gene that is located between heps-1 and the heps-2 of B. stearothermophilus (6). Koike-Takeshita et al. (7) have recently identified the gene product of B. stearothermophilus as MenG, 2-heptaprenyl-1,4-naphthoquinone methyltransferase, and proposed the presence of a novel gene cluster participating in menaquinone-7 biosynthesis. Thus, the gene located between hexs-a and hexs-b is likely to encode the equivalent enzyme, 2-hexaprenyl-1,4-naphthoquinone methyltransferase, of M. luteus B-P 26, thereby forming a similar gene cluster participating in menaquinone-6 biosynthesis in this bacterium.
Ashby and Edwards (1) have isolated the HexPS gene of Saccharomyces cerevisiae, which is able to complement a yeast mutant in coenzyme Q biosynthesis. This gene encodes a 473-amino-acid protein having highly conserved domains characteristic of prenyl diphosphate synthases. However, it is not known whether the yeast protein acts as HexPS by itself or in association with another gene product corresponding to component A of HexPS of M. luteus B-P 26. We have not found any proteins that have conserved sequences in Hexs-a among the open reading frames of the S. cerevisiae whole-genome database. It would be interesting to investigate whether the eukaryotic HexPS has a two-component system similar to that of the prokaryotic HexPS described in this study.
Nucleotide sequence accession numbers.
The nucleotide sequences reported appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB003188 for the fps gene and AB003187 for the DNA containing hexs-a and hexs-b.
Acknowledgments
This work was supported by grants-in-aid for scientific research no. 06240102 and 07680620 from the Ministry of Education, Science, and Culture, Japan.
REFERENCES
- 1.Ashby M N, Edwards P A. Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J Biol Chem. 1990;265:13157–13164. [PubMed] [Google Scholar]
- 2.Fujii H, Koyama T, Ogura K. Hexaprenyl pyrophosphate synthetase from Micrococcus luteus B-P 26. J Biol Chem. 1982;257:14610–14612. [PubMed] [Google Scholar]
- 3.Fujii H, Koyama T, Ogura K. Efficient enzymatic hydrolysis of polyprenyl pyrophosphates. Biochim Biophys Acta. 1982;712:716–718. [PubMed] [Google Scholar]
- 4.Fujii H, Koyama T, Ogura K. Essential protein factors for polyprenyl pyrophosphate synthetases. FEBS Lett. 1983;161:257–260. doi: 10.1016/0014-5793(83)81020-5. [DOI] [PubMed] [Google Scholar]
- 5.Joly A, Edwards P A. Effect of site-directed mutagenesis of conserved aspartate and arginine residues upon farnesyl diphosphate synthase activity. J Biol Chem. 1993;268:26983–26989. [PubMed] [Google Scholar]
- 6.Koike-Takeshita A, Koyama T, Obata S, Ogura K. Molecular cloning and nucleotide sequences of the genes for two essential proteins constituting a novel enzyme system for heptaprenyl diphosphate synthesis. J Biol Chem. 1995;270:18396–18400. doi: 10.1074/jbc.270.31.18396. [DOI] [PubMed] [Google Scholar]
- 7.Koike-Takeshita A, Koyama T, Ogura K. Identification of a novel gene cluster participating in manaquinone (vitamin K2) biosynthesis. Cloning and sequence determination of the 2-heptaprenyl-1,4-naphthoquinone methyltransferase gene of Bacillus stearothermophilus. J Biol Chem. 1997;272:12380–12383. doi: 10.1074/jbc.272.19.12380. [DOI] [PubMed] [Google Scholar]
- 8.Koyama T, Obata S, Osabe M, Takeshita A, Yokoyama K, Uchida M, Nishino T, Ogura K. Thermostable farnesyl diphosphate synthase of Bacillus stearothermophilus: molecular cloning, sequence determination, overproduction, and purification. J Biochem (Tokyo) 1993;113:355–363. doi: 10.1093/oxfordjournals.jbchem.a124051. [DOI] [PubMed] [Google Scholar]
- 9.Koyama T, Tajima M, Sano H, Doi T, Koike-Takeshita A, Obata S, Nishino T, Ogura K. Identification of significant residues in the substrate binding site of Bacillus stearothermophilus farnesyl diphosphate synthase. Biochemistry. 1996;35:9533–9538. doi: 10.1021/bi960137v. [DOI] [PubMed] [Google Scholar]
- 10.Koyama T, Yoshida I, Ogura K. Undecaprenyl diphosphate synthase from Micrococcus luteus B-P 26: essential factors for the enzymatic activity. J Biochem (Tokyo) 1988;103:867–871. doi: 10.1093/oxfordjournals.jbchem.a122363. [DOI] [PubMed] [Google Scholar]
- 11.Marrero P F, Poulter C D, Edwards P A. Effects of site-directed mutagenesis of the highly conserved aspartate residues in domain II of farnesyl diphosphate synthase activity. J Biol Chem. 1992;267:21873–21878. [PubMed] [Google Scholar]
- 12.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 13.Ogura K, Koyama T. Mechanistic enzymology and molecular genetics of chain elongation in isoprenoid biosynthesis. In: Ogura K, Sankawa U, editors. Dynamic aspects of natural products chemistry. Tokyo, Japan: Kodansha Scientific; 1997. pp. 1–23. [Google Scholar]
- 14.Ohnuma S-I, Koyama T, Ogura K. Purification of solanesyl-diphosphate synthase from Micrococcus luteus. A new class of prenyltransferase. J Biol Chem. 1991;266:23706–23713. [PubMed] [Google Scholar]
- 15.Song L, Poulter C D. Yeast farnesyl-diphosphate synthase: site-directed mutagenesis of residues in highly conserved prenyltransferase domains I and II. Proc Natl Acad Sci USA. 1994;91:3044–3048. doi: 10.1073/pnas.91.8.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi I, Ogura K, Seto S. Heptaprenyl pyrophosphate synthetase from Bacillus subtilis. J Biol Chem. 1979;255:4539–4543. [PubMed] [Google Scholar]
- 17.Yoshida I, Koyama T, Ogura K. Dynamic interaction between components of hexaprenyl diphosphate synthase from Micrococcus luteus B-P 26. Biochemistry. 1987;26:6840–6845. doi: 10.1021/bi00395a038. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida I, Koyama T, Ogura K. Formation of a stable and catalytically active complex of the two essential components of hexaprenyl diphosphate synthase from Micrococcus luteus B-P 26. Biochem Biophys Res Commun. 1989;160:448–452. doi: 10.1016/0006-291x(89)92453-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y-W, Koyama T, Ogura K. Two cistrons of the gerC operon of Bacillus subtilis encode the two subunits of heptaprenyl diphosphate synthase. J Bacteriol. 1997;179:1417–1419. doi: 10.1128/jb.179.4.1417-1419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]